- 1Musculoskeletal Health and Ergonomics Laboratory, Department of Occupational Therapy, Tufts University, Medford, MA, United States
- 2US Department of Veterans Affairs, VA Pittsburgh Health System, Pittsburgh, PA, United States
- 3Department of Physical Medicine and Rehabilitation, Tufts Medical Center, Boston, MA, United States
- 4Tufts Applied Cognition Laboratory, Department of Psychology, Tufts University, Medford, MA, United States
- 5Department of Mechanical Engineering, Tufts University, Medford, MA, United States
- 6Pain Management Center, Department of Anesthesia and Pain Management, Brigham and Women’s Hospital / Harvard Medical School, Boston, MA, United States
Immersive virtual reality (IVR) is increasingly used as a treatment for chronic pain. In this crossover randomized pilot study, we examined the effect of 10- and 20-min dosages on back pain intensity, affect, and measures of pain sensitization in people with chronic back pain (CBP). Twenty-one people with CBP were seen for two visits of IVR. Participants were randomly assigned to receive either 10- or 20-min of IVR in Visit 1 and the other dosage in Visit 2. Our primary analyses were effect sizes and simple inferential comparisons for pain intensity, affect, fatigue, and measures of pain sensitization assessed using quantitative sensory testing. Overall, IVR had a moderate, significant effect in reducing back pain intensity, negative affect, and painful aftersensations. When dosage was examined, 20-min had a moderate, significant effect on pain while 10-min had a small, non-significant effect, although the between-dosage difference was non-significant. Interestingly, effects were much larger in Visit 1, particularly for 20-min, but this diminished in Visit 2, and both dosages had a smaller effect in Visit 2. We interpret these results to indicate that pain modulation may be associated with novelty and engagement that can attenuate over time if the IVR encounter is not sufficiently engaging. Moreover, that if participants are engaged in a single session, 20-min may be necessary to obtain sufficient competency with IVR, while in subsequent sessions, 10-min of IVR may be sufficient to affect pain.
1 Introduction
Immersive virtual reality (IVR) has emerged over the last 20-years as an effective and viable method to treat acute pain, and more recently as a treatment for chronic pain (Baker et al., 2022). Chronic back pain (CBP) is one of the most common forms of chronic pain, with a prevalence estimated between 3.9 and 20.3 percent of the population (Meucci et al., 2015). Few studies have examined the efficacy of IVR on CBP, however, outcomes suggest that IVR may be more effective than opioids (Tack, 2021), with studies showing that IVR can achieve a ≥30% overall reduction in pain (Garcia et al., 2021; Garcia et al., 2022), a reduction that is consistent with the magnitude of benefits reported in studies of potent analgesic medications such as opioids (Deyo et al., 2015).
One physiological factor associated with continued presence of pain is central sensitization. Central sensitization is defined as “an amplification of neural signaling within the central nervous system that elicits pain hypersensitivity” (Woolf, 2011). Central sensitization contributes to the experience of allodynia, hyperalgesia, and “wind up” in which repeated noxious stimulation becomes progressively more painful, as well as the phenomenon of painful aftersensations in which the sensation of pain can linger after the stimulus is ended (Woolf, 2011). There is increasing evidence that central sensitization may play a role in CBP (Schuttert et al., 2021; Tack, 2021). Studies estimate that between 40% and 50% of people with CBP have some evidence of central sensitization (Schuttert et al., 2021). People with CBP are over three times more likely to be prescribed opioids and more than 14 times more likely to be prescribed long acting opioids (Gore et al., 1976) than people without CBP, even though opioids have limited effectiveness for people with CBP. IVR may be an effective treatment for central sensitization in people with CBP (Nijs et al., 2011; Tack, 2021; Bazzari and Bazzari, 2022). However, to our knowledge, no study has directly measured the effect of IVR on pain-related sensitization in people with CBP.
The most common mechanism of pain reduction in IVR is distraction (Gupta et al., 2018; Tack, 2021). Because IVR is engaging, it diverts attention away from the pain leaving fewer resources available for pain processing (Loreto-Quijada et al., 2014). One area which has received little attention in the IVR literature is optimal IVR duration. Protocols for acute and chronic IVR session duration ranged from less than 5 min to 45 min or more (Baker et al., 2022). As we develop therapeutic interventions that incorporate IVR, we must determine specific parameters that will lead to best therapeutic results. As therapists have limited time with their patients, they need to ensure that all modalities achieve the desired effect; too little will undermine the goals of treatment, but overuse will waste time, frustrate patients, or even be potentially hazardous.
