- 1Institute for Health Policy Studies, University of California, San Francisco, San Francisco, CA, United States
- 2Department of Urology, University of California, San Francisco, San Francisco, CA, United States
- 3Department of Surgery, University of California, San Francisco, San Francisco, CA, United States
- 4Department of Epidemiology & Biostatistics, University of California, San Francisco, San Francisco, CA, United States
Introduction: A single-arm pre-post pilot study in an academic setting found that pre-consultation decision support was associated with improved patient knowledge among men with early-stage prostate cancer. We now report on exploratory analyses from a controlled study featuring patients from both academic and community settings.
Methods: We enrolled 58 men to usual care and 61 men to the intervention. We evaluated whether the intervention was associated with patients answering key knowledge items correctly just before their urology visit.
Results: Just prior to the urology visit, 39/56 or 70% in the intervention group replied correctly to key knowledge items, compared to 31/55 or 56% in the usual care group (p=0.15). At baseline, the intervention group started with 42/60 or 70% correct and the usual care group started with 28/56 or 50% (p=0.03). This imbalance at baseline created a ceiling effect: more men in the usual care group had room to improve on their knowledge scores. Indeed, seven men moved from incorrect to correct in the usual care group, versus 5 in the intervention group; and five men in the intervention group moved from correct to incorrect versus 3 in the usual care group (p=0.44).
Discussion: In addition to small sample size, reasons for the null findings may include clustering of highly educated participants at the academic site combined with over-representation of academic site participants in the intervention group. We confirmed, from the pilot study, the feasibility of using pre-health student interns as health coaches. Future research should explore whether increasing adoption of telehealth will improve the feasibility of delivering pre-visit decision support in community settings.
1 Introduction
People diagnosed with serious illness generally are at risk for both under-treatment and over-treatment relative to their personal priorities, disease risk characteristics, and the best available medical evidence (1–3). This problem is salient in the case of low-risk prostate cancer patients, who are often over-treated with surgery or radiation relative to what they say they would have preferred if fully informed about all the options and outcomes, including the risks and benefits of active surveillance.
Decision support interventions tailored to specific clinical crossroads have increased patient self-efficacy, knowledge, question-asking, and satisfaction; and decreased decisional conflict, regret, anxiety, and distress (1, 4–16). More informed and involved patients tend to pursue less invasive treatment options than less informed and involved patients (17, 18).
However, in the area of prostate cancer, decision support interventions have not provided personalized risk estimates to patients. The need for personalized risk estimates arises from the potential for an initially low-risk prostate cancer to be reclassified over time as higher-risk. Many patients and physicians avert the risk of reclassification by erring on the side of active treatment (surgery or radiation) from the start. Another reasonable strategy would be to choose active surveillance (implying close monitoring) and switch to active treatment only in those cases where the patient’s risk increased over time.
To support fully informed decision making by patients, our team developed a comprehensive decision support intervention (DSI). Our DSI prompts patients to review their individual risk and other educational materials, as well as make a list of questions in writing for their physicians (6). We also trained a service learning workforce to deliver the intervention remotely, by internet and telephone, in a way that does not require clinics to modify their practices, and that leverages student interns receiving academic credit as part of their academic training (6).
We hypothesized that delivering decision support would increase patient knowledge, and this proximal outcome would ultimately increase the proportion of men making informed decisions regarding their selection of active surveillance, surgery, or radiation for low-risk prostate cancer. Our initial pilot study in an academic setting found our intervention feasible and effective in informing men of two key facts prior to their first urology visit upon diagnosis with low-risk prostate cancer (6). Because our intervention has novel elements, including pre-visit provision of personalized risk estimates delivered by student health coaches, we now report on a study including patients at four community sites.
