
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Urol. , 28 September 2023
Sec. Pediatric, Adolescent and Developmental Urology
Volume 3 - 2023 | https://doi.org/10.3389/fruro.2023.1275276
This article is part of the Research Topic Quality Improvement in Pediatric Urology View all 4 articles
Enhanced Recovery After Surgery (ERAS) is a set of evidence-based, multidisciplinary protocols that aim to improve the perioperative experience for patients by optimizing factors before, during, and after surgery. Originally developed for adult colorectal surgery, these protocols have expanded and been adopted into the pediatric surgical realm, including pediatric urology. Preoperative interventions are directed toward reducing physiologic and emotional stress prior to surgery, including preoperative education and decreased duration of fasting. Intraoperative interventions are designed to support physiologic homeostasis through maintenance of normothermia and euvolemia, use of regional anesthesia, and minimizing placement of drains. Postoperative interventions seek to reduce the physiologic burden of surgery and restore patients to their functional baseline through early oral intake, early mobilization, and opioid-sparing, multimodal analgesia. ERAS has demonstrated efficacy and safety across a wide variety of surgical subspecialties. In pediatric urology, ERAS has led to earlier return of bowel function, decreased opioid utilization, and shorter hospital length of stay, without an increase in complications compared to prior standard of care. ERAS can thus be seen as a system through which quality improvement (QI) initiatives can be designed and tailored to particular settings and patient populations. This review aims to summarize current data in pediatric urology regarding ERAS elements in the context of QI and patient safety. It will discuss the barriers and future directions of this field, including collaboration with implementation science to facilitate adoption of these protocolized measures more widely.
Surgery places physiologic and emotional stress on patients and families. Enhanced Recovery After Surgery (ERAS) is a perioperative, multidisciplinary set of protocols aimed at reducing the physiologic burden of surgery (1). Pioneered by Danish surgeon, Dr. Henrik Kehlet in the late 1990s, the main tenets of ERAS include preoperative education, limiting preoperative fasting, nutritional support, use of minimally invasive approaches, judicious use of intraoperative opioids, minimization of indwelling tubes/catheters, early postoperative mobilization, and use of multimodal analgesia (1, 2). The first protocols were developed in 2001 for adult colorectal surgery and have since expanded to encompass urology, gynecology, and otolaryngology, among other surgical subspecialties (1, 3). Widespread adoption of ERAS in pediatrics was slow, owing in part to unique pediatric physiology, different nutritional demands, and limited ability of patients in this population to communicate (4). Elements of ERAS are currently used in pediatric surgical subspecialties such as colorectal surgery, otolaryngology, and urology (5–7). Within pediatric urology, ERAS pathways have been studied in children undergoing operations ranging from pyeloplasty to complex lower urinary tract reconstructions involving augmentation cystoplasty and/or urinary diversion (8, 9). Additional consideration has been given to children with comorbidities, such as spina bifida or ventriculoperitoneal shunts, to ensure that a greater proportion of patients can benefit from these interventions (10, 11). In the evolving landscape of quality improvement (QI) in healthcare, ERAS is a central component to value-based care and a valuable tool that balances safety, outcomes, and surgical experiences for patients and families. This review will focus on the evidence in support of the efficacy of ERAS in pediatric urology, particularly as it pertains to QI initiatives and advancement of patient safety.
Engaging patients and families through preoperative education is the initial step in ERAS buy-in, regardless of surgical specialty or procedure type. Besides the duty to disclose relevant information to patients, effective preoperative education has been shown to reduce anxiety and improve the overall surgical experience (12, 13). Education should be age-appropriate and include active involvement of older children and adolescents, as appropriate, in order to promote engagement and set expectations (14). In a systematic review of ERAS in pediatric urology, increased preoperative counseling was one of the most common elements implemented, suggesting the importance of this step in the surgical journey (4). Our institution has established a multidisciplinary, virtual preoperative clinic visit involving surgeons, anesthesiologists, psychologists, and nurses to provide comprehensive education and describe the tailored role of ERAS principles in their specific surgery. This is an opportunity for the team to establish a foundational relationship with patients and families.
Parental involvement is a salient component of ERAS in the pediatric population, as they are key stakeholders in the care of the patient. Engaging parents begins early with preoperative education to facilitate buy-in, which may augment patient participation and adherence to ERAS protocols, especially in older children (15). Setting and managing expectations about their roles in patients’ recoveries and emphasizing criteria for discharge based on postoperative milestones rather than length of stay have been cited as important components (16, 17). Education for parents must be robust and comprehensive as they take on the roles of pain control, refeeding, and monitoring for complications, particularly as earlier discharge is becoming the norm with ERAS (15). Specifically, education on pain management at home is vital. One study found that discharge instructions tailored to patients’ analgesic needs in the hospital and guidance on use of multimodal analgesia led to increased parental satisfaction and may have contributed to decreased opioid utilization at home (18). This point was echoed in a study utilizing a focus group to elicit parents’ perspectives for improvement in the surgical neonatal intensive care unit experience, and feedback was ultimately implemented into local ERAS protocols. Parents also noted that excessive paperwork with medical jargon was overwhelming, which prompted transition to readable informational handouts (17). The ERAS principle of a multidisciplinary team approach must necessarily involve parents as core members to facilitate investment, improve satisfaction, and advance patient safety.
The concept of prehabilitation refers to preoperative exercise, nutritional, and psychological support to bolster patients’ ability to tolerate stressors associated with surgery. The majority of work in prehabilitation thus far comes from adult surgical oncology, where nutritional optimization has been studied as a method to counteract cancer cachexia and/or chemotherapy side effects prior to surgical intervention. Suboptimal preoperative nutritional status and frailty contribute to perioperative complications and impaired wound healing, but nutritional supplementation has been demonstrated to improve outcomes (19). Data are promising insofar as they show decreased rates of postoperative infections and hospital length of stay when combined with ERAS principles. Immunonutrition, which involves fortifying nutrition with certain amino acids or fatty acids, is another adjuvant therapy thought to help modulate the host immune response after surgery, although current evidence is limited (20). Preoperative exercise programs have shown similar benefits in reducing postoperative complications through increased physical reserves. In specific cases, there is potential for patients who were previously considered poor surgical candidates due to deconditioning to be considered for surgery with use of these programs (21). Meanwhile, preoperative psychological support aims to encourage behavioral modifications (e.g., smoking cessation) and promote mental well-being through stress and anxiety reduction. While behavioral changes may not be as relevant in pediatrics, there is room to incorporate the latter into preoperative interventions. Currently, there is low quality evidence in support of psychological prehabilitation for patient outcomes (22). Notably, there is a paucity of data on prehabilitation in the pediatric population. While frailty may not be as common in children, those with reduced functional capacity prior to surgery may benefit from additional therapies to build reserves for optimized recovery. Furthermore, insulin resistance and inflammation have been implicated as drivers of postoperative metabolic derangements, so children with proinflammatory conditions may be another target population for these programs (23). Evaluating the use of preoperative psychological support for parents and caretakers may also yield important findings with implications for additional preoperative counseling and services and building this into pediatric ERAS pathways will likely improve the surgical experience for patients and families.