In this crossover, randomized pilot study we evaluated the effect of two different durations of IVR (10- and 20-minutes) on CBP symptoms (pain, fatigue, affect) and pain sensitization. We tested IVR effects on sensitization by evaluating changes in pain ratings in response to mechanical stimuli (Schuttert et al., 2021) using quantitative sensory testing (QST). QST assesses sensory and pain perception pathways (Schuttert et al., 2021). It quantifies the thresholds of detection of cutaneous stimuli, the perceived intensity, and the temporal summation (changes in perception over multiple applications) of pain. These psychophysical testing procedures are widely-accepted, non-invasive, and non-tissue damaging ways to measure central sensitization (Schuttert et al., 2021). We hypothesized that IVR would have a moderate effect CBP symptoms and QST-assessed indices of sensitization and that they would differ by different IVR durations.
2 Materials and methods
2.1 Participants
Our population was people with CBP. Inclusion criteria: aged 20 to 75; with CBP lasting greater than 3 months from non-cancer related injury/illness; pain greater than or equal to 4 (0 = no pain, 10 = worst pain). Exclusion criteria: back pain primarily due to rheumatic disorders (e.g., rheumatoid arthritis), back pain due to central neurological disorders (e.g., stroke, multiple sclerosis, spinal cord injury); contagious disorders or open areas on the face that would come in contact with the headset; previous seizures, loss of awareness, or other symptoms linked to an epileptic condition, used a hearing aid, pacemaker, or defibrillator; insufficient vision to see IVR programs; insufficient upper extremity coordination to operate IVR controls; insufficient cognitive ability to answer questionnaires or learn to use the IVR; unable to understand and respond to English.
Participants were recruited through Tufts Medical Center (TMC) Clinical Trials website, Facebook, letters sent by patient’s physiatrist at TMC, and fliers placed at Brigham & Women’s Chronic Pain Program and Spaulding Outpatient Center. Tufts University Institutional Review Board approved this study. The ClinicalTrial.Gov number was NCT04307446.
2.2 Instruments
Our primary outcomes were changes in symptoms (pain and fatigue), affect, and QST-based indicators of pain sensitization before and after the IVR experience.
2.2.1 Pain Numerical Rating Scale (NRS)
Participants rated their current pain intensity on a scale of 0–10 scale (0 = no pain, 10 = worst) for all body parts including the neck, upper and lower backs.
2.2.2 Fatigue NRS
Participants rated their fatigue level on a scale of 0–10 scale (0 = no fatigue, 10 = worst).
2.2.3 Positive and Negative Affect Schedule (PANAS)
Affect was measured on the PANAS (Thompson, 2007) a 20-item self-report questionnaire that measured self-reported positive (e.g., alert, excited) and negative (e.g., distressed, jittery) affect. Each item was rated on a 5-point Likert scale (1 = not at all, 5 = very much) and overall scores for each type of affect were the sum of 10-items.
2.2.4 Quantitative sensory testing (QST)
We used two QST testing protocols: Mechanical Pressure Pain Threshold (Pressure) and Mechanical Temporal Summation of Pain (Temporal).
2.2.4.1 Mechanical pressure pain threshold
For sensitivity to pressure we used a handheld pressure algometer (Wagner Instruments, Greenwich, CT) to measure mechanical pressure sensation at the right and left trapezius and quadriceps muscles by gradually increasing pressure on the muscle belly until participants indicated that the pressure became painful. Sensitivity to pressure is considered improved if it takes greater pressure to elicit a pain response during post IVR testing than pre IVR testing.
2.2.4.2 Mechanical temporal summation of pain
We determined temporal summation or windup to repeated punctate (pinprick) stimuli and painful aftersensations For temporal summation, 10 punctate mechanical stimuli were applied using PinPrick Simulator Set (MRC Systems GmbH) at the rate of 1 per second on the dorsum of the middle finger of the right hand. Participants rated the painfulness of the first, fifth, and 10th stimulus. To identify painful aftersensations, we asked participants to provide a final rating of remaining pain 15 seconds after discontinuing the punctate stimuli for the temporal summation test. Both tests were considered to improve if participants reported lower pain scores post IVR session than before.
We measured additional outcomes at the Baseline visit and at the beginning of Visits 1 and 2.
