- 1Department of Medicine, Uniformed Services University of the Health Sciences, Bethesda, MD, United States
- 2Center for Neuroscience and Regenerative Medicine, Uniformed Services University of the Health Sciences, Bethesda, MD, United States
- 3Walter Reed National Military Medical Center, Bethesda, MD, United States
- 4The Henry M. Jackson Foundation, Bethesda, MD, United States
- 5National Intrepid Center of Excellence, Walter Reed National Military Medical Center, Bethesda, MD, United States
- 6Department of Psychiatry, Leiden University Medical Center, Leiden, Netherlands
Background and Purpose: PTSD and mTBI are persistent and frequently comorbid after combat, yet current therapies often achieve only modest impact. A novel exposure-based “walk and talk” cognitive therapy, Motion-Assisted, Multi-Modal Memory Desensitization and Reconsolidation (3MDR), featuring participant-selected music and pictures and an eye movement (EM) task in an immersive virtual environment, has shown efficacy in treatment-resistant male veterans, but has not been studied in women or after TBI. The EM task is adapted from eye movement desensitization and reprocessing (EMDR) therapy, but dismantling studies of EMDR have questioned EM benefit. This pilot study assesses 3MDR in male and female veterans with comorbid PTSD and mTBI, and the impact of EM on response. We hypothesized that 3MDR would prove efficacious, both with (EM+) and without EM (EM-).
Design: Participants with probable PTSD (PCL-5 ≥ 34) and mTBI were randomized to EM + or EM-across 10 sessions. Participants provided songs and pictures that they rated on impactfulness. While walking in the 3MDR virtual environment, participants started with a song to bring them back to the time of their trauma, and then traversed two hallways, actively walking toward emotionally evocative pictures that they then discussed with their therapist. Key words or feelings they expressed were superimposed over the picture, then read aloud, whereupon EM + participants recited numbers flashing on a ball crisscrossing the picture. These procedures were repeated for multiple pictures per session. A song to return the participant to present day closed each session. Change in PCL-5 score from pre-to post-intervention was the primary outcome, with additional measures at 3 and 6 months.
Results: Sixteen (80%) of 20 participants completed the intervention (8 EM+, 8 EM-); 9 (6 EM+, 3 EM-) had resolution of PTSD diagnosis and two improved significantly without resolution. Average PCL-5 score declined from 52.0 (95% confidence intervals: 46.3, 57.7) at baseline to 33.6 (24.3, 42.9) post-intervention (p < 0.01). The EM + group achieved statistically significant improvement (p = 0.01) while the EM-did not (p = 0.10).
Conclusion: For veterans with PTSD and comorbid mTBI, 3MDR is effective, and the EM component may add value. Confirmation with larger studies is important.
Introduction
Posttraumatic stress disorder (PTSD) and mild traumatic brain injury (mTBI), also known as concussion, are highly prevalent and frequently comorbid, signature wounds of the Global War on Terror. Mild TBI may alter key brain regions and pathways to facilitate the emergence of PTSD (Kennedy et al., 2010) through alterations in key brain regions and pathways, or reduce resilience by overwhelming compensatory mechanisms such as brain-derived neurotrophic factor. We previously demonstrated that combat mTBI is associated with disruption of white matter tracts, reduced functional connectivity, and PTSD severity (Costanzo et al., 2014), and that comorbid PTSD and mTBI are associated with cognitive impairment (Dunbar et al., 2019). There are currently no proven treatments for mTBI (Minen et al., 2018; Feinberg et al., 2021). For PTSD, Cognitive Processing Therapy (CPT) and Prolonged Exposure (PE) have the strongest evidence base to support their use (VA, DoD, 2017) but even in well-controlled clinical trials with expert therapists, many do not resolve their PTSD (Steenkamp et al., 2015; Rapcencu et al., 2017; Rauch et al., 2017; Foa et al., 2018). Moreover, “talk therapy” is not appealing to many service members (SMs) leading many to either disengage or fail to follow up. Pharmacotherapy has even more dismal response and compliance rates (Lee et al., 2016), highlighting a need for the development and validation of more appealing and effective therapies for PTSD.
To that end, virtual reality exposure therapy (VRET), whether via head-mounted display or immersive “cave” environment, has the potential to take the weight off a patient’s shoulders by facilitating their recall of events through virtual cues. We previously reported that VRET achieves significant improvement on functional magnetic resonance imaging in key brain regions that are altered in PTSD, namely the amygdala, hippocampus, and anterior cingulate cortex (Roy et al., 2010; Roy et al., 2014). VRET has also significantly decreased PTSD symptom severity, social isolation, depression and anger, in Operation Enduring Freedom (OEF) and Operation Iraqi Freedom (OIF) veterans and active duty military personnel (Beidel et al., 2019). VRET has also demonstrated safety and efficacy in treating military sexual trauma (MST)-related PTSD (Loucks et al., 2019). This is significant, because women comprise 14% of the active duty military population, but tend to have somewhat higher rates of PTSD than men, likely because of the greater likelihood of both prior life trauma as well as military sexual trauma (MST). Another alternative therapy, eye movement desensitization and reprocessing (EMDR) (Shapiro, 1989), which combines elements of exposure therapy with an eye movement task, has evidence to support its use for PTSD (Gunter and Bodner, 2008). Working memory theory postulates the brain has limited resources and has been used to articulate the potential therapeutic benefit of a unique element of EMDR: a dual task (following an object with your eyes) will render less memory capacity for other memory processes, so recollection will be less vivid and affect-laden. However, the benefit of the eye movement task has been questioned (Albright and Thyer, 2009bib_albright_and_thyer_2009bib_albright_and_thyer_2009bib_albright_and_thyer_2009).
