
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Astron. Space Sci., 23 July 2021
Sec. Astrobiology
Volume 8 - 2021 | https://doi.org/10.3389/fspas.2021.700579
This article is part of the Research TopicBioregenerative Life-Support Systems for Crewed Missions to the Moon and MarsView all 26 articles
A base on the Moon surface or a mission to Mars are potential destinations for human spaceflight, according to current space agencies’ plans. These scenarios pose several new challenges, since the environmental and operational conditions of the mission will strongly differ than those on the International Space Station (ISS). One critical parameter will be the increased mission duration and further distance from Earth, requiring a Life Support System (LSS) as independent as possible from Earth’s resources. Current LSS physico-chemical technologies at the ISS can recycle 90% of water and regain 42% of O2 from the astronaut’s exhaled CO2, but they are not able to produce food, which can currently only be achieved using biology. A future LSS will most likely include some of these technologies currently in use, but will also need to include biological components. A potential biological candidate are microalgae, which compared to higher plants, offer a higher harvest index, higher biomass productivity and require less water. Several algal species have already been investigated for space applications in the last decades, being Chlorella vulgaris a promising and widely researched species. C. vulgaris is a spherical single cell organism, with a mean diameter of 6 µm. It can grow in a wide range of pH and temperature levels and CO2 concentrations and it shows a high resistance to cross contamination and to mechanical shear stress, making it an ideal organism for long-term LSS. In order to continuously and efficiently produce the oxygen and food required for the LSS, the microalgae need to grow in a well-controlled and stable environment. Therefore, besides the biological aspects, the design of the cultivation system, the Photobioreactor (PBR), is also crucial. Even if research both on C. vulgaris and in general about PBRs has been carried out for decades, several challenges both in the biological and technological aspects need to be solved, before a PBR can be used as part of the LSS in a Moon base. Those include: radiation effects on algae, operation under partial gravity, selection of the required hardware for cultivation and food processing, system automation and long-term performance and stability.
The International Space Station (ISS) has been continuously inhabited for over twenty years. The Life Support System (LSS) on board the station is in charge of providing the astronauts with oxygen, water and food. For that, Physico-Chemical (PC) technologies are used, recycling 90% of the water and recovering 42% of the oxygen (O2) from the carbon dioxide (CO2) that astronauts produce (Crusan and Gatens, 2017), while food is supplied from Earth.
Space agencies currently plan missions beyond Low Earth Orbit, with a Moon base or a mission to Mars as potential future scenarios (ESA Blog 2016; ISEGC 2018; NASA 2020). The higher distance from Earth of a lunar base, compared to the ISS, might require the production of food in-situ, to reduce the amount of resources required from Earth. PC technologies are not able to produce food, which can only be achieved using biological organisms. Several candidates are currently being investigated, with a main focus on higher plants (Kittang et al., 2014; Hamilton et al., 2020) and microalgae (Detrell et al., 2020b; Poughon et al., 2020).
Microalgae, like higher plants, produce O2 and edible biomass through photosynthesis by consuming CO2, nutrients and water. Compared to higher plants, microalgae provide a higher harvest index, biomass productivity and light exploitation, and consume less water (Degen 2003; Schmid-Staiger et al., 2009). However, algae cannot be used as our unique nutrition source, due to their high protein content. To ensure a balanced diet, algae can only substitute part of the human daily consumption, thus they can complement either higher plant technologies or food supplied from Earth. The amount of microalgae recommended will depend on the species and how they have been cultivated, with some estimations achieving a recommended maximum of 35% (Belz et al., 2014).
The selection of the algal species will play an important role in the design of the technology required and the performance of the system. Two microalgae are widely used on Earth as food supplement or biofuel source among others: Chlorella vulgaris and Limnospira indica (Spirulina). Both species have been widely studied for space applications. Chlorella is a spherical unicellular eukaryotic green algae (Figure 1), while Spirulina is a filamentous multicellular prokaryotic cyanobacteria (also called blue-green algae). The main advantages of Chlorella vs. Spirulina are its simple shape and its adaptability to a wide range of cultivation conditions, making it very robust. However, Chlorella’s thick cell wall does not allow the human body to assimilate the nutrients inside the cell, requiring a cell wall breakdown process before human consumption (Mason 2001), which is not required with Spirulina. Both candidates present advantages and disadvantages, and both have the potential to be used for space applications. This paper focuses on the use of Chlorella vulgaris, based on the experience at the Institute of Space Systems (IRS) at the University of Stuttgart, Germany. Chlorella has a mean diameter of 6 μm (Yamamoto et al., 2004). It can grow in a wide range of pH and temperature levels (Ackerman 2007) and CO2 concentrations (Powell et al., 2009). It also shows a high resistance to cross contamination (Lakaniemi Aino-Maija et al., 2012), making it a perfect candidate for long-duration cultivation.
The use of microalgae for space applications requires the design of the equipment for a controlled cultivation, a Photobioreactor (PBR). Besides the physical containment provided by the reactor chamber itself, the system will require other subsystems like lighting, nutrient supply, gas exchange, thermal control, growth medium control, harvesting and processing (Storhas 2000). The PBR will need to provide the required environment for the microalgae to perform as required, considering the constrains and requirements of a space missions, for example power consumption limitations. The size of the required PBR will depend on several parameters: the algal species, the cultivation parameters and the intended amount of oxygen or biomass production rate per day.
This paper first looks at the current state-of-the-art, with focus on research of microalgae for space applications, looking as well to the currently used PC technologies for air management, in Chapter 2. A trade-off analysis of a PC system and a hybrid system (including PC technologies and a microalgae PBR) is evaluated in Chapter 3, justifying the potential of a PBR for a Moon base. Chapter 4 identifies the challenges and open questions that still need to be solved, before a PBR can become a reality in a lunar base.
Recycling LSS technologies have already been used in space in several missions, for water recycling, CO2 removal and O2 production, among other tasks. Several experiments, looking at food production have already taken place in space, but such a technology is still not available as part of the LSS. This chapter focuses on the current state-of-the art of PC recycling technologies for air management onboard the ISS and the research carried out on microalgae for space applications.
The main tasks considered within the air management system for this paper are the CO2 removal, CO2 reduction and O2 production, since those tasks can also be fulfilled using microalgae. Several technologies onboard the ISS and short-term research plans are available from different space agencies, mainly NASA, ESA, JAXA, and Roscosmos.
The main NASA technologies for air management are the Carbon Dioxide Removal Assembly (CDRA), the Sabatier Reactor Assembly (SRA) and the Oxygen Generation Assembly (OGA). CDRA is a regenerative CO2 absorption system based on zeolite sorbent beds. Currently, further technologies are being investigated to substitute CDRA, for example using amine sorbents, which should be tested in the coming years onboard the ISS. The SRA processes the CO2, consuming hydrogen and producing water and methane. It was operating on the ISS until end of 2017, and NASA currently plans an upgrade and return to the ISS. The OGA produces oxygen by water electrolysis. The system is currently in use onboard the ISS and NASA is planning an upgrade based on operational experience. (Shaw et al., 2020).