Extended fasting prior to general anesthesia was rooted in the idea that it decreased gastric volume and acidity and reduced the risk of regurgitation and aspiration of gastric contents during surgery (24). However, a Cochrane review found that children permitted fluids up to two hours prior to surgery did not have higher gastric volumes or lower gastric pH compared to those who fasted. Furthermore, these children were less hungry and thirsty and more comfortable. Among the analysis of more than 2500 children, there was only one reported case of aspiration (25). A shorter duration of fasting has been widely adopted in the pediatric urology realm for nearly all surgical procedures. The safety of this approach has been consistently borne out of data demonstrating no difference in complications before and after implementation (4, 9). Additionally, prolonged fasting has been associated with a catabolic state and insulin resistance (26). The aim of carbohydrate loading prior to surgery aims to reduce these physiologic derangements and lower the stress response (27). Data from the adult perioperative literature have shown that drinking carbohydrate-rich clear liquids up until two hours before surgery resulted in less hunger and thirst compared to those who fasted or drank non-caloric clear liquids (28). Meanwhile, evidence in pediatrics suggests that a clear liquid carbohydrate drink may promote a more stable perioperative metabolic state, particularly for longer operations (29). Based on existing data, a group of pediatric urologists and anesthesiologists have incorporated a preoperative clear liquid complex carbohydrate load into their revised ERAS protocols for lower urinary tract reconstructions (30).
Preoperative mechanical bowel preparation was popularized in the 1970s due to purported benefits of decreasing complications and infections associated with surgeries involving the intestines (31). Efforts to challenge this dogma have been incremental. An early retrospective study comparing outcomes and complications in children undergoing augmentation cystoplasty with versus without mechanical bowel preparation found that those who were spared from bowel preparation had shorter median length of stay (LOS), earlier time to postoperative oral intake, and similar rates of infections and anastomotic leaks (32). Another study looked specifically at 30-day postoperative complications in children undergoing augmentation cystoplasty without bowel preparation. They reported no intraoperative complications and an overall 30-day postoperative complication rate of 9.87%, which was equivalent to existing rates in the literature (33).
There has been concern related to eliminating mechanical bowel preparation in the subset of patients with spina bifida with ventriculoperitoneal (VP) shunts due to the risk of shunt infections, which can be devastating (32). A study of children with VP shunts undergoing augmentation cystoplasty using bowel found no difference in the rate of shunt infections between those who did and did not undergo bowel preparation (34). Similarly, there were no VP shunt infections following introduction of an ERAS protocol for pediatric urinary tract reconstruction that included elimination of a dedicated, preoperative bowel preparation and maintenance of routine outpatient bowel regimens in those with concomitant neurogenic bowel (10). This particular example illustrates the consideration in outcomes among different patient populations when evaluating whether intended effects of ERAS elements are equally distributed. Overall, foregoing this step has been a welcome addition to ERAS protocols for many institutions due to improved perioperative experiences for patients and reductions in costs associated with decreased LOS (9, 30, 31).
Anesthetic-induced inhibition of thermoregulation, in addition to exposure of body surface area to the cooler operating room environment, can lead to perioperative hypothermia, defined as a body temperature of less than 36.0°C during the perioperative period (35). Even a mean decrease in body temperature of 1.5°C has been associated with increased likelihood of requiring a blood transfusion and developing a postoperative infection, with subsequent increased costs of care related to managing these complications (36). A Cochrane review also found a similar benefit of perioperative normothermia in mitigating surgical site infections and complications in patients undergoing abdominal surgery (37). Since younger patients are more susceptible to anesthetic-induced thermodysregulation, maintenance of appropriate body temperature during surgery should be a priority for safety and cost-conscious care (35). The Pediatric Urology Recovery After Surgery Endeavor (PURSUE) multicenter study has incorporated maintenance of normothermia—defined as body temperature of 36°C–38°C during skin-to-skin time—into their revised ERAS protocol for lower urinary tract reconstruction (30).
Fluid management has been a mainstay of ERAS protocols since their inception. Hypovolemia predisposes patients to renal injury, while hypervolemia increases the risk for cardiorespiratory complications, impaired wound healing, and delayed recovery (38). One study found that for each additional liter of fluid given, the risk of postoperative symptoms delaying recovery increased by 16% while the risk of postoperative complications increased by 32% (39). Within pediatric urology, special attention is paid to renal function as many urologic patients have or are at risk of renal injury as a function of their disease processes. Thus, achieving intraoperative euvolemia with the goal of maintaining adequate renal perfusion is of the utmost importance (40). This tenet is reflected in the ubiquitous adoption of minimizing excessive intraoperative fluids in both pediatric colorectal surgery and pediatric urology (4, 7, 10, 41, 42).
Prior standard of care involved administering a fluid bolus to account for presumed fluid deficits in the context of prolonged fasting, followed by intraoperative maintenance fluids based on the Holliday and Segar formula (43). Given minimization of prolonged fasting and the risks associated with hypervolemia, as discussed, goal-directed fluid resuscitation is now commonplace. However, assessing for euvolemia has been imperfect. Methods such as the pleth variability index (PVI), stroke volume index, transesophageal echocardiography, and esophageal doppler have been studied as proxies for hemodynamic variables and fluid responsiveness during surgery (44). While some studies show the predictive value of PVI in guiding goal-directed fluid therapy, research in PVI has generally lacked standardization in types of fluid given, volume administered, and definition of fluid responsiveness, thus limiting the conclusions that can be drawn (45). Currently, no single method has been shown to be superior. Nonetheless, goal-directed fluid therapy has been shown to significantly decrease the volume of intraoperative fluids administered and reduce surgical morbidity (43, 46).
The opioid epidemic has galvanized the medical community into reducing reliance on opioids for pain control. Operative pain management may be the context of children’s first exposure to opioids. This patient population may be more vulnerable to misuse compared to adults due to alterations in the reward and habit centers in the brain, with some data reporting that approximately 5% of opioid-naïve adolescents and young adults continue to fill opioid prescriptions more than 90 days after surgery (47). With evidence demonstrating similar efficacy of NSAIDs to opioids in managing postoperative pain in children, the transition to regional and multimodal analgesia starting in the operating room has become standard for many pediatric surgeries (4, 41, 48, 49).
