- 1Aquatic Toxicology Laboratory, St. Cloud State University, St. Cloud, MN, United States
- 2Institute for Environment and Sanitation Studies, University of Ghana, Accra, Ghana
- 3College of Natural Resources, University of Idaho, Moscow, ID, United States
Contaminants of Emerging Concern (CECs) have been documented across the seven continents, including Antarctica, and are likely an impediment to the sustainable management of natural resources. Most studies to date have relied on sweeping chemistry surveys, reliant upon sophisticated instrumentation. This approach is expensive, relies on limited laboratory capacity, and generates results that are spatially and temporally constrained. Here we review existing approaches that can overcome these limitations by focusing on effects-based monitoring. Passive samplers can generate long-term records regarding the occurrence of CECs. As samples are concentrated, their analysis can be achieved using equipment that will be more common and less expensive. A second approach involves rapid test methods for single compounds, including test strips, ELISA assays, and mobile phone-based analytic tools. These can provide inexpensive CEC presence data for many field sites and can be used to stratify sampling and thereby reduce cost. Identifying the presence of a single compound can often shed light on the likely presence of entire groups of chemicals. Pairing these chemistry-derived approaches with geospatial modeling to predict CEC presence and concentrations across watersheds has already been applied in several large watersheds. Utilizing available ecotoxicological knowledge bases provides an opportunity to link modeled CEC occurrence and concentrations with likely adverse biological responses. Finally, confirmatory on-site exposure experiments can corroborate the presence or absence of biological effects hypothesized from the above chain of evidence to provide natural resource managers with information to make conservation decisions.
1 Introduction
In the 1990s, a series of studies, mostly conducted in Europe (Desbrow et al., 1998; Jobling et al., 1998; Routledge et al., 1998), highlighted the emerging environmental threat caused by trace concentrations of man-made and natural compounds released into aquatic environments. Subsequent advances in analytic chemistry, which lowered detection limits, revealed that surface waters were contaminated by an ever-increasing number of chemical compounds (Kolpin et al., 2002; Elliott et al., 2017; Glassmeyer et al., 2017). These concerted efforts identified a wide range of compounds coined Contaminants of Emerging Concern (CECs) and advanced the theory that adverse biological effects observed in wildlife may be linked to minute concentrations of CECs (Orlando et al., 2004; Kidd et al., 2007; Thrupp et al., 2018). Much of these advancements were made possible by sophisticated machinery and ample funding from government and industry sources for large-scale environmental reconnaissance studies of CEC occurrence and concentrations. In composite, these studies have revealed a grim landscape in which few aquatic ecosystems have remained unscathed from the presence and biological effects of CECs (Nilsen et al., 2019; Du et al., 2020).
Coupling chemical occurrence studies with controlled laboratory exposure studies featuring aquatic sentinel organisms, has provided sufficient information to predictively model the presence of environmental CECs, and also to infer the potential for adverse biological effects (Diamond et al., 2015; Kiesling et al., 2018). Unfortunately, a sufficient density of data for such modeling efforts is largely confined to the high-income countries of the northern hemisphere. In contrast, entire regions, and most of the Global South, especially in low- and middle-income countries (LMIC) are depauperate of such data (Schoenfuss et al., 2022). This data gap presents both difficulties and opportunities, as the lack of data precludes them from following in the footsteps of richer nations, but also provides an opportunity wherein they do not have to reinvent the wheel and make the same mistakes and missteps that have occurred historically.