The Russian technologies onboard the ISS include Vozdukh for CO2 extraction, also a zeolite sorbent system, and Elektron for oxygen production through electrolysis. JAXA future plans include a low temperature Sabatier catalyst, and an amine-based CO2 removal. (Anderson et al., 2016).
The Life Support Rack (LSR), also known as Advanced Closed-Loop System (ACLS), is an European technology, launched to the ISS in September 2018. The LSR is composed by the Carbon Dioxide Concentration Subsystem (CCA), an amine-based CO2 extraction system, the Carbon Dioxide Reprocessing Subsystem (CRA), a Sabatier reactor, and the Oxygen Generation Subsystem (OGA), a fixed alkaline electrolyzer. All technologies are integrated in one Rack and capable to provide in nominal operations for three crew members. (Witt et al., 2020).
The potential of using microalgae for space applications has been considered since the beginning of human spaceflight. Several experiments have taken place both on Earth and in space. Those experiments have either evaluated the potential contribution of microalgae to LSS loop closure, tested the required technology for space applications or investigated the effects of the space environment on microalgae cells.
During the 1960’s, several experiments with closed compartments on Earth with several living organisms cohabitating with humans took place independently in the United States (Gitelson and Lisovsky 2003) and in Russia (Kirensky et al., 1968). For example, one of the test platforms used, which included microalgae among other organisms, was Bios-3. It had a 315 m3 compartment for three inhabitants, with experiments lasting up to 90 days (Gitelson and Lisovsky 2003). In Europe, since the 1990’s, the MELiSSA (Micro-Ecological Life Support System Alternative) project is also aiming to test LSS closure on Earth. The European Space Agency (ESA) initiative is based on a system with separate compartments, each with a specific living organism contributing to the recycling pathway. One of the five compartments for MELiSSA contains Spirulina, which uses the nutrients produced by its predecessor compartment to produce oxygen and biomass for the crew (Lasseur et al., 2010). Experiments on Earth focused on long-term cultivation for space applications have been carried out at the Institute of Space Systems (IRS)—University of Stuttgart since 2010. Their research includes the longest reported experiment with Chlorella for space applications lasting over six years in a Flat Panel Airlift (FPA) reactor from the company Subitec® (Buchert et al., 2012; Helisch et al., 2016; Helisch et al., 2020), Figure 2, and two experiments lasting over 180 days in a microgravity adapted reactor (Keppler et al., 2018; Helisch et al., 2020), Figure 3. The research at IRS also includes the development of hardware for space applications, looking for example at the development of a lighting unit and evaluating its effects on the performance of the PBR.

FIGURE 2. 6 L Flat Panel Airlift (FPA) Reactor from the company Subitec®. The system is illuminated with halogen lamps. The system allows the cultivation of Chlorella vulgaris with growth rates of up to 4 g/l/d.

FIGURE 3. PBR@LSR reactor chamber (Detrell et al., 2019a). The PBR@LSR experiment included two microgravity-adaptet reactors, with a total capacity of 650 ml algae suspension.
Those experiments on Earth have focused on the performance of the algae, the design of the PBR and its interaction with other technologies under Earth conditions. Besides this research, about 50 experiments have already been carried out in space, mostly focusing on biological aspects. Those experiments generally had a short duration (several days) and have used different algal species, including amongst others Chlorella vulgaris (Niederwieser et al., 2018). The first experiments took place during the 1960’s, exposing Chlorella to space conditions in a photosynthetically inactive state, followed by a cultivation back on Earth (Semenenko and Vladimirova 1961; Shevchenko et al., 1967; Ward and Phillips 1968; Antipov et al., 1969). The first actual cultivation of Chlorella in space took place in the 1970’s, lasting two weeks (Moskvitin and Vaulina 1975). Other experiments, including not only algae but also other organisms, have been flown over the last decades, for example the Closed Equilibrated Biological Aquatic System (CEBAS) (Blüm 2003), Omegahab (Anken 2008), and Closed Aquatic Ecosystem (CAES) (Wang et al., 2008). Only two experiments have focused to date on the PBR technology in microgravity conditions, artemiss with 30 days’s cultivation of Spirulina (Poughon et al., 2020) and PBR@LSR with two week’s cultivation of Chlorella (Detrell et al., 2020b).
To evaluate the effects of including a PBR in the LSS, the Equivalent System Mass (ESM) can be used. It considers not only the mass of the system, but also the influence of its volume, required power, cooling and crew time. Equivalency factors, specific for a mission scenario can be used to transform all terms in mass-equivalent (Anderson et al., 2018). Adding a PBR will certainly increase the system mass, but will reduce the amount of food supplied from Earth. At a certain mission duration, the system with a PBR will become most favourable.
A comparison of a PC and a hybrid system’s ESM is carried out by Detrell 2021. The PC system is based on current technologies for CO2 removal, CO2 reduction and O2 production (Figure 4). As a reference technology the LSR is used, since it is the latest full technology brought to the ISS for air management. The LSR has a total mass of 715 kg, a volume of 1.8 m3 and requires a power of 2.1 kW, providing for three astronauts (Kappmaier et al., 2016; Matthias 2018). The system requires an addition of 0.47 kg/d of water per person. This water is spitted though electrolysis into O2 and H2, used by the crew and by Sabatier reactor respectively. In the PC LSS, the food will entirely be brought from Earth. The hybrid system includes the same PC technologies and a PBR system (Figure 5). A PBR system for a lunar base is still not available, and thus a first estimation for the main sizing parameters is done using laboratory data. Current experiments at IRS have shown that Chlorella vulgaris can be cultivated to provide growth rates up to 4 g/l/d. Variations in the growth rate will have a high impact on the system sizing and thus the ESM. Although growth rates of 4 g/l/d have been reported in several experiments, maintaining the system at those levels for long-periods of time in space conditions (that is at reduced gravity and higher space radiation dose) still needs to be demonstrated. Therefore, the study considers a range of reasonable growth rates, between 2 and 4 g/l/d. The algae volume required also depend on the PBR goal, i.e., the amount of food or oxygen that it is expected to be provided. Although first estimations, based on its composition, suggest up to 35% of the daily mass food consumption could be substituted by algae (Belz et al., 2014), such a diet and its potential side effects have still not been reported. In the study, two scenarios are considered for a first estimation, a PBR sized to satisfy 10 and 30% of the daily consumption, which would require a PBR system between 50 and 100 L per person (Detrell 2021). The addition of the PBR will reduce the amount of food that needs to be brought from Earth, but will add the need of nutrients. Figures 6, 7 show the results of the ESM per crew member. The break-even-point at which a PBR will be more favourable occurs at missions lasting between 4.0 and 6.5 years for a 30% food supply, and 4.8 and 7.3 years for a 10% food supply (Detrell 2021). Those mission durations are quite high, but reasonable for scenarios such as a permanently crewed Moon base.
