- Department of Biosciences, School of Bio Sciences and Technology, Vellore Institute of Technology, Vellore, Tamil Nadu, India
The accumulation of heavy metals in the ecosystem due to industrialization has led to toxic effects on various life forms such as flora, fauna and microfauna. Several approaches that are currently used for the removal of heavy metals are not cost-effective and efficient. Therefore, the current study was focused on the bio-removal of chromium (Cr), lead (Pb) and zinc (Zn) using augmentation with strong biofilm forming Bacillus infantis (VITVJ8), which was confirmed by Bacillus infantis augmented to the rhizosphere region of Chrysopogon zizanioides with a percentage of 0.025%. The bioremoval process was catalyzed by superoxide dismutase, chromate reductase and catalase activity with 83U/ml, 51U/ml and 75U/ml, respectively. VITVJ8 was also found to be a plant growth promoting bacterium as it was capable of producing indole acetic acid (IAA) 63µg/ml and siderophores 1.238cm and of solubilizing insoluble phosphate 72.3%. The bacterium could tolerate Cr and Zn up to 1000ppm, whereas for Pb it was 1250ppm. Further, rhizoremediation setup consisting of C. zizanioides augmented with VITVJ8 strain showed enhanced uptake of Zn, Pb and Cr (89% > 86% > 77%) as compared to phytoremediation (60% > 62% > 59%) treated plants. Seed germination assay revealed 75.78% increase in the germination index for set-ups treated with B. infantis, which also confirmed the reduction in heavy metal (HM) toxicity upon treatment with effective strain and enhanced plant growth. Since the uptake was found to be maximum in the roots versus the shoots, vetiver can be considered as a root accumulator of Cr, Pb and Zn when amended with B. infantis.
1 Introduction
Heavy metals are a major threat to the environment due to their extensive usage in various industries. Continuous discharge of these heavy metals from industries into the environment, driven by anthropogenic activities results in adverse effects on both micro and macroorganisms. Several studies have reported the deleterious effects of heavy metals on human health (1, 2). Rapid industrialization and anthropogenic activities have led to increased contamination due to heavy metals such as Cr, Pb and Zn in the various states of India. Ranipet district of Tamil Nadu is known for the presence of numerous tanneries and other industries which in turn increases the pollution of the saturated and unsaturated zones in its vicinity (3, 4). In this region, soil and water are reported to have heavy metals at concentrations exceeding the permissible limits. According to WHO, the permissible limits for Cr, Pb and Zn in water are 0.05ppm, 0.01ppm and 3ppm respectively (5).
The existence of heavy metals such as chromium (Cr), predominantly exists in two forms i.e., trivalent Cr(III) and hexavalent chromium Cr(VI) (6, 7). While Cr(III) is considered as a vital nutrient in trace amounts for animals and humans, Cr(VI) is highly lethal and carcinogenic. The industrial use of chromium, particularly in metal plating, tanning, and dye production, poses significant contamination of soil and water (8). The industrial use of chromium in manufacturing stainless steel, dyes, pigments, and in leather tanning processes has led to widespread environmental contamination (9). Similarly, lead (Pb) is another ubiquitous heavy metal found naturally in trace amount and is introduced into the environment through various activities, including mining, smelting of ores and manufacturing of batteries (10, 11). Lead is extremely toxic, even at minimal exposure levels and is harmful to children, causing cognitive impairments and developmental delays (12).
Zinc (Zn) is a key trace element necessary for the functioning of over 300 enzymes and proteins, but excessive concentrations can be harmful and often referred as double-edged sword. Zinc contamination primarily arises from mining, smelting, industrial discharges (3, 13). Although zinc is less toxic than chromium and lead, it can disrupt the normal metabolic functions of the cells (14, 15).
Heavy metals such as chromium (Cr), lead (Pb), and zinc (Zn) are natural elements with densities greater than 5g/cm³. While trace amounts of these metals can act as micronutrients (16), higher concentrations lead to disorders in various life forms (17). The occurrence of these heavy metals in their free state is more prevalent in terrestrial and aquatic ecosystems. Due to their non-biodegradable and recalcitrant nature, heavy metals tend to get accumulated extensively in the environment. This bioaccumulation can lead to numerous health hazards, including damage to the neural, hepatic, and renal systems, as well as other disorders such as anemia and cancer (18). The mechanism involved in the toxicity of heavy metals also varies depending on the specific heavy metals and its source.
Free radical imbalance caused by oxidative stress contributes to Pb toxicity, while Cr toxicity is attributed to the formation of thiol or methyl groups, which penetrates into cell membranes and subsequently damages DNA and proteins (19, 20). The removal of these heavy metals from the environment has been a major concern for the past two decades. Discharge of effluents from industries such as paint manufacturing, tanneries and textiles are some of the major sources of heavy metal pollution (21). Some of the strategies and mechanism involved during metal-microbe interaction include bioaccumulation, bioleaching and biosorption (20, 22).
Several studies have reported the potential of biological processes in bioremoval or recovery of heavy metals from industrial effluents (7). Some of the major physical and chemical methods such as sorption, chemical dialysis, flocculation, filtration, electrodialysis, reverse osmosis, etc. have been effective in the treatment of heavy metals. However, these processes are often not recommended for metal recovery as it is not cost-efficient. Therefore, bioremediation strategies such as microbial remediation, phyto-rhizoremediation and application of mixed biosystems have gained attention due to their cost effectiveness in the bioremoval of heavy metals (23).
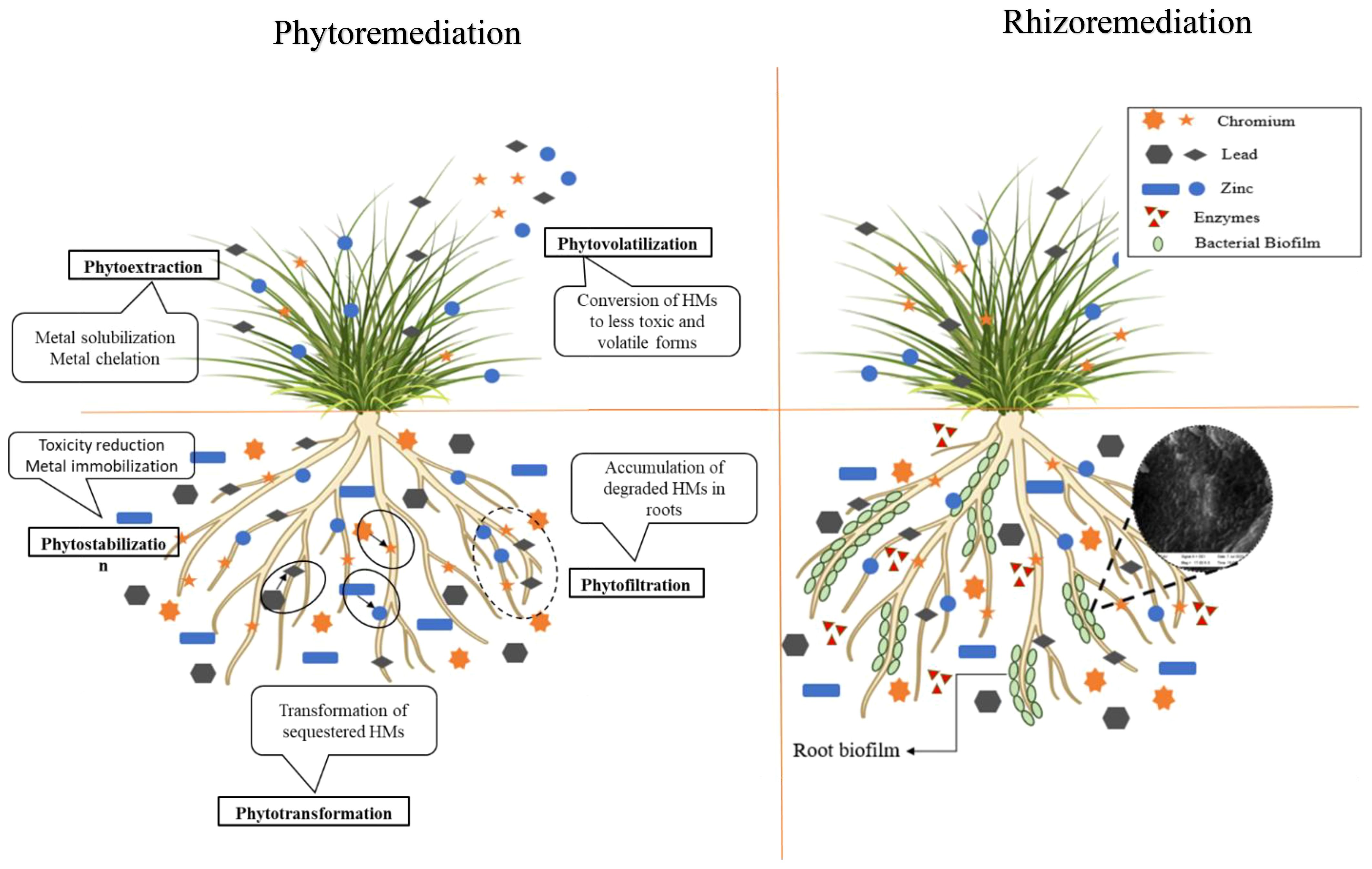
Phyto-rhizoremediation strategy utilizes plants and their associated bacteria to clean-up contaminated environments, improvisation of soil health and restoration of ecosystems. It is an ecofriendly, cost effective and a sustainable alternative to the other remediation techniques (24). Phytoremediation involves application of hyperaccumulator plants to degrade or transform pollutants into its non-toxic form. Plants like Pennisetum purpureum, Chrysopogon zizanioides etc., can take up heavy metals and transform them into non-toxic forms (25, 26).
The plant growth promoting rhizobacteria (PGPR) such as Pseudomonas, Bacillus, Agrobacterium, Azoarcus, Arthobacteria, Klebsiella, Azobacter, Serratia and Enterobacter sp. can promote plant growth in HM contaminated environments (27, 28). C. zizanioides, a perennial grass belonging to the Gramineae family, is known for its ability to survive at extreme conditions (29, 30). This plant is highly tolerant to pH levels and high salinity due to its fibrous root system (31). Studies have shown the effectiveness of vetiver in uptake of metals such as Cd, Zn, Pb and Cr. Das and Osborne (32) have previously reported C. zizanioides to be an effective tool for the removal of Pb through phyto-rhizoremediation, identifying vetiver as a root accumulator for the first time (33–35).
Therefore, the current study was aimed to identify a cost-effective and efficient process for the bioremoval of Cr, Pb and Zn using indigenous bacteria of wetland paddy rhizosphere soil. The isolates were screened for MTC, biofilm and enzymatic reduction of heavy metals. The effective strain obtained was used for phyto and rhizoremediation approach. To understand the efficacy of the dual biosystem strategy used for the bioremoval of Cr, Pb and Zn. To our knowledge, this is the first study to emphasize the role of Bacillus infantis in the bioremoval of chromium (Cr), lead (Pb), and zinc (Zn) by phyto- and rhizoremediation.