We obtained baseline demographics (e.g., gender, race, ethnicity), medical history (e.g., diagnosis, cause of pain, medications, non-medical treatment experiences), and experiences with IVR. Perceptions and attitudes towards pain were obtained using the Pain Catastrophizing Scale (Sullivan et al., 1995). Type of pain was determined using the PainDETECT Questionnaire (Freynhagen et al., 2006) which identifies participants with pain symptoms related to central sensitization, notably those with neuropathic type symptoms (symptoms associated with damage or dysfunction of the central or peripheral nervous system) compared to nociceptive type symptoms (symptoms associated with activation of nociceptors linked to the musculoskeletal system) (Freynhagen et al., 2006). We used Patient-Reported Outcomes Measurement Information System (PROMIS) outcome measures for fatigue (Food and Drug Administration, 2017) and pain interference (HealthMeasures, 2022). We used the user Engagement Scale [UES] (O’Brien et al., 2018), a twelve-item self-report questionnaire, to measure the participants perception of engagement with the experience using a 5-point Likert scale from 1 (strongly disagree) to 5 (strongly agree). An overall score was obtained by summing the results. We measured cybersickness with Simulator Sickness Questionnaire [SSQ] (Kennedy et al., 1993) a 27-item, 0 to 3 likert scaled questionnaire listing symptoms commonly experienced by IVR users.
2.3 Equipment
We used the Oculus Rift S (Meta.com) virtual reality system. The Rift S headset was tethered to a PC (Alienware). It has a per eye, 1,280-by-1,440 resolution with an 80 Hz refresh rate. It uses a 6-degree of freedom built in motion sensor system allowing the device to track both the direction users face (orientation) and position in space (Greenwald, 2020). Participants chose from two commercially available programs: Ocean Rift™ and Nature Trek™. Each program simulated a rich and immersive outdoor environment that had a variety of locations where the IVR user could visit. Participants could travel anywhere in the environment and had the opportunity to interact with and learn more about ocean animals (Ocean Rift™) or make changes to the environment (Nature Trek™). Users picked whichever program they wished during each Visit and whatever location(s) in the program appealed to them during their IVR session. These programs were selected because they had no set end point so they could be used equally well for 10 or 20 min, they had high quality graphics, and they were fairly intuitive so users could quickly learn how to use the controllers to interact in the environment.
2.4 Procedures
Randomization was generated via coin-flip and enrollment and allocation was performed by an independent researcher. Participants were randomly assigned to order of dosage using concealed allocation with some receiving 10-min during Visit 1 and some 20-min. Participants completed three visits. The Baseline Visit was remote, and participants completed informed consent and baseline questionnaires. Visit 1 and 2 data were collected in a lab setting. All participants received both dosages over two visits with at least a 72-h washout period. Immediately before and after the IVR session, current symptoms, affect, and QST were measured, and participants rated their cybersickness and engagement after each IVR session. At the end of Visit 2, participants responded to open ended questions regarding the IVR experience.
2.5 Data analysis
Data were organized to compare 10-min to 20-min regardless of order. Scores for back pain were calculated by adding rating upper and lower back scores. Difference scores for pre and post IVR were calculated by subtracting the pre IVR score from the post IVR score. The PROMIS fatigue and pain interference raw scores were rescaled into standardized T-scores (Food and Drug Administration, 2017; HealthMeasures, 2022) (Mean = 50; SD = 10) so we could compare our sample to the US sample. Average QST pressure scores in kilograms were calculated by taking the mean of combined thigh and shoulder scores. To examine temporal summation, we created a difference score by subtracting the one second pain intensity score from the ten second pain intensity score.
This was an exploratory pilot study and we did not complete a power analysis. Therefore, our data may have been underpowered to prevent Type II error (Sullivan and Feinn, 2012). To avoid this and to provide researchers and clinicians with information on the magnitude of the effect of IVR on pain our primary outcomes were effect sizes, Cohen’s dz. We calculated Cohen’s dz rather than Cohen’s ds (Lakens, 2013) because our two groups were strongly correlated due to our crossover design. In Cohen’s dz the numerator is the difference between the mean of the difference scores and the denominator is the standard deviation of the difference scores. We ran descriptive statistics and completed analyses of normalcy. Our primary outcomes, back pain, affect and fatigue, were normally distributed while QST were not. For the normally distributed data, we calculated effect sizes using means. For non-normally distributed data we first calculated an r by dividing the absolute standardized Wilcoxon signed rank test statistic z by the square root of the number of pairs (Marshall and Marquier, 2019) and then translated it to a d. These effect sizes were interpreted using Cohen’s classification of effect sizes: 0.20 is a small effect, 0.50 is a moderate effect and 0.80 and above is a large effect (Marshall and Marquier, 2019). For ease of interpretation, we coded all Cohen’s dz which demonstrated the expected direction of an outcome (i.e., An “improved” score) as positive and all those that demonstrated the opposite of the expected direction as negative.