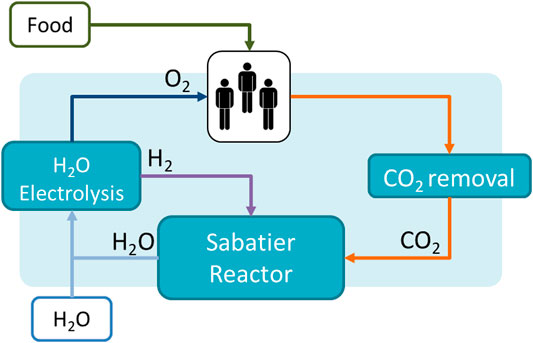
FIGURE 4. PC LSS for Air Management. The system is based on the current LSR technology on board the ISS.
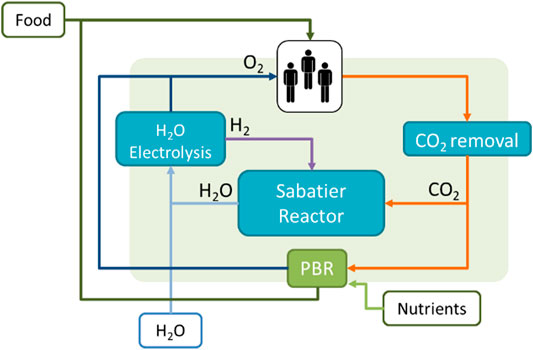
FIGURE 5. Hybrid LSS: PC + PBR. The system includes the technologies based on the LSR and the addition of a PBR, which can provide oxygen and food. Nutrients need to be added to the system.
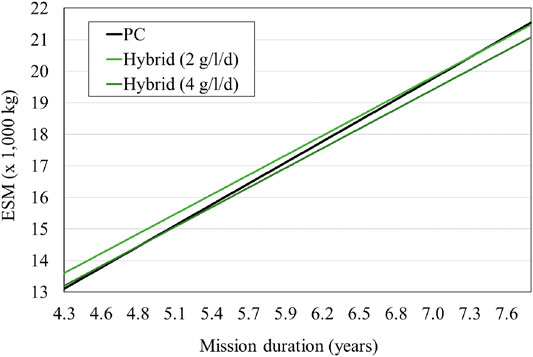
FIGURE 6. ESM comparing PC and Hybrid LSS with microalgae 10% food supply. The PC ESM is based on the LSR published data, while the PBR is based on the IRS laboratory data. A growth rate between 2 and 4 g/l/d is realistic according to current experiments and would provide an ESM that is more favorable for mission durations between 4.8 and 7.3 years (Detrell 2021).
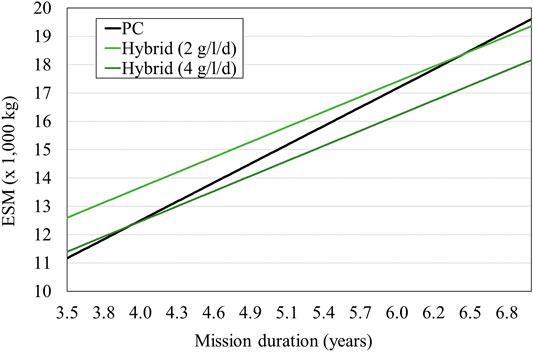
FIGURE 7. ESM comparing PC and Hybrid LSS with microalgae 30% food supply. The PC ESM is based on the LSR published data, while the PBR is based on the IRS laboratory data. A growth rate between 2 and 4 g/l/d is realistic according to current experiments and would provide an ESM that is more favorable for mission durations between 4.0 and 6.5 years (Detrell 2021).
The ESM of the PBR system could potentially be reduced in a Moon-base scenario with In-Situ Resources Utilization (ISRU). A PBR requires a high amount of water, as a medium support for the algae inside the reactor chamber. If the water could be obtained directly on the Moon surface (Honniball et al., 2021), or even the materials to build the reactor chamber (Schleppi et al., 2021), a PBR ESM would be reduce and thus it would be favourable in even shorter mission durations.
Besides the ESM, other parameters such as technology readiness should also be considered. The PC systems have been widely used in a relevant environment for long periods of time. For a PBR technology, still more research and testing is needed, requiring a research effort during the coming years, for it to become a real competitive option to PC technologies. Even if the PBR can only produce part of the required food, it will reduce the amount of food stored for long periods of time, which could have a significant impact on its nutritional value. This aspect will become even more relevant for missions further away, for example to Mars, where resupply intervals will increase substantially (Drysdale et al., 2003).
According to current research, a Chlorella PBR shows a big potential as a biological component in a lunar base to produce oxygen and fresh food supplement. The research at IRS since 2010 has been focused on the usage of microalgae, particularly Chlorella vulgaris, for space applications. The research has focused mainly in two aspects: the long-term stable cultivation and the system design under space conditions. Regarding the PBR design, several studies and experiments have been taken place at IRS considering all potential scenarios, including the microgravity experiment PBR@LSR, launched to ISS in 2019, and Moon/Mars photobioreactor preliminary studies. At IRS cultivation techniques have been investigated, allowing a successful over 6 years non-axenic cultivation, under Earth condition. During this research, several challenges or open questions that need to be solved, before such a component can become a reality, have been identified. Those include the influence of lunar environmental conditions, technical and biological aspects.
No experiments with microalgae have been carried out on the Moon, thus no previous experience exist on the influence of its environment on the algae and the performance of a PBR. The main two aspects to be considered are space radiation and partial gravity.
The experiments with microalgae carried out to date in space were in Low Earth Gravity, i.e., under the Earth’s magnetosphere protection (Niederwieser et al., 2018). Those experiments have shown different results on the effects of space conditions at cell level, still showing that cultivation under space conditions is possible. A series of experiments showed that Chlorella can survive continuous exposure to ionizing radiation while maintaining more than 90% of its original photosynthetic capacity, higher than other species (Rea et al., 2008). Space radiation levels on the Moon will be considerably higher. It is possible to partially test the effects of certain radiation on Earth, however, the long-term effects of space radiation need to be tested in-situ, in the lunar surface. The location of the PBR within the lunar base will play an important role in the amount of radiation received. If the PBR is integrated within the habitable structure of the station, the system will count with the same level of radiation protection than the crew.
Chlorella is an immobile unicellular organism. Gravity causes sedimentation within the reactor, which is avoided by creating a continuous movement of the algae-suspension. The lunar gravity is 1/6th of Earth’s gravity, which will have an effect on the movement of the cells and the gas within the liquid phase, and could eventually have an effect at cell level. The latter can be investigated in the laboratory using a clinostat. The experiments on microgravity have not shown an effect in the algae performance due to lack of gravity. Thus, no effects with partial gravity are expected. The major influence of reduced gravity will be on the system design, requiring a reactor and other subsystems to work as expected under partial gravity. Hardware design and testing in low gravity can be achieved through Computational Fluid Dynamics (CFD) simulations (Detrell et al., 2019b) and lunar-gravity parabolic flight campaign experiments (Pletser et al., 2012), before testing the system in-situ in the lunar environment.
