- 1Department of Urology, University of Florence, Florence, Italy
- 2San Marco Hospital, Catania, Italy
- 3Department of Urology, University Hospital Frankfurt, Goethe University Frankfurt am Main, Frankfurt am Main, Germany
- 4Department of Urology and Andrology, Ospedale di Circolo and Macchi Foundation, Varese, Italy
- 5Department of Urology, Molinette Hospital, A.O.U. Città della Salute e della Scienza di Torino, Turin, Italy
- 6Department of Urology, University Magna Graecia of Catanzaro, Catanzaro, Italy
- 7Urology Section, Department of Surgery, University of Catania, Catania, Italy
Background: Hyaluronic acid (HA) has demonstrated clinical benefits for the treatment of Peyronie’s disease (PD); however, there are no reports that documented long-term outcomes. The aim of the current study is to illustrate the clinical outcomes after 2 years of follow-up in patients who received HA for PD.
Methods: From January 2015 to December 2018, we enrolled 244 patients affected by PD in this multicenter non-randomized clinical study, as previously reported. Patients received intralesional HA (Group A) or verapamil (Group B). Follow-up was undertaken after 3 months, 1 year, and 2 years. International Index of Erectile Function (IIEF-5), PC, and visual analogue scale (VAS) were collected.
Results: Among the whole cohort, 125 patients were included in Group A and 119 in Group B. As PC, the median curvature was 25.0° at 3 months [interquartile range (IQR), 15.0–30.0] (p=0.45) and was maintained at 1 (p=0.87) and 2 years of follow-up (p=0.90) (Group A), while it was 30.0° (IQR, 20.0–35.0) (p=0.67) at 3 months and was stable at 1 (p=0.77) and 2 years of follow-up (p=0.80) (Group B). For VAS score, the median change in Group A was −4.0 (IQR, −4.0, −5.0, p<0.01) after 3 months, −4.0 (IQR, −4.0, −5.0, p<0.01) at 1 year, and −4.0 (IQR, −4.0, −5.0, p<0.01) at 2 years of follow-up, while in Group B, it was −1.0 (IQR, −2.0, −0.5, p=0.25) after 3 months, −3.5 (IQR, −3.5, −2.0, p<0.01) at 1 year, and −4.0 (IQR, −4.0, −5.0, p<0.01) at 2 years of follow-up. Adjusted p-value for between-group comparisons was <0.01 at 3 months and not significant at 1 (p=0.53) and 2 years (p=0.80).
Conclusions: We reported clinical efficacy of intralesional HA in patients with PD after 2 years of follow-up by slightly improving penile curvature (PC) and bother of the disease. These results should be considered during the counseling of the patients especially regarding the lack of worsening over time.
Introduction
Induratio penis plastica or Peyronie’s disease (PD) is disease of unknown etiology that is characterized by the onset of fibrosis in the tunica albuginea of the penis and consequent penile deformity (1, 2). Up to now, many conservative and medical treatments have been used in order to improve penile curvature (PC) or decrease penile pain. Unfortunately, due to the lack of well-defined randomized clinical trial, the only Food and Drug Administration (FDA) drug approved is the collagenase Clostridium histolyticum (CCH), since the study IMPRESS I and II have demonstrated its clinical efficacy in improving all clinical outcomes (3, 4). However, it is important to underline that in European countries, CCH is not distributed anymore, which has consequent clinical impact on treatment decision (5).
In this context, there is an urgent need of finding alternatives to the management of PD, taking into account that many conservative and empirical therapies have been used in the past without meaningful advantages (6). A recent systematic review with meta-analysis demonstrated that in patients with active PD, the existing evidence is inconclusive to support the use of any combination treatment modality (7).
Hyaluronic acid (HA) has been demonstrated to be effective in reverting fibrosis in many clinical indications (8).
Furthermore, many other therapies have been used in the past, like oral treatment (potassium para-aminobenzoate, Potaba), vitamin E, colchicine, tamoxifen, carnitine, pentoxifylline, phosphodi- esterase type 5 inhibitors), or intralesional treatment (steroids, verapamil), with contrasting results such that the European Association of Urology guidelines did not recommend their clinical use (1).
However, an article from Loftus et al. published on 2020 reported that in 2008, pentoxifylline was the most commonly prescribed oral agent, being used in 33% of patients, and 11% received intralesional verapamil (9).
