- 1Department of Urology, San Carlo di Nancy Hospital, Rome, Italy
- 2Interventional Radiology Unit, San Camillo Hospital, Rome, Italy
- 3Urology Unit, Department of Surgery, Tor Vergata University of Rome, Rome, Italy
- 4Department of Life, Health and Environmental Sciences, Urology Unit, University of L’Aquila, Coppito, Italy
- 5Department of Interventional Radiologist, San Carlo di Nancy Hospital, Rome, Italy
Purpose: The study evaluated the effectiveness of prostatic arterial embolization (PAE) in the relief of benign prostatic obstruction (BPO) beyond the patient-reported outcomes.
Methods: Retrospective evaluation of patients who underwent PAE (March 2015–December 2019). All patients underwent prostate MRI to assess prostate volume (PVol), uroflowmetry to assess Qmax, and were administered IPSS + QoL. MRI, and IPSS were repeated 3 months postoperatively. Patients were contacted for urological consultation, including uroflowmetry with post-voiding residual volume (PVR), IPSS + QoL. Additionally, patient satisfaction was assessed. Sexual function, including ejaculation and complications, was recorded.
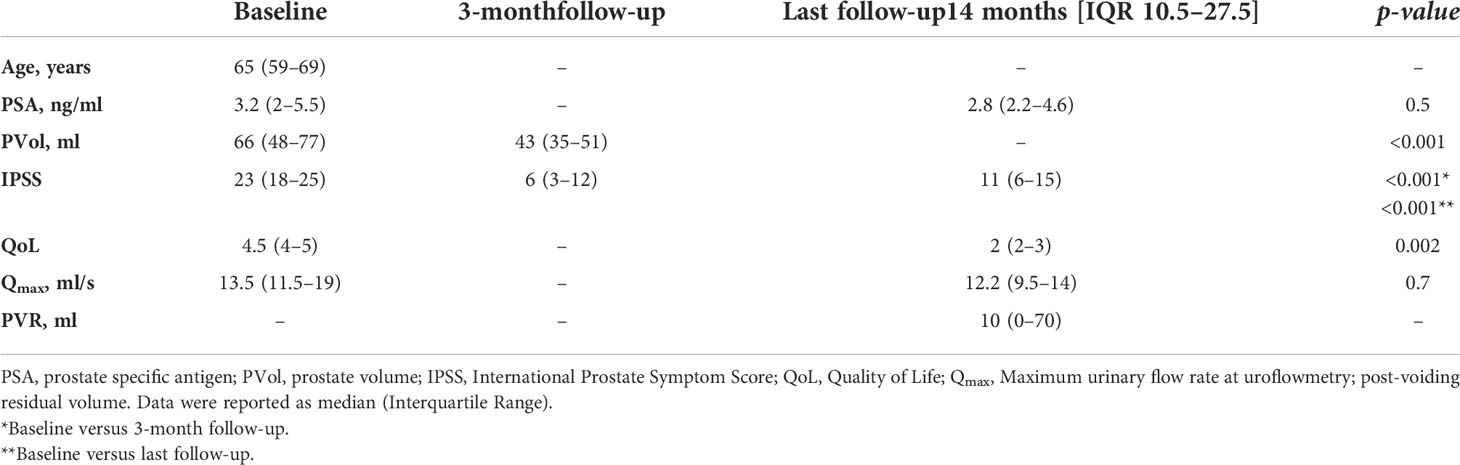
Results: Fifty-two patients were analyzed. At baseline, PVol was 66 ml (IQR 48–67), Qmax 13.5 ml/s (IRQ 11.5–19), IPSS 23 (IQR 18–25), and QoL 4.5 (IQR 4–5). At 3-month follow-up, MRI showed a 27% (IQR 18–36) reduction in PVol (p <0.001). The IPSS decreased by 81% (IQR 58–91, p <0.001). At a median follow-up of 14 months (IQR 10.5–27.5), IPSS decreased by 40% (IQR 26–54, p <0.001) and QoL by 50% (IQR 20–75, p = 0.002) versus baseline. The absolute Qmax was 12.2 ml/s (IQR 9.5–14). Median %variation of Qmax vs baseline was −7.3% (IQR −33.9; +25.5, p = 0.7). All sexually active patients maintained ejaculation. Thirty-eight (73%) were satisfied with the treatment they received. One patient reported post-operative erectile dysfunction. All patients who were counseled for adjuvant endoscopic treatment refused surgery except for one.