The environmental conditions on the Moon explained in the previous section will have an influence on the technology, for example the reduced gravity level will have an influence on the movement of fluids within the reactor. Besides that, other challenges, inherent to the technology itself and the long-term performance also need to be addressed. The main challenges are related to the reactor design, the lighting system, the harvesting and processing unit and the scaling up and automation of the entire process/system.
Several types of reactor geometries are currently being used on Earth, from open ponds to high complex geometry reactors (Płaczek et al., 2017). For space applications, a closed system will be required, since avoiding contamination of the system will be a must. For that reason, systems such as open ponds can be discarded.
A high volumetric efficiency (up to 4 g/l/d with Chlorella) can be obtained with a high complex reactor geometry, with the Flat Panel Airlift (FPA) from the company Subitec®, Figure 2. The air is introduced in the bottom of the reactor and bubbles up in a gravity environment. The complex geometry creates swirls in the sub-chambers of the reactor, ensuring a proper mixing of the algae and providing a homogenous availability of nutrients, CO2 and light. However, the high complex geometry requires an even more complex maintenance. The Subitec® reactor geometry is optimized for an Earth gravity environment, but it can be adapted to lunar conditions, so it provides the same movement as on Earth with 1/6th of gravity (Detrell et al., 2019b). Other reactor types, such as tubular reactors, are easier to build and maintain, but are less efficient with growth rates below 0.1 g/l/d (Martin et al., 2020).
A raceway microgravity adapted reactor, with a FEP membrane for gas exchange and a pump to ensure algae-suspension circulation was designed and used for the PBR@LSR experiment (Detrell et al., 2020a). The growth rates obtained by this design were considerably lower, 0.42 g/l/d (Helisch et al., 2020). An important limitation of this type of reactor is the gas transfer rate of the membrane. While the use of airlift-based reactors was not possible for this experiment due to microgravity conditions, it can be advantageous under lunar reduced gravity conditions.
A trade-off between efficiency and complexity will be required. Volumetric efficiency plays a major role in the selection process if the entire system mass (including reactors and required water to fill them) needs to be brought from Earth. The possibility of using lunar resources, e.g., water from the Moon surface or materials to build the reactors in-situ, might make it possible to consider the use of simpler geometries. In that case, energy requirements and maintenance effort shall also be considered in the geometry selection.
Several reactor geometries could be used for a lunar base, and mission-related parameters, such as the ISRU will play an important role in the reactor geometry selection. The design of the selected geometry will need to consider lunar gravity levels, as explained in section Reduced Gravity.
The usage of direct sunlight is highly dependent on the lunar base location. A base on the lunar equator would experience 14-days-long nights, in which case artificial lighting would be required. Although it is possible to locate a base in areas with high illumination rates, for example on the rims of certain craters in the poles, with sunlight availability over 90% of the time, an artificial lighting system could still be more advantageous. This would allow a better control and adaptability of the lighting to the growth stages and could be used as a non-invasive control tool.
However, power availability is generally a constrain for space systems, thus the lighting system needs to be efficient in terms of energy. Several experiments on Earth have focused on the effect of specific wavelengths and their effect on the cultivation (Blair et al., 2014; Lysenko et al., 2021). Blue and red LEDs, which represent the two main peaks of the light absorption spectrum for Chlorella vulgaris, have been satisfactorily used in laboratory experiments (Bretschneider et al., 2016; Keppler et al., 2017). A lighting system, including more LEDs at different wavelengths could be used to reproduce more precisely the absorption spectrum of Chlorella and would be required to allow a non-invasive control (Martin et al., 2020). Experiments with Chlorella use typically photon flux densities of 200–300 µmol photons/m2/s (Helisch et al., 2020).
The effects of the different lighting concepts and the potential of non-invasive control still need to be further researched. It will be necessary to evaluate the long-term effects on the algae cells and their performance, while reducing the required energy for the lighting. The non-invasive control requires a deep knowledge of the culture and its reaction to different lighting spectrums.
The produced biomass in the reactor needs to be extracted from the system and processed to edible biomass, including a cell wall breakdown process for Chlorella.
The harvesting process requires the separation of the algae biomass from the growth medium, which can be further used in the PBR. Solid-liquid separation technologies are widely used for biomass harvesting in microalgae systems on Earth, with sedimentation, centrifugation and filtration being the main used processes (Singh and Patidar 2018). A key element for space applications is to obtain a high efficient harvesting system, that requires low energy, and does not compromise the biomass to be used as food source. Sedimentation is to expected to occur under lunar gravitational conditions, but it is a slow process. Centrifugation has a high recovery and is a fast process, but requires high amounts of energy and might cause cell damage due to high shear forces. Filtration also provides a high recovery efficiency without the high shear forces, however membranes/filters need to be cleaned or exchanged due to fouling/clogging. Other technologies, like microfluidic systems (Hønsvall et al., 2016) or electrophoresis (Pearsall et al., 2011), should also be considered for a lunar base, but have currently only been tested at small scale on the laboratory. Further research, and scaling up the system is still required.
There are several potential processes for processing, which are known on Earth, including high pressure homogenisation (Halim et al., 2013), freeze drying (Grima et al., 1994), microwave irradiation, and ultrasound (McMillan et al., 2013), but none has been developed or even evaluated for space applications yet. A major problematic of all those methods is the high energy required. As an alternative to direct human consumption, other alternatives such as fish feeding (Carneiro et al., 2020) or enhancing plant growth (Michalak et al., 2016) could also be considered.
The experiments with microalgae for space applications carried out to date have been small experiments, with a couple of litres, while a full PBR for a LSS will require about 100 L per person. A modular PBR system would allow avoiding scaling up effects and at the same time provide redundancy (Xu et al., 2009). In case of contamination occurring at one module, the rest could continue working. Stand-by modus reactors, ready to substitute a failed reactor, could be provided. A scale-up strategy, defining a modular system, ensuring efficient use of resources (e.g., common sensor unit), will be required.
A PBR experiment is generally well monitored and followed by the scientists, in some cases requiring their interaction when off-nominal situations occur. A LSS PBR should be able to work fully automated, to reduce crew time but also the risk of contamination associated with human interaction. The system will require sensors that are reliable for long periods of time or can easily be exchanged/recalibrated with minimal effort. For the system to react in off-nominal situation, those need to be fully understood and easily identifiable with the sensors in the system.
Besides the technological challenges, biological challenges need to be addressed as well. These mainly relate to the long and stable performance of the algae, looking both at the culture composition (axenic/non-axenic) and the long-term cultivation effects.