Nowhere is the significance of this deficiency more evident than it is for the intentionally biologically active CECs, most notably pharmaceuticals, personal care products, and pesticides (LeBlanc, 2023). For example, key pharmaceuticals used to control and prevent arthropod vector-borne infectious diseases, such as malaria, dengue, and chagas disease, may be much more prevalent in LMICs, where these diseases predominate, relative to counties in more temperate climates. Similarly, anti-retroviral drugs are more commonly found in African waterways due to the prevalence of HIV/AIDS (Gwenzi and Chaukura, 2018; Adeola and Forbes, 2022). Furthermore, in many LMICs, these pharmaceuticals are available without a prescription, which contributes to their potential overuse (LeBlanc, 2023). This combination of need and unrestricted availability can result in many pharmaceuticals being found in wastewater (Fekadu et al., 2019). Deficiencies in wastewater treatment result in insufficient removal of these compounds (Asem-Hiablie et al., 2013; Gwenzi and Chaukura, 2018) which leads to concentrations in receiving waters (Fekadu et al., 2019; Huff Chester et al., 2022) far exceeding what is generally found in high-income countries. A similar situation exists relative to agricultural pesticides, as many pesticides remain in use in LMICs that are seldom considered in Northern Hemisphere studies (Matamoros et al., 2020).
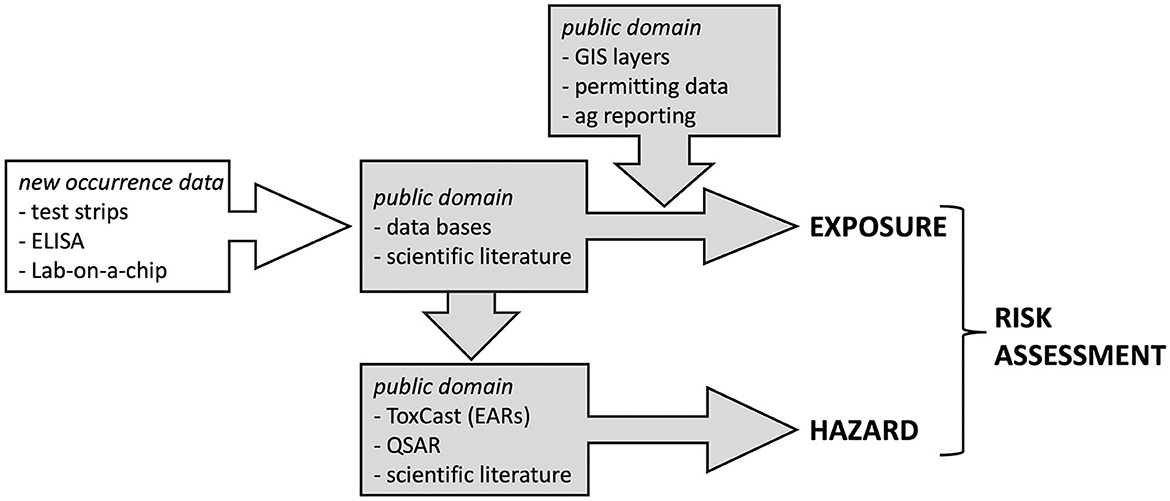
The biological impacts of CECs in the future will undoubtedly be influenced by current changes in worldwide population, urbanization, and the effects of climate change. Yet, despite the grim specter that these chemicals represent, it is neither financially feasible nor scientifically advisable for LMICs to repeat the CEC reconnaissance efforts of the northern hemisphere. In much the same way that cell phone technology leapfrogged landlines in many LMICs (James, 2012) we propose using knowledge, experience, and methodologies developed over the past 30 years to leapfrog the “reconnaissance” stage of CEC studies and to develop ecologically, cost-effective, and highly relevant models for regions of the southern hemisphere (Figure 1). The hope is that rapid implementation of these models will provide guidance to natural resource managers at a time when many southern hemisphere ecosystems have not yet reached the stage of deterioration that resulted in the early findings of endocrine disrupting compounds in the northern hemisphere.
