- 1Bureau of Infectious Disease and Laboratory Sciences, Massachusetts Department of Public Health, Boston, MA, United States
- 2Biobot Analytics, Inc., Cambridge, MA, United States
- 3Massachusetts Executive Office of Health and Human Services, Boston, MA, United States
Introduction: Correctional facilities have environmental, resource, and organizational factors that facilitate SARS-CoV-2 transmission and challenge clinical testing of staff and residents. In Massachusetts, multiple state prisons implemented wastewater surveillance for strategic testing of individuals and isolation of COVID-19 cases early in the course of infection, as recommended by the Centers for Disease Control and Prevention (CDC). Our objective was to quantify the correlation of COVID-19 cases with facility-level wastewater surveillance compared to standard case surveillance in towns in closest geographic proximity to participating correctional facilities.
Materials and methods: Available data included number of reported COVID-19 cases in residents from each of eight participating facilities (labeled A-H for anonymity), wastewater viral concentrations at each facility, and COVID-19 cases reported to routine surveillance in towns geographically nearest each facility. We selected data from December 2020-February 2022. Spearman's rank correlation was calculated at each facility to assess agreement between town cases and facility resident cases, and between wastewater concentrations and facility resident cases. We considered a correlation of ≤0.3 as weak and ≥0.6 as strong.
Results: Facilities housed a mean of 502 individuals (range 54–1,184) with mean staffing of 341 (range 53–547). In 7/8 facilities, the town/resident cases correlation coefficients (ρ) were statistically significant (range 0.22–0.65); in all facilities, the wastewater/facility resident cases correlations were statistically significant (range 0.57–0.82). Consistently, ρ values were higher for facility-specific wastewater/resident cases than for town/resident cases: A (0.65, 0.80), B (0.59, 0.81), C (0.55, 0.70), D (0.61, 0.82), E (0.46, 0.62), F (0.51, 0.70), and H (0.22, 0.57).
Conclusion: We conclude that wastewater surveillance for SARS-CoV-2 can provide an additional signal to objectively supplement existing COVID-19 clinical surveillance for the early detection of cases and infection control efforts at correctional facilities.
1. Introduction
The SARS-CoV-2 pandemic has caused immeasurable suffering in terms of morbidity, mortality, and the effects of economic impacts, with vulnerable populations having been affected disproportionately. In particular, individuals residing in correctional facilities have suffered mortality rates above those of the comparable general population (Marquez et al., 2021); their vulnerabilities are associated with conditions in their communities (e.g., social determinants of health) as well as during confinement (Simpson and Butler, 2020). Specifically, SARS-CoV-2 is most efficiently transmitted in crowded, indoor spaces and the combination of environmental, resource, and organizational factors existent in corrections that facilitate SARS-CoV-2 transmission. At the same time, clinical testing of staff and residents to identify infections early and prevent transmission is challenging in correctional facilities (Hawks et al., 2020; Montoya-Barthelemy et al., 2020; Hassard et al., 2022).
In Massachusetts, approximately 6,500 individuals reside under the supervision of the Massachusetts Department of Correction (DOC) in a wide variety of facility sizes and infrastructure. Part of the DOC mission is to provide appropriate care for residents, and the DOC contracts with a private healthcare service (DOC Coronavirus Information Guide | Mass.gov) to meet those needs. In response to COVID-19, the DOC conducted activities to “minimize opportunities for the SARS-CoV-2 to enter state correctional facilities; to suppress its spread inside of those facilities; and to provide adequate testing to identify, and medical care to treat, residents and staff who contract it” (EOPSS and DOC, 2021).
Unfortunately, screening asymptomatic individuals is challenging because infection control interventions that follow the detection of a positive individual lead to testing refusals (Wurcel et al., 2020). In the absence of systematic screening, asymptomatic cases could easily go undetected and serve as sources of transmission. Because of this, the DOC implemented wastewater surveillance in select facilities in December 2020 with the vision to capture an unbiased sample of residents and use results to target testing and isolate positives early in the course of infection. Our objective was to quantify the correlation of COVID-19 cases with facility-level wastewater surveillance compared to standard case surveillance in towns in closest geographic proximity to participating correctional facilities.
2. Methods
We used several sources of data to measure cases for this study, including resident testing and case data from the DOC, the Massachusetts Department of Public Health surveillance system (Massachusetts Virtual Epidemiologic Network [MAVEN] case data from neighboring towns and surveillance notes), and Biobot Analytics (wastewater data).
Eight correctional facilities participated in wastewater surveillance (Table 1). The average daily population in 2021 at each facility ranged from < 100 residents to >1,000; facilities varied widely in terms of their security and resident demographics.