We explored the generalizability of our results using inferential statistics. We used paired t-tests to compare pre IVR to post IVR scores and to compare scores between the 10- and 20-min groups. We used Wilcoxon signed rank tests for all QST comparisons. We set alpha at .05. For pre/post-test we used a 1-tail test, as we had a directional hypothesis (post test scores would show improvement over pretest scores) and we used a 2-tailed test for the 10- vs. 20-min scores as we did not have a directional hypothesis.
Our primary analysis combined 10- and 20-min dosage regardless of visit order. However, because of the use of the crossover design, we were concerned that there would be an order effect. We therefore completed a secondary analysis which separated the data by visit and examined the results during Visit 1 and Visit 2 separately. As results of these analyses showed different outcomes, we included these results to further clarify using IVR for pain.
3 Results
3.1 Demographics
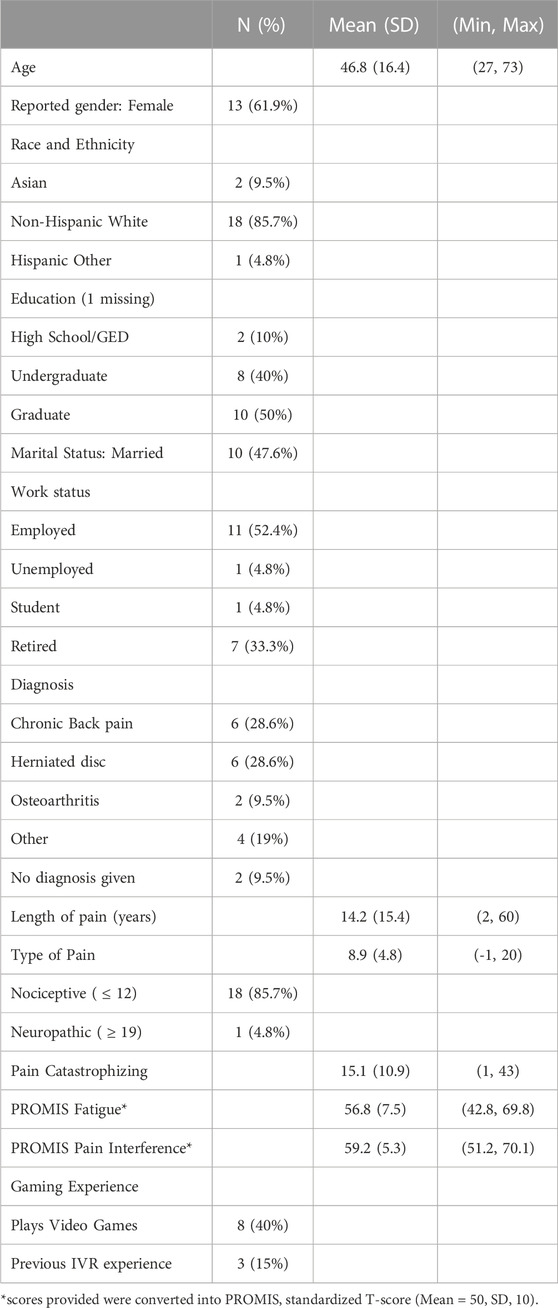
Twenty-three people were consented and enrolled. Two participants withdrew from the study (scheduling conflicts), and one participant only completed the first of two visits. The data from this first visit was included in the study. Table 1 provides the demographics of the sample. Based on the painDETECT score, relatively few participants reported likely neuropathic pain (5%). Only eight participants had experience with video games (40%) and only three had experience with IVR (15%). Average cybersickness score after IVR sessions was 5.4 (SD 4.4) on the SSQ (maximum possible score, 81).