2 Methodologies to detect CECs in aquatic environments
The “gold standard” for measuring CECs in aquatic environments is widely considered gas or liquid chromatography followed by tandem mass spectrometry (hereafter: GC/LC-MS/MS; Glassmeyer et al., 2017). Today, the use of Quadrupole Time of Flight Mass Spectrometry (QTOF-MS) allows for the identification of chemical compounds heretofore unknown to researchers and their reference libraries. However, the reliance on these advanced technologies has several drawbacks. Beyond its cost, data obtained only provide a snapshot of CEC occurrence and concentrations at the time the (grab-)sample was obtained. Consequently, the use of these tools runs the risk of committing a type 2 error, that is the declaration that a single non-detect of a compound suggests that the waterbody is free from contamination relative to that compound. While this may be true in lentic systems, such as ponds or lakes, it is much less likely to be true in lotic systems where flowing water may lead to episodic pulses (e.g., Spalding and Snow, 1989) of chemicals that may be missed due to a failure to match sampling to chemical occurrence. In addition, GC/LC-MS/MS is blind to compounds less water soluble, lost during sample preparation, or interfered with by matrix effects. A combination of other approaches can overcome these limitations (Burki et al., 2006; Thomas et al., 2017) and may be more suitable for remote environments and LMIC where the available instrumentation is limited.
2.1 Integrated passive samplers
Integrated passive samplers are devices that will accumulate compounds well above the concentration found within the water, in accordance with the affinity of the matrix contained within the sampler (Alvarez et al., 2007; Alvarez, 2010). These devices are usually small, inexpensive, and are placed in aquatic field sites for several days or weeks before recovery and extraction of CECs. This methodology has several advantages over extracting CEC occurrence and concentration data from grab- or compound-water samples. Chronic deployment allows for time-integration of CEC occurrence and may capture spikes in CEC concentration, for example, because of a strong precipitation event, that may be missed in a grab sample (Jorgenson et al., 2018). In addition, the logistics of affixing a small sampler at a remote field site is far simpler than collecting, compounding, and transporting larger volumes of water samples back to a laboratory. Lastly, because passive samplers accumulate samples over time, the mass of CEC in an extracted sample is usually much greater than in an extracted grab water sample, thus requiring less sophisticated methodology for analysis (for example HPLC may be sufficient for CEC identification; Alvarez et al., 2007; Barber et al., 2012).
One significant drawback of passive samplers has been the inability to provide data beyond the mass of a compound adherent to the sample matrix at the end of deployment. However, approaches have been developed more recently that allow for the calculation of average aqueous concentration for compounds accumulated on the passive sampler matrix (Bartelt-Hunt et al., 2011; Morin et al., 2012; Ibrahim et al., 2013). The remaining drawbacks of passive samplers are the focused nature of the sampler matrix, as only the compounds with an affinity to the matrix will be accounted for in the subsequent analysis.
2.2 Temporal variation in discharge
One of the underlying assumptions associated with using grab samples or integrative samplers to quantify the presence of CECs in aquatic systems is that the compounds are continuously present in detectable concentrations over time. As mentioned previously, this assumption is likely to hold in lentic environments, or in lotic environments downstream of point source discharge sources, such as wastewater treatment plants or industrial outflow. Interestingly, this assumption may hold regardless of whether the contaminant is a persistent organic pollutant or not. For non-persistent organic pollutants, that is those pollutants that are rapidly transformed into metabolites in the environment, a condition known as pseudo-persistence may develop (Daughton, 2002). In the case of pseudo-persistence, the concentration of a non-persistent organic will remain consistent, for as the “older” chemical is biotransformed into a suite of metabolites, it is continuously being replaced by new chemicals entering the environment.
In an interesting turn of events, the tools used by citizen scientists in developed countries may play a key role in environmental monitoring in LMICs. Home aquarists, as well as community members interested in the quality of their drinking water, have pushed for simplistic, rapid, and inexpensive home detection kits for a variety of different chemicals. Test strips are generally designed to detect a single compound or a group of closely related compounds. Some of the earliest technologies focused on nitrates and phosphates and provided a visual color-based concentration estimation based on the Beer-Lambert law. Other technologies have become available that can positively detect concentrations of organic chemicals at levels that occur in the environment, that cause adverse health impacts to fish and wildlife, and that would be a concern in drinking water supplies (Joseph et al., 2022). Advantages of using these strips, particularly in LMICs, is that the cost on a per-sample basis is very small when compared to grab sampling followed by GC/LC-MS/MS, the results are, for all practical purposes, immediate, and the implementation of the approach does not require considerable training. As a collateral, such studies may also reduce the carbon footprint of the monitoring campaign by employing the local citizenry to be involved in the testing, rather than having to send professionals considerable distances to collect samples. One of the primary disadvantages of developing monitoring campaigns that use these strips is strip availability. Considering that these strips are commercially produced their availability may wax and wane with market demand. While this may be an annoyance in developing countries where the strips are produced, the pipeline of strip supply to LMICs may be tenuous at best.
