
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Astron. Space Sci. , 28 May 2021
Sec. Astrochemistry
Volume 8 - 2021 | https://doi.org/10.3389/fspas.2021.671622
This article is part of the Research Topic Exploring the Chemical Universe View all 10 articles
The abundance of interstellar ice constituents is usually expressed with respect to the water ice because, in denser regions, a significant portion of the interstellar grain surface would be covered by water ice. The binding energy (BE) or adsorption energy of the interstellar species regulates the chemical complexity of the interstellar grain mantle. Due to the high abundance of water ice, the BE of surface species with the water is usually provided and widely used in astrochemical modeling. However, the hydrogen molecules would cover some part of the grain mantle in the denser and colder part of the interstellar medium. Even at around ∼10 K, few atoms and simple molecules with lower adsorption energies can migrate through the surface. The BE of the surface species with H2 substrate would be very different from that of a water substrate. However, adequate information regarding these differences is lacking. Here, we employ the quantum chemical calculation to provide the BE of 95 interstellar species with H2 substrate. These are representative of the BEs of species to a H2 overlayer on a grain surface. On average, we notice that the BE with the H2 monomer substrate is almost ten times lower than the BE of these species reported earlier with the H2O c-tetramer configuration. The encounter desorption of H and H2 was introduced [with
Interstellar grains mainly consist of amorphous silicate and some form of carbonaceous materials (Li, 2004). It is now well established that these grains can significantly constrain the chemical composition of molecular clouds or star-forming regions. In the cloud’s denser regions, where the temperature is reasonably low (∼10 K), the grain surface would be covered by icy layers. A sizable portion of these icy layers may contain water molecules. Thus, providing the binding energies (BEs) with the water as a substrate is proper. In reality, the surface species would face a bare grain in the diffuse region. Some ice layers would grow on the top of this grain surface in the denser medium and host the incoming species. The composition of this ice layer depends on the initial elemental abundance of the species in that region. It would not necessarily always be H2O-dominated. There are ample examples of the presence of a notable portion of CO, CO2, CH4, NH3, CH3OH, etc., on the ice (Gibb et al., 2004; Das et al., 2010; Das and Chakrabarti, 2011; Das et al., 2016; Gorai et al., 2020a).
Hydrogen molecules are ubiquitous in denser regions of the interstellar medium (ISM). Thus, its accretion rate on the grain is much higher in comparison to the others. However, because of the low adsorption energy, it can be easily desorbed from the grain. Despite this, a significant portion of the grain mantle would be covered by molecular hydrogen, especially around the cold and dense interstellar condition. This inhomogeneous surface coverage could influence the mobility of the other surface species. Initially, the encounter desorption mechanism was introduced by Hincelin et al. (2015) to eliminate the overestimation of the abundance of molecular hydrogen on the grain. This desorption process occurs during surface diffusion and is induced by the presence of repulsive inter-H2 forces, effectively reducing the BE of H2. They considered
A substantial amount of BE values are available from the temperature-programmed desorption (TPD) studies on various model substrates like graphite, diamond-like carbon, amorphous or crystalline silica, silicates, water, and other ice surfaces (Collings et al., 2004; Noble et al., 2012; Ward et al., 2012; Dulieu et al., 2013). But, the BE of the species with H2 substrate is yet to be known. Cuppen and Herbst (2007) had estimated the BE of H atom on H2 substrate ∼45 K by following Vidali et al. (1991). They also estimated the BEs of O, OH,
A vital impediment in examining the encounter desorption with other species is the shortage of information about the adsorption energy of these species with H2 molecule. Here, we employ quantum chemical calculations to determine the adsorption energy of these species with H2 molecule. Obtained BE assessments are executed in our Chemical Model of Molecular Cloud (hereafter CMMC) (Das et al., 2015a; Das et al., 2015b; Gorai et al., 2017a; Gorai et al., 2017b; Sil et al., 2018; Gorai et al., 2020b). The encounter desorption effect is vital during the prestellar core phase. Studying the formation of stars is one of the essential intricacies of astrophysics. A complete understanding of the star formation process is yet to be established. However, in brief, stars are formed by a long condensation process (Pagani et al., 2013). In the beginning, warm diffuse material (∼8000 K) converts into a cold neutral atomic gas (∼100 K and ∼10–100 cm−3). After further evolution, it transforms into a more dense region (102–104 cm−3 and ∼10–20 K). If no other heating source is present, then a dense core (>104 cm−3) appears in some places of these turbulent materials. Some of these cores further evolve into prestellar cores (>105 cm−3) (Bergin and Tafalla, 2007; Keto and Caselli, 2008). Prestellar cores further continue their evolution for the formation of the protostar. Due to the accretion of atoms and molecules, gas-phase abundance is depleting, whereas the molecular ice mantles form. The chemical composition of the grain mantle is mainly governed by the addition of atomic hydrogen with the atoms or simple molecules. The chemical composition of the bulk ices further varies with the star formation process associated with it. Depending on this, it is expected that the ice composition would be very different in various places. However, from the infrared observations, it was observed that the significant repositories of interstellar hydrogen, oxygen, carbon, and nitrogen are H2O, CH3OH, H2CO, CO, CO2, CH4, and NH3 (Gibb et al., 2000; Whittet et al., 2007; Öberg et al., 2008; Boogert et al., 2015).
This article is compiled as follows. In Section 2, we confer computational methodology. Discussion and results are presented in Section 3, and finally, in Section 4, we conclude.