2 Materials and methods
2.1 Heavy metals and chemicals
All chemicals used in this study were of 100% purity. Potassium dichromate, lead acetate, and zinc chloride, the heavy metal salts, were sourced from Himedia. The chemicals for media preparation were obtained from Himedia and SRL, India. Reagents for the plant growth-promoting rhizobacteria (PGPR) study were procured from Sigma and SRL, India. Stock solutions for each of the three metals were prepared at a concentration of 50,000mg/l.
2.2 Sample collection
Wetland paddy rhizosphere soil samples were collected from a depth of 20cm from the agricultural lands of Ranipet Industrial Estate (12.550°N–79.170°E). The SIPCOT (State Industries Promotion Corporation of Tamil Nadu) industrial corridor, located near Vellore, Tamil Nadu, India, is primarily dominated by leather, chemical, and heavy metal fabrication industries. The study was conducted using a random sampling method. A total of 12 soil samples were collected in sterile polyethylene bags and were transported to the laboratory within 2h for the isolation of bacteria capable of tolerating Cr, Pb and Zn. The physicochemical properties of the soil such as pH, conductivity and other micronutrients were analyzed and are mentioned in Supplementary Table S1.
2.3 Isolation of Cr, Pb and Zn resistant bacteria by direct and enrichment methods
The collected soil samples were pooled and serially diluted, plating was performed from 10−4 and 10−5 dilutions into modified LGI agar plates supplemented with 100ppm of Cr and 250ppm of Pb and Zn in the form of potassium dichromate, lead acetate and zinc chloride respectively. The concentrations selected was 10 folds higher than the permissible limits suggested by ATSDR, EPA and APHA due to the variation in concentration that is expected to be released from industries. For enrichment technique, the soil samples were transferred into LGI liquid medium supplemented with same metals used for LGI solid medium and the tubes were incubated for 7 days at 120 rpm. Upon enrichment, serial dilution was performed to obtain morphologically distinct colonies which were further purified and maintained at 50% glycerol stock (36).
2.4 Screening for Cr, Pb and Zn resistant effective bacteria
2.4.1 Determination of maximum tolerance concentration by plate and broth assay
For plate assay LGI agar medium was supplemented with Cr, Pb and Zn at concentrations ranging from 100–500ppm. All the isolates were inoculated and the plates were incubated for 48h at 28 ± 2°C. Visible growth on media plates indicated the ability of the isolate to resist and grow at the concentration provided (37).
Similarly, in the broth assay, LGI media was supplemented with varying concentrations of heavy metals (Cr, Pb and Zn) ranging from 100–1500ppm. The media was inoculated with 2% seed culture (0.27 OD) of the isolates and the tubes were incubated in dynamic conditions for 48h at 28 ± 2°C. Drop plate assay was performed to confirm the viability of cells (38).
2.4.2 Detection for biofilm formation by test tube and microtiter plate assay
Test tube assay was conducted to assess the biofilm forming capacity of the bacterial isolate VITVJ8. LGI broth was amended with 100ppm of Cr and 250ppm of Pb and Zn. The samples were inoculated with 2% (v/v) of seed culture (VITVJ8) and were incubated on a rotary shaker at 28 ± 2°C for 48h. Upon incubation, the bacterial broth was carefully discarded, and the test tubes were rinsed thoroughly with sterile distilled water. Crystal violet (0.1%) was added to the tubes to stain the biofilm, if formed on the walls of the test tube. The formation of purple coloration in the tubes indicated the ability of the isolate in forming biofilm.
Microtiter plate assay was performed using a similar protocol as the test tube assay, LGI broth was supplemented with 100ppm of Cr, 250ppm of Pb and Zn in a 96-well microtiter plate. Each well was inoculated with 2% (v/v) of VITVJ8 and incubated on a rotary shaker for 48h. After incubation, the absorbance of the wells was recorded at 620nm, Further, the broth was carefully discarded, and the wells were rinsed thoroughly with sterile distilled water. 0.1% crystal violet was then added to the wells to stain the biofilm and it was recorded at 560nm (39). All experiments were performed in triplicates.
The specific biofilm formation (SBF) was calculated based on the following formula.
AB – OD at 560nm of crystal violet stained bacteria.
CW – OD at 560nm of crystal violet stained control.
G – OD at 620nm of cells before staining.
2.5 Screening for enzymatic activity of the effective strain
The catalase activity was estimated using hydrogen peroxide (H2O2) as described by Das and Osborne (40). The reaction mixture consisting of 0.1ml of cell free broth, 0.9ml of phosphate buffer and 20mM H2O2 solution in sodium potassium phosphate buffer at pH7.0 and the reaction was initiated and incubated for 1min at room temperature. To stop the reaction 1ml of 1mM sulfuric acid (H2So4) was added. Initial and final absorbance after 1 min of incubated was recorded spectrophotometrically at 240nm. The reaction mixture without cell free broth served as control. For determination of chromate reductase activity, diphenyl carbazide (DPC) was used. To measure the reduction of hexavalent chromium (Cr VI) to trivalent chromium (Cr III). The 200µl reaction mixture consists of 80 µl of cell-free broth, 0.1 mM NADH, and 12.5mg/l of K2Cr2O7 (Cr VI) in a potassium phosphate buffer at pH 7.0. A reaction mixture without the cell-free broth serves as the control. The mixture is incubated at 30°C for 30 minutes, after which the residual Cr VI is quantified using the DPC reagent (41). Superoxide dismutase (SOD) activity was performed by adding 0.025mM of Nitro Blue Tetrazolium (NBT) solution (0.1ml), 0.1mM of NADH solution (0.1ml), 0.8ml of sodium phosphate buffer at pH 7.4 and 0.1ml of cell free lysate were mixed thoroughly and the reaction mixture was incubated at room temperature for 5 min and the absorbance was measured at 560nm using spectrophotometer (42).
2.6 Molecular characterization of the effective strain VITVJ8
The effective strain VITVJ8 was characterized morphologically and biochemically by the conventional tests such as gram staining, IMViC, catalase and oxidase (43). Genomic DNA is extracted from VITVJ8 using fluorescent dye terminator technique, ensuring the purity and integrity of the DNA for downstream applications. The 16S rRNA gene, which is highly conserved among bacteria, is amplified using polymerase chain reaction (PCR) with universal primers 27F and 1492R.
● 27F Primer Sequence: 5′-AGA GTT TGA TCC TGG CTC AG-3′
● 1492R Primer Sequence: 5′-GGT TAC CTT GTT ACG ACT/T3′
The amplified 16S rRNA gene PCR product is purified to remove any unincorporated nucleotides, primers, and other contaminants. The purified PCR product is sequenced using automated DNA sequencers to determine the exact nucleotide sequence of the 16S rRNA gene. The obtained sequences are analyzed and compared with NCBI’s GenBank. BLAST (Basic Local Alignment Search Tool) is used to find the closest matches and identify the bacterial isolate. A phylogenetic tree was constructed using the neighbor joining method using mega 2.0 software. The tree was bootstrapped to indicate the evolutionary distance of the effective strain to its neighbor (44, 45).
2.7 Growth kinetics
The impact of heavy metals such as Cr, Pb and Zn on the strain VITVJ8 was studied by comparing the growth conditions in the presence and absence of these metals. Throughout the experiment concentration of Cr was maintained at 100ppm, Pb and Zn concentrations were maintained at 250ppm with 2% bacterial seed culture (0.27 OD). The flask was incubated at 28 ± 2°C and the growth was monitored on 12h intervals until the cells reached the decline phase.
2.8 Optimization of the culture conditions and the bioremoval of Cr, Pb and Zn
The optimization of heavy metal removal parameters included the factors such as carbon source (glucose, sucrose and glycerol), nitrogen source (yeast extract, ammonium sulphate and urea) and pH (ranging from 4.5–8.5). These factors were designed using RSM, a design expert statistical software (V11, stat-Ease Inc. Minneapolis, Mn, USA). Preliminary experiments were carried out to determine the optimal range for each parameter.
Data analysis was performed using a quadratic model approach where one factor was analyzed in reference to the other two factors at three different level +1, 0 and −1. The percentage of heavy metal removal was designated as the response variable (R1) upon 20 experimental runs. Validity of the model and the output response were analyzed based on the results obtained from ANOVA (Analysis of Variance) and 3D contour plots (46).
Using the optimized parameters, the effective bacterial isolate was inoculated into LGI medium at the specified concentrations. The flasks were incubated at 28 ± 2°C and after incubation the cells were harvested by centrifugation at 11,000 rpm for 20 min at 4°C. Concentration of heavy metals in both supernatant and pellet was estimated using Atomic Absorption Spectrometer (AAS) model variant Spectraa 240 (47, 48).
2.9 Toxicity assessment of Cr, Pb and Zn in presence and absence of VITVJ8
2.9.1 Seed germination assay
Toxicity of Cr, Pb and Zn was tested against the seeds of Vigna radiata (VRM (Gg) 1), Vigna mungo (CO6) and Glycine max (CO1) with a sample size of n=3. Seeds were obtained from the VIT School of Agricultural Innovations and Advanced Learning (VAIAL), VIT, Vellore and were surface sterilized after soaking in sterile distilled water.
The following treatments were included
i. Positive control: water
ii. LGI broth
iii. Negative control: untreated heavy metals (100ppm Cr, 250ppm Pb and Zn)
iv. VITVJ1 treated
v. VITVJ1 treated with heavy metals (Cr, Pb and Zn)
vi. VITVJ6 treated
vii. VITVJ6 treated with heavy metals (Cr, Pb and Zn)
viii. VITVJ8 treated
ix. VITVJ8 treated with heavy metals (Cr, Pb and Zn)
The germination rate was monitored over a period of 10 to 14 days, with a 12:12 light–dark cycle. Length of plumule and radicle was measured and the percentage of seed germination was calculated using the following formula reported by Wagh et al. (49).
