- 1Scion, Christchurch, New Zealand
- 2Thünen, Institute of Climate-Smart Agriculture, Braunschweig, Germany
- 3Scion, Rotorua, New Zealand
- 4Faculty of Agriculture and Life Sciences, Lincoln University, Lincoln, New Zealand
- 5Bioprotection Research Centre, School of Biological Sciences, University of Canterbury, Christchurch, New Zealand
- 6Manaaki Whenua–Landcare Research, Lincoln, New Zealand
Factors affecting the deposition of carbon and nitrogen into the rhizosphere soil have important implications for natural and managed ecosystems. These include the invasiveness of plants, extent to which ecosystems sequester soil carbon, through to regulation of N flow within and from agricultural ecosystems. This study determined if the close elemental ratios often measured in soils are evident within the highly active rhizosphere compartment, or rather potentially emerge to a conserved ratio (over time) from different initial rhizosphere states. Toward this, we assessed the rhizosphere C and N content (and C:N ratio) of 37 plant species; these were further grouped into the categories provenance (native or exotic to New Zealand), form (forb, grass, shrub, or tree), root-based nitrogen fixation symbiosis (+/– N-fixation), or mycorrhization type. Furthermore, the potential nitrification rate (PNR) among the plant rhizosphere soils was quantified to explore relationships between nitrate formation and the total C and C:N ratio. Mycorrhization status, provenance, and form had no significant influence on nutrient status nor PNR in rhizosphere soil samples (p > 0.05). However, total C and total N were significantly increased in the rhizosphere of N-fixing species (p < 0.02). These increased in proportion, with the C:N remaining constant for both groups (~12.24; p = 0.79). Rhizosphere PNR did not vary with categories of plants tested and had no correlation to rhizosphere total C, total N, nor C:N ratio (p > 0.3 for all). Overall, this study showed that conservation of nutrient ratios often measured in soils are present within the rhizosphere, where initial inputs of C and N enter the soil ecosystems. With the exception of N-fixing plant species, rhizosphere soils retain remarkably high conservation in C, N, C:P, and PNR among key groupings.
Introduction
Nitrogen is a key nutrient regulating net primary productivity (NPP), particularly in non-managed (typically natural) ecosystems. With most terrestrial ecosystems N limited (1, 2) plants have evolved a range of mechanisms to ensure their N requirements are met (3–5). These include, for example, physiological and developmental mechanisms that enable plants to improve the efficiency of N uptake from N poor soils (scavenging more N from the ecosystem), or capacity to support ongoing metabolism and growth with low cytosolic N concentrations (low N physiology) (6, 7). Plants can improve their ability to scavenge N by altering carbon partitioning to favour root growth, optimising root morphology (e.g., proportion of fine roots), increase soil/root contact (8), and via chemical secretions (9). Many plants also form symbiotic relationships with mycorrhizae and/or N-fixing bacteria. While mycorrhizal fungi (AMF) help increase N uptake by effectively increasing plants ability to explore for N present in the soil solution (10, 11), symbiosis with N-fixing bacteria bypass this by directly converting atmospheric N into plant-available forms. These microbes (mycorrhizal fungi and soil-based nitrogen-fixing bacteria) are especially important in low-N ecosystems and are responsible for c. 5–20% (grassland and savannah) to 80% (temperate and boreal forests) of all nitrogen acquired by plants annually (12). These strategies are influenced by climate and soil factors, but plant species have been found to be of equal or greater importance than abiotic factors when it comes to nutrient cycling (5, 13).
In natural ecosystems, invading plants compete for light, nutrients, and water. Successful establishment, naturalisation, and spread of introduced species is often attributed to their biological characteristics such as rapid growth, high reproductive output, tolerance of environmental conditions, and novel interactions with herbivores and microbes (14, 15). If N is limited, successfully invading plants are often those that have adapted mechanisms to effectively access and efficiently use N. One way of accomplishing this is through influencing the mineralization and immobilisation of N (3, 13).
A key method plants use for reducing mineralisation of N is via biological nitrification inhibition (BNI). BNI occurs through the chemical inhibition of specific soil microorganisms by chemicals secreted from the roots of some plants, subsequently reducing the rate of nitrification in soil adjacent to their root systems (16). This enables the plants to more effectively recapture N in the soil, improving N use efficiency. Biological nitrification inhibition activity can be very specific, varying in strength among plants [e.g., C4 tropical grasses > C3 temperate grasses; (17)]. As well as being important in natural ecosystems, BNI is of applied interest. The introduction or selection-based improvement of this trait into commercial plant cultivars [e.g., (18, 19)], addition of the trait through plant-microbiome manipulation [e.g., (20)], or using non-agricultural plants within productive landscapes for protection of riparian zones or other sensitive areas [e.g., (21)] all offer opportunities to utilise BNI as a means for reducing nitrification rates within agroecosystems (16). Although this can be measured directly, with inhibitor-based assays on target microorganisms (22), BNI is often measured by inference through assessment of changes in potential nitrification rate (PNR) in soils [e.g., (23)] particularly in initial screening-based trials. Similarly, in this study we use measurement of changes in PNR as a proxy for potential BNI activity.
Mineral nitrogen in soil is readily immobilised into the microbial biomass when available carbon is present (24). Over time, this moves soil organic matter toward a state of conservation in the C:N ratio (as well as other elements); a phenomena common to biology in various ecosystems [the “Redfield ratio;” (25)] and typical in plant litter (4, 26) as it is in soil (26). Plant roots contribute to the soil C pool through root turnover, death, and decomposition, but also through sloughing of mucilage from live cells and rhizodeposition of root exudations (27). Of these, the excretion of root exudates typically represents the largest input of C into the soil ecosystem (28, 29). As well as providing energy for the growth and activity of soil microbes, stimulating soil organic matter mineralization, and mobilisation of nutrients into the plant-available fraction (30), these exudates play an important role in N availabity and dynamics in soil ecoystems. Factors affecting rhizodeposition/exudation include environmental (31), soil microbial community (32), but also plant type (29).
