
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Urol. , 12 May 2022
Sec. Urologic Oncology
Volume 2 - 2022 | https://doi.org/10.3389/fruro.2022.903693
This article is part of the Research Topic Insights in Urologic Oncology, Volume I View all 5 articles
Following radical nephroureterectomy for upper tract urothelial carcinoma, bladder tumor recurrence is a common event occurring in up to 22-47% of cases within the first post-operative year. In this review, we summarize the current knowledge on predictors of bladder tumor recurrence after radical nephroureterectomy and methods for reducing the risk of bladder tumor recurrence. Risk factors can be classified as modifiable and non-modifiable. Of these, the treating urologist has the greatest potential to decrease bladder tumor recurrence by focusing on treatment specific risk factors. Procedures which may decrease the risk of bladder tumor recurrence include limiting diagnostic ureteroscopy with biopsy to cases with equivocal diagnosis, use of perioperative intravesical chemotherapy, and complete distal ureterectomy with bladder cuff excision. Ongoing studies evaluating the timing and choice of intravesical chemotherapy during/after radical nephroureterectomy may help to further reduce bladder tumor recurrence in the future.
Urothelial carcinoma is the fourth most common solid malignancy, with cases occurring in both the upper urinary tract (renal calyces, renal pelvis, ureter) and the lower urinary tract (urinary bladder and urethra) (1, 2). Although comprised of the same features histologically, upper tract urothelial carcinomas (UTUC) have been recognized as a distinct disease from lower tract urothelial carcinoma due to anatomical, biological, and molecular differences (2, 3). UTUC currently accounts for 5-10% of all urothelial carcinoma cases compared to 90-95% of cases occurring in the lower tract, with cases of UTUC slowly rising (2, 4, 5). Despite the differences, upper and lower tract urothelial carcinoma remain intertwined as UTUC is associated with a high rate of bladder tumor recurrence (BTR), cited at 22-47% following radical nephroureterectomy (RNU) (2, 6, 7). Risk factors for developing primary UTUC include smoking, phenacetin, exposure to aristocholic acid (known as Chinese herb nephropathy or Balkan endemic nephropathy), Lynch syndrome, and occupational exposures including aromatic amines (8). Current tobacco smoking has been identified as a significant risk factor for UTUC, with the relative risk for developing UTUC among smokers being 2.5 to 7-fold higher than among non-smokers (9). Phenacetin, an analgesic, has also been shown to indirectly cause carcinogenesis by inducing nephrotoxicity through papillary necrosis (10). Balkan endemic nephropathy and Chinese herb nephropathy are the same disease characterized by a mutation of the p53 gene due to exposure to aristolochic acid, which has been specifically linked to UTUC (11). Lynch Syndrome is a genetic disorder characterized by abnormalities in DNA mismatch repair that leads to a 22-fold increased relative risk in developing UTUC, with an 8% risk in developing urologic cancers by 70 years of age (12, 13). Occupational hazards such as exposure to aromatic amines (common in the chemical and rubber industries) and polycyclic aromatic hydrocarbons (used in coal and aluminum manufacturing) may also increase the risk of UTUC (8, 14).
RNU with bladder cuff excision is the standard of care for non-metastatic UTUC (15). Despite recent advances in the care of urothelial carcinoma, the rate of BTR following RNU remains high (4, 7, 16, 17). Multiple studies have examined the risk factors for BTR, but results vary significantly. This may be partly due to UTUC of high stage and grade having the propensity to develop distant metastasis, causing patients to succumb to the original disease before BTR becomes clinically apparent (18). There are two primary theories for the development of BTR following RNU which include the field cancerization hypothesis and clonal expansion of multifocal carcinomas theory. Field cancerization was first proposed in 1953 by Slaughter et al. and was based on observations of the multicentric development of cancers in the oral cavity, with the high impact of carcinogens being associated with lifestyle factors (19). A similar understanding is theorized for the urinary tract as the urothelium is exposed to carcinogens that are present within the urine resulting in development of independent tumors at various sites within the urinary tract (19, 20). The other (and more widely accepted) theory is the clonal expansion of multifocal carcinomas, it proposes that multiple carcinomas in the urinary tract are the result of intraluminal spread from a single lesion, and that multiple carcinomas originate from a single transformed cell via seeding or implantation of cancer cells at different sites (20). Regardless of the origin, BTR following RNU remains an important focus of research due to the associated increased monitoring, treatment and morbidity. This review focuses on risks factors and methods to decrease BTR following RNU.
Multiple predictors of BTR following RNU have been identified. These predictors can be categorized as modifiable and non-modifiable, Table 1. The evidence supporting these predictors and potential measures to mitigate the associated risk of BTR will be presented.
Clinical and standard pathologic features have been evaluated as potential predictors of BTR after RNU. In a retrospective analysis of 1,839 patients by Xylinas et al, advanced age, male gender, ureteral tumor location, use of laparoscopic surgical technique, endoscopic distal ureteral management, prior bladder cancer, higher tumor stage, concomitant CIS and lymph node involvement were all significantly associated with BTR after RNU (21). These features were utilized to developed a nomogram for predicting the probability of BTR over a 3 year period. BTR occurred in 31% of patients included. The accuracy of the nomogram was moderate with concordance index of 67.8% in the training cohort and 69% in the validation cohort. The authors suggest that such predictive models may help to determine use of perioperative intravesical chemotherapy (IVC) and to develop risk-based surveillance regimens.