A prospective case-control study found that children undergoing urologic reconstructive surgery with adherence to ERAS protocols were more likely to receive intraoperative dexmedetomidine, acetaminophen, and NSAIDs, with a resultant decrease in intra- and postoperative opioids compared to historical controls (50). Another study sought to characterize factors associated with same-day discharge for pediatric pyeloplasty between 2008–2020. The authors identified a trend of greater utilization of ketorolac and regional blocks and a decrease in opioid use throughout the years, which was reflected in a shorter LOS (8). Among children with spina bifida undergoing complex lower urinary tract reconstruction, use of regional analgesia resulted in a 70% intraoperative and 78% postoperative, in-hospital reduction in opioid use without higher pain scores. The authors note that use of regional analgesia as part of ERAS protocols confers the benefits of opioid minimization to this subset of patients where neurologic function and sensation may be altered (11).
The burden of the opioid epidemic has increased costs in sectors spanning from healthcare, substance use treatment, criminal justice, and the labor market (51). Viewed through the lens of QI and patient safety, minimizing perioperative opioid use has the implication to mitigate immediate adverse health outcomes as well as far-reaching consequences associated with future opportunity costs for patients.
Placement of tubes and drains at the conclusion of surgery has long been standard practice in order to facilitate surgical site drainage and prevent fluid collections and infections. However, this intervention contributes to postoperative pain and discomfort, limits mobility, and negatively impacts quality of life for patients (1). Evidence from the adult literature does not show a benefit for prophylactic drain placement in a myriad of surgeries (52). Similarly, drain placement has not been associated with improved outcomes in select pediatric surgeries, including upper urinary tract reconstruction (53–55). Numerous studies in pediatric urology have successfully incorporated this component into their enhanced recovery protocols without an increase in adverse events, further supporting the safety of this approach (8, 10, 50, 56). Current evidence seems poised to obviate operative drain placement in many pediatric urologic procedures.
Patients were routinely nil per os (NPO) after surgery to mitigate postoperative nausea and vomiting. In the setting of surgeries that involved the gastrointestinal tract, it was purported that avoiding immediate oral intake would protect anastomoses from the stress of feeding (57). Certain pediatric urologic procedures, such as enterocystoplasty, involve the use of bowel segments to reconstruct the urinary tract. Thus, by extension, children undergoing these types of procedures should also be kept NPO after surgery to preserve bowel integrity. However, a systematic review of eleven randomized controlled trials comparing postoperative NPO versus early oral refeeding within 24 hours after colorectal surgery found no clear benefit to restricting oral intake. While early refeeding did lead to an increased risk of vomiting, it was associated with decreased risk of anastomotic dehiscence and infections of any type, ultimately leading to shorter LOS (57). Early postoperative refeeding has been implemented and studied in numerous pediatric surgical subspecialties and been found to be a welcome addition leading to earlier return of bowel function, an important criterion for hospital discharge (7, 41, 58). In adult urology, early oral intake spared patients undergoing open radical cystectomy from five additional days of fasting and resulted in earlier time to bowel movement (3.64 versus 6 days), without a difference in 90-day complication rates (3). This outcome was reproduced in pediatric urology as well (4). Even amongst children undergoing bladder augmentation and/or urinary diversion, early refeeding has been associated with earlier return of bowel function (9, 10).
Efforts to minimize opioid use begin intraoperatively and continue into the postoperative period. The emphasis on opioid stewardship is particularly important after surgery because of discrepancies in provider prescribing patterns (59, 60). Even as recently as 2017, some children received up to 24–26 days’ supplies of opioids following routine hernia repairs and tonsillectomies (61). Within pediatric urology, one study found that 99% of children were prescribed opioids after surgery (62). One possible driver of this pattern of excessive opioids is concern regarding potential pain crises (63). However, current evidence suggests ERAS patients have well-controlled pain, both in-hospital and after discharge. PACU pain scores were significantly lower in ERAS cohorts who were managed with adjunct non-opioid medications such as acetaminophen and NSAIDs (41, 50). Meanwhile, 80% of children had non-significant levels of pain by postoperative day one (64). In those who were prescribed opioids, most used five doses or less, typically within the first three days after surgery (65). Even a majority of pediatric urologists surveyed did not believe that patients used all opioid doses prescribed (66). Opioid utilization patterns corroborate this belief, with up to 62% of patients reporting unused doses two weeks postoperatively (62). Thus, the duration of recovery after surgery represents a critical period for intervention in reducing opioid use. Reassuringly, studies in the pediatric population have consistently demonstrated successful reduction in postoperative opioid use upon adoption of ERAS protocols (4, 7, 10, 41, 50).
T/he data presented thus far validate the success of ERAS in isolated measures. Whether that is improvement in quality of life by reducing preoperative fasting and eliminating bowel preparation, reducing the risk of infection and hypervolemia by maintaining intraoperative normothermia and euvolemia, or expediting milestones to safe discharge with early postoperative refeeding—current evidence supports the transition away from historical practice to these updated policies. However, assessing the efficacy of ERAS as a whole also involves examining the overall impact of these protocols on global outcomes and complications. Table 1 summarizes interventions and outcomes in select pediatric urology studies.
Perioperative care has been proposed as a significant driver of surgical outcomes, not the surgery itself (1). It has been suggested that mortality may not be an appropriate indicator of success in the pediatric ERAS realm (4). Hence, length of stay has, and continues to be, an important objective measure of the cumulative impact of ERAS elements, as it reflects an improvement in systems of care. Time and time again, ERAS has resulted in reductions in hospital LOS for children across different surgical specialties that are both statistically and clinically significant (7, 9, 41, 42, 58). One study in children undergoing urologic reconstruction noted that the greater the number of ERAS elements implemented, the shorter the LOS (10). This suggests an additive effect of individual elements in contributing to outcomes. It has been observed that there are fewer ERAS elements carried out in pediatrics compared to adults, pointing to potential room for further improvement in reduction of LOS (58). Another meaningful outcome is patient and family satisfaction. While inherently subjective, this measure is arguably just as important since data on patient experience are often gathered during QI initiatives in order to optimize subsequent encounters. Early studies in pediatric ERAS have described high levels of patient and family satisfaction as a result of participation (4, 58). Additional work is necessary to fully characterize the surgical experience and gather more granular data on areas for improvement.
Evaluating ERAS through the lens of patient safety involves scrutinizing complication rates compared to prior standard of care. Studies in pediatric colorectal and minimally invasive surgeries reported similar postoperative complication rates before and after adoption of ERAS (7, 41, 42, 67). Results from pediatric urology also demonstrated complication rates that were not worse compared to pre-ERAS cohorts (33). In fact, ERAS has been associated with fewer bowel-associated and total complications, with similar rates of postoperative emergency department visits, readmissions, and reoperations (4, 9, 10, 56). These robust data support ERAS as a tool to promote QI and patient safety.