2.10 Characterization of PGPR traits
All the isolates were assessed for PGPR traits such as the production of IAA (indole acetic acid), siderophore, ammonia and the ability of the isolate in solubilizing insoluble phosphate. For the indole acetic acid (IAA) production, to the 48h culture free supernatant, Salkowski’s reagent and orthophosphoric acid were added and change in color from yellow to pink indicated the production of IAA which was further quantified spectrophotometrically at 530nm (50). For the siderophore production, supernatant of 48h cell free supernatant of Fiss minimal medium was added in the wells of Chrome Azurol S (CAS) agar and formation of halozone indicated siderophore production (51). For ammonia production, Nessler’s reagent was added to the 48h culture supernatant, change in color from yellow to brown indicated ammonia production (52). The ability of the isolate in converting insoluble tricalcium phosphate to soluble form was studied in Pikovskaya agar plates where the formation of halozone indicated efficient solubilization (53). The solubilization efficiency (SE) was calculated by the formula (49),
2.11 Pot culture studies
Pot culture study was performed to observe the efficiency of the effective isolate in combination with the plants, in enhancing plant growth and thereby increasing the uptake of heavy metals. C. zizanioides (vetiver) was selected for the study due to their fast-growing potential, fibrous root system and their robust nature to survive in extreme conditions like drought and water logging. Plantlets of vetiver were collected from the VIT nursery and were thoroughly washed to remove soil particles. Before planting, the plantlets were pruned to 5cm of root and shoot. The pruned plantlets were then planted in pots containing 2kg of soil. Soil used for the pot culture study was sieved (2mm) to remove the denser particles and was packed into a 2kg LDPE bags.
2.11.1 Phytoremediation treatments for the Cr, Pb and Zn uptake
In phytoremediation setup i.e., without the augmentation of effective bacteria, 2kg of soil was transferred into LDPE and the soil was artificially polluted with 50, 100 and 150ppm of Cr, Pb and Zn and the plants were constantly supplemented only with water so has to compare the growth rate in the presence and absence of VITVJ8. After the preparation of the pots the pruned plantlets were planted. The study was carried out for a period of 45 days at VIT Green house, all the treatments were carried out in triplicates (54). Plants were uprooted at a regular interval of 15 days to assess the various parameters such as plant growth measurements, chlorophyll content and estimation of HM using AAS.
2.11.2 Rhizoremediation treatments for the Cr, Pb and Zn uptake
In the rhizoremediation treatment, effective bacteria was augmented in soil and was artificially polluted with 50, 100 and 150ppm of heavy metals. The rhizoremediation pots were bioaugmented with 5ml of the effective isolate VITVJ8 (1.0 × 1012 cfu/ml) at every 0th and every 15th day of the study to maintain the microbial load. Each pot received the same amount of water and the plants were uprooted at 0th, 15th, 30th and 45th day (40). The various treatments used in the phyto-rhizoremediation study are mentioned in Supplementary Figure S7.
2.11.3 Analysis of various parameters
2.11.3.1 Plant growth measurement
Physical changes in the plants across all the treatments were monitored by uprooting at 15-day intervals. Measurements of root length and shoot height were assessed, and the data were analyzed using ANOVA for statistical significance.
2.11.3.2 Chlorophyll content
Total chlorophyll content (a and b) was estimated using Arnow’s method. One gram of fresh leaves was homogenized with 80% acetone, and the resulting mixture was then centrifuged at 10,000 rpm at 4°C. Absorbance of the supernatant was measured at 645nm and 663nm. Chlorophyll a and b were determined by Arnow’s equation (55).
2.11.3.3 Estimation of heavy metal
Root and shoot samples were dried at 55°C and digested using concentrated nitric acid as per the Environmental Protection Agency (EPA) protocol 3051a. Soil samples were heated to 60–70°C with the addition of concentrated sulphuric acid. The digested samples were filtered (syringe filter) before the assessment of Cr, Pb and Zn concentrations using atomic absorption spectroscopy (AAS), Varian’s Model spectraAA 220.
2.12 Statistical analysis
All the study was carried out in triplicates and the statistical analysis was conducted for the bioremoval of Cr, Pb and Zn by Bacillus infantis in soil and plants based on the time intervals using Origin software version 8.0. The optimization of data was evaluated using Design Expert software version 12.0. Response Surface Methodology (RSM) was employed with a quadratic model approach. Plant growth parameters was analyzed using Analysis of Variance (ANOVA) for the statistical significance.
3 Results and discussion
3.1 Isolation of effective bacteria
A total of eight morphologically distinct colonies VITVJ1 to VITVJ8 were isolated on LGI medium supplemented with 100ppm of Cr, 250ppm of Pb and Zn. Lalhriatpuii et al. (56) revealed the potential of rhizobacteria in the removal of heavy metals such as Cr, Pb and Zn. There are several studies showing the application of MSM for isolation of bacteria capable of surviving high concentrations of heavy metals. Concentration used for the study was based on the previous reports by Das and Osborne (40). For the isolation of heavy metal resistant bacteria wetland paddy rhizosphere soil was chosen due to the presence of increased abundance of bacterial diversity in the rhizosphere (57). Tekaya et al. (58) reported the role of rhizobacteria in restoring metal-contaminated soils, and the mechanisms by which PGPRs assisted in heavy metal bioremediation was studied by Riseh et al. (59).
3.2 Screening of effective bacteria
3.2.1 Maximum tolerance concentration of the isolates
In plate assay, among all the isolates, VITVJ1, VITVJ3, VITVJ6 and VITVJ8 showed higher tolerance up to 500ppm for Cr, Pb and Zn. In one of the study, isolate VITT3 showed a higher tolerance for zinc, as compared to our isolates. However, our isolates were capable of resisting three pollutants. A similar study by Pani et al. (48) reported that the bacterial isolate VITT3 effectively tolerates zinc and manganese concentrations of up to 2000mg/l. Similarly, Wani and Omozele (60) reported that Bacillus sp. (PB5) and Klebsiella sp. (PB6) exhibited the highest tolerance, withstanding up to 1000µg/ml of Cr VI. Our isolates VITVJ1, VITVJ6, and VITVJ8 showed higher tolerance levels for Cr, reaching up to 1500ppm, indicating higher resistance in our strains. Yahaghi et al. (61) found that Bacillus altitudinus (YSP104), Bacillus cereus (YSP69), and Bacillus filamentosus (YSP110) showed the highest tolerance for Pb up to 414mg/l, while YSP104 exhibited a Zn tolerance of 196.14mg/l. Our isolates showed higher tolerance towards Pb and Zn, confirming maximum resistance capabilities as compared to the strains obtained from the previous studies. Yahaghi et al. (61) found that Bacillus altitudinus (YSP104), Bacillus cereus (YSP69), and Bacillus filamentosus (YSP110) showed the highest tolerance for Pb up to 414mg/l and YSP104 exhibited tolerance to Zn up to 196.14mg/l. Our isolates exhibited similar or higher tolerance levels, especially in the case of Pb, underlining their potential for effective bioremediation. VITVJ1, VITVJ3, VITVJ6, and VITVJ8, show significant tolerance to heavy metals, which is consistent with the tolerance levels. Das et al. (43) also described Enterobacter cloacae (VITPASJ1) showed the maximum tolerance of 1000mg/l against Pb. The enhanced tolerance in broth assays, especially for Cr and Pb, indicates that these isolates can thrive in environments with concentrations of Cr, Pb and Zn, making them excellent candidates for bioremediation.
3.2.2 Screening for biofilm formation:
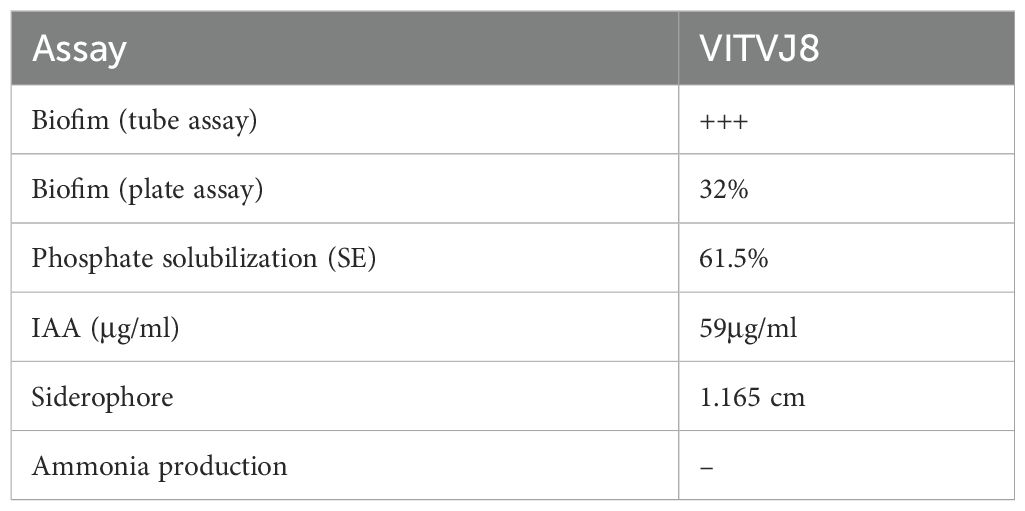
In the qualitative analysis, isolate VITVJ8 showed a dense visible purple color lining on the walls of the test tubes indicating the strong formation of biofilm. Itusha et al. (54) described how isolates VITKKAJ1 and VITKKAJ5 were capable of producing strong biofilm and it was also evident that when HM concentration increased, biofilm formation was gradually reduced. Microtiter plate quantitative assay, exhibited a significant increase in specific biofilm formation and VITVJ8 showed a 32% increase in biofilm formation when exposed to 100ppm of Cr and 250ppm of Pb and Zn (Table 1). Recent studies have emphasized the importance of biofilms, as they are known to facilitate uptake of heavy metals through sorption. In the current study, both test tube and microtiter assays revealed a similar trend i.e., increasing concentrations of heavy metals led to a decrease in biofilm formation (62). Similarly, Wagh et al. (49) also revealed that Bacillus xiamenensis VITMSJ3 can develop strong biofilm in the presence of increased concentrations of heavy metals.
3.3 Enzymatic analysis
There was an increase in enzyme activity in response to various concentrations of Cr, Pb and Zn. Notably, SOD and catalase activities were significantly enhanced with the increase in Pb concentration of up to 150ppm. Catalase activity also showed a positive correlation with Zn concentration of up to 150ppm (Supplementary Figure S2). Kumar et al. (63) reported similar pattern, where increase in SOD activity was directly proportional to the increase in Zn concentration. Similarly, reports by Shi et al. (64) and Abedi and Shahpiri (65) highlighted the crucial role of SOD in scavenging superoxide radicals, which acts as a defense mechanism against oxidative stress by breaking down H2O2 into H2O and O2. Therefore, it can be substantiated that bioremoval process was catalyzed by the activities of superoxide dismutase (SOD), chromate reductase and catalase.
In contrast, chromate reductase activity decreased with a Cr concentration of 150ppm, indicating the inverse proportionality of the isolate in resisting chromium. This finding aligns with the report of Baldiris et al. (66), who also stated the bacterial tolerance to Cr VI at 100ppm (40). The activity of chromate reductase was further elucidated, with expression observed in membrane associated chromate reductase when cells were pre-exposed to Cr VI (67). Therefore, the resistance in isolates is not by a single enzyme but a collective resistance obtained by synthesis of all the three enzymes.