Here, we have utilized the Gaussian 09 suite of programs (Frisch et al., 2013) for quantum chemical calculations. In a periodic treatment of surface adsorption phenomena, the BE is related to the interaction energy, (ΔE), as follows:
For a bounded adsorbate, the BE is a positive quantity and is defined as follows:
where
To find the optimized energy of all structures, we have used a second-order Møller–Plesset (MP2) method with an aug-cc-pVDZ basis set (Dunning and Thom, 1989). We have considered 95 interstellar species for the computation of their BEs with the H2 substrate. To make the calculation more straightforward, we have considered a monomer configuration of the H2 molecule as an adsorbent. The adsorbates noted in Table 1 are placed at a suitable distance from the adsorbent with a weak bond so that a van der Waals interaction occurs during the optimization. All the optimized geometries are provided in the supplementary information. We must confess that the interstellar species considered in this study are often larger than the H2. Since the estimated BEs are different in different locations, this may lead to a fallacious estimation. It is recommended to take the average whenever different binding sites are found. Despite these flaws, it can provide us with a general picture and startup initiative to compare the BE of a species with the water and H2 substrate. Following the BE calculations carried out by Das et al. (2018) (see Tables 2 and 3), here we have not considered the ZPE and BSSE corrections for our BE calculations. All the obtained BE values are noted in Table 1. It is interesting to note that except for phosphorous, the calculated adsorption energy of most of the abundant atoms (H, C, N, O, and S) with the H2 substrate is found to be <100 K.
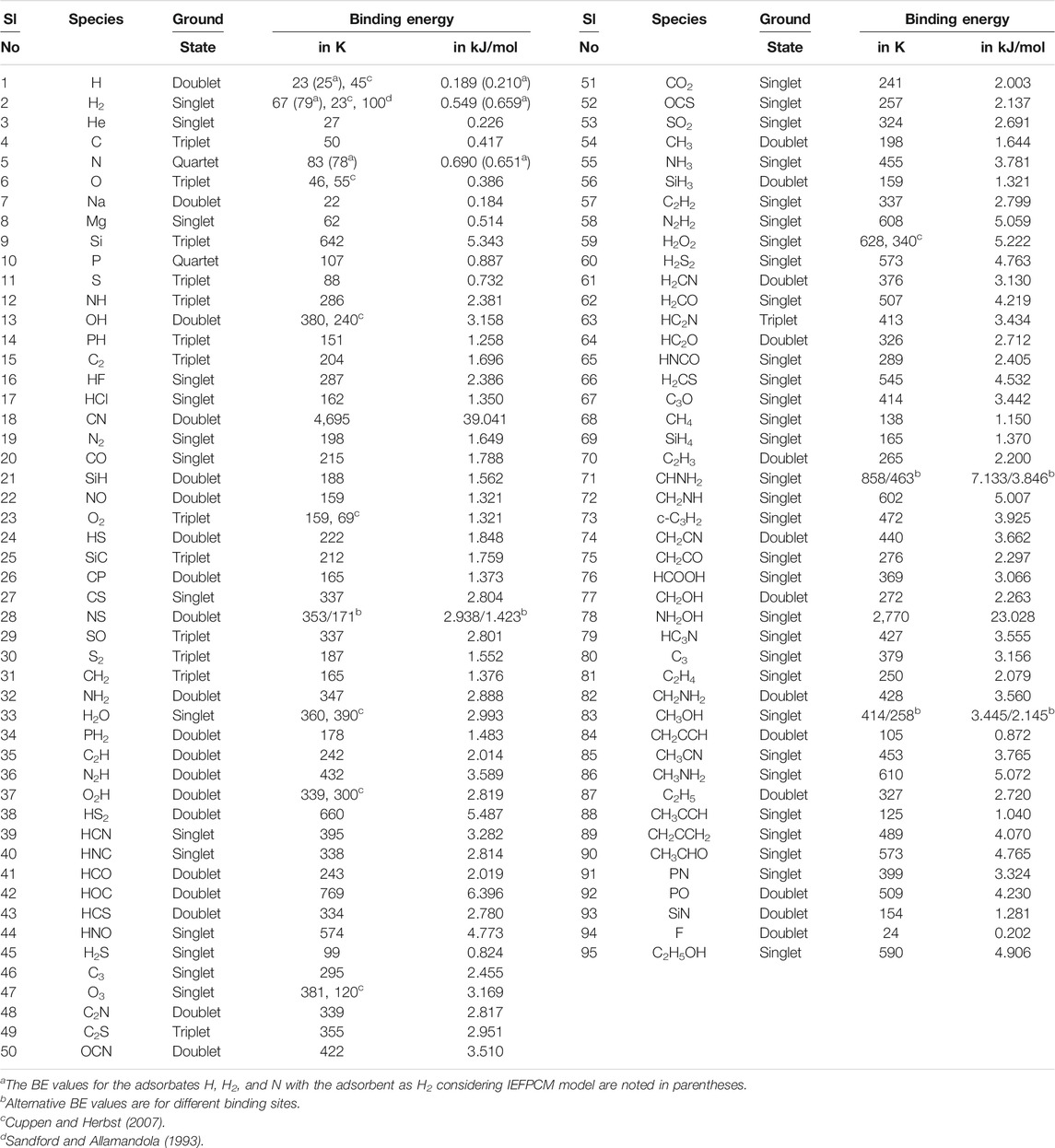
TABLE 1. Calculated binding energy (with MP2/aug-cc-pVDZ) of various species with H2 monomer surface.