While grab samples, passive samplers and strip detectors can all be used successfully in isolation, a particularly attractive experimental design would be to use the strip detectors to identify geographic regions or temporal periods when contaminants are present and then follow up with more expensive sampling and/or the deployment of sentinel organisms. Such an approach has been used with success in Nebraska, USA, where pulses of agrichemicals were detected using an atrazine test strip, which was then followed by grab sampling (Ali and Kolok, 2015) or deployment of POCIS (Knight et al., 2013) as well as the concomitant deployment or exposure of sentinel organisms to the river water.
2.3 ELISA
Another relatively inexpensive, highly sensitive, and rapid method to measure some CECs is the use of enzyme-linked immunoassays (ELISA). This methodology relies on antibodies developed specifically to detect one compound (or a small group of closely related compounds) in an aqueous sample. The infrastructure required for ELISAs is a fraction of the cost of analytical instruments and there are validated kits available for a multitude of CECs. The sensitivity of this methodology approaches that of GC/LC-MS/MS for some compounds, often without the need for sample concentration. In addition, this method can provide information for more selective analytical analysis of only samples with positive detections (Graziano et al., 2006). A trained technician can complete the analysis of dozens of samples in a day at a fraction of the expense of a similar analysis through analytical chemistry.
2.4 Cell-based in-vitro methodologies
A range of cell-based assays (for example YES, ERa-CALUX, MELN, T47D-KBluc, and GeneBLAzer-ERa) to measure naturally occurring estrogens and their mimics in environmental samples have been developed to detect estrogenic compounds and mixtures at low ng/L concentrations (reviewed in Jarosová et al., 2014; Kunz et al., 2017). While often more complex than ELISAs, these assays utilize equipment found in many standard cell- and tissue-culture molecular laboratories. The added advantage of cell-based in-vitro assays is their ability to measure the totality of biological activity associated with one mode of action (most commonly estrogenic activity; Jarosová et al., 2014).
Lab-on-a-chip technology and related paper-based microfluidic devices, made possible by recent advancements in cell phone cameras and computational technology appear as another promising route to gain high-quality, exquisite sensitivity data on CEC occurrence in environmental samples (reviewed in Jang et al., 2011; Zulkkifli et al., 2018). Here, a reader is added to the camera function of a smartphone and a drop of water is placed into an optical read chamber. This technology was first developed to measure nutrients and heavy metals (Pol et al., 2017) but also shows promise for trace organic contaminants including CECs (Jang et al., 2011).
3 Integration of CEC occurrence with biological effects
Neither passive samplers, test strips, ELISAs, or similar methods will provide the broad suite of compound detection possible through GC/LC-MS/MS. However, a full accounting of all CECs in an environment is usually not necessary to obtain sufficient information for natural resource decision-making. Furthermore, the behavior of groups of CECs can often be interfered from the presence of one compound. For example, atrazine is predictably applied in row crop agriculture with other agrochemicals, therefore, occurrence data for the former can also be provided by extrapolation evidence for the presence of the latter (Southwick et al., 2003). In the case of pesticides, and prescription pharmaceuticals, government regulations in many countries require close tracking of the use of these compounds. Monitoring one of three or four large-volume herbicides with similar application patterns may be sufficient to predict the environmental presence and relative concentrations of the entire cohort. This approach is especially applicable when regulatory requirements provide ample use and occurrence data that can be reviewed.
