
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sens., 09 February 2024
Sec. Biosensors
Volume 5 - 2024 | https://doi.org/10.3389/fsens.2024.1302520
 Hui Ma1,2
Hui Ma1,2 Christine Loscher1
Christine Loscher1 Anne Parle-McDermott1
Anne Parle-McDermott1 Jenny Fitzgerald3
Jenny Fitzgerald3 Julie Meneely4
Julie Meneely4 Christopher Elliott4
Christopher Elliott4 Richard Welten4
Richard Welten4 Geofrey J. Mchau5,6
Geofrey J. Mchau5,6 Edna Makule5
Edna Makule5 Revocatus Machunda5
Revocatus Machunda5 Yun Yun Gong7
Yun Yun Gong7 Martin Kimanya5
Martin Kimanya5 Aoife Crawley1
Aoife Crawley1 Ivan Maguire3
Ivan Maguire3 Caroline Murphy1*†
Caroline Murphy1*† Richard O’Kennedy1,8*†
Richard O’Kennedy1,8*†Introduction: Globally, the need for measuring exposure to algal toxins has become urgent due to ever-increasing reports of contamination in sea and freshwater, in shellfish and fish stocks and in aerosols.
Methods: To address this issue, we evaluated the potential of determining autoantibodies to a panel of biomarkers known to be elevated following exposure to the hepatotoxin microcystin leucine-arginine. The presence of autoantibodies, specific to four selected stress-response, metabolomic and chaperone biomarkers, namely, Heat shock protein 1, Triosephosphate isomerase, Peroxiredoxin 1 and Peroxiredoxin 2 was employed in screening 371 serum samples from microcystin-exposed individuals in Tanzania. In addition, the capacity of the LightDeck fluorescence-based detector, a point-of-use device, to monitor these autoantibody responses in comparison to enzyme-linked immunosorbent assay was evaluated.
Results: By using the determination of autoantibodies to this novel panel of biomarkers an altered response was observed following microcystin exposure, with levels generally upregulated. The presence of elevated levels of microcystin leucine-arginine in water, as well as in food sources in Tanzania, may potentially have significant health effects on the population.
Discussion: This novel biomarker panel may have potential for the detection of microcystin leucine-arginine exposure as well as various microcystin exposure-associated cancers (e.g., hepatocellular cancer and colorectal cancer). In addition, the utilisation of the LightDeck point-of-use device proved successful for the rapid analysis of this biomarker panel.
There is a notable worldwide increase in the utilisation of panels of biomarkers for clinical and diagnostic investigations and such studies require biomarkers that have been thoroughly validated, verified and evaluated. Such biomarker panels could reflect responses/changes of individuals to specific exposures to toxic materials (Silins and Högberg, 2011). However, there is no generally agreed classification terminology, validation scheme or systematic review of biomarkers for nutrition, health and food intake currently available (Dragsted et al., 2017). This research aims to study the potential efficacy of a panel of four biomarkers for demonstrating microcystin (MC) exposure using a point-of-use device.
Increased human population and activities, climate change and environmental contamination have led to the eutrophication of the water by cyanobacterial blooms. Cyanotoxins, produced by such cyanobacterial blooms, cause severe water and food contamination, as well as human and animal health issues. Microcystins are highly toxic, and are one of the mostly commonly occurring cyanotoxins. Microcystin leucine-arginine (MC-LR) is one of the most dangerous as it is potentially carcinogenic for both animals and humans, through inhibition of protein phosphatases, which then cause the hyper-phosphorylation of cellular proteins (Lone et al., 2015).
High risk of MC-exposure was found in waterbodies providing drinking water in Africa (Zhao et al., 2023). In many West African countries, like Tazania, surface water is the main drinking water source, and thus it is a potentially hazardous source for MC exposure and can be a common public health problem in these areas. Exposure to MC can also occur through eating contaminated fish (including shellfish) and ingesting health-food supplements containing contaminated algae. Once ingested, MC mainly targets the liver, however, other organs such as lung, bladder, ovary, prostate, kidneys and brain are also affected. It was reported that chronic exposure to MC-LR causes lung barrier damage, kidney structure and function damage, ovarian inflammation and decrease of gonad index (Liu et al., 2021; Liu et al., 2022; Yao et al., 2023). It also induces epithelial hyperplasia and inflammation in mouse bladder, increasing the risk of bladder cancer (Zhang et al., 2022). Moreover, MC-LR exposure induces prostatic toxicity, skin barrier damage, brain tissue inflammation and oxidative stress (He et al., 2022; Pan et al., 2023; Wang et al., 2023). Cancers can be induced and/or promoted by MCs. MC-LRs are purported inducers of hepatocellular carcinoma (HCC) (Zheng et al., 2017; Shi et al., 2021) and colorectal cancer (CRC), the fifth and the third most common malignancies in the world, and their incidence is increasing globally (Megger et al., 2014; Jiang et al., 2023).
Acute intoxications with MCs are very well documented, however, chronic exposure to these toxins is much less well understood and, importantly, long-term exposure, while it has been linked to hepatocellular cancer and colorectal cancer, requires more definitive and non-invasive tests for reliable epidemiological studies (Meneely and Elliott, 2013). The importance of human exposure to MC toxins in relation to the development of cancers is currently not well understood. The published literature is dominated by targeted analytical methods capable of measuring MCs and these can detect acute exposure in humans. Identification of chronic exposure to such toxins is more complicated and difficult. Biomarkers play an important role in helping to understand the relationships between exposure to environmental toxins and the development of chronic human diseases and cancer. Therefore, this research aims to utilise MC exposure-specific biomarkers to accomplish this.
While the body’s immune system produces antibodies in response to the presence of external invaders, it also produces autoantibodies to ‘self’ antigens or autoantigens, which are produced, promoted, or modified by various diseases, especially autoimmune disease and cancer. However, some autoantibodies are also detected in healthy individuals, usually with low levels (Shome et al., 2022). Autoantibodies are usually considered to be harmful, some cause injury directly, while some are believed to play crucial roles in immune responses that causes inflammation and damage (e.g., autoantibodies could promote cancer progression, and drive severe and long COVID-19) (Ma et al., 2022). However, autoantibodies are very suitable to be used as diagnostic biomarkers as they are generally more stable than circulating antigens. Moreover, autoantibodies can be found at detectable levels in the early stages of diseases including cancers (Zaenker and Ziman, 2013; O’Reilly, et al., 2015). In addition, their presence may precede the manifestation of clinical symptoms, making them ideal candidates for pre-symptomatic screening (Li et al., 2005; Ladd et al., 2013; Pedersen et al., 2013; O’Reilly et al., 2015).
In this paper four putative autoantibody-based biomarkers, namely, Heat shock protein 90 (HSP90), Triosephosphate isomerase (TPI), Peroxiredoxin-1 (PRDX1) and Peroxiredoxin-2 (PRDX2) were identified through extensive microcystin exposure literature searches (Welten et al., 2020). The biomarkers were ranked as to whether they were purported to be up or downregulated in response to microcystin-LR exposure, whether they were linked to the development of HCC and/or CRC, and the number of literature references associated with each biomarker.
HSP90 is a chaperone protein and has many roles within the body, for example, it aids the correct folding of other proteins, stabilises proteins from heat stress, and plays a crucial role in protein degradation. As HSP90 stabilises numerous crucial proteins in tumor growth, it has the capacity to be a promising biomarker for diagnosis and prognosis and may be of therapeutic value for monitoring various cancers (Kryeziu et al., 2019). HSP90 is also a potential target for anti-cancer treatment, e.g., HSP90 inhibitors have become popular candidates for anti-cancer therapy (Ernst et al., 2020). Studies have indicated that HSP90 may be positively involved in the development of HCC and may provide a potential biomarker for monitoring advanced HCC (Qin et al., 2019). Also, Wei et al. (2020) concluded that the levels of plasma HSP90α in HCC patients were significantly higher than in healthy donors, and suggested that levels of plasma HSP90α could be used in initial diagnosis. Moser et al. (2007) and Kryeziu et al. (2019) reported that by blocking HSP90, colon cancer growth and hepatic metastasis could be significantly inhibited. Moreover, it was reported that the male red swamp crayfish, Procambarus clarkia, displayed significantly increased levels of HSP90 following exposure to 0.1–100 μg/L MC-LR from 1 to 7 days (Yuan et al., 2016). Dramatically increased levels of HSP90 were also observed in zebrafish larvae exposed to low (200 μg/L) and high (800 μg/L) concentrations of MC-LR from 12 h to 7 days (Li et al., 2008).