3.4 Identification of effective bacteria
The bacterial strain VITVJ8 was identified as a Gram-positive, rod-shaped endospore forming motile bacteria. It was also found to be oxidase negative and catalase positive. The isolate was further characterized and identified through 16S rRNA gene sequencing. Molecular sequencing and phylogenetic tree analysis revealed that VITVJ8 has a close evolutionary relationship with Bacillus infantis. The sequence has been submitted to GenBank with the accession number OM250034 (Supplementary Figure S3).
3.5 Effect of Cr, Pb and Zn on growth kinetics of the bacterium
Growth kinetics of the isolated bacteria, both in the presence and absence of heavy metals (100ppm Cr, 250ppm Pb and Zn), showed an increased lag phase, followed by the steady exponential phase with increased cell growth (Supplementary Figure S4). In the presence of Cr, Pb and Zn, there was no significant changes observed in growth pattern of VITVJ8. There are several studies indicating the presence of extended lag phase upon exposure to heavy metals. Zn and Cu uptake by Pseudomonas sp. has been reported to have a prolonged lag phase upon treatment (68–72).
3.6 Optimization of the growth parameters of VITVJ8
The optimization of bacterial growth was primarily influenced by factors such as carbon (glucose, sucrose and glycerol) and nitrogen sources (yeast extract, ammonium sulphate and urea) and pH levels (ranging from 4.5 to 8.5). Among the tested carbon sources, the highest degradation was achieved when the media was supplemented with 1% of glucose as the primary carbon source and the optimal pH for maximum degradation was determined to be 6.5. Further, for nitrogen source, 1% yeast extract yielded the highest degradation. The results obtained was validated using a Response Surface Methodology (RSM) model. The results corresponded with the experimental trials, confirming the reliability of the optimization study carried out through both experimental and RSM models (Figure 1).
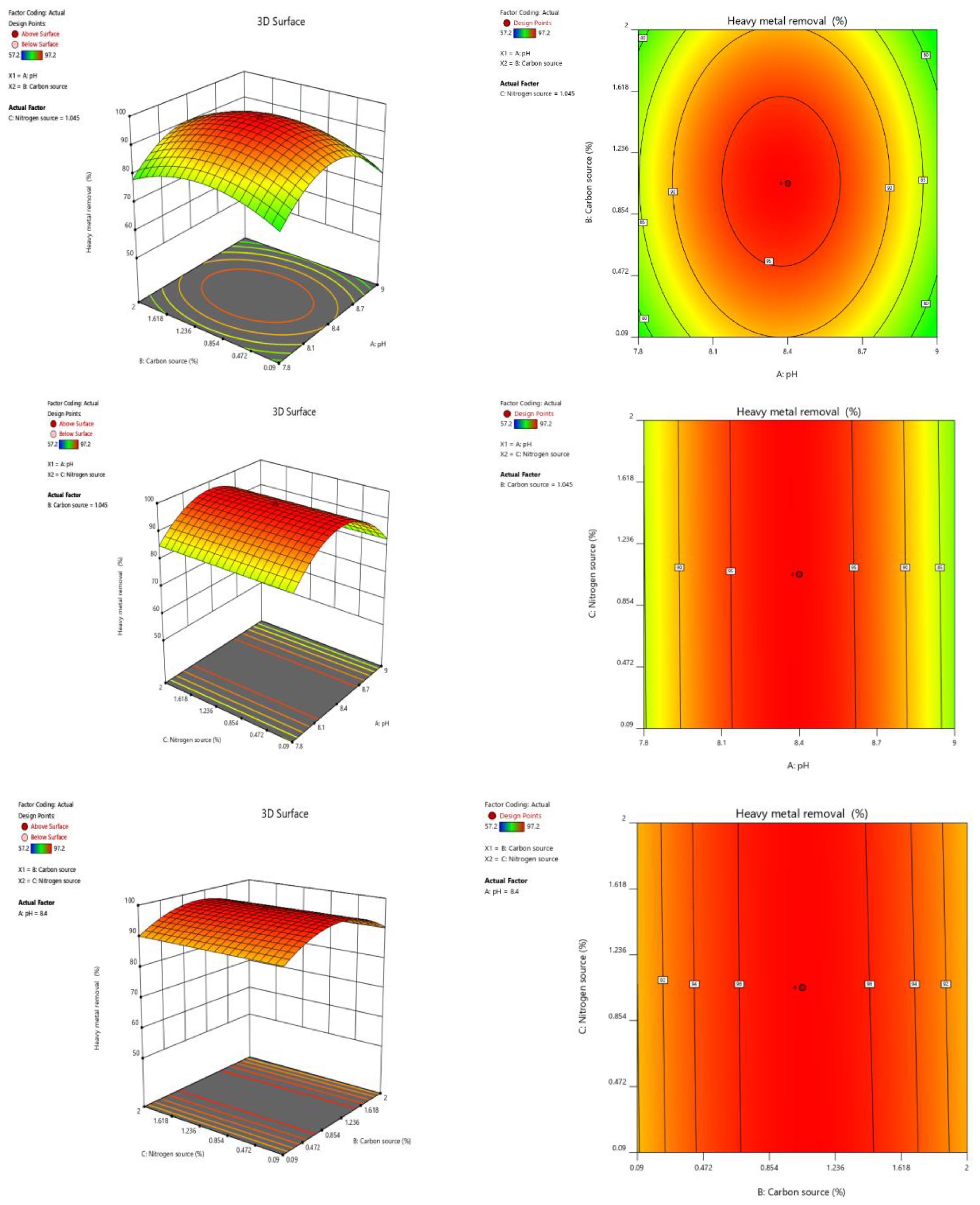
Figure 1. The 3D surface diagram and the contour plots showing the interaction between optimized parameters with respect to heavy metal removal by Bacillus infantis VITVJ8.
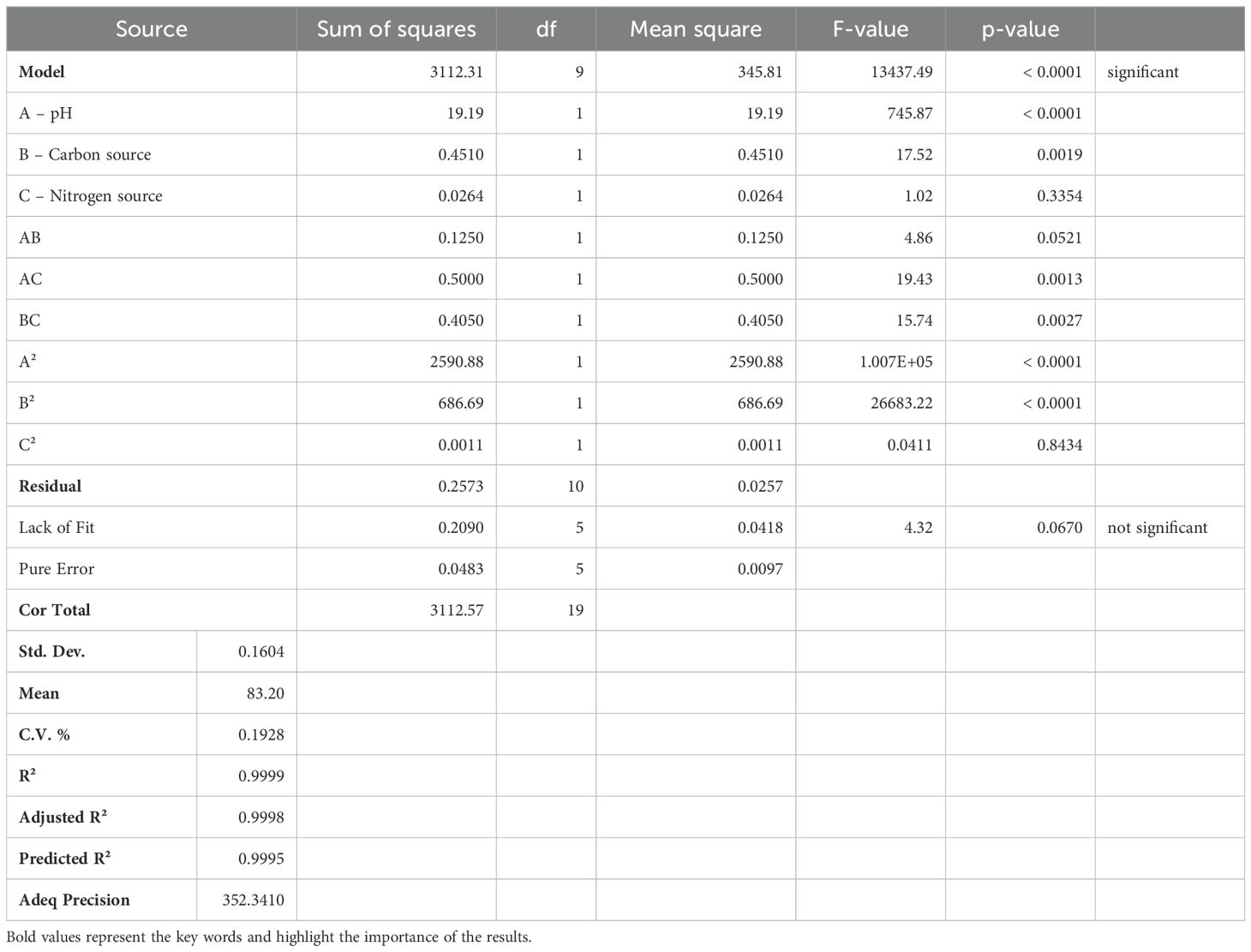
The 3D surface diagrams and contour plots of the Box Behnken Design (BBD) model are illustrated in Figures 1A–F. The actual and predicted output responses were validated using ANOVA quadratic model (Table 2). Table 2 also shows the F values for pH (745.87), carbon source (17.52) and nitrogen source (1.02) with corresponding P values being 0.0001, 0.0019, 0.3354 respectively. The model was validated with two among three responses having P values less than 0.05. The pr.ecision value, calculated as the ratio between signal and noise, was 350.410 which exceeds the adequate precision threshold of 4, indicating a strong signal strength. The 3D diagrams (Figures 1A–C) and contour plots (Figures 1D–F) showed that optimal input variables significantly influenced HM removal, both independently and in combination. Supplementary Table S2 provides the RSM design data, consisting of 20 experimental runs. Among the various factors studied which includes A (pH), B (carbon source) and C (nitrogen source), it was observed that the interactions AB, AC, and BC, as well as the quadratic terms A², B², and C², were significant. The following BBD equation was derived based on the coded factors for heavy metal removal Percentage (%): 97.08 – 1.19A + 0.1817B – 0.0439C – 0.1250AB – 0.2500AC – 0.2250BC – 13.41A2 – 6.90B2 − 0.0086C2.
This equation was used to predict the responses and assess the relative impact of the optimal factors. The results obtained from RSM and the derived statistical values were found to have relative impact within standard parameters, affirming the effective growth of the bacterial isolate VITVJ8 with the optimized parameters. The optimization using RSM for bioremoval studies has been reported in several studies (32, 46, 73, 74).