Motion-Assisted, Multi-Modal Memory Desensitization and Reconsolidation (3MDR) is a novel PTSD therapy founded on dual-task processing and working memory theory (Vermetten et al., 2013; Steenkamp et al., 2015); 3MDR methods have been previously described (van Gelderen et al., 2018). Briefly, 3MDR incorporates new technologies in order to decrease cognitive avoidance and increase engagement, combining the attractive features and previously demonstrated therapeutic principles of VRET and EMDR, within the novel context of the Computer Assisted Rehabilitation Environment (CAREN). The CAREN is technologically sophisticated, featuring a motion platform with embedded treadmill, surrounded by a 180-degree panoramic screen to display virtual environments (VEs) that are synchronized to the speed of the treadmill. The CAREN system was developed as a rehabilitation device (Collins et al., 2015), but we previously demonstrated it can be used to distinguish between service members with TBI alone versus TBI plus PTSD (Onakomaiya et al., 2017).
During 3MDR treatment, participants walk in a virtual environment (VE) on the CAREN’s treadmill, first listening to a self-selected song to bring them back to the time of their trauma, and then actively walk toward self-selected, emotionally evocative pictures displayed within the VE. The CAREN facilitates the multi-modal approach of 3MDR by enabling physical activity (walking), and simultaneous verbal engagement with a therapist, to occur within a fully immersive VE that can be individualized to enable confrontation, and in doing so to overcome avoidance. The physical movement, music and high-affect visuals are intended to diminish emotional avoidance during exposure (Vermetten et al., 2013). Walking may be more than just a distraction, however, as physical activity has been shown to be beneficial in reducing symptoms of PTSD and depression (Robertson et al., 2012; Rosenbaum et al., 2015) perhaps by increasing blood flow to the brain, production of endorphins, and/or other mechanisms. In 3MDR, participants learn how to actively overcome their avoidance (a defining feature of PTSD) by deliberately confronting, representations of their own traumatic memories. This allows the patient to ‘step into the past’, and has been reported to assist in reduction of symptoms and to enhance self-empowerment (Nijdam and Vermetten, 2018). As such, 3MDR may prove to be an effective treatment with specific benefits derived from the unprecedented combination of: 1) virtual reality-based immersion, 2) concurrent physical activity, 3) continual communication with a therapist throughout the treatment period, and 4) lateral eye movement, integrated in a more engaging manner than has previously been the case in traditional EMDR.
Preliminary results regarding the efficacy of the 3MDR approach in veterans with treatment-resistant, combat-related PTSD have been promising. A case study of two veterans with chronic PTSD (Vermetten et al., 2013) documented pre-to post-treatment reductions in PTSD symptoms measured by the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5). A more recent case report documented that two of three participants’ PTSD symptoms reduced so significantly that they no longer met criteria for PTSD after treatment, and these clinically significant results were maintained after additional follow up (Van Gelderen et al., 2018). A pilot study of 3MDR conducted at the Ottawa Hospital provided treatment for five Canadian Armed Forces SMs with treatment-resistant PTSD (Jetly et al., 2017) utilizing the PTSD Checklist for DSM-5 (PCL-5) as the primary outcome measure. In this small sample, PCL-5 scores declined from an average of 47 at baseline, to 33.8 post-treatment, with a decline of 10 or more generally being considered clinically significant (Jetly et al., 2017). Participants also expressed positive impressions of the treatment and exhibited high compliance. Recently, two larger clinical trials have been published (Bisson et al., 2020; van Gelderen et al., 2020), documenting the efficacy of combat-related, treatment-resistant PTSD. The first study randomized 43 Dutch military veterans (42 males) to 10 sessions of 3MDR or a non-specific treatment component control group. A clinically relevant and significantly greater decline in PTSD severity, as measured with the CAPS-5, was evident in the 3MDR group, with 9 of 20 (45%) classified as responders, whereas only 2 of 20 (10%) improved in the control group (van Gelderen et al., 2020). The second study was conducted at the Institute of Psychological Medicine and Clinical Neurosciences at Cardiff University’s School of Medicine, randomizing 42 male British veterans with treatment-resistant, service-connected PTSD, to either immediate 3MDR or a waitlist with subsequent delayed 3MDR. Once again, 3MDR was found to achieve superior improvement in PTSD severity (Bisson et al., 2020). The effect sizes for the two studies were reported to be 0.83 and 0.65, respectively.
