
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Astron. Space Sci. , 18 June 2021
Sec. Astrobiology
Volume 8 - 2021 | https://doi.org/10.3389/fspas.2021.699688
This article is part of the Research Topic Bioregenerative Life-Support Systems for Crewed Missions to the Moon and Mars View all 26 articles
Nitrogen (N) recycling is essential for efficient food production in regenerative life support systems. Crew members with a high workload need 90–100 g of protein per person per day, which is about 14 g of N, or 1 mole of N, per person per day. Most of this N is excreted through urine with 85% as urea. Plants take up N predominantly as nitrate and ammonium, but direct uptake as urea is possible in small amounts. Efficient N recycling requires maintenance of pH of waste streams below about 7 to minimize the volatilization of N to ammonia. In aerobic reactors, continuous aerobic conditions are needed to minimize production and volatilization of nitrous oxide. N is not well recycled on Earth. The energy intensive Haber–Bosh process supplies most of the N for crop production in terrestrial agriculture. Bacterial fixation of dinitrogen to ammonium is also energy intensive. Recycling of N from plant and human waste streams is necessary to minimize the need for N fixation. Here we review approaches and potential for N fixation and recycling in regenerative life support systems. Initial estimates indicate that nearly all the N from human and plant waste streams can be recovered in forms usable for plants.
Human habitation on extraterrestrial surfaces presents a challenge due to their distance from Earth and inhospitable conditions for life. Earth’s moon has virtually no atmosphere, and the thin atmosphere of Mars is dominated by carbon dioxide (CO2) with small amounts of nitrogen (N) and oxygen (Owen et al., 1977). Because of the thin atmospheres of the Moon and Mars, there will be minimal protection from dangerous short-wave radiation from the Sun and incoming meteorites, meaning that early missions will likely take place in largely closed habitats and must rely on supplies brought from Earth to sustain crew members (CMs). This creates a supply dependency, which can lead to problems if unforeseen challenges arise during extended missions. International space agencies have funded decades of research to promote the development of advanced life support systems for CMs to maintain self-sustainability by producing food and oxygen (Wheeler, 2010). Although optimizing plant growth has been a large research topic, little work has been funded to study nutrient recycling. A related study nearly 25 years ago (discussed later) was funded in the United States by the National Aeronautics and Space Administration (NASA) to model mass balance of N in a closed system (Loader et al., 1997).
In the short term, bioavailable N must either be shipped from Earth or fixed from atmospheric dinitrogen gas (N2). We expect it to be highly cost effective to recycle N. N is likely present in small amounts of Lunar and Martian regolith in addition to being a small component of the Martian atmosphere (Kerridge, 2001). Existing surface N may come from the solar wind or from carbonaceous chondrite meteorites, which have been found to contain amino acids (Cronin and Pizzarello, 1983). Changes in atmospheric composition over time may have resulted in higher or lower levels of N in the past, which may now be locked away in untapped deposits (Klingler et al., 1989; Gebauer et al., 2020). These N reserves represent a possible source for N that may be mined in the future once colonies are established on extraterrestrial surfaces. This review focuses on in situ resource utilization of N by fixing it directly from the atmosphere.
Recycling becomes paramount, especially as mission duration and scope increase. N is an essential plant macronutrient that presents a rich opportunity for recycling due to its well-understood transformations between organic and inorganic forms. N recycling must rely on integrated waste collection, separation, and processing components. Waste must be collected and source separated for successful recovery of N from human excreta. Urea must then be removed and concentrated from the liquid phase before being efficiently hydrolyzed to ammonium to minimize volatilization as ammonia gas and maximize N recovery. Plant and human waste can be proportionally fed into a combination of aerobic and anaerobic digesters to promote nitrification and mineralization. These processes produce ammonium and nitrate that can be used as a N source for plants, which produce food, completing the cycle. Optimizing every step in the cycle decreases the amount of N2 that must be recovered from the atmosphere through bacterial N fixation. Here we review approaches for recycling N and progress on microbial N fixation for advanced life support systems.
The N cycle on Earth is shown in Figure 1. The atmosphere of Earth is about 79% inert N2. N2 is fixed into bioavailable ammonium by archaea and bacteria (either free-living or plant-associated) and by the Haber–Bosch process, with the biological and industrial processes each accounting for approximately 50% of globally fixed N. Both approaches are energy intensive and require large amounts of either adenosine triphosphate (ATP) or fossil fuels, respectively (Fowler et al., 2013). This fixed N can be rapidly taken up by plants and assimilated into proteins (Xu et al., 2012).
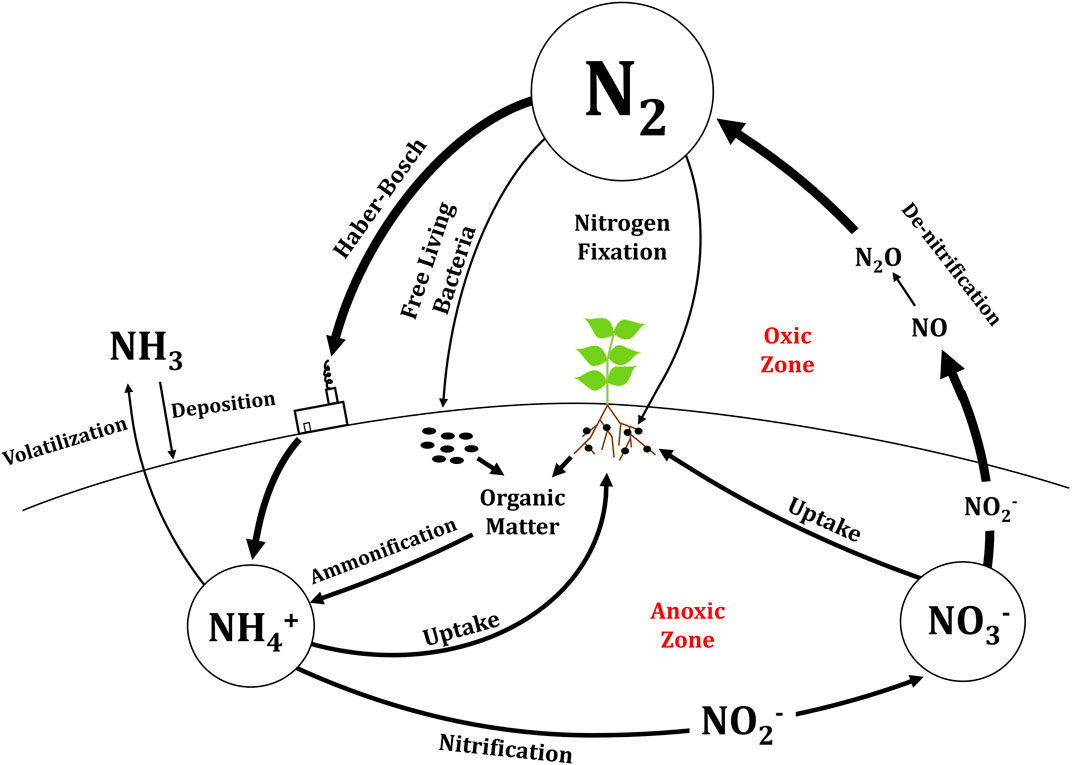
FIGURE 1. Conversions between nitrogen forms in the nitrogen cycle on Earth. The long convex line represents the surface of Earth with anoxic conversions below ground and oxic conversions above ground.
Ammonium that has been fixed from the atmosphere can be converted to nitrate by naturally occurring nitrifying bacteria or through industrial production. Biological nitrification occurs across two steps when Nitrosomonas spp. oxidize ammonium to nitrite and Nitrobacter spp. oxidize nitrite to nitrate (Gee et al., 1990). Plants can then uptake either ammonium or nitrate. N is transferred from plants to animals as proteins. After hydrogen, carbon, and oxygen, N comprises the highest quantity of all essential nutrients on a mole basis in both plants and animals. This is because N is a major component of proteins, as well as nucleic acids, chlorophylls, and defensive compounds (Mu et al., 2016; Stein and Klotz, 2016).
When proteins degrade in mammalian cells, the N is converted to urea and is discarded by the organism through urine and sweat. Waste products also include other nitrogenous compounds in small amounts such as creatinine, ammonium bicarbonate, and ammonium citrate (Verostko et al., 2004). Urea is a stable molecule that will hydrolyze unaided with a half-life of 3.6 years to form two molecules of ammonia and one molecule of carbonic acid (Zerner, 1991). The enzyme urease, both free and in bacteria, catalyzes the conversion of urea to ammonia above pH five and increases the reaction rate by four orders of magnitude compared to unaided hydrolysis (Amtul et al., 2002; Udert et al., 2003). Urease is found in both bacteria and plants, but not in animals. Animals must excrete urea as a waste product before it builds up to toxic concentrations. Urea is the most common N fertilizer applied to crops with almost 60% of all N fertilizers being urea (Davis et al., 2016). Urea applied to the soil is hydrolyzed by urease in naturally occurring bacteria for plant uptake or is taken up in small amounts through plant cells by transporters, where it is later hydrolyzed and assimilated into proteins (Wang et al., 2008).