Conclusion: In our analysis, PAE provided significant improvement in the short-term follow-up patient-reported outcomes. Most patients were satisfied, and ejaculation was maintained. On the other hand, the effectiveness of PAE on the relief of BPO seemed virtually intangible in most of the cases.
Introduction
It is estimated that 25%–60% of men will suffer from benign prostatic hyperplasia (BPH) over their lifetime (1, 2). BPH may cause obstruction, leading to lower urinary tract symptoms (LUTS) and worsen the quality of life in affected patients. Such benign prostatic obstruction (BPO) is initially treated by medical therapy. In those patients affected by LUTS refractory to medical therapy, transurethral resection of the prostate (TURP) and its laser-based transurethral enucleative alternatives have represented the gold-standard approach for decades (3, 4). The issue during daily clinical practice is that patients (particularly when younger) are often reluctant to undergo transurethral “extirpative” techniques given their associated risks, the first and foremost being retrograde ejaculation (5).
In this setting, various less-invasive approaches with ejaculation-sparing intent have been developed and are currently available as alternatives to standard transurethral techniques. The temporary implantable nitinol device is a “micro-invasive” option that showed an encouraging rate of ejaculation preservation (6). Recent systematic reviews evaluated the effects on ejaculatory dysfunction of many other “micro-invasive” or “ultra minimally-invasive” treatment modalities to manage BPO: water vapor thermal therapy, prostatic urethral lift, waterjet ablation, and prostatic arterial embolization (PAE) showed less than 2% rate of retrograde ejaculation at 1-year follow-up (7, 8).
PAE is a relatively new, micro-invasive treatment option, first described by Pisco et al. (9).
It is currently unsupported by the American Urological Association guidelines and weakly recommended by the European Association of Urology (EAU). The EAU guidelines underline that the technique is still under investigation; furthermore, strong collaboration between urologists and trained interventional radiologists is mandatory (10, 11). There is level 1a evidence that PAE is less effective than TURP at improving symptoms and urodynamic parameters such as urinary flow rate (12–15).
Most of the studies demonstrated a significant improvement in the patient-reported outcomes, while a clinically significant impact on the maximum flow rate at uroflowmetry is still debated. This is because either minimal improvement is described, or urodynamic parameters are not investigated when observational studies are conceived within the literature produced within the interventional radiology setting.
To make a contribution in this field, this analysis was conceived.
The primary aim of the study was to report the outcomes of PAE. The secondary aim was to offer a urological insight into the data.
Materials and methods
Data of patients who consecutively underwent PAE performed by a team of experienced interventional radiologists (SM and MG) were retrospectively reviewed. All patients gave their consent before being included and analyzed within the goals of the present study, performed in accordance with the Declaration of Helsinki.
Procedures were performed between March 2015 and December 2019. All patients had enclosed serum prostate-specific antigen (PSA), had undergone prostate magnetic resonance imaging (MRI) to assess prostate volume (PVol), uroflowmetry to assess maximum flow rate (Qmax), and post-voiding residual volume (PVR), and have been administered the International Prostate Symptom Score (IPSS) with the Quality of Life (QoL) (16). Three months postoperatively, MRI and IPSS were repeated. Specifically for the study, patients were contacted for urological consultation, which included repeated PSA, uroflowmetry with assessment of PVR, and re-administration of IPSS and QoL. Eventual missing data were retrieved from the medical records of each patient. Additionally, the satisfaction of the patient with the treatment received was assessed using Question 32 of the Expanded Prostate Cancer Index Composite questionnaire (“How satisfied are you with treatment received for your prostate disease intervention?”) (17).
Information about the ejaculatory function and eventual procedure-related complications that occurred or further treatments required were investigated and recorded.