Clearly an understanding of C and N dynamics in rhizosphere soils, and influences on plants and their root microbiomes on these to improve their collective fitness and competitiveness, is important the functioning of natural ecosystems. However, there are also significant outcomes for ecosystems managed for productive use, particularly agricultural systems for intensified food and/or fibre production. It is often within agricultural systems where C:N ratios typically shift out of natural stoichiometric balance due to inputs of mineral fertilisers, concentrated deposition of nitrogen from livestock urine, or via addition of agricultural “waste”, such as crop residue, into soil and so on (33–35). The outcomes of these practises can lead to events such as N-immobilisation, nitrate leaching, N2O emissions, eutrophication (36). Given this, there is interest in potential use of plants, grown in strategic locations on-farm or within landscapes/catchments, as permanent “engineers” of the soil ecosystem; i.e., can plants, differing in key rhizosphere traits associated with C deposition and/or interruption of nitrate formation through BNI activity, be strategically used to ameliorate impacts of agricultural intensification linked to disruption of soil nutrient cycling?
Another key area where plant driven alteration of rhizosphere geochemistry may alter fitness in the environment and have significant outcomes for ecosystem biodiversity and function is the invasion of plants into new ranges. New Zealand, for example, is one of the weediest countries globally, with naturalised, non-native plants conservatively comprising over half its flora (>2,000 of the 4,000 named plant species) (37). Many of these have become invasive weeds in both natural and managed ecosystems. These naturalised, non-native plant species span trees (e.g., wilding pines such as Pinus contorta) to grasses (38, 39). Despite most New Zealand native species being adapted to infertile soil (40) they are still outcompeted by invading plant species. Gorse (Ulex europaeus), for example, is one of the most invasive weeds of New Zealand as well as being one of the most widespread nitrogen fixing species (41). Its success is likely due to higher N-fixation rates compared with native N-fixers (42). Research into N acquisition in native vs. naturalised plants in New Zealand is often focused on factors such as if a plant forms N-fixing symbiosis or altered root morphology. Other areas such as the links between mycorrhizal associations, the influence of different plant species, the impact of the amount and type of carbon available, and other mechanisms such as biological nitrogen inhibition have on N cycling in the rhizosphere are often overlooked.
This study investigates if plant species per se, or species traits such as mycorrhization, N-fixation, plant form, or native v exotic provenance, are associated with changes in rhizosphere C and N. The overall goals are primarily to (1) to establish if there is potential to use variation in rhizosphere traits associated with C and N among plants as a potential means to reduce the rate of N cycling, and therefore potential ecosystem leakage, from agricultural soils, and also to (2) better understand links, if any, between rhizosphere C and N traits and plants potential ability to extend into new habitats (i.e., invasiveness). A range of native and exotic plants to New Zealand were used to explore this. The species spanned a broad phylogenetic range and differed in mycorrhizal associations and N fixing symbiosis. All plants were planted in a common soil comprised of a composite of New Zealand high country soils; this approach was taken to ensure the natural range of symbionts (e.g., beneficial mutualists) were present for the different taxa and that changes in rhizosphere C and N were expressed within a common soil system.
Methods
Plant Growth and Soil Processing
Plants from 37 species were grown in a blended soil medium. The species (spanning Gymnosperm trees through to monocot grasses; Supplementary Table 1), included a roughly equal proportion of native (n = 18) and exotic species (n = 19), and differed in mycorrhizal and N2-fixing symbiosis associations. To ensure each species could recruit necessary microbiome associations, soils from 12 sub-alpine, grass and shrub-dominated sites that represented the common range of many the species were collected; these sites, soil type, and collection are described in full elsewhere (15). These sieved “live-soils” were blended in equal proportion, and then bulked out by mixing in, with equal portions, steam pasteurised field-collected soil and pasteurised sand (15). The soils were added to 10 l pots and seeds or cuttings of each of the plant species planted. Between 12 and 20 replicate pots for each plant species were established. These were randomly arranged in the glasshouse and watered regularly. During winter supplemental lighting (~5 h per day) was provided. No fertilisers were added. The plants were grown for approximately 10 months and weeds removed on observation.
Depending on availability [i.e., plants that had grown and samples not required for other work; i.e., (15)], rhizosphere soils were collected from between four and seven replicate pots for each plant species. For the majority (24 of the 37 plants), this was six replicate (independent) pots. Rhizosphere soil was collected by pressing open 50 ml centrifuge tubes into the soil adjacent to the stem(s) of the plants in each pot. Three samples were collected from each pot (typically 3 points around the circumference of each plant) and the samples for each pot (plant replicate) bulked (i.e., single samples for each replicate of each plant; n = 213), sieved to 2 mm, and then stored at 4°C. While root material was removed during the process, it was not practicable to remove very fine roots or root hairs that were not visible. It should be noted that these plants were grown on for a short time for other purposes (not described here). On final recovery of the plants, visual inspection of the root-soil systems provided validation that the collection of the material was highly colonised by roots (as is typical of these types of pot assays), operationally defined herein as “rhizosphere” or, at very least, soil under direct influence of root growth and exudates. For each soil, total N and total C were determined by Dumas combustion (RJ Hill Laboratories, NZ). C:N was calculated as the direct ratio between each pair of total C and total N data.
Potential Nitrification Rate
Soil maximum water holding content (MWHC) was determined on a single sample of 5 randomly pooled soils and assessed as the amount of water the soil could hold under gravity (i.e., no suction) (43). Using a single sample for MWHC was appropriate as all plants had been grown in a single (composite) soil.
For each soil sample, the potential nitrification rate (PNR) was determined using the soil-slurry method (44). Approximately 15 g (dry wt.) of soil was added into an Erlenmeyer flask and water added to 50% MWHC. At the same time, a separate sample of each soil was weighted into an aluminium tray and dried overnight at 105°C for reference thermogravimetric moisture determination. The Erlenmeyer flasks were incubated overnight at 25°C and then 100 ml of 1.5 mM NH4 solution added (44). The flasks were placed on an orbital shaker and incubated at 180 rpm for 24 h.