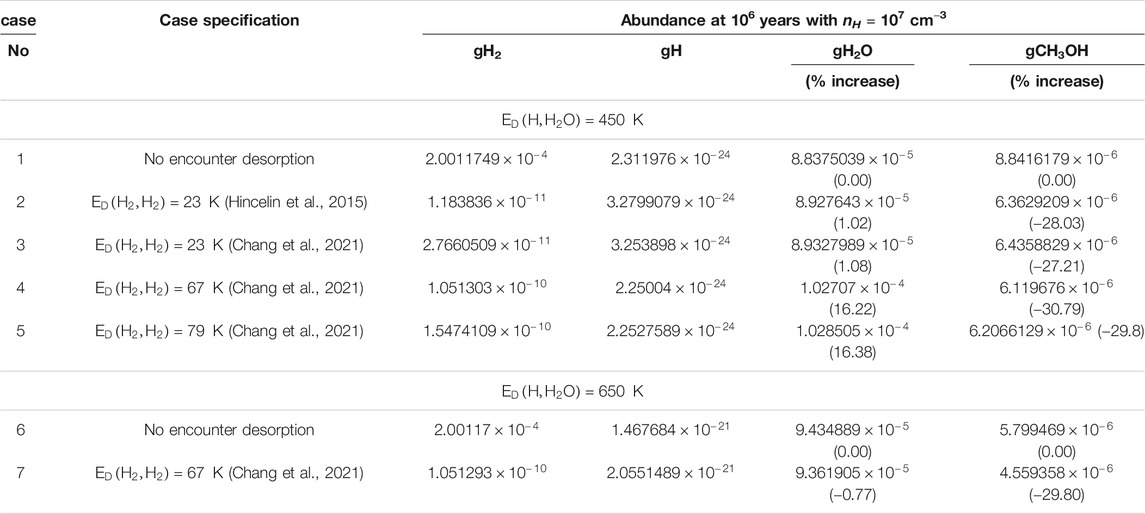
TABLE 3. The obtained abundance of gH2, gH, gH2O, and gCH3OH for the effect of encounter desorption of H2 under various situations with
In Table 1, we have reported the BE values obtained by considering a free-standing H2 interacting with a species. But in reality, this H2 would be pre-adsorbed and can feel the surface. It would yield a different BE than the previous case. To check the effect of condensed H2O in the ice phase, we also have calculated the interaction energy by considering the molecule embedded in a continuum solvation field. For this purpose, we have examined the local effects and the integral equation formalism (IEF) variant of the polarizable continuum model (PCM) (Cancès et al., 1997; Tomasi et al., 2005) with water as a solvent (Gorai et al., 2020a). The obtained values for H, H2, and N with the IEFPCM model are noted in Table 1 (in parentheses). The two calculations significantly differ. For example, with the free-standing H2, we have the BE of H, H2, and N ∼23 K, 67 K, and 83 K, respectively, whereas with the IEFPCM model, we have obtained ∼25 K, 79 K, and 78 K, respectively. So, the free-standing H2 underestimated the BE of H and H2 by 2 and 12 K, whereas it overestimated N’s case by 5 K. We also provided in Table 1 the literature BE values (Sandford and Allamandola, 1993; Cuppen and Herbst, 2007) (if available) for the comparison.
Das et al. (2018) provided BEs of the ∼100 interstellar species considering the c-tetramer configuration of water molecules. Table 1 shows the BE of the roughly same interstellar species with the H2 monomer. We have noticed that the obtained BEs with H2 are much smaller than those of the water tetramer configuration. On average, we have received almost 10 times lower BEs with the H2 surface. Table 1 shows the ground state of the species used to calculate the BE. The values of BE are very much sensitive on the chosen ground state spin multiplicity. To evaluate the ground state spin multiplicity of each species, we have taken the help of Gaussian 09 suite of program. The way to check for the ground state spin multiplicity is to run separate calculations (job type “opt + freq”), each with different spin multiplicities, and then compare the results between them. The lowest energy electronic state solution of the chosen spin multiplicity is the ground state noted for the species in Table 1.
We have included the encounter desorption phenomenon in our CMMC code (Das et al., 2015a; Das et al., 2015b; Das et al., 2016; Gorai et al., 2017a; Gorai et al., 2017b; Sil et al., 2018; Gorai et al., 2020b) to study its effect. The surface chemistry network of our model is mostly adopted from Ruaud et al. (2015), Das et al. (2015b), and Gorai et al. (2020b). The gas-phase network of the CMMC model is mainly adopted from the UMIST database (McElroy et al., 2013). Additionally, we have also included the deuterated gas-phase chemical network from the UMIST. A cosmic ray rate of 1.3 × 10−17 s−1 is considered in all our models. Cosmic ray–induced desorption and nonthermal desorption rates with a fiducial parameter of 0.01 is considered. For all the grain surface species, we have adopted a photodesorption rate of 1 × 104 per incident UV photon (Ruaud et al., 2015). A sticking coefficient of 1.0 is considered for the neutral species except for the H and H2. The sticking coefficients of H and H2 are considered by following the relation proposed by Chaabouni et al. (2012). Following Garrod and Pauly (2011), here we have implemented the competition between diffusion, desorption, and reaction. For the diffusion energy (Eb), we have considered R × adsorption energy (ED). Here, R is a scaling factor that can vary between 0.35 and 0.8 (Garrod et al., 2007). The BE of the species is mostly considered from Wakelam et al. (2017), and a few from Das et al. (2018). Table 2 refers to the adopted initial abundances concerning the total hydrogen nuclei in all forms. Except for HD’s value in Table 2, elemental abundances are taken from Semenov et al. (2010). We considered the initial abundances of HD from Roberts and Millar (2000).