TPI is an enzyme required for cell growth and maintenance (Judith et al., 1985) and is over expressed in many cancers including lung adenocarcinoma, bladder squamous cell carcinoma and breast carcinoma (Montgomerie et al., 1997; Chen et al., 2002; Tamesa et al., 2009). A study by Chen et al. (2017), found that TPI was associated with cancer, and had the potential to be a prognostic indicator of overall survival in gastric cancer (GC) patients. It is involved in migration and invasion of cancer cells (Chen et al., 2017). Husi et al. (2019) identified TPI, as a potential target marker for oesophagastric junction (OGJ) cancer, and hepatocellular carcinoma (Hamaguchi et al., 2008). TPI was found to be upregulated in the nasopharyngeal carcinoma cell line CNE-2 (a human poorly differentiated neural progenitor cell line), and Chen et al. (2015), concluding that among others, TPI and heat shock protein 90, merited further investigation.
PRDX1, is a member of the peroxiredoxin (PRDX) family. The peroxiredoxins are a ubiquitously expressed group of anti-oxidant enzymes which mediate signal transduction in mammalian cells (Huang et al., 2018). Proteins involved in oxidoreductase activity and cytoskeletal processes are persistently affected by microcystin-LR exposure (Welten et al., 2020). PRDX1 participates in cellular anti-oxidation, cellular growth and proliferation. RACK1, RAN and PRDX1 all associate with IgG from tumor cells and may form a large complex (Wang et al., 2013). It is believed that PRDX1 plays a key role in the progression and metastasis of human tumours, thus providing a potential candidate for anti-cancer treatment (Ding et al., 2017). Li et al. (2018) found that PRDX1 promoted tumor metastasis and angiogenesis in colorectal cancer. Sun et al. (2015) identified PRDX1 as being overexpressed in liver cancer tissue. Chen et al. (2010) also indicated that PRDX1 positivity was linked to both a poor response to neoadjuvant therapy and survival in patients with colorectal cancer. Moreover, in cellular and animal experiments, the inhibition of PRDX1 dramatically decreased tumor growth. Sun et al. (2015) reported that PRDX1 was overexpressed in tumor tissues of liver cancer and provided an independent prognostic factor for poor overall survival. Chen et al. (2005) also found out that PRDX1 in mice was upregulated within the first 4 h after exposure to low concentrations of MC-LR.
Similarly to PRDX1, PRDX2 is another member of the peroxiredoxin (PRDX) family. Upregulated levels of PRDX2 were found in rat hippocampus (Li et al., 2012) as well as in mouse liver after MC-LR exposure (Chen et al., 2005). Moreover, increased levels of PRDX2 were also reported in various cancer cells and tissues, including CRC (Lu et al., 2014; Chuerduangphui et al., 2020). In CRC cells, PRDX2 plays a crucial role in oxidation-induced apoptosis regulation, which makes PRDX2 a promising target for CRC treatment. In addition, the upregulation of PRDX2 correlates with tumor progression. Therefore, PRDX2 could also be used as a potential biomarker for the prognosis of CRC (Peng et al., 2017).
Therefore, these four markers were selected and their combination should provide greater efficacy for being able to determine potential MC exposure.
Recombinant human heat shock protein (HSP90), proteins peroxiredoxin 1 (PRDX1), peroxiredoxin 2 (PRDX2) and triosephosphate isomerase protein (TPI) were all sourced from Abcam (United Kingdom). Donkey anti-human (horseradish peroxidase [HRP]-labelled) IgG was purchased from Jackson ImmunoResearch Europe Ltd. (United Kingdom). Nunc 96-well Enzyme-linked immunosorbent assay (ELISA) plates were purchased from GE Healthcare (United Kingdom). All other reagents were sourced from Sigma–Aldrich (United Kingdom), unless stated otherwise.
This study was approved by the National Institute for Medical Research (NIMR) ethical committee with ethical clearance number NIMR/HQ/R.8a/Vol. IX/2426. All human blood samples were obtained with written informed consent. In total, sera from 368 patients were analysed in this study. All samples were retrieved from Tanzania with water sources classed as ‘at risk’ for contamination with microcystin. Blood was obtained from all patients in the Ukerewe District. Serum was prepared and stored at −80°C.
A 5 mL fasting blood sample was collected by a qualified nurse from each subject. The serum was separated following centrifugation for analysis of blood MC-specific biomarkers and stored at −80°C.
An indirect enzyme-linked immunosorbent assay (ELISA) was developed to detect autoantibodies in human serum. In brief, ELISA plates were coated with HSP90, TPI, PRDX1, PRDX2, respectively (overnight incubation (o/n) at 4°C; 100 μL/well; 2 μg/mL for HSP90, TPI, PRDX1; 1 μg/mL for PRDX2) (The coating concentration of each antigen was optimised using ELISA). Plates were washed, blocked (1 h, 37°C), and incubated with human sera (overnight at RT; 100 μL/well; 1 in 200 dilution). Following washing, plates were incubated with horse radish peroxidase (HRP) labelled-donkey anti-human IgG (1 h at 37°C; 100 μL/well; 1 in 20,000 dilution). Tetramethylbenzidine (TMB) was used as substrate for the peroxidase reaction (RT; 100 μL/well) and the enzymatic reaction was stopped after 10 min with 10% (v/v) HCl (RT; 50 μL/well). The optical density (OD) was read at 450 nm (Safire2 plate reader, Tecan Group). Commercial human serum (Sigma, Ireland; S1-100ML; Lot number: 3610512; each lot is produced by collection of 100 L from 400 to 500 donors) was used in quadruplicate as a control across ELISA plates. ELISA ‘cut-offs’ were calculated as the average of the commercial serum relative OD values, which are the mean ± standard deviation (S.D.), where n = 4. Serum samples with values above the ‘cut-off’ were identified as positive for autoantibodies against the protein of interest. All tests were analysed using GraphPad Prism 9 software (GraphPad Software, La Jolla, CA, United States).
The design and production of portable optical-planar waveguide cartridges for the determination of microcystin was carried out in collaboration with LightDeck Diagnostics, Colorado, United States of America (Murphy et al., 2015) (Figure 1). A panel of four biomarkers indicative of microcystin exposure were employed/tested as a diagnostic platform. Biomarkers (HSP90, TPI, PDRX1 and PDRX2) were printed using a Bio-Dot AD3200 robotic arrayer onto microarrays at varying concentrations (5, 20, 100 and 250 μg/mL). Spots with a diameter of 0.5 mm were generated using a Bio-Jet print head which dispensed 20 nL. Each antigen concentration was printed onto a grid with 1 mm centres, blocked with a protein-based blocking agent (0.5% (w/v) casein in PBS (150 mM, pH 7.4) with Proclin300 anti-microbial agent) prior to spin-drying. Microarrays were then assembled into an injection-moulded cartridge with a 5 mm wide fluidic channel (max volume: 30 µL), which contains a single inlet port for the addition of sample and reagents, as well as an outlet port for waste collection. Multiple human serum samples were screened. LightDeck harnesses the power of advanced planar waveguide technology to generate quantitative, lab-quality results almost anywhere in a very short timeframe.
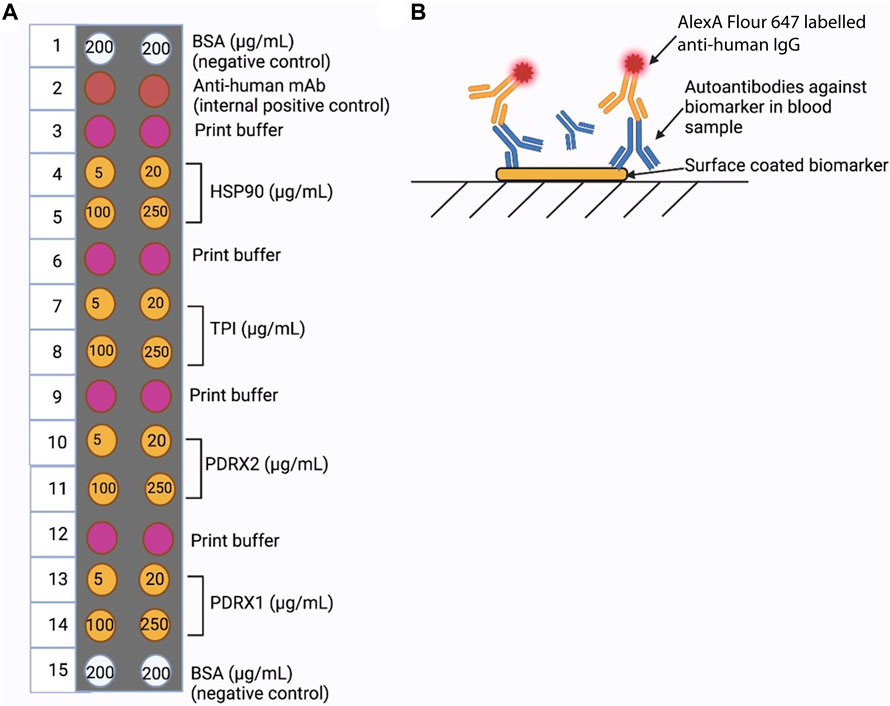
FIGURE 1. LightDeck Platform. (A) The cartridge set-up for autoantibody detection using LightDeck. (B) Strategy for autoantibody detection used with fluorescently labelled (Alexa-647) antibody.
