
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Sens., 25 April 2022
Sec. Biosensors
Volume 3 - 2022 | https://doi.org/10.3389/fsens.2022.873862
This article is part of the Research TopicThought Leaders in Sensor Research: Volume 1View all 5 articles
Electroceuticals is an emerging field that combines the technology in conductive materials with their ability to interface with biological systems. The development of highly conductive electrodes to monitor human health in real-time while simultaneously delivering stimulation promises to revolutionize medical science. Aspects to consider during development include the desired shape, electrode material properties, number of active sites, carriers used, and methods of deployment and activation. Novel organic-conductor based electrode compositions offer properties unattainable with conventional metal electrodes. Emerging innovative deployment strategies communicate directly with target tissues while minimizing damage to the surrounding biological environment. Here we highlight the recent reported technology on platinized graphene fibers (sutrode), a high performance electrode, capable of recording electrophysiological signals from small autonomic nerves, which could bring us closer to the ultimate goal: modulating the activity of individual organs with high selectivity and precision for a therapeutic medical outcome. An in-depth understanding of electrode materials and methods of fabrication and deployment can provide unprecedented opportunities for electroceutical research.
Electrical signals or biopotentials are fundamental to maintaining equilibrium in biological systems. Their functions range from regulating ionic interchange on voltage dependent channels in cell membranes, to electrical processes that control cellular and humoral communication between organs. Diseases or disorders often disrupt this equilibrium either due to environmental stimuli (epigenetic) or internal conditions (genetic or cellular). Electroceuticals operate on the principle of applying standardized electrical currents through a neural interface, artificially modifying the function of strategic targets to achieve balance.
The use of bioelectrical interfaces in medicine improves the quality of life of individuals affected by chronic diseases, debilitating disorders of the brain, spine, limbs, and sensory organs through direct interfacing with the nervous system or organs. The use of electrical devices to modulate organ function dates from the last century (1958) with the development of cardiac pacemakers. These body-implantable sensors have so far been able to monitor imbalances associated with disease states, as exemplified by the implantable glucose biosensor for diabetic patients, which can now provide continuous data for up to 180 days (Heo and Kim 2019; Boscari et al., 2022). This emerging field is currently replacing pharmacological treatments for patients that suffer from debilitating conditions and are unresponsive to conventional drug treatments. For example, vagal nerve stimulation (VNS) has been used to treat drug-resistant epilepsy in more than 100,000 patients and is generally well-tolerated (Fisher et al., 2021). VNS has also shown positive effects in the treatment of rheumatoid arthritis (Koopman et al., 2016) and Crohn’s disease in early human trials (Bonaz et al., 2016).
Central and peripheral nervous systems are prime targets for electroceuticals, as their modulation not only affects a compartmentalized cellular circuit in an organ or tissue, but also humoral modulation of elements that underlie different disorders, such as modulation of cytokines and interleukins during inflammatory reflex by stimulating the vagus or splenic nerves. Sensor research has made great progress in addressing major challenges that promise to revolutionize medical science and make the field possible. These sensors can now monitor neurophysiological signals entering and exiting specific organs (Merrill et al., 2011; Yao et al., 2020; Sharma et al., 2021). Precise positioning of these sensors in nerves at organ entry points, provides information via inter-organ communication which can be analyzed, in order to provide information about organ malfunction during a disease state, and inform on possible interventions (Im and Seo 2016; Trung and Lee 2016; Weber et al., 2020). This use of electrical stimulation has become known as electroceuticals or bioelectronic medicines–a potential alternative to the traditional approach used to treat disease with pharmaceuticals.
This field of electroceuticals is propelled through the development of innovative and sensitive electrodes. The requirements for this type of electrodes differ from those implanted for monitoring chronic diseases. Composition and structure must enable them to make intimate contact with delicate, sometimes hard to access nerves. This places additional demands on the physical properties of the implanted electrode, requiring thin, soft, flexible, yet strong materials for intimate contact with the soft tissue. These may need to operate over extended periods of years, but can also be effective for some conditions within a few weeks in producing valuable organ function information. If we consider that the introduction of the sensors (actually any foreign object) into the human body triggers a cascade of immune and inflammation processes, we can gain some insights into the performance of implantable electrodes, based on the severity of the tissue response, and altered activity over time. Upon insertion, physical trauma and protein adsorption trigger inflammation, and fibrotic encapsulation (Anderson et al., 2008; Lotti et al., 2017; O’Malley et al., 2017; Veiseh and Vegas 2019). This results in increased impedance at the electrode-tissue interface, which compromises signal fidelity and increases power consumption and eventually failure of the device. These biological responses are influenced by the following properties of the sensing material.
Electrode chemical composition determines performance. Platinum and platinum-iridium are conventional implantable electrode materials due to their good electrical conductivity and stability. However, high charge injection over time leads to Platinum dissolution, and the production of chemical radicals that affect the micro-environmental interphase electrode-tissue, increasing the foreign body response (Kumsa et al., 2017; Harris et al., 2018). Current electrode chemistry has strengths and limitations, and several criteria are needed to be considered when defining electrode chemical compositions, since it will impact the resultant signal-to-noise ratio while recording, and the availability of a wide water window (potential window) that avoids electrolysis during stimulation (Cogan 2008; Jalili et al., 2017). The material also needs to be highly processable and adaptable to different manufacturing options, allowing it to be formed into specific structures and integrated within the overall system (Won et al., 2020).