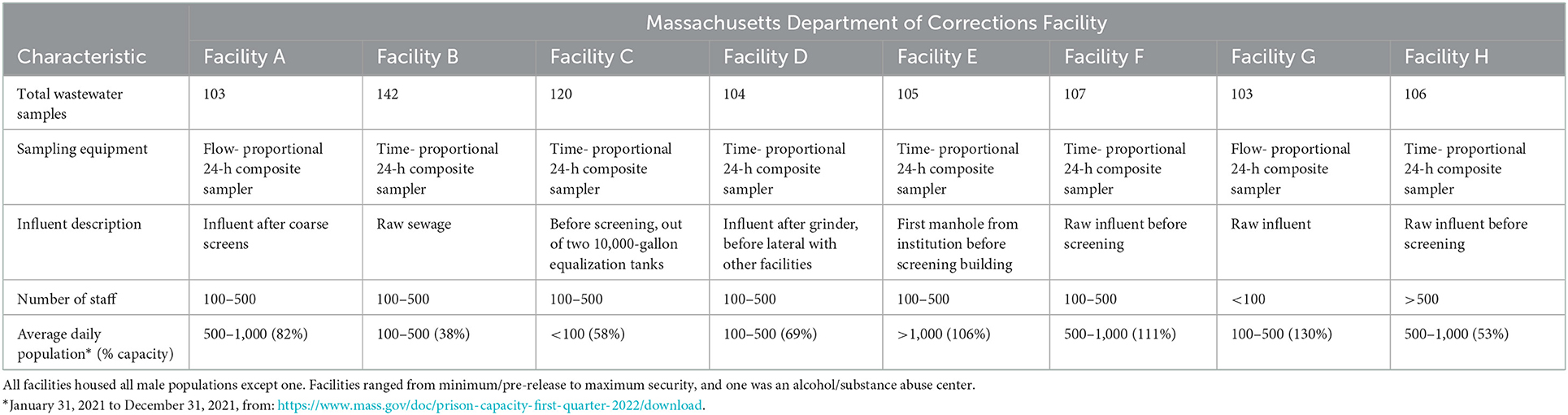
Table 1. Table of Massachusetts Department of Corrections facilities and associated characteristics.
2.1. Case data
Case data are individual level infections that have been reported to surveillance. Facility-level case data for residents of DOC facilities were provided by the DOC for this analysis. An active case at the facility was defined as an individual with a positive PCR or rapid antigen test and symptoms within 10 days of the test. Facility capacity information was procured via the DOC's quarterly report on the status of prison capacity from January 31 to December 31, 2021 (https://www.mass.gov/doc/prison-capacity-first-quarter-2022/download). Massachusetts DOC Tableau dashboards were used to ascertain the capacity, security level, and demographics of facility residents (https://public.tableau.com/app/profile/madoc). These were created by the DOC's Data Analytics Unit under an emergency Massachusetts law to protect health and wellness (Session Law-Acts of 2020 Chapter 93 (malegislature.gov).
Towns in closest geographic proximity to the participating DOC facilities were identified. Town-level case data were obtained from MAVEN, an integrated, web-based disease surveillance and case management system used in surveillance of over 90 reportable infectious diseases (Troppy et al., 2014). The laboratory-initiated electronic reporting of COVID-19 cases followed case definitions from the CDC/CSTE (https://www.cdc.gov/sars/guidance/b-surveillance/app1.html). MAVEN also contains notes from epidemiologists conducting surveillance follow-up to track activities and use free-text notes associated with facility clusters. For this study, all PCR tests were extracted by town, week (1–53 as used by the Morbidity and Mortality Weekly Report), week start and end dates, and test results to compile COVID-19 cases in towns neighboring DOC facilities, and data for the analysis period was provided as a csv file. We also extracted free-text notes to identify dates of any program recommendations to increase clinical testing, and whether these were specified to be in response to wastewater signals.
2.2. Wastewater data
Twenty-four-hour composite samples of raw influent wastewater were collected from each site as part of regular wastewater surveillance provided by Biobot Analytics, using either time- or flow-proportional 24-h composite samplers. Samples were collected at least once per week from December 15, 2020, with the analysis period ending February 12, 2022, for a total of 890 samples across eight sites (Table 1). Samples were collected and analyzed as described previously (Duvallet et al., 2022). Briefly, 15 mL of wastewater was concentrated approximately 100x, and RNA was extracted using RNeasy Mini columns or cassettes. RNA samples eluted from the RNeasy kit were subjected to one-step RT-qPCR analysis in triplicate for N1, N2, Pepper mild mottle virus (PMMoV) and other control amplicons. Ct-values were called from raw fluorescence data using the Cy0 algorithm from the qpcR package (v1.4-1) in R (Guescini et al., 2008), and manually inspected for agreement with the raw traces in the native Biorad software. Standard curves were generated and used to quantify virus amounts from Ct-values. PMMV was used as a fecal indicator for normalization of SARS-CoV-2 concentrations.