All steps were carried out at room temperature, and the excess fluid was pipetted out of the cartridge outlet port after each step. In the initial assay step individual human serum samples (100 µL of 1:100 dilution in PBS) were added to the cartridge coated with the relevant biomarkers, followed by a 10 min incubation. ‘Washing’ was performed by addition of 170 µL of PBS followed by incubation for 3 min. Detection antibody (Alexa Flour™ 647 labelled anti-human IgG antibody (Fisher, Cat. No: 10337882), 100 µL of 1:2,000 dilution in PBS) was then applied to the cartridge and incubated for 10 min, followed by the second wash, by addition of 170 µL of PBS and incubated for 3 min. The cartridge was then inserted into the LightDeck system and the fluorescence response determined immediately.
Commercial human serum samples (Sigma, Ireland; S1-100 mL; Lot number: 3610512; each lot was produced by combining 100 L of sera from 400 to 500 donors) were used, in sextuplicate, as negative controls. LightDeck ‘cut-offs’ were calculated as the average of the negative control serum relative signal values, which are the mean ± standard deviation (S.D.), where n = 6. Serum samples with values above the ‘cut-off’ (negative control) were identified as positive for the presence of autoantibodies against the target protein of interest. The percentage positivity was calculated using the following formula:
V = value determined, Ave = average value.
All tests were analysed using GraphPad Prism 9 software (GraphPad Software, La Jolla, CA, United States of America).
ELISA and LightDeck-based immunoassay ‘cut-offs’ levels were calculated as the average of the commercial serum (negative control) relative values. For ELISA, the negative control ‘cut-off’ are the mean ± standard deviation (S.D.), where n = 4. For LightDeck, the ‘cut-off’ are the mean ± standard deviation (S.D.), where n = 6. Serum samples with values above the ‘cut-off’ were identified as positive for autoantibodies against the protein of interest. All tests were analysed using GraphPad Prism 9 software (GraphPad Software, La Jolla, CA, United States).
Ten serum samples were randomly selected from the high risk exposure group (main source of water is either shallow water or lake water) and 10 from the low risk exposure group (main source of water is either deep water or tap water). Controls were composed of commercially available human sera that consisted of a pooled sample of 400–500 individuals.
For TPI and PRDX2, the autoantibody positivity in high risk exposure individuals is 50% and 60%, whereas in low risk it is 70% and 80%, respectively, when compared with autoantibody levels in controls. In addition, the overall and mean levels of anti-HSP90 and anti-PRDX1 autoantibodies is higher than those of anti-TPI and anti-PRDX2 autoantibodies (Figure 2).
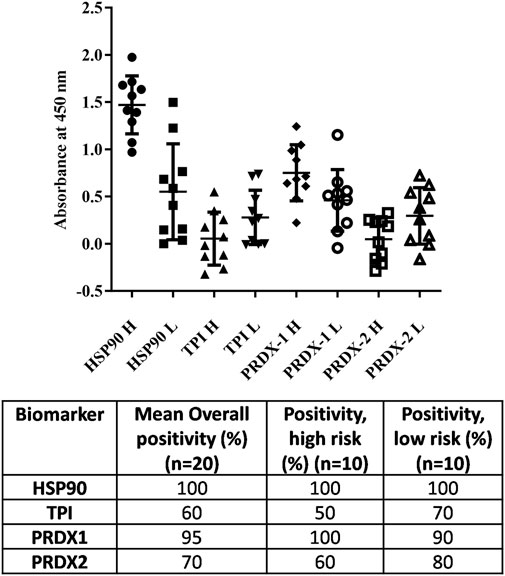
FIGURE 2. Analysis of 20 random blood samples towards the antigens of interest (HSP-90, TPI, PRDX1 and PRDX2) by ELISA. Ten patient serum samples from high risk patients (H) whose main source of water is either shallow water or lake water, and 10 from low risk patients (L) whose main source of water is either deep water or tap water, were used.
When we comparing the ELISA results with the results obtained from LightDeck system, similar conclusions were made. HSP90 and PRDX1 are the two biomarkers with higher positive rates than TPI and PRDX2. However, the overall positive rate detected by the LightDeck system is a little lower when compared with ELISA (Figure 3). This difference may be because the LightDeck system is less sensitive, or is more accurate, providing less false positive results, which in turn require further analyses. However, the use of multiple controls within the cartridge (i.e., negative controls: BSA and print buffer; positive control: anti-human IgG) provided by the LightDeck system in each detection for every sample, would suggest there is good reason to believe that LightDeck system is more accurate with less false positive readings.
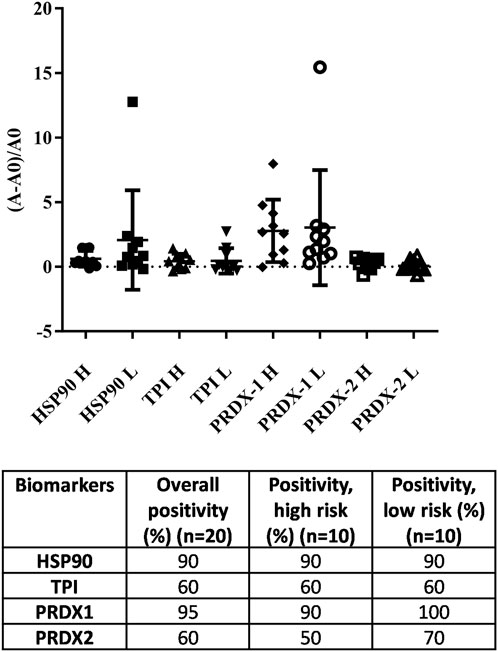
FIGURE 3. Analysis of 20 random blood samples towards the target antigens (HSP-90, TPI, PRDX1 and PRDX2) using the LightDeck system. A = absorbance of each individual exposure sample; A0 = average absorbance of the controls. H = high risk; L = low risk.
The results obtained from ELISA and LightDeck tests are very similar, which demonstrated that LightDeck provided reliable analysis potential with increased speed to assist future diagnosis and treatments. Autoantibody levels against HSP90 and PRDX1 showed 95%–100% positivity levels using both the ELISA and LightDeck tests, when compared with the autoantibody levels in controls. The mean levels of anti-HSP90 and PRDX1 autoantibody levels in high risk exposure individuals are higher than in low risk individuals. While the overall autoantibody positivity levels against TPI (60%) and PRDX2 (60%–80%) are relatively lower than those against HSP90 and PRDX1 determined using both ELISA and LightDeck-based immunoassay (Figure 4).
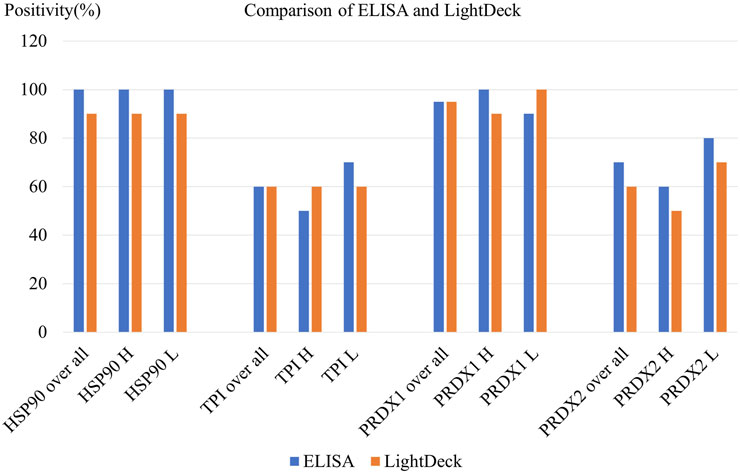
FIGURE 4. Comparison of ELISA and LightDeck results using 20 randomly selected patient serum samples. H = high risk; L = low risk.