For electrodes implants, platinum and platinum-iridium are the gold standards for electrode materials because of their high conductivity and stability most prevalent in the market (Wellman et al., 2018). However, due to the low electroactive surface area, the signal-to-noise ratio during signal recording is limited. For neuromodulation, safely injecting electrical charge into relatively small nerve targets through miniature electrodes is a challenge Therefore, novel electrode materials with low impedance for sensitive signal recording and high charge injection capacity (CIC) for neural stimulation are highly desirable. Relatively large electrodes (i.e., >20 μm diameter) are known to exacerbate initial insertion tissue injury, and damage surrounding tissue by chronic local strain fields induced by micromotion (Spencer et al., 2017; Sharafkhani et al., 2022). Reduced electrode size minimizes the risk of insertion injury (Chen et al., 2017), and small needle electrodes (i.e., <8 µm OD) avoid foreign body response and disruptive local neuro-glial communication, thereby limiting the release of pro-inflammatory cytokines (Chen et al., 2017). To this end, new materials and microfabrication have enabled the development of a novel sixteen-channel amorphous silicon carbide-based sensor with a 23 µm width, 10 µm thick shank and four ultramicro-sized electrodes (8 × 25 µm2) per shank for intraneural interrogation. This intraneural electrode provides enhanced spatial resolution to target small nerve fibers in the rat vagus nerve when compared to conventional electrodes with a large geometric surface area (Ghazavi et al., 2020).
The use of novel materials offers new opportunities to fabricate small, sensitive electrodes with high CIC. Electrodes based on organic conductors, such as graphene or carbon fibres, are favoured for their electrochemical stability (Zhou and Angelo 2013; Wang et al., 2019; Gonzalez-Gonzalez et al., 2021), and reduced foreign body response (Nayagam et al., 2011). As one of the most widely used nanomaterials, graphene has excellent physical and chemical properties and can interact with other biological molecules, such as DNA, enzymes, proteins or peptides through noncovalent adsorption such as π–π stacking, hydrogen bonds, or electrostatic interactions, as well as thorough covalent bindings between free amine proteins and the carboxylic groups of graphene oxide (Li et al., 2016). These interactions will have a subsequent effect on the ability to effect electron transfer and/or to store charge at the electrode solution interface.
Graphene-based materials have been widely used in regenerative medicine (Shin et al., 2016) and tissue engineering due to their excellent mechanical strength and electrical conductivity (Weaver and Cui 2015; Bourrier et al., 2019; Laurencin and Daneshmandi 2020; Devi et al., 2021). A free-standing graphene fibre microelectrode (50 µm OD), has been shown to effectively stimulate retinal ganglion cells in vitro (Apollo et al., 2015). In addition, these highly flexible graphene-fiber microelectrodes can perform high-frequency deep brain stimulation (DBS) on the subthalamic nucleus, effectively reducing movement disorders in Parkinson’s diseased rats (Zhao et al., 2020).
Recently, a novel graphene-based fiber–called a “sutrode”, was developed by adding a platinum layer to enhance current collection ability (Figures 1A,B; (Wang et al., 2019). This novel electrode material provides ultrahigh performance by combining the mechanical properties of a suture with the properties of an electrode. The electrode structure has low impedance and high CIC (10 mC cm−2), which is significantly higher than traditional noble metal and conductive polymer based electrodes (Wang et al., 2019). The diameter of the graphene fiber can be as low as 20 μm, which can effectively reduce the foreign body response (Pancrazio et al., 2017). Electrode arrays fabricated using these fibers are allow to communicate directly with individual neurons and achieve stable single unit recording from the cortex with a high signal-to-noise ratio (SNR) of 9.2 dB (Wang et al., 2019). In addition, this ultra-flexible suture-like electrode can interface with multiple splenic neurovascular plexus (SNVP) and enable selective neural stimulation (Figures 1C,D) (Gonzalez-Gonzalez et al., 2021).
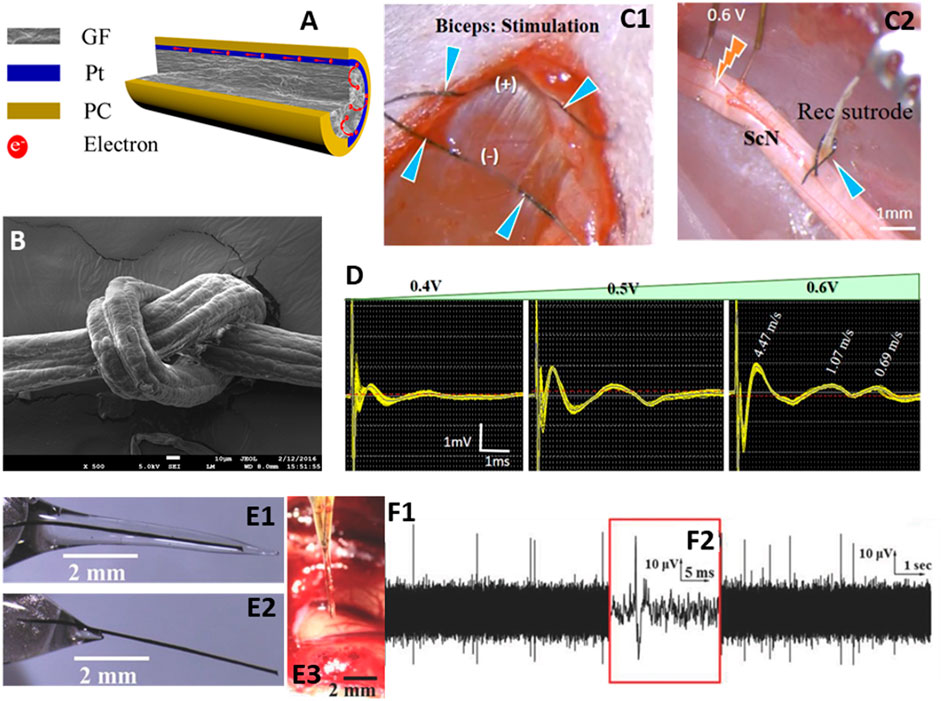
FIGURE 1. (A) structure of graphene microfiber electrode -sutrode, (B) SEM image of sutrode tied into a knot, (C1) Sutrode placed on biceps (blue arrows), (C2) Stimulation of the tibial nerve fascicle with hook electrodes and recording with the sutrode (blue arrow), (D) recording of graded evoked compound nerve action potentials, (E1) Graphene fiber encased in sucrose microneedle, (E2) sutrode after the sucrose microneedles are dissolved, (E3) sucrose microneedle coated sutrode inserted into feline visual cortex, (F1) neural activity recorded within 20 s of implantation, confirming sucrose dissolution, and (F2) magnified image of action potential recorded with sutrode. Adapted with permission from (Apollo et al., 2015; Wang et al., 2019; Gonzalez-Gonzalez et al., 2021).