3.2 Effect of IVR on symptoms and pain
Overall, IVR, regardless of dosage, had a significant moderate beneficial effect on back pain (dz = 0.52) and a small effect on Negative Affect (dz = 0.48) (Figure 1). When analyzed by dosage, the largest effects were seen for 20-min changes in back pain (dz = 0.49) (Figure 1) and PANAS Negative affect (dz = −0.32) and both were significant. Cohen’s dz for between 10-min and 20-min were all negligible to small and non-significant. Overall, 43% of participants reported a 30% or greater change in back pain. When examined by dosage, 42% of participants in the 10-min dosage reported a greater than 30% change in their pain score and 35% of participants in the 20-min dosage reported this change. This comparison was not significantly different. (Specific results of the tests including exact p-values are provided in the Supplementary Materials).
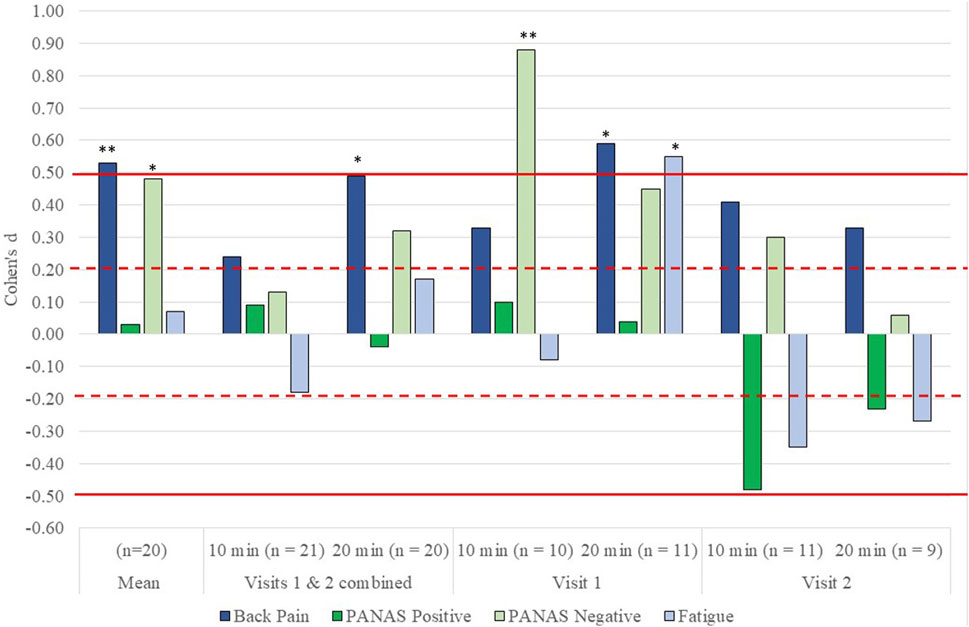
FIGURE 1. Effect size d for the effect of immersive virtual reality (IVR) on back pain, affect and fatigue overall, by dosage (10 min vs. 20 min) and by visit (Visit 1 vs. Visit 2). All results have been adjusted so that a positive d indicates that the score improved, and a negative score indicates that the score worsened after VR. Dotted horizontal lines indicate a small effect size while solid lines indicate a moderate effect size. *indicates a significant change between pre IVR scores and post IVR scores of p ≤ .05 while **indicates a significant change of p ≤ .01. Comparisons between 10 and 20 min of IVR were all non-significant.
3.3 Effect of IVR on QST
IVR, regardless of dosage, had a significant moderate effect reducing reports of pain for painful aftersensations (Figure 2). It had small to moderate effects at increasing temporal summation and sensitivity to pressure; however, these were not significant. When analyzed by dosage, a similar pattern emerged except the effect size for temporal summation was negligible to small and non-significant. Cohen’s dz for between 10-min and 20-min were all negligible to small and non-significant.

FIGURE 2. Effect size d for the effect of immersive virtual reality (IVR) on Quantitative Sensory Testing (QST) scores, by dosage (10 min vs. 20 min) and by visit (Visit 1 vs. Visit 2). All results have been adjusted so that a positive d indicates that the score improved and a negative score that the score worsened after IVR. Dotted horizontal lines indicate a small effect size while solid lines indicate a moderate effect size. *indicates a significant change between pre IVR scores and post IVR scores of p ≤ .05 while **indicates a significant change of p ≤ .01. Comparisons between 10 and 20 min of IVR were all non-significant.