A total of 371 individual esposure samples were analysed for autoantibodies against HSP90, TPI, PRDX1 and PRDX2 and upregulation of autoantibodies against each was identified when compared with autoantibodies in healthy controls, using either 250 μg/mL or 100 μg/mL as the coating concentration of antigens. Similar results were obtained for each autoantibody level determination, as shown in Supplementary Figure S1. The difference between the results obtained when using either 250 μg/mL or 100 μg/mL of coated antigens was small varing from 1% to 3%.
Generally, the individuals who take water from the low risk water source (including tap water and deep water) showed no significantly different outcomes when compared with individuals who use the high risk water source (including lake and shallow water) (Table 1; Figure 5A; Supplementary Figure S2). Interestingly, individuals that sourced the tap water NT (no treatment), which is the low risk water, showed the worst outcomes for both PRDX 1 and 2, indicated by the highest positive rate (100%) (Figure 5B). The low risk water, deep water with other treatment (OT; treatment other than filtered) and high risk water, lake water with NT showed the lowest positive rates (42% and 50%, respectively) against TPI when comparing with individuals taking other water sources (Figures 5C, D). The lake water with OT gave the highest positive rate (78%) (Figure 5D). While high risk water, shallow water [with filtering (F) and OT] result in the lowest positive rates to PRDX1 (75%) and PRDX 2 (53%), respectively (Figure 5E). For HSP90, shallow water NT gave the highest positive rate; while the tap water OT, the lowest.
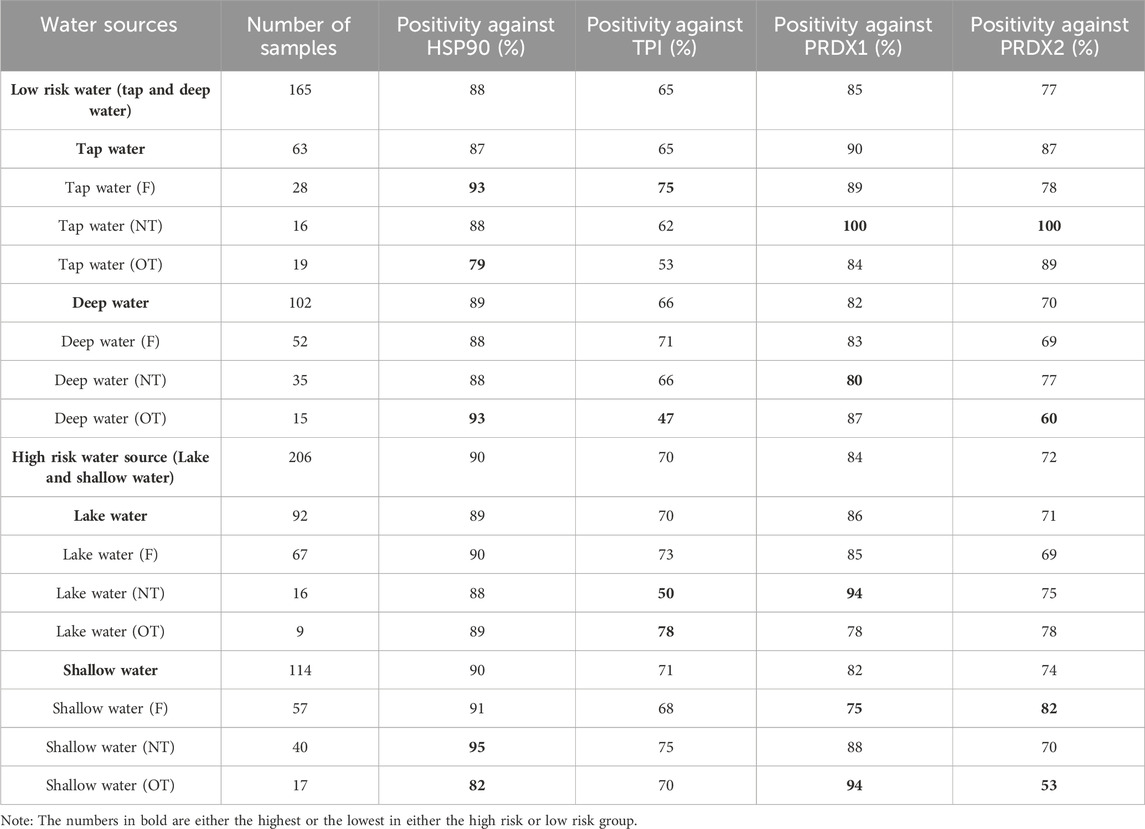
TABLE 1. Comparison of autoantibody levels among individuals drinking from various water sources using LightDeck-derived results (antigen coating concentration = 100 μg/mL). F = Filtered water; NT = water with no treatment; OT = water with other treatment (e.g., boiling, water guard and others).
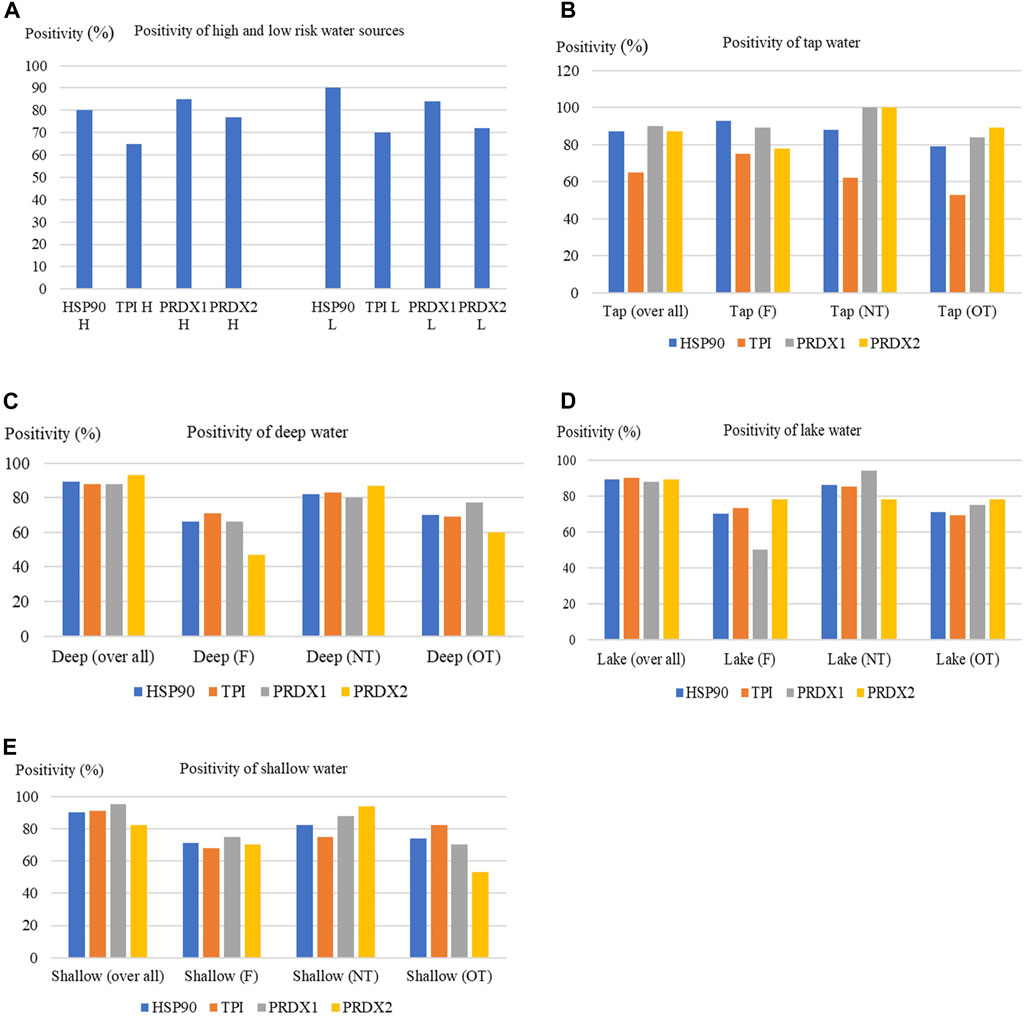
FIGURE 5. Analysis of autoantibody levels [from individuals using high or low risk water (A), which included tap water (B), deep water (C), lake water (D) or shallow water (E), as their main water source] against HSP90, TPI, PRDX1 and PRDX2 using LightDeck system. F = filtered; NT = no treatment; OT = other treatment.