Conducting polymers are also important for microelectrode fabrication in bioelectronic medicines (Balint et al., 2014; Mirabedini et al., 2016; Harris and Wallace 2018; Zhang et al., 2018). Materials such as polypyrrole provide an organic backbone that facilitates intimate interactions with neural cells (Ateh et al., 2006; Moulton and Walace. 2012). This degree of intimacy can be further enhanced by incorporating biologically active molecules as dopants, as illustrated by the enhanced neurite outgrowth and degree of neural branching in vitro (Liu et al., 2009; Liu et al., 2011; Zhang et al., 2018). Such organic electrodes have also proven effective in stimulating protocols used to increase the number of neurites emanating from cortical neurons. In fact, electrodes made of polypyrrole doped with dodecylbenzenesulfonic acid were shown to effectively increase with neuregulin type 1 knockout (model of schizophrenia) (Zhang et al., 2018).
Biocompatible, flexible and stable materials, such as polydimethylsiloxane (PDMS), have been widely used to fabricate soft implantable devices. The excellent electrical insulation properties of PDMS or other materials such as parylene C can be used to isolate current collector wires and connectors from the external environment (Stieglitz et al., 2005).
The duration/degradation of the insulating material needs to be carefully considered when designing electronic implants. It may be necessary to control the degradation of the electrode and carrier materials within a specified time frame. For example, a wireless bioabsorbable electrode was developed to promote nerve regeneration and functional recovery in rodent models (Koo et al., 2018). Through careful selection of electrodes and carrier materials, coupled with appropriate electrode design, bioabsorption begins shortly after implantation. This poly (lactic-co-glycolic acid) (PLGA) and Mg-based electrode dissolves completely in the body after 25 days, avoiding the need to remove the electrode in a second operation, and also reducing foreign body response. Before complete absorption, the device can deliver 100–300 mV stimulation to surrounding nerve tissue, exceeding the threshold voltage for promoting nerve regeneration immediately after surgical implantation. Unlike bioabsorbable electrodes, cochlear implants need to be implanted in the body for decades. Surprisingly, biodegradable polymers can also play an important role in this type of electrode design. Biodegradable polymers such as poly (L-lactide) and poly (4-hydroxybutyrate) (P (4HB)) have been used to coat cochlear implants (Ceschi et al., 2014). As the polymer degrades, these modified cochlear implants have the potential to deliver drugs and growth factors directly to the inner ear after cochlear implantation (Tan et al., 2020).
The choice of methods available for electrode deployment and the appropriate materials for electrode fabrication is defined depending on the location of the target tissue. In order to achieve better positioning and higher fidelity, electrodes need to be rigid enough for easy handling and penetration when needed, yet soft and flexible to minimize foreign body reaction and consequent increases in interfacial impedance. However, inserting soft electrodes into soft tissue is a significant challenge. Some have proposed magnetic insertion of 25 µm Pt-Fe microelectrodes into rat brain, which recorded for 31 days without significant changes in impedance (Dryg et al., 2015). Yim et al. developed a handheld device that can further facilitate the clinical application of magnetic insertion of microelectrodes (Yim et al., 2017). Others have proposed strategies to temporarily increase the stiffness and/or sharpness of implantable electrodes, including using soluble biocompatible coatings, such as silk coated titanium/iridium (Ti/Ir) electrodes (Tien et al., 2013), gelatin coated gold electrodes (Agorelius et al., 2015) or degradable polymer (Poly vinyl acetate) coated Ti/Au (Capadona et al., 2012). Polylactic acid (PLA)/PLGA and polycaprolactone (PCL) coatings on Si CMOS (complementary metal–oxide–semiconductor) electrodes (Hwang et al., 2014) have also been used. Others have simply used freezing of the electrode (Pd) in liquid N2 immediately before insertion (Xie et al., 2015). Recently, a sucrose microneedle was designed to deliver soft graphene-fiber electrodes into deep (i.e., 8 mm) brain target locations (Figures 1E,F) (Apollo et al., 2015; Apollo et al., 2018). The sucrose-coated graphene-fiber electrodes are stiff enough for insertion and become flexible as the sucrose dissolves. It was observed that the insertion force was lower in comparison to that needed for a stainless-steel hypodermic needle. This approach facilitates the use of soft and flexible graphene fibers to record sustainable neural communication for up to 21 days (Apollo et al., 2018). The need for stiffness that varies over time is eliminated by adding a layer of platinum to the graphene fibers. This metallization step provides the rigidity required to insert graphene fiber into the cerebral cortex, while maintaining sufficient flexibility to tie an overhand knot (Wang et al., 2019).
Alternatively, electrodes can be fabricated in-situ, such as polymerized conductive polymer poly (3,4-ethylenedioxythiophene; PEDOT) electrodes in nerve cells (Richardson-Burns et al., 2007) and in the rat hippocampus (Ouyang et al., 2014). More recently, a flowable two-part conductive polymer was injected through a needle and syringe into the space adjacent to neuroanatomical targets, the injectrode® with high electrochemical performance and outstanding sensitivity (Woodington et al., 2021). These strategies enable the efficient integration of electrodes with the in vivo environment, particularly for superficial targets.