In addition to waste products from living organisms, decomposition of dead organisms and organic matter by decomposers (detritivores, bacteria, and fungus) releases organic N as ammonium and nitrate back into the soil. N that has been energetically fixed to ammonium and nitrate can be lost back to the atmosphere through abiotic and biotic processes, respectively. Ammonium can volatilize to ammonia gas under alkaline conditions and escape to the atmosphere. Oxidation of ammonium by ammonia-oxidizing microorganisms produces a hydroxylamine intermediate that can be oxidized to nitric oxide, then to nitrite, and then to nitrate or reduced to nitrous oxide and N2 (Soler-Jofra et al., 2021). The bacterial process of annamox can also oxidize ammonium to N2 using either nitric oxide or nitrite as an oxidant while producing water (Stein and Klotz, 2016). While both nitrification and denitrification can produce nitrous oxide, the major products of nitrification are nitrite and nitrate, and the major product of denitrification is N2 (Heil et al., 2016; Wrage-Mönnig et al., 2018).
The atmosphere on Earth is dominated by N (79% N2 gas), but the Martian atmosphere is dominated by CO2 and contains less than 2% N2 gas by volume (Mahaffy et al., 2013). In space or extraterrestrial surfaces, industrial N2 fixation will not initially be feasible due to the large amount of energy and infrastructure needed. Instead of industrial fixation, early inhabitants must either bring N reserves from Earth or fix N using bacteria. Bringing large initial N stocks from Earth as a N gas (N2 or ammonia) or a solid (nitrate salt) can be challenging due to transport difficulties and overall N percentage. For example, ammonia gas is 82% N on a mass basis, while salts have a much lower percentage. Frequent resupply is currently impractical with extended mission durations, and bacterial N fixation represents the most feasible option. Recovery of N with minimal volatilization is essential to reduce the resources (launch mass/equivalent system mass) necessary to support bacterial N fixation. The volumetric and chemical demands of a biological N fixation system to support a manned mission are expected to be significant, even with high N recovery in fixation, composting, and human waste integration. An infrastructure capable of efficient N recovery at each step will allow N fixation to focus mainly on replenishing unrecovered N and will drastically reduce volumetric, chemical, and energetic demands required for fixation.
An overview of N recycling on Mars is provided in Figure 2. N2 from the Martian atmosphere will be fixed by N-fixing microbes in bioreactors. Biological fixation is still possible even with the much lower percentage of atmospheric N on Mars compared to Earth (Klingler et al., 1989). This step transforms N2 into organic N in the form of proteins. These proteins must be degraded to plant-available N (ammonium) through anaerobic digestion. Small amounts of N are lost during anaerobic digestion as gaseous and recalcitrant forms. Plants may be grown hydroponically or in media and must be fed a combination of ammonium and nitrate to overcome toxicity issues/physiological disorders. Half of the plant biomass is edible, while the other half is inedible and must be composted in an aerobic digester. This aerobic digestor can also take in hydrolyzed urea from human urine and output nitrate for plants, but minor gaseous losses are again possible. Feces can also be composted in a digester to recover N that is fed back to plants. This simple overview shows how recycling of N can greatly reduce the demand for atmospheric N fixation.
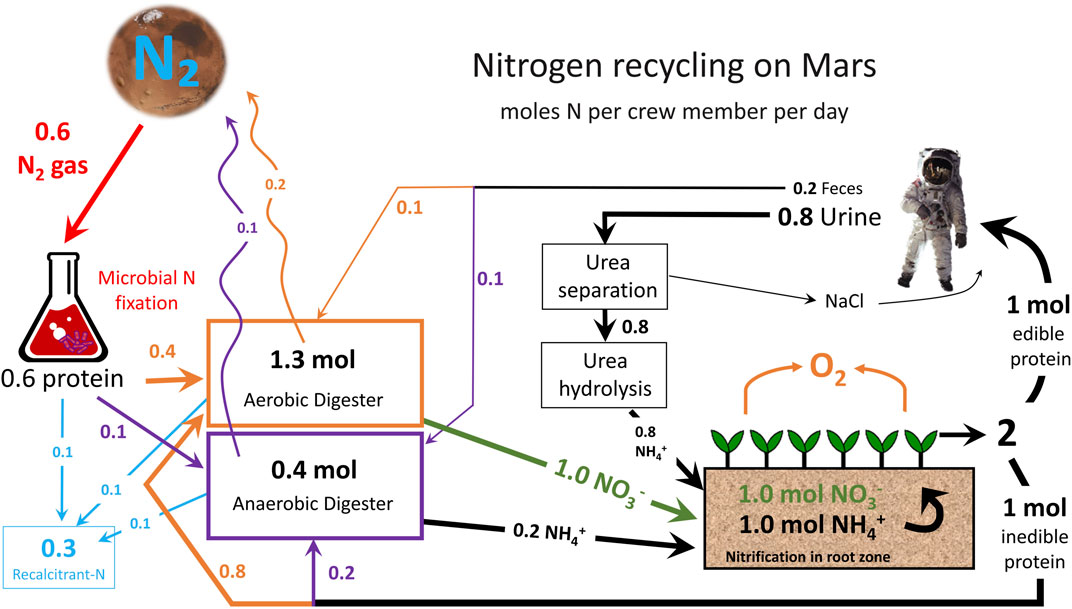
FIGURE 2. The nitrogen (N) cycle on Mars in an advanced life support system. N values are provided in moles of N per person per day and estimated gaseous losses are shown with sinuous arrows. N fixation requirement is higher than the N output based on current recovery values of N from bacterial biomass.
Loader et al. (1997) appear to be the first group to publish a simple model for mass balance of N for regenerative life support. The authors envisioned fixing N from the life-support module atmosphere with an aquaculture unit providing additional protein. Their model suggests that denitrification might be a 12% gaseous loss. A well-designed plant production system with adequate aeration should have minimal denitrification. They did not include an anaerobic reactor, make mention of N recycling from urine, and did not include loss as recalcitrant N. While their separate nitrification and aerobic bioreactors produce nitrate and ammonium, our model specifies separate bioreactors to produce bioavailable N. Their model estimated similar values for plant uptake, harvest index, and human N requirements.
Adult humans excrete about the same amount of N as they ingest on a daily basis. The daily N requirement is determined by the N required to replenish spent proteins and nucleic acids. This can be calculated based on replenishment of excreted N as shown in Table 1 or based on the requirements of ingested nitrogen.
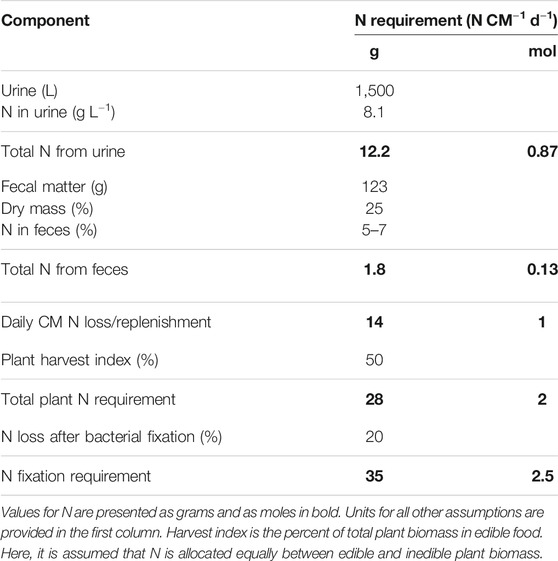
TABLE 1. Daily nitrogen (N) replenishment requirements for a crew member (CM) in an advanced life support system.
About 90% of the nitrogen discarded by humans is found in urine (Heinonen-Tanski and van Wijk-Sijbesma, 2005). CMs are estimated to produce on average 1.5 kg urine CM−1 d−1 (Anderson et al., 2018). The concentration of urea in typical adult human urine is 13.4 gL−1 with ammonium salts contributing 4.1 gL−1 and organic compounds contributing 5.4 gL−1 (Putnam, 1971). The total N concentration across the urine waste products equates to 8.1 gL−1 or 12.2 gN CM−1 d−1. Daily fecal excretion is estimated to be about 123 g CM−1 d−1. Feces is about 5–7% N on a dry mass basis, which represents 25% of the wet mass (Harder et al., 2019). N lost in solid excreta therefore represents an additional requirement of 1.8 gN CM−1 d−1. This equates to a daily N replacement of about 14 g N, or 1 mole of N, per person per day.