Given the large number of samples, and the need to process the soils in a timely manner, nitrification was measured across two time-points only; at setup (time 0), and after 24 h incubation. Thus, any error in calculation of the rate of nitrification across two points directly contributes to the variation among the replicates for each plant (i.e., this is captured). For nitrate measurement, flasks were allowed to stand for 30 min and then 10 ml of supernatant collected into 15 ml tubes. These were centrifuged at 8,000 g for 15 min at 5°C, and then the supernatant passed through 0.22 um syringe-filter to a collection phial. These were snap frozen to −80°C and then moved to storage at −20°C. Nitrate-N was determined by initial reduction to nitrite-N using a cadmium reduction coil (OTCR—open tubular cadmium reactor), followed by the reaction of nitrite-N with sulphanilamide/NED to form an azo-dye compound (spectrophotometrically determined). Nitrate formed was calculated on a “per g soil” basis and then the formation rate determined from the 24 h time period (i.e., PNR = μg NO3-N formed g−1 soil h−1).
Data Analysis
From the experimental replicates (4–7 individual pots) for each plant, the mean PNR in the rhizosphere soil was determined. These were compared across samples using the treatment groups based on: Provenance (native or exotic); functional group (forb, tree, grass, shrub); mycorrhizal association (AMF, EMF, none); and N-fixing (+/–) symbiosis (Supplementary Table 1) using ANOVA. When three or more levels were present in any treatment, mean values for each species were calculated and then compared across levels using Bonferroni's multiple comparisons test. Similar testing was conducted for the total C, and total N in the rhizosphere soil among the samples. Comparisons were tested among soils in which plants were grown, not between soils planted vs. those in a non-planted state. As the intent of this work was to compare the relative influence of different plant species and traits on soil properties, the comparison to an unplanted soil with no plants or root traits etc thus did not comprise a valid reference for comparison.
Relationships between soil total C, total N, and PNR were tested using correlation (Person's two-tailed); these were conducted for both the entire data set (all plants) and again within each of the N-fixing symbiosis groups (given the later findings of the importance of N-fixing traits in affecting these—see later results).
Results
Variation Among Plant Species
Variation in PNR, total C, total N, and C:N ratio across plants are given in Figures 1A–D, respectively. Due to the need to correct for false discovery among the high number of multiple comparisons (Bonferroni's corrections), an effective p < 0.0001 would be required to obtain the conventional significance threshold of 5%. As such, discovery of statistically-meaningful differences by this approach are clearly constrained; results are included for general reference only and should be interpreted as highly conservative.
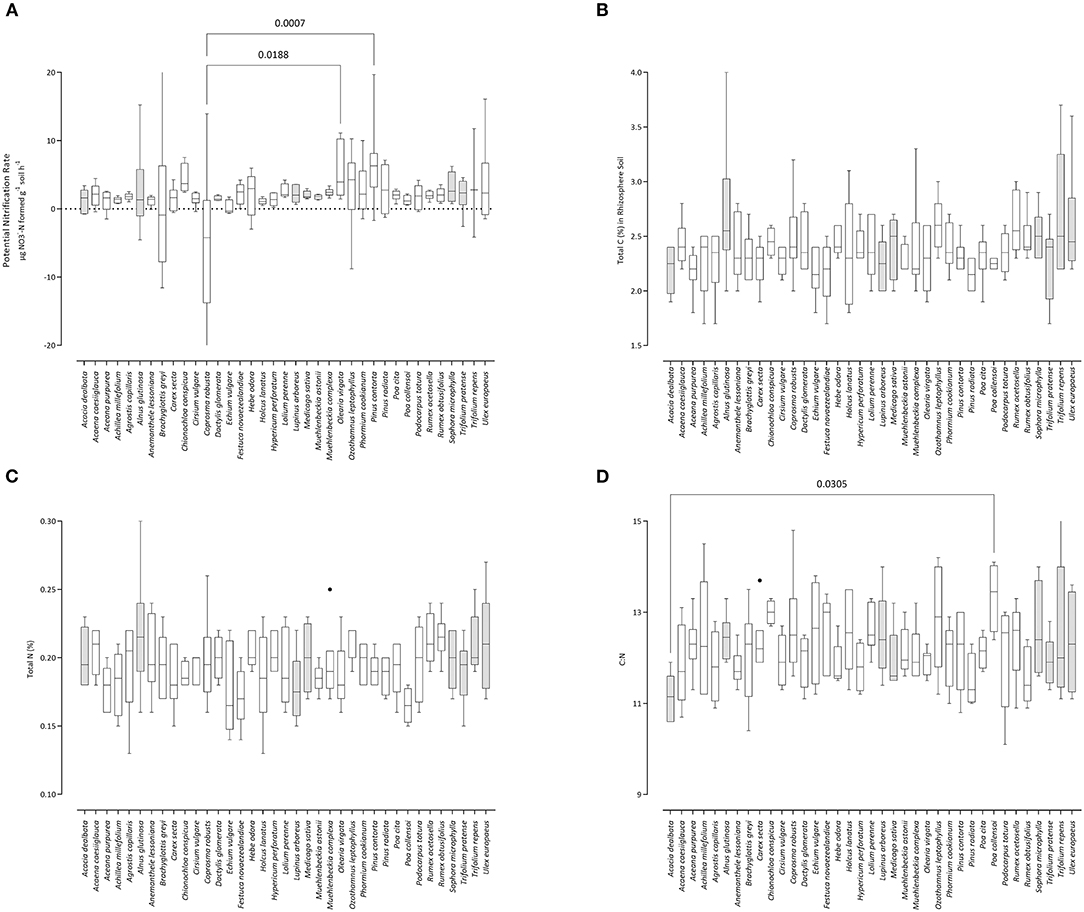
Figure 1. Influence of plant species on (A) potential nitrification rate (PNR), (B) carbon content, (C) nitrogen content, and (D) the C:N ratio in rhizosphere soil samples. Box-and-whisker plots generated using Tukey's method, whereby the middle of the box is plotted at the median, and the whiskers are calculated based on 25th and 75th percentiles. Where whiskers are not capped, individual datum are truncated (i.e., extend beyond the upper or lower Y-axis limits). Shaded boxes are plants known to host N-fixing symbiosis. Pairs of samples connected by lines have statistically significant mean values (p-values given) based on Bonferroni's corrected multiple comparisons approach.