There were several difficulties that had to be confronted during this research. The selection of appropriate controls was problematic. We realised that using the commercial serum as negative control was not the ideal option, however, it was the best choice available. The negative control serum is crucial to the determination of positivity rate and up/downregulation of the relevant biomarker. If the autoantibody level in the serum samples used as negative controls is higher than that in the ideally matched control, then a lower positivity rate will be detected, as well as lower upregulation and higher downregulation. On the other hand, if the autoantibody level in the sera used as negative controls is lower than that in the ideally matched control, then a higher positivity rate will be detected, as well as higher upregulation and lower downregulation. The ideal controls would be individuals living in the same area, definitely consuming uncontaminated (by MCs) water and food, but it is highly likely that they do not exist, as the water and food in entire nation and the neighboring nations may be contaminated by MCs at various levels (Mchau et al., 2019; Tamele and Vasconcelos, 2020; Mutoti et al., 2022).
Other factors that can be improved are within the survey. There are various additional elements included in the survey, but the number of some samples are too small for comparison or to make a conclusion. For instance, there are only 49 samples from patients who smoke in comparison with 322 samples from patients who do not smoke; 32 consumed boiled water, while 339 consumed unboiled water. Therefore, only general comparisons can be made. In order to improve such studies, more samples from these small subgroups should be included. However, it would be very difficult to apply in practice.
Moreover, it was discovered after sampling that there were issues with people switching water source. Hence, the individuals who took part in the survey may have potentially changed their main water sources from low to high risk, or high to low risk, which will also affect the final results. Therefore, more accurate results will be obtained if the survey could include more detailed information on the main water source used in the past few years (e.g., in the past 5 years).
The World Health Organization (WHO) suggested that there are mainly two methods that can be applied to reduce MCs in drinking-water: physical removal of cell-bound MCs and dissolved MCs removal (WHO, 2023). A high proportion of MCs are cell-bound in healthy cyanobacterial cells. Large amounts of MCs are only released into water during cell rupture (e.g., cell lysis) (Wang et al., 2021). Therefore, the majority of MCs can be removed by physical processes. For MCs already dissolved in water, they can be effectively depleted by adsorption onto powdered activated carbon (PAC) or granular activated carbon (GAC) (El Bouaidi et al., 2022).
It has also been suggested that in order to prevent cyanobacterial regrowth, which leads to the production of MCs, water should be protected from light (required for cyanobacteria growth). In addition, chlorine will inhibit cyanobacterial regrowth. Temperature, pH, and nutrient levels also impact the growth and toxicity of microcystin-producing algae. Therefore, there is a potential to use such parameters to forecast bloom severity.
It was reported that temperature controls toxin release from cyanobacterial cells. For instance, cyanobacterial biomass in a lake (temperature ranged from 3°C to 27°C) increased with warming up to 18°C, but declined sharply with further temperature elevation (Walls et al., 2018). Zepernick et al. (2021) found out that increased pH (≥9.2) decreased growth rate and diatom silica deposition. Moreover, pH plays a crucial role in bloom succession, which may lead to prolonging summer microcystin blooms and constrain diatom fall resurgence. It is well-known that nutrients (mainly nitrogen and phosphorus) trigger algal biomass. It appears that the concentration of nitrogen and phosphorus instead of the nitrogen/phosphorus ratio regulated Microcystis dominance (Li et al., 2023). Recently, hydrogen peroxide (7 mg/L) was successfully applied to control cyanobacteria in ponds apparently without influence on beneficial algae and aquatic animals (Ng et al., 2013). Moreover, after the treatment with either hydrogen peroxide (10 mg/L) or hydroxyapatite (40 µm particles at 2.5 g/L), mcyA gene copies and MC levels decreased dramatically (Struewing et al., 2022).
HSP90 and PRDX1 showed the highest (over 90%) positive autoantibody levels in small-scale testing of the four selected biomarkers. Similarly, HSP90 and PRDX1 showed the highest positive autoantibody rates (93% and 86%, respectively) in large-scale analysis. Both the small and large scale tests indicated that these four selected biomarkers can be upregulated on long-term exposure to MC, by consuming MC-contanimated water and food in Tanzania. Interestingly, based on the results of the large-scale testing, these four biomarkers can also be downregulated when examining exposure to MC (via consuming MC-contanimated water and food in Tanzania) in about 10%–35% of the patients analysed. Similar results were also reported by Chen et al. (2005), who found out that the PRDX1 in mice was upregulated in the first 4 h after exposure to a low concentration of MC-LR (50 μg/kg) and then downregulated to some extent, while increasing concentrations of MC-LR resulted in the downregulation of PRDX1. Therefore, different concentrations of MC-LR exposure with different terms of exposure (long or short) may lead to very different levels for different biomarkers. More research is required in this area.
Another interesting result arising from this study is that low risk and high risk groups showed very little difference in positive autoantibody levels against these four biomarkers. Moreover, individuals using lake water, which is a high risk water source, generally showed better outcomes (less positivity) against these four biomarkers, similar to results for the deep water (with filtering) group, a group of low risk. However, not all the results surprised us. Shallow water source users, a high risk group, showed the worst outcomes (highest autoantibody positive rates) for all the four biomarkers.
Importantly, these results must also be considered amongst other factors and circumstances, for example, influences such as food source and lifestyle habit need to be taken into consideration. Especially important is food consumption, which includes fish, crops, vegetables and any other food in the daily diet. In our survey for the 371 candidates in this research, 100% of individuals eat one to two fish per week. Over 90% of fish consumed are lake fish, whose living enviroment is badly contaminated by MC (Mchau et al., 2019) and it was not surprising to have found high concentrations of MC in lake fish (Rumisha and Nehemia, 2013). All of the 371 candidates consumed maize flour/maize as their main food, while about 36% also take other crops (e.g., rice, cassava and cassava flour). Unfortunately, irrigation with MC-LR-contanimated water raises the potential risk of the presence of MC-LR in crops. Cao et al. (2018) investigated the bioaccumulation of MCs congeners in a soil-plant system. They reported that the accumulation of MCs in soils, roots, leaves and grains (rice) was detected in the natural cyanobacteria bloom-containing lake water region (Chen et al., 2012; Cao et al., 2018). Due to their high chemical stability and low molecular weight, MCs can survive in high quantities in various parts of plants (roots, stems, and leaves), which leads to the increasing of health risks for consumers of agricultural products (Melaram et al., 2022).
Zhang et al. (2021) examined the phytotoxicity and bioconcentration of MCs in agricultural plants finding that durum wheat, corn, white mustard and garden cress showed the greatest phytotoxicity, while vegetables could bioconcentrate about 3 times more microcystins than other plants. Moreover, cyanotoxins could also transfer to plants from surface irrigating waters. Therefore, plants like lettuce and cabbage that require spray irrigation could have significant potential for contamination by cyanotoxins (Liu et al., 2016). In addition, cyanotoxin contamination might also be occurring via application of cyanotoxin-contaminated water during food processing (Mutoti et al., 2022). Therefore, water is only one among the many factors which affect the definition of low or high risk of MC exposure. This may explain why the low risk and high risk group showed very little difference in autoantibody positivity rates against the selected four biomarkers.
Point-of-care/use testing enables fast (usually 10–30 min), accurate, sensitive and non-invasive diagnosis/detection which may assist in the selection of the most appropriate disease treatment or demonstration of contamination (Hayes et al., 2018). ELISA is accurate and sensitive, but it is relatively slow (usually 3–4 h) and there is an ongoing need for rapid generation of results. Moreover, point-of-care/use tests can be applied a non-laboratory environment and are easy to use, with very little or no training required (Ma et al., 2021). ELISA, however, requires a laboratory environment, professional training and advanced laboratory practice for good performance. The combination of our novel biomarker panel and a point-of-care/use device could address these issues. The findings of our research will also aid the development of other autoantibody-based point-of-use testing for various diseases or contaminant exposures (e.g., cardiovascular disease and polluted food/water) (Patel et al., 2022). The initial limitation of the LightDeck point-of-use device is the cost of purchase of the device and cartridges, while systems for ELISA are quite commonly available and plates can easily be generated in-house. For LightDeck, cartridges may need to be customised for particular applications and this will be an additional cost.