Maximizing quality of care while minimizing cost is a considerable motivation for QI initiatives within the realm of surgery and it has been suggested that ERAS protocols play a part through reductions in hospital LOS and patient morbidity (68). While it can be difficult to quantify exact cost savings, limited studies have demonstrated that ERAS implementation is associated with cost savings for patients (1). A systematic review of costs in the adult surgical literature showed reduced in-hospital costs for those undergoing esophageal, gastric, pancreatic, colorectal, bariatric, vascular, and gynecologic surgeries with ERAS protocols (68). There is a paucity of data for pediatric surgeries but a decrease in hospital costs has been reported for children undergoing colorectal surgery (4, 7). Two other studies found that costs were not higher after implementation of ERAS for pediatric surgeries (69, 70). Meanwhile, a QI initiative at a pediatric ambulatory surgical center noted that their cost reduction was driven by substitution of intravenous acetaminophen with ketorolac (71). While additional cost analyses are necessary, particularly in pediatric urology, it should be acknowledged that in-hospital economic evaluations cannot adequately account for indirect societal costs associated with factors such as children’s need for postoperative, in-home support leading to potential reductions in caregiver wages (68). Thus, future studies that incorporate these indirect costs will illustrate a more comprehensive picture of the true cost impacts from ERAS. Nonetheless, current evidence indicates that ERAS provides good value-based care (1).
The extent of published evidence demonstrates the safety and effectiveness of ERAS protocols for the pediatric population, however, adoption in pediatric urology remains slow (72). A survey of pediatric urologists found that 38% of respondents lacked familiarity with enhanced recovery pathways. Among those with familiarity, lack of consensus with other pediatric urologists (62%), lack of administrative support (56%), difficulty initiating and maintaining pathways (38%), and lack of anesthesia support (31%) were among the most commonly cited barriers to implementation and/or standardization (73). Lack of administrative support was echoed as a barrier in a study of pediatric surgeons (74). A QI initiative to decrease perioperative opioid use in children also noted inadequate care team buy-in as a limiting factor to optimal implementation (71). Meanwhile, 90% of pediatric urology survey respondents were willing to implement some elements of ERAS into their practice, suggesting a willingness, or even demand, for such pathways (73).
While the obstacles to implementing such an extensive protocol may seem numerous, they must be weighed against the benefits for patient care and safety. A QI initiative to implement enhanced recovery pathways in 20 children undergoing bladder reconstruction demonstrates the feasibility of such an endeavor and highlights the assets of this approach (56). Designated champions communicated changes to their teams and met regularly for audits. Time from planning to first implementation was seven months and protocol elements led to a significant decrease in LOS (4 versus 9 days, p<0.05) without an increase in 30-day complications. A median of 16 out of 24 ERAS elements (67%) were implemented, which was below their goal of 80% adherence, but it still resulted in significant improvements in their outcome measures.
Pioneers in ERAS claim that 70–80% adherence to ERAS elements is necessary to improve outcomes (1). The current literature reveals that additional work is necessary to achieve this standard, with adherence rates ranging from 45–62%; meanwhile only 16% of cohorts were able to achieve at least 75% adherence in one study (42, 73, 74). However, the improvements in patient outcomes despite suboptimal adherence to all elements lends support to the effectiveness of these protocols. Furthermore, while some studies cite low ERAS implementation rates among pediatric surgeons, many currently implement individual elements in their practice but do not label it as ERAS, suggesting a degree of underreporting (41, 73, 74).
The future of ERAS in pediatric urology will likely involve additions or alterations in the elements implemented. For example, recent data from the adult surgical literature have shown that patients allowed to drink clear liquids up until arrival in the operating room did not have worse outcomes, opening up the potential for future adoption in the pediatric domain to further decrease discomfort associated with fasting (75). In fact, the European Society of Anesthesiology and Intensive Care recently put forth pediatric guidelines permitting intake of clear fluids up until one hour before surgery (76). Another major avenue for future improvement involves identifying and addressing barriers to adoption of ERAS among a larger proportion of pediatric urologists. This will require collaboration with experts in implementation science, a field that studies and develops strategies to promote adoption of evidence-based interventions into clinical practice, with a focus on how to optimize delivery to achieve the most impact (77). Replicating successful implementation from one setting to another can be difficult due to various contextual factors, so asking why an initiative was successfully implemented is just as important as asking how an initiative produces improved outcomes (78).
Popular frameworks within implementation science include Consolidated Framework for Implementation Research (CFIR); Exploration, Preparation, Implementation, Sustainment (EPIS); and Normalization Process Theory (NPT) (Table 2) (77). NPT—which focuses on behaviors and attitudes that allow an intervention to become incorporated into routine practice and no longer seen as an intervention—has been utilized in the setting of implementing several ERAS protocols (79). Lam and colleagues implemented an ERAS protocol for neonatal intestinal resection and outlined recommendations for successful adoption in alternate settings as viewed through an NPT lens: identifying champions from different teams, gathering multidisciplinary stakeholder buy-in, tailoring interventions to local needs, eliciting patient and family engagement, and performing audits for process improvements (80). Another study applied the NPT framework to understand factors that promoted ERAS implementation in adult thoracic, colorectal, and head and neck surgeries. The authors found that differentiating ERAS from prior standard of care and positive beliefs in the value of ERAS, both individually and as a team, facilitated successful implementation (81). The CFIR framework has also been used to identify effective team handoffs, robust post-discharge support, and promotion of patients’ self-efficacy in recovery as factors associated with successful implementation of ERAS in adult orthopedic surgery (82).
As ERAS continues to gain traction in pediatric urology, drawing from work in implementation science will be an important component to overcome barriers to successful expansion. Figure 1 illustrates how general implementation science principles can be harnessed to facilitate tailored adoption of an intervention, such as ERAS, which can then be studied and revised locally using QI frameworks.