3.4 Sensitivity analysis
Our sensitivity analyses compared 10- and 20-min results by visit (Figure 1, 2). There were larger positive effects for symptoms and affect during Visit 1, with the greatest effects occurring for those who received the 20-min dosages. During Visit 2, we continued to see positive effects for back pain and negative affect, but these were smaller than Visit 1. The effects on positive affect and fatigue indicated that participants reported decreased positive affect and greater fatigue during Visit 2, with those engaging in 10-min reporting larger, worse outcomes.
There was little clear pattern for the QST scores when analyzed by visit (Figure 2). In general, there were worsened results for temporal summation, which increased and pressure pain threshold which decreased after IVR, and improved results for painful aftersensations which diminished. Notably, there was a significant large effect on pressure pain threshold during the 20-min IVR for Visit 2, with participants reporting lower pain threshold to pressure after IVR than before, indicating it took less pressure to elicit a painful response after IVR than before.
4 Discussion
We evaluated the magnitude of the effects of IVR on pain, fatigue, affect and pain sensitization and whether effects differed for two different durations of IVR, 10-min and 20-min. We found that, overall, IVR had a moderate positive effect in reducing back pain, painful aftersensations and negative affect, a negligible effect on fatigue and positive affect, and a small sensitizing effect on pressure pain threshold and temporal summation. Over 40% of participants reported a 30% or greater reduction in back pain intensity after IVR, comparable to studies using opioids for pain reduction (Deyo et al., 2015).
There were no significant differences between 10- and 20-min of IVR. However, a detailed analysis indicates some valuable information for both clinical application and future research trials in IVR. When comparing 10- and 20-min of IVR without considering whether the visit was first or second, 20-min of IVR appeared more effective for CBP and negative affect than 10-min of IVR. However, when we examined dosage by first or second visit, we found that 20-min appeared more effective for back pain and fatigue in Visit 1, but the two dosages were similar in Visit 2. Ten minutes seemed to have a greater, albeit small, effect, on negative affect than 20-min in Visit 1, but not Visit 2.
These results suggest that there might be a threshold of time at the first visit to adjust to the logistics of using IVR before full engagement occurs. This threshold may be less on subsequent visits, allowing participants to achieve reduced symptoms in a shorter timeframe. The drop in scores for Visit 2 may also reflect the importance of using interesting and engaging programs. We chose simple experiences that required relatively short times to achieve competence. While this simplicity allowed participants to adjust to the IVR program quickly, programs may have become less interesting in Visit 2, particularly for the 20-min duration, reducing engagement and concomitant reductions in symptoms. This hypothesis was supported by several participants who reported that they were bored with programs by Visit 2. These results suggest that engagement is a key part of the mechanism for distraction.
IVR had only limited effects on indices of central sensitization. Overall, painful aftersensations improved, but temporal summation and sensitivity to pressure pain increased slightly. This effect may be explained by the overall nature of pain in this sample. Most symptoms were nociceptive not neuropathic, and the sample at baseline did not appear highly sensitized when evaluating QST responses. There may have been something of a ceiling effect in which no consistent reduction could be obtained. Further evaluation of IVR on people with neuropathic pain syndromes or who have known central sensitization would better determine whether IVR is an effective means to reduce central sensitization. Additionally, participants completed only two visits. It may require multiple applications of IVR to reduce central sensitization in people with CBP.
This study indicates that the question of what dosage is best for optimal reduction of symptoms in people with CBP is dependent on many factors. Longer dosages may be useful for a single visit. It may also be more optimal if the IVR experience is highly engaging, and people with CBP wish to continue with the experience. However, shorter doses appear effective, particularly over several visits, and may be more practical in a clinical situation where a therapist has a limited amount of time to see a patient.
This is a small pilot study and had multiple limitations. The sample size was not selected to obtain power so it is possible that a larger sample would have obtained significant results. There was a high heterogeneity in the age of participants. We did not test for central sensitization before starting the evaluation, it was not one of our inclusion criteria, leading to the potential for a ceiling effect in our participants. The IVR that we chose might have been better if it had been more interactive and engaging. Further, while the two IVR program used in the study were similar they were not identical in their level of engagement, therefore difference in pain may have been due to the type of program used rather than the dosage. Further tests on types of IVR are highly recommended to determine which have the greatest engagement and if this engagement is correlated with pain modulation. Adding a control condition would also increase our ability to interpret effects.