Technique for prostatic arterial embolization
The technique to perform PAE included proximal embolization first, then distal embolization, by using calibrated 300–500 l-trisacryl microspheres (Embosphere, Merit Medical, Salt Lake City, USA). Prior to intervention, all patients had received 4,000 IU of low-molecular-weight heparin and antibiotic therapy with cefazolin (2 g). A Foley catheter was introduced into the bladder and filled with a mixture of iodinate contrast medium (20%) and saline solution (80%). The procedure was performed under local anesthesia, via a unilateral (usually right-side) trans-femoral approach.
For this purpose, a 5 Fr sheath and an F5 Cobra-shaped (C2) or Roberts Uterine Artery Catheter are introduced via the common femoral artery to catheterize the hypogastric artery and then its anterior division. Once the catheter is in the anterior division of the hypogastric artery, a digital subtraction angiography is performed in left anterior oblique projection (35°) and caudal–cranial angulation (10°) to visualize the anatomy of the prostatic arteries. Contrast media is injected, then a road map of the artery from which the prostatic artery takes origin is obtained. Afterwards, the prostatic vessels are catheterized with a 3 F coaxial microcatheter. When the catheterization of the prostatic vessels is achieved, manual angiography is performed to confirm the position of the catheter in the ostium of the prostatic artery and to visualize the vascular anatomy of the prostate in the same oblique and frontal position. Then, the microcatheter was advanced distally into the prostatic artery and embolization was performed (Figure 1).
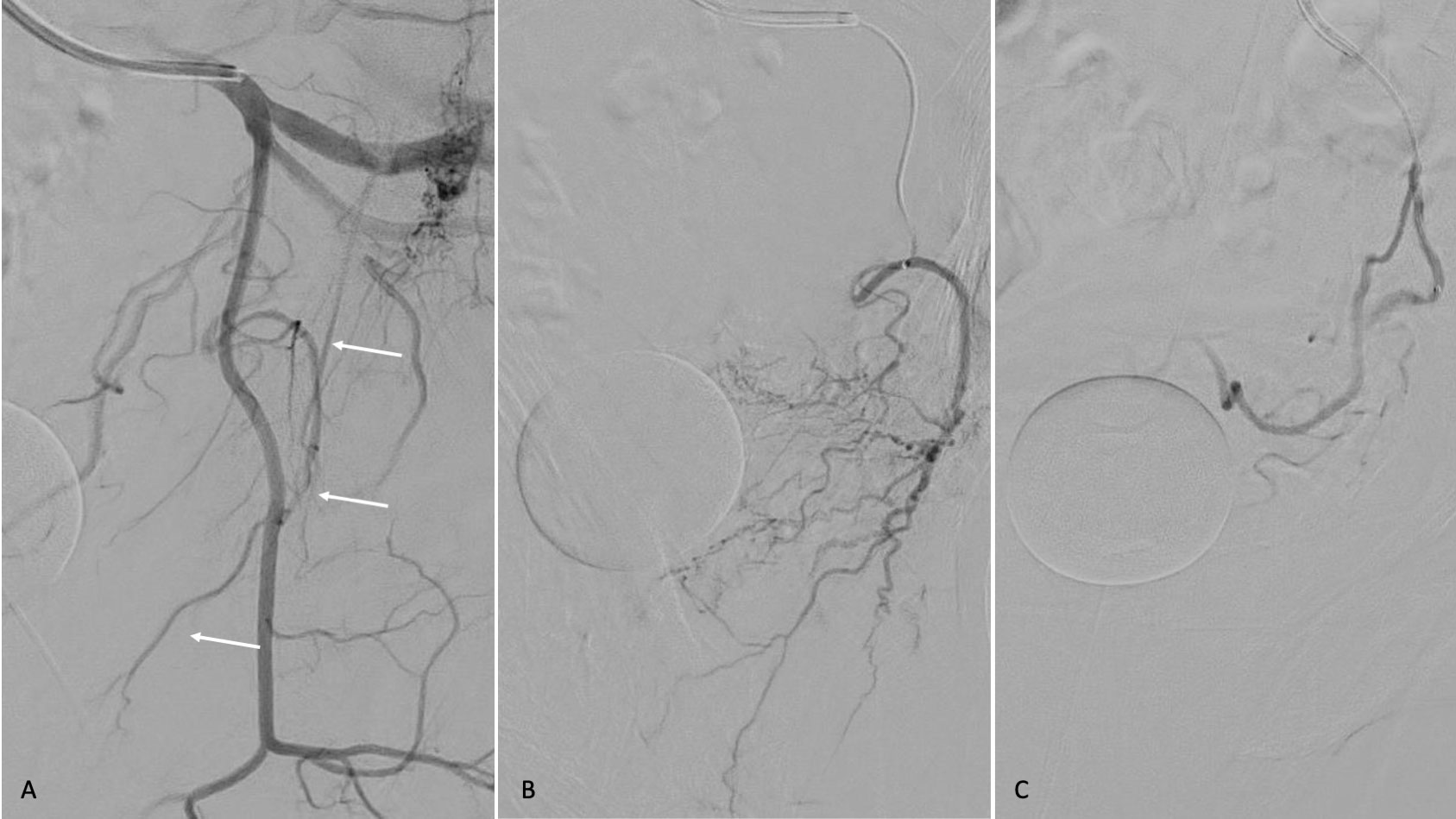
Figure 1 Digital subtraction angiography, oblique projection, (A) of the anatomy of the prostatic artery angiography (B) after selective catheterization demonstrating the prostate gland. (C) Prostatic artery angiography after embolization; decreased vascularity of the prostate is shown.