In February 2022, we updated our laboratory methodology improve sample processing time, throughput, and sensitivity, and to account for supply chain availability, adopting the current protocol, which uses Ceres NanoTrap particles (SKU 44202) to capture viruses essentially as described in the Ceres Nanosciences APP-030 protocol. Viral particles were captured from 9.6 mL of wastewater, immediately lysed with the MagMAX Lysis Solution (Applied Biosystems A52606), and 400 μL of the resulting lysate were used for nucleic acid isolation with MagMAX DNA/RNA binding beads. Samples eluted from the magnetic beads were subjected to one-step RT-qPCR analysis as described above.
Laboratory controls were run with all methods, including positive synthetic SARS-CoV-2 RNA controls and two no-template controls included on every qPCR plate. For positive and negative controls, Ct values outside the expected ranges trigger a re-run of the qPCR plate. One set of extraction blank controls was also run each day. Additionally, a recovery control was spiked into every sample and measured in parallel to PMMV. Matrix inhibition was assessed manually by reviewing raw qPCR curves and by an internal qPCR control measured in parallel to PMMV. Finally, PMMV was used as a proxy measure for per-plate recovery, and qPCR plates with unusually low PMMV were flagged values for further review and potential plate re-run. Only results which passed all quality controls are included in this dataset.
2.3. Statistical analyzes
Our hypotheses were that: (1) SARS-CoV-2 concentrations measured in the wastewater of facilities would correlate strongly with COVID-19 cases in residents of DOC facilities (i.e., individuals who were incarcerated), as residents in facilities are, largely, a closed population, with all waste deposited at facilities; and (2) resident and town cases would correlate moderately, because staff often live in neighboring communities and might bring SARS-CoV-2 into the target correctional facilities from community exposures.
To assess correlations between COVID-19 cases in DOC facilities, neighboring towns, and SARS-CoV-2 concentrations in wastewater, we analyzed time series for all locations between December 15, 2020 and February 12, 2022. Variables were: wastewater SARS-CoV-2 concentrations, town-level case data, and facility-level case data. However, these were all reported at different frequencies, so epidemiologic week was used as a common timescale to compare across data sources. Wastewater SARS-CoV-2 concentrations and case counts were averaged by epidemiologic week, using the geometric mean and standard mean, respectively. To characterize the relationship between wastewater SARS-CoV-2 concentrations and COVID-19 cases, we computed cross-correlations between three time series: weekly, average facility-level wastewater SARS-CoV-2 concentrations (hereafter “wastewater”), weekly, average facility-level recorded DOC resident COVID-19 cases (“resident cases”), weekly, and weekly, town-level COVID-19 cases among the general population (“town cases”).
Spearman's rank correlation coefficient tests (ρ) were performed to assess correlations; p < 0.05 were considered statistically significant; ρ ≥ 0.6 was considered strong, ρ ≤ 0.3 was weak. Sensitivity analyzes were performed to evaluate the impact of COVID-19 waves (pre-Omicron vs. all samples) on correlations. All statistical analyzes were conducted in R Studio, Version 4.2.0 (R Core Team, 2022).
This analysis was a secondary use of data and was not considered human subjects research by the Massachusetts Department of Public Health.
3. Results
Between December 15, 2020 and February 12, 2022, a total of 1,246 COVID-19 cases were recorded across eight Massachusetts DOC facilities in residents, with 34,781 rapid antigen tests administered, 1,273 of which were positive. A total of 32,908 COVID-19 cases were recorded in the eight towns most closely geographically associated with each facility. In the same time frame, 890 wastewater samples were collected across all facilities (Table 1).
3.1. Correlations
Results varied across facilities, but overall, correlations between residents and wastewater correlations were strong (p > 0.6) and resident and town cases correlations were moderate (Figure 1). Consistently, correlation values were higher for facility-specific wastewater/resident cases than for town/resident cases (Table 2). We conducted sensitivity analyzes (Supplementary material) to assess correlations for: (1) days where at least five COVID-19 tests were administered; (2) weeks where at least five COVID-19 tests were administered; and (3) the time period before the Omicron wave (December 2020-November 2021). As expected, resident cases and wastewater concentrations were more strongly correlated across facilities for the first two sensitivity analyzes (Supplementary Tables S1, S2). Town case counts were more strongly correlated with resident case counts when at least an average of five COVID-19 tests were administered weekly at facilities; this might be indicative of overall pandemic dynamics and disease waves affecting community and facility-level COVID-19 activity. Notably, analyzing data from before the Omicron wave in December 2021 showed attenuated correlations among residents, wastewater, and town cases across facilities. Correlations of resident cases and wastewater were moderate in several facilities; correlations of resident and town cases were weak, and most were not statistically significant (Supplementary Table S3).