4.1 Conclusion
Immersive virtual reality is an effective method to address acute pain, and there is increasing evidence that it is also effective with chronic pain. This study was an initial examination of a more nuanced application of virtual reality by examining dosage. Our results suggest that differing dosages can have different effects but was inconclusive and did not identify that one dosage was superior to another. The characteristics of the IVR such as level of engagement in the immersive environment, the type of task, and the level of competence with the IVR are as likely as dosage to alter the effects on pain, affect, and pain sensitization. Further study is needed to fully understand what characteristics of IVR engagement affect pain and other symptoms and what type of dosage may be needed depending upon circumstances. Future work should also examine the effect of IVR on specific causes of pain as well as by differing levels of pain beliefs such as pain catastrophization. Our study also used QST to examine pain sensitization, which can often play an important contributing role in shaping CBP symptomatology. We did not detect a reduction in pain sensitization from pre-to post-IVR; however, further evaluation is needed as there are still many questions that need to be answered about virtual reality and its impact on central sensitization.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://clinicaltrials.gov/study/NCT04307446.
Ethics statement
The studies involving humans were approved by The Health Sciences Institutional Review Board Tufts University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
NB: Conceptualization, Formal-analysis, Funding-acquisition, Methodology, Project-administration, Supervision, Visualization, Writing–original draft, Writing–review and editing. AP: Investigation, Project-administration, Supervision, Writing–original draft, Writing–review and editing. MK: Conceptualization, Methodology, Writing–review and editing. RB: Resources, Writing–review and editing. NW: Methodology, Writing–review and editing. JI: Methodology, Writing–review and editing. RE: Conceptualization, Resources, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding from 2020 Tufts CTSI Pilot Studies Program Through Tufts CTSI NIH Clinical and Translational Science Award (UL1TR002544).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frvir.2023.1260313/full#supplementary-material
References
Baker, N. A., Polhemus, A. H., Haan Ospina, E., Feller, H., Zenni, M., Deacon, M., et al. (2022). The state of science in the use of virtual reality in the treatment of acute and chronic pain: a systematic scoping review. Clin. J. Pain 38 (6), 424–441. doi:10.1097/ajp.0000000000001029
Bazzari, A. H., and Bazzari, F. H. (2022). Advances in targeting central sensitization and brain plasticity in chronic pain. Egypt. J. Neurology Psychiatry Neurosurg. 58 (1), 38. doi:10.1186/s41983-022-00472-y
Deyo, R. A., Von Korff, M., and Duhrkoop, D. (2015). Opioids for low back pain. BMJ Clin. Res. 350, g6380. doi:10.1136/bmj.g6380
Food and Drug Administration, (2017). PROMIS fatigue scoring manual. Patient Reported Outcome Measurement Information System. Available at: https://www.fda.gov/media/137977/download.
Freynhagen, R., Baron, R., Gockel, U., and Tolle, T. R. (2006). painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr. Med. Res. Opin. 22 (10), 1911–1920. doi:10.1185/030079906x132488
Garcia, L., Birckhead, B., Krishnamurthy, P., Mackey, I., Sackman, J., Salmasi, V., et al. (2022). Durability of the treatment effects of an 8-week self-administered home-based virtual reality program for chronic low back pain: 6-month follow-up study of a randomized clinical trial. J. Med. Internet Res. 24 (5), e37480. doi:10.2196/37480
Garcia, L. M., Birckhead, B. J., Krishnamurthy, P., Sackman, J., Mackey, I. G., Louis, R. G., et al. (2021). An 8-week self-administered at-home behavioral skills-based virtual reality program for chronic low back pain: double-blind, randomized, placebo-controlled trial conducted during COVID-19. J. Med. Internet Res. 23 (2), e26292. doi:10.2196/26292
Gore, M., Sadosky, A., Stacey, B. R., Tai, K.-S., and Leslie, D. (2012). The burden of chronic low back pain: clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine (Philadelphia, Pa 1976) 37 (11), E668–E677. doi:10.1097/brs.0b013e318241e5de
Greenwald, W. (2020). Oculus quest 2 vs. Oculus Rift S: which VR headset should you buy?. PCMag. Available at: https://www.pcmag.com/comparisons/oculus-quest-vs-oculus-rift-s-which-vr-headset-should-you-buy#.