Statistical analysis
Continuous variables are presented as median and interquartile ranges (IQR). Categorical variables are presented as frequencies and proportions. The Mann–Whitney U test was used when comparing two groups of dependent variables, while non-parametric Friedman ANOVA and Kendall’s concordance were used to compare differences among more than two groups. A comparison of categorical variables was performed using the Fisher’s exact test.
A p-value of <0.05 was considered statistically significant. Statistical analysis was performed using “Statistic” 8.0 Software (StatSoft, Tulsa, OK, United States).
Results
The datasets of fifty-two patients who underwent PAE were analyzed. The age at surgery was 65 years (IQR 59–69). Prior to treatment, PVol was 66 ml (IQR 48–67), Qmax 13.5 ml/s (IRQ 11.5–19), IPSS 23 (IQR 18–25), and QoL 4.5 (IQR 4–5).
At the 3-month follow-up, MRI showed a 27% (IQR 18–36) reduction in PVol (p <0.001). The IPSS was found to have decreased by 81% (IQR 58–91, p <0.001), with a median value of 6 (IQR 3–12).
Urological consultations were performed at a median follow-up of 14 months (IQR 10.5–27.5).
At this time-point, with respect to baseline, IPSS was found to have decreased by 40% (IQR 26–54, p <0.001), with a median value of 11 (IQR 6–15) and QoL decreased by 50% (IQR 20–75, p = 0.002), with a median value of 2 (2, 3). Uroflowmetry was performed; absolute Qmax was 12.2 ml/s (IQR 9.5–14). Percentage median variation of Qmax versus baseline was a non-statistically significant 7.3% decrease (IQR −33.9; +25.5, p = 0.7). Median PVR was 10 ml (IQR 0–70). Table 1 reports complete data at the different time-points evaluated. Figure 2 plots the median (IQR) IPSS at the different time-points.