The results generated in this study show low levels of difference between the point-of-use device, LightDeck, and ELISA. The general positivity measured by ELISA is slightly higher than that measured by LightDeck. Taking into consideration the multiple controls in each cartridge and beside each coating spot (e.g., BSA control, control with buffer only and the anti-human IgG positive) included in the LightDeck system for individual sample detection, this 0%–10% slight difference could highly possibily be caused by higher accuracy of LightDeck, which provides less false positive results. Moreover, for ELISA, which included limited negative controls (i.e., four negative controls) for the whole plate (e.g., while testing 92 samples), will provide less accuracy due to the time difference for addition of each sample. For instance, the time difference of preparation/addition between the first sample and the last sample (96th sample) could be 10–30 min, which may lead to incubation time differences (10–30 min) of samples on the same plate, and this may cause differences in the binding of each sample (in this case binding between the autoantibody and coated antigen). However, for the LightDeck system, each sample is detected independently in an individual cartridge. Therefore, the point-of-use (LightDeck) device may provide more accurate and rapid diagnosis/detection when compared with traditional ELISA testing.
In summary, upregulation of the four selected biomarkers (HSP90, TP1, PRDX1 and PRDX2) was successfully detected following long-term exposure to MC, by LightDeck and by ELISA. Due to the correlation of chronic exposure to MCs and various cancers (e.g., hepatocellular cancer and colorectal cancer), this novel panel of biomarkers has potential to assist cancer diagnostics and therapeutics for population in MCs-polluted area. Similarly, the autoantibodies against these biomarkers can also be applied as natural, stable and safe biomarkers. There are several benefits for using autoantibodies as biomarkers, which have been reported to be produced, promoted, or modified by various diseases (e.g., cancers and cardiovascular diseases). Firstly, the longer half-lives (when compared with autoantigens) of autoantibody makes their detection easier than autoantigen detection. Secondly, autoantibodies enable earlier diagnosis, as many can be detected as early as 2 years before the clinical manifestations of the disease (O’Reilly et al., 2015). This novel panel of biomarkers can also be employed for monitoring of occupational health, particularly for workers potentially exposed to MC-LR in specific work environments.
While there are issues arising in the sampling of patients based on their potential MC exposure, the utility of using panels of targets to highlight long-term exposure to toxins is clearly as very valuable strategy. The feasibility of this approach was demonstrated by the deployment of a rapid, highly sensitive and accurate point-of-use device for the enumeration of autoantibodies to selected target autoantigens, thus eliminating potential disadvantages associated with ELISA.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the National Institute for Medical Research (NIMR) ethical committee with ethical clearance number NIMR/HQ/R.8a/Vol. IX/2426. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
HM: Conceptualization, Investigation, Methodology, Resources, Writing–original draft. CL: Investigation, Project administration, Resources, Writing–original draft. AP-M: Investigation, Resources, Writing–original draft. JF: Investigation, Methodology, Resources, Writing–original draft. JM: Investigation, Resources, Writing–original draft. CE: Funding acquisition, Investigation, Resources, Writing–original draft. RW: Investigation, Resources, Writing–original draft. GM: Investigation, Resources, Writing–original draft. EM: Investigation, Resources, Writing–original draft. RM: Investigation, Resources, Writing–original draft. YG: Investigation, Resources, Writing–original draft. MK: Investigation, Resources, Writing–original draft. AC: Investigation, Resources, Writing–original draft. IM: Investigation, Resources, Writing–original draft. CM: Conceptualization, Methodology, Project administration, Resources, Supervision, Writing–review and editing. RO: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Science Foundation Ireland [Grant number 14/IA/2646].
Dr. Sarah Bickman, senior scientist and product manager of LightDeck Diagnostics, is thanked for her technical support on LightDeck related work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsens.2024.1302520/full#supplementary-material
Cao, Q., Steinman, A. D., Wan, X., and Xie, L. (2018). Bioaccumulation of microcystin congeners in soil-plant system and human health risk assessment: a field study from Lake Taihu region of China. Environ. Pollut. 240, 44–50. doi:10.1016/j.envpol.2018.04.067
Chen, G., Gharib, T. G., Huang, C. C., Thomas, D. G., Shedden, K. A., Taylor, J. M., et al. (2002). Proteomic analysis of lung adenocarcinoma: identification of a highly expressed set of proteins in tumors. Clin. Cancer Res. 8 (7), 2298–2305.
Chen, J., Han, F. X., Wang, F., Zhang, H., and Shi, Z. (2012). Accumulation and phytotoxicity of microcystin-LR in rice (Oryza sativa). Ecotoxicol. Environ. Saf. 76 (2), 193–199. doi:10.1016/j.ecoenv.2011.09.022
Chen, M. F., Lee, K. D., Yeh, C. H., Chen, W. C., Huang, W. S., Chin, C. C., et al. (2010). Role of peroxiredoxin I in rectal cancer and related to p53 status. Int. J. Radiat. Oncol. Biol. Phys. 78 (3), 868–878. doi:10.1016/j.ijrobp.2010.05.025
Chen, T., Huang, Z., Tian, Y., Lin, B., He, R., Wang, H., et al. (2017). Clinical significance and prognostic value of Triosephosphate isomerase expression in gastric cancer. Med. Baltim. 96 (19), e6865. doi:10.1097/MD.0000000000006865
Chen, T., Wang, Q., Cui, J., Yang, W., Shi, Q., Hua, Z., et al. (2005). Induction of apoptosis in mouse liver by microcystin-LR: a combined transcriptomic, proteomic, and simulation strategy. Mol. Cell Proteomics 4 (7), 958–974. doi:10.1074/mcp.M400185-MCP200
Chen, Z. T., Liang, Z. G., and Zhu, X. D. (2015). A review: proteomics in nasopharyngeal carcinoma. Int. J. Mol. Sci. 16 (7), 15497–15530. doi:10.3390/ijms160715497
Chuerduangphui, J., Ekalaksananan, T., Heawchaiyaphum, C., Vatanasapt, P., and Pientong, C. (2020). Peroxiredoxin 2 is highly expressed in human oral squamous cell carcinoma cells and is upregulated by human papillomavirus oncoproteins and arecoline, promoting proliferation. PLoS One 15 (12), e0242465. doi:10.1371/journal.pone.0242465
Ding, C., Fan, X., and Wu, G. (2017). Peroxiredoxin 1 - an antioxidant enzyme in cancer. J. Cell Mol. Med. 21 (1), 193–202. doi:10.1111/jcmm.12955
Dragsted, L. O., Gao, Q., Praticò, G., Manach, C., Wishart, D. S., Scalbert, A., et al. (2017). Dietary and health biomarkers-time for an update. Genes Nutr. 12, 24. doi:10.1186/s12263-017-0578-y
El Bouaidi, W., Enaime, G., Loudiki, M., Yaacoubi, A., Douma, M., Ounas, A., et al. (2022). Adsorbents used for microcystin removal from water sources: current knowledge and future prospects. Processes 10 (7), 1235. doi:10.3390/pr10071235
Ernst, A., Hennel, R., Krombach, J., Kapfhammer, H., Brix, N., Zuchtriegel, G., et al. (2020). Priming of anti-tumor immune mechanisms by radiotherapy is augmented by inhibition of heat shock protein 90. Front. Oncol. 10, 1668. doi:10.3389/fonc.2020.01668
Hamaguchi, T., Iizuka, N., Tsunedomi, R., Hamamoto, Y., Miyamoto, T., Iida, M., et al. (2008). Glycolysis module activated by hypoxia-inducible factor 1alpha is related to the aggressive phenotype of hepatocellular carcinoma. Int. J. Oncol. 33 (4), 725–731.
Hayes, B., Murphy, C., Crawley, A., and O'Kennedy, R. (2018). Developments in point-of-care diagnostic technology for cancer detection. Diagn. (Basel) 8 (2), 39. doi:10.3390/diagnostics8020039
He, J., Shu, Y., Dai, Y., Gao, Y., Liu, S., Wang, W., et al. (2022). Microcystin-leucine arginine exposure induced intestinal lipid accumulation and MC-LR efflux disorder in Lithobates catesbeianus tadpoles. Toxicology 465, 153058. doi:10.1016/j.tox.2021.153058
Huang, W. S., Huang, C. Y., Hsieh, M. C., Kuo, Y. H., Tung, S. Y., Shen, C. H., et al. (2018). Expression of PRDX6 correlates with migration and invasiveness of colorectal cancer cells. Cell. Physiology Biochem. 51 (6), 2616–2630. doi:10.1159/000495934
Husi, H., Fernandes, M., Skipworth, R. J., Miller, J., Cronshaw, A. D., Fearon, K. C. H., et al. (2019). Identification of diagnostic upper gastrointestinal cancer tissue type-specific urinary biomarkers. Biomed. Rep. 10 (3), 165–174. doi:10.3892/br.2019.1190
Jiang, X., Zhang, H., Zhang, H., Wang, F., Wang, X., Ding, T., et al. (2023). Microcystin-LR-induced interaction between M2 tumor-associated macrophage and colorectal cancer cell promotes colorectal cancer cell migration through regulating the expression of TGF-β1 and CST3. Int. J. Mol. Sci. 24 (13), 10527. doi:10.3390/ijms241310527
Judith, R. B., Ira, D., and James, R. K. (1985). Characterization of the functional gene and several processed pseudogenes in the human triosphosphate isomerase gene family. Mol. Cell Biol., 1694–1706.
