- 1School of Engineering, Deakin University, Waurn Ponds, VIC, Australia
- 2Centre for Regional and Rural Futures, School of Life and Environmental Sciences, Deakin University, Burwood, VIC, Australia
Microbial communities play a vital role in nitrogen (N) removal in constructed wetlands (CWs). However, the lack of studies on microbial characteristics of wetland systems designed to treat stormwater demonstrates the importance of comprehensive investigation on microbial response to wetland fluctuations. Moreover, the observed inconsistency in N removal, and detected links between microbial shifts and wetland water level fluctuations is an area of research interest perculiar to stormwater applications. This study surveyed nearly 150 publications to provide a summary and evaluation of N removal efficiency in different types of CWs where microbial communities and their behavior have been correlated to regulating factors. Factors such as flow regime, plants, and physico-chemical properties (e.g., temperature, dissolved oxygen, pH, and nitrogen concentration) were found to significantly influence microbial diversity and composition. Although many studies have analyzed microbial N removal, a majority conducted their studies in bioretention systems. Accordingly, some of the microbial pathways in CWs designed for stormwater treatment have not been investigated. As such, it is suggested that pathways, such as dissimilatory nitrate reduction to ammonium (DNRA) and comammox activity and their changes over dry-wet cycles in stormwater constructed wetlands be investigated. This information could assist engineers to take advantage of the presence of other N transforming communities which could improve microbial diversity within wetland systems. Moreover, it is recommended to track microbial functional genes and their changes over wetland water fluctuation to develop an ecosystem with conditions favorable for microbial pathways with higher N removal potential. In conclusion, the findings of the current literature review reinforce the importance of stormwater runoff treatment and the implementation of new design strategies that are able to enhance microbial activity and diversity leading to a better treatment outcome.
Introduction
Excessive urbanization has led to increases in the volume and rate of stormwater runoff due to the increase in imperviousness (Janke et al., 2017; Ma et al., 2021). Moreover, excessive levels of nutrients contained in stormwater have caused hypoxia, eutrophication (Hobbie et al., 2017), and algal blooms in water bodies (Glibert and Burford, 2017; Paerl et al., 2018). Urban water management approaches have therefore been designed for the harvesting and reusing stormwater via environmentally sustainable approaches (Prüss, 1998; Johnson et al., 2003; Hörman et al., 2004; Noble et al., 2006; USEPA, 2010; Converse et al., 2011). Thus, constructed stormwater wetlands as a green and cost-effective approach have been widely used to eliminate stormwater pollutants to safely discharge treated urban runoff into natural water bodies (Malaviya and Singh, 2012; Tippler et al., 2012; Fu et al., 2016; Hu et al., 2016).
Constructed wetlands time have the potential to reduce peak flow rates and eliminate stormwater pollutants (Converse et al., 2011; Garfí et al., 2012; Sani et al., 2013; Vymazal, 2014; Wu et al., 2015; Fu et al., 2016; Hu et al., 2016; Huang et al., 2018). The system mitigates the effects of added nitrogen via physical, chemical, and biological processes including filtration, sedimentation, volatilization, plant uptake, and microbial mediated mechanisms. While some of the CWs have been shown to exhibit high N removal rates, there is a wide variation in removal rates (Table 1). It has been demonstrated that N removal mechanisms are influenced by factors such as type of CW, wetland vegetation, wetland hydrology, physico-chemical and wetland biological properties (Malaviya and Singh, 2012; Tippler et al., 2012; Fu et al., 2016; Hu et al., 2016; Griffiths and Mitsch, 2020; Zhao and Piccone, 2020; Wei et al., 2021).
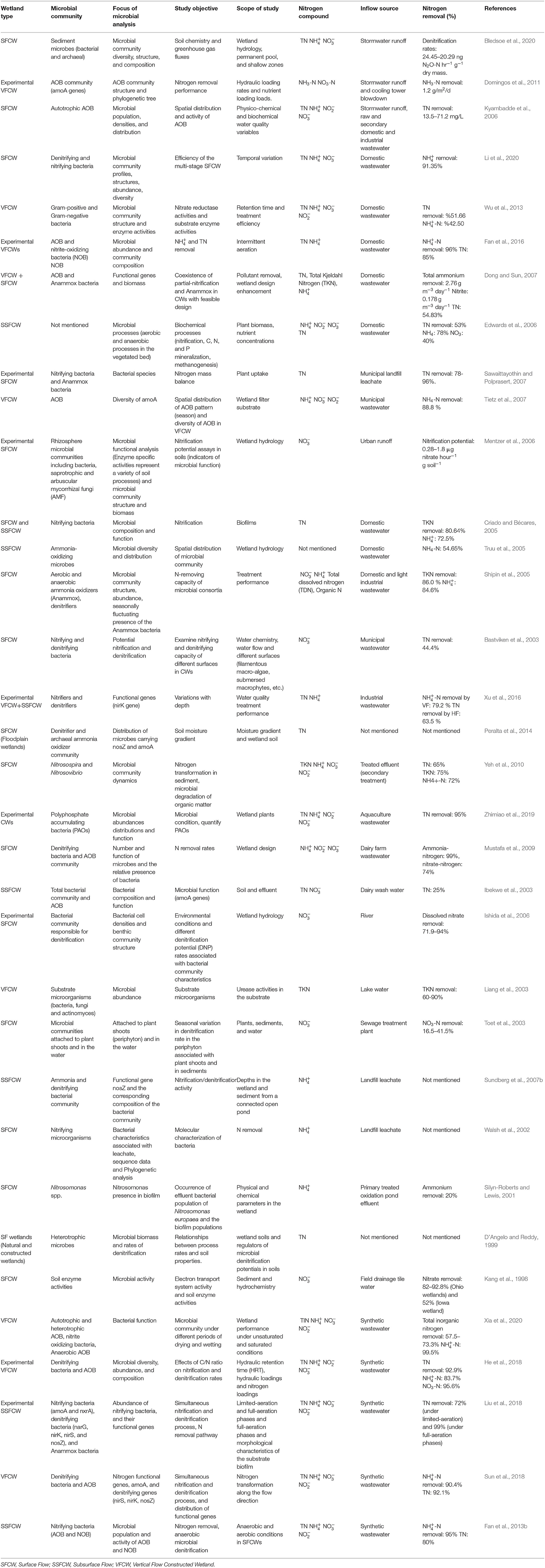
Table 1. Summary of studies investigating microbial communities responsible for N transformation in different types of CWs.
Microbial pathways play a primary role in N removal, and their diversity and community composition reflect the proper functioning and maintenance of CWs. Consequently, the inconsistency in N removal rates could be correlated to the variation in microbial community and their response to the wetland design factors and operational condition. Thus, a review of the literature was undertaken with the aim of identifying microbial communities present and their role in the treatment processes in CWs designed to capture stormwater. The objectives of this study were: (i) Provide a literature review where microbiological studies have been carried out for constructed wetlands, (ii) Understand the function of microbial communities and their role in the treatment process, and (iii) Identify research gaps and provide recommendations for future studies. The overall result of this review will assist water managers to improve urban water management to meet water quality standards.
Treatment of Stormwater Runoff
Previous studies have analyzed microbial pathway of N removal, communities present, and their response to wetland hydrology, hydraulic loading rates, nutrient concentrations, physico-chemical parameters, retention time, intermittent aeration, and plant biomass using advanced molecular techniques. However, a large portion of the literature deals with the treatment of domestic and industrial wastewater, while the current study focuses mainly on CWs that are designed to treat stormwater. The review therefore has been expanded to include these systems, highlighting features that are peculiar to stormwater as it is unique in many respects compared to other influents (Lucke et al., 2018; Wang et al., 2022). The total nitrogen concentration in stormwater is usually 10 times lower than in domestic wastewater; this difference will be higher when compared against industrial wastewater and agricultural runoff as they are often linked to the specific land use of the catchment (Lucke et al., 2018). Another feature of stormwater that is unique is the intermittent nature since inflows occur only after rain events. A summary of studies investigating microbial communities responsible for N transformation in surface flow, subsurface flow, and vertical flow CWs is listed in Table 1.
The reported N removal rates for various types of CWs in the literature indicate the variation in wetland N removal efficiency. The reason(s) behind the inconsistency could be linked to the variations in microbial communities, suggesting that CWs are not in optimal performance, but also differences in application, which makes comparison difficult. As shown in Table 1, individual studies have focussed on different types of wetland systems and different microbial communities. The variations of microbial N removal are also linked to the types and quality of inflow source including raw or treated domestic wastewater, industrial effluent, and urban runoff.
A majority of studies on N transforming microbial community in CWs have been carried out for domestic (Bastviken et al., 2003; Criado and Bécares, 2005; Shipin et al., 2005; Truu et al., 2005; Dong and Sun, 2007; Sawaittayothin and Polprasert, 2007; Tietz et al., 2007; Wu et al., 2013; Fan et al., 2016; Li et al., 2020) or synthetic wastewater (Fan et al., 2013b; He et al., 2018; Liu et al., 2018; Sun et al., 2018; Xia et al., 2020). There are limited studies focussing on microbial community in CWs treating stormwater and the influence of wetland design features on N transforming microbes (Kyambadde et al., 2006; Domingos et al., 2011; Bledsoe et al., 2020). For example, Bledsoe et al. (2020) emphasized the importance of microbial controls on biogeochemical processes in stormwater constructed wetlands in order to improve water quality. They found that reduction of flooded areas and increasing shallow land area in a stormwater constructed wetland can enhance N removal while reducing greenhouse gas emissions. Changes in water chemistry were also examined to assess nutrient transformations following rewetting and recovery of microbial communities in CWs treating stormwater (Macek et al., 2020). Wang et al. (2022) have investigated N composition in stormwater runoff samples by studying the chemical properties before and after storm events in order to improve N pollution management. Another study conducted by Walaszek et al. (2018) considered the impact of dry/wet weather on variations in hydrologic conditions, physico-chemical parameters, and heavy metals loads.
Microbial diversity and its effect on N removal have been investigated in different types of treatment systems including bioretention cells (Zuo et al., 2020; Biswal et al., 2021), stormwater detention basin (Morse et al., 2017). Other studies such as Sun et al. (2018) focused on microbial functional genes considering denitrifying bacteria, ammonium oxidizing bacteria (AOB), and their distribution to analyse N removal efficiency in a vertical flow CW. Bledsoe et al. (2020) studied denitrification rates and microbial community structure within permanently flooded and temporarily flooded areas of a surface flow constructed wetland treating stormwater runoff to examine N removal potential. A recently discovered microbial pathway, dissimilatory nitrate reduction to ammonium (DNRA) in CWs treating stormwater has also been proposed by Rahman et al. (2019a,b,c).
Regarding stormwater control measures, it has been found that there is insufficient studies focused on constructed wetlands to understand how the types of microbial community present and their behavior over different cycles of wet and dry conditions affect the N removal rates in these systems (Gold et al., 2019). The majority of studies investigate nitrogen removal from stormwater in systems such as bioretention cells (Chen et al., 2013; Norton et al., 2017; Waller et al., 2018; Biswal et al., 2021), wet ponds (Blaszczak et al., 2018; Gold et al., 2021), wet/dry detention basins (McPhillips and Walter, 2015; Morse et al., 2017) or filtration/infiltration basins (Bettez and Groffman, 2012) (Table 1). Stormwater control measures such as bioretention cells have been assessed for the impact of operational conditions, climatic conditions, wet-dry alternation, influent loads, N concentration, and hydraulic residence time on wetland microbial community. The measures or strategies for increasing N removal are then proposed from the perspectives of structural improvement of the bioretention system, optimization of media composition, and enhancement of the N removal reaction processes. Moreover, while different types of CWs have been analyzed for microbial N removal from wastewaters and correlating behavior with wetland design factors and operational condition, there is an apparent lack of studies focused on constructed wetlands designed for stormwater treatment.
Nitrogen Transformation in CWs
Constructed wetlands contain diverse groups of microorganisms which are critical for the proper functioning and maintenance of the system (Sims et al., 2012; Adrados et al., 2014; Ligi et al., 2014; Cao et al., 2017; Zhang et al., 2020). However, the extent of treatment depend upon microbial N transforming processes (Fernandes et al., 2015; Ibekwe et al., 2016; Cao et al., 2017; Fu et al., 2017; Gold et al., 2019; Zhang et al., 2020). Microbial communities and their responses to the wetland types, hydrologic dynamics and physico-chemical variables have been investigated in previous studies to understand their role in N removal. The presence of diverse microbial groups enables CWs to better cope with environmental changes and by the proliferation of organisms which are better adapted to the new conditions (Ibekwe et al., 2003).
One of the major N treatment mechanisms in CWs occurs via microbial interactions with different forms of nitrogen. A significant proportion of pollutants containing nitrogen are firstly converted to inorganic ammonia nitrogen (-N) during ammonification process in both aerobic and anaerobic conditions. Thereafter, -N is mainly removed by nitrification-denitrification processes in CWs under sequential oxidative stages. Nitrification implies a chemolithoautotrophic oxidation of ammonia to nitrate (NO) (ammonia oxidation) performed by microbes such as Nitrosomonas under strict aerobic conditions. Then, denitrifying bacteria (denitrifiers) such as Psuedomonas, Micrococcus, Achromobactor and Bacillus reduce nitrite and nitrate into nitrogen gas under anaerobic or anoxic conditions (Lee et al., 2009). The comammox bacteria, members of the genus Nitrospira are also involved in N transformation (Daims et al., 2016). This genus contains full genetic complement for both ammonia and nitrite oxidation which could directly convert ammonium and nitrite to nitrate without nitrous oxide production. However, there are limited studies investigating the presence of comammox pathway in CWs (van Kessel et al., 2015; Pelissari et al., 2018; Sun et al., 2020; Zhang et al., 2021) and further research is needed to understand the relative importance of comammox process in N removal in CWs in comparison to other removal mechanisms (Zhang et al., 2021). Microbial N removal via denitrification accounts for as much as 90% of overall N removal (Lin et al., 2002; Faulwetter et al., 2009; Li et al., 2018a). Anammox (anaerobic ammonium oxidation) process has also been identified as an alternative processes in total nitrogen removal (Mulder et al., 1995). Anammox bacteria perform denitrification and transform -N into nitrogen gas without the need for aeration and addition of an external carbon source (Ward, 2003). Relatively high TN removals via this process have been reported (Dong and Sun, 2007; Sun and Austin, 2007). Stormwater does not contain sufficient biodegradable carbon or an external organic source to carry out denitrification. The co-function of different groups of N transforming microbes in CWs along with optimal operating parameters could potentially enhance the N removal performance of CWs (Third et al., 2001).