The highest average total C was present in the rhizosphere of Alnus glutinosa (2.72%) and the lowest in Pinus radiata (2.15%). This was a spread of ~0.57 across the 37 plant species, or 21% compared with the maximum value. The maximum total N was also highest under Alnus glutinosa (0.218%), but the lowest content was it the rhizosphere soil of the grass Poa collensoi (0.165%). This comprised a spread of 0.053, or 25%. The widest C:N ratio was in soil under Poa collensoi (13.35); this effect was driven by its relatively low N content. The narrowest C:N was under Acacia dealbata (11.15). The range of C:N among plants was therefore 2.2, or 16.5%.
Potential nitrification rate was highest under Pinus contorta (6.37), an invasive tree species in New Zealand (39). The lowest PNR was measured in rhizosphere soil from under the New Zealand native plant Coprosma robusta (−7.0). Overall, PNR rates spanned a range of ~210% across the panel of plant species tested.
Provenance
Plants from native or exotic provenance did not differ in rhizosphere PNR (p = 0.448), total C content (0.318), nor C:N ratio (p = 0.133). On initial inspection of the results, plants from exotic provenance had higher rhizosphere soil N-content (p = 0.031), However, of the 19 exotic species, seven had N-fixing traits, where for natives (n = 18) N-fixation was only present in a single species, Sophora microphylla (Supplementary Table 1). After excluding these eight N-fixing species, repeated analysis of the data found no effect of provenance on rhizosphere soil N content (p = 0.217).
Functional Group
The plants were grouped into forbs (n = 15), grasses (n = 10), shrubs (n = 7), and trees (n = 5) (Supplementary Table 1). Among these groups, no differences in total C (p = 0.093), C:N ratio (0.161), or PNR (p = 0.466) were evident in the rhizosphere soils.
Total N was found to differ among the functional groups (main ANOVA test p = 0.008). Inspection of the pair-wise comparisons found this was only associated with differences between grasses and forbs (p = 0.008). Similarly for results of the provenance testing, this outcome was biassed on differences in N fixers present in these groups. Root-symbiotic N-fixation traits are not present in grasses, but were present in 4 of the 15 forbs (~27%) thereby driving this finding.
Mycorrhizal Association
The three types of mycorrhizal associations, arbuscular (AMF), ectomycorrhizal (EMF), nor non-mycorrhizal (NM) did not associate with changes in rhizosphere soil total C (p = 0.433), total N (0.345), C:N ratio (p = 0.667), or potential nitrification rate (p = 0.0931).
N-Fixing Symbiosis
Potential nitrification rate, total C, total N, and the C:N of rhizosphere soil between N-fixing and non N-fixing groups of plants are given in Figures 2A–D, respectively. Total rhizosphere carbon (p = 0.015) and nitrogen (p = 0.018) were greater in soils from under plants with N-fixing symbiosis traits (Figures 2B,C). For carbon, the mean content in the rhizosphere soils of non N-fixing species was 2.34, and this increased to 2.47 for N-fixing species (i.e., a 5.6% increase). For nitrogen, the magnitude increase was similar (5.3%), with N increasing from 0.19 (non N-fixing) to 0.20. Given both C and N increased by similar proportions, there was no attendant impact on C:N ratio (p = 0.667). Similarly, the potential nitrification rate in the rhizosphere soil of the two groups did not vary (p = 0.555).
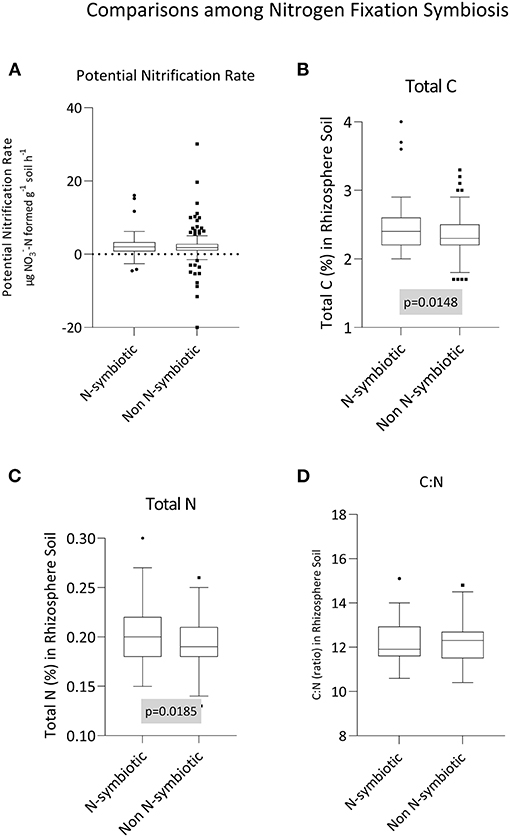
Figure 2. Influence of N-fixing symbiosis on (A) potential nitrification rate, (B) carbon content, (C) nitrogen content, and (D) the C:N ratio in rhizosphere soil samples. Box-and-whisker plots generated using Tukey's method, whereby the middle of the box is plotted at the median, and the whiskers are calculated based on 25th and 75th percentiles. Statistical testing between +/– N fixing groups was conducted using unpaired, two-tailed t-tests.
Relationships Among PNR, Total C, Total N, and C:N
Correlations among the potential nitrification rate (PNR), total C, total N, and the C:N ratio of the rhizosphere soil samples are given in Table 1. Analysis were conducted across the entire data set, and then between groups of plants identified as forming N-fixing symbiosis or not (Table 1). This approach was based on the influence that N-fixation, as a species trait, had on rhizosphere soil properties, enabling the partitioning of the effects of N-fixing plants from that of the wider dataset.