3.7 Bioaccumulation of Cr, Pb and Zn by effective strain VITVJ8
3.7.1 Bioremoval of heavy metals using AAS analysis:
Bacterial cells of VITVJ8 were capable of absorbing Cr: Zn : Pb with a maximum concentration of 81%:90.8%:93.6% (Supplementary Figure S5). Similarly, Wagh et al. (49) reported that Bacillus xiamenesis achieved 80% of Pb through the sequestration of extracellular polysaccharides (EPS) (75). VITVJ8 strain showed better bioremoval efficiency towards Pb and Zn, indicating it to be an effective strain that can be used for bioremediation. Similarly, Mwandira et al. (76) and Rizvi et al. (77) showed that bacterial strains can effectively biosorb Cr and Zn. Pagnucco et al. (78) revealed the metal tolerance and biosorption capabilities of Serratia sp. and Raoultella sp. in single and multi-metal solutions. VITVJ8 strain showed similar biosorption capabilities, highlighting its potential for bioremediation in diverse environments.
3.7.2 Seed germination assay
Germination of Vigna radiata, Vigna mungo and Glycine max seeds was observed after 24h. Among all the treatments, seeds treated with distilled water exhibited 100% germination, with both plumule and radicle formation. In contrast, other setups treated with Cr, Pb and Zn showed germination index of 75.78%. Seeds supplemented with only bacteria achieved the highest germination index of 89.47% (Supplementary Table S3).
However, there was a reduction in phytotoxicity and an increased germination index in seeds augmented with the effective strain (Supplementary Figure S6). The decrease in toxicity can be attributed to the biosorption or bioaccumulation carried out by the bacteria. A study by Nouren et al. (79) reported that the accumulation of toxic compounds in seeds can enter the food chain, posing a potential threat to ecosystem. Whereas, our findings align with this study, highlighting the risk of heavy metal accumulation in untreated seeds and the subsequent benefits of bacterial treatment in reducing this risk. Reddy and Osborne (80) revealed that seeds directly exposed to reactive dyes were more toxic than those treated with effective bacteria, this is consistent with our observations where seeds treated with bacteria exhibited lower phytotoxicity and higher germination rates, emphasizing the protective role of bacteria against heavy metal toxicity. A study by Shahid et al. (81) examined the effects of Cr, Cd, Pb and Zn on germination of Vigna unguiculata, the seed germination percentage was significantly reduced when treated with 50 and 100ppm and the present study aligns with these findings showing a similar trend in reducing the plumule and radicle length when treated with heavy metals. Siddiqui et al. (82) investigated the toxic effects of Cd, Cr, and Pb on seeds and found that seeds soaked in heavy metals exhibited less toxicity during the germination of Brassica rapa var. turnip compared to seeds that were not soaked. Our results also show a reduction in phytotoxicity in seeds treated with heavy metals when bacteria were introduced, confirm that pre-treatment with bacteria can reduce heavy metal toxicity. Our study’s reveals that the effective bacterial strain significantly enhances seed germination and reduces phytotoxicity in heavy metal-treated seeds. This is consistent with the reports which highlight the benefits of bacterial treatments in reducing the adverse effects of heavy metals.
3.8 Analysis of plant growth promoting traits
The strain VITVJ8 showed the ability to produce siderophores, IAA and solubilize the insoluble phosphate, exhibiting its plant growth promoting traits (PGPR) (Table 1). The quantitative assay revealed that 59μg/ml of IAA was produced upon supplementation with 5mg/ml of tryptophan. Ahemad and Kibert (83) reported that IAA can also be produced by bacteria such as Azospirillum, Pseudomonas, Bacillus and Cyanobacteria. The presence of halozone around VITVJ8 in NBRIP medium indicated the solubilization of insoluble phosphate to its soluble form with an efficiency of 61.5%. Similarly, halozone formation of 1.165cm in CAS agar plates indicated siderophore production, which can be used for mobilization of iron. Hou et al. (84) reported an increase in the siderophore production in iron-depleted conditions. Tsotetsi et al. (85) and Gupta et al. (11) reported that Bacillus sp. are prominent PGPR which have the ability to tolerate stress and produce various biomolecules that can contribute to PGPR traits. Similarly, Itusha et al. (54) reported Aeromonas sp. (VITJAN13) as an efficient plant growth-promoting rhizobacterium (PGPR) capable of producing 46.5µg/ml of indole-3-acetic acid (IAA). The bacteria also demonstrated the ability to produce siderophores, which was confirmed by the formation of halo zones. Several strains such as Enterobacter cloacae (VITPASJ1), Bacillus xiamenensis (VITMSJ3), Klebsiella sp. (VITAJ23) have proven to be effective PGPR (43, 49, 86).
3.9 Pot culture studies
Pot culture study was performed using C. zizanioides to understand the uptake of heavy metals in the presence and absence of VITVJ8 in the rhizospheric region.
3.9.1 Effect of heavy metal (Cr, Pb and Zn) on the growth and total chlorophyll content of C. zizanioides
Plants augmented with PGPR bacteria VITVJ8 showed positive effects from the 15th day interval onwards, although no significant difference in root length was observed as compared to the phytoremediation setup. Slight variation in shoot length was observed at higher concentrations of heavy metals. In a similar study by Vaishnavi and Osborne (87), negligible difference in the root length was observed after the 15th day of study. However, at the 30th and 45th day intervals, significant differences were observed in the rhizoremediation setup as compared to phytoremediation (Figure 2A). Dey et al. (89) reported that augmentation with a PGPR strain enhanced root length in Arachis hypogea seedling after the 30th day interval (88). The pot culture study clearly indicated the tolerance capacity of C. zizanioides up to 100ppm Cr and 250ppm Pb and Zn. The growth parameters were enhanced in soil augmented with VITVJ8. Chlorophyll content was found to be decreased in the phytoremediation setup with increase in concentration of tested heavy metals, whereas in the rhizoremediation setup, chlorophyll content was found to be increased (Figure 2B). Das et al. (40) reported that application of Enterobacter cloacae (VITPASJ1) enhanced the chlorophyll content as compared to non-treated Pennisetum purpureum. Therefore, model plant C. zizanioides used in the study showed the ability to tolerate heavy metals at concentrations of 100ppm of Cr, 250ppm of Pb and Zn in phyto and rhizoremediation studies.
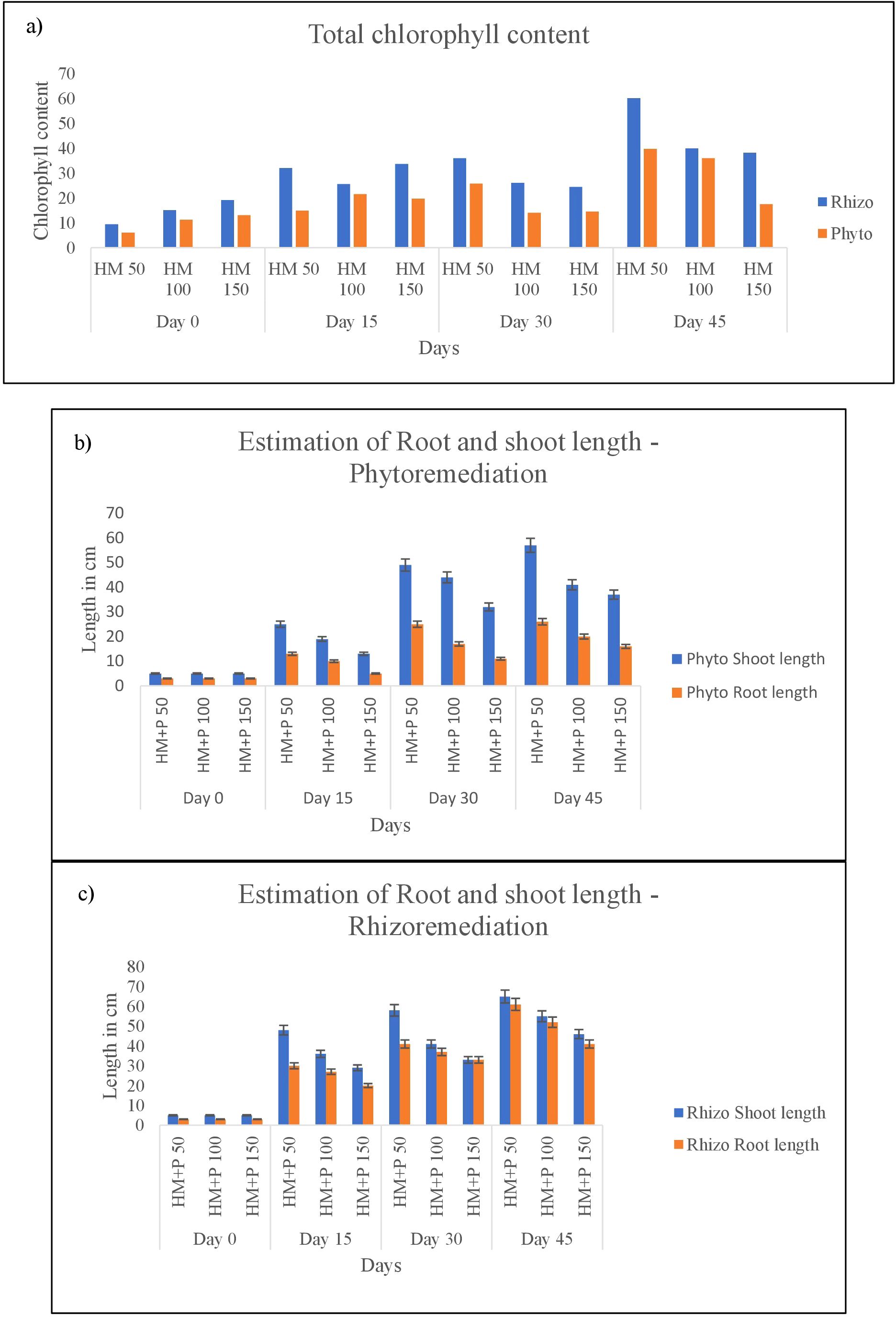
Figure 2. (A) Total chlorophyll content (B) Estimation of root length (C) Estimation of shoot length.