Among the environmental variables, pH, DO, and nitrogen concentration could potentially change the spatial distribution of microbial community structures, contributing to a change in microbial composition (Wang et al., 2020a). Wetland characteristics and components such as plants, organic carbon content (Sun et al., 2012; Liao et al., 2019), DO (Jianlong and Ning, 2004), redox condition (Faulwetter et al., 2009), temperature and pH have significant influences on microbial N removal (Kozub and Liehr, 1999; Sirivedhin and Gray, 2006). For example, denitrification rates strongly depend on NO concentration, type and quality of organic carbon sources, periods of wetting and drying, plant residues, redox potential, soil type, the presence of overlying water, temperature, and pH (Vymazal, 1995; Bastviken et al., 2005; Sirivedhin and Gray, 2006). Denitrifying bacteria have been identified as the main bacteria group at elevated levels of organic carbon in CWs while ammonium oxidizing bacteria (AOB) are the main bacteria group in the absence of organic carbon (Sun et al., 2012). Nutrient and organic carbon concentrations have been considered as one of the main drivers of diversity of microbial communities. The predominant anoxic–anaerobic condition within CWs increase the level of denitrification which is a key process of N removal (Vymazal, 2007). Observed variations in microbial communities have also been correlated with dry-weather flow or additional inter-wetland pollution sources (Staley et al., 2015; Huang et al., 2018) as the function, diversity and abundance of N transforming microbes depend on type of CWs, their design and operation conditions (Andersson et al., 2002; Kadlec, 2009; Peralta et al., 2010; Pelissari et al., 2017).
Factors Regulating Microbial Communities in CWs
Type of Constructed Wetland
Constructed wetlands have traditionally been categorized based on their hydrological regime and the resultant flow characteristic (Vymazal, 2007). The type and magnitude of N removal processes can vary based on the type of CWs as microbial growth and activity depend on wetland design and operational condition (Vymazal, 2013b). For example, N removal rates can vary from 40 to 50% with removed loads ranging between 250 and 630 g N m−2 yr−1 (Vymazal, 2007). The influence of wetland flow characteristics on microbial structure and function have been previously investigated by Peralta et al. (2010) who found that natural wetlands had significantly different assemblages of denitrifiers in comparison to restored wetlands. The comparison of denitrifying genes (narG, nirS, nosZ) in sediments within two different types of wetlands, estuarine vs. wastewater effluent-fed CWs, indicated that nitrite-reducing gene nirS was dominant over narG and nosZ in the effluent-fed wetland (Chon et al., 2011). Moreover, the abundance of specific microbial communities such as Nitrosomonas spp. as the N transforming bacteria can vary based on the type of CWs (Flood et al., 1999; Silyn-Roberts and Lewis, 2001). The population and composition of AOB were found to be different in vertical and horizontal CWs, with a higher percentage of Nitrosospira-like sequences (Ibekwe et al., 2003; Gorra et al., 2007; Tietz et al., 2007) in comparison to an overland flow system which did not show any changes in AOB composition (Sundberg et al., 2007a).
Specific design features of conventional wetland systems can bring about changes to N removal and alter the main removal mechanisms in wetlands. Vertical subsurface flow CWs (VSSF-CWs) with partially saturated conditions operating under low organic loads favored simultaneous nitrification and denitrification processes (Pelissari et al., 2017). Moreover, VFCWs with high redox potentials favor aerobic microbial processes such as ammonification and nitrification (Kadlec et al., 2000). The comparison between VF, SF and HFCWs showed a significantly higher biochemical oxygen demand removal and nitrification but relatively lower denitrification in VF-CWs than the SF and HFCWs (Vymazal, 2007). Nitrification and denitrification processes have been shown to be the major N removal mechanisms in HSSF-CWs rather than volatilization, adsorption, and plant uptake (Cooper et al., 1997; Vymazal, 2007).
Plants
A wide range of technologies from biofiltration systems (Shirdashtzadeh et al., 2017; Wu et al., 2017) to CWs (Wu et al., 2016) have employed plants to treat wastewater because of their complex interactions with pollutants and microorganisms (Harris et al., 1996; Abal et al., 2002; Brix, 2020). Indeed, plants are one of the most ubiquitous components of CWs, and can directly uptake and assimilate nutrients (Vymazal, 2007, 2013a; López et al., 2016), stabilize the bed surface, serve as substrate for microbial attachment, release oxygen (Peng et al., 2014) and stimulate the growth of N transforming microbes (Li et al., 2018b; Han et al., 2020). Plants also regulate hydraulic conditions and reduce wind stress, which supports sedimentation and prevents re-suspension (Dieter, 1990; Vymazal, 2013a). Nitrogen attenuation by plants is a process encompassing several steps including uptake, assimilation, translocation and as the plant is aging, recycling and remobilization while the main process is the absorbance of nitrogen from the soil in the form of nitrate (NO) and ammonium (-N). However, the studies on N take up by plants and microorganisms reveal that microbes may take up different N forms than plants which may lead to less competition (Schimel and Chapin, 1996; Kaye and Hart, 1997; Hodge et al., 2000; Lipson and Näsholm, 2001).
Biofilms created by submerged plants (or a part of plants) provide large surfaces for microcolonies which are composed of different bacterial communities such as denitrifiers (Zhang et al., 2016). In this regard, microbial communities of planted wetland systems have been found to have higher number of soil microbes and higher activities of soil enzymes (Salvato et al., 2012) accompanied by higher N removal rates (Lin et al., 2002; Yousefi and Mohseni-Bandpei, 2010), compared to unplanted wetlands (Lai et al., 2011; Huang et al., 2012; Boog et al., 2014). The enhanced microbial density, activity, and diversity in the plant rhizosphere create an effective N transformation zone (Picard et al., 2005; Kadlec and Wallace, 2008; Faulwetter et al., 2009; Truu et al., 2009) due to the abundance of organic matter (Gagnon et al., 2007; Truu et al., 2009; Xu et al., 2017) and the presence of the oxygenated root zone (Lai et al., 2012; Peng et al., 2014). The oxygenated root zone supports aerobic processes even under flooded conditions with a bulk of influent NO reduced in the vicinity of plant roots (Han et al., 2020).
Microbial functional genes involved in NO reduction processes such as denitrification, Anammox, dissimilatory nitrate reduction to ammonium (DNRA), and denitrifying anaerobic methane oxidation (DAMO)] (Wang et al., 2020b) and nitrification (Stottmeister et al., 2003) have been found in the plant rhizosphere. The rhizosphere mosaic characteristic of aerobic and anaerobic zones assist the process of nitrification and denitrification (Zhu et al., 2010). For example, greater numbers of nitrifying bacteria and higher bacterial activity were detected on plant roots compared to the bulk matrix (Kyambadde et al., 2006). The rhizosphere denitrifying bacteria had different community structure from bulk sediment revealed by PCR-denaturing gradient gel electrophoresis (DGGE) of nosZ. The results demonstrate the effect of vegetation on the functioning and structure of bacterial communities involved in N removal in CWs (Ruiz-Rueda et al., 2009).
Plants also play a significant role in the removal of via nitrification-denitrification pathways (Coban et al., 2015). However, the rates of N removal such as denitrification can vary significantly among vegetation communities especially in less aerated systems (Vymazal, 1995; Toet et al., 2003; Bastviken et al., 2005; Sirivedhin and Gray, 2006; Hernandez and Mitsch, 2007). Wetland vegetation density has been found to control microbial community composition (Toscano et al., 2015; Zheng et al., 2016; Han et al., 2020), diversity (Ibekwe et al., 2007) and distribution (e.g., ammonia oxidizers and denitrifiers) (Zhang et al., 2017) via enhancing wetland retention time (Jadhav and Buchberger, 1995; Vymazal, 2013a).
Organic Carbon and Nutrient Concentration
Nutrient concentration can affect diversity, composition, and structure of microbial communities (Hu et al., 2014). It has been reported that nutrient availability can increase the richness of microorganisms, as water samples from natural sites contain higher bacterial richness and diversity than sediment and surface water sampled from urban runoff (Logue et al., 2012). However, other studies reported that nutrient availability can reduce the diversity of microbial communities (Van Horn et al., 2011). Among the different types of nutrients, total phosphorus (TP) and NO have been recognized as the main factors affecting the composition of microbial communities in urban aquatic systems (Wang et al., 2018). A higher concentration of NO has been correlated with increased occurrence of denitrifying genes (Zhang et al., 2016), higher denitrification rates in sediments (Vymazal, 1995; Bastviken et al., 2005; Sirivedhin and Gray, 2006) and more vigorous and robust populations of denitrifiers within wetland sediments (Vymazal, 1995; Bastviken et al., 2005; Sirivedhin and Gray, 2006).
Microbial denitrification is considered as the dominant N removal process at high NO loading rates (Spieles and Mitsch, 1999; Xu et al., 2017) while low concentration of NO results in coupled nitrification-denitrification as the major removal process in CWs. The rate of DNRA in CWs has been related to NO loss (Kendall et al., 2007), while the presence of both and NO can inhibit NO uptake by microorganisms (Coban et al., 2015). Nitrosospira spp. has been detected as the prevalent group in low-ammonia () environments (Kowalchuk et al., 2000; Bäckman et al., 2003), while Nitrosomonas species is the prevalent bacterial group at high concentrations (Schramm et al., 1996; Juretschko et al., 1998; Okabe et al., 1999; Purkhold et al., 2003; Sundberg et al., 2007a; Tietz et al., 2007).
The abundance of N transforming microbes has a significant correlation with organic matter content (Vymazal, 1995; Hernandez and Mitsch, 2007; Sundberg et al., 2007b; Kim et al., 2016), their availability and degradability potential (Kozub and Liehr, 1999; Sirivedhin and Gray, 2006). Dissolved organic matter (DOM) has been found as one of the major contributing factor to the microbial variation, composition and structure in surface waters (Logue and Lindström, 2008; Pang et al., 2014). The variation in loading rates of organic carbon has been found to influence microbial diversity (e.g., nitrifiers) and increase the competition among different microbial groups (Thompson et al., 1995; Schramm et al., 1996; Grunditz et al., 1998; Kowalchuk et al., 2000; Webster et al., 2002; Truu et al., 2005), with an uneven distribution of available organic matter known to cause a sparse and heterogeneous distribution of microbes in CWs. The chemical oxygen demand to nitrogen ratio (COD/N) has been used as an assessment method to represent the efficiency of wetland N removal (Wu et al., 2009; Rodziewicz et al., 2019).
Higher levels of diversity and abundance of AOB have been detected at higher COD (Prosser, 1990; Van Niel et al., 1993; Hunt et al., 2003; Zhao et al., 2011; Fan et al., 2013a; Si et al., 2019). Heterotrophic denitrification is negatively influenced by low carbon-to-nitrogen ratios (mol/mol) (Lee et al., 2009; Wu et al., 2009), while denitrification in some CWs have been significantly enhanced with increased COD/N because of enrichment by denitrifiers in CWs (Xu et al., 2017). The limitation of COD and the presence of favor Anammox bacteria, where the abundance of amoA has been correlated with N removal efficiency (Zhang et al., 2017). Microbial N transforming processes in wetland bed sediments such as ammonification, nitrification and denitrification depend on characteristics of bed sediment such as nutrient availability (Vymazal, 1995; Bastviken et al., 2005; Sirivedhin and Gray, 2006; Truu et al., 2009; Repert et al., 2014). Soil nutrient concentration can significantly impact N removal, for example, wetlands with mineral soils showed more rapid N removal rates compared to those with organic soils (Gale et al., 1993). Moreover, some types of soil can provide better sources of carbon to promote microbial processes in CWs (Dordio and Carvalho, 2013).
Temperature
Temperature correlates strongly with N removal in CWs by regulating microbial processes as metabolic rates at higher temperatures generally result in higher microbial growth and activity (Zhang et al., 2008; Faulwetter et al., 2009). Different functional groups of microbes have different growth temperature optima, for example, ammonia-oxidizing bacteria grow faster than nitrite oxidizing bacteria at temperatures above 15°C while nitrite oxidizing bacteria growth can be inhibited at 25°C (Paredes et al., 2007). Nitrosomonas and Nitrobacter require temperatures above 5°C for their growth (Cooper et al., 1996) while temperatures lower than 10°C inhibit nitrification (Herskowitz et al., 1987; Xie et al., 2003). Studies have reported different optimal temperatures for nitrification (Vymazal, 1995; Paul, 2014). Truu et al. (2009) reported a range of 28–36°C for nitrification while other studies reported optimal temperature to be as low as 0 to 5°C (Sundblad and Wittgren, 1991; Sundberg et al., 2007a). However, nitrifying communities can adapt to temperature variations and may maintain their activity at lower temperatures via their metabolic adaptation (Cookson et al., 2002).
Seasonal temperature increases of more than 6°C have been found to have a significant impact on and total inorganic N removal via simultaneous partial nitrification and Anammox process (He et al., 2012). It has been reported that bacteria conducting nitrification and denitrification are sensitive to lower temperatures. However, bacteria in the upper layers were more sensitive to the temperature changes than bacteria in the lower layers of vertical flow filter systems (VSSF-CW) (Truu et al., 2009). Denitrification can only occur above 5°C in CWs (Herskowitz et al., 1987; Brodrick et al., 1988; Werker et al., 2002) with complete denitrification accomplished at 52°C (Liao et al., 2018). The increase of temperature from 4 to 25°C was found to have the largest positive effect on the potential rate of denitrification (increasing from 0.0021 to 0.8100 kg N2O/kg sediment per day) (Sirivedhin and Gray, 2006). Although low temperatures (<5°C) can suppress denitrification in CWs (Werker et al., 2002; Burchell et al., 2007), no seasonal or spatial influences were observed on bacterial abundance or diversity, and lower temperatures did not change the N removal rates during winter period (Kern, 2003).