A CM is expected to consume about 3,000 kcal (12,550 kJ) per day due to high mission workloads (Cooper et al., 2011). If 16% of the daily intake is protein, and protein provides a gross energy of 5.1 kcalg−1 protein, a daily intake of about 94 g protein would be required (Hormoz, 2013; Anderson et al., 2018). The daily N requirement is therefore 15 gN CM−1 d−1assuming 16% N in protein. These values are based on food exported from Earth (Anderson et al., 2018). Ideally, this caloric intake would be split between initial food supply and food grown in transit or on extraterrestrial surfaces. Mission costs increase when the initial supply is increased (Cahill and Hardiman, 2020), and optimal food production reduces dependence on food resupply. The calculated N requirement based on both crew input and output is about 14 gN CM−1 d−1.
Assuming 14 gN per CM per day, four CMs over a standard 967-days mission would require 54 kg of N to replace that lost to urine excretion (Anderson et al., 2018).
However, about half of the N in plant biomass is not in an edible form. Harvest index is the ratio of edible to total biomass and varies from 20% in peanut (Arachis hypogaea) and sweet potato (Ipomoea batatas) to 90% in lettuce (Lactuca sativa) and spinach (Spinacia oleracea), with an average value of 50% for common crops (Wheeler et al., 2003; Anderson et al., 2018). This means that the N required to grow the food is doubled to 28 gN CM−1 d−1. This must come primarily from ammonium and nitrate.
Without recovery, capture of N must come directly and entirely from the atmosphere. On Earth, this process is split between microbial production and Haber-Bosch industrial production. The energy and mass requirements to fix N using the Haber-Bosch process are likely prohibitively expensive. A more efficient approach, especially on a small scale, is to use bacterial fixation through the enzyme nitrogenase. This can be accomplished by fixing N from the atmosphere of an enclosed life support system or from the Martian atmosphere, although the lower partial pressure of N2 must be considered (Klingler et al., 1989; Mahaffy et al., 2013).
Nitrogenase uses ATP to reduce N2 to two molecules of ammonia, consuming electrons in the process (Newton, 2007). ATP required to fix N2 can be produced through consumption of reduced carbon compounds or light reactions, depending on chemotrophic or phototrophic metabolism (Soundararajan et al., 2019). At a biologically relevant pH, ammonia is in the protonated ammonium form and is assimilated to glutamate by glutamate and glutamine synthetase for use in proteins (Nagatani et al., 1971). N fixation efficiency of nitrogenase is largely determined by oxygen concentrations with anaerobic conditions leading to the highest efficiency (Mortenson, 1978).
Plant-associated bacteria, such as rhizobia in legumes, have long been known to be productive N fixers (Franche et al., 2009). They form associations with plant roots and form nodules, which provide the host plant with N. Growing non-leguminous plants in regenerative life support systems requires free-living N-fixing bacteria, such as heterotrophic Azotobacter spp. or photosynthetic cyanobacteria (Meeks and Elhai, 2002; Inomura et al., 2017). Early work with cyanobacteria in regenerative life support systems was carried out at NASA Ames by Packer et al. (1986). Major cellular components, such as protein, glycogen, and sugars, were higher when the cyanobacteria Nostoc muscorum were grown under white light compared to blue light. They also characterized extreme oxygen sensitivity to nitrogenase as stated above. Cyanobacteria have continued to be a promising resource for regenerative life support systems, though recent work has focused on producing carbon compounds such as ethanol instead of fixing N (Zhou and Gibbons, 2015). Cyanobacteria are capable of N fixation under aerobic conditions and would be valuable for integration with oxygen-containing components of life support systems (Stal, 2015). Combining cyanobacterial means of N fixation with anaerobic methods can add to life support system versatility.
Multiple species of purple non-sulfur bacteria (PNSB) are also ideal for N fixation, especially because of their accumulation of compounds beneficial for plants and their ability to switch between multiple metabolic methods (Sakarika et al., 2020). Rhodopseudomonas palustris TN110, a strain of a widespread PNSB, was shown by Sakpirom et al. (2017) to contain all three nitrogenase isozymes (Mo–Fe, V–Fe, and Fe–Fe) and released the highest concentration of ammonium among 235 tested isolates. This makes R. palustris a valuable asset to a potential biological N fixation system and has been the basis for the N fixation and recovery systems described below.
Once fixed by bacteria, N must be transformed into bioavailable forms for plant uptake. When applied to the root-zone, both living and dead PNSB cells have been shown to increase edible biomass across many species (Sakarika et al., 2020). In addition to supplying N, it is possible that PNSB may indirectly promote plant growth by promoting the growth of other microorganisms which release plant growth promoting substances (PGPS), such as auxins. Kondo et al. (2008) found reduced growth of spinach (S. oleracea) and komatsuna (Brassica rapa var. perviridis) when applying PNSB to sterilized soil, demonstrating a possible interaction with other soil microorganisms. Rice (Oryza sativa) yield was enhanced when a biofertilizer comprising of PNSB at a concentration of 108 cells g−1 was applied at 0.75 kgha−1, although no distinction was made between growth promotion due to release of N or other PGPS (Kantachote et al., 2016). Substitution of PNSB for synthetic N did not significantly change the fruit mass of tomato, but malic and phosphoric acid content were increased, contributing to higher fruit quality (Kondo et al., 2010).
Few studies have quantified the recovery of N from PNSB for use as a fertilizer. The most closely related studies have focused on determining quantities and rates of ammonia production in ruminants. Hyper-ammonia-producing (HAB) gram-positive bacteria isolated from the intestines of ruminants can ferment amino acids to ammonia (Bento et al., 2015). Eschenlauer et al. (2002) reported ammonia production rates of 1.8–19.7 nmol ammonia per mg of protein per min from HAB grown on trypticase. The ammonia production rate of HAB in ruminants varies considerably based on diet and has been found to be higher in vitro than in vivo (Taghavi-Nezhad et al., 2014). Capture of ammonia under acidic conditions will lead to protonation, preventing loss of N through volatilization.
Preliminary studies from our laboratory indicate 80% of total N from R. palustris can be recovered as ammonium if the pH is rigorously controlled at 7. If we assume 28 gN CM−1 d−1 from plants combined with an 80% recovery efficiency from PNSB this leads to a final total of 35 gN CM−1 d−1 that must be fixed by bacteria. This number can be significantly reduced if N is recycled.
Optimizing N recovery involves increasing the efficiency of recovery from excreta and maximizing the recovery of bioavailable N (ammonium and nitrate) from anaerobic and aerobic digestion of human and plant wastes. Minimizing volatile losses and/or maximizing recovery of volatiles further improves N recovery. Selecting for crop cultivars with a high harvest index minimizes inedible plant materials and helps to improve the recovery efficiency.
The simplest method to apply N to plants from urine is direct application following sterilization. Direct application of urine has been shown to improve plant growth compared to no fertilizer controls, but its use is complicated by low levels of bioavailable N, odor, and salt build-up in the substrate (Salisbury et al., 1997; Pandorf et al., 2019). N must be extracted from the urea in urine and transformed to bioavailable forms to alleviate these issues. This consists of four steps: sterilization, volume reduction, stabilization, and recovery (Maurer et al., 2006). Many methods have been developed to accomplish these tasks, though they vary in simplicity, expense, volume requirements, and efficiency.
The N from protein degradation is converted into urea, which is the dominant N form in the waste stream. Urea comprises over one half of the total dissolved solids and 75–90% of the discarded N in human urine (Rose et al., 2015; Simha and Ganesapillai, 2017). The remaining N is bound in other N-containing compounds such as creatinine and ammonium salts. Urea excretion from the human body varies based on diet, with the average adult human producing about 500 L of urine per year, equating to about 20 g urea CM−1 d−1 (Lind et al., 2001; Amtul et al., 2002). Urea can be fed directly to plants in the root zone, but it is taken up very slowly. Unaided urea hydrolysis is slow, but will eventually proceed due to bacterial contamination (Elliot, 1986). Supplementing the growing media with small amounts of urease has the potential to significantly increase the rate of hydrolysis (Figure 3).
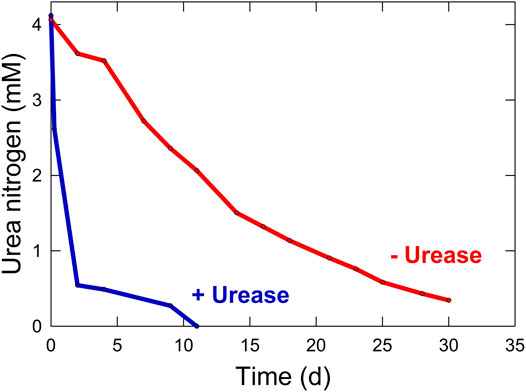
FIGURE 3. Rate of urea hydrolysis to ammonium with (3.2 enzyme units L−1) and without (0 enzyme units L−1) the addition of urease from Jack bean (Canavalia ensiformis).