To date, all published 3MDR studies have incorporated the full treatment paradigm, but the eye movement element, patterned after EMDR with rapid horizontal back-and-forth eye movements, has been a controversial feature within the EMDR paradigm itself. Some assessments of the role of eye movement tasks have been conducted in non-clinical, undergraduate or healthy participant populations (Schubert et al., 2011; van den Hout et al., 2010; Patel and McDowall, 2016; Samara et al., 2011), which may not necessarily be generalizable to combat veterans with PTSD. A 2013 meta-analysis of the contribution of eye movements, encompassing 15 clinical trials that compared EMDR therapy with and without eye movements, did identify a moderate effect size for the eye movement element of EMDR (Lee and Cuijpers, 2013), but there were methodologic limitations to many of the studies that had been conducted. A subsequent randomized controlled trial compared three different treatment groups of participants with PTSD: 1) exposure while fixating on the therapist’s moving hand, 2) exposure fixating on a non-moving hand, and 3) a control group (Sack et al., 2016). This more rigorously designed study found no significant differences in PTSD symptom improvements between both treatment groups, suggesting that eye movement tasks did not significantly enhance the benefit afforded by the other elements of EMDR. Therefore, the purpose of the current pilot study was to gather an initial assessment of whether the eye movement task adds significant value to the 3MDR treatment paradigm. We also targeted 50% women to provide preliminary evidence of the benefit of 3MDR across gender.
Materials and methods
Participants and setting
For this pilot study, 20 participants were enrolled, evenly divided between female and male active duty SMs or veterans, with a diagnosis of probable PTSD as manifest by a Posttraumatic Stress Disorder Checklist for DSM5 (PCL-5) score of at least 34. Participants were also required to have a history of mTBI, documented by administration of the Ohio State TBI identification method, with a period of at least 3 months since their most recent concussion. Exclusion criteria were: a diagnosis of moderate or severe TBI; a psychotic disorder, bipolar disorder, or active suicidal or homicidal ideations; regular use of benzodiazepines in the previous 30 days; inability to walk continuously at a normal pace for 60 min; active pregnancy; and inability to demonstrate the capacity for informed consent. The study was conducted in the CAREN Laboratory at the National Intrepid Center of Excellence, Walter Reed National Military Medical Center (WRNMMC), Bethesda, MD. All procedures were approved by institutional review boards at WRNMMC and Uniformed Services University (USU), both in Bethesda, MD. All participants completed written informed consent administered by the principal investigator before taking part in any study procedures.
Procedures
Once enrolled, participants were randomly assigned to the Eye Movement group (EM+) or the No Eye Movement group (EM-) and were paired with a single study therapist for the duration of the intervention. Each study therapist was responsible for contacting their participant to schedule their sessions. Our 3MDR procedures are consistent with those previously described (Mert & Vermetten, 2011; Vermetten et al., 2013). Briefly, all participants were required to attend 10 sessions: three preparatory, six intervention, and one consolidation.
The purpose of the first preparatory session was to establish rapport, educate the participant on the elements of 3MDR, facilitate the selection of their music and pictures, and to establish familiarity with the CAREN system. This session included psychoeducation about PTSD, an explanation of the 3MDR VE and the principle of reconsolidation, and how the various elements are designed to improve their symptoms. The therapist also discussed walking on the treadmill and how they can choose to adjust their pace. Finally, the therapist discussed possible reactions to the exposure to their pictures, emphasized the importance of allowing oneself to feel the associated emotions, and noted that distress is normal and is expected to decrease over the course of the intervention. Participants were instructed on how to select pictures that appropriately evoked memories of their traumatic experience(s), as well as songs to recall the period of the trauma as well as bring them back to present day. The second preparatory session centered on a discussion of the pictures and music selected. The therapist first reviewed the pictures with the participant to ensure their suitability in facilitating recall of the traumatic event. The participant was asked to rate each picture on the Subjective Units of Distress scale (SUDS), ranging from 1-10 based on the level of distress that each picture evoked. The therapist also reviewed the participant’s music selections, discussed why they were chosen, and tried to ensure that the songs selected were suitable to differentiate past from present. The third preparatory session introduced the participant to the CAREN and the 3MDR VE. Participants were provided with a safety brief and fit with a full-body harness. They were acclimated to the system through a battery of preliminary VEs, over the course of approximately 10-15 min, to ensure they felt comfortable weight-shifting and walking on the CAREN and that no dizziness or disorientation occurred. The therapist then joined the participant on the platform, standing to the left of the treadmill, for a practice run of the actual 3MDR VE, using a neutral picture (e.g., a fruit basket) and neutral music (i.e., instrumental music). Upon immediate verbal questioning, no participants reported discomfort with these procedures.