Although previous studies have demonstrated clinical benefits of HA therapy for PD, there are no reports that documented long-term outcomes in terms of PC and penile pain for this disease. For this reason, the aim of the current study is to illustrate the clinical outcomes after 2 years of follow-up in patients that received intralesional HA or verapamil for PD.
Material and Methods
From January 2015 to December 2018, we enrolled 244 patients affected by PD in this multicenter Italian non-randomized clinical study, as previously reported (10). Patients received intralesional HA or verapamil without randomization on the basis of patients and physician preferences.
The study was carried out after the realization of a specific informed consent, with the international approval of Donatello Private Hospital Committee (MED-2019-VD).
All patients were >18 years old, sexually active men, and affected by PD for <6 months and in the active phase. Before the enrollment, patients underwent clinical visit documenting the presence of PD plaque. Excluded patients were those with ventral curvature, hourglass deformity, initial curvature <15°, and calcified penile plaques (high calcification according to Cocci et al. (11). Patients received weekly intraplaque HA (0.8% highly purified sodium salt HA 16 mg/2 mL, IBSA Farmaceutici Italia SRL, Lodi, Italy) (Group A) or verapamil (10 mg in 5 ml of normal saline water) for 8 weeks (Group B).
All patients underwent Duplex Doppler ultrasonography in the basal condition and after the induction of penile erection by using 10 mcg of alprostadil. Plaque size was measured according to the longest diameter (mm). The degree of PC was evaluated during a full erection using a goniometer protractor.
Follow-up was undertaken after 3 months, 1 year, and 2 years. The following information were collected: Short-International Index of Erectile Function (IIEF-5) (12) and PC. Visual analogue scale (VAS) was applied to verify the presence of penile pain before and after treatment. The primary outcome of the study was the change in penile pain, while the secondary outcomes were the change in PC and erectile function.
Statistical Analysis
Data are reported by the use of medians (and interquartile ranges, IQRs) and were tested according to their normal distribution (normality of variable distribution was tested using the Kolmogorov–Smirnov test). Categorical variables were tested with the chi-squared test. Mann–Whitney test has been applied to test differences between two independent groups. For paired analysis, we applied a paired Kruskal–Wallis test to estimate the median difference in the change from one timepoint to the other between the two groups. For the comparison between groups, we applied the Bonferroni test.
All statistical analyses were completed using Stata software ver. 14 (Stata Corp., College Station, TX, USA). For all statistical comparisons, a significance level of p<0.05 was considered to show differences between the groups.
Results
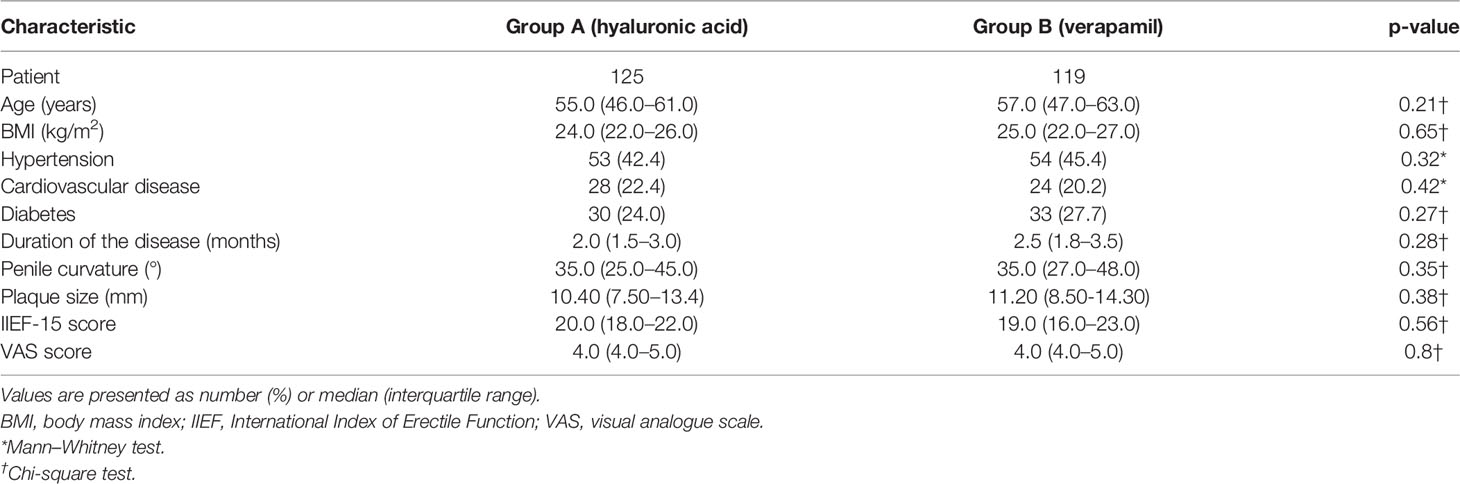
Among the whole cohort, 125 patients were included in Group A and 119 in Group B. Table 1 shows the baseline characteristic of the study cohort. All patients completed the study follow-up.