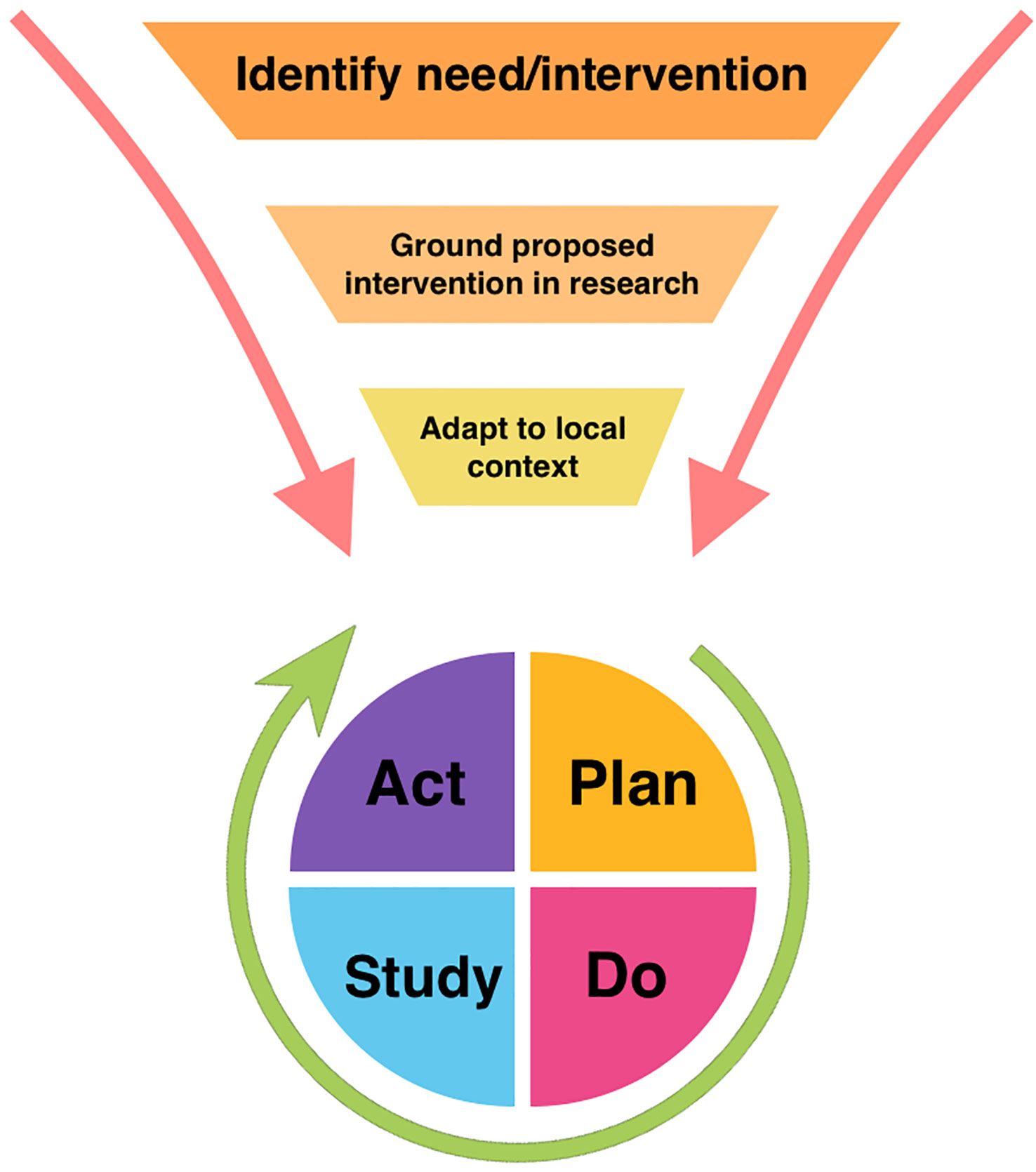
Figure 1 Model of relationship between broad implementation science principles and Plan-Do-Study-Act quality improvement framework.
Surgery can be a considerable undertaking for patients and families. ERAS protocols were developed to address and optimize the patient experience at every step of the surgical pathway. Studies repeatedly illustrate the safety and efficacy of ERAS in decreasing patient discomfort, perioperative opioid use, and hospital length of stay without increased complications compared to prior standard of care. The iterative process of implementation and audits demonstrates the role of ERAS in driving QI initiatives and promoting patient safety. This is a rapidly growing sector of research, particularly within pediatric urology, as providers strive to improve process measures through consideration and adoption of additional elements. As the field of pediatric urology ERAS continues to advance, the paradigm is shifting from the sole focus on its merits as a tool to optimize patient outcomes and safety to one that includes addressing barriers and facilitators to widespread implementation of these evidence-based interventions.
DH: Writing – original draft, Writing – review & editing. KH: Writing – review & editing. MB: Writing – review & editing. KR: Writing – review & editing.
The research reported in this publication was supported by the Pediatric Urology Research Enterprise (PURE), Pediatric Urology, Children’s Hospital Colorado.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: A review. JAMA Surg (2017) 152(3):292–8. doi: 10.1001/jamasurg.2016.4952
2. Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth (1997) 78(5):606–17. doi: 10.1093/bja/78.5.606
3. Palumbo V, Giannarini G, Crestani A, Rossanese M, Calandriello M, Ficarra V. Enhanced recovery after surgery pathway in patients undergoing open radical cystectomy is safe and accelerates bowel function recovery. Urology (2018) 115:125–32. doi: 10.1016/j.urology.2018.01.043
4. Fung AC, Chu FY, Chan IH, Wong KK. Enhanced recovery after surgery in pediatric urology: current evidence and future practice. J Pediatr Urol (2023) 19(1):98–106. doi: 10.1016/j.jpurol.2022.07.024
5. Kitchin S, Raman VT, Javens T, Jatana KR. Enhanced recovery after surgery: A quality improvement approach. Otolaryngol Clin North Am (2022) 55(6):1271–85. doi: 10.1016/j.otc.2022.07.011
6. Rove KO, Brockel MA, Brindle ME, Scott MJ, Herndon CDA, Ljungqvist O, et al. Embracing change-the time for pediatric enhanced recovery after surgery is now. J Pediatr Urol (2019) 15(5):491–3. doi: 10.1016/j.jpurol.2019.04.005
7. Su Y, Xu L, Hu J, Musha J, Lin S. Meta-analysis of enhanced recovery after surgery protocols for the perioperative management of pediatric colorectal surgery. J Pediatr Surg (2022) 58(9):1686–93. doi: 10.1016/j.jpedsurg.2022.11.017
8. Rickard M, Chua M, Kim JK, Keefe DT, Milford K, Hannick JH, et al. Evolving trends in peri-operative management of pediatric ureteropelvic junction obstruction: working towards quicker recovery and day surgery pyeloplasty. World J Urol (2021) 39(9):3677–84. doi: 10.1007/s00345-021-03621-9
9. Haid B, Karl A, Koen M, Mottl W, Haid A, Oswald J. Enhanced recovery after surgery protocol for pediatric urological augmentation and diversion surgery using small bowel. J Urol (2018) 200(5):1100–6. doi: 10.1016/j.juro.2018.06.011
10. Rove KO, Brockel MA, Saltzman AF, Dönmez MI, Brodie KE, Chalmers DJ, et al. Prospective study of enhanced recovery after surgery protocol in children undergoing reconstructive operations. J Pediatr Urol (2018) 14(3):252.e1–.e9. doi: 10.1016/j.jpurol.2018.01.001
11. Moore RP, Burjek NE, Brockel MA, Strine AC, Acks A, Boxley PJ, et al. Evaluating the role for regional analgesia in children with spina bifida: A retrospective observational study comparing the efficacy of regional versus systemic analgesia protocols following major urological surgery. Reg Anesth Pain Med (2023) 48(1):29–36. doi: 10.1136/rapm-2022-103823
12. Bondy LR, Sims N, Schroeder DR, Offord KP, Narr BJ. The effect of anesthetic patient education on preoperative patient anxiety. Reg Anesth Pain Med (1999) 24(2):158–64. doi: 10.1016/s1098-7339(99)90078-0
13. Egbert LD, Battit GE, Welch CE, Bartlett MK. Reduction of postoperative pain by encouragement and instruction of patients. A Study Doctor-Patient Rapport. N Engl J Med (1964) 270:825–7. doi: 10.1056/nejm196404162701606
14. Roberts K, Brindle M, McLuckie D. Enhanced recovery after surgery in paediatrics: A review of the literature. BJA Educ (2020) 20(7):235–41. doi: 10.1016/j.bjae.2020.03.004
15. Salaün JP, Ecoffey C, Orliaguet G. Enhanced recovery in children: how could we go further? World J Pediatr Surg (2021) 4(2):e000288. doi: 10.1136/wjps-2021-000288