Urine exits the body as a sterile liquid (Simha, 2013). Pathogens from unhealthy humans and fecal contamination from incomplete source separation compromise sterility (Höglund et al., 2002; Santos et al., 2004). Sterilization involves processes to eliminate pathogens from the waste stream to reduce contamination of downstream products. Urine may be sterilized by heat, pressure, or ultraviolet light, but these methods require energy. Alternatively, storage at low pH for weeks to months can inactivate viruses, inhibit pathogens, combat the rise in pH due to urea hydrolysis, and reduce ammonia volatilization (Hellström et al., 1999; Patel et al., 2020). If acidic conditions are maintained, no N is lost during the sterilization process.
The volume of urine must be condensed following sterilization to both recycle water and collect the valuable metabolites. Evaporation energy requirements to remove water can be minimized by vapor-compression distillation to generate additional heat for evaporation and to recover 85% of the energy used compared to standard evaporation (Wood, 1982). Urea can also be separated from the liquid phase through freeze/thaw cycles. When urine begins to freeze, the ice that forms has minimal solutes. These solutes, such as urea, remain dissolved in the unfrozen portion. Removal of the distilled frozen water effectively concentrates the dissolved solids while concurrently purifying water for other uses. Lind et al. (2001) found that freezing and melting urine samples could concentrate more than 80% of the original N into a much lower volume. Reverse osmosis may also be used to remove water from urine and concentrate solutes. Ammonia recovery during reverse osmosis has been found to be about 70%, but higher recoveries may be possible if acidification is used to prevent volatilization (Thörneby et al., 1999). An 80% recovery rate means 16 g urea CM−1 d−1 can be recovered as ammonium following volume reduction.
Stabilization of condensed urine is essential to minimize volatilization of ammonium to ammonia gas. Released ammonia causes unpleasant odors and becomes difficult to capture in the gas phase. When urea is hydrolyzed in water, ammonium, CO2, and hydroxide are released, causing the pH of the solution to increase. Alkaline pH causes ammonium to de-protonate and volatilize to ammonia gas. Acidification is therefore necessary to both maintain neutral pH for optimal microbial activity in an aqueous system and prevent the loss of ammonia gas as shown in Figure 4 (Hellström et al., 1999; Maurer et al., 2006). CO2 must be vented or additionally recycled during this step to prevent build-up to toxic levels.
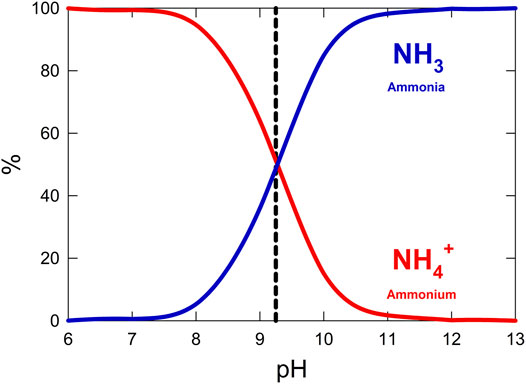
FIGURE 4. Percent abundance of ammonia and ammonium across a range of pH values in a closed system. The pKa of ammonium is shown as a dashed line.
Recovering urea and/or ammonium from urine is the most important step in N recovery from human waste. The pH must be rigorously controlled during the processes to prevent escape of ammonia gas. Current systems take the urea in urine and convert it to ammonium, nitrate, or a mix that can be fed to plants as their principal N sources. A recent example is the two-step Valorization of Urine Nutrients in Africa (VUNA) process where aeration is used for oxidation of organic matter and nitrification, converting half the ammonium in the urine into nitrate, a step that is completed when the pH has fallen to 6.20–6.25. At this point, the VUNA process uses vacuum distillation for recovery of water and a solution that is rich in ammonium nitrate (Udert and Wächter, 2012; Fumasoli et al., 2016).
Although energy consumption is high for recovery, volume reduction of urine can reduce the energy requirement by 58% (Maurer et al., 2003). Tun et al. (2016) studied filters for reducing water volume and concentrating ammonium from urine using membrane distillation following acidification. They found a combination PTFE/PP filter reduced the amount of ammonium transferred in relation to water to as low as 6.91 × 10–5 g-N/g-H2O. Some of these processes have been implemented in long-term studies to quantify N recovery from urine for simulated space missions. Beler-Baykal et al. (2011) utilized the natural conversion of urea to ammonium over six weeks to transform the N in urine into a bioavailable form before passing it through a column of clinoptilolite for separation to recover about 86% of the original N. An additional benefit of the use of clinoptilolite was the elimination of salinity from the waste stream, a valuable attribute to eliminate salt stress in plants. Simha et al. (2018) used a regenerative activated carbon column to achieve 90% recovery of urea from urine concentrate after multiple passes through their system. Fu et al. (2016) studied a 105 days crewed simulated closed ecosystem and were able to recover 20.5% of N from urine using simple distillation. However, this recovered N was stored instead of fed to plants, which were fertilized with pre-formed plant minerals stored from the beginning of the study.
Recent work has used synthetic microbial communities to complete nitrification directly in fresh diluted urine. This method bypasses the separate aerobic digestion step where nitrification may normally be present. Though previous stabilization steps still apply, Christiaens et al. (2019) were able to achieve N production rates of 29 mg nitrate L−1 d−1 in 10% diluted urine even under high sodium pressures. Udert and Wächter (2012) developed a system combining distillation with nitrification to completely recover nutrients from urine. Controlling pH for optimal nitrification led to 97% N recovery in the form of ammonium nitrate. Feng et al. (2008) showed up to 95% of the original ammonium could be converted to nitrate under high dissolved oxygen levels with rigorous solution pH control. These processes present alternative methods that may save space and resources in comparison to separate dedicated bioreactors.
Significant nitrification occurs in all agricultural soils and there is great potential to enhance nitrification in the root zone in a regenerative system. Controlled watering would optimize aeration, and the process could be supplemented by adding nitrifying organisms.
We are currently optimizing small scale bioreactors to convert the urea in urine into ammonium. A peristaltic pump is used to move solution from a bottom reservoir to the top of the column where it flows through perlite under continuous recirculation (Figure 5). Initial results show that ammonia volatilization is minimized when the pH is controlled near neutral (Figure 6). Our diagram assumes that we can achieve 100% recovery of ammonium from urine (Figure 2).

FIGURE 5. Recirculating columns controlled at pH 7 with perlite as a substrate for the conversion of urea into ammonium.
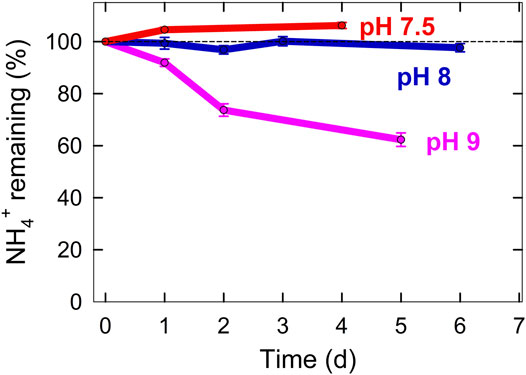
FIGURE 6. Ammonia gas volatilization over time determined by the amount of ammonium remaining in solution controlled at 3 pH values.
The most bioavailable forms of N for plants are ammonium and nitrate. Their interaction in plant nutrition is complex, and optimal ratios may vary among species. Plants commonly show no deficiencies or toxicities when only fed nitrate as a nitrogen source, but they often exhibit toxicity effects when ammonium is the sole source (Britto and Kronzucker, 2002; Miller et al., 2007; Esteban et al., 2016). This may be due to nutrient imbalances caused by a preference for ammonium uptake over nitrate uptake leading to pH imbalance (Imas et al., 1997). Savvas et al. (2006) studied ammonium as a nitrogen source from 0 to 30% of total nitrogen and found 30% ammonium produced plants with the highest dry mass, though all treatments appeared healthy with no noticeable deficiencies or toxicities. Yield among some species can decrease as the fraction of N from ammonium is increased beyond 25% (Britto and Kronzucker, 2002). In a previous NASA funded project at Utah State University, Hooten (1998) found no decrease in the yield of wheat under 80% ammonium as an N source when the pH in the hydroponic solution was rigorously controlled at pH 6. If this approach can be expanded to other crops, it would significantly decrease the amount of N that must be nitrified.