At 1-year follow-up, plaque size decreased by −1.45 mm (IQR = -−2.0, 1.30; p=0.56) in Group A and −1.10 mm (IQR, −1.8, 1.2; p=0.42) in Group B, showing no statistically significant differences between the two treatment schedules (p=0.10). At 1 year, plaque size decreased by −1.0 mm (IQR = −2.2, 1.0; p=0.59) in Group A and −1.10 mm (IQR, −1.8, 1.2; p=0.37) in Group B, showing no statistically significant differences between the two treatment schedules (p=0.25), while at 2 years, plaque size decreased by −1.7 mm (IQR = −2.2, 1.5; p=0.40) in Group A and −1.6 mm (IQR, −2.3, 1.2; p=0.62) in Group B, showing no statistically significant differences between the two treatment schedules (p=0.47).
As regards PC, the median curvature was 25.0° at 3 months (IQR, 15.0–30.0) (p=0.45) and was maintained at 1 (p=0.87) and 2 years of follow-up (p=0.90) (Group A), while it was 30.0° (IQR, 20.0–35.0) (p=0.67) at 3 months and was stable at 1 (p=0.77) and 2 years of follow-up (p=0.80) (Group B) (Figure 1). Adjusted p-value for between-group comparisons was not significant at 3 months (p=0.44), 1 year (p=0.62), and 2 years (p=0.78).
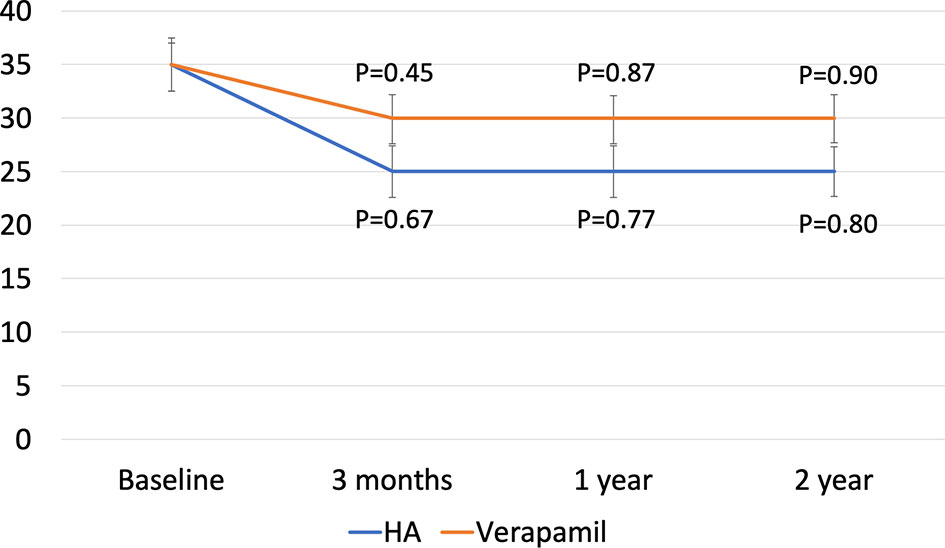
Figure 1 Median penile curvature from baseline to final follow-up. P-value are expressed as difference against baseline. Values were derived using Bonferroni test.
When assessing VAS score, the median change in Group A was −4.0 (IQR, −4.0, −5.0, p<0.01) after 3 months, −4.0 (IQR, −4.0, −5.0, p<0.01) at 1 year, and −4.0 (IQR, −4.0, −5.0, p<0.01) at 2 years of follow-up, while in Group B, it was −1.0 (IQR, −2.0, −0.5, p=0.25) after 3 months, −3.5 (IQR, −3.5, −2.0, p<0.01) at 1 year, and −4.0 (IQR, −4.0, −5.0, p<0.01) at 2 years of follow-up (Figure 2). Adjusted p-value for between-group comparisons was <0.01 at 3 months and not significant at 1 year (p=0.53) and 2 years (p=0.80).