16. Moon JK, Hwang R, Balis FM, Mattei P. An enhanced recovery after surgery protocol in children who undergo nephrectomy for wilms tumor safely shortens hospital stay. J Pediatr Surg (2022) 57(10):259–65. doi: 10.1016/j.jpedsurg.2022.05.020
17. Lam JY, Howlett A, Stephen LM, Brindle ME. Parental perceptions and experiences of care in the surgical neonatal intensive care unit. Pediatr Surg Int (2023) 39(1):210. doi: 10.1007/s00383-023-05484-0
18. Moffitt JK, Cepeda A Jr., Ekeoduru RA, Teichgraeber JF, Nguyen PD, Greives MR. Enhanced recovery after surgery protocol for primary cleft palate repair: improving transition of care. J Craniofac Surg (2021) 32(1):e72–e6. doi: 10.1097/scs.0000000000006985
19. Ljungqvist O, de Boer HD, Balfour A, Fawcett WJ, Lobo DN, Nelson G, et al. Opportunities and challenges for the next phase of enhanced recovery after surgery: A review. JAMA Surg (2021) 156(8):775–84. doi: 10.1001/jamasurg.2021.0586
20. De Luca R, Gianotti L, Pedrazzoli P, Brunetti O, Rizzo A, Sandini M, et al. Immunonutrition and prehabilitation in pancreatic cancer surgery: A new concept in the era of eras® and neoadjuvant treatment. Eur J Surg Oncol (2023) 49(3):542–9. doi: 10.1016/j.ejso.2022.12.006
21. Sanchez-Lorente D, Navarro-Ripoll R, Guzman R, Moises J, Gimeno E, Boada M, et al. Prehabilitation in thoracic surgery. J Thorac Dis (2018) 10(Suppl 22):S2593–s600. doi: 10.21037/jtd.2018.08.18
22. Gillis C, Ljungqvist O, Carli F. Prehabilitation, Enhanced Recovery after Surgery, or Both? A narrative Review. Br J Anaesth (2022) 128(3):434–48. doi: 10.1016/j.bja.2021.12.007
23. Chabot K, Gillis C, Carli F. Prehabilitation: metabolic considerations. Curr Opin Clin Nutr Metab Care (2020) 23(4):271–6. doi: 10.1097/mco.0000000000000663
24. Andersson H, Schmitz A, Frykholm P. Preoperative fasting guidelines in pediatric anesthesia: are we ready for a change? Curr Opin Anaesthesiol (2018) 31(3):342–8. doi: 10.1097/aco.0000000000000582
25. Brady M, Kinn S, Ness V, O'Rourke K, Randhawa N, Stuart P. Preoperative fasting for preventing perioperative complications in children. Cochrane Database Syst Rev (2009) 4):Cd005285. doi: 10.1002/14651858.CD005285.pub2
26. Rove KO, Edney JC, Brockel MA. Enhanced recovery after surgery in children: promising, evidence-based multidisciplinary care. Paediatr Anaesth (2018) 28(6):482–92. doi: 10.1111/pan.13380
27. Fearon KC, Ljungqvist O, Von Meyenfeldt M, Revhaug A, Dejong CH, Lassen K, et al. Enhanced recovery after surgery: A consensus review of clinical care for patients undergoing colonic resection. Clin Nutr (2005) 24(3):466–77. doi: 10.1016/j.clnu.2005.02.002
28. Joshi GP, Abdelmalak BB, Weigel WA, Harbell MW, Kuo CI, Soriano SG, et al. 2023 American society of anesthesiologists practice guidelines for preoperative fasting: carbohydrate-Containing clear liquids with or without protein, chewing gum, and pediatric fasting duration-a modular update of the 2017 american society of anesthesiologists practice guidelines for preoperative fasting. Anesthesiology (2023) 138(2):132–51. doi: 10.1097/aln.0000000000004381
29. Laird A, Bramley L, Barnes R, Englin A, Winderlich J, Mount E, et al. Effects of a preoperative carbohydrate load on postoperative recovery in children: A randomised, double-blind, placebo-controlled trial. J Pediatr Surg (2023) 58(9):1824–31. doi: 10.1016/j.jpedsurg.2023.05.004
30. Rove KO, Strine AC, Wilcox DT, Vricella GJ, Welch TP, VanderBrink B, et al. Design and development of the pediatric urology recovery after surgery endeavor (Pursue) multicentre pilot and exploratory study. BMJ Open (2020) 10(11):e039035. doi: 10.1136/bmjopen-2020-039035
31. Weatherly DL, Szymanski KM, Whittam BM, Bennett WE Jr., King S, Misseri R, et al. Comparing inpatient versus outpatient bowel preparation in children and adolescents undergoing appendicovesicostomy. J Pediatr Urol (2018) 14(1):50.e1–.e6. doi: 10.1016/j.jpurol.2017.07.013
32. Gundeti MS, Godbole PP, Wilcox DT. Is bowel preparation required before cystoplasty in children? J Urol (2006) 176(4 Pt 1):1574–6. doi: 10.1016/j.juro.2006.06.034
33. Víctor D, Burek C, Corbetta JP, Sentagne A, Sager C, Weller S, et al. Augmentation cystoplasty in children without preoperative mechanical bowel preparation. J Pediatr Urol (2012) 8(2):201–4. doi: 10.1016/j.jpurol.2011.01.015
34. Casperson KJ, Fronczak CM, Siparsky G, O'Donnell C, Gundeti MS, Campbell JB, et al. Ventriculoperitoneal shunt infections after bladder surgery: is mechanical bowel preparation necessary? J Urol (2011) 186(4 Suppl):1571–5. doi: 10.1016/j.juro.2011.03.074
35. Esnaola NF, Cole DJ. Perioperative normothermia during major surgery: is it important? Adv Surg (2011) 45:249–63. doi: 10.1016/j.yasu.2011.03.007
36. Mahoney CB, Odom J. Maintaining intraoperative normothermia: A meta-analysis of outcomes with costs. AANA J (1999) 67(2):155–63.