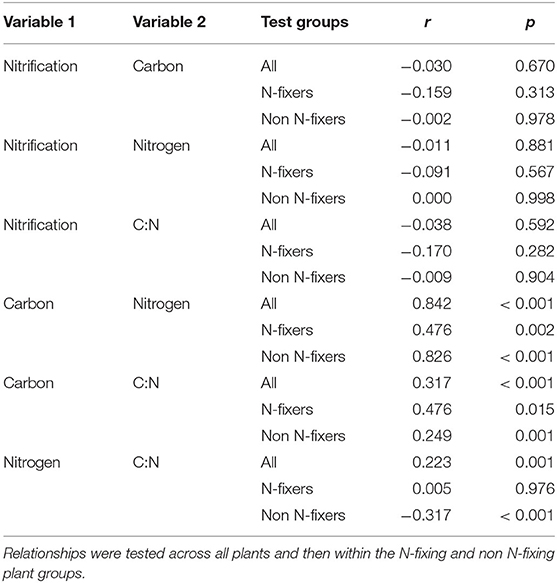
Table 1. Summary person correlations statistics (r) and significance (p) for associations among rhizosphere potential nitrification rate, total C, total N, and C:N ratio.
The PNR in rhizosphere soil had no association with total C, total N, nor the soil C:N ratios (p = 0.67, p = 0.881, and p = 0.592, respectively). Furthermore, these test outcomes were unaffected where data were analysed across all the plants, or independently within the two N-fixing groups (Table 1).
Total carbon and total N were closely related (r = 0.84; p < 0.001) across all plants. The association was strongest for non-N fixing plants (r = 0.826) compared with N-fixing species (r = 0.476; p = 0.002); i.e., for plants that can fix their own nitrogen, the relationship between soil total C and N contents was considerably weaker than for species that don't have this trait (Table 1).
The C:N ratio was more strongly associated with soil C content than soil N content (comparing sets of r-values between the “Total C and C:N,” with “Total N and C:N” groups; Table 1). The relationship between total C and C:N was strongest in the N-fixing group, and the total N and C:N relationship strongest in the non-N fixing group. In order to better understand these relationships, the data were modelled using linear regression and these trends plotted (Supplementary Figure 4). For both N-fixing and non-fixing plants, total C and C:N increased at nearly equal rates (similar slopes). For total N, however, the relationships between N-fixing and non N-fixing plants are dissimilar (Supplementary Figure 4B). For N fixers, the relationship is stable; i.e., as N increases the C:N remains ~12.2. However, for non N-fixing plants, increased soil N is associated with a reduction in the C:N ratio. As stated before, for both plant groups, these relationships between total N and C are significantly correlated, however this is strongest for the non-fixing species (e.g., compare confidence bands on Supplementary Figure 4B).
Discussion
The aim of this study was to investigate if traits such as mycorrhization, N fixation, growth form (grass, herb, tree, etc), or native v exotic provenance, are associated with changes in rhizosphere soil total C content, total N content, and N mineralisation rate. This is important as the ability to manipulate rhizosphere C and N deposition and N mineralization could have many consequences for ecosystem function. Understanding how these vary among plants and via plant traits may provide insights into ecology of both managed and natural ecosystems. For example, strategies to alter N in the rhizosphere soil may affect plants ability to compete and survive in N limited environments such as infertile soils (e.g., highly weathered/leached or early chronosequence), thereby influencing trajectories of ecological succession through to invasion success (establishment of exotic species). In agricultural ecosystems, the ability to manipulate rhizosphere C deposition and N mineralization and nitrification could lead to opportunities to alter soil C cycling and N dynamics (45). For example, if plants can recapture N already present in the soil ecosystem (i.e., a more closed N cycle), this would both increase N use efficiency and reduce the amount of N lost to the environment (e.g., waterways or atmosphere).
C and N are tightly coupled in the rhizosphere of all plants, irrespective of if they are native or exotic, nature of their mycorrhizal associations, or functional group (forbs, grasses, shrubs, and trees). Total C, C:N ratio, and potential nitrification rate in the rhizosphere soil did not differ between any of the plants in this study; i.e., although the plant species evaluated varied widely in many traits, their rhizosphere soils generally maintained a similar equilibrium in elemental stoichiometry. This agrees with the findings of Kirkby et al. (46) who found that the elemental ratios in soil organic matter (C:N:P:S) were remarkably similar across a wide range of soils from Australia and around the world. Cleveland and Liptzin (47) also conducted a review on global patterns of soil and soil microbial biomass C:N:P ratios. They found that C:N:P ratios in both soil (186:13:1) and the soil microbial biomass (60:7:1) are generally well-constrained at the global scale. This relationship between the elemental composition of organisms and the ecosystems they inhabit is critical to ecosystem function (48), and thus is highly conserved [e.g., (25)].
The single plant-associated factor influencing C and N associations was N-fixing symbiosis type. N fixers had, on average, higher levels of both N and C levels in their rhizosphere soil. The symbiotic relationship between plants with rhizobia and actinorhizal root-nodule forming bacteria (e.g. Rhizobium, Frankia) enhances soil N via N2 fixation (49). This increase in soil N results in increased biomass yields which when returned to the soil increases soil organic carbon (50), and increased N is also thought to increase rhizodeposition (29). N-fixers have a competitive advantage in N limited systems (51) and can be a contributing factor to the success of plants in low N environments and of invading species, e.g., gorse (Ulex europaeus) in New Zealand and the fire tree (Myrica faya) in Hawaii (41, 52).
A missing consideration in this work is the potential for non-nodule forming N-fixing root associations to be contributing to rhizosphere geochemical processes. These diazotrophic associations can occur in and on root the roots of many plant species (53, 54). However, the contribution of these toward soil N fertility are variable, dependant on the system under investigation [e.g., (55)], and can be difficult to assess (56). These associations were not formally considered in this study, but may play an important role in rhizosphere N and C content and cycling in these soils.
Although N fixing plants had higher average total N in the rhizosphere soil, this had no effect on PNR nor the C:N ratio. This provides further evidence that soil ecosystems maintain an equilibrium of N and C, regardless of the plant species or species traits. The C:N ratio is maintained by plants and microbes in the soil. The microbial community (biomass) has a relatively fixed C:N ratio and this largely controls the C:N ratio of soil organic matter within a given ecosystem type (e.g., agricultural pasture or native forest) (57). Adaptability of plant form and function, plant species diversity, and regulation of biological N fixation all contribute to stabilise the C:N ratio of organic matter inputs to soil. Soil processes such as the priming effect and nitrate leaching tend to restore stoichiometry by releasing elements in excess (58). Fontaine et al. (59) suggests the soils may regulate nutrient and carbon sequestration by lowering the priming effect when nutrient availability is high, allowing for sequestration of nutrients and carbon, and when nutrient availability is low microbes release nutrients from soil organic matter.