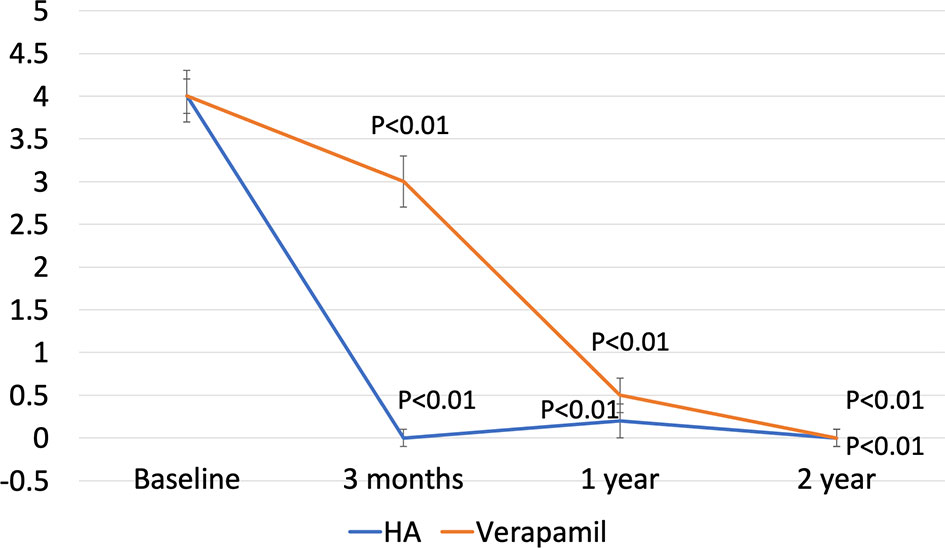
Figure 2 Median VAS from baseline to final follow-up. P-value are expressed as difference against baseline. Values were derived using Bonferroni test.
When assessing IIEF-5 score, the median change in Group A was 0.2 (IQR, 0.2–0.3, p=0.87) after 3 months, 0.5 (IQR, 0.3–0.6, p=0.82) at 1 year, and 0.5 (IQR, 0.3–0.6, p=0.79) at 2 years of follow-up, while in Group B, it was −1.0 (IQR, −2.0, 0.5, p=0.45) after 3 months, −0.8 (IQR, −1.0, −0.6, p=0.59) at 1 year, and −0.5 (IQR, −1.0, −0.7, p=0.88) at 2 years. Adjusted p-value for between-group comparisons were not significant. We did not observe any complication after therapy.
Discussion
In the present study, we reported the clinical efficacy data of HA therapy 2 years after administration in patients with PD (10). The results reported that this therapy determines a stable result over time without any clinical worsening in terms of pain and PC. However, several assessments must be taken into account, and these results interpreted with due caution. When analyzing literature data, a previous study reported that HA injections seem to provide an effective minimally invasive option in the acute phase of PD and might have the potential to lower penile pain and ameliorate erectile function, as compared with other intralesional agents (10).
Zucchi et al. recently conducted a study comparing the effect of combined oral administration and intralesional injection of HA with intralesional injections alone, in patients with early onset of PD. Eighty-one patients were randomized into two groups. Group A consisted of 41 patients receiving oral administration of HA in combination with weekly intralesional injection of HA for 6 weeks. Group B consisted of 40 patients who received weekly intralesional injections of HA for 6 weeks only. The authors reported that oral administration combined with intralesional treatment with HA had greater efficacy to improve PC and overall sexual satisfaction in comparison with intralesional HA treatment alone (13).
In the first network meta-analysis published by Russo et al., the authors demonstrated that with regard to PC (°) improvement, HA and verapamil showed worse outcomes when compared with CCH (−6.66° and −2.30°) and interferon α-2b (−6.75° and −2.38°). When considering improvement in erectile function, HA, verapamil, and interferon α-2b showed a slight increase in mean change when compared with CCH (+2.39, +1.77, and +0.65). Moreover, verapamil and interferon α-2b showed slightly worse mean change in comparison to HA (+0.62 and +1.74), whereas interferon α-2b was worse than verapamil (−1.12) (14).