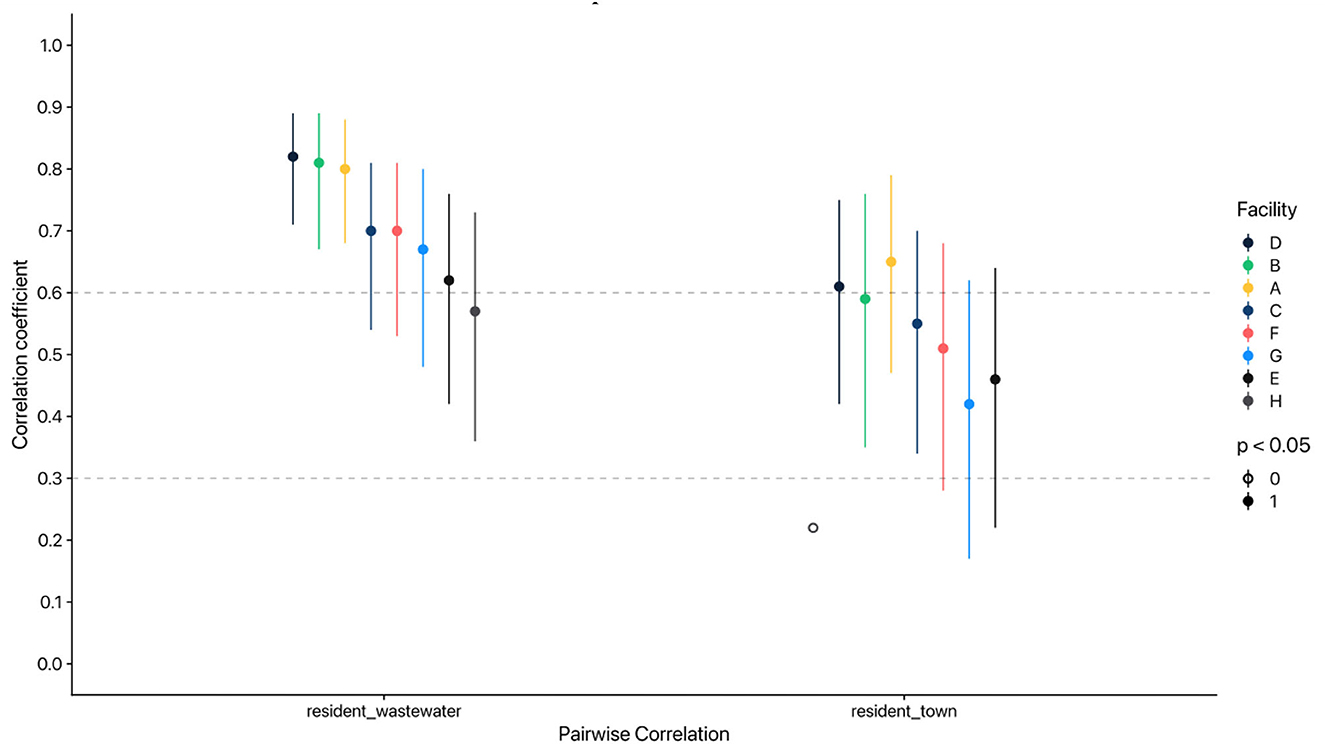
Figure 1. Pairwise correlations by resident and town cases and SARS-CoV-2 concentration in wastewater across Massachusetts Department of Corrections facilities. Spearman's rank correlation coefficient used; lines represent 95% confidence intervals. Case counts and wastewater SARS-CoV-2 virus concentrations were averaged by MMWR week. wastewater = weekly geometric mean of SARS-CoV-2 effective concentrations in copies/L; town = weekly average reported COVID-19 cases by neighboring town; resident = weekly average reported active COVID-19 cases among residents in Mass DOC facilities.
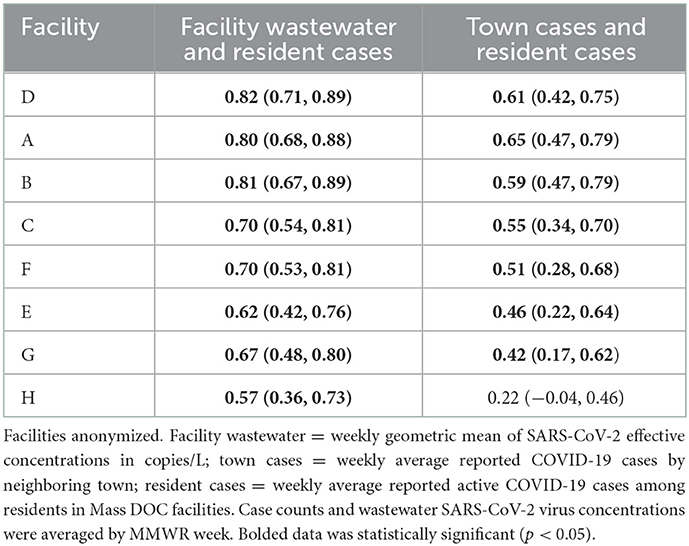
Table 2. Table of Spearman's rank correlation coefficients by Massachusetts Department of Corrections facility for facility wastewater, resident active COVID-19 cases, and town COVID-19 cases, December 2020–February 2022.