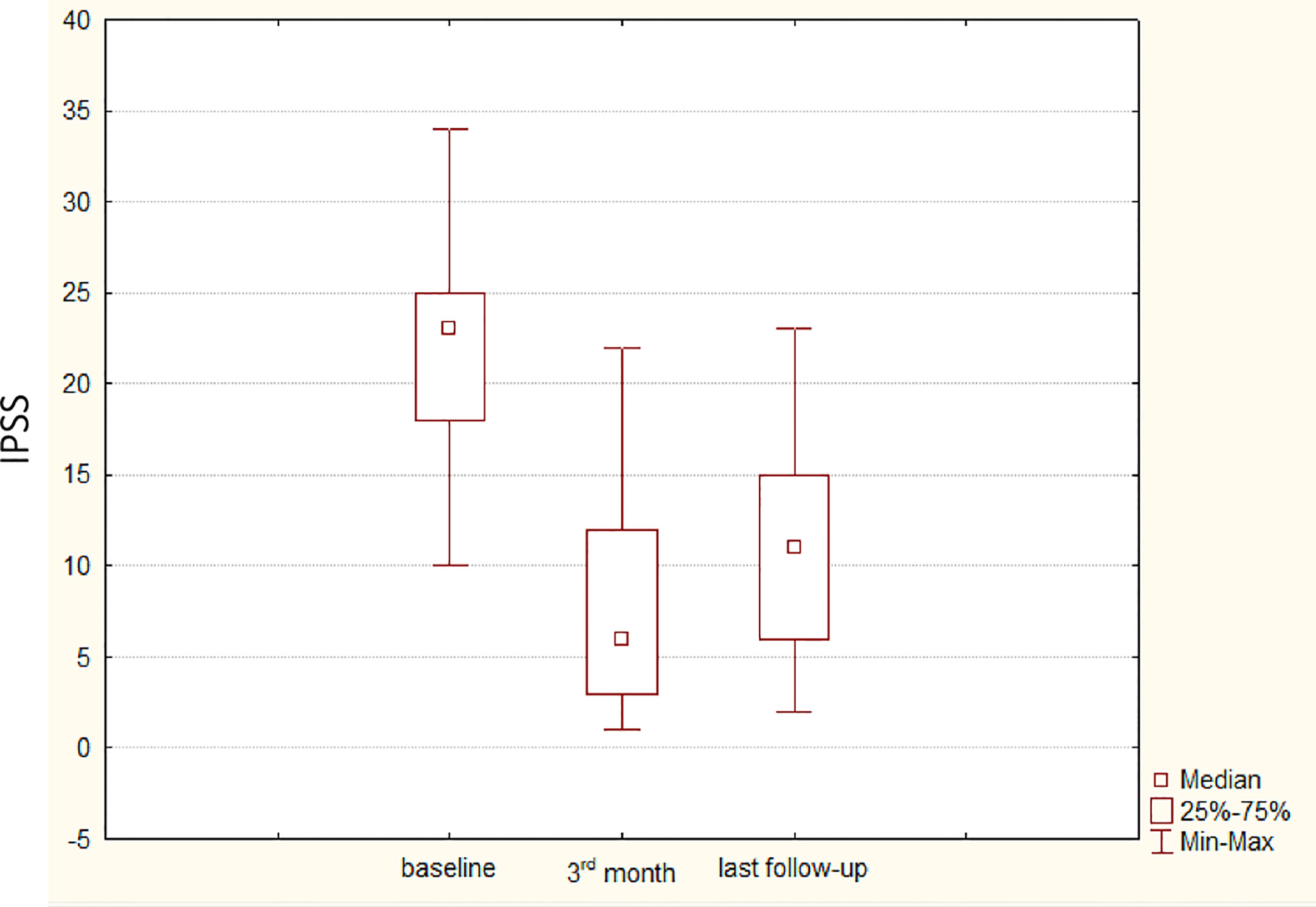
Figure 2 Box & Whisker plots depicting the median, the 25%–75% interquartile range, the minimum, and the maximum of the distribution of the International Prostate Symptom Score (IPSS), assessed at different time points: baseline, third month postoperatively, and last follow-up. The median last follow-up was 14 months; interquartile range was 10.5–27.5 months.
One hundred percent of patients who were still sexually active at the time of urological consultation referred maintained ejaculation. One patient reported post-operative erectile dysfunction. Overall, 38 patients (73%) were satisfied with the treatment received according to EPIC Question 32.
Forty-six patients (88.5%) did not discontinue or re-start medical therapy. Among them, patients with severely pathologic uroflowmetry (<10 ml/s, 21 patients) who were counseled for adjuvant endoscopic treatment after PAE refused surgery except by one during the follow-up.
Discussion
Our analysis showed that PAE provides a significant improvement in IPSS when assessed three months post-operatively. Re-assessment at longer term follow-up showed an initial worsening of IPSS, although the difference was not significant compared to the third month assessment. The quality of life was significantly improved.
No significant adverse events were reported except in one patient who suffered from erectile dysfunction. Most patients were satisfied with treatment, with ejaculation maintained in all cases.