Once sufficient CEC occurrence data have been gathered through the above-described methods, existing databases can be consulted to determine whether a CEC or mixture of CECs is likely to cause biological effects in exposed biota. ToxCast (EPA; https://www.epa.gov/chemical-research/toxicity-forecasting); COMPTOX (EPA, https://www.epa.gov/chemical-research/comptox-chemicals-dashboard); ECOTOX (EPA; https://cfpub.epa.gov/ecotox/help.cfm); NORMAN (https://www.norman-network.com/nds/susdat/); provide effects based data for many thousands of compounds and allow for the calculation of exposure-activity ratios (EARs) through secondary software packages such as ToxEval (USGS, https://www.usgs.gov/software/toxeval). EAR values >1 for any given compound may suggest that this compound could alter biological processes in exposed organisms at a field site. By summing EARs for multiple compounds, field sites can be ranked to establish natural resource management priorities.
Using occurrence data established through environmental monitoring and data interfered from the scientific literature models of CEC presence and likelihood of environmental impact can be developed for entire regions. By pairing CEC occurrence data with geophysical characteristics available in existing GIS databases (slope, vegetation, population density, point sources) and climate data from meteorological archives (temperature, precipitation, prevailing weather patterns) predictions can be derived for CEC presence and concentrations in similar, but previously unstudied, watersheds. To further extend the reach of these models, occurrence data can then be converted into biological effects data using the available ecotox databases and EAR approaches described above.
As is the case for any modeling effort, ground truthing is required as a final step to validate these models. Using predictions generated by the models, scientists can select a few field sites with a gradient of inferred CEC presence and ecological effects and apply passive samplers, test kits, and ELISA to validate prediction. If possible, collections of wild fish, or the deployment of caged fish, also allow validation of the predicted ecological impacts of CEC occurrence. The resultant validated models can then be used by natural resource managers to identify sites in greatest need of mitigation efforts, sites of greatest promise for restoration, and sites where CEC presence may present obstacles for conservation efforts.
4 Conclusions
CECs are of worldwide concern but require regional knowledge to manage and mitigate. Using readily available technology, freely available databases, and software, scientists in LMIC can begin to fill the existing void in CEC occurrence and biological effects data without the need for prohibitively expensive analytical infrastructure. While by no means a simple task, the methods, and workflows outlined in this manuscript may provide a roadmap to rapidly gain critical information to serve natural resource managers at a time when aquatic ecosystems worldwide, and especially in the southern hemisphere are under threat by environmental pollution.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
HS: Conceptualization, Methodology, Writing—original draft, Writing—review & editing. AK: Conceptualization, Methodology, Writing—original draft, Writing—review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adeola, A. O., and Forbes, P. B. C. (2022). Antiretroviral drugs in South African surface waters: prevalence, analysis, and potential remediation. Environ. Toxicol. Chem. 41, 247–262. doi: 10.1002/etc.5127
Ali, J., and Kolok, A. S. (2015). On-site, serial exposure of female fathead minnows to the Elkhorn River, Nebraska, USA, spring agrichemical pulse. Environ. Toxicol. Chem. 34, 1354–1361. doi: 10.1002/etc.2928
Alvarez, D. A. (2010). Guidelines for the Use of the Semipermeable Membrane Device (SPMD) and the Polar Organic Chemical Integrative Sampler (POCIS) in Environmental Monitoring Studies: U.S. Geological Survey, Techniques and Methods 1–D4. Reston, VA: USGS.
Alvarez, D. A., Huckins, J. N., Petty, J. D., Jones-Lepp, T., Stuer-Lauridsen, F., Getting, D. T., et al. (2007). Tool for monitoring hydrophilic contaminants in water: polar organic chemical integrative sampler (POCIS). Comp. Analyt. Chem. 48, 171–197 doi: 10.1016/S0166-526X(06)48008-9
Asem-Hiablie, S., Church, C. D., Elliott, H. A., Shappell, N. W., Schoenfuss, H. L., Drechsler, P., et al. (2013). Serum estrogenicity and biological responses in African catfish raised in wastewater ponds in Ghana. Sci. Total Environ. 463–464, 1182–1191. doi: 10.1016/j.scitotenv.2013.06.032
Barber, L. B., Writer, J. H., Keefe, S. H., Brown, G. K., Ferrey, M. L., Jahns, N. D., et al. (2012). Endocrine Disrupting Chemicals in Minnesota Lakes—Water-Quality and Hydrological Data from 2008 and 2010: U.S. Geological Survey Open-File Report 2012–1124. Reston, VA: USGS.