2 Methods
2.1 Context, setting, population, study design, and sample
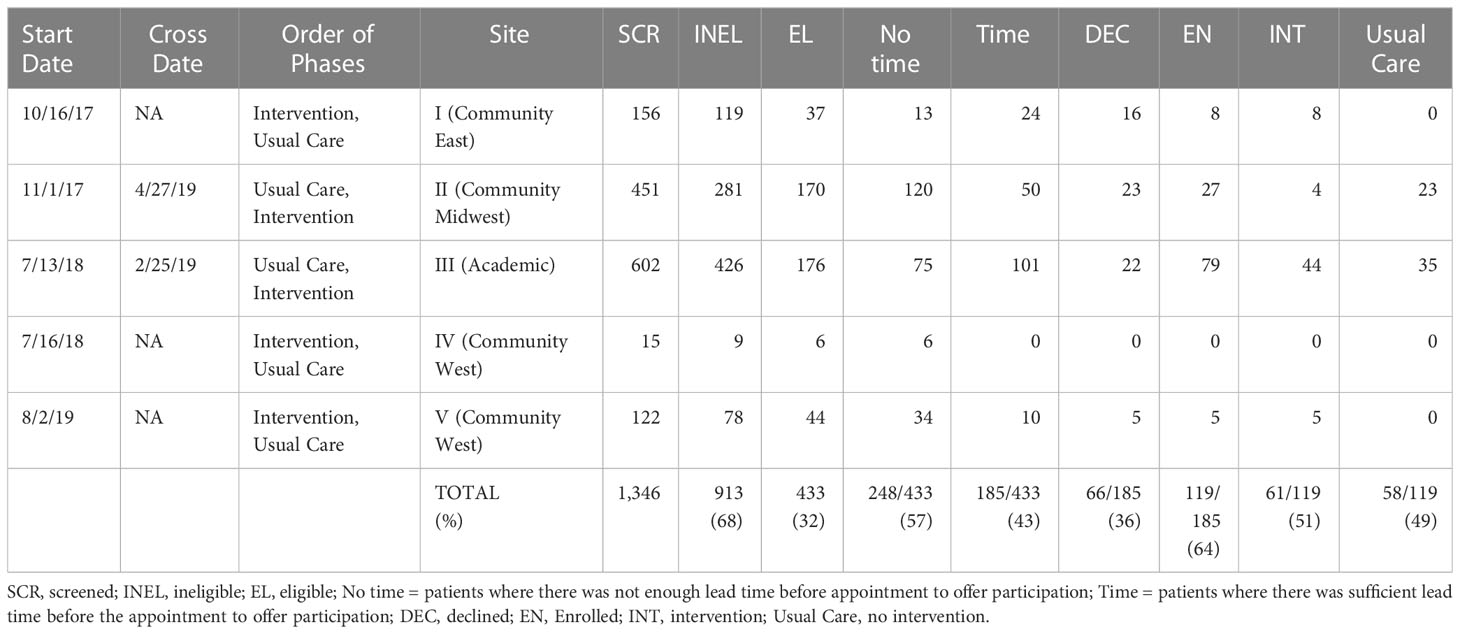
We set out to recruit men with low-risk prostate cancer at four community sites. We chose a convenience sample of four community sites from active enrolling sites (2016-2017) of the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) study (19), representing different geographic regions of the United States. We designed a four-site, site-randomized, two-period, cluster-crossover study and planned to enroll 160 participants, allowing for attrition (12) and non-response (12), leaving 136 fully evaluable participants (ClinicalTrials.gov registry NCT03397160). We randomized sites in pairs (blocks size 2) to start period 1 as usual care or intervention, with the starting allocation also randomized. We planned to cross over each site to intervention or usual care in period 2 after they met a priori accrual goals (i.e., N~40 per site, 20 men per period). We budgeted funds for a research coordinator at each site to screen men into the following eligibility criteria and then offer enrollment and guide participation in the trial.
Men were considered eligible for this study if they were diagnosed with localized low-risk prostate cancer defined as: diagnostic PSA <= 15 ng/ml; clinical stage cT1/2, N0, M0; and Gleason sum 2-6 (or Gleason score 3 + 4 with <=33% cores positive for adenocarcinoma) based on a diagnostic biopsy with a minimum of 10 cores. Additionally, men had to have not received or already decided on any treatment for prostate cancer, have had no prior use of 5-alpha reductase inhibitors with 6 months of the biopsy (criteria intended for ancillary correlative science study), have English language proficiency and ability to sign an informed consent form; and be considered candidates for active surveillance at their institution by their treating urologist.