On the other hand, the “urological” effectiveness of PAE on the relief of BPO seemed virtually intangible in most of the cases, according to Qmax at uroflowmetry. As such, Qmax remained almost stable over time when comparing the baseline data versus that at the last follow-up available. At a median follow-up of 14 months, most patients did not discontinue or re-start medical therapy for BPO. Among them, 21 patients with severely pathologic uroflowmetry who were counseled for adjuvant endoscopic treatment after PAE refused surgery except for one.
Notwithstanding their intrinsic limitations, IPSS and QoL are important patient-reported outcomes used to grade the level of bother perceived by the patients affected by LUTS (2, 3, 6, 11).
The results from the case series reported herein are in line with the previously published single-center experiences and meta-analyses of studies suggesting that PAE improves LUTS according to the pooled analysis of IPSS and QoL scores.
Indeed, according to a recently published systematic review and meta-analysis of randomized controlled trials, the average improvement in IPSS ranged from −16.33 to −13.1 points, while the average improvement in QoL ranged from −2.36 (−3.08, −1.63) to −3.11 (−3.55, −2.67) within the postoperative 24-month follow-up (13). On the other hand, IPSS reassessment at the time of urological consultation allowed us to detect an initial worsening of symptoms if compared to the third month assessment.
Regarding sexual function, the effect of PAE on erectile and ejaculatory functions is still a matter of debate (18). In agreement with the findings of a single-center retrospective study focused on the sexual function bothers conducted on 129 patients who underwent PAE, in our experience, only one patient (2%) reported erectile dysfunction after the procedure. On the other hand, all patients who maintained valid erectile function had their ejaculatory function preserved in our hands.
Finally, no adverse events related to PAE were reported. No patient required intervention to manage procedure-related complications.
One of the more interesting points of debate arising from our analysis is the finding of the absence of an objective improvement in Qmax after PAE on the one hand, while almost all patients who were found with severe BPO at uroflowmetry refused further treatment on the other hand.
Although the assessment of Qmax at uroflowmetry is affected by intrinsic limitations, with patients reporting pathological Qmax having no obstruction according to pressure/flow studies (19), Qmax is an objective, non-invasive measure commonly used to quantify the relief from the BPO after treatment.
Previous experiences comparing PAE versus TURP have already underlined how changes in Qmax after PAE are marginal. A randomized controlled trial by Abt et al., a multi-center matched pair prospective analysis by Ray et al., and a retrospective single-center study by Carnevale et al. reported improvements in Qmax averaging 3 to 5 ml/s after PAE (20–22).
A major limitation of the available evidence on the “PAE versus TURP challenge” is that the de-obstructive effect of PAE is evaluated using canonical criteria established for standard procedures. Moreover, we can consider the improvement of Qmax as the “knock-out” argument, which awards conventional and radical approaches. On the other hand, it has been proven that patients affected by BPH are primarily bothered by storage LUTS, above all, urge incontinence and nocturia, which mostly impact on their quality of life. Paradoxically, despite its high prevalence, a weak urinary stream is a less bothersome lower urinary tract symptom (23).
Accordingly, although almost all patients were found affected by BPO at our urological consultation when uroflowmetry was performed, and thus counseled for an adjuvant treatment aimed to de-obstruct, only one accepted the side-effect risk of a permanent ejaculatory dysfunction and underwent transurethral laser vaporesection.
This finding suggests the potential existence of an unmeasurable bias, which is intrinsic to studies investigating the effectiveness of micro-invasive, alternative techniques to treat BPO.
Maybe we are wrong, but the typical patient candidate for such treatments refers to a strong will to maintain ejaculation. The assessment of this patient could be confounded by hyper-positive answers in the patient-reported outcome questionnaires, which could supersize the effect of the treatment.
We recognize other limitations of this study. First, it was a retrospective design. Although patients were recalled for urological consultation in person, we were unable to retrieve missing paramount information such as third-month uroflowmetry that remained unperformed. Moreover, we acknowledge that information about sexual and ejaculatory function was not assessed in a standardized fashion at baseline. Second, the sample size was relatively small, thus limiting the consistency of our conclusion. Lastly, as aforementioned, we believe that an unmeasurable bias was introduced by the patients enrolled in this analysis, who all had a strong will to maintain ejaculation and then could have supersized the overall impact of the procedure itself on the patient-reported outcomes. But this is both a limitation and a thought-provoking finding, stimulating further debate in the field.