The encounter desorption effect was first introduced by Hincelin et al. (2015). The rate of encounter desorption of H2 on the surface of H2 is defined as follows:
where gH2 is the surface concentration of H2 molecules in cm−3,
In the above equation,
There would be various desorption factors (by thermal, reactive, cosmic ray, etc.). The thermal desorption is defined as
where
First of all, we have benchmarked our model with Hincelin et al. (2015). In Figure 1, we have compared our results with those obtained in Hincelin et al. (2015). For this comparison, following Hincelin et al. (2015), we have used T = 10 K,
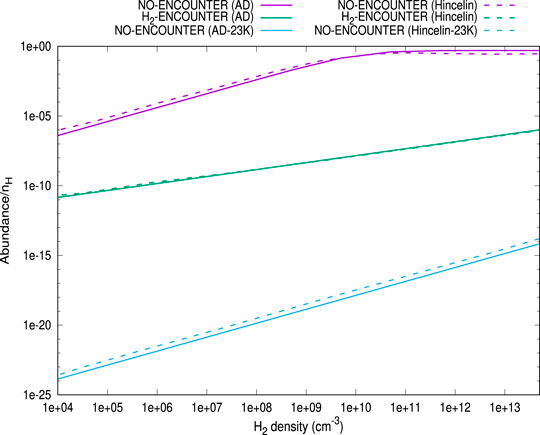
FIGURE 1. The comparison between Figure 2 of Hincelin et al. (2015) and the cases obtained here. We have extracted Figure 2 of Hincelin et al. (2015) by using the online tool of Rohatgi (2020). Three cases are shown: (A) no encounter desorption is considered with
Figure 2 shows the time evolution of gH2 by considering
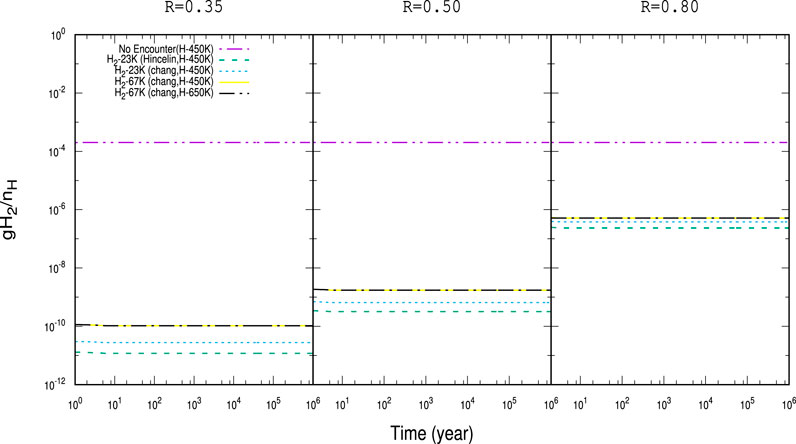
FIGURE 2. Time evolution of the abundances of gH2 with
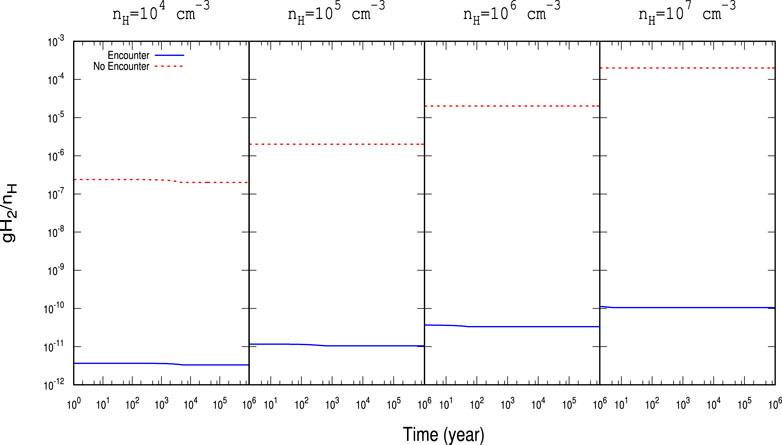
FIGURE 3. Time evolution of gH2 with
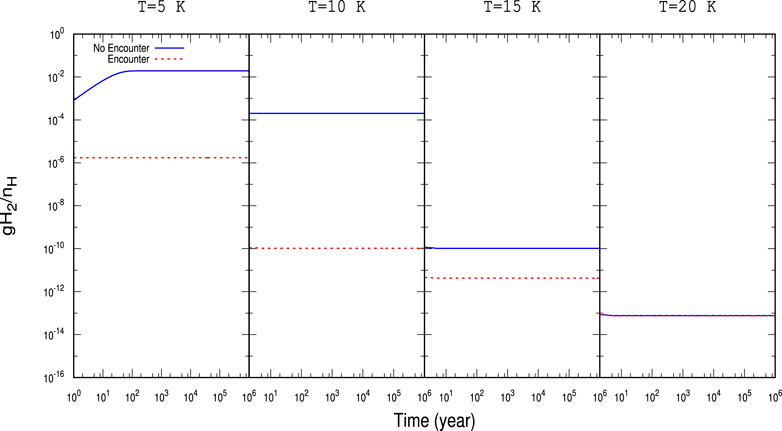
FIGURE 4. Time evolution of gH2 with
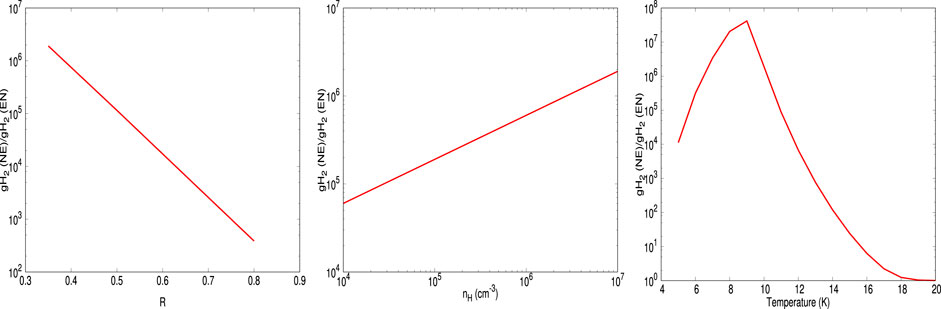
FIGURE 5. The ratio between the final abundances of gH2 obtained with the no encounter (NE) desorption and encounter desorption (EN) is shown. From left to right, it shows the variation of this ratio with R,
For a better illustration, the obtained abundances with
The obtained abundance of gH is noted in Table 3. The gH abundance is marginally decreased in Chang et al. (2021) compared to Hincelin et al. (2015). The use of higher
The effect of the encounter desorption on the other major surface species (gH2O and gCH3OH) is also shown in Table 3. In the bracketed term, we have noted the percentage increase in their abundances from the case where no encounter desorption was considered [for
The idea of encounter desorption Hincelin et al. (2015) primarily arose to eliminate the enhanced surface coverage of H2 in the relatively denser and colder medium. Since H2 has lower adsorption energy with the water surface (∼440 K), it could move on the surface very fast and occupy a position on the top of another H2 molecule. Comparatively, in the denser and colder region, the chances of this occurrence enhance. Since the H2 molecule on H2 has negligible BE [23 K used in Cuppen and Herbst (2007), Hincelin et al. (2015)], it could easily desorb back to the gas phase. Other surface species can, of course, meet with H2, but the idea of this encounter desorption arises when the species can occupy a position on the top of the H2 molecule. For example, a carbon atom is having a BE of 10,000 K (Wakelam et al., 2017). H2 could quickly meet one C atom on the grain surface, but due to the lower mobility of atomic carbon at a low temperature, every time H2 will be on the top of the carbon atom. Since the whole C-H2 system is attached to the water substrate, this will not satisfy the encounter desorption