In practice, we encountered problems implementing the study: turnover of staff at community sites; fewer than expected patients met the eligibility criteria; and we depleted time and budget on screening out large numbers of ineligible patients. Therefore, in July 2018, after 9 months of slow enrollment, we added the University of California, San Francisco (UCSF), which was the site of the prior academically-based pilot study. Our rationale was that, while our site-randomized crossover trial was proving infeasible, we could still seek to replicate our uncontrolled pilot study results in a controlled study. In addition, given depletion of original funding, the slow accrual, and the onset of the COVID-19 pandemic, we had to close the study prior to reaching the original accrual goal of 160, after accruing 119 participants. We now report briefly on our findings, which should be considered exploratory, and illustrate lessons learned from attempting to translate a pre-visit intervention for low-risk patients from an academic pilot study to community settings.
2.2 Outcomes, measures, and instruments
2.2.1 Primary outcome: knowledge of two key facts
The primary endpoint was the proportion of patients who responded accurately to two survey items from the Prostate Cancer Decision Quality Instrument (20), administered at the clinic by a study coordinator just before the consultation with the urologist. The two items assessed whether, among men diagnosed with early-stage prostate cancer, most will die of prostate cancer; about half will die of prostate cancer; or most will die of something else (answer: most will die of something else); and whether waiting three months to make a treatment decision will affect their survival a lot; somewhat; or a little or not at all (answer: a little or not at all).
Knowledge of these key facts is an important endpoint because patients often associate the word cancer with high mortality risks, and urgency to act. Patients also often associate more invasive treatment with greater benefit.
In the case of early-stage, low-risk prostate cancer, patients would ideally recognize that they are more likely to die of other causes than prostate cancer and that most can safely take three months to make decisions. This knowledge potentially opens patients up to consideration of active surveillance along with surgery and radiation therapies.
Our intervention aimed to educate patients about their low mortality risk and the time available for decision-making after their cancer diagnosis and before they saw their urologist for primary treatment counseling. Therefore, we assessed this endpoint just prior to the first urology appointment after a biopsy confirmed early-stage low-risk cancer. We compared results at this timepoint between the usual care and intervention groups. We also assessed the knowledge endpoint at baseline to evaluate within-subject changes in knowledge; and repeated the knowledge assessment after the consultation with the urologist.
2.3 Intervention
As reported earlier (6), we developed a decision aid to present patients with the following estimates: survival and quality of life outcomes from the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) outcomes database for patients who underwent active surveillance, surgery, or radiation; and risk of reclassification (“upstaging” or “upgrading”) based on demographic, clinical, and pathologic parameters (i.e., age, prostate volume, prostate tumor grade, percent positive cores from biopsy, PSA) using a model developed at UCSF and tested using UCSF and CaPSURE data (21).
After reviewing the decision aid, either via a secure website, mailed screenshots, or emailed screenshots, intervention group patients spoke with a pre-health student acting as a coach. Per the pilot study intervention, we trained four coaches to be neutral and non-directive in reviewing the contents of the decision aid and in helping the study participants make a list of questions (4). The coach reviewed the decision aid screen by screen with the patient, reviewed built-in help text with the patient when appropriate, and wrote down (without answering) each patient’s questions for the physician. The coach saved a word-processed document with the patient’s questions for the physician, and sent that file to the site study coordinator, who printed copies for the patient, family, and physician and made those copies available to all parties at the time of the clinic visit. We assured fidelity of the coaches to the intervention by recording and reviewing role-plays with simulated patients.
For the low-risk patients eligible for our study, survival projections were relatively high, and risk of reclassification was relatively low. We expected the intervention to communicate to patients that they were more likely to die of causes other than prostate cancer and that they had time to make decisions.