The six intervention sessions (Sessions 4-9), where the participant and therapist worked through trauma(s) related to the pictures provided, had three phases: warm-up, intervention, and cool-down. The warm-up phase began with the participant on the treadmill, and the therapist standing next to the treadmill, on the CAREN platform. Once the participant was brought up to their comfortable walking pace, while their pre-selected music was played to bring them back to the time of the trauma. During this phase, an outdoor scene with a bright sky and a blue honeycomb pathway was displayed on the panoramic screen with the visuals linked directly to the participant’s walking speed. Once the first song was played through in its entirety, the visual on the screen then shifted to a darkened sky and a red honeycomb pathway with a set of metal doors shown in the distance. This transition indicated a shift to the intervention phase of the session. During this phase, the participant continued to walk through the doors, into a hallway. They continued along the hallway until they reached a second set of double doors. These doors opened to a second hallway, at the end of which appeared one of their pre-selected pictures. At first, the picture was in the distance but increased in size as the participant continued to walk toward it. Once they reached the end of the hallway and the picture was at full size, the visual on the screen came to a stop so the picture remained large in front of the participant while they continued to walk on the treadmill. At this time, the therapist would query the participant about what the picture represented, associated memories, and any physiological symptoms they might have been feeling as they discussed the picture (e.g., heart racing, tension, sweating). During this discussion, the therapist relayed to the engineer key phrases or emotions (e.g., frustration, guilt), expressed by the participant, to superimpose them over the image. When the therapist felt the discussion was sufficiently complete, typically after 10-15 min, they asked the participant to read the superimposed words aloud and then the words faded away. If the participant was in the EM + group, after the words faded, a red ball with a white number bounced back-and-forth across the screen, superimposed over the picture, for approximately 30 s. Participants were asked to read aloud the number they saw on the ball, as the number changed each time the ball reached either edge of the screen. If a participant was in the EM-group, they were asked to continue to look at their image for another 30 s, thinking about the words and discussion. The picture would then fade away and the participant was asked to provide their SUDS score on a scale from 1-10. This process of walking into and through the two hallways, was repeated for up to seven pictures per session. After the final picture, they transitioned to the cool-down phase of the session where the final SUD was obtained. During this phase, the visual changed back to the blue honeycomb pathway and the participant’s second piece of music was played in its entirety to help them mentally return to the present time. Then the treatment was complete, the therapist and participant were escorted off the CAREN platform to debrief about how the session went and what lingering thoughts or emotions still needed to be processed. The participant was then asked to write, diary-style, about the day’s experience. The therapist did the same.
The final consolidation session was conducted with the therapist and principal investigator. The participant was asked to complete a battery of questionnaires and the therapist reviewed the progress made, highlighting significant improvements in symptoms and SUDs with select pictures, as well as quotes from their written entries. Finally, there was a discussion of what additional therapy, if any, might be helpful to supplement the gains made during the study, and how it might be obtained. Figures illustrating the CAREN system and 3MDR VE, are included in the supplemental materials (Supplementary Appendix A).
Measures
The primary outcome measure was the well-validated PCL-5 to assess self-reported PTSD symptom severity. The PCL-5 was administered at baseline, after the completion of three intervention sessions (to assess midway progress), post-therapy at the final session, and at 3- and 6-month follow-up. Secondary measures consisted of several self-report measures questionnaires that assess conditions that are frequently comorbid with PTSD: the Neurobehavioral Symptom Inventory (NSI) to assess post-concussive symptom severity, the Patient Health Questionnaire Depression module (PHQ-9) to assess depression symptom severity, and the Insomnia Severity Index (ISI) to evaluate insomnia and sleep concerns. Secondary measures were administered at baseline, post-therapy, and at 3- and 6- month follow-ups.
Analytic plan
Student’s t-tests, 2-tailed for paired samples, were used to assess improvement in PTSD symptom severity on the PCL-5 for the entire study population and within each arm and the effect size for change in PCL-5 score over time (both groups combined). The Shapiro-Wilk test was conducted to assess the normality assumption of the pre- and post-intervention PCL-5 scores. As an additional step, non-parametric tests of the paired samples, using the Paired Wilcoxon Signed-Rank test, were applied. The Cohen’s f was computed for the fixed effect of time from a mixed model, to derive the effect size for the entire study population. Given the small sample size, rather than comparing the EM+ and EM-groups using conventional statistical tests, we performed tests of group differences in PCL-5 pre vs. post-intervention, based on small sample methods (i.e., Fisher’s exact test, permutation test), to determine if the observed data between the two groups were different from a randomly distributed pattern.
Results
Demographics
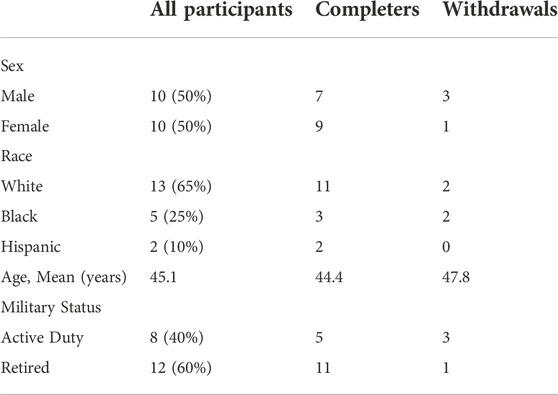
By design, equal numbers of males and females were recruited to participate in the study. Demographic details for all participants, with comparisons between those completing vs. withdrawing from the study, are presented in Table 1. The 16 participants (80%) who completed the full intervention were equally distributed between those randomized to perform the eye movement task (EM+) and those not performing this task (EM-). The four who did not follow through with the intervention identified changes in their schedules as the reason for their withdrawal; all withdrew prior to the CAREN sessions, and the withdrawal of three of the four was directly and entirely attributable to the impact of the COVID-19 pandemic, which forced the shutdown of the study for approximately 6 months.