37. Madrid E, Urrútia G, Roqué i Figuls M, Pardo-Hernandez H, Campos JM, Paniagua P, et al. Active body surface warming systems for preventing complications caused by inadvertent perioperative hypothermia in adults. Cochrane Database Syst Rev (2016) 4(4):Cd009016. doi: 10.1002/14651858.CD009016.pub2
38. Makaryus R, Miller TE, Gan TJ. Current concepts of fluid management in enhanced recovery pathways. Br J Anaesth (2018) 120(2):376–83. doi: 10.1016/j.bja.2017.10.011
39. Gustafsson UO, Hausel J, Thorell A, Ljungqvist O, Soop M, Nygren J. Adherence to the Enhanced Recovery after Surgery Protocol and Outcomes after Colorectal Cancer Surgery. Arch Surg (2011) 146(5):571–7. doi: 10.1001/archsurg.2010.309
40. Jones DR, Lee HT. Perioperative renal protection. Best Pract Res Clin Anaesthesiol (2008) 22(1):193–208. doi: 10.1016/j.bpa.2007.08.005
41. Dagorno C, Montalva L, Ali L, Brustia R, Paye-Jaquen A, Pio L, et al. Enhancing recovery after minimally invasive surgery in children: A systematic review of the literature and meta-analysis. J Pediatr Surg (2021) 56(12):2157–64. doi: 10.1016/j.jpedsurg.2021.04.004
42. Reppucci ML, Wehrli LA, Schletker J, Nolan MM, Rieck J, Fares S, et al. The effect of an enhanced recovery protocol in pediatric patients who undergo colostomy closure and malone procedures. Pediatr Surg Int (2022) 38(12):1701–7. doi: 10.1007/s00383-022-05213-z
43. Mathew PJ, Sharma S, Bhardwaj N, Ashok V, Malik MA. Goal-directed fluid therapy guided by plethysmographic variability index (Pvi) versus conventional liberal fluid administration in children during elective abdominal surgery: A randomized controlled trial. J Pediatr Surg (2023) 58(4):735–40. doi: 10.1016/j.jpedsurg.2022.11.015
44. Kendrick JB, Kaye AD, Tong Y, Belani K, Urman RD, Hoffman C, et al. Goal-directed fluid therapy in the perioperative setting. J Anaesthesiol Clin Pharmacol (2019) 35(Suppl 1):S29–s34. doi: 10.4103/joacp.JOACP_26_18
45. Weber F, Rashmi BK, Karaoz-Bulut G, Dogger J, de Heer IJ, Prasser C. The predictive value of the pleth variability index on fluid responsiveness in spontaneously breathing anaesthetized children-a prospective observational study. Paediatr Anaesth (2020) 30(10):1124–31. doi: 10.1111/pan.13991
46. Hamilton MA, Cecconi M, Rhodes A. A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg (2011) 112(6):1392–402. doi: 10.1213/ANE.0b013e3181eeaae5
47. Yaster M, McNaull PP, Davis PJ. The opioid epidemic in pediatrics: A 2020 update. Curr Opin Anaesthesiol (2020) 33(3):327–34. doi: 10.1097/aco.0000000000000865
48. Chauhan RD, Idom CB, Noe HN. Safety of ketorolac in the pediatric population after ureteroneocystostomy. J Urol (2001) 166(5):1873–5. doi: 10.1016/S0022-5347(05)65710-2
49. Lieh-Lai MW, Kauffman RE, Uy HG, Danjin M, Simpson PM. A randomized comparison of ketorolac tromethamine and morphine for postoperative analgesia in critically ill children. Crit Care Med (1999) 27(12):2786–91. doi: 10.1097/00003246-199912000-00030
50. Han DS, Brockel MA, Boxley PJ, Dönmez M, Saltzman AF, Wilcox DT, et al. Enhanced recovery after surgery and anesthetic outcomes in pediatric reconstructive urologic surgery. Pediatr Surg Int (2021) 37(1):151–9. doi: 10.1007/s00383-020-04775-0
51. Kawasaki S, Sharfstein JM. The cost of the opioid epidemic, in context. Am J Manag Care (2019) 25(13 Suppl):S241–s2.
52. Kosins AM, Scholz T, Cetinkaya M, Evans GRD. Evidence-based value of subcutaneous surgical wound drainage: the largest systematic review and meta-analysis. Plast Reconstr Surg (2013) 132(2):443–50. doi: 10.1097/PRS.0b013e3182958945
53. Sforza S, Di Maida F, Mari A, Zaccaro C, Cini C, Tellini R, et al. Is a drainage placement still necessary after robotic reconstruction of the upper urinary tract in children? Experience from a tertiary referral center. J Laparoendosc Adv Surg Tech A (2019) 29(9):1180–4. doi: 10.1089/lap.2019.0302
54. Neville JJ, Aldeiri B. Drain placement in paediatric complicated appendicitis: A systematic review and meta-analysis. Pediatr Surg Int (2023) 39(1):171. doi: 10.1007/s00383-023-05457-3
55. Alexiades NG, Ahn ES, Blount JP, Brockmeyer DL, Browd SR, Grant GA, et al. Development of best practices to minimize wound complications after complex tethered spinal cord surgery: A modified delphi study. J Neurosurg Pediatr (2018) 22(6):701–9. doi: 10.3171/2018.6.Peds18243
56. Chan YY, Chu DI, Hirsch J, Kim S, Rosoklija I, Studer A, et al. Implementation and sustainability of an enhanced recovery pathway in pediatric bladder reconstruction: flexibility, commitment, teamwork. J Pediatr Urol (2021) 17(6):782–9. doi: 10.1016/j.jpurol.2021.08.023
57. Lewis SJ, Egger M, Sylvester PA, Thomas S. Early enteral feeding versus "Nil by mouth" after gastrointestinal surgery: systematic review and meta-analysis of controlled trials. BMJ (2001) 323(7316):773–6. doi: 10.1136/bmj.323.7316.773
58. Pearson KL, Hall NJ. What is the role of enhanced recovery after surgery in children? A scoping review. Pediatr Surg Int (2017) 33(1):43–51. doi: 10.1007/s00383-016-3986-y
59. Rizeq YK, Many BT, Vacek JC, Silver I, Goldstein SD, Abdullah F, et al. Trends in perioperative opioid and non-opioid utilization during ambulatory surgery in children. Surgery (2019) 166(2):172–6. doi: 10.1016/j.surg.2019.04.005
60. Denning NL, Kvasnovsky C, Golden JM, Rich BS, Lipskar AM. Inconsistency in opioid prescribing practices after pediatric ambulatory hernia surgery. J Surg Res (2019) 241:57–62. doi: 10.1016/j.jss.2019.03.043
61. Horton JD, Munawar S, Corrigan C, White D, Cina RA. Inconsistent and excessive opioid prescribing after common pediatric surgical operations. J Pediatr Surg (2019) 54(7):1427–31. doi: 10.1016/j.jpedsurg.2018.07.002