2.4 Analysis plan
The primary statistical analysis compared, for assessments made just before the patient consultation with their urologist, the proportion of patients in intervention versus control groups responding correctly to both key knowledge items; and the proportion of patients whose answers changed from at least one incorrect at baseline to both correct before the urologist visit. We used the chi-square test (and p-values) to compare proportions across groups; and conditional logistic regression (and 95% confidence intervals (CI)) for comparing the changes within subjects. A threshold of 0.05 was used to determine statistical significance. We excluded from each analysis any patients who were missing a required response.
3 Results
3.1 Study sample
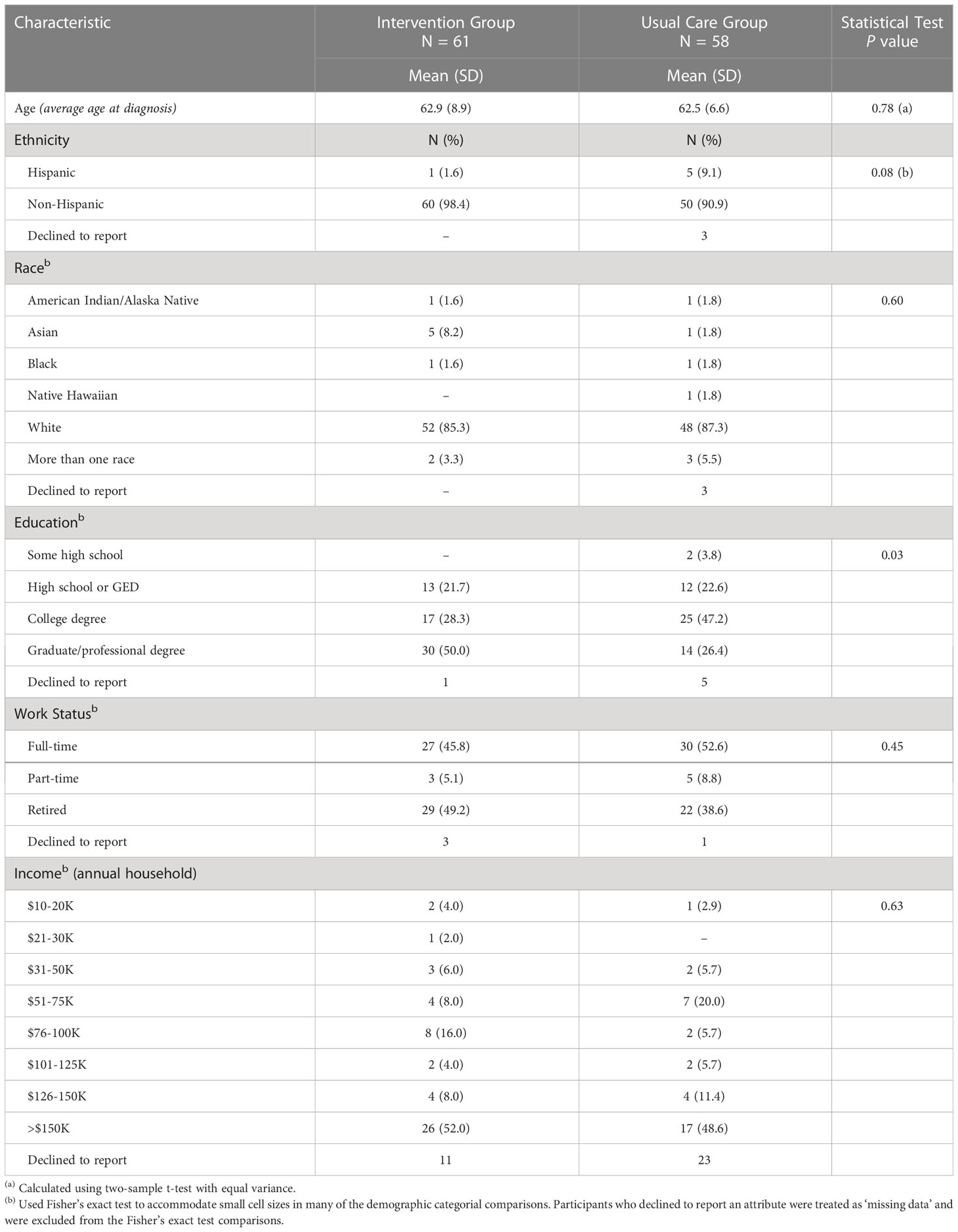
Among the four sites that enrolled patients, we screened 1,346 patients, of whom 913 (68%) did not meet our study’s eligibility criteria. The main reasons for ineligibility were for not meeting clinical criteria of low-risk disease (e.g., ~40% of 855 men screened did not meet the Gleason grade criteria described above). This left 433 potentially eligible patients. Research coordinators were unable to reach 249 of these patients ahead of their appointment in time to offer them participation, leaving 185 patients who were both eligible and available. Of these, 66 (36%) declined and 119 (64%) enrolled. Study participants received the intervention or usual care based on the study phase their site was in at the time of their enrollment. After sites I and II accrued participants very slowly, we added our academic site in the next block (III, IV). When site IV failed to accrue participants, we replaced it with site V, which also accrued slowly. In the original design, all sites were to cross over after accruing their share of patients; in practice, only sites II and III accrued sufficiently to cross over and contribute to a controlled comparison. See Figure 1 and Table 1 for details of the participant allocation and ascertainment by site. Screening began 10/16/2017 and concluded on 3/20/2020. Table 2 summarizes the sample demographics. Given these deviations from our original enrollment plans, the intervention and control groups were not balanced between the academic site and the community sites, and not balanced in terms of participant characteristics (most notably education level), possibly due to clustering of participants in the different settings (Tables 1, 2). While the slow accrual meant that we could not implement our original study design, for ethical reasons we report on our exploratory analyses of the data as collected.
3.2 Exploratory analyses