Outcomes
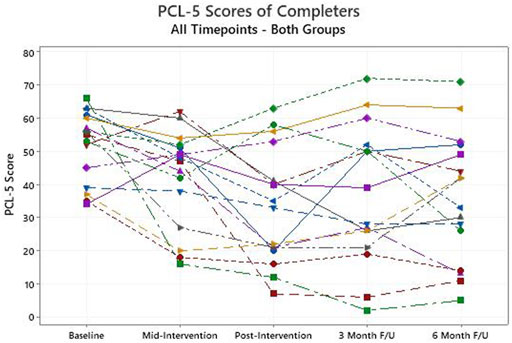
Among those that completed the intervention, the primary outcome measure, the PCL-5 score, declined from a baseline average of 52.0 (95% confidence intervals: 46.3, 57.7) to 33.6 (24.3, 42.9) after the intervention (p < 0.01, Figure 1). For the PCL-5, a change of 10 or more is generally considered clinically significant. When those who withdrew are included in an intention to treat analysis, and their baseline scores are carried forward and used unchanged as post-intervention scores, the average improvement on the PCL-5 remains clinically and statistically significant, dropping from 52.6 at baseline to 37.9 (p < 0.01). Those in the EM + group achieved statistically significant improvement on average (p = 0.01) while those in the EM-group did not (p = 0.10), but the numbers are small in each group (Figure 2). Non-parametric assessments of change in PCL-5 scores indicated a similar pattern of significance (i.e., overall change in PCL-5, p < 0.01; change in EM + group, p < 0.04; change in EM-group, p < 0.11). For the 16 completers, seven (5 EM+, two EM-) had resolution of their PTSD diagnosis, four had significant improvement in symptom severity without resolution (1 EM+, 3 EM-), and five (2 EM+, 3 EM-) did not achieve significant symptom reduction but did feel that they had made significant progress in opening up about their symptoms. Overall, 10 (5 EM+, 5 EM-) of the 16 had a clinically significant decline on the PCL-5, and there was no evidence of a significant difference in the response between the EM+ and EM-groups with the Fisher’s Exact Test or the Permutation Test, which both account for the small sample size. The Cohen’s f that was computed to assess the effect size for change in PCL-5 score over time in both groups (EM+,EM-) combined was 0.3. This value represents a medium to large effect size on the Cohen’s f scale -- i.e., Cohen’s f = Cohen’s d/2. The improvements observed in completers at the end of the intervention were largely sustained at the 3- and 6-month follow-up assessments, with average PCL-5 scores of 37.0 and 36.0 respectively (Figure 1).
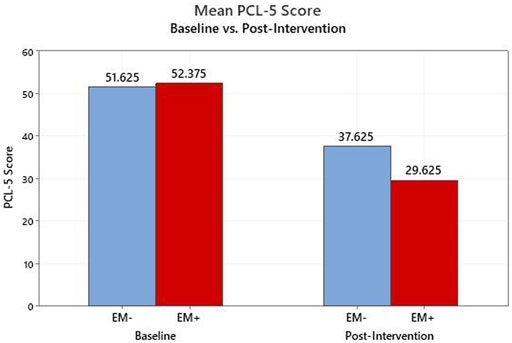
FIGURE 2. PCL-5 Scores from baseline to post-intervention for completers; Breakdown by eye movement group (EM+, EM−).
Gender did not significantly impact response to treatment, with the nine female completers dropping from an average baseline PCL-5 score of 53.7 to 33.4 post-intervention, while the average score for the 7 male completers’ declined from 49.9 to 33.9. More women completed treatment, and they had a larger magnitude of improvement, on average.
Most participants had a history of multiple traumas, with combat and sexual traumas predominant. The most common themes addressed by those with combat trauma were anger, frustration and closure, while those with sexual trauma most often addressed anger, disgust, frustration, fear, and regret. Individual participants completing the study expressed greater understanding and clarity post-intervention: “I can notice exactly what I was feeling and why. Things seem more clear now.”; “I am able to make connections not previously thought possible”. Others noted significant cognitive improvement: “strengthened but not hardened”; “…able to describe … more difficult images in detail and not feel overwhelmed by them and the memories”; “very relaxed and positive … more at peace with my circumstances than I have been in years”.
Discussion
This is a relatively small pilot study, but it breaks new ground in several important ways with regard to demonstrating the efficacy of 3MDR therapy for PTSD. It is the first study to move beyond treatment-resistant, combat-related PTSD that has been the focus of prior studies in Dutch and British veterans, as this study allowed PTSD from any source, though all participants were either active duty or military veterans. In addition, it is the first study to include a significant number of female participants, as prior studies almost exclusively treated male veterans. Further, all participants in this study had a history of mTBI in addition to probable PTSD. The results of this study, demonstrating clinically and statistically significant improvement in PTSD symptom severity across the study population, therefore expand the evidence base for 3MDR, indicating that it may benefit all military SMs with PTSD, with or without mTBI, both male and female. Moreover, it is important to also highlight the enthusiasm with which participants embraced the 3MDR approach. Dropout rates are historically quite high (20-50%) in PTSD treatment trials, ostensibly largely because a defining feature of PTSD is avoidance of reminders of one’s trauma, and engaging in therapy inevitably results in confronting those memories in one way or another. The unique features of 3MDR seem particularly engaging to participants, perhaps in part because of the novelty of the VE, and the distraction or additional stimulation of walking on a treadmill and engaging in an eye movement task, but probably more significantly the fact that they invested themselves by choosing music and pictures that are integrated into the VE. While our numbers are small, the fact that no one withdrew after starting therapy in the CAREN is compelling, and while the overall 20% dropout rate is at the lower end of what might be expected in a PTSD treatment trial, the fact that three of the four withdrawals occurred in the context of COVID-19 protocols, without which the dropout rate almost certainly would have been less than historical norms. The prior Dutch (n = 43) and British studies (n = 42) had significantly larger sample sizes, and each had only one drop-out during the intervention phase, while we had no drop-outs during the CAREN sessions, so that across the three studies with more than 100 participants, even in the face of the COVID pandemic that impacted our study, it is clear that 3MDR is able to achieve significantly higher compliance than other PTSD therapies.