Bartelt-Hunt, D., Snow, D., Damon-Powell, T., Brown, D., Schwarz, M., and Kolok, A. S. (2011). Quantitative evaluation of laboratory uptake rates for pesticides, pharmaceuticals and steroid hormones using polar organic chemical integrative samplers. Environ. Toxicol. Chem. 30, 1412–1420. doi: 10.1002/etc.514
Burki, R., Vermeirssen, E. L. M., Korner, O., Joris, C., Burkhardt-Holm, P., and Segner, H. (2006). Assessment of estrogenic exposure in brown trout (Salmo trutta) in a Swiss midland river: integrated analysis of passive samplers, wild and caged fish, and vitellogenin mRNA and protein. Environ. Toxicol. Chem. 25, 2077. doi: 10.1897/05-545R.1
Daughton, C. G. (2002). Environmental stewardship and drugs as pollutants. Lancet 360, 9339. doi: 10.1016/S0140-6736(02)11176-7
Desbrow, C., Routledge, E. J., Brighty, G. C., Sumpter, J. P., and Waldock, M. (1998). Identification of estrogenic chemicals in STW effluent. 1. Chemical fractionation and in vitro biological screening. Environ. Sci. Technol. 32, 1549–58. doi: 10.1021/es9707973
Diamond, J., Munkittrick, K., Kapo, K. E., and Flippin, J. (2015). A framework for screening sites ar risk from contaminants of emerging concern. Environ. Toxicol. Chem. 34, 2671–2681. doi: 10.1002/etc.3177
Du, B., Fan, G., Yu, W., Yang, S., Zhou, J., and Luo, J. (2020). Occurrence and risk assessment of steroid estrogens in environmental water samples: a five-year worldwide perspective. Environ. Poll. 267, 115405. doi: 10.1016/j.envpol.2020.115405
Elliott, S. M., Brigham, M. E., Lee, K. E., Banda, J. A., Choy, S. J., and Gefell, D. J. (2017). Contaminants of emerging concern in tributaries to the Laurentian Great Lakes: I. Patterns of occurrence. PLoS ONE 12, e0182868. doi: 10.1371/journal.pone.0182868
Fekadu, S., Alemayehu, E., Dewil, R., and Van der Bruggen, B. (2019). Pharmaceuticals in freshwater aquatic environments: a comparison of the African and European challenge. Sci. Total. Environ. 654, 324–337. doi: 10.1016/j.scitotenv.2018.11.072
Glassmeyer, S. T., Furlong, E. T., Kolpin, D. W., Batt, A. L., Benson, R., Boone, J. S., et al. (2017). Nationwide reconnaissance of contaminants of emerging concern in source and treated drinking waters of the United States. Sci. Total Environ. 581–582, 909–922. doi: 10.1016/j.scitotenv.2016.12.004
Graziano, N., McGuire, M. J., Roberson, A., Adams, C., Jiang, H., and Blute, N. (2006). 2004 National atrazine occurrence monitoring program using the abraxis ELISA method. Environ. Sci. Technol. 40, 1163–1171. doi: 10.1021/es051586y
Gwenzi, W., and Chaukura, N. (2018). Organic contaminants in African aquatic systems: current knowledge, health risks, and future research. Sci. Total Environ. 619–620, 1493–1514. doi: 10.1016/j.scitotenv.2017.11.121
Huff Chester, A., Gordon, C., Hartmann, H. A., Bartell, S. E., Ansah, E., Yan, T., et al. (2022). Contaminants of emerging concern in the Lower Volta River, Ghana, West Africa: the agriculture, aquaculture, and urban development nexus. Environ. Toxicol. Chem. 41, 369–381. doi: 10.1002/etc.5279