Microalgae cohabit with other living organisms on Earth. However, for space applications, the use of a closed PBR is preferred to an open system, to allow control of the population inside the PBR. That allows an axenic cultivation, which would require a complex hardware to ensure no other organisms enter the system during the required interactions for biomass extraction and nutrient insertion. A closed PBR also allows the use of a defined and well-established ecosystem, with the microalgae as dominant species. This has the potential to prevent the invasion of other species or suppress their upsurge during cultivation (Zhang et al., 2018). The use of a defined non-axenic culture has been discussed in several publications, with some results showing growth promotion (Cho et al., 2015; Ramanan et al., 2016), while others report system contamination (Wang et al., 2013). No evidence has been found in literature of a stable axenic cultivation for long periods of time, over years (Detrell et al., 2020a). Ensuring an axenic cultivation for long periods of time would require a highly complex system and procedures. An interaction with the exterior of the PBR will be required for air exchange, nutrient insertion and biomass. To ensure the axenicity in the PBR, it is necessary that the system is able to ensure that no other organisms can enter the system or that treatments (for example use of antibiotics) can be applied to selectively eliminate those (Mustapa et al., 2016). But the usage of a non-axenic cultivation comes with its challenges too. It is crucial not only to guarantee the predominance of the microalgae over time, but also of the associated community. If the biomass is to be used as a food source, it is required to ensure that it is edible and no organisms harmful for humans have entered the system. Microbial community analyses have shown the prevalence of Chlorella as the main species in non-axenic cultivation experiments (Haberkorn et al., 2020). A modular approach and proper analysis would allow to identify and reject any running reactor that might not fulfil the requirements for human consumption. Automated flow cytometry with advanced data analysis relying on phenotypic fingerprinting could contribute to a continuous monitoring of the microbial community (Haberkorn et al., 2021). The use of flow cytometry in a known non-axenic culture can enable the understanding of population dynamics and their response to external events.
A PBR for a LSS will need to work continuously for long-periods of time, since it would be responsible for providing part of the food and oxygen required by the crew. However, most of the experiments carried out for space applications to date have lasted only a few days or weeks (Niederwieser et al., 2018; Helisch et al., 2020). Three long-term cultivation laboratory experiments on non-axenic cultivation for space applications have been reported so far: a Subitec® FPA reactor experiment lasting over 6 years (Buchert et al., 2012; Helisch et al., 2016; Helisch et al., 2020), and two microgravity adapted reactor experiments over 180 days (Keppler et al., 2018; Helisch et al., 2020). Those experiments have demonstrated the feasibility of long-term cultivation and performance of the PBR. Besides the long-term performance of the culture, related to the non-axenic cultivation already mentioned, changes at cell level over time, influences in the composition, as well as biofilm formation are the main concerns for long-term cultivation. As mentioned in Light/Energy Availability, the lighting system can have a high influence on the culture. Thus, this can be used to influence the composition of the biomass. Similarly, the nutrient supply, including composition and supply interval will also have an impact in the culture.
Long-duration cultivation increases the probability of biofilm formation, which can be caused by direct adhesion of cells, biological deposits (e.g., extracellular polysaccharides–EPS) or cellular debris. The biofilm formation can result in an inhomogeneous availability of nutrients and dispersion of light energy influx, influencing the PBR performance. Some of the parameters showing a high influence on biofilm formation include light intensity and temperature, availability of carbon, nitrogen and phosphorous, as well as stress response to bacteria and mechanical forces (Wang et al., 2013; Helisch et al., 2016). Further long-term experiments are required to further understand, prevent or minimise the effects of biofilm formation, before a PBR can be used long-term in a LSS.
The use of microalgae for space applications has been widely investigated for several decades. One of the potential candidate species is Chlorella vulgaris, due to its robustness and adaptability. The feasibility of a microalgae based system depends both on biological and technological aspects. Besides the cultivation strategies, the required hardware, the Photobioreactor, will play an important role.
Several experiments with microalgae have already taken place in space. Those experiments were mostly short (several weeks) and small (several milliliters). Although those experiments have provided highly valuable results, further research is still required. Microalgae for a LSS will need to work continuously for long periods of time and will require a much higher volume.
A first estimation for a Chlorella PBR, based on laboratory data, suggests a reactor size of 100 L per person, to fulfil 30% of the human daily food requirements. The PBR would reduce the amount of required food supplied from Earth, but would require the addition of the PBR system itself, with a certain mass, but also other resources such as power or nutrients. A comparison of the PC LSR technology, with the same system including a PBR, shows that in terms of ESM, the PBR would be more favorable in missions lasting at least four years. This is a plausible scenario for a lunar base.
The work carried out at IRS serves as an initial base for the PBR research for a lunar base, but several challenges have been identified, which require further research, before a PBR can be used as part of a LSS in a lunar base. The main identified challenges include the higher space radiation and lower gravity on the Moon surface, as well as technical and biological aspects. A PBR design for the Moon does not exist yet, and several aspects need to be consider for the design: the reactor geometry (which will define volumetric efficiency, and needs to be adapted to lunar gravity), the lighting system (that will be defined by the power requirement) and the harvesting and processing (which shall allow the continuous cultivation and food production). Modularity and system automation (particularly looking at sensors and off-nominal scenarios) will also be crucial during the technology design. The design should also consider biological aspects, like the biofilm formation and its effects, and the effects of a non-axenic cultivation, to ensure a long-term stable performance.
GD is the main and only author of this paper.
This paper looks at the next steps in the use of microalgae, based on the experience and research carried out since 2010 at the IRS LSS research group, with projects funded by DLR/BMWi, the Friedrich and Elisabeth Boysen Foundation and the Dubai Future Foundation. This publication was supported by the German Research Foundation (DFG) within the funding programme Open Access Publishing.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The work presented in this paper would not have been possible without the contribution of current and former team members over the years, which include besides the author of this paper: Stefan Belz, Britta Ganzer, Melanie Buchert, Jens Bretschneider, Emil Nathanson, Harald Helisch, Jochen Keppler, Johannes Martin, and Andreas Dannenberg, and the support professors: Ernst Messerschmid, Stefanos Fasoulas, and Reinhold Ewald.
ACLS, Advanced Closed-Loop System; CCA, Carbon Dioxide Concentration Subsystem; CDRA, Carbon Dioxide Removal Assembly; CRA, Carbon Dioxide Reprocessing Subsystem; ESM, Equivalent System Mass; FPA, Flat Panel Airlift; IRS, Institute of Space Systems (Institut für Raumfahrtsysteme); ISRU, In-situ Resources Utilization; ISS, International Space Station; LSR, Life Support Rack; LSS, Life Support System; OGA, Oxygen Generation Assembly; PBR, Photobioreactor; PC, Phyisico-chemical; SRA, Sabatier Reactor Assembly.
Ackerman, U. (2007). Mikrotechniken für eine effiziente Bioenergieerzeugung. Dresden, Germany: VDI/VDE-IT. doi:10.1002/9780470034590.emrhp0004
Anderson, M., Gatens, R., Ikeda, T., Ito, T., Witt, J., and Hovland, S. (2016). “Life Support and Environmental Monitoring International System Maturation Team Considerations,” in 46th International Conference on Environmental Systems. Available online at https://ttu-ir.tdl.org/bitstream/2346/67736/1/ICES_2016_447.pdf.