Based on these results, it seems crucial to understand how HA can lead to its efficacy. A very interesting study conducted by Watanabe et al. performed immunohistochemistry analysis followed by digital quantification to evaluate transforming growth factor beta (TGF-β), heparanases, and metalloproteinases (MMPs) in plaque derived from PD patients. Pathological features showed decreased apoptosis and blood vessel number in Peyronie’s tissues. TGF-β and interleukin (IL)-6 were significantly increased in PD. There was an increased expression of heparanases, although no alteration was observed for MMPs. HA and hyaluronic acid synthases, hyaluronidases, and dermatan sulfate were not changed, while the level of chondroitin sulfate was significantly increased in Peyronie’s samples (15).
These results highlight the need for alternative therapies for the treatment of PD, and in this context, intralesional HA together with low-intensity shockwave therapy (LIST) may offer significant contribution. As reported by Sokolakis et al. in a long-term sham-controlled trial on LIST for PD management, improvement of pain was reported in 23 participants at 4 weeks and in 22 at 3 years and, furthermore, a mean difference of 2.2 points in the VAS at 4 weeks and a mean difference of 2.5 points at 3 years (16). Although no data have been reported on the combination with HA and LIST, further research can be conducted to elucidate their efficacy.
Before concluding, we would like to highlight some limitations including the lack of a placebo group, randomization, and biomarkers used to assess intralesional efficacy of HA. In particular, results obtained by our cohort can be secondary to the stabilization of the disease, and the use of placebo can be essential for this demonstration. Second, we did not collect data on surgical treatment after intralesional HA, and it is important to underline that the measurement of plaque size is not reliable and cannot serve as primary endpoint to future studies. Furthermore, we did not assess the clinical efficacy at 6 months of therapy, and we would like to highlight that all patients completed the study protocol. These latter aspects in fact should be considered for further research.
Conclusions
In this study, we reported clinical efficacy of intralesional HA in patients with PD after 2 years of follow-up by slightly improving PC and bother of the disease. In a short period, HA was demonstrated to be superior to verapamil in decreasing penile pain, but both treatments were similar at 1 and 2 years. Furthermore, long follow-up reported stable erectile function and plaque size. These results should be considered during the counseling of the patients especially regarding the lack of worsening over time.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Donatello Private Hospital Committee (MED-2019-VD). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AC and GR designed the study. All authors collected data. AC and GR drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The submission fees were supported by IBSA Farmaceutici Italia SRL. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Salonia A, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, et al. European Association of Urology Guidelines on Sexual and Reproductive Health—2021 Update: Male Sexual Dysfunction. Eur Urol (2021) 80:333–57. doi: 10.1016/j.eururo.2021.06.007
2. Minhas S, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, et al. European Association of Urology Guidelines on Male Sexual and Reproductive Health: 2021 Update on Male Infertility. Eur Urol (2021) 80(5):603–20. doi: 10.1016/j.eururo.2021.08.014
3. Gelbard M, Goldstein I, Hellstrom WJG, McMahon CG, Smith T, Tursi J, et al. Clinical Efficacy, Safety and Tolerability of Collagenase Clostridium Histolyticum for the Treatment of Peyronie Disease in 2 Large Double-Blind, Randomized, Placebo Controlled Phase 3 Studies. J Urol (2013) 190:199–207. doi: 10.1016/j.juro.2013.01.087