Ibrahim, I., Togola, A., and Gonzalez, C. (2013). In-situ calibration of POCIS for the sampling of polar pesticides and metabolites in surface water. Talanta 116, 495–500. doi: 10.1016/j.talanta.2013.07.028
James, J. (2012). The distributional effects of leapfrogging in mobile phones. Telem. Inform. 29, 294–301. doi: 10.1016/j.tele.2011.09.001
Jang, A., Zou, Z., Lee, K. L., Ahn, C. H., and Bishop, P. L. (2011). State-of-the-art lab chip sensors for environmental water monitoring. Meas. Sci. Technol. 22, e032001. doi: 10.1088/0957-0233/22/3/032001
Jarosová, B., Bláha, L., Giesy, J. P., and Hilscherová, K. (2014). What level of estrogenic activity determined by in vitro assays in municipal waste waters can be considered as safe? Environ. Internat. 64, 98–109. doi: 10.1016/j.envint.2013.12.009
Jobling, S., Nolan, M., Tyler, C. R., Brighty, G., and Sumpter, J. P. (1998). Widespread sexual disruption in wild fish. Environ. Sci. Technol. 32, 2498–2506. doi: 10.1021/es9710870
Jorgenson, Z. G., Thomas, L., Elliott, S. M., Cavallin, J. E., Randolph, E. C., Choy, S. J., et al. (2018). Contaminants of emerging concern presence and adverse effects in fish: a case study in the laurentian great lakes. Environ. Poll. 236, 718–733. doi: 10.1016/j.envpol.2018.01.070
Joseph, N., Sangster, J., Topping, M., Bartelt-Hunt, S., and Kolok, A. S. (2022). Evaluating the impact of turbidity, precipitation, and land use on nutrient levels and atrazine concentrations in Illinois surface water as determined by citizen scientists. Sci. Total Environ. 850, 158081. doi: 10.1016/j.scitotenv.2022.158081
Kidd, K. A., Blanchfield, P. J., Mills, K. H., Palace, V. P., Evans, R. E., Lazorchak, J. M., et al. (2007). Collapse of a fish population after exposure to a synthetic estrogen. Proc. Natl. Acad. Sci. U. S. A. 104, 8897–8901. doi: 10.1073/pnas.0609568104
Kiesling, R. L., Elliott, S. M., Kammel, L. E., Choy, S. J., and Hummel, S. L. (2018). Predicting the occurrence of chemicals of emerging concern in surface water and sediment across the U.S. portion of the Great Lakes Basin. Sci. Total Environ. 651, 838. doi: 10.1016/j.scitotenv.2018.09.201
Knight, L. A., Christenson, M. K., Trease, A. J., Davis, P. H., and Kolok, A. S. (2013). The spring runoff in Nebraska's Elkhart River and its impact on two sentinel species. Environ. Toxicol. Chem. 32, 1544–1551. doi: 10.1002/etc.2220
Kolpin, D. W., Furlong, E. T., Meyer, M. T., Thurman, E. M., Zaugg, S. D., Barber, L. B., et al. (2002). Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: a national reconnaissance. Environ. Sci. Technol. 36, 1202–1211. doi: 10.1021/es011055j
Kunz, P. Y., Simon, E., Creusot, N., Jayasinghe, B. S., Kienle, C., Maletz, S., et al. (2017). Effect-based tools for monitoring estrogenic mixtures: evaluation of five in vitro bioassays. Water Res. 110, 378–388. doi: 10.1016/j.watres.2016.10.062
LeBlanc, G. A. (2023). Everyday Chemicals: Understanding the Risks. New York, NY: Columbia University Press.