Anderson, M., Keener, J., and Ewert, M. (2018). Life Support Baseline Values and Assumptions Document. NASA/TP-2015-218570, Rev1. Available online at https://ntrs.nasa.gov/api/citations/20180001338/downloads/20180001338.pdf. doi:10.1093/oso/9780198755821.003.0003
Anken, Ralf. (2008). Effect of Long-Term Microgravity on the Mineralisation of Inner Ear Otoliths of Fish - a Spaceflight Study. In 37th COSPAR Scientific Assembly.
Antipov, V. V., Delone, N. L., Nikitin, M. D., Parfyonov, G. P., and Saxonov, P. P. (1969). Some Results of Radiobiological Studies Performed on Cosmos-110 Biosatellite In Life Sciences and Space Research, 207–208. Available online at https://europepmc.org/article/med/12197540.
Belz, S., Buchert, M., Bretschneider, J., Nathanson, E., and Fasoulas, S. (2014). Physicochemical and Biological Technologies for Future Exploration Missions. Acta Astronautica. 101, 170–179. doi:10.1016/j.actaastro.2014.04.023
Blair, M. F., Kokabian, B., and Gude, V. G. (2014). Light and Growth Medium Effect on Chlorella Vulgaris Biomass Production. J. Environ. Chem. Eng. 2 (1), 665–674. doi:10.1016/j.jece.2013.11.005
Blüm, V. (2003). Aquatic Modules for Bioregenerative Life Support Systems: Developmental Aspects Based on the Space Flight Results of the C.E.B.A.S. Mini-Module. Adv. Space Res. 31 (7), 1683–1691. doi:10.1016/S0273-1177(03)80015-7
Bretschneider, J., Henn, N., Belz, S., Detrell, G., Keppler, J., Fasoulas, S., et al. (2016). “Functionality and Setup of the Algae Based ISS Experiment PBR@LSR,” in 46th International Conference on Environmental Systems, ICES16-203. Available online at https://ttu-ir.tdl.org/handle/2346/67593.
Buchert, M., Belz, S., Messerschmid, E., and Fasoulas, S. (2012). Cultivating Chlorella Vulgaris for Nutrition and Oxygen Production during Long-Term Manned Space Missions. 63rd Int. Astronautical Congress., IAC12–A16.4.
Carneiro, W. F., Castro, T. F. D., Orlando, T. M., Meurer, F., Paula, D. A. d. J., de Jesus, A., et al. (2020). Replacing Fish Meal by Chlorella Sp. Meal: Effects on Zebrafish Growth, Reproductive Performance, Biochemical Parameters and Digestive Enzymes. Aquaculture. 528, 735612. doi:10.1016/j.aquaculture.2020.735612
Cho, D.-H., Ramanan, R., Heo, J., Lee, J., Kim, B.-H., Oh, H.-M., et al. (2015). Enhancing Microalgal Biomass Productivity by Engineering a Microalgal-Bacterial Community. Bioresour. Technology 175, 578–585. doi:10.1016/j.biortech.2014.10.159
Crusan, J., and Gatens, R. (2017). Cislunar Habitation & Environmental Control & Life Support Systems. Washington DC: NASA Advisory Council, Human Exploration & Operations Committee. 1–42.
Degen, J. (2003). Entwicklung eines Photobioreaktors mit verbesserter Lichtnutzung für Mikroalgen. Stuttgart, Germany: Fraunhofer Verlag.
Detrell, G., Helisch, H., Keppler, J., Martin, J., Angerer, O., Adrian, A., et al. (2019a). “PBR@LSR: the Algae-Based Photobioreactor Experiment at the ISS – Configuration and Operations,” in 49th International Conference on Environmental Systems, ICES19-95. Available online at https://ttu-ir.tdl.org/handle/2346/84416.
Detrell, G., Martin, J., Keppler, J., and Helisch, H. (2019b). Algae on Moon and Mars Ensure Astronaut Survival. Final Report MBR Space Settlement Challenge Grant No: MBR012. Stuttgart, Germany: University of Stuttgart. IRS-18-P5.
Detrell, G., Helisch, H., Keppler, J., Martin, J., and Henn, N. (2020a). Microalgae for Combined Air Revitalization and Biomass Production for Space Applications. In Chapter 20 from: Biofiltration to Promising Options in Gaseous Fluxes Biotreatment. Elsevier, 419–445. doi:10.1016/B978-0-12-819064-7.00020-0
Detrell, G., Keppler, J., Helisch, H., Martin, J., Henn, N., Reinhold, E., et al. (2020b). PBR@LSR: The Algae-Based Photobioreactor Experiment at the ISS – Operations and Result in 2020 International Conference on Environmental Systems, ICES20-25. Available online at https://ttu-ir.tdl.org/handle/2346/86331.
Detrell, G. (2021). “Microalgae-based Hybrid Life Support System from Simulations to Flight Experiment,” in 50th International Conference on Environmental Systems, ICES21–185.
Drysdale, A. E., Ewert, M. K., and Hanford, A. J. (2003). Life Support Approaches for Mars Missions. Adv. Space Res. 31 (1), 51–61. doi:10.1016/S0273-1177(02)00658-0
ESA Blog (2016). Moon Village: A Vision for Global Cooperation and Space 4.0. Available online at checked on 3/1/2021 http://blogs.esa.int/janwoerner/2016/11/23/moon-village.
Gitelson, J. I., and Lisovsky, G. M. (2003). Man-Made Closed Ecological Systems. Hoboken: Taylor & Francis.
Grima, E. M., Medina, A. R., Giménez, A. G., Sánchez Pérez, J. A., Camacho, F. G., and García Sánchez, J. L. (1994). Comparison between Extraction of Lipids and Fatty Acids from Microalgal Biomass. J. Am. Oil Chem. Soc. 71 (9), 955–959. doi:10.1007/BF02542261
Haberkorn, I., Off, C. L., Besmer, M. D., Buchmann, L., and Mathys, A. (2021). Automated Online Flow Cytometry Advances Microalgal Ecosystem Management as In Situ, High-Temporal Resolution Monitoring Tool. Front. Bioeng. Biotechnol. 9, 642–671. doi:10.3389/fbioe.2021.642671
Haberkorn, I., Walser, J. C., Helisch, H., Böcker, L., Belz, S., Schuppler, M., et al. (2020). Characterization of Chlorella Vulgaris (Trebouxiophyceae) Associated Microbial Communities 1. J. Phycol. 56 (5), 1308–1322. doi:10.1111/jpy.13026
Halim, R., Rupasinghe, T. W. T., Tull, D. L., and Webley, P. A. (2013). Mechanical Cell Disruption for Lipid Extraction from Microalgal Biomass. Bioresour. Technology. 140, 53–63. doi:10.1016/j.biortech.2013.04.067
Hamilton, T., Castro, V., Ott, C. M., and Oubre, C. (2020). Culture-Based Environmental Microbiology Monitoring of Crop-Based Space Food Systems (Veggie Monitoring). Galveston, TX: JSC-E-DAA-TN77285.