Liao et al. (2018) investigated denitrifying genes (nirK, nirS, narG, and nosZ) in CWs and found that temperature changes can cause shifts in microbial structure, diversity, and abundance. It has also been reported that sensitivity of microbial processes in CWs to temperature variations are higher in dry conditions compared to flooded conditions (Nurk et al., 2005). Low temperature was found to have a negative effect on the number of nitrifying bacteria and nitrification in a HFCW during the winter period (Kern, 2003). However, the impact of temperature on nitrification can be limited because low temperatures can enhance other environmental factors which are beneficial to the nitrifiers (e.g., increased redox potential). Heterotrophic activity of microorganisms is not likely to be impacted by temperature variation probably due to the mixed populations contributing to activities at different temperatures (Tao et al., 2007). Lastly, the sensitivity of N removal processes to temperature may depend on wastewater properties (Truu et al., 2009).
Dissolved Oxygen and pH
Microbial function and diversity depend on presence or absence of oxygen in CWs. Dissolved Oxygen (DO) is negatively correlated with the relative abundances of denitrifying genes (Zhang et al., 2016) and plays a significant role in N availability as it determines the oxidized and reduced forms of nitrogen. High DO levels was reported to have led to the increase in abundance of nitrifying bacteria, denitrifying bacteria, and anammox bacteria (Liu et al., 2018). DO is necessary for ammonification in the micro-gradient of aerobic and anaerobic zones of CWs, and also affects the steep redox gradients in wetlands leading to changes in the spatial distribution of microbial biomass in wetlands (Reddy and D'angelo, 1997; Scholz and Lee, 2005; Truu et al., 2009). Furthermore, the microsites with steep oxygen gradients within wetlands allow denitrification and nitrification to occur in sequence in very close proximity to each other (Kadlec and Wallace, 2008).
Aerobic nitrifying bacteria perform complete nitrification at the upper layers of a water body (Robertson and Kuenen, 1984; Kern, 2003; Nurk et al., 2005) or the oxygenated zones of rhizosphere (Zhu and Sikora, 1995; Martin et al., 1999; Münch et al., 2005) because of the presence of adequate amount of oxygen (Hammer, 1986; Tchobanoglous et al., 1991; Vymazal, 1995). At the water-sediment interface, which is characterized by an oxygen gradient, aerobic reactions can take place near the surface while anaerobic reactions take place at the deeper layer of bottom sediments (Reddy et al., 1984). Thus, the depth that microbial communities occupy in the bed sediments of CWs plays a significant role in defining the aerobic/anaerobic processes (Wei et al., 2021).
pH plays a vital role in development of microbial communities (Paredes et al., 2007; Vymazal, 2007; Mayes et al., 2009; Saeed and Sun, 2012), as ammonification, nitrification, denitrification and Anammox are all pH dependent processes (Vymazal, 1995; Bastviken et al., 2005; Sirivedhin and Gray, 2006; Wang et al., 2017). The optimal pH for ammonification was found to be 6.5 to 8.5 (Reddy et al., 1984; Vymazal, 1995; Saeed and Sun, 2012). The ideal pH for nitrifying bacteria ranges between 7.2 and 9.0 (Tchobanoglous et al., 1991; Cooper et al., 1996; Paredes et al., 2007; Vymazal, 2007), while pH between 7.5 and 7.8 can result in partial nitrification over nitrite oxidation (He et al., 2012). The optimal pH of denitrification ranges from 6 to 8, while it reduces at pH 5, and is negligible below pH 4 (Vymazal, 2007). Although pH can affect nitrification rates, its influence is reported to be negligible under neutral pH conditions (Reddy et al., 1984). Microbial mediated processes can also change the pH, as denitrification can increase pH by leading to higher wetland alkalinity.
Hydrology and Hydraulics of Constructed Wetlands
Constructed wetlands experience different hydrologic and hydraulic behavior as well as different degrees of drying and re-wetting conditions. The impact of dry-weather condition and storm events on N transforming microbes in CWs have been studied by Converse et al. (2011), Vymazal (2014), Wu et al. (2015), Huang et al. (2018). Variations in wetland hydrology and hydraulics can account for the observed inconsistency in treatment rates and shifts in microbial communities (Chen et al., 2013; Bernardin-Souibgui et al., 2018; Huang et al., 2018; Kan, 2018; Rose et al., 2018).
Foulquier et al. (2013) reported that the structure of bacterial communities under dry–wet cycles differ from those found under permanently inundated conditions. Moreover, a series of dry–wet stress cycles and the reduction in soil respiration rates have been linked to microbial community composition (Fierer et al., 2003). Thus, effective N removal requires stability in hydraulic response which influences retention, high contact between water and wetland components and flow distribution (Schimel et al., 2007; Wallenstein and Hall, 2012). The retention time, a key parameter is CW design, is a measure of the average amount of time that inflow to the wetland is retained before final discharge and has been shown to affect microbial community structure, composition, and abundance (Toet et al., 2005; Faulwetter et al., 2009).
Water level fluctuations is one of the key characteristics of CWs that can cause shifts in microbial communities as microbial composition, structure, and function are sensitive to the depth, frequency, and duration of fluctuations. The dynamics of wetland hydrology not only alter the response of wetland systems, but also alter the microbial genes responsible for N removal pathways (Bambauer et al., 1998; Fritsche et al., 1999; Kämpfer et al., 1999; Doronina et al., 2010; Drury et al., 2013; Eichmiller et al., 2013; Liu et al., 2013; Chen et al., 2014; Mo et al., 2016; Isabwe et al., 2018). Systems with high water levels contained significantly higher DO concentrations in their bed sediment which can change the microbial community structure and composition (Han et al., 2020; Huang et al., 2020). In addition, during periods of drawdown, enhanced diffusion of oxygen can lead to the promotion of nitrification process over denitrification (Baldwin and Mitchell, 2000; Knorr et al., 2008). The rates of microbial functions can be continually affected in seasonally dewatered zones compared to the permanently inundated areas. Saturated conditions limit the diffusion of oxygen in sediment and promote anaerobic processes such as denitrification over aerobic processes (e.g., nitrification) as the hydrological regime can regulate gas exchange between the atmosphere and sediments (Hefting et al., 2004; Reddy and DeLaune, 2008; Siljanen et al., 2011). Moreover, hydraulic fluctuations affect the steep redox gradients in CWs which control microbial mediated mechanisms (Reddy and D'angelo, 1997; Scholz and Lee, 2005). Periodic drying and wetting conditions can cause changes in microbial community dynamics (Huang et al., 2018; Kan, 2018; Rose et al., 2018). Previous studies have investigated the processes of microbial assembly and dynamics under wet and dry condition within CWs, creek, and watershed (Bambauer et al., 1998; Fritsche et al., 1999; Kämpfer et al., 1999; Doronina et al., 2010; Drury et al., 2013; Eichmiller et al., 2013; Liu et al., 2013; Chen et al., 2014; Mo et al., 2016; Huang et al., 2018; Isabwe et al., 2018; Kan, 2018; Rose et al., 2018).
During storm events, overland flow introduce new groups of microbes to the wetland, which can change microbial abundance and composition (Sundberg et al., 2007a). While drying and wetting conditions cause immediate physiological stresses to microorganisms, repeated dry–wet cycles can cause long-term effects at community level through the selection of taxa (Fierer et al., 2003; Mikha et al., 2005; Schimel et al., 2007; Xiang et al., 2008). Thus, storm events can change the microbial function, with the potential for alteration of subsequent biogeochemical transformations. Kan (2018) who tracked changes of bacterial community before, during, and after storm events found that the diversity of bacterial community significantly increased during the peak discharge, with the starting bacterial community being very different from the storm community. There are also differences in the distribution patterns of bacterial community, which are event specific, with each bacterial phylum showing distinct response to the event (Kan, 2018). During large storm events, functional genes changed notably and distinct bacterial groups (e.g., nitrifying and denitrifying genes) became the dominant groups (Merbt et al., 2015). During dry weather, the microbial communities have been shown to transform from heterotrophic at the inlet to predominantly autotrophic at the outlet (Huang et al., 2018) indicating a high spatial variation in composition of bacterial communities during dry weather (Petersen et al., 2005; Sercu et al., 2008; Parks and VanBriesen, 2009). Moreover, dry period water samples have been found with differences in the abundance of distinct bacterial families (Isabwe et al., 2018) with less diversity in comparison to the wet weather samples (Huang et al., 2018). The inconsistency of denitrification rates between the sampling locations have been best described by concentration of nitrogen in sediments and hydrological processes, including water residence times (Kjellin et al., 2007). The fluctuation of water regime reduces microbial mediated processes due to the negative impact on wetland vegetation (Greenway et al., 2007; Raulings et al., 2010; Vanderbosch and Galatowitsch, 2011; Webb et al., 2012).
Conclusions and Recommendations
There is a large body of literature investigating treatment efficiencies in CWs with less attention paid to associating microbial processes with treatment processes in constructed wetlands. The conclusions that can be stated from this review are:
(i) There is a disproportionate number of studies understanding microbial community dynamics in constructed wetlands designed to treat stormwater as there are for wastewater. Of the 34 studies of functioning constructed wetlands including surface, vertical and subsurface flow configurations, only four studies dealt specifically with microbial analysis and constructed wetlands treating stormwater.
(ii) The hydrology and hydrologic behavior of stormwater wetlands are complicated with frequent emptying and drying and fluctuations of the water surface. Such perturbations can cause significant changes in the oxic state in the wetland which can alter the microbial genes responsible for the N removal pathways and may ultimately be responsible for the variability in treatment effectiveness reported.
(iii) The influence of other physico-chemical parameters such as organic carbon, nutrients, temperature (seasonal), dissolved oxygen and pH do not vary by as large an extent and are less likely to cause large shifts in microbial communities as compared to wetland hydraulics and hydrology. Nevertheless, as nutrient concentrations in stormwater are comparatively low compared to wastewater, the response of microbial communities under low nutrient and organic carbon condition requires further assessment.
This review has also highlighted pertinent questions that require further investigation:
(i) How does N transforming microbes respond to the wetland physico-chemical features?
Previous studies have investigated microbial response to CWs' physico-chemical variables in different types of CWs. The environmental variables explained microbial shifts, however their impact on N removal could be insignificant depending on wetland condition. They could potentially change the spatial distribution of the microbial community and microbial compositions; thus, we observe different rates of N removal. Studies of N removal performance and mechanisms under physico-chemical variations have identified main pathway(s) of N removal in CWs. These findings could assist to determine the optimal conditions where different pathways could co-exist while different groups of microbes perfume their role at different levels of N removal.
(ii) How do microbial communities respond to the changes in wetland hydrology?
Microbial shifts were associated with variations in wetland hydrology and hydraulics which can account for the observed wetland performance inconsistency. The microbial responses to wetland hydrology such as water level fluctuations caused shifts in community composition, structure, and function. The dynamics of wetland hydrology alter the microbial genes; thus, the effectiveness of N removal depends on stability in wetland hydrology.
(iii) How do microbial communities differ under wet and dry weather conditions?
Investigation of microbial assembly and dynamics under wet and dry condition showed that during storm events, new groups of microbes could be introduced to CWs which can change the microbial abundance and composition. Periodic drying and rewetting conditions could cause long-term effects at the community level through the selection of taxa with immediate physiological stresses to microorganisms. Tracking bacterial dynamic before, during, and after storm events demonstrated the differences in distribution patterns of bacterial community at each storm event where each bacterial phylum representing distinct response to the event. As the fluctuation in water regime could inhibit microbial mediated processes, diversity and abundance of N transforming microbes could be used to assess the CWs treatment performance.
(iv) Can microbial shifts be used to assess the CWs' N removal performance?
It has been revealed that microbial characterization, their structure, diversity, and composition could indicate wetland health and its ability to function as intended. The abundance of genes that are involved in N transformation were positively or negatively correlated with total N removal following rain events. Moreover, the presence of top microbial phyla with the highest degree of relative richness have been used as reliable alternative indicators of wetland treatment performance. Indeed, previous works have strongly supported the implication of new designs in CWs to promote bacterial diversity which is essential for N removal while the microbial shifts could be used as ecological indicators.
Although some of these questions have been raised by others in previous research studies, the general lack of information about the microbial shifts in CWs that treat stormwater needs to be addressed. Many studies have analyzed microbial N removal, and bacterial community but a majority have conducted their studies in bioretention systems. It is therefore recommended, in the context of stormwater constructed wetlands to:
(i) Study less investigated N transformation pathways such as DNRA and comammox processes and focus on microbial community involved in these processes and their changes over dry-wet cycles. This information could assist engineers to take advantage of the presence of other N transforming communities leading to higher microbial diversity within CWs treating stormwater.
(ii) Track microbial recovery in terms of their structure, composition, and distribution in CWs to reveal the impact of the flow regime in CWs. The results should be carefully analyzed and interpreted because the extent of microbial reaction to fluctuations depends on a wide range of factors.
(iii) Employ advanced molecular techniques to link microbial properties of stormwater wetlands to the transport and sources of nitrogen. Tracking the microbial functional genes assists scientists to develop ecosystems that are favorable to the establishment of pathways with higher N removal potential in stormwater treatment.
Lastly, the findings of the current literature review reinforce the importance of stormwater runoff treatments and implementation of new design into CWs to enhance microbial activity and diversity leading to better treatment outcomes.
Author Contributions
MS took the lead in writing the manuscript with support from LC and LB. LC and LB provided critical feedback and helped shape the research, analysis, and manuscript. All authors contributed to the article and approved the submitted version.
Funding
MS was a recipient of Deakin University Postgraduate Research (DUPR) scholarship. The provision of this financial support is gratefully acknowledged.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abal, E., Moore, K. B., Gibbes, B. R., and Dennison, W., (eds.). (2002). State of South-East Queensland Waterways Report 2001. Brisbane, QLD: Moreton Bay Waterways and Catchments Partnership.
Adrados, B., Sánchez, O., Arias, C. A., Becares, E., Garrido, L., Mas, J., et al. (2014). Microbial communities from different types of natural wastewater treatment systems: vertical and horizontal flow constructed wetlands and biofilters. Water Res. 55, 304–312. doi: 10.1016/j.watres.2014.02.011
Andersson, J., Wittgren, H., Kallner, S., Ridderstolpe, P., and Hägermark, I. (2002). Wetland Oxeloesund, Sweden- the first five years of operation. Adv. Ecol. Sci. 12, 9–28.