3.9.2 Estimation of the total heavy metal content on roots, shoots and soil
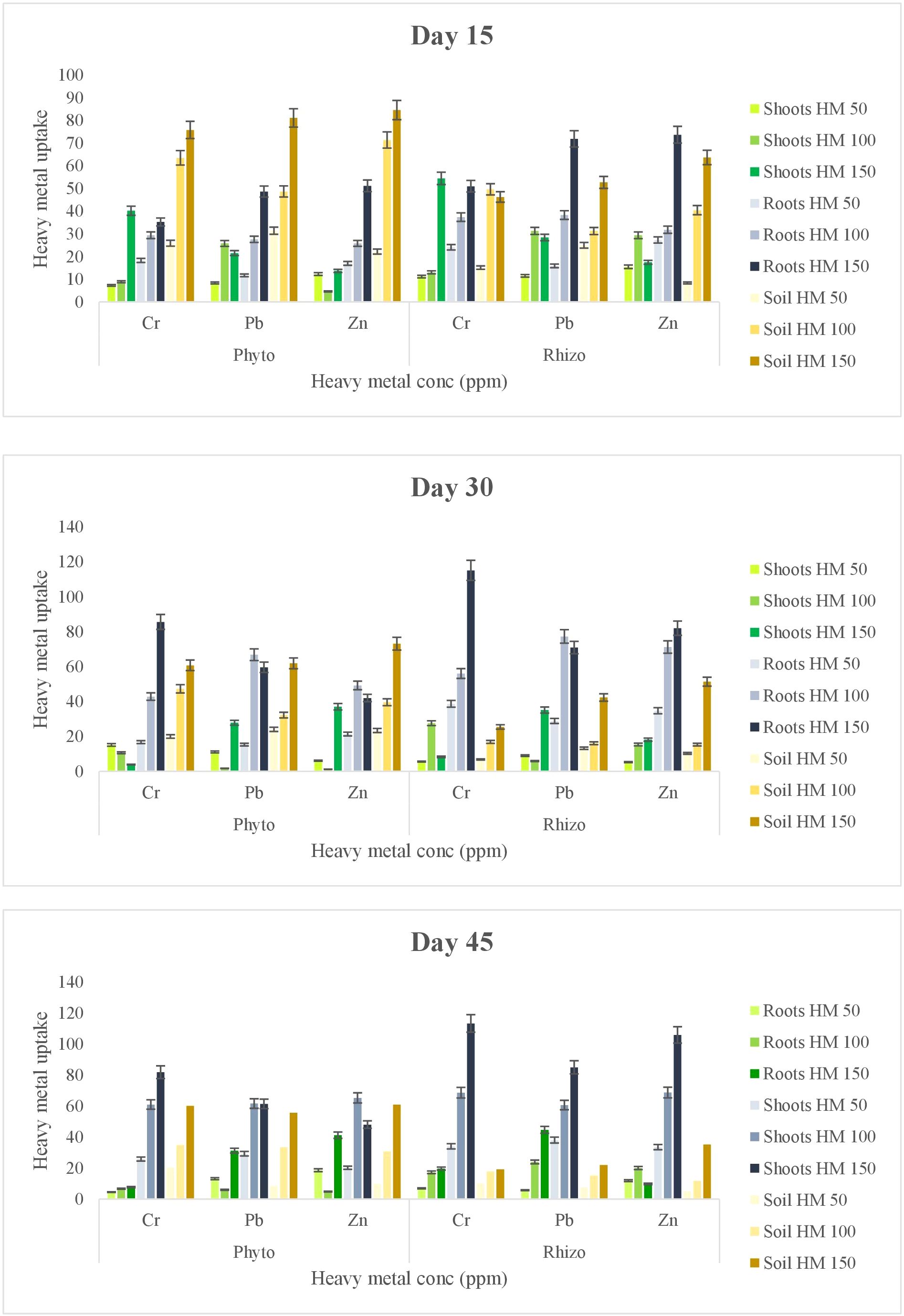
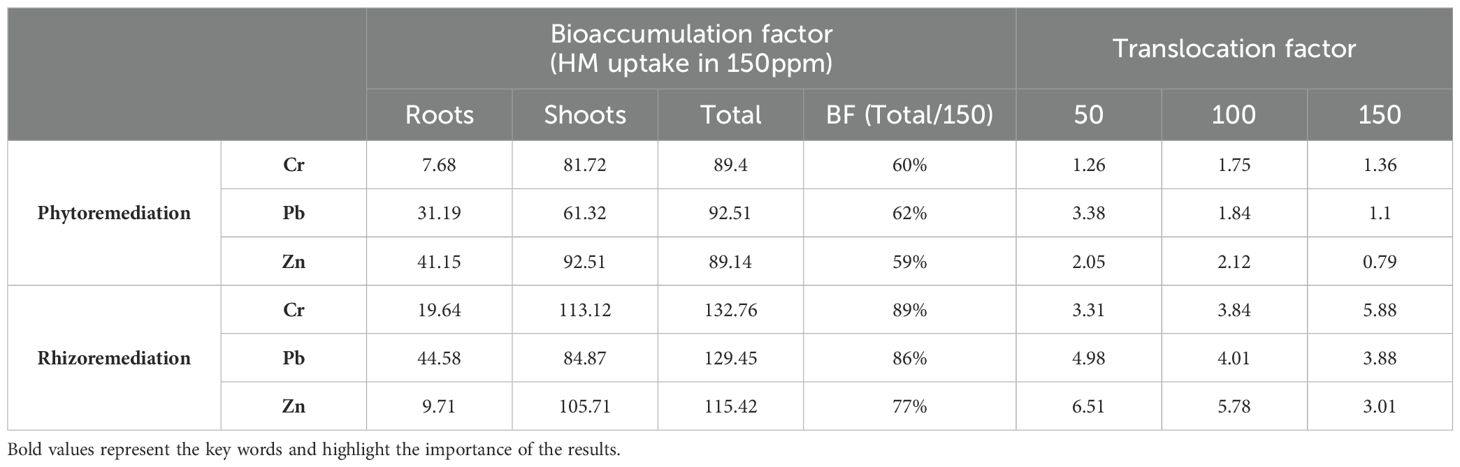
Accumulation of heavy metals in the plant root and shoots solely depends on the heavy metals present in the rhizosphere soil and their interaction with the plant root system. In this study, an increase in heavy metal concentration was observed in roots, particularly in the rhizoremediation setup as compared to the phytoremediation. This could be attributed to the minimal translocation of heavy metals to the shoots, likely due to the fibrous root system of vetiver (Figure 3). The uptake of heavy metal was higher in the rhizoremediation setup than in phytoremediation setup, indicating that the PGPR strain VITVJ8 has the potential to solubilize available heavy metals such as Cr, Pb and Zn in the rhizosphere and enhance plant uptake. In contrast, phytoremediation setup retained the heavy metals in the rhizosphere region of the soil. The bioaccumulation factor of heavy metals using VITVJ8 in the rhizoremediation approach showed a 23.66% higher enhancement in heavy metal uptake as compared to the phytoremediation setups. The uptake percentage of Cr, Pb and Zn in rhizoremediation was found to be 89>86>77% as compared to that of phytoremediation 60>62>59% (Table 3), with the translocation factor mentioned in Table 3. Das and Osborne (40) described the bioremoval of Pb using multiple biosystems like Pennisetum purpureum a hyperaccumulator plant, treated with Lumbricus terrestris (earthworm) augmented with a Pb resistant bacteria (VITPASJ1). Similarly, in this study the plant-bacterial biosystem showed enhanced uptake of Cr, Pb and Zn and can be further used for the large-scale bioremoval of heavy metals.
4 Conclusion
In the current study, the effective strain VITVJ8 obtained from rhizosphere soil was found to be tolerance to Cr, Pb and Zn. The strain was also found to be a PGPR and 16S rRNA gene sequencing confirmed VITVJ8 to be the closest neighbor of B. infantis. The rhizoremediation setup in pot culture studies revealed enhanced uptake of Zn, Pb and Cr as compared to that of phytoremediation setup (Figure 4). Therefore, from the current study it can be concluded that the isolate VITVJ8 along with C. zizanioides can be effectively used for bioremediation of Zn, Pb and Cr. Since the isolate is a soil microbe it can be used for the removal of heavy metals onsite. The effective train VITVJ8 along with C. zizanioides can be used for the treatment of Pb, Cr and Zn contaminated soil. The shoot of the plants can be harvested at regular intervals to reduce the concentration of metals from the soil as the plants to be proven to be a hyperaccumulator of tested metals.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
JV: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JO: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by Indian Council of Medical Research (ICMR), New Delhi (3/1/2(13)/Env/2021-NCD-II).
Acknowledgments
Authors are grateful to acknowledge Indian Council for Medical Research (ICMR), New Delhi, India for providing Senior Research Fellowship (ICMR-SRF). We are also grateful to VIT management for providing their support and VIT-TBI (Technology Business Incubator) for providing AAS facility.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsoil.2025.1484039/full#supplementary-material
References
1. Širić I, Eid EM, El-Morsy MH, Osman HE, Adelodun B, Abou Fayssal S, et al. Health risk assessment of hazardous heavy metals in two varieties of mango fruit (Mangifera indica L. var. Dasheri and Langra). Horticulturae. (2022) 8:832. doi: 10.3390/horticulturae8090832
2. Al-Huqail AA, Kumar P, Abou Fayssal S, Adelodun B, Širić I, Goala M, et al. Sustainable use of sewage sludge for marigold (Tagetes erecta L.) cultivation: experimental and predictive modeling studies on heavy metal accumulation. Horticulturae. (2023) 9:447. doi: 10.3390/horticulturae9040447
3. Paramasivam K, Ramasamy V, Suresh G, Vimalathithan RM. Magnetic susceptibility as proxy for metal concentrations and their risk levels in vaigai river sediment, tamilnadu, India: horizontal and vertical approach. Soil Sediment Contam: Int J. (2024) 33:594–611. doi: 10.1080/15320383.2023.2226753
4. Pradhoshini KP, Santhanabharathi B, Suhail Ahmed M, Priyadharshini M, Palanivel M, Saranya P, et al. Natural radioactivity estimation and heavy metals concentration in commercial tea brands–a baseline study on human health risk hazards due to tea consumption in Tamilnadu, India. Int J Environ Anal Chem. (2024), 1–19. doi: 10.1080/03067319.2024.2307978
5. FAO/WHO (Food and Agriculture Organization/World Health Organization). Summary of Evaluations Performed by the Joint FAO/WHO Expert Committee on Food Additives (JECFA 1956–2003), (first through sixty-first meetings). ILSI Press International Life Sciences Institute (2004).
6. Costa M. Potential hazards of hexavalent chromate in our drinking water. Toxicol Appl Pharmacol. (2003) 188:1–5. doi: 10.1016/S0041-008X(03)00011-5
7. Gupta A, Khan F, Pandey P, Tripathi M, Pathak N. A comprehensive review on the role of biosurfactants in remediation of heavy metals from contaminated environment. Bioremed J. (2024), 1–27. doi: 10.1080/10889868.2024.2427076
8. Costa M, Klein CB. Toxicity and carcinogenicity of chromium compounds in humans. Crit Rev Toxicol. (2006) 36:155–63. doi: 10.1080/10408440500534032
9. Nriagu JO, Nieboer E eds. Chromium in the natural and human environments Vol. 20. Hoboken, New Jersey, US: John Wiley & Sons (1988).