Kryeziu, K., Bruun, J., Guren, T. K., Sveen, A., and Lothe, R. A. (2019). Combination therapies with HSP90 inhibitors against colorectal cancer. Rev. Cancer 1871 (2), 240–247. doi:10.1016/j.bbcan.2019.01.002
Ladd, J. J., Chao, T., Johnson, M. M., Qiu, J., Chin, A., Israel, R., et al. (2013). Autoantibody signatures involving glycolysis and splicesome proteins precede a diagnosis of breast cancer among postmenopausal women. Cancer Res. 73 (5), 1502–1513. doi:10.1158/0008-5472.CAN-12-2560
Li, G., Yan, W., Qiao, Q., Chen, J., Cai, F., He, Y., et al. (2012). Global effects of subchronic treatment of microcystin-LR on rat splenetic protein levels. J. Proteomics 77, 383–393. doi:10.1016/j.jprot.2012.09.012
Li, H. X., Sun, X. Y., Yang, S. M., Wang, Q., and Wang, Z. Y. (2018). Peroxiredoxin 1 promoted tumor metastasis and angiogenesis in colorectal cancer. Pathol. Res. Pract. 214 (5), 655–660. doi:10.1016/j.prp.2018.03.026
Li, J., Xian, X., Xiao, X., Li, S., and Yu, X. (2023). Dynamic characteristics of total and microcystin-producing Microcystis in a large deep reservoir. Environ. Pollut. 335, 122256. doi:10.1016/j.envpol.2023.122256
Li, Y., Karjalainen, A., Koskinen, H., Hemminki, K., Vainio, H., Shnaidman, M., et al. (2005). p53 autoantibodies predict subsequent development of cancer. Int. J. Cancer 114 (1), 157–160. doi:10.1002/ijc.20715
Li, Y., Sun, B., Wu, H., and Nie, P. (2008). Effects of pure microcystin-LR on the transcription of immune related genes and heat shock proteins in larval stage of zebrafish (Danio rerio). Aquaculture 289, 154–160. doi:10.1016/j.aquaculture.2008.12.029
Liu, H., Tian, Z., Guo, Y., Liu, X., Ma, Y., Du, X., et al. (2021). Microcystin-leucine arginine exposure contributes to apoptosis and follicular atresia in mice ovaries by endoplasmic reticulum stress-upregulated Ddit3. Sci. Total Environ. 756, 144070. doi:10.1016/j.scitotenv.2020.144070
Liu, H., Zeng, X., Wang, Y., Losiewicz, M. D., Chen, X., Du, X., et al. (2022). Chronic exposure to environmentally relevant concentrations of microcystin-leucine arginine causes lung barrier damage through PP2A activity inhibition and Claudin1 ubiquitination. J. Agric. Food Chem. 70 (35), 10907–10918. doi:10.1021/acs.jafc.2c05207
Liu, Y. Y., Wang, Y., Walsh, T. R., Yi, L. X., Zhang, R., Spencer, J., et al. (2016). Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect. Dis. 16 (2), 161–168. doi:10.1016/S1473-3099(15)00424-7
Lone, Y., Koiri, R. K., and Bhide, M. (2015). An overview of the toxic effect of potential human carcinogen Microcystin-LR on testis. Toxicol. Rep. 2, 289–296. doi:10.1016/j.toxrep.2015.01.008
Lu, W., Fu, Z., Wang, H., Feng, J., Wei, J., and Guo, J. (2014). Peroxiredoxin 2 is upregulated in colorectal cancer and contributes to colorectal cancer cells' survival by protecting cells from oxidative stress. Mol. Cell Biochem. 387 (1-2), 261–270. doi:10.1007/s11010-013-1891-4
Ma, H., Cassedy, A., and O'Kennedy, R. (2021). The role of antibody-based troponin detection in cardiovascular disease: a critical assessment. J. Immunol. Methods 497, 113108. doi:10.1016/j.jim.2021.113108
Ma, H., Murphy, C., Loscher, C. E., and O'Kennedy, R. (2022). Autoantibodies - enemies, and/or potential allies? Front. Immunol. 13, 953726. doi:10.3389/fimmu.2022.953726
Mchau, G. J., Makule, E., Machunda, R., Gong, Y. Y., and Kimanya, M. (2019). Harmful algal bloom and associated health risks among users of Lake Victoria freshwater: Ukerewe Island, Tanzania. J. Water Health 17 (5), 826–836. doi:10.2166/wh.2019.083
Megger, D. A., Naboulsi, W., Meyer, H. E., and Sitek, B. (2014). Proteome analyses of hepatocellular carcinoma. J. Clin. Transl. Hepatol. 2 (1), 23–30. Epub 2014 Mar 15. doi:10.14218/JCTH.2013.00022
Melaram, R., Newton, A. R., and Chafin, J. (2022). Microcystin contamination and toxicity: implications for agriculture and public health. Toxins (Basel) 14 (5), 350. doi:10.3390/toxins14050350
Meneely, J. P., and Elliott, C. T. (2013). Microcystins: measuring human exposure and the impact on human health. Biomarkers 18 (8), 639–649. doi:10.3109/1354750X.2013.841756
Montgomerie, J. Z., Gracy, R. W., Holshuh, H. J., Keyser, A. J., Bennett, C. J., and Schick, D. G. (1997). The 28K protein in urinary bladder, squamous metaplasia and urine is triosephosphate isomerase. Clin. Biochem. 30 (8), 613–618. doi:10.1016/s0009-9120(97)00115-x
Moser, C., Lang, S. A., Kainz, S., Gaumann, A., Fichtner-Feigl, S., Koehl, G. E., et al. (2007). Blocking heat shock protein-90 inhibits the invasive properties and hepatic growth of human colon cancer cells and improves the efficacy of oxaliplatin in p53-deficient colon cancer tumors in vivo. Mol. Cancer Ther. 6 (11), 2868–2878. doi:10.1158/1535-7163.MCT-07-0410
Murphy, C., Stack, E., Krivelo, S., McPartlin, D. A., Byrne, B., Greef, C., et al. (2015). Detection of the cyanobacterial toxin, microcystin-LR, using a novel recombinant antibody-based optical-planar waveguide platform. Biosens. Bioelectron. 67, 708–714. doi:10.1016/j.bios.2014.10.039
Mutoti, M., Gumbo, J., and Jideani, A. I. O. (2022). Occurrence of cyanobacteria in water used for food production: a review. Phys. Chem. Earth, Parts A/B/C. 125, 103101. doi:10.1016/j.pce.2021.103101
Ng, P. H., Cheng, T. H., Man, K. Y., Huang, L., Cheng, K. P., Lim, K. Z., et al. (2013). Hydrogen peroxide as a mitigation against Microcystis sp. bloom. Aquaculture 577, 739932. doi:10.1016/j.aquaculture.2023.739932
O'Reilly, J. A., Fitzgerald, J., Fitzgerald, S., Kenny, D., Kay, E. W., O'Kennedy, R., et al. (2015). Diagnostic potential of zinc finger protein-specific autoantibodies and associated linear B-cell epitopes in colorectal cancer. PLoS One 10 (4), e0123469. doi:10.1371/journal.pone.0123469
Pan, C., Qin, H., Yan, M., Qiu, X., Gong, W., Luo, W., et al. (2023). Environmental microcystin exposure triggers the poor prognosis of prostate cancer: evidence from case-control, animal, and in vitro studies. J. Environ. Sci. (China) 127, 69–81. doi:10.1016/j.jes.2022.05.051
Patel, A. J., Tan, T. M., Richter, A. G., Naidu, B., Blackburn, J. M., and Middleton, G. W. (2022). A highly predictive autoantibody-based biomarker panel for prognosis in early-stage NSCLC with potential therapeutic implications. Br. J. Cancer 126 (2), 238–246. doi:10.1038/s41416-021-01572-x
Pedersen, J. W., Gentry-Maharaj, A., Fourkala, E. O., Dawnay, A., Burnell, M., Zaikin, A., et al. (2013). Early detection of cancer in the general population: a blinded case-control study of p53 autoantibodies in colorectal cancer. Br. J. Cancer 108 (1), 107–114. doi:10.1038/bjc.2012.517
Peng, L., Wang, R., Shang, J., Xiong, Y., and Fu, Z. (2017). Peroxiredoxin 2 is associated with colorectal cancer progression and poor survival of patients. Oncotarget 8 (9), 15057–15070. doi:10.18632/oncotarget.14801
Qin, L., Huang, H., Huang, J., Wang, G., Huang, J., Wu, X., et al. (2019). Biological characteristics of heat shock protein 90 in human liver cancer cells. Am. J. Transl. Res. 11 (4), 2477–2483.