At baseline, the intervention group started with 42/60 or 70% correct and the usual care group started with 28/56 or 50% correct. This difference was statistically significant (p=0.03). Before the consultation with their urologist, after intervention 39/56 or 70% in the intervention group answered correctly compared to 31/55 or 56% in usual care. The difference (our primary analysis) was not statistically significant (p=0.15). After the consultation with the urologist, 45/55 or 82% in the intervention group were correct, and 37/56 or 66% in the usual care group. The difference was not statistically significant (p=0.06).
Of the 10 men in each group who changed their responses between baseline and post-intervention assessment of knowledge, 5/10 (50%) moved from incorrect to correct in the intervention group versus 7/10 (70%) in the usual care group. This difference was not statistically significant (p=0.44).
4 Discussion
4.1 Discussion of primary outcome
The intervention group reported higher knowledge at all three time points: at baseline, immediately before, and immediately after consultation with the urologist. In this respect, this controlled study replicated the findings of the pilot study. However, the magnitude of the differences were smaller than in the pilot study (6), and our comparisons to usual care did not show statistically significant differences. One possible explanation is that at baseline, before any intervention, the intervention group answered 70% correct whereas the usual care group was 50% correct. This difference was likely due to imbalances in accrual by site type, and clustering of similar, highly educated participants at the academic site. Specifically, more intervention patients came from the academic site than community sites; and more participants at the academic site had higher levels of education. For example, 50% of the participants in the academic site had graduate or professional degrees, compared to 26% in the usual care site. The ensuing differences in intervention and control groups may have resulted from the difference in their starting knowledge scores, i.e. a ceiling effect. The intervention group had few participants with room to improve.
4.2 Lessons learned
After it became clear that our study would not accrue sufficient patients in its original community site crossover design, we added the academic site from the pilot study. This allowed us to conduct a controlled exploratory analysis, and use the data collected from community site patients who contributed their participation and deserved our best efforts to leverage their participation into insights. We learned several lessons that may be valuable for clinicians and researchers, summarized below.