The assembly protocols used to create organic electrode structures provide a way to integrate bioactive motifs into the electrode structure. In the case of nanostructured carbons, bioactive binder molecules such as chitosan (Buaki-Sogó et al., 2021), alginate (Cirillo et al., 2021) and hyaluronic acid (Zheng et al., 2019) may be included. As mentioned above, biological macromolecules can be integrated into conducting polymers at the time of synthesis.
Smaller molecules such as drugs or growth factors can also be introduced into these organic electrode structures. For example, enhanced cell attachment density and neurite outgrowth were observed on laminin coated diamond electrodes (Sikder et al., 2021). Using an immobilized neural adhesion protein (L1) improves neural cell adhesion and controls inflammation response when using silicon electrodes, and improves chronic electrophysiologic recording performance in vivo (Woeppel and Cui 2021). Meanwhile, dexamethasone has been loaded into polypyrrole (Leprince et al., 2010) or graphene oxide (Weaver et al., 2014) electrodes to counter inflammation upon implantation. Brain-derived neurotrophic factor (BDNF) (Thompson et al., 2010) and neurotrophin-3 (NT-3) (Thompson et al., 2011) were incorporated into polypyrrole-based electrodes to improve nerve regeneration. These bioactive motifs can then be released exactly at the point they are needed in order to modulate the electrode-tissue interface.
Beginning with single-wire microelectrodes used to record electrical activity from nerves in the 1950s, various neural electrodes with different geometries and configurations have been developed. Multiple electrode arrays may be fabricated by assembling wire electrodes together into bundles. In 1970 Wise et al. reported the first micro-electromechanical systems (MEMS) based multielectrode-microprobe for biopotential recording (Wise et al., 1970). Following this pioneering work, a new field called neural-MEMS was created (Hajjhassan et al., 2008; Seymour et al., 2017). With the development of MEMS fabrication technology, silicon-based microprobes were fabricated by combing soft lithography, transfer-printing, and 3D packaging technologies. The miniaturization of neural probes has enabled the realisation of multi-electrode arrays (MEAs) for neural recording and stimulation (Seymour et al., 2017).
The development of hybrid neural electrodes has recently attracted attention. This involves the combination of electrical activity with bioactive motifs. For example, functional polymer fibers were integrated into poly (acrylamide)-alginate hydrogel to achieve stable electrophysiological activity recording for up to 6 months (Park et al., 2021). 3D printing also offers a way to fabricate hybrid neural implants (Ni et al., 2019). These novel fabrication methods can further facilitate the development of electroceutical electrodes.
The organic electrode compositions described above can be used to fabricate high-performance electrode systems. These electrodes, for instance, can be expanded to release multiple biological components using simple coatings with multiple molecules with differential release profiles. For example, the release of anti-inflammatory drugs immediately after implantation could be followed by the release of biofactors that minimise scarring and others that promote growth of appropriate tissue around the electrodes.
3D printing is an established fabrication technology that allows the production of tissue-compatible electrodes. Electrodes, structural materials, and bioactive materials (drugs, growth factors, and even living cells) can be integrated using 3D printing and can be further developed to strategically distribute molecules and cells in implantable devices. Eventually, such methods can be developed into 4D printing systems, adding the dimension of changes in composition or response as a factor of time.
Ultimately, the combination of sensing and stimulation electrodes can be integrated as closed-loop systems will dramatically improve the performance of electroceutical devices (Lee et al., 2020). As we learn more about electrophysiological-based diagnostics, we will be able to develop more accurate and targeted systems that can automatically modulate organ function in response to internal or external stimuli. This will require rapid interpretation of large volumes of data and undoubtedly the machine learning tidal wave we are currently riding will have an impact here.
The continued development of novel power sources, such as implantable/biodegradable power sources (Jia et al., 2016; Jia et al., 2017) and wirelessly systems (Hernandez-Reynoso et al., 2019), offers numerous advantages over traditional wired and batterie powered systems. This area has been somewhat neglected but we expect to see great advances here in coming years.
Finally, it is important to consider that the miniature and electrical systems being developed will face regulatory and perhaps ethical questions not faced in the development of more traditional medical approaches to treating disease. Therefore, early integration of such issues is imperative for the effective development of electroceuticals medical devices and their impact on human health.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor DD declared a past co-authorship with the author(s) GW.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors wish to acknowledge funding from the Australian Research Council (ARC) Centre of Excellence Scheme (CE140100012). XL gratefully acknowledges the support of the ARC Industrial Transformation Training Centre in Additive Biomanufacturing (ABM) (IC160100026), Australian National Fabrication Facility (ANFF)—Materials Node and The Translational Research Initiative for Cellular Engineering and Printing (TRICEP).