3.2. Case studies
Wastewater monitoring for SARS-CoV-2 is most beneficial to building-level facilities when used as a tool to provide situational awareness and/or an early warning for COVID-19 outbreaks. Despite small overall reported case counts in the majority of the facilities, correlations between SARS-CoV-2 concentrations in wastewater and COVID-19 case counts were apparent (Figure 2). Because of this, we also selected three individual facilities to evaluate in separate case studies based on information available about responses to wastewater monitoring signals.
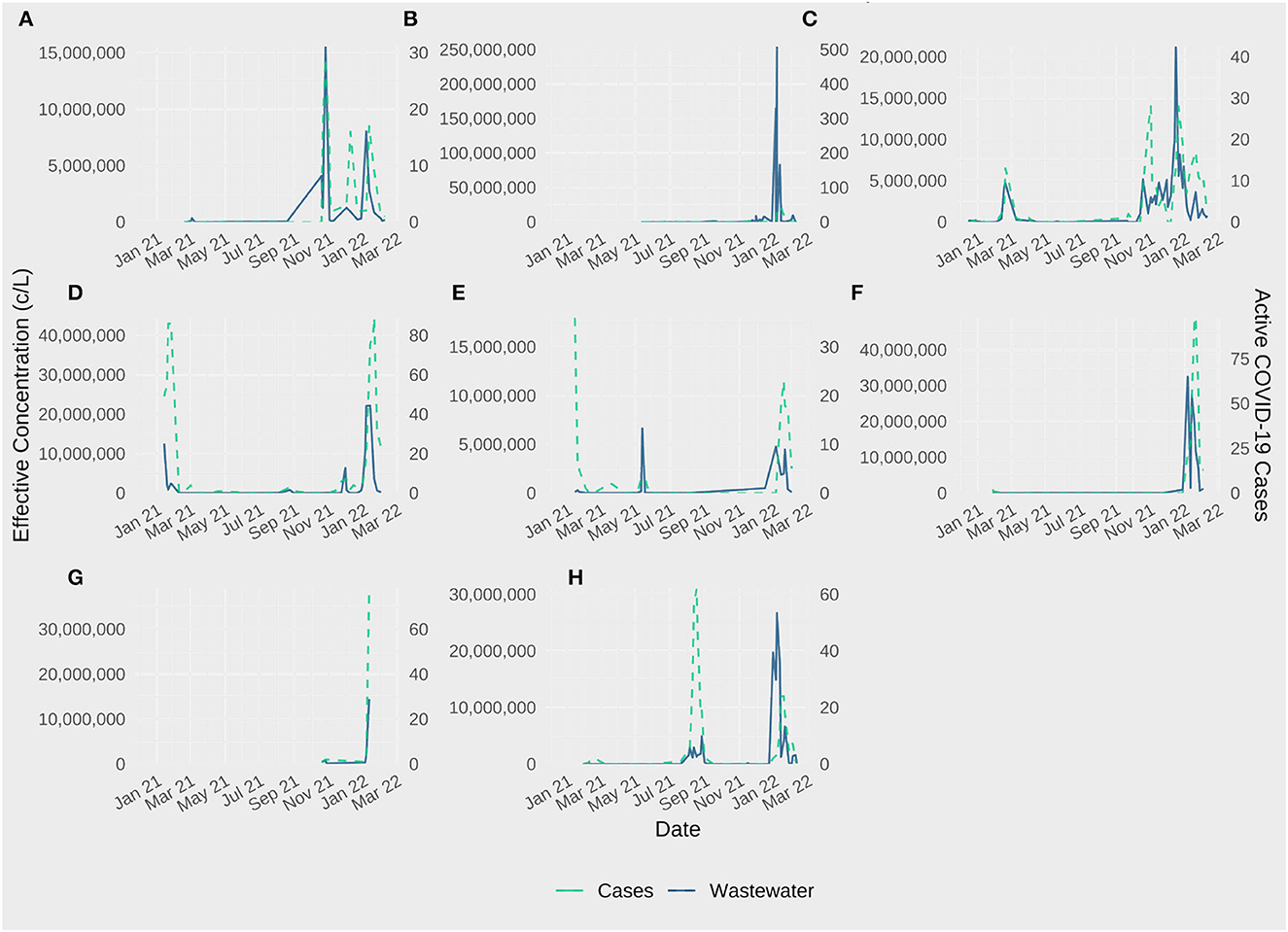
Figure 2. Effective SARS-CoV-2 concentration and active resident COVID-19 cases in Eight Massachusetts Department of Corrections Facilities (A–H), December 2020–February 2022.