4.2.1 Cautionary lessons learned about the challenges of priming patients with education before visits to urologists
Our intervention attempted to screen and contact patients before they saw a urologist to discuss treatment options for a diagnosis of early-stage, low-risk prostate cancer. Priming patients for a visit should logically improve their participation (e.g. question-asking) and recall of information at the visit. Studies in academic settings have shown such effects, including in the domain of prostate cancer (8–10). At the time of the study, however, our community partners were more used to screening patients and interacting with them after their first in-person clinical visit, not before. This is an important distinction that clinicians and researchers should consider when translating pre-visit interventions from academic to community settings. In this study, the academic site was able to approach 101 out of 176 eligible patients (57%), whereas the community sites approached only 84 out of 257 eligible patients (33%). This is likely because academic sites build up a strong capacity to recruit patients to a variety of studies, including those that require interaction before any visit takes place.
Looking to the future, it may be that pre-visit interventions are more feasible in settings with more telehealth capabilities. Our study took place before the massive increase in telehealth surrounding COVID. Community settings are now embracing telehealth in the wake of the response to COVID-19. In addition, with the recent advances in use of electronic health records, future studies could leverage patient portals to screen for eligibility, offer electronic consenting, and deliver educational interventions.
4.2.2 Cautionary lessons learned about the challenges of identifying and intervening with low-risk patients
Another insight from our study that may help future clinicians and researchers relates to low-risk patients. In the domain of prostate cancer, low-risk patients may seek or accept unnecessarily aggressive treatment which they would avoid if they fully understood their risk profile. Our screening process, looking for low-risk patients, yielded smaller than expected numbers of eligible patients. One explanation may be that clinician attitudes to diagnosing prostate cancer were evolving – i.e., clinicians became more concerned with over-diagnosing prostate cancer and were waiting for higher prostate-specific antigen test scores before conducting biopsies. More patients than expected became ineligible for our study because either their prostate-specific antigen scores or tumor Gleason scores exceeded our low-risk eligibility threshold by the time they had a biopsy and were ready to consider treatment. This raises the question of when and how to identify low-risk patients and educate them. Our intervention was novel in attempting to educate patients after a biopsy but before their appointment with a urologist to discuss treatment options. Perhaps educational interventions should be aimed even further upstream, at patients with rising prostate-specific antigen scores who are being followed to consider biopsies. Essentially these patients are starting with active surveillance and could begin to consider how they might respond given possible biopsy results. Here again, patient education for cohorts of patients that are still being seen in primary care will likely be easier with new advances in telehealth, including patient portals.
4.3 Strengths and limitations
The final study implementation was an academic and community based, unblinded, controlled trial that did not meet original accrual goals. This outcome left us susceptible to several biases. Our study may reflect sampling bias and clustering. Because sites followed a different diagnostic protocol, they had different lead times for study recruitment, which differentially affected study accrual rates across the sites, another form of sampling bias. Most participants came from an academic site where a potential ceiling effect in the primary outcome, knowledge, was more pronounced at baseline than at community sites. This may have been due to the imbalance in characteristics (notably, education level) of participants who clustered around academic versus community sites.
We enrolled higher numbers of usual care participants early in the study compared to intervention group participants, so the maturation (passage of time) bias was different in each group. We believe the passage of time was associated with changing diagnostic and treatment practices among clinicians and could have been associated with different levels of knowledge among study participants. Specifically, the intervention group participants may have started at a higher level of knowledge about their mortality risk simply because clinicians and the media were paying more attention over time to the need to avoid overdiagnosis and overtreatment among men with low-risk prostate cancer.
While our study was underpowered to detect differences between the groups, especially in the context of a ceiling effect in the intervention group, this experience offers a few valuable lessons. We replicated the feasibility, in our pilot study, of using undergraduate student interns as coaches. They earned academic credit through their participation in a service learning program, the Patient Support Corps (6). In course ratings, students expressed satisfaction with the service-learning experience. Using students as workforce extenders helped the study conserve budgetary resources while still providing personal attention to study participants. Students were able to coach patients by telephone while the patient looked at a decision aid over the internet.