Agorelius, J., Tsanakalis, F., Friberg, A., Thorbergsson, P. T., Pettersson, L. M. E., and Schouenborg, J. (2015). An Array of Highly Flexible Electrodes with a Tailored Configuration Locked by Gelatin during Implantation-Initial Evaluation in Cortex Cerebri of Awake Rats. Front. Neurosci. 9, 331. doi:10.3389/fnins.2015.00331
Anderson, J. M., Rodriguez, A., and Chang, D. T. (2008). Foreign Body Reaction to Biomaterials. Semin. Immunol. 20, 86–100. doi:10.1016/j.smim.2007.11.004
Apollo, N. V., Jiang, J., Cheung, W., Baquier, S., Lai, A., Mirebedini, A., et al. (2018). Development and Characterization of a Sucrose Microneedle Neural Electrode Delivery System. Adv. Biosys. 2, 1700187. doi:10.1002/adbi.201700187
Apollo, N. V., Maturana, M. I., Tong, W., Nayagam, D. A. X., Shivdasani, M. N., Foroughi, J., et al. (2015). Soft, Flexible Freestanding Neural Stimulation and Recording Electrodes Fabricated from Reduced Graphene Oxide. Adv. Funct. Mater. 25, 3551–3559. doi:10.1002/adfm.201500110
Ateh, D. D., Navsaria, H. A., and Vadgama, P. (2006). Polypyrrole-based Conducting Polymers and Interactions with Biological Tissues. J. R. Soc. Interf. 3, 741–752. doi:10.1098/rsif.2006.0141
Balint, R., Cassidy, N. J., and Cartmell, S. H. (2014). Conductive Polymers: Towards a Smart Biomaterial for Tissue Engineering. Acta Biomater. 10, 2341–2353. doi:10.1016/j.actbio.2014.02.015
Bonaz, B., Sinniger, V., Hoffmann, D., Clarençon, D., Mathieu, N., Dantzer, C., et al. (2016). Chronic Vagus Nerve Stimulation in Crohn's Disease: a 6-month Follow-Up Pilot Study. Neurogastroenterol. Motil. 28, 948–953. doi:10.1111/nmo.12792
Boscari, F., Vettoretti, M., Cavallin, F., Amato, A. M. L., Uliana, A., Vallone, V., et al. (2022). Implantable and Transcutaneous Continuous Glucose Monitoring System: a Randomized Cross over Trial Comparing Accuracy, Efficacy and Acceptance. J. Endocrinol. Invest. 45, 115–124. doi:10.1007/s40618-021-01624-2
Bourrier, A., Shkorbatova, P., Bonizzato, M., Rey, E., Barraud, Q., Courtine, G., et al. (2019). Monolayer Graphene Coating of Intracortical Probes for Long‐Lasting Neural Activity Monitoring. Adv. Healthc. Mater. 8, 1801331. doi:10.1002/adhm.201801331
Buaki-Sogó, Mireia., García-Carmona, L., Gil-Agustí, Mayte., García-Pellicer, Marta., and Quijano-López, Alfredo. (2021). 'Flexible and Conductive Bioelectrodes Based on Chitosan-Carbon Black Membranes: Towards the Development of Wearable Bioelectrodes. Nanomaterials 11, 2052. doi:10.3390/nano11082052,
Capadona, J. R., Tyler, D. J., Zorman, C. A., Rowan, S. J., and Weder, C. (2012). Mechanically Adaptive Nanocomposites for Neural Interfacing. MRS Bull. 37, 581–589. doi:10.1557/mrs.2012.97
Ceschi, P., Bohl, A., Sternberg, K., Neumeister, A., Senz, V., Schmitz, K. P., et al. (2014). Biodegradable Polymeric Coatings on Cochlear Implant Surfaces and Their Influence on Spiral Ganglion Cell Survival. J. Biomed. Mater. Res. 102, 1255–1267. doi:10.1002/jbm.b.33110
Chen, R., Canales, A., and Anikeeva, P. (2017). Neural Recording and Modulation Technologies. Nat. Rev. Mater. 2, 16093. doi:10.1038/natrevmats.2016.93
Cirillo, G., Pantuso, E., Curcio, M., Vittorio, O., Leggio, A., Iemma, F., et al. (2021). Alginate Bioconjugate and Graphene Oxide in Multifunctional Hydrogels for Versatile Biomedical Applications. Molecules 26, 1355. doi:10.3390/molecules26051355
Cogan, S. F. (2008). Neural Stimulation and Recording Electrodes. Annu. Rev. Biomed. Eng. 10, 275–309. doi:10.1146/annurev.bioeng.10.061807.160518
Devi, M., Vomero, M., Fuhrer, E., Castagnola, E., Gueli, C., Nimbalkar, S., et al. (2021). Carbon-based Neural Electrodes: Promises and Challenges. J. Neural Eng. 18, 041007. doi:10.1088/1741-2552/ac1e45
Dryg, I. D., Ward, M. P., Qing, K. Y., Mei, H., Schaffer, J. E., and Irazoqui, P. P. (2015). Magnetically Inserted Neural Electrodes: Tissue Response and Functional Lifetime. IEEE Trans. Neural Syst. Rehabil. Eng. 23, 562–571. doi:10.1109/tnsre.2015.2399856
Fisher, B., DesMarteau, J. A., Koontz, E. H., Wilks, S. J., and Melamed, S. E. (2020). Responsive Vagus Nerve Stimulation for Drug Resistant Epilepsy: A Review of New Features and Practical Guidance for Advanced Practice Providers. Front. Neurol. 11, 610379. doi:10.3389/fneur.2020.610379
Ghazavi, A., González-González, M. A., Romero-Ortega, M. I., Cogan, S. F., and Cogan, Stuart. F. (2020). Intraneural Ultramicroelectrode Arrays for Function-specific Interfacing to the Vagus Nerve. Biosens. Bioelectron. 170, 112608. doi:10.1016/j.bios.2020.112608
Gonzalez-Gonzalez, M. A., Bendale, G. S., Wang, K., Wallace, G. G., Romero-Ortega, M., and Romero-Ortega, Mario. (2021). Platinized Graphene Fiber Electrodes Uncover Direct Spleen-Vagus Communication. Commun. Biol. 4, 1097. doi:10.1038/s42003-021-02628-7