Helisch, H., Keppler, J., Bretschneider, J., Belz, S., Henn, N., Fasoulas, S., et al. (2016). “Preparatory Ground-Based Experiments on Cultivation of Chlorella Vulgaris for the ISS Experiment PBR@LSR,” in 46th International Conference on Environmental Systems, ICES16-205. Available online at https://ttu-ir.tdl.org/handle/2346/67595.
Helisch, H., Keppler, J., Detrell, G., Belz, S., Ewald, R., Fasoulas, S., et al. (2020). High Density Long-Term Cultivation of Chlorella Vulgaris SAG 211-12 in a Novel Microgravity-Capable Membrane Raceway Photobioreactor for Future Bioregenerative Life Support in Space. Life Sci. Space Res. 24, 91–107. doi:10.1016/j.lssr.2019.08.001
Honniball, C. I., Lucey, P. G., Li, S., Shenoy, S., Orlando, T. M., Hibbitts, C. A., et al. (2021). Molecular Water Detected on the Sunlit Moon by SOFIA. Nat. Astron. 5 (2), 121–127. doi:10.1038/s41550-020-01222-x
Hønsvall, B. K., Altin, D., and Robertson, L. J. (2016). Continuous Harvesting of Microalgae by New Microfluidic Technology for Particle Separation. Bioresour. Technology 200, 360–365. doi:10.1016/j.biortech.2015.10.046
ISEGC (2018). The Global Exploration Roadmap, January 2018. Available online at updated on January, 2018, checked on 3/1/2021 https://www.globalspaceexploration.org/wordpress/wp-content/isecg/GER_2018_small_mobile.pdf.
Kappmaier, F., Witt, J., and Matthias, C. (2016). “Carbon Dioxide Reprocessing Subsystem for Loop Closure as Part of the Regenerative Life Support System ACLS,” in 46th International Conference on Environmental Systems. Available online at https://ttu-ir.tdl.org/handle/2346/67702.
Keppler, J., Helisch, H., Belz, S., Bretschneider, J., Detrell, G., Henn, N., et al. (2017). “From Breadboard to Protoflight Model – the Ongoing Development of the Algae-Based ISS Experiment PBR@LRS,” in 47th International Conference on Environmental Systems, ICES17-180. Available online atdoi:10.1051/jtsfen/2017nuc08
Keppler, J., Helisch, H., Detrell, G., Belz, S., Martin, J., Fasoulas, S., et al. (2018). “Microalgae Cultivation in Space for Future Exploration Missions: A Summary of the Development Progress of the Spaceflight Experiment PBR@LSR on the International Space Station ISS,” in 69th International Astronautical Congress, IAC18-A.1.7.4.
Kirensky, L. V., Terskov, I. A., Gitelson, I. I., Lisovsky, G. M., Kovrov, B. G., and Okladnikov, Y. N. (1968). Experimental Biological Life Support System. II. Gas Exchange between Man and Microalgae Culture in a 30-day Experiment. Life Sci. Space Res. 6, 37–40. Available online at https://europepmc.org/article/med/11982027.
Kittang, A.-I., Iversen, T.-H., Fossum, K. R., Mazars, C., Carnero-Diaz, E., Boucheron-Dubuisson, E., et al. (2014). Exploration of Plant Growth and Development Using the European Modular Cultivation System Facility on the International Space Station. Plant Biol. J. 16 (3), 528–538. doi:10.1111/plb.12132
Lakaniemi, Aino-Maija, A.-M., Hulatt, C. J., Wakeman, K. D., Thomas, D. N., and Puhakka, J. A. (2012). Eukaryotic and Prokaryotic Microbial Communities during Microalgal Biomass Production. Bioresour. Technology 124, 387–393. doi:10.1016/j.biortech.2012.08.048
Lasseur, C., Brunet, J., de Weever, H., Dixon, M., Dussap, G., Godia, F., et al. (2010). MELiSSA: The European Project of Closed Life Support System. Gravit. Space Biol. 23 (2), 23–32.
Lysenko, V., Kosolapov, A., Usova, E., Tatosyan, M., Varduny, T., Dmitriev, P., et al. (2021). Chlorophyll Fluorescence Kinetics and Oxygen Evolution in Chlorella Vulgaris Cells: Blue vs. Red Light. J. Plant Physiol. 258-259, 153392. doi:10.1016/j.jplph.2021.153392
Martin, J., Dannenberg, A., Detrell, G., Fasoulas, S., and Ewald, R. (2020). “Noninvasive Process Control of a Microalgae-Based System for Automated Treatment of Polluted Agricultural Ground Water Transferred from the Development of a Bioloigcal Life Support System,” in 2020 International Conference on Environmental Systems, ICES20–21.
Mason, R. (2001). Chlorella and Spirulina: Green Supplements for Balancing the Body. Altern. Complement. Therapies. 7 (3), 161–165. doi:10.1089/107628001300303691
Matthias, C. (2018). “ACLS – the Life Support Rack – for Accommodation on the ISS,” in Deutscher Luft- und Raumfahrtkongress. Friedrichshafen. doi:10.14361/9783839440889
McMillan, J. R., Watson, I. A., Ali, M., and Jaafar, W. (2013). Evaluation and Comparison of Algal Cell Disruption Methods: Microwave, Waterbath, Blender, Ultrasonic and Laser Treatment. Appl. Energ. 103, 128–134. doi:10.1016/j.apenergy.2012.09.020
Michalak, I., Chojnacka, K., Dmytryk, A., Wilk, R., Gramza, M., and Rój, E. (2016). Evaluation of Supercritical Extracts of Algae as Biostimulants of Plant Growth in Field Trials. Front. Plant Sci. 7, 1591. doi:10.3389/fpls.2016.01591
Moskvitin, E. V., and Vaulina, E. N. (1975). Experiment with a Physiologically Active Chlorella Culture on the Soyuz-9 Spaceship. Space Biol. Aerospace Med. 9 (3), 8–13. doi:10.1007/bf00856007
Mustapa, M., Sallehudin, N. J., Mohamed, M. S., Noor, N. M., and Raus, R. A. (2016). Decontamination of Chlorella Sp. Culture Using Antibiotics and Antifungal Cocktail Treatment. ARPN J. Eng. Appl. Sci. 11, 104–109. Available online at https://www.researchgate.net/profile/normawaty_mohammad_noor/publication/304254551_decontamination_of_chlorella_sp_culture_using_antibiotics_and_antifungal_cocktail_treatment/links/576b2ae708ae5b9a62b3a8bc/decontamination-of-chlorella-sp-culture-using-antibiotics-and-antifungal-cocktail-treatment.pdf.