10. Hernberg S. Lead poisoning in a historical perspective. Am J Ind Med. (2000) 38:244–54. doi: 10.1002/1097-0274(200009)38:3<244::AID-AJIM3>3.0.CO;2-F
11. Gupta R, Khan F, Alqahtani FM, Hashem M, Ahmad F. Plant growth–promoting Rhizobacteria (PGPR) assisted bioremediation of Heavy Metal Toxicity. Appl Biochem Biotechnol. (2024) 196:2928–56. doi: 10.1007/s12010-023-04545-3
13. Alloway BJ. Soil processes and the behaviour of metals. Heavy metals soils. (1995) 13:3488. doi: 10.1007/978-94-011-1344-1
14. Plum LM, Rink L, Haase H. The essential toxin: impact of zinc on human health. Int J Environ Res Public Health. (2010) 7:1342–65. doi: 10.3390/ijerph7041342
15. Reddy S, Osborne WJ. Heavy metal determination and aquatic toxicity evaluation of textile dyes and effluents using Artemia salina. Biocatal Agric Biotechnol. (2020) 25:101574. doi: 10.1016/j.bcab.2020.101574
16. Gupta P, Diwan B. Bacterial exopolysaccharide mediated heavy metal removal: a review on biosynthesis, mechanism and remediation strategies. Biotechnol Rep. (2017) 13:58–71. doi: 10.1016/j.btre.2016.12.006
17. Goni MA, Abdullah-Al-Mamun M, Khan AS, Hosen L, Khatun MS, Rahman M, et al. Heavy metal distribution and ecological pollution assessments in water bodies and sediments in rural areas of Bangladesh. Environ Nanotechnol Monit Manage. (2024) 21:100937. doi: 10.1016/j.enmm.2024.100937
18. Abou Fayssal S, Kumar P, Popescu SM, Sardar H, Ahmad R, Gupta D, et al. Health risk assessment of heavy metals in saffron (Crocus sativus L.) cultivated in domestic wastewater and lake water irrigated soils. Heliyon. (2024) 10(5):e27138. doi: 10.1016/j.heliyon.2024.e27138
19. Jomova K, Alomar SY, Nepovimova E, Kuca K, Valko M. Heavy metals: Toxicity and human health effects. Arch Toxicol. (2024) 99:1–57. doi: 10.1007/s00204-024-03903-2
20. Rizvi A, Ahmed B, Umar S, Khan MS. Comprehensive insights into sorghum (Sorghum bicolor) defense mechanisms unveiled: Plant growth-promoting rhizobacteria in combating Burkholderia-induced bacterial leaf stripe disease. Plant Stress. (2024) 11:100397. doi: 10.1016/j.stress.2024.100397
21. Oladimeji TE, Oyedemi M, Emetere ME, Agboola O, Adeoye JB, Odunlami OA. Review on the impact of heavy metals from industrial wastewater effluent and removal technologies. Heliyon. (2024) 10(23):e40370. doi: 10.1016/j.heliyon.2024.e40370
22. Kiran Kumar Reddy G, Nancharaiah YV. Microbes, Metal (Loid) s and Microbe–Metal (Loid) Interactions in the Context of Mining Industry. In: Biotechnological Innovations in the Mineral-Metal Industry. Springer International Publishing, Cham (2024). p. 1–22.
23. Castiglione S, Cicatelli A, Ferrol N, Rozpadek P. Effects of plant-microbiome interactions on phyto-and bio-remediation capacity. Front Media SA. (2019) 10. doi: 10.3389/978-2-88945-932-2
24. Abdullahi SSA, Mohammad REA, Jagaba AH, Musa H, Birniwa AH. Natural, synthetic, and composite materials for industrial effluents treatment: a mini review on current practices, cost-effectiveness, and sustainability. Case Stud Chem Environ Eng. (2024) 9:100570. doi: 10.1016/j.cscee.2023.100570
25. Mishra I, Arora NK. Rhizoremediation: a sustainable approach to improve the quality and productivity of polluted soils. Phyto rhizo remed. (2019) 9:33–66. doi: 10.1007/978-981-32-9664-0_2
26. Sherman N. Microbiology, a Laboratory Manual. Reading, Mass. Boston, MA: Addison-Wesley Publishing Company (1986).
27. Enebe MC, Babalola OO. The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress: a survival strategy. Appl Microbiol Biotechnol. (2018) 102:7821–35. doi: 10.1007/s00253-018-9214-z
28. Siddiqui MM, Abbasi BH, Ahmad N, Ali M, Mahmood T. Toxic effects of heavy metals (Cd, Cr and Pb) on seed germination and growth and DPPH-scavenging activity in Brassica rapa var. turnip. Toxicol Ind Health. (2014) 30:238–49. doi: 10.1177/0748233712452605
29. Nokande SE, Razavi SM, Mohammadian MA. The capacity of heavy metal remediation by Cyperus alternifolius, Chrysopogon zizanioides (L.) Roberty, and Aloe vera (L.) Burm. f. under industrial and urban wastewater treatment. Chiang Mai Univ J Natural Sci. (2022) 21:e2022057. doi: 10.12982/CMUJNS.2022.057
30. Xiong J, He Z, Liu D, Mahmood Q, Yang X. The role of bacteria in the heavy metals removal and growth of Sedum alfredii Hance in an aqueous medium. Chemosphere. (2008) 70:489–94. doi: 10.1016/j.chemosphere.2007.06.028
31. Bhatt P, Pandey SC, Joshi S, Chaudhary P, Pathak VM, Huang Y, et al. Nanobioremediation: A sustainable approach for the removal of toxic pollutants from the environment. J Hazard Mater. (2022) 427:128033. doi: 10.1016/j.jhazmat.2021.128033
32. Das A, Osborne JW. Bioremediation of heavy metals. Nanotechnol Food Secur Water Treat. (2018) 11:277–311. doi: 10.1007/978-1-4614-8600-8_11
33. Bhadwal SS, Verma S, Hassan S, Kaur S. Unraveling the potential of hydrogen sulfide as a signaling molecule for plant development and environmental stress responses: A state-of-the-art review. Plant Physiol Biochem. (2024) 212:108730. doi: 10.1016/j.plaphy.2024.108730
34. Charagh S, Hui S, Wang J, Raza A, Zhou L, Xu B, et al. Unveiling innovative approaches to mitigate metals/metalloids toxicity for sustainable agriculture. Physiol Plant. (2024) 176:e14226. doi: 10.1111/ppl.v176.2
35. Yadav G, Sharma N, Goel A, Varma A, Mishra A, Kothari SL, et al. Trichoderma mediated metal chelator and its role in Solanum melongena growth under heavy metals. J Plant Growth Regul. (2024) 43:178–200. doi: 10.1007/s00344-023-11072-2
36. Dash DM, Osborne WJ. Rapid biodegradation and biofilm-mediated bioremoval of organophosphorus pesticides using an indigenous Kosakonia oryzae strain-VITPSCQ3 in a Vertical-flow Packed Bed Biofilm Bioreactor. Ecotoxicol Environ Saf. (2020) 192:110290. doi: 10.1016/j.ecoenv.2020.110290
37. Samata H, Tanaka S, Mizusaki S, Nagata Y, Ozawa TC, Sato A, et al. Synthesis and characterization of caPd 3 O 4 crystals. J Crystall Process Technol. (2012) 2:16. doi: 10.4236/jcpt.2012.21003
38. El-Sayed MH, Abdellatif MM, Mostafa HM, Elsehemy IA, Kobisi AENA. Biodegradation and antimicrobial capability-induced heavy metal resistance of the marine-derived actinomycetes Nocardia harenae JJB5 and Amycolatopsis marina JJB11. World J Microbiol Biotechnol. (2024) 40:1–23. doi: 10.1007/s11274-024-04006-x
39. Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM, et al. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. (1985) 22:996–1006. doi: 10.1128/jcm.22.6.996-1006.1985
40. Das A, Osborne JW. Monitoring the stress resistance of Pennisetum purpureum in Pb (II) contaminated soil bioaugmented with Enterobacter cloacae as defence strategy. Chemosphere. (2018) 210:495–502. doi: 10.1016/j.chemosphere.2018.07.050
41. APHA. (1992). Standard Methods for the Examination of Water and Wastewater. 18th Edition,American Public Health Association (APHA), American Water Works Association (AWWA) and Water Pollution Control Federation (WPCF), Washington DC.
42. Chen S, Guo X, Yu S, Zhou Y, Li Z, Sun Y. Metabolic syndrome and serum liver enzymes in the general Chinese population. Int J Environ Res Public Health. (2016) 13:223. doi: 10.3390/ijerph13020223
43. Das A, Belgaonkar P, Raman AS, Banu S, Osborne JW. Bioremoval of lead using *Pennisetum purpureum* augmented with *Enterobacter cloacae*-VITPASJ1: A pot culture approach. Environ Sci Pollut Res Int. (2017) 24(18):15444–53. doi: 10.1007/s11356-017-8988-3
44. Das A, Osborne JW. Enhanced bioremoval of lead by earthworm-Lumbricus terrestris co-cultivated with bacteria-Klebsiella variicola. J Photochem Photobiol B. (2017) 175:65–72. doi: 10.1016/j.jphotobiol.2017.08.031
45. Buragohain T, Dey P, Osborne WJ. In vitro studies on the inhibition of microbial pathogens by PPDHMP synthesized by Bacillus sp.; an endophyte of Citrus limon (Kaji nemu). Food Biosci. (2023) 55:103003. doi: 10.1016/j.fbio.2023.103003
46. Selvi A, Aruliah R. A statistical approach of zinc remediation using acidophilic bacterium via an integrated approach of bioleaching enhanced electrokinetic remediation (BEER) technology. Chemosphere. (2018) 207:753–63. doi: 10.1016/j.chemosphere.2018.05.144
47. Zhong WS, Ren T, Zhao LJ. Determination of Pb (Lead), Cd (Cadmium), Cr (Chromium), Cu (Copper), and Ni (Nickel) in Chinese tea with high-resolution continuum source graphite furnace atomic absorption spectrometry. J Food Drug Anal. (2016) 24:46–55. doi: 10.1016/j.jfda.2015.04.010
48. Pani T, Das A, Osborne JW. Bioremoval of zinc and manganese by bacterial biofilm: a bioreactor-based approach. J Photochem Photobiol B: Biol. (2017) 175:211–8. doi: 10.1016/j.jphotobiol.2017.08.039
49. Wagh MS, Sivarajan S, Osborne JW. Deciphering the enhanced translocation of Pb, Ni and Cd from artificially polluted soil to Chrysopogon zizanioides augmented with Bacillus xiamenensis VITMSJ3. 3 Biotech. (2024) 14:180. doi: 10.1007/s13205-024-04001-x
50. Gordon SA, Weber RP. Colorimetric estimation of indoleacetic acid. Plant Physiol. (1951) 26:192. doi: 10.1104/pp.26.1.192
51. Schwyn B, Neilands J. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. (1987) 160:47–56. doi: 10.1016/0003-2697(87)90612-9
52. Verma C, Singh P, Kumar R. Isolation and characterization of heavy metal resistant PGPR and their role in enhancement of growth of wheat plant under metal (cadmium) stress condition. Arch Appl Sci Res. (2015) 7:37–43.