No plant is completely edible (100% harvest index). Roots, stems, and in the case of fruiting plants, leaves, are inedible biomass and frequently discarded on Earth. This inedible plant waste must be digested to recover the N. Composting plant material relies on bacteria to convert the unusable biomass (proteins) into useful nutrients (ammonium and nitrate). Heat, moisture, and sometimes oxygen are required to sustain the microbial breakdown. Methane, nitrous oxide, and ammonia are all released as byproducts by microbes during the composting process (Brown et al., 2008). The N in ammonia must be captured to optimize recovery efficiency. This may be achieved by capturing the gas and bubbling it through an acidic solution to protonate the ammonia to ammonium. Current field composting studies of plant material indicate poor N recovery. Hartz et al. (2000) studied over 30 compost amendments ranging from animal manure to plant yard waste. N recovery in manure was about 15–16% while N recovery in plant waste was only 1–2%. Chalk et al. (2013) reviewed recent literature on N recovery in compost and found a maximum recovery of N from manure to be 38% and N recovery form plant waste to range from 8–26%. The composting process is also slow in addition to being inefficient.
Alternative and more efficient means of composting involve both aerobic and anaerobic digestion. These processes are currently being investigated and implemented on Earth for food waste recycling to both produce useable compost and generate clean energy. Both digestion procedures are more rigorous, efficient, and contained composting methods to capture the maximum number of useable byproducts possible with minimal wasted energy or heat losses.
Aerobic digestion utilizes aerobic microorganisms to break down proteins and oxidizes N to nitrate while releasing CO2 and water. The digestion process requires constant agitation and aeration to maintain aerobic conditions and promote microbial degradation of waste (Layden et al., 2007). Aerobic digestion is commonly used in waste water treatment facilities on Earth to stabilize fecal matter, food wastes, and microbial bio-solids (Khalili et al., 2000). Additional volatile losses of nitrous oxide present a considerable recovery challenge and can add an additional 20% daily loss of N (Figure 2). The product is a stable material (no objectionable odor, acceptable and slow decay rate, disinfected) that can facilitate conversion of Martian regolith into soil. During NASA’s Breadboard project, potato yield of plants grown with liquid effluent from aerobic digestion of inedible plant biomass were within 10% of control yields (Mackowiak et al., 1997). Recalcitrant nutrients accumulated over time, but final potato composition was comparable to control tubers. While N was not specifically studied, this project illustrates the compatibility of aerobic digestion effluent with crops in regenerative life support systems.
Anaerobic digestion also uses anaerobic microorganisms to break down wastes into ammonium while releasing methane as a byproduct. The process can be dry or wet depending on the level of moisture. Dry fermentation anaerobic digestion has higher methane yields, lower water inputs, and reduced energy requirements compared to wet fermentation (Luning et al., 2003). The generated methane can be used as a clean energy source and the ammonium can be fed to plants or into additional nitrification digesters to be converted to nitrate. Microbes, much like plants, often show toxicity symptoms and reproductive challenges at high levels of ammonium. This feedback loop poses challenges for anaerobic digestion if it is to sustain a continuous ammonium output without negatively impacting microbial populations (Shapovalov et al., 2020). The remaining bio-solids from digesters in advanced life support systems can be added to plant substrates for slow, extended release of nutrients or tilled into the Martian regolith to begin developing a soil base for future in situ agriculture. This recalcitrant N can be 10% from anaerobic digestion on a daily basis that must be replaced through bacterial fixation.
A visual comparison of aerobic and anaerobic digestion is shown in Table 2. Aerobic digestion is much faster than anaerobic digestion, but it requires higher energy inputs to maintain adequate oxygen levels. A combination of these two systems allows production of both nitrate and ammonium for plant fertilizers. Their input quantities can be manipulated to produce the desired ratios of both N species. An important consideration in both anaerobic and aerobic digestion is the use of batch or continuous throughput methods. Batch digestion involves adding wastes, processing through digestion over a set time period, and removing the desired byproducts before restarting the process. Continuous throughput methods would have an initial waste supply and continue adding additional waste while simultaneously removing byproducts for fuel or fertilizers. Most research has focused on the efficiency of methane produced from both systems. Batch systems with dual solid and liquid phases have shown increased methane production compared to continuous systems when unwanted contaminants were separated from bulk waste prior to digestion (Chowdhury and Fulford, 1992; Zhang et al., 2013). Ammonium is likely to follow this trend as another valuable microbial byproduct.
A combination of both methods will likely be the best approach due to the inevitable buildup of recalcitrant bio-solids that must eventually be eliminated from the digesters. This method is termed semi-continuous and involves coordinated input of waste and removal of byproducts over a set time frame before cleaning and restarting the digester. The process is similar to industrial aquaponics systems on Earth that use continuous throughput mineralization tanks to convert organic fish wastes into inorganic N forms useable by plants.
The biomass of N-fixing bacteria is rich in nutrients essential for plant growth. They can serve as a valuable N source and fertilizer supplement if applied to plants. Although little work has been done to quantify the release of N from N-fixing bacteria, quantitative studies have compared plant harvest parameters in response to applications of live and dead cells. Sakarika et al. (2020) compiled data from many studies to compare effects of PNSB used as a biofertilizer. The application of PNSB generally increased crop yield, but there was no difference in the effects of living or dead cells. Tomato yield and lycopene content were significantly enhanced from the application of both living and dead cells (21–98% and 42–50%, respectively) (Lee et al., 2008).
The N fixation rates of most PNSB are high. Rhodopseudomonas palustris is a diazotroph that possesses three different nitrogenase enzymes and photoheterotrophic metabolic capability. It also has high metabolic versatility and is capable of photoheterotrophic, diazotrophic, photoautotrophic, and non-diazotrophic metabolism (Soundararajan et al., 2019). Since CO2 fixation and N fixation compete for ATP, carbon sources can be provided to maximize N fixation (Larimer et al., 2004). Rhodopseudomonas palustris NifA* has been genetically engineered to have reduced inhibition of nitrogenase at high ammonium concentrations (Adessi et al., 2012). This strain is typically cultured under photoheterotrophic conditions to maximize biomass N content. Although photons provided from sunlight are an option to drive bacterial photosynthesis, this would require a system that utilizes solar fiber optics and concentrating mirrors, which has a higher equivalent system mass than a system that utilizes light-emitting diodes (LEDs) and photovoltaics (Hardy et al., 2020). LED systems can be designed to output specific wavelengths of photons, and R. palustris appears to be able to use photons with wavelengths between 400 and 900 nm for photosynthesis with relatively similar efficiencies (Soundararajan et al., 2019). Acetate or wastewater organics are provided as a readily available food source along with ample N2 gas. Maximizing N biomass output will raise demand for available organic compounds, but these compounds can be obtained from waste, heterotrophic metabolism of R. palustris may stand to be an overall benefit.
Rhodopseudomonas palustris can obtain N through urease-facilitated urea hydrolysis or N fixation via nitrogenase (Malofeeva, 1979). This suggests that N remediation and fixation may occur simultaneously. In non-engineered strains, nitrogenase activity would decrease during urease catabolism as ammonium levels rise and prevent nitrogenase expression (Adessi et al., 2012). It is unlikely that urease or its activity inhibits R. palustris NifA* given its nitrogenase desensitization to elevated ammonium concentrations. Since urease activity is not limited by ATP, whereas nitrogenase activity is, it is likely that nitrogenase would be deprioritized under energy limiting conditions. Urea degradation can occur if urea is readily available. Once urease catalysis ceases, N fixation will become the primary means of N acquisition, though both processes can occur simultaneously if urea and N2 are present. Remediation of N via urea hydrolysis can directly serve to treat wastewater, and N losses can be compensated by nitrogenase activity. This forms a valuable part of the Martian N cycle in which N fixation and remediation can occur in tandem. The major barrier to this process and the implementation of such a system is the removal of inhibitory components, such as those present in sludge or wastewater soap content. Preparing wastewater for integration with N fixation and recovery systems will therefore likely demand considerable upstream processing.
In regenerative life support systems, N-fixing bacterial biomass would serve as a principal N source for crops. Within cells, most of the N is contained in amino acids. Bacterial cells must be broken down by other microbes which mineralize the organic N to ammonium. The pH of this process must be controlled to both allow for an optimal bacterial environment and prevent the volatilization of ammonia gas. Culturing R. palustris is optimized when it is provided with an input of N2 (80%) and CO2 (20%) to facilitate N fixation and pH control. The system must also account for lighting conditions, stirring mechanisms, heating, and the energy required to extract and replenish media during and after harvest. Our current culture system (Figure 7.) has a total power demand of approximately 100 WL−1 of media to provide these conditions, from which approximately 0.1 and 0.05 g of N per liter day can be produced from acetate and wastewater media, respectively. We have demonstrated the N recovery process from bacteria can take about 6 weeks without the intentional inoculation of additional microbes and recover 80% of the N. Eventual recovery systems will use both anaerobic and aerobic digesters to achieve desired products and implement specific bacterial strains with high digestion capabilities to increase both the speed and recovery efficiency of biological N fixation.

FIGURE 7. Photobioreactors used for nitrogen (N) recycling. Wastewater reactor (left) obtains N from atmospheric dinitrogen gas and wastewater while fixing reactor (right) obtains N from atmospheric gas alone.