Hajjhassan, M., Chodavarapu, V., and Musallam, S. (2008). NeuroMEMS: Neural Probe Microtechnologies. Sensors 8, 6704–6726. doi:10.3390/s8106704
Harris, A. R., Newbold, C., Carter, P., Cowan, R., Wallace, G. G., and Wallace, (2018). Measuring the Effective Area and Charge Density of Platinum Electrodes for Bionic Devices. J. Neural Eng. 15, 046015. doi:10.1088/1741-2552/aaba8b
Harris, A. R., and Wallace., G. G. (2018). Organic Electrodes and Communications with Excitable Cells. Adv. Funct. Mater. 28, 1700587. doi:10.1002/adfm.201700587
Heo, Y. J., and Kim, S.-H. (2019). Toward Long-Term Implantable Glucose Biosensors for Clinical Use. Appl. Sci. 9, 2158. doi:10.3390/app9102158
Hernandez-Reynoso, A. G., Nandam, S., O’Brien, J. M., Kanneganti, A., Cogan, S. F., Freeman, D. K., et al. (2019). Miniature Electroparticle-Cuff for Wireless Peripheral Neuromodulation. J. Neural Eng. 16, 046002. doi:10.1088/1741-2552/ab1c36
Hwang, S.-W., Song, J.-K., Huang, X., Cheng, H., Kang, S.-K., Kim, B. H., et al. (2014). High-Performance Biodegradable/Transient Electronics on Biodegradable Polymers. Adv. Mater. 26, 3905–3911. doi:10.1002/adma.201306050
Im, C., and Seo, J.-M. (2016). A Review of Electrodes for the Electrical Brain Signal Recording. Biomed. Eng. Lett. 6, 104–112. doi:10.1007/s13534-016-0235-1
Jalili, R., Kanneganti, A., Romero-Ortega, M. I., and Wallace, G. G. (2017). Implantable Electrodes. Curr. Opin. Electrochemistry 3, 68–74. doi:10.1016/j.coelec.2017.07.003
Jia, X., Wang, C., Ranganathan, V., Napier, B., Yu, C., Chao, Y., et al. (2017). A Biodegradable Thin-Film Magnesium Primary Battery Using Silk Fibroin-Ionic Liquid Polymer Electrolyte. ACS Energ. Lett. 2, 831–836. doi:10.1021/acsenergylett.7b00012
Jia, X., Wang, C., Zhao, C., Ge, Y., and Wallace, G. G. (2016). Toward Biodegradable Mg-Air Bioelectric Batteries Composed of Silk Fibroin-Polypyrrole Film. Adv. Funct. Mater. 26, 1454–1462. doi:10.1002/adfm.201503498
Koo, J., Macewan, M. R., Kang, S.-K., Won, S. M., Stephen, M., Gamble, P., et al. (2018). Wireless Bioresorbable Electronic System Enables Sustained Nonpharmacological Neuroregenerative Therapy. Nat. Med. 24, 1830–1836. doi:10.1038/s41591-018-0196-2
Koopman, F. A., Chavan, S. S., Miljko, S., Grazio, S., Sokolovic, S., Schuurman, P. R., et al. (2016). Vagus Nerve Stimulation Inhibits Cytokine Production and Attenuates Disease Severity in Rheumatoid Arthritis. Proc. Natl. Acad. Sci. U.S.A. 113, 8284–8289. doi:10.1073/pnas.1605635113
Kumsa, D. W., Bhadra, N., Hudak, E. M., and Mortimer, J. T. (2017). Electron Transfer Processes Occurring on Platinum Neural Stimulating Electrodes: Pulsing Experiments for Cathodic-First, Charge-Balanced, Biphasic Pulses for 0.566 ⩽ K ⩽ 2.3 in Rat Subcutaneous Tissues. J. Neural Eng. 14, 056003. doi:10.1088/1741-2552/aa7a4a
Laurencin, C. T., and Daneshmandi., L. (2020). Graphene for Regenerative Engineering. Int. Jnl Ceram. Engine Sci 2, 140–143. doi:10.1002/ces2.10045
Lee, S. J., Nah, H., Heo, D. N., Kim, K.-H., Seok, J. M., Heo, M., et al. (2020). Induction of Osteogenic Differentiation in a Rat Calvarial Bone Defect Model Using an In Situ Forming Graphene Oxide Incorporated Glycol Chitosan/oxidized Hyaluronic Acid Injectable Hydrogel. Carbon 168, 264–277. doi:10.1016/j.carbon.2020.05.022
Leprince, L., Dogimont, A., Magnin, D., and Demoustier-Champagne, S. (2010). Dexamethasone Electrically Controlled Release from Polypyrrole-Coated Nanostructured Electrodes. J. Mater. Sci. Mater. Med. 21, 925–930. doi:10.1007/s10856-010-4008-6
Li, D., Zhang, W., Yu, X., Wang, Z., Su, Z., and Wei, G. (2016). When Biomolecules Meet Graphene: from Molecular Level Interactions to Material Design and Applications. Nanoscale 8, 19491–19509. doi:10.1039/c6nr07249f
Liu, X., Gilmore, K. J., Moulton, S. E., and Wallace, G. G. (2009). Electrical Stimulation Promotes Nerve Cell Differentiation on Polypyrrole/poly (2-methoxy-5 Aniline Sulfonic Acid) Composites. J. Neural Eng. 6, 065002. doi:10.1088/1741-2560/6/6/065002
Liu, X., Yue, Z., Higgins, M. J., and Wallace, G. G. (2011). Conducting Polymers with Immobilised Fibrillar Collagen for Enhanced Neural Interfacing. Biomaterials 32, 7309–7317. doi:10.1016/j.biomaterials.2011.06.047
Lotti, F., Ranieri, F., Vadalà, G., Zollo, L., and Di Pino, G. (2017). Invasive Intraneural Interfaces: Foreign Body Reaction Issues. Front. Neurosci. 11, 497. doi:10.3389/fnins.2017.00497
Merrill, D. R., Lockhart, J., Troyk, P. R., Weir, R. F., and Hankin, D. L. (2011). Development of an Implantable Myoelectric Sensor for Advanced Prosthesis Control. Artif. Organs 35, 249–252. doi:10.1111/j.1525-1594.2011.01219.x