probability. Among the various key elements considered in this study, gH, gN, and gF have the BE of 650 K (Wakelam et al., 2017), 720 K (Wakelam et al., 2017), and 800 K (listed in the original OSU gas-grain code from Eric Herbst group in 2006), respectively, with the water ice. It yields a reasonable time scale for hopping even at a low grain temperature (∼10 K). Since the initial elemental abundance of F is negligible, we can neglect its contribution. The hopping time scale is heavily dependent on the assumed value of R. For example, by considering
In Figure 6, we have shown the time evolution of the abundances of gH, gH2, gN, gD, and gHD with
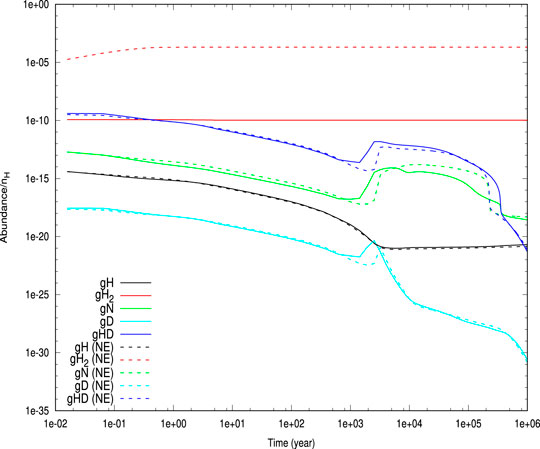
FIGURE 6. Time evolution of the abundances of H, H2, D, HD, and N obtained from our simulation is shown. Solid curves represent the cases by considering the encounter desorption [with
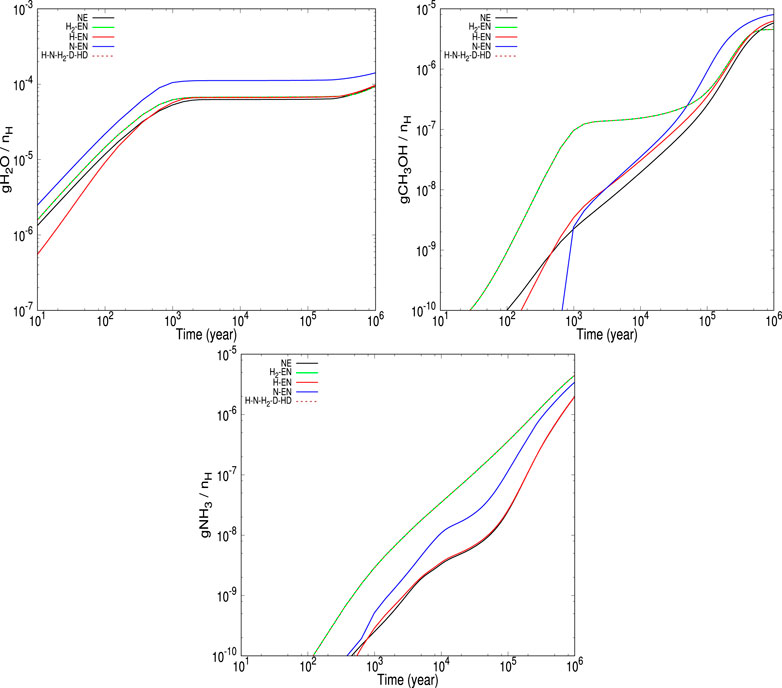
FIGURE 7. The time evolution of the abundances of ice phase water (first panel), methanol (second panel), and ammonia (third panel) is shown for
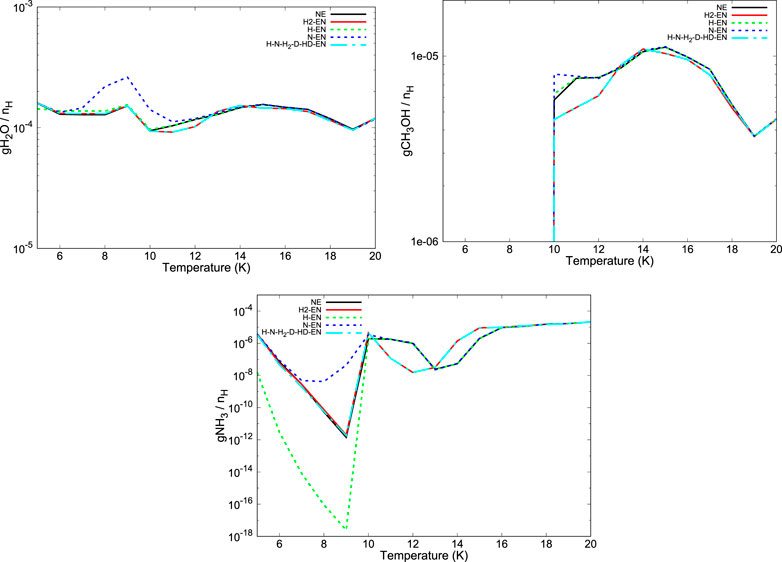
FIGURE 8. Temperature variation of the abundances of ice phase water (first panel), methanol (second panel), and ammonia (third panel) is shown for
Here, we have provided realistic BEs of the interstellar species with the H2 substrate. Supported with these BE values, we further have implemented our CMMC model to check the encounter desorption effect of H2, H, and N on the interstellar ices. Following are the major highlights of this study.
• Our quantum chemical calculation finds a lower BE value (∼10 times) of all the species than it was obtained with the water (Das et al., 2018) substrate.
• Earlier in the literature,
• Our modeling results suggest that the inclusion of the encounter desorption of the H, H2, and N can affect the abundances of the major surface constituents like water, methanol, and ammonia. The cumulative effect roughly resembles a similar abundance with that obtained with the H2’s encounter desorption only. For a bit higher temperature (∼20 K), when the encounter desorption effect of H2 ceases, the encounter desorption of the cumulative cases exactly matches with the no encounter desorption case.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
MS acknowledges DST, the Government of India, for providing financial assistance through the DST-INSPIRE Fellowship [IF160109] scheme. SA and SS acknowledge Indian Center for Space Physics for allowing them to continue their M. Sc. project work. This research was possible in part due to a Grant-In-Aid from the Higher Education Department of the Government of West Bengal.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fspas.2021.671622/full#supplementary-material
Bergin, E. A., and Tafalla, M. (2007). Cold Dark Clouds: The Initial Conditions for Star Formation. Annu. Rev. Astron. Astrophysics 45, 339–396. doi:10.1146/annurev.astro.45.071206.100404
Boogert, A. C. A., Gerakines, P. A., and Whittet, D. C. B. (2015). Observations of the Icy Universe. Annu. Rev. Astron. Astrophysics 53, 541–581. doi:10.1146/annurev-astro-082214-122348