Bäckman, J. S., Hermansson, A., Tebbe, C. C., and Lindgren, P.-E. (2003). Liming induces growth of a diverse flora of ammonia-oxidising bacteria in acid spruce forest soil as determined by SSCP and DGGE. Soil Biol. Biochem. 35, 1337–1347. doi: 10.1016/S0038-0717(03)00213-X
Baldwin, D. S., and Mitchell, A. (2000). The effects of drying and re-flooding on the sediment and soil nutrient dynamics of lowland river–floodplain systems: a synthesis. Regul. Rivers. 16, 457–467. doi: 10.1002/1099-1646(200009/10)16:5<457::AID-RRR597>3.0.CO;2-B
Bambauer, A., Rainey, F. A., Stackebrandt, E., and Winter, J. J. A. (1998). Characterization of Aquamicrobium defluvii gen. nov. sp. nov., a thiophene-2-carboxylate-metabolizing bacterium from activated sludge. 1 Arch. Microbiol. 69, 293–302. doi: 10.1007/s002030050575
Bastviken, S. K., Eriksson, P., Premrov, A., and Tonderski, K. (2005). Potential denitrification in wetland sediments with different plant species detritus. Ecol. Eng. 25, 183–190. doi: 10.1016/j.ecoleng.2005.04.013
Bastviken, S. K., Eriksson, P. G., Martins, I., Neto, J. M., Leonardson, L., and Tonderski, K. (2003). Potential nitrification and denitrification on different surfaces in a constructed treatment Wetland. J. Environ. Qual. 32, 2414–2420. doi: 10.2134/jeq2003.2414
Bernardin-Souibgui, C., Barraud, S., Bourgeois, E., Aubin, J.-B., Becouze-Lareure, C., and Wiest, L. (2018). Incidence of hydrological, chemical, and physical constraints on bacterial pathogens, Nocardia cells, and fecal indicator bacteria trapped in an urban stormwater detention basin in Chassieu, France. J. Environ. Sci. Pollut. Res. 25, 24860–24881. doi: 10.1007/s11356-018-1994-2
Bettez, N. D., and Groffman, P. M. (2012). Denitrification potential in stormwater control structures and natural riparian zones in an urban landscape. Environ. Sci. Technol. 46, 10909–10917. doi: 10.1021/es301409z
Biswal, B. K., Vijayaraghavan, K., Adam, M. G., Lee Tsen-Tieng, D., Davis, A. P., and Balasubramanian, R. (2021). Biological nitrogen removal from stormwater in bioretention cells: a critical review. Crit. Rev. Biotechnol. doi: 10.1080/07388551.2021.1969888. [Epub ahead of print].
Blaszczak, J. R., Steele, M. K., Badgley, B. D., Heffernan, J. B., Hobbie, S. E., Morse, J. L., et al. (2018). Sediment chemistry of urban stormwater ponds and controls on denitrification. Ecosphere 9:e02318. doi: 10.1002/ecs2.2318
Bledsoe, R. B., Bean, E. Z., Austin, S. S., and Peralta, A. L. (2020). A microbial perspective on balancing trade-offs in ecosystem functions in a constructed stormwater wetland. Ecol. Eng. 158:106000. doi: 10.1101/2020.04.01.020776
Boog, J., Nivala, J., Aubron, T., Wallace, S., van Afferden, M., and Müller, R. A. (2014). Hydraulic characterization and optimization of total nitrogen removal in an aerated vertical subsurface flow treatment wetland. Bioresour. Technol. 162, 166–174. doi: 10.1016/j.biortech.2014.03.100
Brix, H.. (2020). Constructed wetlands for water quality improvement (CRC Press), 9–22. doi: 10.1201/9781003069997-3
Brodrick, S. J., Cullen, P., and Maher, W. (1988). Denitrification in a natural wetland receiving secondary treated effluent. Water Res. 22, 431–439. doi: 10.1016/0043-1354(88)90037-1
Burchell, M. R., Skaggs, R. W., Lee, C. R., Broome, S., Chescheir, G. M., and Osborne, J. (2007). Substrate organic matter to improve nitrate removal in surface-flow constructed wetlands. J. Environ. Qual. 36, 194–207. doi: 10.2134/jeq2006.0022
Cao, Q., Wang, H., Chen, X., Wang, R., and Liu, J. (2017). Composition and distribution of microbial communities in natural river wetlands and corresponding constructed wetlands. Ecol. Eng. 98, 40–48. doi: 10.1016/j.ecoleng.2016.10.063
Chen, N., Wu, Y., Wu, J., Yan, X., and Hong, H. J. J. (2014). Natural and human influences on dissolved silica export from watershed to coast in Southeast China. JSR Biogeosci. 119, 95–109. doi: 10.1002/2013JG002429
Chen, X., Peltier, E., Sturm, B.S., and Young, C.B. (2013). Nitrogen removal and nitrifying and denitrifying bacteria quantification in a stormwater bioretention system. Water Res. 47, 1691–1700. doi: 10.1016/j.watres.2012.12.033
Chon, K., Chang, J.-S., Lee, E., Lee, J., Ryu, J., and Cho, J. J. E. E. (2011). Abundance of denitrifying genes coding for nitrate (narG), nitrite (nirS), and nitrous oxide (nosZ) reductases in estuarine versus wastewater effluent-fed constructed wetlands. https://www.researchgate.net/journal/Ecological-Engineering-0925-8574 Ecol. Eng. 37, 64–69. doi: 10.1016/j.ecoleng.2009.04.005
Coban, O., Kuschk, P., Wells, N. S., Strauch, G., and Knoeller, K. (2015). Microbial nitrogen transformation in constructed wetlands treating contaminated groundwater. Environ. Sci. Pollut. Res. Int. 22, 12829–12839. doi: 10.1007/s11356-014-3575-3
Converse, R. R., Piehler, M. F., and Noble, R. T. J. W. (2011). Contrasts in concentrations and loads of conventional and alternative indicators of fecal contamination in coastal stormwater. Water Res. 45, 5229–5240. doi: 10.1016/j.watres.2011.07.029
Cookson, W., Cornforth, I., and Rowarth, J. (2002). Winter soil temperature (2–15 C) effects on nitrogen transformations in clover green manure amended or unamended soils; a laboratory and field study. Soil Biol. Biochem. 34, 1401–1415. doi: 10.1016/S0038-0717(02)00083-4
Cooper, P., Job, G., Green, M., and Shutes, R. J. E. (1997). Reed beds and constructed wetlands for wastewater treatment. J. Eur. Water Pollut. Control 6, 49.
Cooper, P. F., Job, G., Green, M., and Shutes, R. (1996) Reed Beds Constructed Wetlands for Wastewater Treatment. Swindon: Water Research Centre.
Criado, C., and Bécares, E. (2005). Characterization of bacterial communities of a constructed wetland in cold conditions. J. Gen. Appl. Microbiol. 51, 197–201. doi: 10.2323/jgam.51.197
Daims, H., Lücker, S., and Wagner, M. (2016). A new perspective on microbes formerly known as nitrite-oxidizing bacteria. Trends Microbiol. 24, 699–712. doi: 10.1016/j.tim.2016.05.004
D'Angelo, E. M., and Reddy, K. (1999). Regulators of heterotrophic microbial potentials in wetland soils. Soil Biol. Biochem. 31, 815–830. doi: 10.1016/S0038-0717(98)00181-3
Dieter, C. D.. (1990). The importance of emergent vegetation in reducing sediment resuspension in wetlands. J. Freshw. Ecol. 5, 467–473. doi: 10.1080/02705060.1990.9665263
Domingos, S. S., Dallas, S., Skillman, L., Felstead, S., and Ho, G. (2011). Nitrogen removal and ammonia-oxidising bacteria in a vertical flow constructed wetland treating inorganic wastewater. Water Sci. Technol. 64, 587–594. doi: 10.2166/wst.2011.565
Dong, Z., and Sun, T. (2007). A potential new process for improving nitrogen removal in constructed wetlands—promoting coexistence of partial-nitrification and ANAMMOX. Ecol. Eng. 31, 69–78. doi: 10.1016/j.ecoleng.2007.04.009
Dordio, A. V., and Carvalho, A. J. P. (2013). Organic xenobiotics removal in constructed wetlands, with emphasis on the importance of the support matrix. J. Hazard. Mater. 252, 272–292. doi: 10.1016/j.jhazmat.2013.03.008
Doronina, N. V., Kaparullina, E. N., Trotsenko, Y. A., Nörtemann, B., Bucheli-Witschel, M., Weilenmann, H.-U., et al. (2010). Chelativorans multitrophicus gen. nov., sp. nov. and Chelativorans oligotrophicus sp. nov., aerobic EDTA-degrading bacteria. Int. J. Syst. Evol. Microbiol. 60, 1044–1051. doi: 10.1099/ijs.0.003152-0
Drury, B., Rosi-Marshall, E., and Kelly, J. J. J. A. E. M. (2013). Wastewater treatment effluent reduces the abundance and diversity of benthic bacterial communities in urban and suburban rivers. Appl. Environ. Microbiol. 79, 1897–1905. doi: 10.1128/AEM.03527-12
Edwards, K. R., CiŽková, H., Zemanová, K., and Šantručková, H. (2006). Plant growth and microbial processes in a constructed wetland planted with Phalaris arundinacea. Ecol. Eng. 27, 153–165. doi: 10.1016/j.ecoleng.2006.02.004
Eichmiller, J. J., Hicks, R. E., and Sadowsky, M. J. J. E. (2013). Distribution of genetic markers of fecal pollution on a freshwater sandy shoreline in proximity to wastewater effluent. Environ. Sci. Technol. 47, 3395–3402. doi: 10.1021/es305116c
Fan, J., Wang, W., Zhang, B., Guo, Y., Ngo, H. H., Guo, W., et al. (2013a). Nitrogen removal in intermittently aerated vertical flow constructed wetlands: impact of influent COD/N ratios. Bioresour. Technol. 143, 461–466. doi: 10.1016/j.biortech.2013.06.038
Fan, J., Zhang, B., Zhang, J., Ngo, H. H., Guo, W., Liu, F., et al. (2013b). Intermittent aeration strategy to enhance organics and nitrogen removal in subsurface flow constructed wetlands. Bioresour. Technol. 141, 117–122. doi: 10.1016/j.biortech.2013.03.077
Fan, J., Zhang, J., Guo, W., Liang, S., and Wu, H. (2016). Enhanced long-term organics and nitrogen removal and associated microbial community in intermittently aerated subsurface flow constructed wetlands. Bioresour. Technol. 214, 871–875. doi: 10.1016/j.biortech.2016.05.083
Faulwetter, J. L., Gagnon, V., Sundberg, C., Chazarenc, F., Burr, M. D., Brisson, J., et al. (2009). Microbial processes influencing performance of treatment wetlands: a review. Ecol. Eng. 35, 987–1004. doi: 10.1016/j.ecoleng.2008.12.030
Fernandes, J. P., Almeida, C. M. R., Pereira, A. C., Ribeiro, I. L., Reis, I., Carvalho, P., et al. (2015). Microbial community dynamics associated with veterinary antibiotics removal in constructed wetlands microcosms. Bioresour. Technol. 182, 26–33. doi: 10.1016/j.biortech.2015.01.096
Fierer, N., Schimel, J., and Holden, P. (2003). Influence of drying–rewetting frequency on soil bacterial community structure. Microb. Ecol. 45, 63–71. doi: 10.1007/s00248-002-1007-2
Flood, J. A., Ashbolt, N. J., and Pollard, P. C. (1999). Complementary independent molecular, radioisotopic and fluorogenic techniques to assess biofilm communities in two wastewater wetlands. Water Sci. Technol. 39, 65–70. doi: 10.2166/wst.1999.0329
Foulquier, A., Volat, B., Neyra, M., Bornette, G., and Montuelle, B. J. F. M. E. (2013). Long-term impact of hydrological regime on structure and functions of microbial communities in riverine wetland sediments. FEMS Microbiol. Ecol. 85, 211–226. doi: 10.1111/1574-6941.12112
Fritsche, K., Auling, G., Andreesen, J. R., and Lechner, U. J. S. (1999). Defluvibacter lusatiae gen. nov., sp. nov., a new chlorophenol-degrading member of the α-2 subgroup of proteobacteria. Syst. Appl. Microbiol. 22, 197–204. doi: 10.1016/S0723-2020(99)80066-6
Fu, G., Huangshen, L., Guo, Z., Zhou, Q., and Wu, Z. (2017). Effect of plant-based carbon sources on denitrifying microorganisms in a vertical flow constructed wetland. Bioresour. Technol. 224, 214–221. doi: 10.1016/j.biortech.2016.11.007
Fu, G., Yu, T., Ning, K., Guo, Z., and Wong, M.-H. J. E. (2016). Effects of nitrogen removal microbes and partial nitrification-denitrification in the integrated vertical-flow constructed wetland. Ecol. Eng. 95, 83–89. doi: 10.1016/j.ecoleng.2016.06.054
Gagnon, V., Chazarenc, F., Comeau, Y., and Brisson, J. (2007). Influence of macrophyte species on microbial density and activity in constructed wetlands. Water Sci. Technol. 56, 249–254. doi: 10.2166/wst.2007.510
Gale, P., Devai, I., Reddy, K., and Graetz, D. (1993). Denitrification potential of soils from constructed and natural wetlands. Ecol. Eng. 2, 119–130. doi: 10.1016/0925-8574(93)90034-D
Garfí, M., Pedescoll, A., Bécares, E., Hijosa-Valsero, M., Sidrach-Cardona, R., García, J. J. S., et al (2012). Effect of climatic conditions, season and wastewater quality on contaminant removal efficiency of two experimental constructed wetlands in different regions of Spain. https://www.researchgate.net/journal/Science-of-The-Total-Environment-0048-9697 Sci Total Environ. 437, 61–67. doi: 10.1016/j.scitotenv.2012.07.087
Glibert, P. M., and Burford, M. A. (2017). Globally changing nutrient loads and harmful algal blooms: recent advances, new paradigms, and continuing challenges. Oceanography 30, 58–69. doi: 10.5670/oceanog.2017.110
Gold, A. C., Thompson, S. P., and Piehler, M. F. (2019). Nitrogen cycling processes within stormwater control measures: a review and call for research. Water Res. 149, 578–587. doi: 10.1016/j.watres.2018.10.036
Gold, A. C., Thompson, S. P., and Piehler, M. F. (2021). Seasonal variation in nitrate removal mechanisms in coastal stormwater ponds. Water Resour. Res. 57:e2021WR029718. doi: 10.1029/2021WR029718
Gorra, R., Coci, M., Ambrosoli, R., and Laanbroek, H. (2007). Effects of substratum on the diversity and stability of ammonia-oxidizing communities in a constructed wetland used for wastewater treatment. J. Appl. Microbiol. 103, 1442–1452. doi: 10.1111/j.1365-2672.2007.03357.x
Greenway, M., Jenkins, G., and Polson, C. (2007). Macrophyte zonation in stormwater wetlands: getting it right! a case study from subtropical Australia. Water Sci. Technol. 56, 223–231. doi: 10.2166/wst.2007.494
Griffiths, L. N., and Mitsch, W. J. (2020). Nutrient retention via sedimentation in a created urban stormwater treatment wetland. Sci. Total Environ. 727:138337. doi: 10.1016/j.scitotenv.2020.138337
Grunditz, C., Gumaelius, L., and Dalhammar, G. (1998). Comparison of inhibition assays using nitrogen removing bacteria: application to industrial wastewater. Water Res. 32, 2995–3000. doi: 10.1016/S0043-1354(98)00050-5
Hammer, M. J.. (1986). Water and Wastewater Technology. Delhi: Prentice Hall India Learning Private Limited.