N recycling is essential to efficient regenerative life support. Recycling of N is complex and requires many steps, but can be accomplished with high efficiency. Microbial systems can recover N from the atmosphere, and well-designed bioreactors can efficiently recover N from inedible plant waste, urine, and feces. Efficient N recovery can reduce the amount of atmospheric N2 that must be fixed by nearly 10-fold (Table 1 compared to Figure 2). N losses will inevitably occur, but recycling systems can minimize losses and maximize self-sustainability for long-term space missions.
Many areas of N recycling in an advanced life support system are prime candidates for future research to increase N recovery. Urea hydrolysis requires acidic conditions to avoid volatile ammonia losses. Further work is needed to improve both the speed and recovery efficiency of N as ammonium. The extent to which composting can occur in the root zone is also not well studied. The requirement for nitrification is minimized if plants can be fed higher levels of ammonium. Optimization of both speed and efficiency are paramount to this effort. Improving N fixation efficiency can reduce the resources required for bacterial N fixation. Future research will seek to achieve 100% N recovery for a fully regenerative life support system.
NL, PK, and TW contributed to the original draft of the manuscript. CC, LS, and BB guided the editing process, literature search, and design of figures.
This research was supported by the Utah Agricultural Experiment Station, Utah State University, and approved as journal paper number 9465; the NASA-CUBES (Grant Number NNX17AJ31G).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Adessi, A., McKinlay, J. B., Harwood, C. S., and De Philippis, R. (2012). A Rhodopseudomonas Palustris nifA* Mutant Produces H2 from -containing Vegetable Wastes. Int. J. Hydrogen Energ. 37, 15893–15900. doi:10.1016/j.ijhydene.2012.08.009
Amtul, Z., Atta-ur-Rahman, B. S. P., Siddiqui, R., and Choudhary, M. (2002). Chemistry and Mechanism of Urease Inhibition. CMC 9, 1323–1348. doi:10.2174/0929867023369853
Anderson, M. S., Ewert, M. K., and Keener, J. F. (2018). Life Support Baseline Values and Assumptions Document. Available at: https://ntrs.nasa.gov/api/citations/20180001338/downloads/20180001338.pdf (Accessed April 22, 2021).
Beler-Baykal, B., Allar, A. D., and Bayram, S. (2011). Nitrogen Recovery from Source-Separated Human Urine Using Clinoptilolite and Preliminary Results of its Use as Fertilizer. Water Sci. Technol. 63, 811–817. doi:10.2166/wst.2011.324
Bento, C., de Azevedo, A., Detmann, E., and Mantovani, H. (2015). Biochemical and Genetic Diversity of Carbohydrate-Fermenting and Obligate Amino Acid-Fermenting Hyper-Ammonia-Producing Bacteria from Nellore Steers Fed Tropical Forages and Supplemented with Casein. BMC Microbiol. 15, 28. doi:10.1186/s12866-015-0369-9
Britto, D. T., and Kronzucker, H. J. (2002). NH4+ Toxicity in Higher Plants: a Critical Review. J. Plant Physiol. 159, 567–584. doi:10.1078/0176-1617-0774
Brown, S., Kruger, C., and Subler, S. (2008). Greenhouse Gas Balance for Composting Operations. J. Environ. Qual. 37, 1396–1410. doi:10.2134/jeq2007.0453
Cahill, T., and Hardiman, G. (2020). Nutritional Challenges and Countermeasures for Space Travel. Nutr. Bull. 45, 98–105. doi:10.1111/nbu.12422
Chalk, P. M., Magalhães, A. M. T., and Inácio, C. T. (2013). Towards an Understanding of the Dynamics of Compost N in the Soil-Plant-Atmosphere System Using 15N Tracer. Plant Soil 362, 373–388. doi:10.1007/s11104-012-1358-5
Chowdhury, R. B. S., and Fulford, D. J. (1992). Batch and Semi-continuous Anaerobic Digestion Systems. Renew. Energ. 2, 391–400. doi:10.1016/0960-1481(92)90072-B
Christiaens, M. E. R., De Paepe, J., Ilgrande, C., De Vrieze, J., Barys, J., Teirlinck, P., et al. (2019). Urine Nitrification with a Synthetic Microbial Community. Syst. Appl. Microbiol. 42, 126021. doi:10.1016/j.syapm.2019.126021
Cooper, M., Douglas, G., and Perchonok, M. (2011). Developing the NASA Food System for Long-Duration Missions. J. Food Sci. 76, R40–R48. doi:10.1111/j.1750-3841.2010.01982.x
Cronin, J. R., and Pizzarello, S. (1983). Amino Acids in Meteorites. Adv. Space Res. 3, 5–18. doi:10.1016/0273-1177(83)90036-4
Davis, A. M., Tink, M., Rohde, K., and Brodie, J. E. (2016). Urea Contributions to Dissolved 'organic' Nitrogen Losses from Intensive, Fertilised Agriculture. Agric. Ecosyst. Environ. 223, 190–196. doi:10.1016/j.agee.2016.03.006
Eschenlauer, S. C. P., McKain, N., Walker, N. D., McEwan, N. R., Newbold, C. J., and Wallace, R. J. (2002). Ammonia Production by Ruminal Microorganisms and Enumeration, Isolation, and Characterization of Bacteria Capable of Growth on Peptides and Amino Acids from the Sheep Rumen. Appl. Environ. Microbiol. 68, 4925–4931. doi:10.1128/aem.68.10.4925-4931.2002
Esteban, R., Ariz, I., Cruz, C., and Moran, J. F. (2016). Review: Mechanisms of Ammonium Toxicity and the Quest for Tolerance. Plant Sci. 248, 92–101. doi:10.1016/j.plantsci.2016.04.008
Feng, D., Wu, Z., and Xu, S. (2008). Nitrification of Human Urine for its Stabilization and Nutrient Recycling. Bioresour. Technol. 99, 6299–6304. doi:10.1016/j.biortech.2007.12.007
Fowler, D., Coyle, M., Skiba, U., Sutton, M. A., Cape, J. N., Reis, S., et al. (2013). The Global Nitrogen Cycle in the Twenty-First century. Phil. Trans. R. Soc. B 368, 20130164. doi:10.1098/rstb.2013.0164
Franche, C., Lindström, K., and Elmerich, C. (2009). Nitrogen-fixing Bacteria Associated with Leguminous and Non-leguminous Plants. Plant Soil 321, 35–59. doi:10.1007/s11104-008-9833-8
Fu, Y., Li, L., Xie, B., Dong, C., Wang, M., Jia, B., et al. (2016). How to Establish a Bioregenerative Life Support System for Long-Term Crewed Missions to the Moon or Mars. Astrobiology 16, 925–936. doi:10.1089/ast.2016.1477
Fumasoli, A., Etter, B., Sterkele, B., Morgenroth, E., and Udert, K. M. (2016). Operating a Pilot-Scale Nitrification/distillation Plant for Complete Nutrient Recovery from Urine. Water Sci. Technol. 73, 215–222. doi:10.2166/wst.2015.485
Gebauer, S., Grenfell, J. L., Lammer, H., de Vera, J.-P. P., Sproß, L., Airapetian, V. S., et al. (2020). Atmospheric Nitrogen when Life Evolved on Earth. Astrobiology 20, 1413–1426. doi:10.1089/ast.2019.2212
Gee, C. S., Pfeffer, J. T., and Suidan, M. T. (1990). Nitrosomonas and NitrobacterInteractions in Biological Nitrification. J. Environ. Eng. 116, 4–17. doi:10.1061/(asce)0733-9372(1990)116:1(4)
Harder, R., Wielemaker, R., Larsen, T. A., Zeeman, G., and Öberg, G. (2019). Recycling Nutrients Contained in Human Excreta to Agriculture: Pathways, Processes, and Products. Crit. Rev. Environ. Sci. Technol. 49, 695–743. doi:10.1080/10643389.2018.1558889
Hardy, J. M., Kusuma, P., Bugbee, B., Wheeler, R., and Ewert, M. (2020). “Providing Photons for Food in Regenerative Life Support: A Comparative Analysis of Solar Fiber Optic and Electric Light Systems,” in International Conference on Environmental Systems, 1–12. Available at: https://ttu-ir.tdl.org/handle/2346/86378 (Accessed April 22, 2021).