53. Deshwal VK, Kumar P. Effect of Heavy metals on Growth and PGPR activity of Pseudomonads. J Academia Ind Res (JAIR). (2013) 2:286.
54. Itusha A, Osborne WJ, Vaithilingam M. Enhanced uptake of Cd by biofilm forming Cd resistant plant growth promoting bacteria bioaugmented to the rhizosphere of Vetiveria zizanioides. Int J phytoremed. (2019) 21:487–95. doi: 10.1080/15226514.2018.1537245
55. Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenol Beta vulgaris Plant Physiol. (1949) 24:1. doi: 10.1104/pp.24.1.1
56. Lalhriatpuii M, Chatterjee A, Dutta TK, Mohammad A, Patra AK. The effects of dietary inorganic and organic chromium supplementation on blood metabolites, hormones, and mineral composition of blood and internal organs in black bengal goats. Biol Trace Element Res. (2024) 202:2547–63. doi: 10.1007/s12011-023-03856-0
57. Lee SY, Cho KS. Isolation of heavy metal-tolerant and anti-phytopathogenic plant growth-promoting bacteria from soils. J Microbiol Biotechnol. (2024) 34:2252. doi: 10.4014/jmb.2407.07013
58. Tekaya SB, Tipayno S, Kim K, Subramanian P, Sa T. Rhizobacteria: Restoration of heavy metal-contaminated soils. Physiol Mech Adapt Strat Plants Under Changing Environ. (2014) 2:297–323. doi: 10.1007/978-1-4614-8600-8_11
59. Riseh RS, Vazvani MG, Hajabdollahi N, Thakur VK. Bioremediation of heavy metals by Rhizobacteria. Appl Biochem Biotechnol. (2023) 195:4689–711. doi: 10.1007/s12010-022-04177-z
60. Wani PA, Omozele AB. Cr (VI) removal by indigenous Klebsiella species PB6 isolated from contaminated soil under the influence of various factors. Curr Res Bacteriol. (2015) 8:62. doi: 10.3923/crb.2015.62.69
61. Yahaghi Z, Shirvani M, Nourbakhsh F, de la Peña TC, Pueyo JJ, Talebi M. Isolation and characterization of Pb-solubilizing bacteria and their effects on Pb uptake by Brassica juncea: implications for microbe-assisted phytoremediation. J Microbiol Biotechnol. (2018) 28(7):1156–67. doi: 10.4014/jmb.1712.12038
62. Pan H, Zhao X, Zhou X, Yan H, Han X, Wu M, et al. Research progress on the role of biofilm in heavy metals adsorption-desorption characteristics of microplastics: A review. Environ pollut. (2023) 336:122448. doi: 10.1016/j.envpol.2023.122448
63. Kumar A, Khushboo, Pandey R, Sharma B. Modulation of superoxide dismutase activity by mercury, lead, and arsenic. Biol Trace element Res. (2020) 196:654–61. doi: 10.1007/s12011-019-01957-3
64. Shi Y, Ma J, Hanigan D, Chen Y, Qian Y, Guo J, et al. Magnetically recoverable Fe3O4/MoS2/BiOI microspheres for visible light water disinfection: Molecular mechanism and transcriptomic insights. Separation Purific Technol. (2023) 320:124140. doi: 10.1016/j.seppur.2023.124140
65. Abedi H, Shahpiri A. Functional characterization of a manganese superoxide dismutase from Avicennia marina: insights into its role in salt, hydrogen peroxide, and heavy metal tolerance. Sci Rep. (2024) 14:406. doi: 10.1038/s41598-023-50851-5
66. Baldiris R, Acosta-Tapia N, Montes A, Hernández J, Vivas-Reyes R. Reduction of hexavalent chromium and detection of chromate reductase (ChrR) in Stenotrophomonas maltophilia. Molecules. (2018) 23:406. doi: 10.3390/molecules23020406
67. Wu SC, Hsiao W-C, Zhao Y-C, Wu L-F. Hexavalent chromate bioreduction by a magnetotactic bacterium Magnetospirillum gryphiswaldense MSR-1 and the effect of magnetosome synthesis. Chemosphere. (2023) 330:138739. doi: 10.1016/j.chemosphere.2023.138739
68. Reens AL, Cosetta CM, Saur R, Trofimuk O, Brooker SL, Lee ML, et al. Tunable control of B. infantis abundance and gut metabolites by co-administration of human milk oligosaccharides. Gut Microbes. (2024) 16:2304160. doi: 10.1080/19490976.2024.2304160
69. Ghaima KK, Al Draghi WA, Lateef NS. Study the heavy metals tolerance, biosorption and antibiotic resistance of Bacillus cereus isolated from diesel fuel polluted soil. Int J Biol Pharm Res. (2013) 4:502–6.
70. Khullar G, Karami Z, Prakitchaiwattana C. Development of microencapsulated dried Bacillus sp. 63-11 with enhanced shelf stability and bioactivity for use as a food supplement. Int J Food Sci Technol. (2024) 59:1291–8. doi: 10.1111/ijfs.16853
71. Silva MDA, Silva JAGD, Enciso J, Sharma V, Jifon J. Yield components as indicators of drought tolerance of sugarcane. Sci Agricola. (2008) 65:620–7. doi: 10.1590/S0103-90162008000600008
72. Wu H, Huang L, Li J, Yang D, Shang C. Optimization of the conditions for the growth and ethanol production of strain bacillus sp. EtOH. J Biobased Mater Bioenergy. (2024) 18:333–8. doi: 10.1166/jbmb.2024.2375
73. Jaafari J, Yaghmaeian K. Optimization of heavy metal biosorption onto freshwater algae (Chlorella coloniales) using response surface methodology (RSM). Chemosphere. (2019) 217:447–55. doi: 10.1016/j.chemosphere.2018.10.205
74. Vaishnavi J, Devanesan S, AlSalhi MS, Rajasekar A, Selvi A, Srinivasan P, et al. Biosurfactant mediated bioelectrokinetic remediation of diesel contaminated environment. Chemosphere. (2021) 264:128377. doi: 10.1016/j.chemosphere.2020.128377
75. Bhat RA, Mir MY, Dar GH, Dervash MA. Aquatic Environmental Bioengineering: Monitoring and Remediation of Contamination. Hoboken, New Jersey, US: John Wiley & Sons (2022).
76. Mwandira W, Nakashima K, Kawasaki S, Arabelo A, Banda K, Nyambe I, et al. Biosorption of Pb (II) and Zn (II) from aqueous solution by Oceanobacillus profundus isolated from an abandoned mine. Sci Rep. (2020) 10:21189. doi: 10.1038/s41598-020-78187-4
77. Rizvi A, Ahmed B, Zaidi A, Khan MS. Biosorption of heavy metals by dry biomass of metal tolerant bacterial biosorbents: an efficient metal clean-up strategy. Environ Monit Assess. (2020) 192:1–21. doi: 10.1007/s10661-020-08758-5
78. Pagnucco G, Overfield D, Chamlee Y, Shuler C, Kassem A, Opara S, et al. Metal tolerance and biosorption capacities of bacterial strains isolated from an urban watershed. Front Microbiol. (2023) 14:1278886. doi: 10.3389/fmicb.2023.1278886
79. Nouren S, Bhatti HN, Iqbal M, Bibi I, Kamal S, Sadaf S, et al. By-product identification and phytotoxicity of biodegraded Direct Yellow 4 dye. Chemosphere. (2017) 169:474–84. doi: 10.1016/j.chemosphere.2016.11.080
80. Reddy S, Osborne J. An insight on the advancements of biological technologies in the bioremediation of textile effluents. Urban Water J. (2022) 19:468–80. doi: 10.1080/1573062X.2022.2030369
81. Shahid MA, Sarkhosh A, Khan N, Balal RM, Ali S, Rossi L, et al. Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy. (2020) 10:938. doi: 10.3390/agronomy10070938
82. Siddiqui MH, Al-Whaibi MH, Sakran AM, Basalah MO, Ali HM. Effect of calcium and potassium on antioxidant system of *Vicia faba* L. under cadmium stress. Int J Mol Sci. (2012) 13(6):6604–19. doi: 10.3390/ijms13066604
83. Ahemad M, Kibret M. Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J King saud University-sci. (2014) 26:1–20. doi: 10.1016/j.jksus.2013.05.001
84. Hou J, Liu W, Wang B, Wang Q, Luo Y, Franks AE. PGPR enhanced phytoremediation of petroleum contaminated soil and rhizosphere microbial community response. Chemosphere. (2015) 138:592–8. doi: 10.1016/j.chemosphere.2015.07.025
85. Tsotetsi T, Nephali L, Malebe M, Tugizimana F. Bacillus for plant growth promotion and stress resilience: what have we learned? Plants. (2022) 11:2482. doi: 10.3390/plants11192482
86. Sinha A, Lulu S, Vino S, Osborne WJ. Reactive green dye remediation by Alternanthera philoxeroides in association with plant growth promoting Klebsiella sp. VITAJ23: a pot culture study. Microbiol Res. (2019) 220:42–52. doi: 10.1016/j.micres.2018.12.004
87. Vaishnavi J, Osborne JW. Biodegradation of monocrotophos, cypermethrin & fipronil by *Proteus myxofaciens* VITVJ1: A plant - microbe based remediation. Heliyon. (2024) 10(18):e37384. doi: 10.1016/j.heliyon.2024.e37384
88. Barra Caracciolo A, Terenzi V. Rhizosphere microbial communities and heavy metals. Microorganisms. (2021) 9:1462. doi: 10.3390/microorganisms9071462
Keywords: heavy metals, environment, bioremediation, biofilm, phytotoxicity, phyto-rhizoremediation
Citation: Vaishnavi J and Osborne W. J (2025) Phyto-rhizoremediation potential of C. zizanioides augmented with Bacillus infantis (VITVJ8) for the uptake of heavy metals (Cr, Pb and Zn). Front. Soil Sci. 5:1484039. doi: 10.3389/fsoil.2025.1484039
Received: 10 September 2024; Accepted: 27 January 2025;
Published: 11 March 2025.
Edited by:
Asit Mandal, Indian Institute of Soil Science (ICAR), IndiaReviewed by:
Sami Abou Fayssal, University of Forestry, BulgariaSobia Ashraf, Government College University, Pakistan
Copyright © 2025 Vaishnavi and Osborne W.. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jabez Osborne W., amFiZXoudml0QGdtYWlsLmNvbQ==
 Jeevanandam Vaishnavi
Jeevanandam Vaishnavi Jabez Osborne W.*
Jabez Osborne W.*