Han, J. Y., Kim, D.-H., Oh, S., and Moon, H. S. (2020). Effects of water level and vegetation on nitrate dynamics at varying sediment depths in laboratory-scale wetland mesocosms. Sci. Total Environ. 703:134741. doi: 10.1016/j.scitotenv.2019.134741
Harris, G., Batley, G., Fox, D., Hall, D., Jernakoff, P., Molloy, et al. (1996). Port Phillip Bay Environmental Study Final Report. Canberra, ACT: CSIRO.
He, S., Wang, Y., Li, C., Li, Y., and Zhou, J. (2018). The nitrogen removal performance and microbial communities in a two-stage deep sequencing constructed wetland for advanced treatment of secondary effluent. Bioresour. Technol. 248, 82–88. doi: 10.1016/j.biortech.2017.06.150
He, Y., Tao, W., Wang, Z., and Shayya, W. (2012). Effects of pH and seasonal temperature variation on simultaneous partial nitrification and anammox in free-water surface wetlands. J. Environ. Manage. 110, 103–109. doi: 10.1016/j.jenvman.2012.06.009
Hefting, M., Clément, J.-C., Dowrick, D., Cosandey, A.-C., Bernal, S., Cimpian, C., et al. (2004). Water table elevation controls on soil nitrogen cycling in riparian wetlands along a European climatic gradient. Biogeochemistry 67, 113–134. doi: 10.1023/B:BIOG.0000015320.69868.33
Hernandez, M. E., and Mitsch, W. J. (2007). Denitrification potential and organic matter as affected by vegetation community, wetland age, and plant introduction in created wetlands. J. Environ. Qual. 36, 333–342. doi: 10.2134/jeq2006.0139
Herskowitz, J., Black, S., and Lewandowski, W. (1987). Listowel Artificial Marsh Treatment Project. Rome: FAO.
Hobbie, S. E., Finlay, J. C., Janke, B. D., Nidzgorski, D. A., Millet, D. B., and Baker, L. A. (2017). Contrasting nitrogen and phosphorus budgets in urban watersheds and implications for managing urban water pollution. Proc. Nat. Acad. Sci. U.S.A. 114, 4177–4182. doi: 10.1073/pnas.1618536114
Hodge, A., Robinson, D., and Fitter, A. (2000). Are microorganisms more effective than plants at competing for nitrogen? Trends Plant Sci. 5, 304–308. doi: 10.1016/S1360-1385(00)01656-3
Hörman, A., Rimhanen-Finne, R., Maunula, L., von Bonsdorff, C.-H., Torvela, N., Heikinheimo, A., et al. (2004). Campylobacter spp., Giardia spp., Cryptosporidium spp., noroviruses, and indicator organisms in surface water in southwestern Finland, 2000-2001. Appl. Environ. Microbiol. 70, 87–95. doi: 10.1128/AEM.70.1.87-95.2004
Hu, A., Yang, X., Chen, N., Hou, L., Ma, Y., and Yu, C.-P. (2014). Response of bacterial communities to environmental changes in a mesoscale subtropical watershed, Southeast China. Sci. Total Environ. 472, 746–756. doi: 10.1016/j.scitotenv.2013.11.097
Hu, Y., He, F., Ma, L., Zhang, Y., and Wu, Z. (2016). Microbial nitrogen removal pathways in integrated vertical-flow constructed wetland systems. Bioresour. Technol. 207, 339–345. doi: 10.1016/j.biortech.2016.01.106
Huang, L., Gao, X., Liu, M., Du, G., Guo, J., and Ntakirutimana, T. (2012). Correlation among soil microorganisms, soil enzyme activities, and removal rates of pollutants in three constructed wetlands purifying micro-polluted river water. Ecol. Eng. 46, 98–106. doi: 10.1016/j.ecoleng.2012.06.004
Huang, X., Rippy, M. A., Mehring, A. S., Winfrey, B. K., Jiang, S. C., and Grant, S. B. (2018). Shifts in dissolved organic matter and microbial community composition are associated with enhanced removal of fecal pollutants in urban stormwater wetlands. Water Res. 137, 310–323. doi: 10.1016/j.watres.2018.03.020
Huang, Y.-T., Chen, S.-S., Jetten, M. S., and Lin, J.-G. J. J. (2020). Nanoarchitectured structure and population dynamics of anaerobic ammonium oxidizers in a wastewater treatment plant. J. Hazard. Mater. 396:122714. doi: 10.1016/j.jhazmat.2020.122714
Hunt, P. G., Matheny, T. A., and Szögi, A. A. (2003). Denitrification in constructed wetlands used for treatment of swine wastewater. J. Environ. Qual. 32, 727–735. doi: 10.2134/jeq2003.7270
Ibekwe, A., Ma, J., Murinda, S., and Reddy, G. (2016). Bacterial community dynamics in surface flow constructed wetlands for the treatment of swine waste. Sci. Total Environ. 544, 68–76. doi: 10.1016/j.scitotenv.2015.11.139
Ibekwe, A. M., Grieve, C. M., and Lyon, S. R. (2003). Characterization of microbial communities and composition in constructed dairy wetland wastewater effluent. https://journals.asm.org/journal/aem Appl. Environ. Microbiol. 69, 5060–5069. doi: 10.1128/AEM.69.9.5060-5069.2003
Ibekwe, A. M., Lyon, S., Leddy, M., and Jacobson-Meyers, M. J. J. (2007). Impact of plant density and microbial composition on water quality from a free water surface constructed wetland. J. Appl. Microbiol. 102, 921–936. doi: 10.1111/j.1365-2672.2006.03181.x
Isabwe, A., Yang, J. R., Wang, Y., Liu, L., Chen, H., and Yang, J. (2018). Community assembly processes underlying phytoplankton and bacterioplankton across a hydrologic change in a human-impacted river. Sci. Total Environ. 630, 658–667. doi: 10.1016/j.scitotenv.2018.02.210
Ishida, C. K., Kelly, J. J., and Gray, K. A. J. (2006). Effects of variable hydroperiods and water level fluctuations on denitrification capacity, nitrate removal, and benthic-microbial community structure in constructed wetlands. Ecol. Eng. 28, 363–373. doi: 10.1016/j.ecoleng.2006.06.010
Jadhav, R. S., and Buchberger, S. G. (1995). Effects of vegetation on flow through free water surface wetlands. Ecol. Eng. 5, 481–496. doi: 10.1016/0925-8574(95)00039-9
Janke, B. D., Finlay, J. C., and Hobbie, S. E. (2017). Trees and streets as drivers of urban stormwater nutrient pollution. Environ. Sci. Technol. 51, 9569–9579. doi: 10.1021/acs.est.7b02225
Jianlong, W., and Ning, Y. (2004). Partial nitrification under limited dissolved oxygen conditions. Process Biochem. 39, 1223–1229. doi: 10.1016/S0032-9592(03)00249-8
Johnson, J. Y., Thomas, J., Graham, T., Townshend, I., Byrne, J., Selinger, L., et al. (2003). Prevalence of Escherichia coli O157: H7 and Salmonella spp. in surface waters of southern Alberta and its relation to manure sources. Can. J. Microbiol. 49, 326–335. doi: 10.1139/w03-046
Juretschko, S., Timmermann, G., Schmid, M., Schleifer, K.-H., Pommerening-Röser, A., Koops, H.-P., et al. (1998). Combined molecular and conventional analyses of nitrifying bacterium diversity in activated sludge: nitrosococcus mobilis and Nitrospira-like bacteria as dominant populations. Appl. Environ. Microbiol. 64, 3042–3051. doi: 10.1128/AEM.64.8.3042-3051.1998
Kadlec, R., Knight, R., Vymazal, J., Brix, H., Cooper, P., and Haberl, R.. (2000) Constructed Wetlands for Pollution Control: Processes, Performance, Design Operation. www.google.com/search?sxsrf=AOaemvLy02th3VBSHXrayfAbbQbfrzTd2Q:1641401192359&q=London&stick=H4sIAAAAAAAAAOPgE-LVT9c3NEypKCg2NULUeLQz9U3MMkqyNEyyii30kOz8lJTS7JzMTzy9KT8zLrEoEcYqtMlITUwpLE4tKUouKFXLyk8HCi1jZfPLzUvLzdrAyAgCyPSL5WwAAAA&sa=X&ved=2ahUKEwiCh4T4h5v1AhUXIbcAHWhJBUsQmxMoAXoECCEQAw London: IWA Publishing.
Kadlec, R. H.. (2009). Comparison of free water and horizontal subsurface treatment wetlands. Ecol. Eng. 35, 159–174. doi: 10.1016/j.ecoleng.2008.04.008
Kämpfer, P., Müller, C., Mau, M., Neef, A., Auling, G., Busse, H.-J., et al. (1999). Description of Pseudaminobacter gen. nov. with two new species, Pseudaminobacter salicylatoxidans sp. nov. and Pseudaminobacter defluvii sp. nov. Int. J. Syst. Bacteriol.49, 887–897. doi: 10.1099/00207713-49-2-887
Kan, J.. (2018). Storm events restructured bacterial community and their biogeochemical potentials. J. Geophys. Res. Biogeosci. 123, 2257–2269. doi: 10.1029/2017JG004289
Kang, H., Freeman, C., Lee, D., and Mitsch, W. J. (1998). Enzyme activities in constructed wetlands: implication for water quality amelioration. Hydrobiologia 368, 231–235. doi: 10.1023/A:1003219123729
Kaye, J. P., and Hart, S. C. (1997). Competition for nitrogen between plants and soil microorganisms. Trends Ecol. Evol. 12, 139–143. doi: 10.1016/S0169-5347(97)01001-X
Kendall, C., Elliott, E. M., and Wankel, S. D. (2007). Tracing anthropogenic inputs of nitrogen to ecosystems. Stable Isotopes Ecol. Environ. Sci. 2, 375–449. doi: 10.1002/9780470691854.ch12
Kern, J.. (2003). Seasonal Efficiency of a Constructed Wetland for Treating Dairy Farm Wastewater. Billerica, MA: Computational Mechanics Inc., 197–214.