Hartz, T. K., Mitchell, J. P., and Giannini, C. (2000). Nitrogen and Carbon Mineralization Dynamics of Manures and Composts. HortSci 35, 209–212. doi:10.21273/HORTSCI.35.2.209
Heil, J., Vereecken, H., and Brüggemann, N. (2016). A Review of Chemical Reactions of Nitrification Intermediates and Their Role in Nitrogen Cycling and Nitrogen Trace Gas Formation in Soil. Eur. J. Soil Sci. 67, 23–39. doi:10.1111/ejss.12306
Heinonen-Tanski, H., and van Wijk-Sijbesma, C. (2005). Human Excreta for Plant Production. Bioresour. Technol. 96, 403–411. doi:10.1016/j.biortech.2003.10.036
Hellström, D., Johansson, E., and Grennberg, K. (1999). Storage of Human Urine: Acidification as a Method to Inhibit Decomposition of Urea. Ecol. Eng. 12, 253–269. doi:10.1016/S0925-8574(98)00074-3
Höglund, C., Stenström, T. A., and Ashbolt, N. (2002). Microbial Risk Assessment of Source-Separated Urine Used in Agriculture. Waste Manag. Res. 20, 150–161. doi:10.1177/0734242X0202000207
Hooten, T. (1998). Ammonium and Nitrate Effects on Growth, Development and Nutrient Uptake of Hydroponic Wheat. Available at: https://digitalcommons.usu.edu/etd/6748/ (Accessed April 22, 2021).
Hormoz, S. (2013). Amino Acid Composition of Proteins Reduces Deleterious Impact of Mutations. Sci. Rep. 3, 2919. doi:10.1038/srep02919
Imas, P., Bar-Yosef, B., Kafkafi, U., and Ganmore-Neumann, R. (1997). Release of Carboxylic Anions and Protons by Tomato Roots in Response to Ammonium Nitrate Ratio and pH in Nutrient Solution. Plant Soil 191, 27–34. doi:10.1023/A:1004214814504
Inomura, K., Bragg, J., and Follows, M. J. (2017). A Quantitative Analysis of the Direct and Indirect Costs of Nitrogen Fixation: A Model Based on Azotobacter Vinelandii. ISME J. 11, 166–175. doi:10.1038/ismej.2016.97
Kantachote, D., Nunkaew, T., Kantha, T., and Chaiprapat, S. (2016). Biofertilizers from Rhodopseudomonas Palustris Strains to Enhance rice Yields and Reduce Methane Emissions. Appl. Soil Ecol. 100, 154–161. doi:10.1016/j.apsoil.2015.12.015
Kerridge, J. F. (2001). Isotopic Variability of Nitrogen in Lunar Regolith. Sci 293, 1947a. doi:10.1126/science.293.5537.1947a
Khalili, N. R., Chaib, E., Parulekar, S. J., and Nykiel, D. (2000). Performance Enhancement of Batch Aerobic Digesters via Addition of Digested Sludge. J. Hazard. Mater. 76, 91–102. doi:10.1016/S0304-3894(00)00172-2
Klingler, J. M., Mancinelli, R. L., and White, M. R. (1989). Biological Nitrogen Fixation under Primordial Martian Partial Pressures of Dinitrogen. Adv. Space Res. 9, 173–176. doi:10.1016/0273-1177(89)90225-1
Kondo, K., Nakata, N., and Nishihara, E. (2008). Effect of Purple Non-sulfur Bacterium (Rhodobacter Sphaeroides) Application on the Growth and Quality of Spinach and Komatsuna. Jpn. Soc. Agr. Technol. Mgt. 14, 198–203. doi:10.17660/actahortic.2007.761.81
Kondo, K., Nakata, N., and Nishihara, E. (2010). Effect of the Purple Non-sulfur Bacterium (Rhodobacter Sphaeroides) on the Brix, Titratable Acidity, Ascorbic Acid, Organic Acid, Lycopene, and β-carotene in Tomato Fruit. J. Food Agr. Environ. 8, 743–746.
Larimer, F. W., Chain, P., Hauser, L., Lamerdin, J., Malfatti, S., Do, L., et al. (2004). Complete Genome Sequence of the Metabolically Versatile Photosynthetic Bacterium Rhodopseudomonas Palustris. Nat. Biotechnol. 22, 55–61. doi:10.1038/nbt923
Layden, N. M., Mavinic, D. S., Kelly, H. G., Moles, R., and Bartlett, J. (2007). Autothermal Thermophilic Aerobic Digestion (ATAD) - Part I: Review of Origins, Design, and Process Operation. J. Environ. Eng. Sci. 6, 665–678. doi:10.1139/S07-015
Lee, K.-H., Koh, R.-H., and Song, H.-G. (2008). Enhancement of Growth and Yield of Tomato by Rhodopseudomonas Sp. Under Greenhouse Conditions. J. Microbiol. 46, 641–646. doi:10.1007/s12275-008-0159-2
Lind, B.-B., Ban, Z., and Bydén, S. (2001). Volume Reduction and Concentration of Nutrients in Human Urine. Ecol. Eng. 16, 561–566. doi:10.1016/S0925-8574(00)00107-5
Loader, C. A., Garland, J. L., Raychaudhuri, S., and Wheeler, R. M. (1997). A Simple Mass Balance Model of Nitrogen Flow in a Bioregenerative Life Support System. Life Support. Biosph. Sci. 4, 31–41.
Luning, L., van Zundert, E. H. M., and Brinkmann, A. J. F. (2003). Comparison of Dry and Wet Digestion for Solid Waste. Water Sci. Technol. 48, 15–20. doi:10.2166/wst.2003.0210
Mackowiak, C. L., Wheeler, R. M., Stutte, G. W., Yorio, N. C., and Sager, J. C. (1997). Use of Biologically Reclaimed Minerals for Continuous Hydroponic Potato Production in a CELSS. Adv. Space Res. 20, 1815–1820. doi:10.1016/S0273-1177(97)00846-6
Mahaffy, P. R., Webster, C. R., Atreya, S. K., Franz, H., Wong, M., Conrad, P. G., et al. (2013). Abundance and Isotopic Composition of Gases in the Martian Atmosphere from the Curiosity Rover. Science 341, 263–266. doi:10.1126/science.1237966
Maurer, M., Pronk, W., and Larsen, T. A. (2006). Treatment Processes for Source-Separated Urine. Water Res. 40, 3151–3166. doi:10.1016/j.watres.2006.07.012
Maurer, M., Schwegler, P., and Larsen, T. A. (2003). Nutrients in Urine: Energetic Aspects of Removal and Recovery. Water Sci. Technol. 48, 37–46. doi:10.2166/wst.2003.0011
Meeks, J. C., and Elhai, J. (2002). Regulation of Cellular Differentiation in Filamentous Cyanobacteria in Free-Living and Plant-Associated Symbiotic Growth States. MMBR 66, 94–121. doi:10.1128/MMBR.66.1.94-121.2002
Miller, A. J., Fan, X., Orsel, M., Smith, S. J., and Wells, D. M. (2007). Nitrate Transport and Signalling. J. Exp. Bot. 58, 2297–2306. doi:10.1093/jxb/erm066
Mortenson, L. E. (1978). Regulation of Nitrogen Fixation. Curr. Top. Cell. Regul 13. (Elsevier), 179–232. doi:10.1016/B978-0-12-152813-3.50010-0
Mu, X., Chen, Q., Chen, F., Yuan, L., and Mi, G. (2016). Within-leaf Nitrogen Allocation in Adaptation to Low Nitrogen Supply in maize during Grain-Filling Stage. Front. Plant Sci. 7, 699. doi:10.3389/fpls.2016.00699
Nagatani, H., Shimizu, M., and Valentine, R. C. (1971). The Mechanism of Ammonia Assimilation in Nitrogen Fixing Bacteria. Archiv. Mikrobiol. 79, 164–175. doi:10.1007/BF00424923
Newton, W. E. (2007). “Physiology, Biochemistry, and Molecular Biology of Nitrogen Fixation,” in Biology of the Nitrogen Cycle (Amsterdam: Elsevier), 109–129. doi:10.1016/B978-044452857-5.50009-6
Owen, T., Biemann, K., Rushneck, D. R., Biller, J. E., Howarth, D. W., and Lafleur, A. L. (1977). The Composition of the Atmosphere at the Surface of Mars. J. Geophys. Res. 82, 4635–4639. doi:10.1029/JS082i028p04635
Packer, L., Fry, I., and Belkin, S. (1986). Application of Photosynthetic N2-Fixing Cyanobacteria to the CELSS Program. Available at: https://ntrs.nasa.gov/citations/19860010458 (Accessed April 22, 2021).
Pandorf, M., Hochmuth, G., and Boyer, T. H. (2019). Human Urine as a Fertilizer in the Cultivation of Snap Beans (Phaseolus vulgaris) and Turnips (Brassica Rapa). J. Agric. Food Chem. 67, 50–62. doi:10.1021/acs.jafc.8b06011
Patel, A., Mungray, A. A., and Mungray, A. K. (2020). Technologies for the Recovery of Nutrients, Water and Energy from Human Urine: A Review. Chemosphere 259, 127372. doi:10.1016/j.chemosphere.2020.127372
Putnam, D. F. (1971). Composition and Concentrative Properties of Human Urine. Available at: https://ntrs.nasa.gov/api/citations/19710023044/downloads/19710023044.pdf (Accessed April 22, 2021).