Mirabedini, A., Foroughi, J., and Wallace, G. G. (2016). Developments in Conducting Polymer Fibres: from Established Spinning Methods toward Advanced Applications. RSC Adv. 6, 44687–44716. doi:10.1039/c6ra05626a
Moulton, S. E., Higgins, M. J., Kapsa, R. M. I., and Wallace, G. G. (2012). Organic Bionics: A New Dimension in Neural Communications. Adv. Funct. Mater. 22, 2003–2014. doi:10.1002/adfm.201102232
Nayagam, D. A. X., Williams, R. A., Chen, J., Magee, K. A., Irwin, J., Tan, J., et al. (2011). Biocompatibility of Immobilized Aligned Carbon Nanotubes. Small 7, 1035–1042. doi:10.1002/smll.201002083
Ni, J., Ling, H., Zhang, S., Wang, Z., Peng, Z., Benyshek, C., et al. (2019). Three-dimensional Printing of Metals for Biomedical Applications. Mater. Today Bio 3, 100024. doi:10.1016/j.mtbio.2019.100024
O’Malley, J. T., Burgess, B. J., Galler, D., and Nadol, J. B. (2017). 'Foreign Body Response to Silicone in Cochlear Implant Electrodes in the Human. Otology & Neurotology 38, 970–977. doi:10.1097/mao.0000000000001454
Ouyang, L., Shaw, C. L., Kuo, C.-c., Griffin, A. L., and Martin, D. C. (2014). In Vivopolymerization of Poly(3,4-Ethylenedioxythiophene) in the Living Rat hippocampus Does Not Cause a Significant Loss of Performance in a Delayed Alternation Task. J. Neural Eng. 11, 026005. doi:10.1088/1741-2560/11/2/026005
Pancrazio, J. J., Deku, F., Ghazavi, A., Stiller, A. M., Rihani, R., Frewin, C. L., et al. (2017). Thinking Small: Progress on Microscale Neurostimulation Technology. Neuromodulation: Tech. Neural Interf. 20, 745–752. doi:10.1111/ner.12716
Park, S., Yuk, H., Zhao, R., Yim, Y. S., Woldeghebriel, E. W., Kang, J., et al. (2021). Adaptive and Multifunctional Hydrogel Hybrid Probes for Long-Term Sensing and Modulation of Neural Activity. Nat. Commun. 12, 3435. doi:10.1038/s41467-021-23802-9
Richardson-Burns, S. M., Hendricks, J. L., Foster, B., Povlich, L. K., Kim, D.-H., Martin, D. C., et al. (2007). Polymerization of the Conducting Polymer Poly(3,4-Ethylenedioxythiophene) (PEDOT) Around Living Neural Cells. Biomaterials 28, 1539–1552. doi:10.1016/j.biomaterials.2006.11.026
Seymour, J. P., Wu, F., Wise, K. D., and Yoon, E. (2017). State-of-the-art MEMS and Microsystem Tools for Brain Research. Microsyst Nanoeng 3, 16066. doi:10.1038/micronano.2016.66
Sharafkhani, N., Kouzani, A. Z., Adams, S. D., Long, J. M., Lissorgues, G., Rousseau, L., et al. (2022). Neural Tissue-Microelectrode Interaction: Brain Micromotion, Electrical Impedance, and Flexible Microelectrode Insertion. J. Neurosci. Methods 365, 109388. doi:10.1016/j.jneumeth.2021.109388
Sharma, N., Prakash, A., Sahi, A. K., Sharma, N., and Sharma, S. (2021). Multimodal Sensor to Measure the Concurrent Electrical and Mechanical Activity of Muscles for Controlling a Hand Prosthesis. Instrumentation Sci. Tech. 49, 146–163. doi:10.1080/10739149.2020.1804932
Shin, S. R., Li, Y.-C., Jang, H. L., Khoshakhlagh, P., Akbari, M., Nasajpour, A., et al. (2016). Graphene-based Materials for Tissue Engineering. Adv. Drug Deliv. Rev. 105, 255–274. doi:10.1016/j.addr.2016.03.007
Sikder, M. K. U., Tong, W., Pingle, H., Kingshott, P., Needham, K., Shivdasani, M. N., et al. (2021). Laminin Coated diamond Electrodes for Neural Stimulation. Mater. Sci. Eng. C 118, 111454. doi:10.1016/j.msec.2020.111454
Spencer, K. C., Sy, J. C., Falcón-Banchs, R., and Cima, M. J. (2017). A Three Dimensional In Vitro Glial Scar Model to Investigate the Local Strain Effects from Micromotion Around Neural Implants. Lab. Chip 17, 795–804. doi:10.1039/c6lc01411a
Stieglitz, T., Schuetter, M., and Koch, K. P. (2005). Implantable Biomedical Microsystems for Neural Prostheses. IEEE Eng. Med. Biol. Mag. 24, 58–65. doi:10.1109/memb.2005.1511501
Tan, F., Zhu, Y., Ma, Z., and Al-Rubeai, M. (2020). Recent Advances in the Implant-Based Drug Delivery in Otorhinolaryngology. Acta Biomater. 108, 46–55. doi:10.1016/j.actbio.2020.04.012
Thompson, B. C., Moulton, S. E., Richardson, R. T., and Wallace, G. G. (2011). Effect of the Dopant Anion in Polypyrrole on Nerve Growth and Release of a Neurotrophic Protein. Biomaterials 32, 3822–3831. doi:10.1016/j.biomaterials.2011.01.053
Thompson, B. C., Richardson, R. T., Moulton, S. E., Evans, A. J., O'Leary, S., Clark, G. M., et al. (2010). Conducting Polymers, Dual Neurotrophins and Pulsed Electrical Stimulation - Dramatic Effects on Neurite Outgrowth. J. Controlled Release 141, 161–167. doi:10.1016/j.jconrel.2009.09.016