Our analysis showed that PAE can provide a significant improvement in the short-term follow-up of patient-reported outcomes. Most patients are satisfied with the treatment, and ejaculation is maintained. On the other hand, the effectiveness of PAE on the relief of BPO seems virtually intangible in most cases if assessed by the urinary flow rate. An unmeasurable bias introduced by the patient with a strong will to maintain ejaculation (thus refusing further treatments) could supersede the positive effect of the treatment on IPSS and QoL.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not provided for this study on human participants because retrospective study. The patients/participants provided their written informed consent to participate in this study.
Author contributions
RB and SM contributed to conception and design of the study. CC organized the database. RB performed the statistical analysis. RB wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Egan KB. The epidemiology of benign prostatic hyperplasia associated with lower urinary tract symptoms: prevalence and incident rates. Urol Clin North Am (2016) 43(3):289–97.
2. Arafa MA, Farhat K, Aqdas S, Al-Atawi M, Rabah DM. Assessment of lower urinary tract symptoms in Saudi men using the international prostate symptoms score. Urol Ann (2015) 7(2):221–5.
3. Cornu JN, Ahyai S, Bachmann A, et al. A systematic review and meta-analysis of functional outcomes and complications following transurethral procedures for lower urinary tract symptoms resulting from benign prostatic obstruction: An update. Eur Urol (2015) 67(6):1066–96. doi: 10.1016/j.eururo.2014.06.017
4. Bertolo R, Dalpiaz O, Bozzini G, et al. Thulium laser enucleation of prostate versus laparoscopic trans-vesical simple prostatectomy in the treatment of large benign prostatic hyperplasia: head-to-head comparison. Int Braz J Urol (2022) 48(2):328–35. doi: 10.1590/S1677-5538.IBJU.2021.0726
5. Marra G, Sturch P, Oderda M, Tabatabaei S, Muir G, Gontero P. Systematic review of lower urinary tract symptoms/benign prostatic hyperplasia surgical treatments on men’s ejaculatory function: time for a bespoke approach? Int J Urol (2016) 23(1):22–35.
6. Bertolo R, Fiori C, Amparore D, Porpiglia F. Follow-up of temporary implantable nitinol device (TIND) implantation for the treatment of BPH: a systematic review. Curr Urol Rep (2018) 19(6):44. doi: 10.1007/s11934-018-0793-0
7. Lokeshwar SD, Valancy D, Lima TFN, et al. A systematic review of reported ejaculatory dysfunction in clinical trials evaluating minimally invasive treatment modalities for BPH. Curr Urol Rep (2020) 21(12):54. doi: 10.1007/s11934-020-01012-y
8. Checcucci E, Veccia A, De Cillis S, et al. New ultra-minimally invasive surgical treatment for benign prostatic hyperplasia: A systematic review and analysis of comparative outcomes. Eur Urol Open Sci (2021), 33:28–41. doi: 10.1016/j.euros.2021.08.009
9. Pisco JM, Pinheiro LC, Bilhim T, et al. Prostatic arterial embolization to treat benign prostatic hyperplasia. J Vasc Interv Radiol (2011) 22(1):11–9. doi: 10.1016/j.jvir.2010.09.030
10. Parsons JK, Dahm P, Köhler TS, et al. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline amendment 2020. J Urol (2020) 204(4):799–804. doi: 10.1097/JU.0000000000001298
11. . Available at: https://uroweb.org/guideline/treatment-of-non-neurogenic-male-luts/ (Accessed February 5th, 2021).