Kim, H., Bae, H.-S., Reddy, K. R., and Ogram, A. (2016). Distributions, abundances and activities of microbes associated with the nitrogen cycle in riparian and stream sediments of a river tributary. Water Res. 106, 51–61. doi: 10.1016/j.watres.2016.09.048
Kjellin, J., Hallin, S., and Wörman, A. (2007). Spatial variations in denitrification activity in wetland sediments explained by hydrology and denitrifying community structure. Water Res. 41, 4710–4720. doi: 10.1016/j.watres.2007.06.053
Knorr, K.-H., Oosterwoud, M. R., and Blodau, C. (2008). Experimental drought alters rates of soil respiration and methanogenesis but not carbon exchange in soil of a temperate fen. Soil Biol. Biochem. 40, 1781–1791. doi: 10.1016/j.soilbio.2008.03.019
Kowalchuk, G. A., Stienstra, A. W., Heilig, G. H. J., Stephen, J. R., and Woldendorp, J. W. (2000). Molecular analysis of ammonia-oxidising bacteria in soil of successional grasslands of the Drentsche A (The Netherlands). FEMS Microbiol. Ecol. 31, 207–215. doi: 10.1111/j.1574-6941.2000.tb00685.x
Kozub, D., and Liehr, S. (1999). Assessing denitrification rate limiting factors in a constructed wetland receiving landfill leachate. Water Sci. Technol. 40, 75–82. doi: 10.2166/wst.1999.0140
Kyambadde, J., Kansiime, F., and Dalhammar, G. (2006). Distribution and activity of ammonium-oxidizing bacteria in Nakivubo wastewater channel and wastewater treatment wetland, Uganda. Acta Hydrochim. Hydrobiol. 34, 137–145. doi: 10.1002/aheh.200400617
Lai, W.-L., Wang, S.-Q., Peng, C.-L., and Chen, Z.-H. (2011). Root features related to plant growth and nutrient removal of 35 wetland plants. Water Res. 45, 3941–3950. doi: 10.1016/j.watres.2011.05.002
Lai, W.-L., Zhang, Y., and Chen, Z.-H. (2012). Radial oxygen loss, photosynthesis, and nutrient removal of 35 wetland plants. Ecol. Eng. 39, 24–30. doi: 10.1016/j.ecoleng.2011.11.010
Lee, C. G., Fletcher, T. D., and Sun, G. (2009). Nitrogen removal in constructed wetland systems. https://www.researchgate.net/journal/Engineering-in-Life-Sciences-1618-2863 Eng. Life Sci. 9, 11–22. doi: 10.1002/elsc.200800049
Li, B., Chen, J., Wu, Z., Wu, S., Xie, S., and Liu, Y. (2018a). Seasonal and spatial dynamics of denitrification rate and denitrifier community in constructed wetland treating polluted river water. Int. Biodeterior. Biodegrad. 126, 143–151. doi: 10.1016/j.ibiod.2017.10.008
Li, X., Li, Y., Lv, D., Li, Y., and Wu, J. (2020). Nitrogen and phosphorus removal performance and bacterial communities in a multi-stage surface flow constructed wetland treating rural domestic sewage. Science of The Total Environment 709, 136235. doi: 10.1016/j.scitotenv.2019.136235
Li, X., Zhang, M., Liu, F., Chen, L., Li, Y., Li, Y., et al. (2018b). Seasonality distribution of the abundance and activity of nitrification and denitrification microorganisms in sediments of surface flow constructed wetlands planted with Myriophyllum elatinoides during swine wastewater treatment. Bioresour. Technol. 248, 89–97. doi: 10.1016/j.biortech.2017.06.102
Liang, W., Wu, Z. B., Cheng, S. P., Zhou, Q. H., and Hu, H. Y. (2003). Roles of substrate microorganisms and urease activities in wastewater purification in a constructed wetland system. Ecol. Eng. 21, 191–195. doi: 10.1016/j.ecoleng.2003.11.002
Liao, H., Yu, K., Duan, Y., Ning, Z., Li, B., He, L., et al. (2019). Profiling microbial communities in a watershed undergoing intensive anthropogenic activities. Sci. Total Environ. 647, 1137–1147. doi: 10.1016/j.scitotenv.2018.08.103
Liao, R., Miao, Y., Li, J., Li, Y., Wang, Z., Du, J., et al. (2018). Temperature dependence of denitrification microbial communities and functional genes in an expanded granular sludge bed reactor treating nitrate-rich wastewater. RSC Adv. 8, 42087–42094. doi: 10.1039/C8RA08256A
Ligi, T., Oopkaup, K., Truu, M., Preem, J.-K., Nõlvak, H., Mitsch, W. J., et al. (2014). Characterization of bacterial communities in soil and sediment of a created riverine wetland complex using high-throughput 16S rRNA amplicon sequencing. Ecol. Eng. 72, 56–66. doi: 10.1016/j.ecoleng.2013.09.007
Lin, Y.-F., Jing, S.-R., Lee, D.-Y., and Wang, T.-W. (2002). Nutrient removal from aquaculture wastewater using a constructed wetlands system. Aquaculture 209, 169–184. doi: 10.1016/S0044-8486(01)00801-8
Lipson, D., and Näsholm, T. (2001). The unexpected versatility of plants: organic nitrogen use and availability in terrestrial ecosystems. Oecologia 128, 305–316. doi: 10.1007/s004420100693
Liu, H., Hu, Z., Zhang, Y., Zhang, J., Xie, H., and Liang, S. (2018). Microbial nitrogen removal of ammonia wastewater in poly (butylenes succinate)-based constructed wetland: effect of dissolved oxygen. Appl. Microbiol. Biotechnol. 102, 9389–9398. doi: 10.1007/s00253-018-9386-6
Liu, L., Yang, J., Yu, X., Chen, G., and Yu, Z. J. P. O. (2013). Patterns in the composition of microbial communities from a subtropical river: effects of environmental, spatial and temporal factors. PLoS ONE. 8:e81232. doi: 10.1371/journal.pone.0081232
Logue, J. B., Langenheder, S., Andersson, A. F., Bertilsson, S., Drakare, S., Lanzén, A., et al. (2012). Freshwater bacterioplankton richness in oligotrophic lakes depends on nutrient availability rather than on species–area relationships. ISME J. 6:1127. doi: 10.1038/ismej.2011.184
Logue, J. B., and Lindström, E. S. (2008). Biogeography of bacterioplankton in inland waters. J. Freshwater Rev. 1, 99–115. doi: 10.1608/FRJ-1.1.9
López, D., Sepúlveda, M., and Vidal, G. (2016). Phragmites australis and Schoenoplectus californicus in constructed wetlands: Development and nutrient uptake. J. Soil Sci. Plant Nutr. 16, 763–777. doi: 10.4067/S0718-95162016005000055
Lucke, T., Drapper, D., and Hornbuckle, A. (2018). Urban stormwater characterisation and nitrogen composition from lot-scale catchments—New management implications. Sci. Total Environ. 619–620, 65–71. doi: 10.1016/j.scitotenv.2017.11.105
Ma, Y., Wang, S., Zhang, X., and Shen, Z. (2021). Transport process and source contribution of nitrogen in stormwater runoff from urban catchments. Environ. Pollut. 289:117824. doi: 10.1016/j.envpol.2021.117824
Macek, C. L., Hale, R. L., and Baxter, C. V. (2020). Dry wetlands: nutrient dynamics in ephemeral constructed stormwater wetlands. Environ. Manage. 65, 32–45. doi: 10.1007/s00267-019-01227-x
Malaviya, P., and Singh, A. (2012). Constructed wetlands for management of urban stormwater runoff. J. Crit. Rev. Environ. Sci. Technol. 42, 2153–2214. doi: 10.1080/10643389.2011.574107
Martin, C. D., Johnson, K. D., and Moshiri, G. A. (1999). Performance of a constructed wetland leachate treatment system at the Chunchula landfill, Mobile County, Alabama. Water Sci. Technol. 40, 67–74. doi: 10.2166/wst.1999.0139
Mayes, W., Batty, L., Younger, P., Jarvis, A., Kõiv, M., Vohla, C., et al. (2009). Wetland treatment at extremes of pH: a review. Sci. Total Environ. 407, 3944–3957. doi: 10.1016/j.scitotenv.2008.06.045
McPhillips, L., and Walter, M. T. (2015). Hydrologic conditions drive denitrification and greenhouse gas emissions in stormwater detention basins. Ecol. Eng. 85, 67–75. doi: 10.1016/j.ecoleng.2015.10.018
Mentzer, J. L., Goodman, R. M., and Balser, T. C. (2006). Microbial response over time to hydrologic and fertilization treatments in a simulated wet prairie. Plant Soil 284, 85–100. doi: 10.1007/s11104-006-0032-1
Merbt, S. N., Auguet, J.-C., Blesa, A., Mart,í, E., and Casamayor, E. O. J. M. (2015). Wastewater treatment plant effluents change abundance and composition of ammonia-oxidizing microorganisms in Mediterranean urban stream biofilms. Microb. Ecol. 69, 66–74. doi: 10.1007/s00248-014-0464-8
Mikha, M. M., Rice, C. W., and Milliken, G. A. (2005). Carbon and nitrogen mineralization as affected by drying and wetting cycles. Soil Biol. Biochem. 37, 339–347. doi: 10.1016/j.soilbio.2004.08.003
Mo, Q., Chen, N., Zhou, X., Chen, J., and Duan, S. J. E. S. P. (2016). Ammonium and phosphate enrichment across the dry–wet transition and their ecological relevance in a subtropical reservoir, China. Environ. Sci. Process. Impacts. 18, 882–894. doi: 10.1039/C6EM00225K
Morse, N. R., McPhillips, L. E., Shapleigh, J. P., and Walter, M. T. (2017). The role of denitrification in stormwater detention basin treatment of nitrogen. Environ. Sci. Technol. 51, 7928–7935. doi: 10.1021/acs.est.7b01813
Mulder, A., Van de Graaf, A. A., Robertson, L., and Kuenen, J. (1995). Anaerobic ammonium oxidation discovered in a denitrifying fluidized bed reactor. FEMS Microbiol. Ecol. 16, 177–183. doi: 10.1111/j.1574-6941.1995.tb00281.x
Münch, C., Kuschk, P., and Röske, I. (2005). Root stimulated nitrogen removal: only a local effect or important for water treatment? Water Sci. Technol. 51, 185–192. doi: 10.2166/wst.2005.0316
Mustafa, A., Scholz, M., Harrington, R., and Carroll, P. (2009). Long-term performance of a representative integrated constructed wetland treating farmyard runoff. Ecol. Eng. 35, 779–790. doi: 10.1016/j.ecoleng.2008.12.008
Noble, R. T., Griffith, J. F., Blackwood, A. D., Fuhrman, J. A., Gregory, J. B., Hernandez, X., et al (2006). Multitiered approach using quantitative PCR to track sources of fecal pollution affecting Santa Monica Bay, California. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1392893/ Appl. Environ. Microbiol. 72, 1604–1612. doi: 10.1128/AEM.72.2.1604-1612.2006
Norton, R. A., Harrison, J. A., Kent Keller, C., and Moffett, K. B. (2017). Effects of storm size and frequency on nitrogen retention, denitrification, and N2O production in bioretention swale mesocosms. Biogeochemistry 134, 353–370. doi: 10.1007/s10533-017-0365-2
Nurk, K., Truu, J., Truu, M., and M. A. N. D. E. R. Ü. (2005). Microbial characteristics and nitrogen transformation in planted soil filter for domestic wastewater treatment. J. Environ. Sci. Health 40, 1201–1214. doi: 10.1081/ESE-200055659
Okabe, S., Satoh, H., and Watanabe, Y. (1999). In situ analysis of nitrifying biofilms as determined by in situ hybridization and the use of microelectrodes. Appl. Environ. Microbiol. 65, 3182–3191. doi: 10.1128/AEM.65.7.3182-3191.1999
Paerl, H. W., Otten, T. G., and Kudela, R. (2018). Mitigating the Expansion of Harmful Algal Blooms Across the Freshwater-to-Marine Continuum. Washington, DC: ACS Publications.
Pang, X., Shen, H., Niu, Y., Sun, X., Chen, J., Xie, P. J. C., et al. (2014). Dissolved organic carbon and relationship with bacterioplankton community composition in 3 lake regions of Lake Taihu, China. Can. J. Microbiol. 60, 669–680. doi: 10.1139/cjm-2013-0847
Paredes, D., Kuschk, P., Mbwette, T., Stange, F., Müller, R., and Köser, H. (2007). New aspects of microbial nitrogen transformations in the context of wastewater treatment–a review. Eng. Life Sci. 7, 13–25. doi: 10.1002/elsc.200620170
Parks, S. L. I., and VanBriesen, J. M. J. J. (2009). Evaluating temporal variability in bacterial indicator samples for an urban watershed. J. Environ. Eng. 135, 1294–1303. doi: 10.1061/(ASCE)EE.1943-7870.0000104
Paul, E.. (2014). Soil Microbiology, Ecology and Biochemistry. www.google.com/search?sxsrf=AOaemvJdtvsAF0WHw0EOEzysiKRhKp5hKQ:1641401993428&q=Cambridge,+Massachusetts&stick=H4sIAAAAAAAAAOPgE-LUz9U3MM8rzi5X4gAxDQszzLW0spOt9POL0hPzMqsSSzLz81A4VhmpiSmFpYlFJalFxYtYJZwTc5OKMlPSU3UUfBOLixOTM0qLU0tKinewMgIAvcQbN2EAAAA&sa=X&ved=2ahUKEwjYh4H2ipv1AhVkSGwGHSgCBbIQmxMoAXoECCcQAw Cambridge, MA: Academic Press.
Pelissari, C., Ávila, C., Trein, C. M., García, J., de Armas, R. D., and Sezerino, P.H. (2017). Nitrogen transforming bacteria within a full-scale partially saturated vertical subsurface flow constructed wetland treating urban wastewater. Sci. Total Environ. 574, 390–399. doi: 10.1016/j.scitotenv.2016.08.207
Pelissari, C., Guivernau, M., Viñas, M., García, J., Velasco-Galilea, M., Souza, S. S., et al. (2018). Effects of partially saturated conditions on the metabolically active microbiome and on nitrogen removal in vertical subsurface flow constructed wetlands. Water Res. 141, 185–195. doi: 10.1016/j.watres.2018.05.002
Peng, L., Hua, Y., Cai, J., Zhao, J., Zhou, W., Zhu, D. J. E., et al. (2014). Effects of plants and temperature on nitrogen removal and microbiology in a pilot-scale integrated vertical-flow wetland treating primary domestic wastewater. Ecol. Eng. 64, 285–290. doi: 10.1016/j.ecoleng.2013.12.036
Peralta, A. L., Matthews, J. W., and Kent, A. D. (2010). Microbial community structure and denitrification in a wetland mitigation bank. https://journals.asm.org/journal/aem Appl. Environ. Microbiol. 76, 4207–4215. doi: 10.1128/AEM.02977-09
Peralta, A. L., Matthews, J. W., and Kent, A. D. (2014). Habitat specialization along a wetland moisture gradient differs between ammonia-oxidizing and denitrifying microorganisms. Microb. Ecol. 68, 339–350. doi: 10.1007/s00248-014-0407-4
Petersen, T. M., Rifai, H. S., Suarez, M. P., and Stein, A. R. J. J. (2005). Bacteria loads from point and nonpoint sources in an urban watershed. J. Environ. Eng. 131, 1414–1425. doi: 10.1061/(ASCE)0733-9372(2005)131:10(1414)
Picard, C. R., Fraser, L. H., and Steer, D. J. B. (2005). The interacting effects of temperature and plant community type on nutrient removal in wetland microcosms. Bioresour. Technol. 96, 1039–1047. doi: 10.1016/j.biortech.2004.09.007
Prosser, J. I.. (1990). Advances in Microbial Physiology. www.google.com/search?sxsrf=AOaemvKbTmmOgvexHeCg8y3R9ouCbxJofQ:1641402178189&q=Amsterdam&stick=H4sIAAAAAAAAAOPgE-LUz9U3MDJLSYpXYgcxs40LtLSyk63084vSEMyqxJLMvPzUDhWGamJKYWliUUlqUXFi1g5HXOLgayUxNwdrIwA9iXLVlEAAAA&sa=X&ved=2ahUKEwiIpo7Oi5v1AhX-SWwGHfMKA7IQmxMoAXoECEoQAw Amsterdam: Elsevier, 125–181.