One purpose of the study design, randomizing participants in equal numbers to EM+ and EM-arms, was to gather preliminary evidence of whether the eye movement task adds value to the overall 3MDR approach, given some debate about whether it is a positive contributor to the success achieved with EMDR. Again with the caveat of small numbers, our results lend support to the inclusion of the eye movement task, as a greater number in the EM + group had resolution of their PTSD diagnosis, and they had statistically significant improvement in their PTSD symptom severity. On the other hand, the EM-group, while not achieving statistical significance, did have a trend favoring improvement in symptoms despite the small numbers, and both the EM+ and EM-groups had clinically significant improvement in symptoms on average. There was no evidence of a significant difference in response between the two groups. While further study may be helpful in this regard, at this juncture it seems worthwhile, but not imperative, to include an eye movement task in future studies of 3MDR.
Another groundbreaking aspect of this study is the inclusion of women, since prior studies were almost exclusively conducted in men. Although not statistically significantly different, the fact that a larger percentage of women completed the study, and had a larger magnitude of decline in their PCL-5 scores, provides compelling evidence that 3MDR can be as confidently chosen as therapy for women as for men.
There are several limitations that must be acknowledged with this report. First, as noted already, this was a small pilot study, and larger studies are needed to corroborate the results reported. Second, the PCL-5 is a 20-item self-report measure, and while it compares favorably with the results of the more detailed and time-consuming CAPS-5 administration by a trained professional, it does not have the gold standard status of the CAPS-5.
In addition to the conduct of larger studies to corroborate the results of this pilot work, future studies should evaluate the optimal number of intervention sessions, particularly given that non-responders in this study felt that they had made significant progress and that additional sessions would have been helpful. The CAREN system is quite expensive and is only available in scattered locations around the world, so future research could also assess the efficacy of less expensive delivery mechanisms that could make 3MDR available to more individuals across a variety of settings. Several methods have been suggested, including the pairing of a conventional treadmill with several screens, a curved television screen, or a head-mounted display.
In conclusion, the 3MDR approach was shown to be feasible, beneficial and well received by male and female active duty SMs and veterans with both PTSD and mTBI.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Uniformed Services University Institutional Review Board; Walter Reed National Military Medical Center Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author contributions
EV developed the 3MDR therapeutic approach, and he trained and supervised the therapists for the study, PB and KD. The study protocol was written by MR. SK was the engineer. HA was the study coordinator. Statistical analyses were conducted by TH, MR, and SK. This manuscript was written by MR, PB, SK, KD, and HA. All authors then reviewed and made edits to the manuscript to achieve the final form.
Funding
This study was funded by the Center for Rehabilitation Sciences Research, and was provided with referrals and informatics support from the Center for Neuroscience and Regenerative Medicine, both at Uniformed Services University, and with logistical support and infrastructure at the National Intrepid Center of Excellence, Walter Reed National Military Medical Center (WRNMMC). The study design was approved by the WRNMMC Institutional Review Board at Walter Reed National Military Medical Center, protocol # WRNMMC-2018-0201, and was posted on clinicaltrials.gov, # NCT03796936.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frvir.2022.1005774/full#supplementary-material
References
Albright, D. L., and Thyer, B. (2009). Does EMDR reduce post-traumatic stress disorder symptomatology in combat veterans? Behav. Interv. 25 (1), 1–19. doi:10.1002/bin.295
Beidel, D. C., Frueh, B. C., Neer, S. M., Bowers, C. A., Trachik, B., Uhde, T. W., et al. (2019). Trauma management therapy with virtual-reality augmented exposure therapy for combat-related PTSD: A randomized controlled trial. J. anxiety Disord. 61, 64–74. [online] 61. doi:10.1016/j.janxdis.2017.08.005
Bisson, J. I., Deursen, R., Hannigan, B., Kitchiner, N., Barawi, K., Jones, K., et al. (2020). Randomized controlled trial of multi-modular motion-assisted memory desensitization and reconsolidation (3MDR) for male military veterans with treatment-resistant post-traumatic stress disorder. Acta Psychiatr. Scand. 142 (2), 141–151. doi:10.1111/acps.13200
Collins, J. D., Markham, A., Service, K., Reini, S., Wolf, E., and Sessoms, P. (2015). A systematic literature review of the use and effectiveness of the Computer Assisted Rehabilitation Environment for research and rehabilitation as it relates to the wounded warrior. Work 50 (1), 121–129. doi:10.3233/wor-141927