3.2.1. Facility C
Facility C is unique in that it serves a short-term population with specific medical supervision needs. There are no security staff and no visitors; the flow of individuals in and out is less controlled than other correctional facilities. SARS-CoV-2 testing was conducted on intake, but other than that, no routine screening for COVID-19 was conducted among residents. Wastewater signals observed in March 2021 and over the winter 2021/2022 were noted, but did not appear to trigger active testing.
3.2.2. Facility D
Facility D is a maximum-security facility. Residents with COVID-19 symptoms were tested. Visitation was controlled and visitors screened and tested. SARS-CoV-2 testing of staff consisted of random, unannounced use of a mobile testing unit about once per month. Positive results were not routinely reported to DPH. Wastewater signals from winter months of 2021 were observed and noted, but it is unclear to what extent individuals were tested.
3.2.3. Facility E
Facility E is a large, medium security facility where symptomatic residents were also tested as needed. Visitors were screened and tested for SARS-CoV-2. Wastewater signals from June 2021 were documented with notes that testing was not increased as a result.
4. Discussion
While wastewater surveillance has documented advantages for monitoring and controlling COVID-19 at the community (Gonzalez et al., 2020; Wu et al., 2022) and college-campus levels (Harris-Lovett et al., 2021; Wong et al., 2021), to our knowledge, this is the first study to quantify a relationship at correctional facilities. Furthermore, the case studies presented here document the application and direct utility of wastewater surveillance in a variety of correctional facility settings. Cases reported to routine surveillance, as captured in our town-level data, are affected by testing patterns and unless identified by screening, underestimate asymptomatic cases. Towns and correctional facilities have dynamic interactions in that staff and visitors frequently live in neighboring towns, and residents are released to towns (Montoya-Barthelemy et al., 2020; Hassard et al., 2022); however, this study found stronger correlations between wastewater surveillance at the correctional facilities and resident cases compared to town-level surveillance and resident cases.
A special feature of building-level wastewater surveillance for controlling COVID-19 is that it is actionable in a short feedback loop (Wong et al., 2021). New evidence indicates that it can also be affordable (Liu et al., 2022), which would allow further expansion in limited resource settings such as correctional facilities. In addition, since the programmatic efforts and costs of proactive clinical screening to detect infected individuals can be challenging (Brinkley-Rubinstein et al., 2021), our finding that wastewater monitoring at correctional facilities provides value beyond town- or city-level surveillance suggests this makes a good use case. The implementation of action in response to wastewater signals requires partnerships between public health and correctional facility leadership and staff. Wastewater surveillance in this setting can be an additional signal to aid infection control efforts at correctional facilities.
Evidence of town and resident case associations were described in a study of Massachusetts prisons where crowding was also a factor (Leibowitz et al., 2021). The movement of staff and visitors in and out of correctional facilities creates a challenge in interpretating wastewater data. Safety is a high priority of the Massachusetts DOC, and visits were managed. Additionally, as recommended by others (Hawks et al., 2020; Wang et al., 2020), an attempt to reduce the number of residents in 2020 was implemented to reduce COVID-19 transmission risk. Officials began dispensing the first round of doses of the COVID-19 vaccine for DOC residents and staff in January 2021 (EOPSS and DOC, 2021). However, there are many other barriers to controlling infection transmission in correctional facilities, including physical and structural challenges (Wang et al., 2020), and facilities have different capacities in implementing mitigation efforts.
Detection of SARS-CoV-2 in wastewater is an unequivocal result of infection somewhere. However, the many sources of variability in the detection of SARS-CoV-2 in wastewater (Wade et al., 2022) create challenges in interpretating individual results and frustrations for infection control. Smaller populations (i.e., building-level) under surveillance result in uncertainty in the quantitative correlation between wastewater concentrations and cases reported. The number of infected individuals who might contribute to the wastewater on a given day is hard to quantify; in addition, viral shedding in stool is highly variable from a single individual (Acer et al., 2022). As such, we caution partners to avoid overinterpreting a single observation of high concentrations of SARS-CoV-2 in wastewater. However, our observation that high viral concentration values were usually correlated with case clusters suggest a potential value in exploring guidance for interpretation based on certain cutoffs (e.g., percent increase). In addition, the availability and implementation of rapid, on-site testing have reduced the window of timing that wastewater offered earlier in the pandemic. This reinforces the value of combining these with other sources of surveillance data.