In conclusion, we did not observe a statistically significant increase in patient knowledge with the implementation of a decision support intervention incorporating tailored risk education and health coaches. The breakdown in study design, resulting imbalances between groups, lack of sufficient statistical power, secular trends in prostate cancer screening and diagnosis, and ceiling effects in the primary outcome may partially explain these null findings.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by UCSF Human Research Protection Program Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author contributions
The Author Contributions section is mandatory for all articles, including articles by sole authors. The Author Contributions statement must describe the contributions of individual authors referred to by their initials and, in doing so, all authors agree to be accountable for the content of the work. JKB: study conception and design, data analysis and interpretation, manuscript drafting and revisions. JMB: study conception and design, data collection, data analysis and interpretation, manuscript drafting and revisions. JN: study conception and design, data analysis and interpretation, manuscript review and revisions. AZ: study conception and design, data collection, manuscript review and revisions. TW: study conception and design, data collection, manuscript review and revisions. SK: study conception and design, manuscript review and revisions, JW: data analysis interpretation, manuscript review. EB: study conception and design, manuscript review and revisions. MC: study conception and design, manuscript review and revisions. PC: study conception and design, manuscript review and revisions. JC: study conception and design, data analysis and interpretation, manuscript review and revisions. All authors contributed to the article and approved the submitted version.
Funding
Funded by the US Department of Defense grant number W81XWH-13-2-0074, PI: PC.
Acknowledgments
We are grateful to Dawn Bauerly-Pieper, Mark Bridge, Erik Chan, Andre Dempsey, Landon Haller, Carmel Malvar, Lori Pinke, Stan Rosenfeld, Ada Sanchez; Karen Sepucha, Paul Sieber, Karen Stevenson, Lauren Stupar, Imelda Tenggara, Shelley Volz, Daniel Yao, and all study participants.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Adler NE, A. Page, and National Institute of Medicine (U.S.). Committee on psychosocial services to cancer patients / families in a community setting. In: Cancer care for the whole patient: meeting psychosocial health needs, vol. xxii. . Washington, D.C: National Academies Press (2008). p. 429.
2. Brownlee S. Overtreated : why too much medicine is making us sicker and poorer. 1st U.S. ed Vol. 343. New York: NY: Bloomsbury (2007).
3. Hadler NM. Worried sick : a prescription for health in an overtreated America. Chapel Hill, NC: University of North Carolina Press (2008). p. 376.
4. Belkora J, Miller M, Crawford B, Coyne K, Stauffer M, Buzaglo J, et al. Evaluation of question-listing at the cancer support community. Trans Behav Med (2013) 3(2):162–71. doi: 10.1007/s13142-012-0186-8
5. Belkora J, Stupar L, O'Donnell S, Loucks A, Moore D, Jupiter C, et al. Decision support by telephone: randomized controlled trial in a rural community setting. Patient Educ Couns (2012) 89(1):134–42. doi: 10.1016/j.pec.2012.06.009
6. Belkora J, Volz S, Loth M, Teng A, Zarin-Pass M, Moore D, et al. Coaching patients in the use of decision and communication aids: RE-AIM evaluation of a patient support program. BMC Health Serv Res (2015) 15(1):209. doi: 10.1186/s12913-015-0872-6
7. Belkora JK, Loth MK, Chen DF, Chen JY, Volz S, Esserman LJ. Monitoring the implementation of consultation planning, recording, and summarizing in a breast care center. Patient Educ Couns (2008) 73(3):536–43. doi: 10.1016/j.pec.2008.07.037
8. Dimoska A, Tattersall MH, Butow PN, Shepherd H, Kinnersley P. Can a "prompt list" empower cancer patients to ask relevant questions? Cancer (2008) 113(2):225–37. doi: 10.1002/cncr.23543