Costanzo, M. E., Chou, Y.-Y., Leaman, S., Pham, D. L., Keyser, D., Nathan, D. E., et al. (2014). Connecting combat-related mild traumatic brain injury with posttraumatic stress disorder symptoms through brain imaging. Neurosci. Lett. 577, 11–15. doi:10.1016/j.neulet.2014.05.054
Dunbar, K. E., Raboy, A. L., Kirby, Z. M., Taylor, P. L., and Roy, M. J. (2019). Distinguishing the relative impact of post-traumatic stress disorder and traumatic brain injury on iPad-measured cognitive function. Cyberpsychology, Behav. Soc. Netw. 22 (12), 761–765. doi:10.1089/cyber.2019.0296
Feinberg, C., Carr, C., Zemek, R., Yeates, K. O., Master, C., Schneider, K., et al. (2021). Association of pharmacological interventions with symptom burden reduction in patients with mild traumatic brain injury. JAMA Neurol. 78 (5), 596. doi:10.1001/jamaneurol.2020.5079
Foa, E. B., McLean, C. P., Zang, Y., Rosenfield, D., Yadin, E., Yarvis, J. S., et al. (2018). Effect of prolonged exposure therapy delivered over 2 Weeks vs 8 Weeks vs present-centered therapy on PTSD symptom severity in military personnel. JAMA 319 (4), 354. doi:10.1001/jama.2017.21242
Gunter, R. W., and Bodner, G. E. (2008). How eye movements affect unpleasant memories: Support for a working-memory account. Behav. Res. Ther. 46 (8), 913–931. doi:10.1016/j.brat.2008.04.006
Jetly, C. R., Meakin, L. C. C., and Sinitski, E. H. (2017). “Multi-modal virtual-reality based treatment for members with combat related posttraumatic stress disorder: Canadian armed Forces pilot study,” in 2017 international conference on virtual rehabilitation (Montreal, Canada: ICVR), 1–2. doi:10.1109/ICVR.2017.8007474
Kennedy, J. E., Leal, F. O., Lewis, J. D., Cullen, M. A., and Amador, R. R. (2010). Posttraumatic stress symptoms in OIF/OEF service members with blast-related and non-blast-related mild TBI. NeuroRehabilitation 26 (3), 223–231. doi:10.3233/nre-2010-0558
Lee, C., and Cuijpers, P. (2013). A meta-analysis of the contribution of eye movements in processing emotional memories. J. Behav. Ther. Exp. Psychiatry 44 (2), 231–239. doi:10.1016/j.jbtep.2012.11.001
Lee, D. J., Schnitzlein, C. W., Wolf, J. P., Vythilingam, M., Rasmusson, A. M., and Hoge, C. W. (2016). Psychotherapy versus pharmacotherapy for posttraumatic stress disorder: Systematic review and meta-analyses to determine first-line treatments. Depress. Anxiety 33 (9), 792–806. doi:10.1002/da.22511
Loucks, L., Yasinski, C., Norrholm, S. D., Maples-Keller, J., Post, L., Zwiebach, L., et al. (2019). You can do that? Feasibility of virtual reality exposure therapy in the treatment of PTSD due to military sexual trauma. J. Anxiety Disord. 61, 55–63. doi:10.1016/j.janxdis.2018.06.004
Mert, A., and Vermetten, E. (2011). Military motion-assisted memory desensitization and reprocessing (3MDR): A novel treatment for PTSD – proof of concept. J. CyberTherapy Rehabilitation 4 (2).
Minen, M., Jinich, S., and Vallespir Ellett, G. (2018). Behavioral therapies and mind-body interventions for posttraumatic headache and post-concussive symptoms: A systematic review. Headache J. Head Face Pain 59 (2), 151–163. doi:10.1111/head.13455
Nijdam, M. J., and Vermetten, E. (2018). Moving forward in treatment of posttraumatic stress disorder: Innovations to exposure-based therapy. Eur. J. Psychotraumatology 9 (1), 1458568. doi:10.1080/20008198.2018.1458568
Onakomaiya, M. M., Kruger, S. E., Highland, K. B., Kodosky, P. N., Pape, M. M., and Roy, M. J. (2017). Expanding clinical assessment for traumatic brain injury and comorbid post-traumatic stress disorder: A retrospective analysis of virtual environment tasks in the computer-assisted rehabilitation environment. Mil. Med. 182 (S1), 128–136. doi:10.7205/milmed-d-16-00054
Patel, G. J., and McDowall, J. (2016). The role of eye movements in EMDR: Conducting eye movements while concentrating on negative autobiographical memories results in fewer intrusions. J. EMDR Prac. Res. 10 (1), 13–22. doi:10.1891/1933-3196.10.1.13
Rapcencu, A. E., Gorter, R., Kennis, M., van Rooij, S. J. H., and Geuze, E. (2017). Pre-treatment cortisol awakening response predicts symptom reduction in posttraumatic stress disorder after treatment. Psychoneuroendocrinology 82, 1–8. doi:10.1016/j.psyneuen.2017.04.010
Rauch, S. A. M., Cigrang, J., Austern, D., and Evans, A. (2017). Expanding the reach of effective PTSD treatment into primary care: Prolonged exposure for primary care. Focus (Am. Psychiatr. Publ. 15 (4), 406–410. doi:10.1176/appi.focus.20170021
Robertson, R., Robertson, A., Jepson, R., and Maxwell, M. (2012). Walking for depression or depressive symptoms: A systematic review and meta-analysis. Ment. Health Phys. Activity 5 (1), 66–75. doi:10.1016/j.mhpa.2012.03.002
Rosenbaum, S., Tiedemann, A., Stanton, R., Parker, A., Waterreus, A., Curtis, J., et al. (2015). Implementing evidence-based physical activity interventions for people with mental illness: An Australian perspective. Australas. Psychiatry 24 (1), 49–54. doi:10.1177/1039856215590252
Roy, M. J., Costanzo, M. E., Blair, J. R., and Rizzo, A. A. (2014). Compelling evidence that exposure therapy for PTSD normalizes brain function. Stud. Health Technol. Inf. 199, 61–65.