Potential nitrification rate, an important trait investigated to reduce impacts of N deposition in soils, was not related to any plant traits tested. However, there was evidence that some species, and particularly Coprosma, may be able to inhibit ammonia oxidation (sensu BNI in this paper, though indirectly tested via PNR).
It is possible that although the total C, C:N ratio, or potential nitrification rate were not influenced by plant type (with the exception of Coprosma spp., as noted above), the type of C or the C-cycling ecophysiology may shift depending on plant type. The rate or type of C-associated biogeochemistry in soils can affect N pools and flux (58). This has an impact on both N availability and C sequestration (60). In a study investigating soils with high and low nitrate levels, Wakelin et al. (45) found that total C content and extractable organic C of soils with low and high nitrate content were similar. However, soils with anomalously low-nitrate N had shifts in genetic potential for different C-cycling pathways. They concluded that alteration of inputs of soil C or changes in soil C–cycling ecophysiology may shift allocation of N between the total and mineral pools. It is possible that under the different plants in this study similar shifts in C-cycling ecophysiology have occurred.
We chose to be deliberately conservative in the approach to statistical analysis of our results. This was primarily as the experimental system was established to be exploratory, with a view toward investigating the primary factors associated with rhizosphere C and N properties. Indeed, this approach has enabled us to have high confidence in the outcomes presented. Moreover, deeper testing of these data, and particular multi-level interactions among traits (e.g., mycorrhization × N-fixation × form × provenance) are not possible given lack of replication at some levels; e.g., N-fixation entirely absent among grass species, or unbalanced mycorrhizal associations among other trait groups. While mixed-model effects, structural equation modelling, or other testing is certainly possible, this was certainly not the original intent; i.e., exploring the first-order driver(s) of this system. We propose, instead, that more tailored experimental systems be designed to approach exploration of secondary and interaction effects, and that these can now be appropriately guided via the outcomes of this work.
A limitation to this study is that only one soil type was tested. Different root exudates may be released from the plants in this study plants under different conditions and this could impact C and N cycling. An increase in clay and loam content in soil has been found to increase rhizodeposition and therefore C into the soil (29). This is due to the properties of clay and loam that favour microbial activity and nutrient cycling such as water retention, organic matter stabilisation, and high cation exchange capacity.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
SW and KW prepared the original draught. AM undertook sampling, experimental work. SW, AM, and LG undertook analysis and interpretation of the data. ID, LW, and SW contributed to study design. ID, SW, and DW were responsible for funding acquisition. All authors contributed to the revisions of the original draught, contributed to the article, and approved this for submission.
Funding
This work was supported by funding from the Ministry of Business, Innovation and Employment (MBIE contract C09X1610: Reducing nitrogen losses from farms), with support from the NZ Tertiary Education Commission Centres of Research Excellence funding to the BioProtection Centre.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsoil.2021.762510/full#supplementary-material
References
1. Vitousek PM, Howarth RW. Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry. (1991) 13:87–115. doi: 10.1007/BF00002772
2. LeBauer DS, Treseder KK. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology. (2008) 89:371–9. doi: 10.1890/06-2057.1
3. Wedin DA, Tilman D. Species effects on nitrogen cycling: a test with perennial grasses. Oecologia. (1990) 84:433–41. doi: 10.1007/BF00328157
4. Finzi AC, Van Breemen N, Canham CD. Canopy tree–soil interactions within temperate forests: species effects on soil carbon and nitrogen. Ecol Appl. (1998) 8:440–6. doi: 10.1890/1051-0761(1998)008(0440:CTSIWT)2.0.CO;2
5. Dijkstra FA, Hobbie SE, Reich PB. Soil processes affected by sixteen grassland species grown under different environmental conditions. Soil Sci Soc Am J. (2006) 70:770–7. doi: 10.2136/sssaj2005.0088
6. Chapin F III, Eviner V. Biogeochemistry of terrestrial net primary production. Treat Geochem. (2003) 8:682. doi: 10.1016/B0-08-043751-6/08130-5[10.1016/B0-08-043751-6/08130-5]
7. Nacry P, Bouguyon E, Gojon A. Nitrogen acquisition by roots: physiological and developmental mechanisms ensuring plant adaptation to a fluctuating resource. Plant Soil. (2013) 370:1–29. doi: 10.1007/s11104-013-1645-9
8. Hermans C, Hammond JP, White PJ, Verbruggen N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. (2006) 11:610–7. doi: 10.1016/j.tplants.2006.10.007
9. Clarkson DT. Factors affecting mineral nutrient acquisition by plants. Ann Rev Plant Physio. (1985) 36:77–115. doi: 10.1146/annurev.pp.36.060185.000453
10. George E, Marschner H, Jakobsen I. Role of arbuscular mycorrhizal fungi in uptake of phosphorus and nitrogen from soil. Crit Rev Biotechnol. (1995) 15:257–70. doi: 10.3109/07388559509147412