Matamoros, V., Caiola, N., Rosales, V., Hernandez, O., and Ibanez, C. (2020). The role of rice fields and constructed wetlands as a source and a sink of pesticides and contaminants of emerging concern: full-scale evaluation. Ecol. Eng. 156, 105971. doi: 10.1016/j.ecoleng.2020.105971
Morin, N., Miege, C., Coquery, M., and Random, J. (2012). Chemical calibration, performance, validation and application of the polar organic chemical integrative sampler (POCIS) in aquatic environments. Trends Anal. Chem. 36, 144–175. doi: 10.1016/j.trac.2012.01.007
Nilsen, E., Smalling, K. L., Ahrens, L., Gros, M., Miglioranza, K. S. B., Pico, Y., et al. (2019). Critical Review: grand challenges in assessing the adverse effects of contaminants of emerging concern on aquatic food webs. Environ. Toxicol. Chem. 38, 46–60. doi: 10.1002/etc.4290
Orlando, E. F., Kolok, A. S., Binzcik, G. A., Gates, J. L., Horton, M. K., Lambright, C. S., et al. (2004). Endocrine disrupting effects of cattle feedlot effluent on an aquatic sentinel species, the fathead minnow. Environ. Health Perspect. 112, 353–358. doi: 10.1289/ehp.6591
Pol, R., Cespedes, F., Gabriel, D., and Baeza, M. (2017). Microfluid lab-on-a-chip platforms for environmental monitoring. Trends Anal. Chem. 95, 62–68. doi: 10.1016/j.trac.2017.08.001
Routledge, E. J., Sheahan, D., Desbrow, C., Brighty, G. C., Waldock, M., and Sumpter, J. P. (1998). Identification of oestrogenic chemicals in STW effluent. 2. In vivo responses in trout and roach. Environ. Sci. Technol. 32, 1559–1565. doi: 10.1021/es970796a
Schoenfuss, H. L., Propper, C. R., Kolok, A. S., and Forbes, P. B. C. (2022). Terra (aqua) Incognito: knowledge gaps in global ecotoxicology. Environ. Toxicol. Chem. 41, 245–246. doi: 10.1002/etc.5159
Southwick, L. M., Grigg, B. C., Fouss, J. L., and Kornecki, T. S. (2003). Atrazine and metolachlor in surface runoff under typical rainfall conditions in Southern Louisiana. J. Agric. Food Sci. 51, 5355–5361. doi: 10.1021/jf034049a
Spalding, R. F., and Snow, D. D. (1989). Stream levels of agrichemicals during a spring discharge event. Chemosphere 19, 1129–1140. doi: 10.1016/0045-6535(89)90061-1
Thomas, L. M., Jorgenson, Z. G., Brigham, M. E., Choy, S. J., Moore, J. N., Banda, J. A., et al. (2017). Contaminants of emerging concern in tributaries to the Laurentian Great Lakes: II. Biological consequences of exposure. PLoS ONE 12, e0184725. doi: 10.1371/journal.pone.0184725
Thrupp, T. J., Runnalls, T. J., Scholze, M., Kugathas, S., Kortenkamp, A., and Sumpter, J. P. (2018). The consequences of exposure to mixtures of chemicals: something from ‘nothing' and ‘a lot from a little' when fish are exposed to steroid hormones. Sci. Total Environ. 619–620, 1482–1492. doi: 10.1016/j.scitotenv.2017.11.081
Keywords: effects-based approach, rapid assessment, ecology, toxicology, environment
Citation: Schoenfuss HL and Kolok AS (2023) An ecotoxicologically relevant approach to water quality monitoring for contaminants of emerging concern. Front. Water 5:1333165. doi: 10.3389/frwa.2023.1333165
Received: 04 November 2023; Accepted: 27 November 2023;
Published: 18 December 2023.
Edited by:
Timothy Sullivan, University College Cork, IrelandReviewed by:
Jason T. Magnuson, United States Geological Survey, United StatesCopyright © 2023 Schoenfuss and Kolok. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heiko L. Schoenfuss, aHNjaG9lbmZ1c3MmI3gwMDA0MDtzdGNsb3Vkc3RhdGUuZWR1
 Heiko L. Schoenfuss
Heiko L. Schoenfuss Alan S. Kolok
Alan S. Kolok