Prüss, A.. (1998). Review of epidemiological studies on health effects from exposure to recreational water. Int. J. Epidemiol. 27, 1–9. doi: 10.1093/ije/27.1.1
Purkhold, U., Wagner, M., Timmermann, G., Pommerening-Röser, A., and Koops, H.-P. (2003). 16S rRNA and amoA-based phylogeny of 12 novel betaproteobacterial ammonia-oxidizing isolates: extension of the dataset and proposal of a new lineage within the nitrosomonads. J. Med. Microbiol. 53, 1485–1494. doi: 10.1099/ijs.0.02638-0
Rahman, M., Grace, M. R., Roberts, K. L., Kessler, A. J., and Cook, P. L. M. (2019c). Effect of temperature and drying-rewetting of sediments on the partitioning between denitrification and DNRA in constructed urban stormwater wetlands. Ecol. Eng. 140, 105586. doi: 10.1016/j.ecoleng.2019.105586
Rahman, M.M., Roberts, K.L., Warry, F., Grace, M.R., and Cook, P.L. (2019b). Factors controlling dissimilatory nitrate reduction processes in constructed stormwater urban wetlands. Biogeochemistry 142, 375–393. doi: 10.1007/s10533-019-00541-0
Rahman, M. M., Roberts, K. L., Grace, M. R., Kessler, A. J., and Cook, P. L. M. (2019a). Role of organic carbon, nitrate and ferrous iron on the partitioning between denitrification and DNRA in constructed stormwater urban wetlands. Sci. Total Environ. 666, 608–617. doi: 10.1016/j.scitotenv.2019.02.225
Raulings, E. J., Morris, K., Roache, M. C., and Boon, P. I. (2010). The importance of water regimes operating at small spatial scales for the diversity and structure of wetland vegetation. Freshw. Biol. 55, 701–715. doi: 10.1111/j.1365-2427.2009.02311.x
Reddy, K., and D'angelo, E. (1997). Biogeochemical indicators to evaluate pollutant removal efficiency in constructed wetlands. Water Sci. Technol. 35, 1–10. doi: 10.2166/wst.1997.0152
Reddy, K., Patrick, W., and Broadbent, F. (1984). Nitrogen transformations and loss in flooded soils and sediments. Crit. Rev. Environ. Sci. Technol. 13, 273–309. doi: 10.1080/10643388409381709
Reddy, K. R., and DeLaune, R. D. (2008). Biogeochemistry of Wetlands: Science and Applications. Boca Raton, FL: CRC Press.
Repert, D. A., Underwood, J. C., Smith, R. L., and Song, B. J. J. (2014). Nitrogen cycling processes and microbial community composition in bed sediments in the Yukon River at Pilot Station. J. Geophys. Res. Biogeosci. 119, 2328–2344. doi: 10.1002/2014JG002707
Robertson, L. A., and Kuenen, J. G. (1984). Aerobic denitrification: a controversy revived. Arch. Microbiol. 139, 351–354. doi: 10.1007/BF00408378
Rodziewicz, J., Ostrowska, K., Janczukowicz, W., and Mielcarek, A. (2019). Effectiveness of nitrification and denitrification processes in biofilters treating wastewater from de-icing airport runways. Water 11:630. doi: 10.3390/w11030630
Rose, L., Karwan, D., and Aufdenkampe, A. J. J. (2018). Sediment fingerprinting suggests differential suspended particulate matter formation and transport processes across hydrologic regimes. https://www.researchgate.net/journal/Journal-of-Geophysical-Research-Biogeosciences-2169-8961 J. Geophys. Res. Biogeosci. 123, 1213–1229. doi: 10.1002/2017JG004210
Ruiz-Rueda, O., Hallin, S., and Bañeras, L. (2009). Structure and function of denitrifying and nitrifying bacterial communities in relation to the plant species in a constructed wetland. FEMS Microbiol. Ecol. 67, 308–319. doi: 10.1111/j.1574-6941.2008.00615.x
Saeed, T., and Sun, G. (2012). A review on nitrogen and organics removal mechanisms in subsurface flow constructed wetlands: dependency on environmental parameters, operating conditions and supporting media. J. Environ. Manage. 112, 429–448. doi: 10.1016/j.jenvman.2012.08.011
Salvato, M., Borin, M., Doni, S., Macci, C., Ceccanti, B., Marinari, S., et al. (2012). Wetland plants, micro-organisms and enzymatic activities interrelations in treating N polluted water. Ecol. Eng. 47, 36–43. doi: 10.1016/j.ecoleng.2012.06.033
Sani, A., Scholz, M., and Bouillon, L. (2013). Seasonal assessment of experimental vertical-flow constructed wetlands treating domestic wastewater. Bioresour. Technol. 147, 585–596. doi: 10.1016/j.biortech.2013.08.076
Sawaittayothin, V., and Polprasert, C. (2007). Nitrogen mass balance and microbial analysis of constructed wetlands treating municipal landfill leachate. Bioresour. Technol. 98, 565–570. doi: 10.1016/j.biortech.2006.02.002
Schimel, J., Balser, T. C., and Wallenstein, M. (2007). Microbial stress-response physiology and its implications for ecosystem function. Ecology 88, 1386–1394. doi: 10.1890/06-0219
Schimel, J. P., and Chapin, I. I. I. F. S. (1996). Tundra plant uptake of amino acid and NH4+ nitrogen in situ: plants complete well for amino acid N. Ecology 77, 2142–2147. doi: 10.2307/2265708
Scholz, M., and Lee, B. (2005). Constructed wetlands: a review. Int. J. Environ. Stud. 62, 421–447. doi: 10.1080/00207230500119783
Schramm, A., Larsen, L. H., Revsbech, N. P., Ramsing, N. B., Amann, R., and Schleifer, K.-H. (1996). Structure and function of a nitrifying biofilm as determined by in situ hybridization and the use of microelectrodes. Appl. Environ. Microbiol. 62, 4641–4647. doi: 10.1128/aem.62.12.4641-4647.1996
Sercu, B., Werfhorst, L. C. V. D., Murray, J., and Holden, P. A. (2008). Storm drains are sources of human fecal pollution during dry weather in three urban southern California watersheds. Environ. Sci. Technol. 43, 293–298. doi: 10.1021/es801505p
Shipin, O., Koottatep, T., Khanh, N., and Polprasert, C. (2005). Integrated natural treatment systems for developing communities: low-tech N-removal through the fluctuating microbial pathways. Water Sci. Technol. 51, 299–306. doi: 10.2166/wst.2005.0488
Shirdashtzadeh, M., Chandrasena, G. I., Henry, R., and McCarthy, D. T. (2017). Plants that can kill; improving E. coli removal in stormwater treatment systems using Australian plants with antibacterial activity. Ecol. Eng. 107, 120–125. doi: 10.1016/j.ecoleng.2017.07.009
Si, Z., Song, X., Cao, X., Wang, Y., Wang, Y., Zhao, Y., et al. (2019). Nitrate removal to its fate in wetland mesocosm filled with sponge iron: impact of influent COD/N ratio. Front. Environ. Sci. Eng. 14:4. doi: 10.1007/s11783-019-1183-7
Siljanen, H. M., Saari, A., Krause, S., Lensu, A., Abell, G. C., Bodrossy, L., et al. (2011). Hydrology is reflected in the functioning and community composition of methanotrophs in the littoral wetland of a boreal lake. FEMS Microbiol. Ecol. 75, 430–445. doi: 10.1111/j.1574-6941.2010.01015.x
Silyn-Roberts, G., and Lewis, G. (2001). In situ analysis of Nitrosomonas spp. in wastewater treatment wetland biofilms. Water Res. 35, 2731–2739. doi: 10.1016/S0043-1354(00)00544-3
Sims, A., Gajaraj, S., and Hu, Z. (2012). Seasonal population changes of ammonia-oxidizing organisms and their relationship to water quality in a constructed wetland. Ecol. Eng. 40, 100–107. doi: 10.1016/j.ecoleng.2011.12.021
Sirivedhin, T., and Gray, K. A. (2006). Factors affecting denitrification rates in experimental wetlands: field and laboratory studies. Ecol. Eng. 26, 167–181. doi: 10.1016/j.ecoleng.2005.09.001
Spieles, D. J., and Mitsch, W. J. (1999). The effects of season and hydrologic and chemical loading on nitrate retention in constructed wetlands: a comparison of low-and high-nutrient riverine systems. Ecol. Eng. 14, 77–91. doi: 10.1016/S0925-8574(99)00021-X
Staley, C., Gould, T. J., Wang, P., Phillips, J., Cotner, J. B., Sadowsky, M. J. J. S., et al. (2015). Species sorting and seasonal dynamics primarily shape bacterial communities in the Upper Mississippi River. Sci. Total Environ. 505, 435–445. doi: 10.1016/j.scitotenv.2014.10.012
Stottmeister, U., Wießner, A., Kuschk, P., Kappelmeyer, U., Kästner, M., Bederski, O., et al. (2003). Effects of plants and microorganisms in constructed wetlands for wastewater treatment. Biotechnol. Adv. 22, 93–117. doi: 10.1016/j.biotechadv.2003.08.010
Sun, D., Tang, X., Zhao, M., Zhang, Z., Hou, L., Liu, M., et al. (2020). Distribution and Diversity of Comammox Nitrospira in Coastal Wetlands of China. Front. Microbiol. 11:2480. doi: 10.3389/fmicb.2020.589268
Sun, G., and Austin, D. (2007). Completely autotrophic nitrogen-removal over nitrite in lab-scale constructed wetlands: Evidence from a mass balance study. Chemosphere 68, 1120–1128. doi: 10.1016/j.chemosphere.2007.01.060
Sun, G., Zhu, Y., Saeed, T., Zhang, G., and Lu, X. (2012). Nitrogen removal and microbial community profiles in six wetland columns receiving high ammonia load. Chem. Eng. J. 203, 326–332. doi: 10.1016/j.cej.2012.07.052
Sun, H., Yang, Z., Wei, C., and Wu, W. (2018). Nitrogen removal performance and functional genes distribution patterns in solid-phase denitrification sub-surface constructed wetland with micro aeration. Bioresour. Technol. 263, 223–231. doi: 10.1016/j.biortech.2018.04.078
Sundberg, C., Stendahl, J. S., Tonderski, K., and Lindgren, P.-E. (2007a). Overland flow systems for treatment of landfill leachates—potential nitrification and structure of the ammonia-oxidising bacterial community during a growing season. Soil Biol. Biochem. 39, 127–138. doi: 10.1016/j.soilbio.2006.06.016
Sundberg, C., Tonderski, K., and Lindgren, P.-E. (2007b). Potential nitrification and denitrification and the corresponding composition of the bacterial communities in a compact constructed wetland treating landfill leachates. Water Sci. Technol. 56, 159–166. doi: 10.2166/wst.2007.524
Sundblad, K., and Wittgren, H.-B. (1991). Ecological Engineering for Wastewater Treatment. Kärna: Bokskogen Göteborg. 190–198.
Tao, W., Hall, K. J., and Duff, S. J. (2007). Microbial biomass and heterotrophic production of surface flow mesocosm wetlands treating woodwaste leachate: responses to hydraulic and organic loading and relations with mass reduction. Ecol. Eng. 31, 132–139. doi: 10.1016/j.ecoleng.2007.06.007
Tchobanoglous, G., Burton, F. L., and Stensel, H. (1991). Wastewater engineering. Management 7, 1–4.
Third, K., Sliekers, A. O., Kuenen, J., and Jetten, M. (2001). The CANON system (completely autotrophic nitrogen-removal over nitrite) under ammonium limitation: interaction and competition between three groups of bacteria. Syst. Appl. Microbiol. 24, 588–596. doi: 10.1078/0723-2020-00077
Thompson, S. P., Paerl, H. W., and Go, M. C. (1995). Seasonal patterns of nitrification and denitrification in a natural and a restored salt marsh. Estuaries 18, 399–408. doi: 10.2307/1352322
Tietz, A., Hornek, R., Langergraber, G., Kreuzinger, N., and Haberl, R. (2007). Diversity of ammonia oxidising bacteria in a vertical flow constructed wetland. Water Sci. Technol. 56, 241–247. doi: 10.2166/wst.2007.505
Tippler, C., Wright, I. A., and Hanlon, A. J. W. (2012). Is catchment imperviousness a keystone factor degrading urban waterways? A case study from a partly urbanised catchment (Georges River, south-eastern Australia). Water Air Soil Pollut. 223, 5331–5344. doi: 10.1007/s11270-012-1283-5
Toet, S., Huibers, L. H., Van Logtestijn, R. S., and Verhoeven, J. T. (2003). Denitrification in the periphyton associated with plant shoots and in the sediment of a wetland system supplied with sewage treatment plant effluent. Hydrobiologia 501, 29–44. doi: 10.1023/A:1026299017464
Toet, S., Van Logtestijn, R. S., Kampf, R., Schreijer, M., and Verhoeven, J. T. (2005). The effect of hydraulic retention time on the removal of pollutants from sewage treatment plant effluent in a surface-flow wetland system. Wetlands 25, 375–391. doi: 10.1672/13
Toscano, A., Marzo, A., Milani, M., Cirelli, G. L., and Barbagallo, S. (2015). Comparison of removal efficiencies in Mediterranean pilot constructed wetlands vegetated with different plant species. Ecol. Eng. 75, 155–160. doi: 10.1016/j.ecoleng.2014.12.005
Truu J. Nurk K. Juhanson J. and M. A. N. D. E. R. (2005). Variation of microbiological parameters within planted soil filter for domestic wastewater treatment. J. Environ. Sci. Health 40, 1191–1200. doi: 10.1081/ESE-200055636
Truu, M., Juhanson, J., and Truu, J. (2009). Microbial biomass, activity and community composition in constructed wetlands. Sci. Total Environ. 407, 3958–3971. doi: 10.1016/j.scitotenv.2008.11.036
USEPA (2010). Quantitative Microbial Risk Assessment to Estimate Illness in Freshwater Impacted by Agricultural Animal Sources of Fecal Contamination. https://www.google.com/search?sxsrf=AOaemvJWD5EpkkodNxrmrZJj1h8l6znjJw:1641402807679&q=Washington,+DC&stick=H4sIAAAAAAAAAOPgE-LQz9U3SDfIyVACs4oyzLK1tLKTrfTzi9IT8zKrEksy8_NQOFYZqYkphaWJRSWpRcWLWPnCE4szMvPSS_LzdBRcnHewMgIAAScQu1YAAAA&sa=X&ved=2ahUKEwi-tqP6jZv1AhW8UWwGHaZBBLMQmxMoAXoECCMQAw Washington, DC: U.S. Environmental Protection Agency.