Cancès, E., Mennucci, B., and Tomasi, J. (1997). A New Integral Equation Formalism for the Polarizable Continuum Model: Theoretical Background and Applications to Isotropic and Anisotropic Dielectrics. J. Chem. Phys. 107, 3032–3041. doi:10.1063/1.474659
Chaabouni, H., Bergeron, H., Baouche, S., Dulieu, F., Matar, E., Congiu, E., et al. (2012). Sticking Coefficient of Hydrogen and Deuterium on Silicates under Interstellar Conditions. Astron. Astrophysics 538, A128. doi:10.1051/0004-6361/201117409
Chakrabarti, S. K., Das, A., Acharyya, K., and Chakrabarti, S. (2006a). Effective Grain Surface Area in the Formation of Molecular Hydrogen in Interstellar Clouds. Astron. Astrophysics 457, 167–170. doi:10.1051/0004-6361:20065335
Chakrabarti, S. K., Das, A., Acharyya, K., and Chakrabarti, S. (2006b). Recombination Efficiency of Molecular Hydrogen on Interstellar Grains-II. A Numerical Study. Bull. Astronom. Soc. India 34, 299. https://ui.adsabs.harvard.edu/abs/2006BASI...34..299C/abstract
Chang, Q., Zheng, X. L., Zhang, X., Quan, D. H., Lu, Y., Meng, Q. K., et al. (2021). On the Encounter Desorption of Hydrogen Atoms on an Ice Mantle. Res. Astron. Astrophys. 21, 039. doi:10.1088/1674-4527/21/2/39
Collings, M. P., Anderson, M. A., Chen, R., Dever, J. W., Viti, S., Williams, D. A., et al. (2004). A Laboratory Survey of the Thermal Desorption of Astrophysically Relevant Molecules. Monthly Notices R. Astronom. Soc. 354, 1133–1140. doi:10.1111/j.1365-2966.2004.08272.x
Cuppen, H. M., and Herbst, E. (2007). Simulation of the Formation and Morphology of Ice Mantles on Interstellar Grains. Astrophysical J. 668, 294–309. doi:10.1086/521014
Das, A., Acharyya, K., Chakrabarti, S., and Chakrabarti, S. K. (2008a). Formation of Water and Methanol in Star Forming Molecular Clouds. Astron. Astrophysics 486, 209–220. doi:10.1051/0004-6361:20078422
Das, A., Acharyya, K., and Chakrabarti, S. K. (2010). Effects of Initial Condition and Cloud Density on the Composition of the Grain Mantle. Monthly Notices R. Astronomical Soc. 409, 789–800. doi:10.1111/j.1365-2966.2010.17343.x
Das, A., and Chakrabarti, S. K. (2011). Composition and Evolution of Interstellar Grain Mantle under the Effects of Photodissociation. Monthly Notices R. Astronomical Soc. 418, 545–555. doi:10.1111/j.1365-2966.2011.19503.x
Das, A., Majumdar, L., Chakrabarti, S. K., and Sahu, D. (2015b). Deuterium Enrichment of the Interstellar Medium. New Astron. 35, 53–70. doi:10.1016/j.newast.2014.07.006
Das, A., Majumdar, L., Sahu, D., Gorai, P., Sivaraman, B., and Chakrabarti, S. K. (2015a). Methyl Acetate and its Singly Deuterated Isotopomers in the Interstellar Medium. Astrophys. J. 808, 21. doi:10.1088/0004-637X/808/1/21
Das, A., Sahu, D., Majumdar, L., and Chakrabarti, S. K. (2016). Deuterium Enrichment of the Interstellar Grain Mantle. Monthly Notices R. Astronomical Soc. 455, 540–551. doi:10.1093/mnras/stv2264
Das, A., Sil, M., Gorai, P., Chakrabarti, Si. K., and Loison, J. C. (2018). An Approach to Estimate the Binding Energy of Interstellar Species. Astrophys. J. Suppl. Ser. 237, 9. doi:10.3847/1538-4365/aac886