The greatest limitation of this study is the lack of biological evidence of infection in the population over time. Our data were observational and incomplete because our outcome (cases) underestimated infections for several reasons, including disincentives associated with the identification of a positive individual, inconsistent implementation of testing in response to wastewater signals, and undetected cases among staff and residents under mostly symptom-based testing. This means that the correlation analysis might not be ideal with cases as an outcome, and that our findings are conservative.
Formal model-based assessments of the cost benefit of using wastewater surveillance to monitor SARS-CoV-2 in correctional facilities are currently being evaluated through collaboration and support of CDC with a goal to develop an interactive tool similar to COVIDTracer and COVIDTracer Advanced | CDC.
The unique population vulnerabilities, along with environmental and structural conditions of correctional facilities that make clinical testing a challenge, suggest wastewater surveillance is beneficial in this setting. In Massachusetts, a partnership between DOC and DPH intends to expand wastewater surveillance to more facilities (EOPSS and DOC, 2021), and is consistent with CDC guidance on preventing transmission of SARS-CoV-2 in correctional facilities to include consideration for wastewater surveillance at the facility level (https://www.cdc.gov/coronavirus/2019-ncov/community/correction-detention/guidance-correctional-detention.html). Our findings of consistently higher correlation values between wastewater concentration and resident infections than town cases and resident infections offer robust support for conducting wastewater surveillance as an unbiased safety net of information about facilities where testing is challenging.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
RMK contributed to the conception of the work, interpretation of the data for the work, drafted and implemented revisions to the work, provided approval for publication of the content, and is accountable for all aspects of the work. CY and SO contributed to the design of the work, analysis and interpretation of the data for the work, drafted the work, provided approval for publication of the content, and is accountable for all aspects of the work. AO, DC, JM, LC, TS, and KC contributed substantially to the conception of the work, the acquisition and interpretation of data for the work, revision of the draft critically for important intellectual content, provided approval for publication of the content, and are accountable for all aspects of the work. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors appreciate the efforts of Phu Duong Program Coordinator, Research and Planning Division and Jeffrey Fisher Assistant Deputy Commissioner at the Department of Corrections, and Lauren Anderson, Director of the Office of the Ombudsman for the Department of Corrections.
Conflict of interest
CY and SO were employed by Biobot Analytics, Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frwa.2023.1083316/full#supplementary-material
References
Acer, P. T., Kelly, L. M., Lover, A. A., and Butler, C. S. (2022). Quantifying the relationship between SARS-CoV-2 wastewater concentrations and building-level COVID-19 prevalence at an isolation residence: a passive sampling approach. Int. J. Environ. Res. Public Health 19, 11245. doi: 10.3390/ijerph191811245
Brinkley-Rubinstein, L., Peterson, M., Martin, R., Chan, P., and Berk, J. (2021). Breakthrough SARS-CoV-2 infections in prison after vaccination. N. Engl. J. Med. 385, 1051–1052. doi: 10.1056/NEJMc2108479
Duvallet, C., Wu, F., McElroy, K. A., Imakaev, M., Endo, N., Xiao, A., et al. (2022). Nationwide trends in COVID-19 Cases and SARS-CoV-2 RNA wastewater concentrations in the United States. ACS EST Water 2022, 434. doi: 10.1021/acsestwater.1c00434
EOPSS DOC (2021). DOC Launches Vaccination, Electronic Monitoring Programs as Health and Safety Strategies. Available online at: https://www.mass.gov/guides/doc-coronavirus-information-guide (accessed September 30, 2022).