Van Horn, D., Sinsabaugh, R., Takacs-Vesbach, C., Mitchell, K., and Dahm, C. J. A. M. E. (2011). Response of heterotrophic stream biofilm communities to a gradient of resources. Aquat. Microb. Ecol. 64, 149–161. doi: 10.3354/ame01515
van Kessel, M. A. H. J., Speth, D. R., Albertsen, M., Nielsen, P. H., Op den Camp, H. J. M., Kartal, B., et al. (2015). Complete nitrification by a single microorganism. Nature 528, 555–559. doi: 10.1038/nature16459
Van Niel, E., Robertson, L., and Kuenen, J. (1993). A mathematical description of the behaviour of mixed chemostat cultures of an autotrophic nitrifier and a heterotrophic nitrifier/aerobic denitrifier; a comparison with experimental data. FEMS Microbiol. Ecol. 11, 99–108. doi: 10.1111/j.1574-6968.1993.tb05801.x
Vanderbosch, D., and Galatowitsch, S. M. (2011). Factors affecting the establishment of Schoenoplectustabernaemontani (CC Gmel.) Palla in urban lakeshore restorations. Wetlands Ecol. Manag. 19, 35–45. doi: 10.1007/s11273-010-9198-7
Vymazal, J.. (2007). Removal of nutrients in various types of constructed wetlands. J. Sci. Total Environ. 380, 48–65. doi: 10.1016/j.scitotenv.2006.09.014
Vymazal, J.. (2013a). Emergent plants used in free water surface constructed wetlands: a review. Ecol. Eng. 61, 582–592. doi: 10.1016/j.ecoleng.2013.06.023
Vymazal, J.. (2013b). The use of hybrid constructed wetlands for wastewater treatment with special attention to nitrogen removal: a review of a recent development. Water Res. 47, 4795–4811. doi: 10.1016/j.watres.2013.05.029
Vymazal, J. J. E. E.. (2014). Constructed wetlands for treatment of industrial wastewaters: a review. https://www.sciencedirect.com/science/journal/09258574 Ecol. Eng. 73, 724–751. doi: 10.1016/j.ecoleng.2014.09.034
Walaszek, M., Bois, P., Laurent, J., Lenormand, E., and Wanko, A. (2018). Urban stormwater treatment by a constructed wetland: Seasonality impacts on hydraulic efficiency, physico-chemical behavior and heavy metal occurrence. Sci. Total Environ. 637–638, 443–454. doi: 10.1016/j.scitotenv.2018.04.325
Wallenstein, M. D., and Hall, E. K. (2012). A trait-based framework for predicting when and where microbial adaptation to climate change will affect ecosystem functioning. Biogeochemistry 109, 35–47. doi: 10.1007/s10533-011-9641-8
Waller, L. J., Evanylo, G. K., Krometis, L.-A. H., Strickland, M. S., Wynn-Thompson, T., and Badgley, B. D. (2018). Engineered and environmental controls of microbial denitrification in established bioretention cells. Environ. Sci. Technol. 52, 5358–5366. doi: 10.1021/acs.est.7b06704
Walsh, K. A., Hill, T. C., Moffett, B. F., Harris, J. A., Shaw, P. J., and Wallace, J. S. (2002). Molecular characterisation of bacteria in a wetland used to remove ammoniacal-N from landfill leachate. Waste Manag. Res. 20, 529–535. doi: 10.1177/0734242X0202000606
Wang, J., Wang, Y., Bai, J., Liu, Z., Song, X., Yan, D., et al. (2017). High efficiency of inorganic nitrogen removal by integrating biofilm-electrode with constructed wetland: autotrophic denitrifying bacteria analysis. Bioresour. Technol. 227, 7–14. doi: 10.1016/j.biortech.2016.12.046
Wang, L., Pang, Q., Peng, F., Zhang, A., Zhou, Y., Lian, J., et al. (2020a). Response characteristics of nitrifying bacteria and archaea community involved in nitrogen removal and bioelectricity generation in integrated tidal flow constructed wetland-microbial fuel cell. Front. Microbiol. 11:1385. doi: 10.3389/fmicb.2020.01385
Wang, L., Zhang, J., Li, H., Yang, H., Peng, C., Peng, Z., et al. (2018). Shift in the microbial community composition of surface water and sediment along an urban river. Sci. Total Environ. 627, 600–612. doi: 10.1016/j.scitotenv.2018.01.203
Wang, S., Ma, Y., Zhang, X., and Shen, Z. (2022). Transport and sources of nitrogen in stormwater runoff at the urban catchment scale. Sci. Total Environ. 806:150281. doi: 10.1016/j.scitotenv.2021.150281
Wang, S., Pi, Y., Jiang, Y., Pan, H., Wang, X., Wang, X., et al. (2020b). Nitrate reduction in the reed rhizosphere of a riparian zone: from functional genes to activity and contribution. Environ. Res. 180:108867. doi: 10.1016/j.envres.2019.108867
Ward, B. B.. (2003). Significance of anaerobic ammonium oxidation in the ocean. Trends Microbiol. 11, 408–410. doi: 10.1016/S0966-842X(03)00181-1
Webb, J. A., Wallis, E. M., and Stewardson, M. J. (2012). A systematic review of published evidence linking wetland plants to water regime components. Aquat. Bot. 103, 1–14. doi: 10.1016/j.aquabot.2012.06.003
Webster, G., Embley, T. M., and Prosser, J. I. (2002). Grassland management regimens reduce small-scale heterogeneity and species diversity of β-proteobacterial ammonia oxidizer populations. Appl. Environ. Microbiol. 68, 20–30. doi: 10.1128/AEM.68.1.20-30.2002
Wei, J.-,m., Cui, L.-,j., Li, W., Ping, Y.-,m., and Li, W. (2021). Denitrifying bacterial communities in surface-flow constructed wetlands during different seasons: characteristics and relationships with environment factors. Sci. Rep. 11:4918. doi: 10.1038/s41598-021-82438-3
Werker, A., Dougherty, J., McHenry, J., and Van Loon, W. (2002). Treatment variability for wetland wastewater treatment design in cold climates. Ecol. Eng. 19, 1–11. doi: 10.1016/S0925-8574(02)00016-2
Wu, H., Lin, L., Zhang, J., Guo, W., Liang, S., and Liu, H. (2016). Purification ability and carbon dioxide flux from surface flow constructed wetlands treating sewage treatment plant effluent. Bioresour. Technol. 219, 768–772. doi: 10.1016/j.biortech.2016.08.030
Wu, H., Wang, X., He, X., Zhang, S., Liang, R., and Shen, J. (2017). Effects of root exudates on denitrifier gene abundance, community structure and activity in a micro-polluted constructed wetland. Sci. Total Environ. 598, 697–703. doi: 10.1016/j.scitotenv.2017.04.150
Wu, H., Zhang, J., Ngo, H. H., Guo, W., Hu, Z., Liang, S., et al. (2015). A review on the sustainability of constructed wetlands for wastewater treatment: design and operation. Bioresour. Technol. 175, 594–601. doi: 10.1016/j.biortech.2014.10.068
Wu, J., Zhang, J., Jia, W., Xie, H., Gu, R. R., Li, C., et al. (2009). Impact of COD/N ratio on nitrous oxide emission from microcosm wetlands and their performance in removing nitrogen from wastewater. Bioresour. Technol. 100, 2910–2917. doi: 10.1016/j.biortech.2009.01.056
Wu, S.-,q., Chang, J.-,j., Dai, Y., Wu, Z.-,b., and Liang, W. (2013). Treatment performance and microorganism community structure of integrated vertical-flow constructed wetland plots for domestic wastewater. Environ. Sci. Pollut. Res. 20, 3789–3798. doi: 10.1007/s11356-012-1307-0
Xia, Z., Liu, G., She, Z., Gao, M., Zhao, Y., Guo, L., et al. (2020). Performance and bacterial communities in unsaturated and saturated zones of a vertical-flow constructed wetland with continuous-feed. Bioresour. Technol. 315:123859. doi: 10.1016/j.biortech.2020.123859
Xiang, S.-R., Doyle, A., Holden, P. A., and Schimel, J. P. (2008). Drying and rewetting effects on C and N mineralization and microbial activity in surface and subsurface California grassland soils. Soil Biol. Biochem. 40, 2281–2289. doi: 10.1016/j.soilbio.2008.05.004
Xie, S. G., Zhang, X. J., and Wang, Z.-S. (2003). Temperature effect on aerobic denitrification and nitrification. J. Environ. Sci. 15, 669–673.
Xu, D., Xiao, E., Xu, P., Zhou, Y., He, F., Zhou, Q., et al. (2017). Performance and microbial communities of completely autotrophic denitrification in a bioelectrochemically-assisted constructed wetland system for nitrate removal. Bioresour. Technol. 228, 39–46. doi: 10.1016/j.biortech.2016.12.065
Xu, M., Liu, W., Li, C., Xiao, C., Ding, L., Xu, K., et al. (2016). Evaluation of the treatment performance and microbial communities of a combined constructed wetland used to treat industrial park wastewater. Environ. Sci. Pollut. Res. 23, 10990–11001. doi: 10.1007/s11356-016-6181-8
Yeh, T., Pan, C., Ke, T., and Kuo, T. (2010). Organic matter and nitrogen removal within field-scale constructed wetlands: reduction performance and microbial identification studies. Water Environ. Res. 82, 27–33. doi: 10.2175/106143009X447957
Yousefi, Z., and Mohseni-Bandpei, A. (2010). Nitrogen and phosphorus removal from wastewater by subsurface wetlands planted with Iris pseudacorus. Ecol. Eng. 36, 777–782. doi: 10.1016/j.ecoleng.2010.02.002
Zhang, L., Scholz, M., Mustafa, A., and Harrington, R. (2008). Assessment of the nutrient removal performance in integrated constructed wetlands with the self-organizing map. Water Res. 42, 3519–3527. doi: 10.1016/j.watres.2008.04.027
Zhang, M., Huang, J.-C., Sun, S., Ur Rehman, M. M., He, S., and Zhou, W. (2021). Impact of functional microbes on nitrogen removal in artificial tidal wetlands in the Yangtze River estuary: evidence from molecular and stable isotopic analyses. J. Clean. Prod. 287:125077. doi: 10.1016/j.jclepro.2020.125077
Zhang, M., Luo, P., Liu, F., Li, H., Zhang, S., Xiao, R., et al. (2017). Nitrogen removal and distribution of ammonia-oxidizing and denitrifying genes in an integrated constructed wetland for swine wastewater treatment. Ecol. Eng. 104, 30–38. doi: 10.1016/j.ecoleng.2017.04.022
Zhang, S., Pang, S., Wang, P., Wang, C., Guo, C., Addo, F. G., et al. (2016). Responses of bacterial community structure and denitrifying bacteria in biofilm to submerged macrophytes and nitrate. Sci. Rep. 6:36178. doi: 10.1038/srep36178
Zhang, X., Ji, Z., Shao, Y., Guo, C., Zhou, H., Liu, L., et al. (2020). Seasonal variations of soil bacterial communities in Suaeda wetland of Shuangtaizi River estuary, Northeast China. J. Environ. Sci. 97, 45–53. doi: 10.1016/j.jes.2020.04.012
Zhao, H., and Piccone, T. (2020). Large scale constructed wetlands for phosphorus removal, an effective nonpoint source pollution treatment technology. Ecol. Eng. 145:105711. doi: 10.1016/j.ecoleng.2019.105711
Zhao, Y. J., Hui, Z., Chao, X., Nie, E., Li, H. J., He, J., et al. (2011). Efficiency of two-stage combinations of subsurface vertical down-flow and up-flow constructed wetland systems for treating variation in influent C/N ratios of domestic wastewater. Ecol. Eng. 37, 1546–1554. doi: 10.1016/j.ecoleng.2011.06.005
Zheng, Y., Wang, X., Dzakpasu, M., Zhao, Y., Ngo, H. H., Guo, W., et al. (2016). Effects of interspecific competition on the growth of macrophytes and nutrient removal in constructed wetlands: a comparative assessment of free water surface and horizontal subsurface flow systems. Bioresour. Technol. 207, 134–141. doi: 10.1016/j.biortech.2016.02.008
Zhimiao, Z., Xiao, Z., Zhufang, W., Xinshan, S., Mengqi, C., Mengyu, C., et al. (2019). Enhancing the pollutant removal performance and biological mechanisms by adding ferrous ions into aquaculture wastewater in constructed wetland. Bioresour. Technol. 293:122003. doi: 10.1016/j.biortech.2019.122003
Zhu, G., Jetten, M. S., Kuschk, P., Ettwig, K. F., and Yin, C. (2010). Potential roles of anaerobic ammonium and methane oxidation in the nitrogen cycle of wetland ecosystems. Appl. Microbiol. Biotechnol. 86, 1043–1055. doi: 10.1007/s00253-010-2451-4
Zhu, T., and Sikora, F. (1995). Ammonium and nitrate removal in vegetated and unvegetated gravel bed microcosm wetlands. Water Sci. Technol. 32, 219–228. doi: 10.2166/wst.1995.0144
Keywords: constructed wetlands, stormwater, microbial communities, nitrogen removal, regulating factors
Citation: Shirdashtzadeh M, Chua LHC and Brau L (2022) Microbial Communities and Nitrogen Transformation in Constructed Wetlands Treating Stormwater Runoff. Front. Water 3:751830. doi: 10.3389/frwa.2021.751830
Received: 02 August 2021; Accepted: 30 December 2021;
Published: 16 February 2022.
Edited by:
Yun-Ya Yang, University of Maryland, College Park, United StatesReviewed by:
Hong Liu, Suzhou University of Science and Technology, ChinaYijing Shi, South China Normal University, China
Copyright © 2022 Shirdashtzadeh, Chua and Brau. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maryam Shirdashtzadeh, bS5zaGlyZGFzaHR6YWRlaEBkZWFraW4uZWR1LmF1
 Maryam Shirdashtzadeh
Maryam Shirdashtzadeh Lloyd H. C. Chua1
Lloyd H. C. Chua1