Rose, C., Parker, A., Jefferson, B., and Cartmell, E. (2015). The Characterization of Feces and Urine: A Review of the Literature to Inform Advanced Treatment Technology. Crit. Rev. Environ. Sci. Technol. 45, 1827–1879. doi:10.1080/10643389.2014.1000761
Sakarika, M., Spanoghe, J., Sui, Y., Wambacq, E., Grunert, O., Haesaert, G., et al. (2020). Purple Non‐sulphur Bacteria and Plant Production: Benefits for Fertilization, Stress Resistance and the Environment. Microb. Biotechnol. 13, 1336–1365. doi:10.1111/1751-7915.13474
Sakpirom, J., Kantachote, D., Nunkaew, T., and Khan, E. (2017). Characterizations of Purple Non-sulfur Bacteria Isolated from Paddy fields, and Identification of Strains with Potential for Plant Growth-Promotion, Greenhouse Gas Mitigation and Heavy Metal Bioremediation. Res. Microbiol. 168, 266–275. doi:10.1016/j.resmic.2016.12.001
Salisbury, F. B., Gitelson, J. I., and Lisovsky, G. M. (1997). Bios-3: Siberian Experiments in Bioregenerative Life Support. BioScience 47, 575–585. doi:10.2307/1313164
Santos, R. L. S., Manfrinatto, J. A., Cia, E. M. M., Carvalho, R. B., Quadros, K. R. S., Alves-Filho, G., et al. (2004). Urine Cytology as a Screening Method for Polyoma Virus Active Infection. Transplant. Proc. 36, 899–901. doi:10.1016/j.transproceed.2004.03.111
Savvas, D., Passam, H. C., Olympios, C., Nasi, E., Moustaka, E., Mantzos, N., et al. (2006). Effects of Ammonium Nitrogen on Lettuce Grown on Pumice in a Closed Hydroponic System. HortSci 41, 1667–1673. doi:10.21273/HORTSCI.41.7.1667
Shapovalov, Y., Zhadan, S., Bochmann, G., Salyuk, A., and Nykyforov, V. (2020). Dry Anaerobic Digestion of Chicken Manure: A Review. Appl. Sci. 10, 7825–7849. doi:10.3390/app10217825
Simha, P., and Ganesapillai, M. (2017). Ecological Sanitation and Nutrient Recovery from Human Urine: How Far Have We Come? A Review. Sustain. Environ. Res. 27, 107–116. doi:10.1016/j.serj.2016.12.001
Simha, P. (2013). Nutrient Recovery Systems for Human Urine—Ways to Realize Closed Loop Sanitation and Future Sustainable Agricultural Systems. Intl. J. Sci. Res. Publ. 3, 22–27. doi:10.1007/bf00150148
Simha, P., Zabaniotou, A., and Ganesapillai, M. (2018). Continuous Urea-Nitrogen Recycling from Human Urine: A Step towards Creating a Human Excreta Based Bio-Economy. J. Clean. Prod. 172, 4152–4161. doi:10.1016/j.jclepro.2017.01.062
Soler-Jofra, A., Pérez, J., and van Loosdrecht, M. C. M. (2021). Hydroxylamine and the Nitrogen Cycle: A Review. Water Res. 190, 116723. doi:10.1016/j.watres.2020.116723
Soundararajan, M., Ledbetter, R., Kusuma, P., Zhen, S., Ludden, P., Bugbee, B., et al. (2019). Phototrophic N2 and CO2 Fixation Using a Rhodopseudomonas Palustris-H2 Mediated Electrochemical System with Infrared Photons. Front. Microbiol. 10, 1817. doi:10.3389/fmicb.2019.01817
Stal, L. J. (2015). “Nitrogen Fixation in Cyanobacteria,” in eLS. Editors W John, and L Sons (Chichester, UK: John Wiley & Sons), 1–9. doi:10.1002/9780470015902.a0021159.pub2
Stein, L. Y., and Klotz, M. G. (2016). The Nitrogen Cycle. Curr. Biol. 26, R94–R98. doi:10.1016/j.cub.2015.12.021
Taghavi-Nezhad, M., Alipour, D., Flythe, M. D., Zamani, P., and Khodakaramian, G. (2014). The Effect of Essential Oils of Zataria Multiflora and Mentha Spicata on the In Vitro Rumen Fermentation, and Growth and Deaminative Activity of Amino Acid-Fermenting Bacteria Isolated from Mehraban Sheep. Anim. Prod. Sci. 54, 299. doi:10.1071/AN12244
Thörneby, L., Persson, K., and Trägårdh, G. (1999). Treatment of Liquid Effluents from Dairy Cattle and Pigs Using Reverse Osmosis. J. Agric. Eng. Res. 73, 159–170. doi:10.1006/jaer.1998.0405
Tun, L. L., Jeong, D., Jeong, S., Cho, K., Lee, S., and Bae, H. (2016). Dewatering of Source-Separated Human Urine for Nitrogen Recovery by Membrane Distillation. J. Membr. Sci. 512, 13–20. doi:10.1016/j.memsci.2016.04.004
Udert, K. M., Larsen, T. A., Biebow, M., and Gujer, W. (2003). Urea Hydrolysis and Precipitation Dynamics in a Urine-Collecting System. Water Res. 37, 2571–2582. doi:10.1016/S0043-1354(03)00065-4
Udert, K. M., and Wächter, M. (2012). Complete Nutrient Recovery from Source-Separated Urine by Nitrification and Distillation. Water Res. 46, 453–464. doi:10.1016/j.watres.2011.11.020
Verostko, C. E., Carrier, C., and Finger, B. W. (2004). Ersatz Wastewater Formulations for Testing Water Recovery Systems. London: SAE International, Technical Paper 2004-01-2448. doi:10.4271/2004-01-2448
Wang, W.-H., Köhler, B., Cao, F.-Q., and Liu, L.-H. (2008). Molecular and Physiological Aspects of Urea Transport in Higher Plants. Plant Sci. 175, 467–477. doi:10.1016/j.plantsci.2008.05.018
Wheeler, M., Sager, J. C., Prince, R. P., Knott, W. M., Mackowiak, C. L., Stutte, G. W., et al. (2003). Crop Production for Advanced Life Support Systems - Observations from the Kennedy Space Center Breadboard Project. Available at: https://ntrs.nasa.gov/api/citations/20030032422/downloads/20030032422.pdf (Accessed April 22, 2021).
Wheeler, R. (2010). Plants for Human Life Support in Space: From Myers to Mars. Gravit. Space Biol 23, 25–36. doi:10.2514/6.2008-7922
Wood, F. C. (1982). The Changing Face of Desalination - A Consulting Engineer's Viewpoint. Desalination 42, 17–25. doi:10.1016/S0011-9164(00)88737-8
Wrage-Mönnig, N., Horn, M. A., Well, R., Müller, C., Velthof, G., and Oenema, O. (2018). The Role of Nitrifier Denitrification in the Production of Nitrous Oxide Revisited. Soil Biol. Biochem. 123, A3–A16. doi:10.1016/j.soilbio.2018.03.020
Xu, G., Fan, X., and Miller, A. J. (2012). Plant Nitrogen Assimilation and Use Efficiency. Annu. Rev. Plant Biol. 63, 153–182. doi:10.1146/annurev-arplant-042811-105532
Zerner, B. (1991). Recent Advances in the Chemistry of an Old Enzyme, Urease. Bioorg. Chem. 19, 116–131. doi:10.1016/0045-2068(91)90048-T
Zhang, C., Su, H., and Tan, T. (2013). Batch and Semi-continuous Anaerobic Digestion of Food Waste in a Dual Solid-Liquid System. Bioresour. Technol. 145, 10–16. doi:10.1016/j.biortech.2013.03.030
Zhou, R., and Gibbons, W. (2015). Genetically Engineered Cyanobacteria. Available at: https://ntrs.nasa.gov/citations/20150004038 (Accessed April 22, 2021).
Keywords: nitrogen, nitrogen recycling, regenerative life support, nitrogen fixation, nitrogen on Mars
Citation: Langenfeld NJ, Kusuma P, Wallentine T, Criddle CS, Seefeldt LC and Bugbee B (2021) Optimizing Nitrogen Fixation and Recycling for Food Production in Regenerative Life Support Systems. Front. Astron. Space Sci. 8:699688. doi: 10.3389/fspas.2021.699688
Received: 24 April 2021; Accepted: 07 June 2021;
Published: 18 June 2021.
Edited by:
Cyprien Verseux, University of Bremen, GermanyReviewed by:
Ruddy Wattiez, University of Mons, BelgiumCopyright © 2021 Langenfeld, Kusuma, Wallentine, Criddle, Seefeldt and Bugbee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Noah J. Langenfeld, bm9haC5sYW5nZW5mZWxkQHVzdS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.