9. Hacking B, Wallace L, Scott S, Kosmala-Anderson J, Belkora J, McNeill A. Testing the feasibility, acceptability and effectiveness of a 'decision navigation' intervention for early stage prostate cancer patients in Scotland - a randomised controlled trial. Psychooncology (2013) 22(5):1017–24. doi: 10.1002/pon.3093
10. Kinnersley P, Edwards A, Hood K, Cadbury N, Ryan R, Prout H, et al. Interventions before consultations for helping patients address their information needs. Cochrane Database Syst Rev (2007) 3):CD004565. doi: 10.1002/14651858.CD004565.pub2
11. Pitkethly M, MacGillivray S, Ryan R. Recordings or summaries of consultations for people with cancer. Cochrane Database Systematic Rev (2008). doi: 10.1002/14651858.CD001539.pub2
12. Roter DL. Patient participation in the patient-provider interaction: the effects of patient question asking on the quality of interaction, satisfaction and compliance. Health Educ Monogr (1977) 5(4):281–315. doi: 10.1177/109019817700500402
13. Roter DL. Patient question asking in physician-patient interaction. Health Psychol (1984) 3(5):395–409. doi: 10.1037/0278-6133.3.5.395
14. Sepucha KR, Belkora JK, Mutchnick S, Esserman LJ. Consultation planning to help breast cancer patients prepare for medical consultations: effect on communication and satisfaction for patients and physicians. J Clin Oncol (2002) 20(11):2695–700. doi: 10.1200/JCO.2002.10.068
15. Sepucha KR, Belkora JK, Tripathy D, Esserman LJ. Building bridges between physicians and patients: results of a pilot study examining new tools for collaborative decision making in breast cancer. J Clin Oncol (2000) 18(6):1230–8. doi: 10.1200/JCO.2000.18.6.1230
16. Stacey D, Bennett CL, Barry MJ, Col NF, Eden KB, Holmes-Rovner Mc , et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev (2011) (10):CD001431. doi: 10.1002/14651858.CD001431.pub3
17. Griffin SJ, Kinmonth AL, Veltman MW, Gillard S, Grant J, Stewart M. Effect on health-related outcomes of interventions to alter the interaction between patients and practitioners: a systematic review of trials. Ann Fam Med (2004) 2(6):595–608. doi: 10.1370/afm.142
18. Harrington J, Noble LM, Newman SP. Improving patients' communication with doctors: a systematic review of intervention studies. Patient Educ Couns (2004) 52(1):7–16. doi: 10.1016/S0738-3991(03)00017-X
19. Cooperberg MR, Carroll PR. Trends in management for patients with localized prostate cancer, 1990-2013. JAMA (2015) 314(1):80–2. doi: 10.1001/jama.2015.6036
20. Sepucha K. Prostate cancer decision quality instrument v 1.0. Massachusetts General Hospital (2013).
21. Braun A, Chan J, Neuhaus J, Cowan J, Kenfield S, Van Blarigan E, Tenggara I, et al. (2023). MP38-02 the impact of genomic biomarkers on a validated clinical risk prediction model for upgrading/upstaging among men with low-risk prostate. Cancer J Urol. 209(Supplement 4):e523. doi: 10.1097/JU.0000000000003276.02
Keywords: patient education, personalized health care, prostate cancer, active surveillance, health coaching, decision aid, decision support, service learning internships and co-ops
Citation: Belkora JK, Broering JM, Neuhaus J, Zargham A, Weinberg T, Witte JS, Kenfield SA, Van Blarigan EL, Cooperberg MR, Carroll PR and Chan JM (2023) Controlled trial of decision support for men with early-stage prostate cancer: brief research report of effects on patient knowledge. Front. Urol. 3:1127089. doi: 10.3389/fruro.2023.1127089
Received: 19 December 2022; Accepted: 28 April 2023;
Published: 17 May 2023.
Edited by:
Oliver Brunckhorst, King’s College London, United KingdomReviewed by:
Sakineh Hajebrahimi, Tabriz University of Medical Sciences, IranSeetharam Bhat Kulthe Ramesh, Upstate Medical University, United States
Copyright © 2023 Belkora, Broering, Neuhaus, Zargham, Weinberg, Witte, Kenfield, Van Blarigan, Cooperberg, Carroll and Chan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeffrey K. Belkora, amVmZi5iZWxrb3JhQHVjc2YuZWR1
†These authors share senior authorship
 Jeffrey K. Belkora
Jeffrey K. Belkora Jeanette M. Broering
Jeanette M. Broering John Neuhaus4
John Neuhaus4