62. Garren BR, Lawrence MB, McNaull PP, Sutherland R, Bukowski TP, Nielsen ME, et al. Opioid-prescribing patterns, storage, handling, and disposal in postoperative pediatric urology patients. J Pediatr Urol (2019) 15(3):260. doi: 10.1016/j.jpurol.2019.02.009
63. Hecht S, Halstead NV, Boxley P, Brockel MA, Rove KO. Opioid prescribing patterns following implementation of enhanced recovery after surgery (Eras) protocol in pediatric patients undergoing lower tract urologic reconstruction. J Pediatr Urol (2021) 17(1):84.e1–.e8. doi: 10.1016/j.jpurol.2020.10.029
64. Schröder A, Campbell FA, Farhat WA, Salle JLP, Bägli DJ, Lorenzo AJ, et al. Postoperative pain and analgesia administration in children after urological outpatient procedures. J Pediatr Urol (2018) 14(2):171.e1–.e6. doi: 10.1016/j.jpurol.2017.11.014
65. Bilgutay AN, Hua H, Edmond M, Blum ES, Smith EA, Elmore JM, et al. Opioid utilization is minimal after outpatient pediatric urologic surgery. J Pediatr Urol (2020) 16(1):108.e1–.e7. doi: 10.1016/j.jpurol.2019.10.021
66. Ahn JJ, Ellison JS, Merguerian PA. A societies for pediatric urology survey of opioid prescribing practices after ambulatory pediatric urology procedures. J Pediatr Urol (2019) 15(5):451–6. doi: 10.1016/j.jpurol.2019.04.025
67. Arena S, Di Fabrizio D, Impellizzeri P, Gandullia P, Mattioli G, Romeo C. Enhanced recovery after gastrointestinal surgery (Eras) in pediatric patients: A systematic review and meta-analysis. J Gastrointest Surg (2021) 25(11):2976–88. doi: 10.1007/s11605-021-05053-7
68. Stowers MD, Lemanu DP, Hill AG. Health economics in enhanced recovery after surgery programs. Can J Anaesth (2015) 62(2):219–30. doi: 10.1007/s12630-014-0272-0
69. Schukfeh N, Reismann M, Ludwikowski B, Hofmann AD, Kaemmerer A, Metzelder ML, et al. Implementation of fast-track pediatric surgery in a german nonacademic institution without previous fast-track experience. Eur J Pediatr Surg (2014) 24(5):419–25. doi: 10.1055/s-0033-1352528
70. Sangkhathat S, Patrapinyokul S, Tadyathikom K. Early enteral feeding after closure of colostomy in pediatric patients. J Pediatr Surg (2003) 38(10):1516–9. doi: 10.1016/s0022-3468(03)00506-2
71. Franz AM, Martin LD, Liston DE, Latham GJ, Richards MJ, Low DK. In pursuit of an opioid-free pediatric ambulatory surgery center: A quality improvement initiative. Anesth Analg (2021) 132(3):788–97. doi: 10.1213/ane.0000000000004774
72. Cain MP. Enhanced recovery after surgery protocols in pediatric urology-how are we doing and what should we be doing? J Urol (2018) 200(5):952–3. doi: 10.1016/j.juro.2018.08.037
73. Chan YY, Rosoklija I, Meade P, Burjek NE, Raval MV, Yerkes EB, et al. Utilization of and barriers to enhanced recovery pathway implementation in pediatric urology. J Pediatr Urol (2021) 17(3):294.e1–.e9. doi: 10.1016/j.jpurol.2021.01.044
74. Short HL, Taylor N, Thakore M, Piper K, Baxter K, Heiss KF, et al. A survey of pediatric surgeons' Practices with enhanced recovery after children's surgery. J Pediatr Surg (2018) 53(3):418–30. doi: 10.1016/j.jpedsurg.2017.06.007
75. Marsman M, Kappen TH, Vernooij LM, van der Hout EC, van Waes JA, van Klei WA. Association of a liberal fasting policy of clear fluids before surgery with fasting duration and patient well-being and safety. JAMA Surg (2023) 158(3):254–63. doi: 10.1001/jamasurg.2022.5867
76. Frykholm P, Disma N, Andersson H, Beck C, Bouvet L, Cercueil E, et al. Pre-operative fasting in children: A guideline from the european society of anaesthesiology and intensive care. Eur J Anaesthesiol (2022) 39(1):4–25. doi: 10.1097/eja.0000000000001599
77. Yi JA, Hakimi A, Vavra AK. Application of dissemination and implementation science frameworks to surgical research. Semin Vasc Surg (2022) 35(4):456–63. doi: 10.1053/j.semvascsurg.2022.10.001
78. Sarkies MN, Francis-Auton E, Long JC, Pomare C, Hardwick R, Braithwaite J. Making implementation science more real. BMC Med Res Methodol (2022) 22(1):178. doi: 10.1186/s12874-022-01661-2
79. May CR, Mair F, Finch T, MacFarlane A, Dowrick C, Treweek S, et al. Development of a theory of implementation and integration: normalization process theory. Implement Sci (2009) 4:29. doi: 10.1186/1748-5908-4-29
80. Lam JY, Howlett A, McLuckie D, Stephen LM, Else SDN, Jones A, et al. Developing implementation strategies to adopt enhanced recovery after surgery (Eras®) guidelines. BJS Open (2021) 5(2):zraa011. doi: 10.1093/bjsopen/zraa011
81. Sutton E, Herbert G, Burden S, Lewis S, Thomas S, Ness A, et al. Using the normalization process theory to qualitatively explore sense-making in implementation of the enhanced recovery after surgery programme: "It's not rocket science". PloS One (2018) 13(4):e0195890. doi: 10.1371/journal.pone.0195890
Keywords: enhanced recovery after surgery, opioid reduction, length of stay, quality improvement, implementation science
Citation: Ha D, Harris KT, Brockel MA and Rove KO (2023) The role of enhanced recovery after surgery (ERAS) in promoting quality improvement and patient safety in pediatric urology. Front. Urol. 3:1275276. doi: 10.3389/fruro.2023.1275276
Received: 09 August 2023; Accepted: 11 September 2023;
Published: 28 September 2023.
Edited by:
Janelle Fox, Children’s Hospital of The King’s Daughters, United StatesReviewed by:
Liang Qu, University of Melbourne, AustraliaCopyright © 2023 Ha, Harris, Brockel and Rove. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kyle O. Rove, kyle.rove@childrenscolorado.org
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.