Dulieu, F., Congiu, E., Noble, J., Baouche, S., Chaabouni, H., Moudens, A., et al. (2013). How Micron-Sized Dust Particles Determine the Chemistry of Our Universe. Sci. Rep. 3, 1338. doi:10.1038/srep01338
Dunning J Thom, H. (1989). Gaussian Basis Sets for Use in Correlated Molecular Calculations. I. The Atoms Boron through Neon and Hydrogen. J. Chem. Phys. 90, 1007–1023. doi:10.1063/1.456153
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., et al. (2013). Gaussian 09 Revision D.01. Wallingford CT: Gaussian Inc.
Garrod, R. T., and Pauly, T. (2011). On the Formation of CO2 and Other Interstellar Ices. Astrophysical J. 735, 15. doi:10.1088/0004-637X/735/1/15
Garrod, R. T., Wakelam, V., and Herbst, E. (2007). Non-thermal Desorption from Interstellar Dust Grains via Exothermic Surface Reactions. Astron. Astrophys. 467, 1103–1115. doi:10.1051/0004-6361:20066704
Gibb, E. L., Whittet, D. C. B., Boogert, A. C. A., and Tielens, A. G. G. M. (2004). Interstellar Ice: The Infrared Space Observatory Legacy. Astrophys. J. Suppl. Ser. 151, 35–73. doi:10.1086/381182
Gibb, E. L., Whittet, D. C. B., Schutte, W. A., Boogert, A. C. A., Chiar, J. E., Ehrenfreund, P., et al. (2000). An Inventory of Interstellar Ices toward the Embedded Protostar W33a. Astrophysical J. 536, 347–356. doi:10.1086/308940
Gorai, P., Bhat, B., Sil, M., Mondal, S. K., Ghosh, R., Chakrabarti, S. K., et al. (2020b). Identification of Prebiotic Molecules Containing Peptide-like Bonds in a Hot Molecular Core, G10.47+0.03. Astrophys. J. 895, 86. doi:10.3847/1538-4357/ab8871
Gorai, P., Das, A., Das, A., Sivaraman, B., Etim, E. E., and Chakrabarti, Si. K. (2017a). A Search for Interstellar Monohydric Thiols. Astrophys. J. 836, 70. doi:10.3847/1538-4357/836/1/70
Gorai, P., Das, A., Majumdar, L., Chakrabarti, S. K., Sivaraman, B., and Herbst, E. (2017b). The Possibility of Forming Propargyl Alcohol in the Interstellar Medium. Mol. Astrophys. 6, 36–46. doi:10.1016/j.molap.2017.01.004
Gorai, P., Sil, M., Das, A., Sivaraman, B., Chakrabarti, S. K., Ioppolo, S., et al. (2020a). Systematic Study on the Absorption Features of Interstellar Ices in the Presence of Impurities. ACS Earth Space Chem. 4, 920–946. doi:10.1021/acsearthspacechem.0c00098
Hasegawa, T. I., Herbst, E., and Leung, C. M. (1992). Models of Gas-Grain Chemistry in Dense Interstellar Clouds with Complex Organic Molecules. Astrophys. J. Suppl. Ser. 82, 167. doi:10.1086/191713
Hincelin, U., Chang, Q., and Herbst, E. (2015). A New and Simple Approach to Determine the Abundance of Hydrogen Molecules on Interstellar Ice Mantles. Astron. Astrophys. 574, A24. doi:10.1051/0004-6361/201424807
Keto, E., and Caselli, P. (2008). The Different Structures of the Two Classes of Starless Cores. Astrophysical J. 683, 238–247. doi:10.1086/589147
Li, A. (2004). Interaction of Nanoparticles with Radiation. in Astrophysics of Dust. Editors A. N. Witt, G. C. Clayton, and B. T. Draine (Astronomical Society of the Pacific Conference Series), Estes Park, CO, 309, 417.