Gupta, A., Scott, K., and Dukewich, M. (2018). Innovative technology using virtual reality in the treatment of pain: does it reduce pain via distraction, or is there more to it? Pain Med. 19 (1), 151–159. doi:10.1093/pm/pnx109
HealthMeasures, (2022). PROMIS pain interference scoring manual. Available at: https://www.healthmeasures.net/images/PROMIS/manuals/Scoring_Manual_Only/PROMIS_Pain_Interference_Scoring_Manual_03June2022.pdf.
Kennedy, R. S., Lane, N. E., Berbaum, K. S., and Lilienthal, M. G. (1993). Simulator Sickness Questionnaire: an enhanced method for quantifying simulator sickness. Int. J. Aviat. Psychol. 3 (3), 203–220. doi:10.1207/s15327108ijap0303_3
Lakens, D. (2013). Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front. Psychol. 4, 863. doi:10.3389/fpsyg.2013.00863
Loreto-Quijada, D., Gutiérrez-Maldonado, J., Nieto, R., Gutiérrez-Martínez, O., Ferrer-García, M., Saldaña, C., et al. (2014). Differential effects of two virtual reality interventions: distraction versus pain control. Cyberpsychology, Behav. Soc. Netw. 17 (6), 353–358. doi:10.1089/cyber.2014.0057
Marshall, E., and Marquier, B. (2019). Wilcoxon signed-rank test in SPSS. Sheffield, UK: University of Sheffield. ND.
Meucci, R. D., Fassa, A. G., and Faria, N. M. X. (2015). Prevalence of chronic low back pain: systematic review. Rev. Saude Publica 49, 1. doi:10.1590/s0034-8910.2015049005874
Nijs, J., Meeus, M., Van Oosterwijck, J., Roussel, N., De Kooning, M., Ickmans, K., et al. (2011). Treatment of central sensitization in patients with 'unexplained' chronic pain: what options do we have? Expert Opin. Pharmacother. 12 (7), 1087–1098. doi:10.1517/14656566.2011.547475
O’Brien, H. L., Cairns, P., and Hall, M. (2018). A practical approach to measuring user engagement with the refined User Engagement Scale (UES) and new UES short form. Int. J. human-computer Stud. 112, 28–39. doi:10.1016/j.ijhcs.2018.01.004
Schuttert, I., Timmerman, H., Petersen, K. K., McPhee, M. E., Arendt-Nielsen, L., Reneman, M. F., et al. (2021). The definition, assessment, and prevalence of (human assumed) central sensitisation in patients with chronic low back pain: a systematic review. J. Clin. Med. 10 (24), 5931. doi:10.3390/jcm10245931
Sullivan, G. M., and Feinn, R. (2012). Using effect size-or why the P value is not enough. J. Grad. Med. Educ. 4 (3), 279–282. doi:10.4300/jgme-d-12-00156.1
Sullivan, M. J. L., Bishop, S. R., and Pivik, J. (1995). The Pain Catastrophizing Scale: development and validation. Psychol. Assess. 7 (4), 524–532. doi:10.1037/1040-3590.7.4.524
Tack, C. (2021). Virtual reality and chronic low back pain. Disabil. Rehabil. Assist. Technol. 16 (6), 637–645. doi:10.1080/17483107.2019.1688399
Thompson, E. R. (2007). Development and validation of an internationally reliable short-form of the Positive And Negative Affect Schedule (PANAS). J. Cross-Cultural Psychol. 38 (2), 227–242. doi:10.1177/0022022106297301
Keywords: chronic pain, dosage, emotion, fatigue, functional somatic syndrome
Citation: Baker NA, Polhemus A, Kenney M, Bloch R, Ward N, Intriligator J and Edwards R (2023) Examining the difference between 10- and 20-min of immersive virtual reality on symptoms, affect, and central sensitization in people with chronic back pain. Front. Virtual Real. 4:1260313. doi: 10.3389/frvir.2023.1260313
Received: 17 July 2023; Accepted: 13 October 2023;
Published: 24 November 2023.
Edited by:
Marientina Gotsis, University of Southern California, United StatesReviewed by:
Vangelis Lympouridis, University of Southern California, United StatesMiguel Angel Padilla-Castaneda, National Autonomous University of Mexico, Mexico
Copyright © 2023 Baker, Polhemus, Kenney, Bloch, Ward, Intriligator and Edwards. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nancy A. Baker, bmFuY3kuYmFrZXJAdHVmdHMuZWR1
 Nancy A. Baker
Nancy A. Baker Augusta Polhemus1
Augusta Polhemus1 Nathan Ward
Nathan Ward James Intriligator
James Intriligator