4. Goldstein I, Lipshultz LI, McLane M, Hu Y, Xiang Q, Liu G, et al. Long-Term Safety and Curvature Deformity Characterization in Patients Previously Treated With Collagenase Clostridium Histolyticum for Peyronie’s Disease. J Urol (2020) 203(6):1191–7. doi: 10.1097/JU.0000000000000743
5. Cocci A, Russo GI, Salamanca JIM, Ralph D, Palmieri A, Mondaini N. The End of an Era: Withdrawal of Xiapex (Clostridium Histolyticum Collagenase) From the European Market. Eur Urol (2019) 77(5):660–1. doi: 10.1016/j.eururo.2019.11.019
6. Russo GI, Milenkovic U, Hellstrom W, Levine LA, Ralph D, Albersen M. Clinical Efficacy of Injection and Mechanical Therapy for Peyronie’s Disease: A Systematic Review of the Literature. Eur Urol (2018) 74:767–81. doi: 10.1016/j.eururo.2018.07.005
7. Pyrgidis N, Yafi FA, Sokolakis I, Dimitriadis F, Mykoniatis I, Russo GI, et al. Assessment of Conservative Combination Therapies for Active and Stable Peyronie’s Disease: A Systematic Review and Meta-Analysis. Eur Urol Focus (2021) 17:S2405-4569(21)00313-8. doi: 10.1016/j.euf.2021.12.003
8. Zucchi A, Costantini E, Cai T, Cavallini G, Liguori G, Favilla V, et al. Intralesional Injection of Hyaluronic Acid in Patients Affected With Peyronie’s Disease: Preliminary Results From a Prospective, Multicenter, Pilot Study. Sex Med (2016) 4:e83–8. doi: 10.1016/j.esxm.2016.01.002
9. Loftus CJ, Rajanahally S, Holt SK, Raheem OA, Ostrowski KA, Walsh TJ. Treatment Trends and Cost Associated With Peyronie’s Disease. Sex Med (2020) 8:673–8. doi: 10.1016/j.esxm.2020.08.003
10. Cocci A, Di Maida F, Cito G, Verrienti P, Laruccia N, Campi R, et al. Comparison of Intralesional Hyaluronic Acid vs. Verapamil for the Treatment of Acute Phase Peyronie’s Disease: A Prospective, Open-Label Non-Randomized Clinical Study. World J Mens Health (2021) 39:352. doi: 10.5534/wjmh.190108
11. Cocci A, Russo GI, Briganti A, Salonia A, Cacciamani G, Capece M, et al. Predictors of Treatment Success After Collagenase Clostridium Histolyticum Injection for Peyronie’s Disease: Development of a Nomogram From a Multicentre Single-Arm, Non-Placebo Controlled Clinical Study. BJU Int (2018) 122:680–7. doi: 10.1111/bju.14410
12. Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Peña BM. Development and Evaluation of an Abridged, 5-Item Version of the International Index of Erectile Function (IIEF-5) as a Diagnostic Tool for Erectile Dysfunction. Int J Impot Res (1999) 11:319–26. doi: 10.1038/sj.ijir.3900472
13. Cai T, Tiscione D, Favilla V, Puglisi M, Palumbo F, Zucchi A, et al. Oral Administration and Intralesional Injection of Hyaluronic Acid Versus Intralesional Injection Alone in Peyronie’s Disease: Results From a Phase III Study. World J Mens Health (2021) 39:526–32. doi: 10.5534/wjmh.200048
14. Russo GI, Cacciamani G, Cocci A, Kessler TM, Morgia G, Serefoglu EC, et al. Comparative Effectiveness of Intralesional Therapy for Peyronie’s Disease in Controlled Clinical Studies: A Systematic Review and Network Meta-Analysis. J Sex Med (2019) 16:289–99. doi: 10.1016/j.jsxm.2018.12.011
15. Watanabe MS, Theodoro TR, Coelho NL, Mendes A, Leonel MLP, Mader AM, et al. Extracellular Matrix Alterations in the Peyronie’s Disease. J Adv Res (2017) 8:455–61. doi: 10.1016/j.jare.2017.06.004
Keywords: Peyronie’s disease, drugs, induration penis plastica, penile curvature, collagenase Clostridium hystoliticum
Citation: Cocci A, Di Mauro M, Kluth L, Capogrosso P, Falcone M, Mondaini N and Russo GI (2022) Long-Term Outcomes (2 Years) After Hyaluronic Acid Therapy for Peyronie’s Disease. Front. Urol. 2:929367. doi: 10.3389/fruro.2022.929367
Received: 26 April 2022; Accepted: 16 May 2022;
Published: 14 June 2022.
Edited by:
Afonso Morgado, Centro Hospitalar Universitário de São João (CHUSJ), PortugalReviewed by:
Nikolaos Pyrgidis, Martha-Maria Hospital Nuremberg, GermanyCeleste Manfredi, University of Campania Luigi Vanvitelli, Italy
Copyright © 2022 Cocci, Di Mauro, Kluth, Capogrosso, Falcone, Mondaini and Russo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giorgio Ivan Russo, Z2lvcmdpb2l2YW4ucnVzc29AdW5pY3QuaXQ=
 Andrea Cocci
Andrea Cocci Marina Di Mauro
Marina Di Mauro Luis Kluth3
Luis Kluth3 Giorgio Ivan Russo
Giorgio Ivan Russo