McElroy, D., Walsh, C., Markwick, A. J., Cordiner, M. A., Smith, K., and Millar, T. J. (2013). The UMIST Database for Astrochemistry 2012. Astron. Astrophysics 550, A36. doi:10.1051/0004-6361/201220465
Noble, J. A., Congiu, E., Dulieu, F., and Fraser, H. J. (2012). Thermal Desorption Characteristics of CO, O2 and CO2 on Non-porous Water, Crystalline Water and Silicate Surfaces at Submonolayer and Multilayer Coverages. Monthly Notices R. Astronomical Soc. 421, 768–779. doi:10.1111/j.1365-2966.2011.20351.x
Öberg, K. I., Boogert, A. C. A., Pontoppidan, K. M., Blake, G. A., Evans, N. J., Lahuis, F., et al. (2008). The C2dspitzerspectroscopic Survey of Ices Around Low-Mass Young Stellar Objects. III. CH4. Astrophys. J. 678, 1032–1041. doi:10.1086/533432
Pagani, L., Lesaffre, P., Roueff, E., Jorfi, M., Honvault, P., González-Lezana, T., et al. (2013). Philosophical Trans. R. Soc. A: Math. Phys. Eng. Sci. 370, 5201–5212. doi:10.11.1098/rsta.2012.0027
Roberts, H., and Millar, T. J. (2000). Modelling of Deuterium Chemistry and its Application to Molecular Clouds. Astron. Astrophysics 361, 388–398. https://ui.adsabs.harvard.edu/abs/2000A%26A...361..388R/abstract
Ruaud, M., Loison, J. C., Hickson, K. M., Gratier, P., Hersant, F., and Wakelam, V. (2015). Modelling Complex Organic Molecules in Dense Regions: Eley-Rideal and Complex Induced Reaction. Monthly Notices R. Astronomical Soc. 447, 4004–4017. doi:10.1093/mnras/stu2709
Sandford, S. A., and Allamandola, L. J. (1993). H2 in Interstellar and Extragalactic Ices: Infrared Characteristics, Ultraviolet Production, and Implications. Astrophys. J. Lett. 409, L65. doi:10.1086/186861
Semenov, D., Hersant, F., Wakelam, V., Dutrey, A., Chapillon, E., Guilloteau, S., et al. (2010). Chemistry in Disks. IV. Benchmarking Gas-Grain Chemical Models with Surface Reactions. Astron. Astrophys. 522, A42. doi:10.1051/0004-6361/201015149
Sil, M., Gorai, P., Das, A., Bhat, B., Etim, E. E., and Chakrabarti, S. K. (2018). Chemical Modeling for Predicting the Abundances of Certain Aldimines and Amines in Hot Cores. Astrophys. J. 853, 139. doi:10.3847/1538-4357/aa984d
Sil, M., Gorai, P., Das, A., Sahu, D., and Chakrabarti, S. K. (2017). Adsorption Energies of H and H: a Quantum-Chemical Study. Eur. Phys. J. D 71, 45. doi:10.1140/epjd/e2017-70610-4
Tomasi, J., Mennucci, B., and Cammi, R. (2005). Quantum Mechanical Continuum Solvation Models. Chem. Rev. 105, 2999–3094. doi:10.1021/cr9904009PMID: 16092826
Vidali, G., Ihm, G., Kim, H. Y., and Cole, M. W. (1991). Potentials of Physical Adsorption. Surf. Sci. Rep. 12, 135–181. doi:10.1016/0167-5729(91)90012-M
Wakelam, V., Loison, J. C., Mereau, R., and Ruaud, M. (2017). Binding Energies: New Values and Impact on the Efficiency of Chemical Desorption. Mol. Astrophys. 6, 22–35. doi:10.1016/j.molap.2017.01.002
Ward, M. D., Hogg, I. A., and Price, S. D. (2012). Thermal Reactions of Oxygen Atoms with CS at Low Temperatures on Interstellar Dust. Monthly Notices R. Astronom. Soc. 425, 1264–1269. doi:10.1111/j.1365-2966.2012.21520.x
Keywords: astrochemistry, binding energy, numerical, ISM, star formation, chemical model, Monte Carlo (MC) algorithm
Citation: Das A, Sil M, Ghosh R, Gorai P, Adak S, Samanta S and Chakrabarti SK (2021) Effect of Binding Energies on the Encounter Desorption. Front. Astron. Space Sci. 8:671622. doi: 10.3389/fspas.2021.671622
Received: 24 February 2021; Accepted: 30 April 2021;
Published: 28 May 2021.
Edited by:
Ryan C. Fortenberry, University of Mississippi, United StatesReviewed by:
Marco Minissale, Centre National de la Recherche Scientifique (CNRS), FranceCopyright © 2021 Das, Sil, Ghosh, Gorai, Adak, Samanta and Chakrabarti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ankan Das, YW5rYW4uZGFzQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.