11. Miransari M. Arbuscular mycorrhizal fungi and nitrogen uptake. Arch Microbiol. (2011) 193:77–81. doi: 10.1007/s00203-010-0657-6
12. Van Der Heijden MG, Bardgett RD, Van Straalen NM. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett. (2008) 11:296–310. doi: 10.1111/j.1461-0248.2007.01139.x
13. Hobbie SE. Effects of plant species on nutrient cycling. Trends Ecol Evol. (1992) 7:336–9. doi: 10.1016/0169-5347(92)90126-V
14. Peltzer DA, Bellingham PJ, Kurokawa H, Walker LR, Wardle DA, Yeates GW. Punching above their weight: low-biomass non-native plant species alter soil properties during primary succession. Oikos. (2009) 118:1001–14. doi: 10.1111/j.1600-0706.2009.17244.x
15. Waller LP, Allen WJ, Barratt BIP, Condron LM, França FM, Hunt JE, et al. Biotic interactions drive ecosystem responses to exotic plant invaders. Science. (2020) 368:967–72. doi: 10.1126/science.aba2225
16. Coskun D, Britto DT, Shi W, Kronzucker HJ. Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nat Plants. (2017) 3:17074. doi: 10.1038/nplants.2017.74
17. Subbarao G, Rondon M, Ito O, Ishikawa T, Rao IM, Nakahara K, et al. Biological nitrification inhibition (BNI)—is it a widespread phenomenon? Plant Soil. (2007) 294:5–18. doi: 10.1007/s11104-006-9159-3
18. Subbarao GV, Nakahara K, Hurtado MP, Ono H, Moreta DE, Salcedo AF, et al. Evidence for biological nitrification inhibition in brachiaria pastures. Proc Natl Acad Sci USA. (2009) 106:17302–7. doi: 10.1073/pnas.0903694106
19. Villegas D, Arevalo A, Nuñez J, Mazabel J, Subbarao G, Rao I, et al. Biological nitrification inhibition (BNI): phenotyping of a core germplasm collection of the tropical forage grass megathyrsus maximus under greenhouse conditions. Front. Plant Sci. (2020) 11:820. doi: 10.3389/fpls.2020.00820
20. Bowatte S, Barrett B, Luscombe C, Hume DE, Luo D, Theobald P, et al. Effect of grass species and fungal endophyte on soil nitrification potential. New Zeal J Agr Res. (2011) 54:275–84. doi: 10.1080/00288233.2011.606325
21. Franklin HM, Robinson BH, Dickinson NM. Plants for nitrogen management in riparian zones: a proposed trait-based framework to select effective species. Ecol Manag Restor. (2019) 20:202–13. doi: 10.1111/emr.12380
22. O'Sullivan CA, Duncan EG, Whisson K, Treble K, Ward PR, Roper MM. A colourimetric microplate assay for simple, high throughputassessment of synthetic and biological nitrification inhibitors. Plant Soil. (2017) 413:275–87. doi: 10.1007/s11104-016-3100-1
23. Zhou Y, Lambrides CJ, Li J, Xu Q, Toh R, Tian S, et al. Nitrifying microbes in the rhizosphere of perennial grasses are modified by biological nitrification inhibition. Microorganisms. (2020) 8:1687. doi: 10.3390/microorganisms8111687
24. Ladd JN, Oades JM, Amato M. Microbial biomass formed from 14C, 15N labelled plant material decomposing in soils in the field. Soil Biol Biochem. (1981) 13:119–26. doi: 10.1016/0038-0717(81)90007-9
25. Redfield AC. The biological control of chemical factors in the environment. Am Sci. (1958) 46:205–21.
26. Steltzer H, Bowman WD. Differential influence of plant species on soil nitrogen transformations within moist meadow alpine tundra. Ecosystems. (1998) 1:464–74. doi: 10.1007/s100219900042
27. Van Veen J, Liljeroth E, Lekkerkerk L, Van de Geijn S. Carbon fluxes in plant-soil systems at elevated atmospheric CO2 levels. Ecol Appl. (1991) 1:175–81. doi: 10.2307/1941810
28. Jones MB, Donnelly A. Carbon sequestration in temperate grassland ecosystems and the influence of management, climate and elevated CO2. New Phytol. (2004) 164:423–39. doi: 10.1111/j.1469-8137.2004.01201.x
29. Nguyen C. Rhizodeposition of organic C by plant: mechanisms and controls. In: Lichtfouse E, Navarrete M, Debaeke P, Véronique S, Alberola C, editors. Sustainable Agriculture. Netherlands: Springer (2009). p. 97–123.
30. Shahzad T, Chenu C, Genet P, Barot S, Perveen N, Mougin C, et al. Contribution of exudates, arbuscular mycorrhizal fungi and litter depositions to the rhizosphere priming effect induced by grassland species. Soil Biol Biochem. (2015) 80:146–55. doi: 10.1016/j.soilbio.2014.09.023
31. Grayston S, Vaughan D, Jones D. Rhizosphere carbon flow in trees, in comparison with annual plants: the importance of root exudation and its impact on microbial activity and nutrient availability. Appl Soil Ecol. (1997) 5:29–56. doi: 10.1016/S0929-1393(96)00126-6
32. Clemmensen KE, Finlay RD, Dahlberg A, Stenlid J, Wardle DA, Lindahl BD. Carbon sequestration is related to mycorrhizal fungal community shifts during long-term succession in boreal forests. New Phytol. (2015) 205:1525–36. doi: 10.1111/nph.13208
33. Haynes RJ, Williams PH. Nutrient cycling and soil fertility in the grazed pasture ecosystem. Adv Agron. (1993) 49:119–99. doi: 10.1016/S0065-2113(08)60794-4
34. Edmeades DC. The long-term effects of manures and fertilisers on soil productivity and quality: a review. Nutr Cycl Agroecosys. (2003) 66:165–80. doi: 10.1023/A:1023999816690
35. Knorr M, Frey SD, Curtis PS. Nitrogen additions and litter decomposition: a meta-analysis. Ecology. (2005) 86:3252–7. doi: 10.1890/05-0150
36. Galloway JN, Aber JD, Erisman JW, Seitzinger SP, Howarth RW, Cowling EB, et al. The nitrogen cascade. Bioscience. (2003) 53:341–56. doi: 10.1641/0006-3568(2003)053(0341:TNC)2.0.CO;2
37. Wilton A, Breitwieser I. Composition of the New Zealand seed plant flora. New Zeal J Bot. (2000) 38:537–49. doi: 10.1080/0028825X.2000.9512703
38. Rose A, Platt K, Frampton C. Vegetation change over 25 years in a New Zealand short-tussock grassland: effects of sheep grazing and exotic invasions. New Zeal J Ecol. (1995) 19:163–74.