12. Xu XJ, Li J, Huang XZ, Liu Q. An updated meta-analysis of prostatic arterial embolization versus transurethral resection of the prostate in the treatment of benign prostatic hyperplasia. World J Urol (2020) 38(10):2455–68. doi: 10.1007/s00345-019-03044-7
13. Xiang P, Guan D, Du Z, et al. Efficacy and safety of prostatic artery embolization for benign prostatic hyperplasia: a systematic review and meta-analysis of randomized controlled trials. Eur Radiol (2021). doi: 10.1007/s00330-020-07663-2
14. Knight GM, Talwar A, Salem R, Mouli S. Systematic review and meta-analysis comparing prostatic artery embolization to gold-standard transurethral resection of the prostate for benign prostatic hyperplasia. Cardiovasc Intervent Radiol (2021) 44(2):183–93. doi: 10.1007/s00270-020-02657-5
15. Jung JH, McCutcheon KA, Borofsky M, et al. Prostatic arterial embolization for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database Syst Rev (2020) 12:CD012867. doi: 10.1002/14651858.CD012867.pub2
16. Bertolo R, Cipriani C, Pozzi L, Bove P. A simplified Italian translation of the international prostate symptom score twists the reality in the aging male with lower urinary tract symptoms. Prostate Cancer Prostatic Dis (2020) 23(3):534–6. doi: 10.1038/s41391-020-0203-9
17. Wei JT, Dunn RL, Litwin MS, et al. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology (2000) 56:899–905.
18. Marzano L, Thiounn N, Pereira H, et al. Prostatic artery embolization allows to maintain full sexual activity in patients suffering from bothersome lower urinary tracts symptoms related to benign prostatic hyperplasia. Cardiovasc Intervent Radiol (2020) 43(8):1202–7. doi: 10.1007/s00270-020-02520-7
19. Wadie BS. How correlated is BOO with different objective parameters commonly used in evaluation of BPH: a prospective study. Int Urol Nephrol (2020). doi: 10.1007/s11255-020-02707-4
20. Ray AF, Powell J, Speakman MJ, et al. Efficacy and safety of prostate artery embolization for benign prostatic hyperplasia: an observational study and propensity-matched comparison with transurethral resection of the prostate (the UK-ROPE study). BJU Int (2018) 122(2):270–82.
21. Abt D, Hechelhammer L, Mullhaupt G, et al. Comparison of prostatic artery embolisation (PAE) versus transurethral resection of the prostate (TURP) for benign prostatic hyperplasia: randomised, open label, non-inferiority trial. BMJ (2018) 361:k2338.
22. Carnevale FC, Iscaife A, Yoshinaga EM, et al. Transurethral resection of the prostate (TURP) versus original and PErFecTED prostate artery embolization (PAE) due to benign prostatic hyperplasia (BPH): Preliminary results of a single center, prospective, urodynamic-controlled analysis. Cardiovasc Intervent Radiol (2016) 39(1):44–52. doi: 10.1007/s00270-015-1202-4
Keywords: prostatic arterial embolization, benign prostatic obstruction (BPO), patient-reported outcomes (PROs), lower urinary tract symptoms (LUTS), benign prostatic hyperplasia (BPH)
Citation: Bertolo R, Cipriani C, Giuliani MS, Maiorino F, Vittori M, Carilli M, Signoretti M, Minucci S and Bove P (2022) Prostatic arterial embolization as a micro-invasive treatment option for benign prostatic obstruction: A subtle balance between short-term follow-up patient-reported outcomes and de-obstructive effectiveness. Front. Urol. 2:960875. doi: 10.3389/fruro.2022.960875
Received: 03 June 2022; Accepted: 20 July 2022;
Published: 12 August 2022.
Edited by:
Enrico Checcucci, Candiolo Cancer Institute (IRCCS), ItalyReviewed by:
Riccardo Campi, Careggi Hospital, ItalyGianluca Sampogna, University of Milan, Italy
Daniele Amparore, San Luigi Gonzaga University Hospital, Italy
Copyright © 2022 Bertolo, Cipriani, Giuliani, Maiorino, Vittori, Carilli, Signoretti, Minucci and Bove. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Riccardo Bertolo, cmljY2FyZG9iZXJ0b2xvQGhvdG1haWwuaXQ=
†These authors have contributed equally to this work and share senior authorship
 Riccardo Bertolo
Riccardo Bertolo Chiara Cipriani
Chiara Cipriani Maria Silvia Giuliani2
Maria Silvia Giuliani2 Francesco Maiorino
Francesco Maiorino