Roy, M. J., Francis, J., Friedlander, J., Banks-Williams, L., Lande, R. G., Taylor, P., et al. (2010). Improvement in cerebral function with treatment of posttraumatic stress disordera. Ann. N. Y. Acad. Sci. 1208 (1), 142–149. doi:10.1111/j.1749-6632.2010.05689.x
Sack, M., Zehl, S., Otti, A., Lahmann, C., Henningsen, P., Kruse, J., et al. (2016). A comparison of dual attention, eye movements, and exposure only during eye movement desensitization and reprocessing for posttraumatic stress disorder: Results from a randomized clinical trial. Psychother. Psychosom. 85 (6), 357–365. doi:10.1159/000447671
Samara, Z., Elzinga, B. M., Slagter, H. A., and Nieuwenhuis, S. (2011). Do horizontal saccadic eye movements increase interhemispheric coherence? Investigation of a hypothesized neural mechanism underlying EMDR. Front. Psychiatry 2, 4. doi:10.3389/fpsyt.2011.00004
Schubert, S. J., Lee, C. W., and Drummond, P. D. (2011). The efficacy and psychophysiological correlates of dual-attention tasks in eye movement desensitization and reprocessing (EMDR). J. Anxiety Disord. 25 (1), 1–11. doi:10.1016/j.janxdis.2010.06.024
Shapiro, F. (1989). Efficacy of the eye movement desensitization procedure in the treatment of traumatic memories. J. Trauma. Stress 2 (2), 199–223. doi:10.1002/jts.2490020207
Steenkamp, M. M., Litz, B. T., Hoge, C. W., and Marmar, C. R. (2015). Psychotherapy for military-related PTSD: A review of randomized clinical trials. JAMA 314 (5), 489–500. [online]. doi:10.1001/jama.2015.8370
VA/DoD (2017). Clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Available at: https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal012418.pdf.
van den Hout, M. A., Engelhard, I. M., Smeets, M. A. M., Hornsveld, H., Hoogeveen, E., de Heer, E., et al. (2010). Counting during recall: Taxing of working memory and reduced vividness and emotionality of negative memories. Appl. Cogn. Psychol. 24 (3), 303–311. doi:10.1002/acp.1677
van Gelderen, M. J., Marieke, J., Nijdam, M. J., Haagen, J. F. G., and Vermetten, E. (2020). Interactive motion-assisted exposure therapy for veterans with treatment-resistant posttraumatic stress disorder: A randomized controlled trial. Psychother. Psychosom. 89 (4), 215–227. doi:10.1159/000505977
van Gelderen, M. J., Nijdam, M. J., and Vermetten, E. (2018). An innovative framework for delivering psychotherapy to patients with treatment-resistant posttraumatic stress disorder: Rationale for interactive motion-assisted therapy. Front. Psychiatry 9, 176. doi:10.3389/fpsyt.2018.00176
Vermetten, E., Meijer, L., van der Wurff, P., and Mert, A. (2013). The effect of military motion-assisted memory desensitization and reprocessing treatment on the symptoms of combat-related posttraumatic stress disorder: First preliminary results. Stud. Health Technol. Inf. 191, 125–127. PMID: 23792857.
Keywords: combat stress, posttraumatic stress (PTSD), traumatic brain injury, virtual reality, eye movement
Citation: Roy MJ, Bellini P, Kruger SE, Dunbar K, Atallah H, Haight T and Vermetten E (2022) Randomized controlled trial of motion-assisted exposure therapy for posttraumatic stress disorder after mild traumatic brain injury, with and without an eye movement task. Front. Virtual Real. 3:1005774. doi: 10.3389/frvir.2022.1005774
Received: 28 July 2022; Accepted: 28 October 2022;
Published: 22 November 2022.
Edited by:
Regis Kopper, University of North Carolina at Greensboro, United StatesReviewed by:
Lihua You, Bournemouth University, United KingdomMatti Pouke, University of Oulu, Finland
Copyright © 2022 Roy, Bellini, Kruger, Dunbar, Atallah, Haight and Vermetten. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael J. Roy, TURNaWNoYWVsLnJveUB1c3Vocy5lZHU=
 Michael J. Roy
Michael J. Roy Paula Bellini1,2,4
Paula Bellini1,2,4 Kerri Dunbar
Kerri Dunbar Eric Vermetten
Eric Vermetten