Rumisha, C., and Nehemia, A. (2013). Feeding selectivity of wild and pond-cultured nile tilapia (Oreochromis niloticus) in the lake Victoria basin in Mara, Tanzania. Afr. J. Aquat. Sci. 38 (1), 55–60. doi:10.2989/16085914.2013.784698
Shi, L., Du, X., Liu, H., Chen, X., Ma, Y., Wang, R., et al. (2021). Update on the adverse effects of microcystins on the liver. Environ. Res. 195, 110890. doi:10.1016/j.envres.2021.110890
Shome, M., Chung, Y., Chavan, R., Park, J. G., Qiu, J., and LaBaer, J. (2022). Serum autoantibodyome reveals that healthy individuals share common autoantibodies. Cell Rep. 39 (9), 110873. doi:10.1016/j.celrep.2022.110873
Silins, I., and Högberg, J. (2011). Combined toxic exposures and human health: biomarkers of exposure and effect. Int. J. Environ. Res. Public Health 8 (3), 629–647. doi:10.3390/ijerph8030629
Struewing, I., Sienkiewicz, N., Zhang, C., Dugan, N., and Lu, J. (2022). Effective early treatment of Microcystis exponential growth and microcystin production with hydrogen peroxide and hydroxyapatite. Toxins (Basel). 15 (1), 3. doi:10.3390/toxins15010003
Sun, Y. L., Cai, J. Q., Liu, F., Bi, X. Y., Zhou, L. P., and Zhao, X. H. (2015). Aberrant expression of peroxiredoxin 1 and its clinical implications in liver cancer. World J. Gastroenterol. 21 (38), 10840–10852. doi:10.3748/wjg.v21.i38.10840
Tamele, I. J., and Vasconcelos, V. (2020). Microcystin incidence in the drinking water of Mozambique: challenges for public health protection. Toxins (Basel) 12 (6), 368. doi:10.3390/toxins12060368
Tamesa, M. S., Kuramitsu, Y., Fujimoto, M., Maeda, N., Nagashima, Y., Tanaka, T., et al. (2009). Detection of autoantibodies against cyclophilin A and triosephosphate isomerase in sera from breast cancer patients by proteomic analysis. Electrophoresis 30 (12), 2168–2181. doi:10.1002/elps.200800675
Walls, J. T., Wyatt, K. H., Doll, J. C., Rubenstein, E. M., and Rober, A. R. (2018). Hot and toxic: temperature regulates microcystin release from cyanobacteria. Sci. Total Environ. 610-611, 786–795. doi:10.1016/j.scitotenv.2017.08.149
Wang, J., Lin, D., Peng, H., Huang, Y., Huang, J., and Gu, J. (2013). Cancer-derived immunoglobulin G promotes tumor cell growth and proliferation through inducing production of reactive oxygen species. Cell death Dis. 4 (12), e945. doi:10.1038/cddis.2013.474
Wang, S., Yang, S., Zuo, J., Hu, C., Song, L., Gan, N., et al. (2021). Simultaneous removal of the freshwater bloom-forming cyanobacterium Microcystis and cyanotoxin microcystins via combined use of algicidal bacterial filtrate and the microcystin-degrading enzymatic agent, MlrA. MlrA. Microorg. 9 (8), 1594. doi:10.3390/microorganisms9081594
Wang, W., Zhang, H., Wei, L., Ma, Y., Jiang, H., Yuen, C. N. T., et al. (2023). Microcystin-leucine arginine causes brain injury and functional disorder in Lithobates catesbeianus tadpoles by oxidative stress and inflammation. Aquat. Toxicol. 258, 106509. doi:10.1016/j.aquatox.2023.106509
Wei, W., Liu, M., Ning, S., Wei, J., Zhong, J., Li, J., et al. (2020). Diagnostic value of plasma HSP90α levels for detection of hepatocellular carcinoma. BMC Cancer 20 (1), 6. doi:10.1186/s12885-019-6489-0
Welten, R. D., Meneely, J. P., and Elliott, C. T. (2020). A comparative review of the effect of microcystin-LR on the proteome. Expo. Health 12, 111–129. doi:10.1007/s12403-019-00303-1
WHO (2023). Publications, WHO-guidelines. Cyanobacterial toxins: microcystins Background document for development of WHO Guidelines for drinking-water quality and Guidelines for safe recreational water environment. Available at: https://iris.who.int/bitstream/handle/10665/338066/WHO-HEP-ECH-WSH-2020.6-eng.pdf.
Yao, X., Liu, Y., Yang, Y., Li, Y., Hu, N., Song, F., et al. (2023). Microcystin-LR-exposure-induced kidney damage by inhibiting MKK6-mediated mitophagy in mice. Toxins (Basel) 15 (6), 404. doi:10.3390/toxins15060404
Yuan, J., Gu, Z., Zheng, Y., Zhang, Y., Gao, J., Chen, S., et al. (2016). Accumulation and detoxification dynamics of microcystin-LR and antioxidant responses in male red swamp crayfish Procambarus clarkii. Aquat. Toxicol. 177, 8–18. doi:10.1016/j.aquatox.2016.05.004
Zaenker, P., and Ziman, M. R. (2013). Serologic autoantibodies as diagnostic cancer biomarkers--a review. Cancer Epidemiol. Biomarkers Prev. 22 (12), 2161–2181. doi:10.1158/1055-9965.EPI-13-0621
Zepernick, B. N., Gann, E. R., Martin, R. M., Pound, H. L., Krausfeldt, L. E., Chaffin, J. D., et al. (2021). Elevated pH conditions associated with Microcystis spp. blooms decrease viability of the cultured diatom Fragilaria crotonensis and natural diatoms in Lake Eyrie. Front. Microbiol. 12, 598736. doi:10.3389/fmicb.2021.598736
Zhang, S., Wu, W., Peng, Y., Liu, L., Zhang, Y., Wang, R., et al. (2022). Chronic exposure to microcystin-leucine-arginine induces epithelial hyperplasia and inflammation in the mouse bladder. Ecotoxicol. Environ. Saf. 244, 114033. doi:10.1016/j.ecoenv.2022.114033
Zhang, Y., Whalen, J. K., and Sauvé, S. (2021). Phytotoxicity and bioconcentration of microcystins in agricultural plants: meta-analysis and risk assessment. Environ. Pollut. 272, 115966, 115966. doi:10.1016/j.envpol.2020.115966
Zhao, X., Liu, Y., Guo, Y. M., Xu, C., Chen, L., Codd, G. A., et al. (2023). Meta-analysis reveals cyanotoxins risk across African inland waters. J. Hazard. Mater. 451, 131160. doi:10.1016/j.jhazmat.2023.131160
Keywords: Biomarkers, autoantibody, microcystin, LightDeck, point-of-use, diagnostic
Citation: Ma H, Loscher C, Parle-McDermott A, Fitzgerald J, Meneely J, Elliott C, Welten R, Mchau GJ, Makule E, Machunda R, Gong YY, Kimanya M, Crawley A, Maguire I, Murphy C and O’Kennedy R (2024) Evaluation of a point-of-use device used for autoantibody analysis and its potential for following microcystin leucine-arginine exposure. Front. Sens. 5:1302520. doi: 10.3389/fsens.2024.1302520
Received: 26 September 2023; Accepted: 12 January 2024;
Published: 09 February 2024.
Edited by:
Valtencir Zucolotto, University of São Paulo, BrazilReviewed by:
Kalil Cristhian Figueiredo Toledo, University of São Paulo, BrazilCopyright © 2024 Ma, Loscher, Parle-McDermott, Fitzgerald, Meneely, Elliott, Welten, Mchau, Makule, Machunda, Gong, Kimanya, Crawley, Maguire, Murphy and O’Kennedy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caroline Murphy, Y2Fyb2xpbmUucy5tdXJwaHlAZGN1Lmll; Richard O’Kennedy, cmljaGFyZG9rZW5uZWR5QGdtYWlsLmNvbQ==
†These authors share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.