Gonzalez, R., Curtis, K., Bivins, A., Bibby, K., Weir, M. H., Yetka, K., et al. (2020). COVID-19 surveillance in Southeastern Virginia using wastewater-based epidemiology. Water Res. 186, 116296. doi: 10.1016/j.watres.2020.116296
Guescini, M., Sisti, D., Rocchi, M. B., Stocchi, L., and Stocchi, V. A. (2008). A new real-time PCR method to overcome significant quantitative inaccuracy due to slight amplification inhibition. BMC Bioinform. 9, 326. doi: 10.1186/1471-2105-9-326
Harris-Lovett, S., Nelson, K. L., Beamer, P., Bischel, H. N., Bivins, A., Bruder, A., et al. (2021). Wastewater surveillance for SARS-CoV-2 on college campuses: initial efforts, lessons learned, and research needs. Int. J. Environ. Res. Public Health 18, 4455. doi: 10.3390/ijerph18094455
Hassard, F., Smith, T. R., Boehm, A. B., Nolan, S., O'Mara, O., Di Cesare, et al. (2022). Wastewater surveillance for rapid identification of infectious diseases in prisons. Lancet Microbe. 3, e556–e557. doi: 10.1016/S2666-5247(22)00154-9
Hawks, L., Woolhandler, S., and McCormick, D. (2020). COVID-19 in Prisons and Jails in the United States. JAMA Intern. Med. 180, 1041–1042. doi: 10.1001/jamainternmed.2020.1856
Leibowitz, A. I., Siedner, M. J., Tsai, A. C., and Mohareb, A. M. (2021). Association between prison crowding and COVID-19 incidence rates in massachusetts prisons, April 2020-January 2021. JAMA Intern. Med. 181, 1315–1321. doi: 10.1001/jamainternmed.2021.4392
Liu, P., Ibaraki, M., VanTassell, J., Geith, K., Cavallo, M., Kann, R., et al. (2022). A sensitive, simple, and low-cost method for COVID-19 wastewater surveillance at an institutional level. Sci. Total Environ. 807 (Pt 3), 151047. doi: 10.1016/j.scitotenv.2021.151047
Marquez, N., Ward, J. A., Parish, K., Saloner, B., and Dolovich, S. (2021). COVID-19 incidence and mortality in federal and state prisons compared with the US population, April 5, 2020 to April 3, 2021. JAMA 326, 1865–1867. doi: 10.1001/jama.2021.17575
Montoya-Barthelemy, A. G., Lee, C. D., Cundiff, D. R., and Smith, E. B. (2020). COVID-19 and the correctional environment: the american prison as a focal point for public health. Am. J. Prev. Med. 58, 888–891. doi: 10.1016/j.amepre.2020.04.001
R Core Team (2022). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: https://www.R-project.org/ (accessed September 14, 2022).
Simpson, P. L., and Butler, T. G. (2020). COVID-19, prison crowding, and release policies. BMJ 369, m1551. doi: 10.1136/bmj.m1551
Troppy, S., Haney, G., Cocoros, N., and Cranston, K. (2014). Alfred DeMaria Jr. infectious disease surveillance in the 21st Century: an integrated web-based surveillance and case management system. Public Health Rep. 129, 132–8. doi: 10.1177/003335491412900206
Wade, M. J., Lo Jacomo, A., Armenise, E., Brown, M. R., Bunce, J. T., Cameron, G. J., et al. (2022). Understanding and managing uncertainty and variability for wastewater monitoring beyond the pandemic: lessons learned from the United Kingdom national COVID-19 surveillance programmes. J. Hazard Mater. 424(Pt B), 127456. doi: 10.1002/essoar.10507606.2
Wang, E. A., Western, B., and Berwick, D. M. (2020). COVID-19, Decarceration, and the role of clinicians, health systems, and payers: a report from the national academy of sciences, engineering, and medicine. JAMA 324, 2257–2258. doi: 10.1001/jama.2020.22109
Wong, T. E., Thurston, G. M., Barlow, N., Cahill, N. D., Carichino, L., Maki, K., et al. (2021). Evaluating the sensitivity of SARS-CoV-2 infection rates on college campuses to wastewater surveillance. Infect. Dis. Model 6, 1144–1158. doi: 10.1016/j.idm.2021.09.003
Wu, F., Xiao, A., Zhang, J., Moniz, K., Endo, N., Armas, F., et al. (2022). SARS-CoV-2 RNA concentrations in wastewater foreshadow dynamics and clinical presentation of new COVID-19 cases. Sci. Total Environ. 805, 150121. doi: 10.1016/j.scitotenv.2021.150121
Keywords: SARS-CoV-2, correctional facility, wastewater-based epidemiology, disease surveillance, COVID-19
Citation: Klevens RM, Young CCW, Olesen SW, Osinski A, Church D, Muten J, Chou L, Segal T and Cranston K (2023) Evaluation of wastewater surveillance for SARS-CoV-2 in Massachusetts correctional facilities, 2020–2022. Front. Water 5:1083316. doi: 10.3389/frwa.2023.1083316
Received: 28 October 2022; Accepted: 06 January 2023;
Published: 26 January 2023.
Edited by:
Ariel-Kushmaro, Ben-Gurion University of the Negev, IsraelReviewed by:
Nuhu Amin, University of Technology Sydney, AustraliaSihem Jebri, National Center for Nuclear Science and Technology, Tunisia
Copyright © 2023 Klevens, Young, Olesen, Osinski, Church, Muten, Chou, Segal and Cranston. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: R. Monina Klevens,  bW9uaW5hLmtsZXZlbnNAbWFzcy5nb3Y=
bW9uaW5hLmtsZXZlbnNAbWFzcy5nb3Y=
 R. Monina Klevens
R. Monina Klevens Cristin C. W. Young2
Cristin C. W. Young2 Scott W. Olesen
Scott W. Olesen Anthony Osinski
Anthony Osinski