Tien, L. W., Wu, F., Tang-Schomer, M. D., Yoon, E., Omenetto, F. G., and Kaplan, D. L. (2013). Silk as a Multifunctional Biomaterial Substrate for Reduced Glial Scarring Around Brain-Penetrating Electrodes. Adv. Funct. Mater. 23, 3185–3193. doi:10.1002/adfm.201203716
Trung, T. Q., and Lee, N.-E. (2016). Flexible and Stretchable Physical Sensor Integrated Platforms for Wearable Human-Activity Monitoringand Personal Healthcare. Adv. Mater. 28, 4338–4372. doi:10.1002/adma.201504244
Veiseh, O., and Vegas, A. J. (2019). Domesticating the Foreign Body Response: Recent Advances and Applications. Adv. Drug Deliv. Rev. 144, 148–161. doi:10.1016/j.addr.2019.08.010
Wang, K., Frewin, C. L., Esrafilzadeh, D., Yu, C., Wang, C., Pancrazio, J. J., et al. (2019). High‐Performance Graphene‐Fiber‐Based Neural Recording Microelectrodes. Adv. Mater. 31, 1805867. doi:10.1002/adma.201805867
Weaver, C. L., and Cui., X. T. (2015). Directed Neural Stem Cell Differentiation with a Functionalized Graphene Oxide Nanocomposite. Adv. Healthc. Mater. 4, 1408–1416. doi:10.1002/adhm.201500056
Weaver, C. L., Larosa, J. M., Luo, X., and Cui, X. T. (2014). Electrically Controlled Drug Delivery from Graphene Oxide Nanocomposite Films. ACS Nano 8, 1834–1843. doi:10.1021/nn406223e
Weber, D. J., Hao, M., Urbin, M. A., Schoenewald, C., and Lan, N. (2020). “'Chapter Twenty One - Sensory Information Feedback for Neural Prostheses,” in Biomedical Information Technology. Editor D. D. Feng. Second Edition (Academic Press).
Wellman, S. M., Eles, J. R., Eles, K. A., Ludwig, K. A., Seymour, J. P., Michelson, N. J., et al. (2018). A Materials Roadmap to Functional Neural Interface Design. Adv. Funct. Mater. 28, 1701269. doi:10.1002/adfm.201701269
Wise, K. D., Angell, J. B., and Starr, A. (1970). An Integrated-Circuit Approach to Extracellular Microelectrodes. IEEE Trans. Biomed. Eng. BME-17, 238–247. doi:10.1109/tbme.1970.4502738
Woeppel, K. M., and Cui., X. T. (2021). Nanoparticle and Biomolecule Surface Modification Synergistically Increases Neural Electrode Recording Yield and Minimizes Inflammatory Host Response. Adv. Healthc. Mater. 10, 2002150. doi:10.1002/adhm.202002150
Won, S. M., Song, E., Reeder, J. T., and Rogers, J. A. (2020). Emerging Modalities and Implantable Technologies for Neuromodulation. Cell 181, 115–135. doi:10.1016/j.cell.2020.02.054
Woodington, B. J., Curto, V. F., Yu, Y. L., Martínez-Domínguez, H., Coles, L., Malliaras, G. G., et al. (2021). Electronics with Shape Actuation for Minimally Invasive Spinal Cord Stimulation. Sci. Adv. 7, eabg7833. doi:10.1126/sciadv.abg7833
Xie, C., Liu, J., Fu, T.-M., Dai, X., Zhou, W., and Lieber, C. M. (2015). Three-dimensional Macroporous Nanoelectronic Networks as Minimally Invasive Brain Probes. Nat. Mater 14, 1286–1292. doi:10.1038/nmat4427
Yao, G., Yin, C., Wang, Q., Zhang, T., Chen, S., Lu, C., et al. (2020). Flexible Bioelectronics for Physiological Signals Sensing and Disease Treatment. J. Materiomics 6, 397–413. doi:10.1016/j.jmat.2019.12.005
Yim, S., Hwang, D., Ihn, Y. S., Jeong, J., Oh, S., and Kim, K. (2017). “A Handheld Device for Magnetically Inserting a Neural Interface into a Peripheral Nervous System,” in 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), 226–229. doi:10.1109/embc.2017.8036803
Zhang, Q., Beirne, S., Shu, K., Esrafilzadeh, D., Huang, X.-F., Wallace, G. G., et al. (2018). Electrical Stimulation with a Conductive Polymer Promotes Neurite Outgrowth and Synaptogenesis in Primary Cortical Neurons in 3D. Sci. Rep. 8, 9855. doi:10.1038/s41598-018-27784-5
Zhao, S., Li, G., Tong, C., Chen, W., Wang, P., Dai, J., et al. (2020). Full Activation Pattern Mapping by Simultaneous Deep Brain Stimulation and fMRI with Graphene Fiber Electrodes. Nat. Commun. 11, 1788. doi:10.1038/s41467-020-15570-9
Zheng, T., Xu, N., Kan, Q., Li, H., Lu, C., Zhang, P., et al. (2019). Wet-Spinning Assembly of Continuous, Highly Stable Hyaluronic/Multiwalled Carbon Nanotube Hybrid Microfibers. Polymers 11, 867. doi:10.3390/polym11050867
Keywords: electroceuticals, sensing electrodes, stimulation electrodes, biofabrication, electrode material
Citation: Liu X, Wang K, González-González MA, Romero-Ortega M and Wallace GG (2022) Sensing and Stimulating Electrodes for Electroceuticals. Front. Sens. 3:873862. doi: 10.3389/fsens.2022.873862
Received: 11 February 2022; Accepted: 30 March 2022;
Published: 25 April 2022.
Edited by:
Dermot Diamond, Dublin City University, IrelandReviewed by:
Antonio J. Ricco, National Aeronautics and Space Administration (NASA), United StatesCopyright © 2022 Liu, Wang, González-González, Romero-Ortega and Wallace. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gordon G. Wallace, Z3dhbGxhY2VAdW93LmVkdS5hdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.