39. Peltzer DA. Ecology and consequences of invasion by non-native (wilding) conifers in New Zealand. J New Zeal Grasslands. (2018) 80:39–46. doi: 10.33584/jnzg.2018.80.359
40. Wardle P. Environmental influences on the vegetation of New Zealand. New Zeal J Bot. (1985) 23:773–88. doi: 10.1080/0028825X.1985.10434242
41. Magesan GN, Wang H, Clinton PW. Nitrogen cycling in gorse-dominated ecosystems in New Zealand. New Zeal J Ecol. (2012) 36:21–8. Available online at: https://newzealandecology.org/nzje/3013.pdf
42. Wardle D, Greenfield L. Release of mineral nitrogen from plant root nodules. Soil Biol Biochem. (1991) 23:827–32. doi: 10.1016/0038-0717(91)90093-Y
43. Jenkinson DS, Powlson DS. The effects of biocidal treatments on metabolism in soil V. A method for measuring soil biomass. Soil Biol Biochem. (1976) 8:209–13. doi: 10.1016/0038-0717(76)90005-5
44. Hart SC, Stark JM, Davidson EA, Firestone MK. Nitrogen mineralization, immobilization, and nitrification. In: Weaver R. Angle J, Bottomley B, editor. Methods of Soil Analysis. Part 2. Microbiological and Biochemical Properties. Madison, WI: Soil Science Society of America (1994).
45. Wakelin S, Maclean P, Cave V, Zhou J, Grelet GA, Whitehead D, et al. Characterising the soil ecosystem phenotype associated with relatively low nitrate-N concentrations. Appl Soil Ecol. (2019) 142:189–98. doi: 10.1016/j.apsoil.2019.04.012
46. Kirkby C, Kirkegaard J, Richardson A, Wade L, Blanchard C, Batten G. Stable soil organic matter: a comparison of C:N:P:S ratios in Australian and other world soils. Geoderma. (2011) 163:197–208. doi: 10.1016/j.geoderma.2011.04.010
47. Cleveland CC, Liptzin D. C:N:P stoichiometry in soil: is there a “Redfield ratio” for the microbial biomass? Biogeochemistry. (2007) 85:235–52. doi: 10.1007/s10533-007-9132-0
49. Ardley J, Sprent J. Evolution and biogeography of actinorhizal plants and legumes: a comparison. J Ecol. (2021) 109:1098–121. doi: 10.1111/1365-2745.13600
50. Christopher SF, Lal R. Nitrogen management affects carbon sequestration in North American cropland soils. Crit Rev Plant Sci. (2007) 26:45–64. doi: 10.1080/07352680601174830
51. Hartwig UA. The regulation of symbiotic N2 fixation: a conceptual model of N feedback from the ecosystem to the gene expression level. Perspect Plant Ecol. (1998) 1:92–120. doi: 10.1078/1433-8319-00054
52. Vitousek PM, Walker LR, Whiteaker LD, Mueller-Dombois D, Matson PA. Biological invasion by myrica faya alters ecosystem development in Hawaii. Science. (1987) 238:802–4. doi: 10.1126/science.238.4828.802
53. Kirchhof G, Reis VM, Baldani JI, Eckert B, Dobereiner J, Hartmann A. Occurrence, physiological and molecular analysis of endophytic diazotrophic bacteria in gramineous energy plants. Plant Soil. (1997) 194:45–55. doi: 10.1023/A:1004217904546
54. Gupta VVSR, Zhang B, Penton CR, Yu J, Tiedje JM. Diazotroph diversity and nitrogen fixation in summer active perennial grasses in a mediterranean region agricultural soil. Front Mol Biosci. (2019) 6:115. doi: 10.3389/fmolb.2019.00115
55. Chen XD, Dunfield KE, Fraser TD, Wakelin SA, Richardson AE, Condron LM. Soil biodiversity and biogeochemical function in managed ecosystems. Soil Res. (2020) 58:1–20. doi: 10.1071/SR19067
56. Unkovich M, Herridge D, Peoples M, Cadisch G, Boddey R, Giller K, et al. Measuring Plant-Associated Nitrogen Fixation in Agricultural Systems. ACIAR Monograph No. (2008). Available online at: https://www.aciar.gov.au/sites/default/files/legacy/node/10169/MN136%20Part%201.pdf
57. Manzoni S, Jackson RB, Trofymow JA, Porporato A. The global stoichiometry of litter nitrogen mineralization. Science. (2008) 321:684–6. doi: 10.1126/science.1159792
58. Soussana JF, Lemaire G. Coupling carbon and nitrogen cycles for environmentally sustainable intensification of grasslands and crop-livestock systems. Agr Ecosyst Environ. (2014) 190:9–17. doi: 10.1016/j.agee.2013.10.012
59. Fontaine S, Henault C, Aamor A, Bdioui N, Bloor J, Maire V, et al. Fungi mediate long term sequestration of carbon and nitrogen in soil through their priming effect. Soil Biol Biochem. (2011) 43:86–96. doi: 10.1016/j.soilbio.2010.09.017
Keywords: rhizosphere, carbon, nitrogen, potential nitrification rate, mycorrhizae, N-fixation, provenance, plant traits
Citation: Wakelin SA, Matson A, Wigley K, Waller L, Dickie IA, Whitehead D and Garrett L (2021) High Maintenance of Rhizosphere Soil C and N Equilibrium Regardless of Plant Species or Species Traits. Front. Soil Sci. 1:762510. doi: 10.3389/fsoil.2021.762510
Received: 22 August 2021; Accepted: 28 September 2021;
Published: 25 October 2021.
Edited by:
Emily B. Graham, Pacific Northwest National Laboratory (DOE), United StatesReviewed by:
Joseph E. Knelman, University of Colorado Boulder, United StatesMianhai Zheng, South China Botanical Garden, Chinese Academy of Sciences (CAS), China
Copyright © 2021 Wakelin, Matson, Wigley, Waller, Dickie, Whitehead and Garrett. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Steve A. Wakelin, c3RldmUuYS53YWtlbGluQHNjaW9ucmVzZWFyY2guY29t
 Steve A. Wakelin
Steve A. Wakelin A. Matson
A. Matson K. Wigley
K. Wigley L. Waller4
L. Waller4 David Whitehead
David Whitehead L. Garrett
L. Garrett