NASA (2020). Explore Science 2020-2024 A Vision for Science Excellence. Available online at updated on 5/27/2020, checked on 3/16/2021https://science.nasa.gov/science-red/s3fs-public/atoms/files/2020-2024_Science.pdf.
Niederwieser, T., Kociolek, P., and Klaus, D. (2018). A Review of Algal Research in Space. Acta Astronautica. 146, 359–367. doi:10.1016/j.actaastro.2018.03.026
Pearsall, R., Connelly, R., Fountain, M., Hearn, C., Werst, M., Hebner, R., et al. (2011). Electrically Dewatering Microalgae. IEEE Trans. Dielect. Electr. Insul. 18 (5), 1578–1583. doi:10.1109/TDEI.2011.6032827
Pletser, V., Winter, J., Duclos, F., Bret-Dibat, T., Friedrich, U., Clervoy, J.-F., et al. (2012). The First Joint European Partial-G Parabolic Flight Campaign at Moon and Mars Gravity Levels for Science and Exploration. Microgravity Sci. Technol. 24 (6), 383–395. doi:10.1007/s12217-012-9304-y
Poughon, L., Laroche, C., Creuly, C., Dussap, C.-G., Paille, C., Lasseur, C., et al. (2020). Limnospira Indica PCC8005 Growth in Photobioreactor: Model and Simulation of the ISS and Ground Experiments. Life Sci. Space Res. 25, 53–65. doi:10.1016/j.lssr.2020.03.002
Powell, E. E., Mapiour, M. L., Evitts, R. W., and Hill, G. A. (2009). Growth Kinetics of Chlorella Vulgaris and its Use as a Cathodic Half Cell. Bioresour. Technology 100 (1), 269–274. doi:10.1016/j.biortech.2008.05.032
Płaczek, M., Patyna, A., and Witczak, S. (2017). Technical Evaluation of Photobioreactors for Microalgae Cultivation. E3s Web Conf. 19, 2032. doi:10.1051/e3sconf/20171902032
Ramanan, R., Kim, B.-H., Cho, D.-H., Oh, H.-M., and Kim, H.-S. (2016). Algae-bacteria Interactions: Evolution, Ecology and Emerging Applications. Biotechnol. Adv. 34 (1), 14–29. doi:10.1016/j.biotechadv.2015.12.003
Rea, G., Esposito, D., Damasso, M., Serafini, A., Margonelli, A., Faraloni, C., et al. (2008). Ionizing Radiation Impacts Photochemical Quantum Yield and Oxygen Evolution Activity of Photosystem II in Photosynthetic Microorganisms. Int. J. Radiat. Biol. 84 (11), 867–877. doi:10.1080/09553000802460149
Schleppi, J., Bromiley, G., Odling, N., and Bennett, N. S. (2021). In-situ Resource Utilisation Manufacturing of Optically Transparent Glass from Lunar Regolith Simulant. J. Mater. Sci. 56 (21), 12132–12153. doi:10.1007/s10853-021-06059-x
Schmid-Staiger, U., Preisner, R., Trösch, W., and Marek, P. (2009). Kultivierung von Mikroalgen im Photobioreaktor zur stofflichen und energetischen Nutzung. Chem. Ingenieur Technik 81 (11), 1783–1789. doi:10.1002/cite.200900079
Semenenko, V., and Vladimirova, M. (1961). Effect of Cosmic Flight Conditions in the Sputnik-Ship on the Viability of Chlorella. Physiol. Plants (8), 743–749.
Shaw, L. A., Garr, J. D., Gavin, L. L., Matty, C. M., Ridley, A., Salopek, M. J., et al. (2020). International Space Station as a Testbed for Exploration Environmental Control and Life Support Systems–2020 Status in 2020 International Conference on Environmental Systems. Available online at https://ttu-ir.tdl.org/bitstream/handle/2346/86400/ices-2020-299.pdf?sequence=1.
Shevchenko, V. A., Sakovich, I. S., Meshcheryakova, L. K., and Petrovnin, M. G. (1967). Study of the Development of Chlorella during Space Flight. Environmental Space Science. 1, 25–28.
Singh, G., and Patidar, S. K. (2018). Microalgae Harvesting Techniques: A Review. J. Environ. Manage. 217, 499–508. doi:10.1016/j.jenvman.2018.04.010
Storhas, W. (2000). Bioreaktoren und periphere Einrichtungen: Ein Leitfaden für die Hochschulausbildung, für Hersteller und Anwender. Berlin, Germany: Springer.
Wang, G., Liu, Y., Li, G., Hu, C., Zhang, D., and Li, X. (2008). A Simple Closed Aquatic Ecosystem (CAES) for Space. Adv. Space Res. 41 (5), 684–690. doi:10.1016/j.asr.2007.09.020
Wang, H., Zhang, W., Chen, L., Wang, J., and Liu, T. (2013). The Contamination and Control of Biological Pollutants in Mass Cultivation of Microalgae. Bioresour. Technology 128, 745–750. doi:10.1016/j.biortech.2012.10.158
Ward, C. H., and Phillips, J. N. (1968). Stability of Chlorella Following High-Altitude and Orbital Space Flight. Developments in Industrial Microbiology, 9, 345–354.
Witt, J., Hovland, S., Laurini, D., Matthias, C., Boettcher, F., Bevilacqua, T., et al. (2020). “On-orbit Testing of the Advanced Closed Loop System ACLS,” in 2020 International Conference on Environmental Systems. Available online at https://ttu-ir.tdl.org/bitstream/handle/2346/86479/ices-2020-510.pdf?sequence=1.
Xu, L., Weathers, P. J., Xiong, X.-R., and Liu, C.-Z. (2009). Microalgal Bioreactors: Challenges and Opportunities. Eng. Life Sci. 9 (3), 178–189. doi:10.1002/elsc.200800111
Yamamoto, M., Fujishita, M., Hirata, A., and Kawano, S. (2004). Regeneration and Maturation of Daughter Cell walls in the Autospore-Forming green Alga Chlorella Vulgaris (Chlorophyta, Trebouxiophyceae). J. Plant Res. 117 (4), 257–264. doi:10.1007/s10265-004-0154-6
Keywords: life support system, Moon base, photobioreactor, oxygen and food production, microalgae
Citation: Detrell G (2021) Chlorella Vulgaris Photobioreactor for Oxygen and Food Production on a Moon Base—Potential and Challenges. Front. Astron. Space Sci. 8:700579. doi: 10.3389/fspas.2021.700579
Received: 26 April 2021; Accepted: 09 July 2021;
Published: 23 July 2021.
Edited by:
Cyprien Verseux, University of Bremen, GermanyReviewed by:
Josep M. Trigo-Rodríguez, Consejo Superior de Investigaciones Científicas (CSIC), SpainCopyright © 2021 Detrell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gisela Detrell, ZGV0cmVsbEBpcnMudW5pLXN0dXR0Z2FydC5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.