A meta-analysis, by Seisen et al. presents three categories of predictors of BTR including patient, tumor, and treatment specific factors (22). Patient specific factors identified included male gender, previous bladder cancer, smoking, and preoperative CKD (5, 22). Tumor specific factors included multifocality, invasive pathologic T stage, ureteral location, positive pre-operative urinary cytology, and the presence of necrosis (22). Lastly, treatment specific factors increasing the risk of BTR included laparoscopic approach, extravesical bladder cuff removal, positive surgical margins, and diagnostic ureteroscopy (dURS) (17, 22).
Several molecular markers have been suggested to be associated with BTR. Whole-exome sequencing of DNA and RNA and protein analysis from UTUC tumor specimens revealed (23) FGFR3 to be the most commonly mutated gene (74%) in both low grade (92%) and high-grade (60%) disease. UTUC specimens were divided into 4 subtypes based on RNA expression, with Cluster 3 (categorized by having 100% FGFR3 mutations, 71% PIK3CA, no TP53 mutations, high tobacco use) having the highest rates of BTR. Notably, Cluster 3 was the only UTUC subgroup with PIK3CA mutations, with no TP53 mutations identified (23). While this data suggests an increased risk of BTR associated with Cluster 3 tumors, the small sample size of study does not permit statistical analysis and future evaluation of larger data sets are required prior to considering risk of BTR based upon these molecular features.
Additional large multi-institutional retrospective studies have noted that Ki-67, HER-2, EGFR, N-cadherin, AIB1 and EIF5A2 are associated with BTR after RNU (24, 25). Additionally, preoperative monocyte-to-lymphocyte ratio (MLR) has also been shown to be associated with BTR (26). While not currently used in clinical practice, such molecular biomarkers along with preoperative MLR have the potential for helping determine which patients are more susceptible to BTR after RNU (24, 26), and may help to develop risked based surveillance plans.
dURS is important when UTUC is suspected on CT imaging or atypical urine cytology. However, dURS and biopsy for the diagnosis of UTUC is a potential risk factor for BTR. This is believed to be related to cell implantations from the primary tumor resulting from tumor manipulation. A 2018 systemic review pooled data from eight independent retrospective studies with a total of 3,340 patients. Meta-analysis of the hazard ratios demonstrated an increased risk of BTR associated with dURS prior to RNU, HR 1.53 (95% CI 1.31–1.77) (27). Multiple other studies published since this meta-analysis have noted similar results, Table 2. All of these studies were retrospective and only one evaluated data from multiple institutions (17, 28–30). Use of IVC during the perioperative period of RNU was only reported in one of these studies and none reported the use of IVC at the time of dURS. dURS was noted to be an independent predictor of BTR in all but one of these series. Furthermore, it is worth noting that dURS was the most common independent predictor across these series. Due to this association it is reasonable to limit dURS when possible and evaluate additional measures to decrease BTR associated with dURS (17, 28–30).
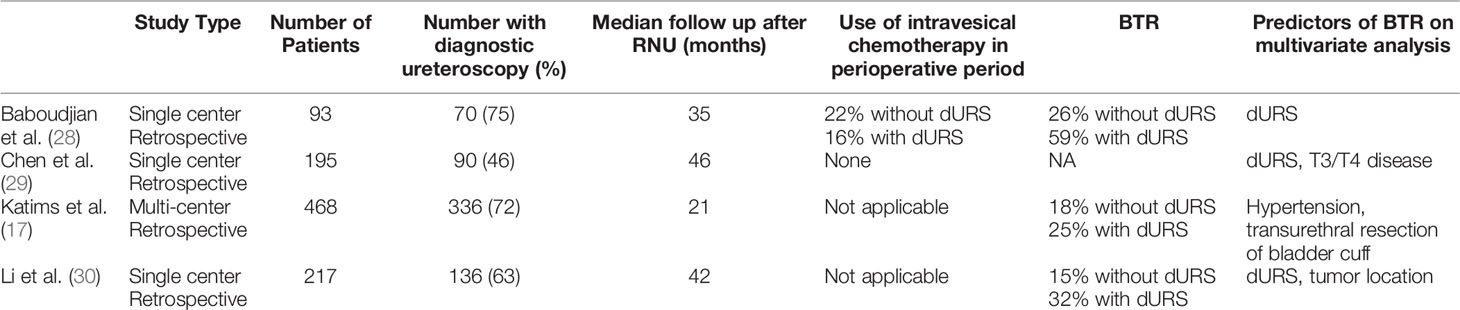
Table 2 Contemporary series evaluating association of diagnostic ureteroscopy and BTR following RNU.
A potential method to decrease BTR when dURS is performed is the administration of IVC immediately following dURS. The potential for this approach has been evaluated by Gallioli et al. in a prospective series of 26 patents. Patients with UTUC were treated with endoscopic tumor ablation followed by a single dose of mitomycin instilled in renal pelvis. The single dose of mitomycin C was associated with a 7.7-fold lower risk of urothelial cancer recurrence (upper tract or bladder) compared to the historical control group (31). While this study did not evaluate BTR following RNU, it does demonstrate the potential for an intervention to decrease BTR that may be associated dURS.
IVC during the perioperative period of RNU has the potential to decrease BTR by destroying circulating tumor cells released into the urine during surgery which may result in tumor seeding. The efficacy of postoperative IVC has been evaluated in two important randomized trials, summarized in Table 3. The ODMIT-C trial by O’Brien et al. evaluated the impact of a single post-operative dose of 40mg of mitomycin C (MMC) in reducing BTR within the first 12 months following RNU (32). MMC was administered at the time of urinary catheter removal which typically occurred 7-10 days following RNU. Important exclusion criteria included a prior history of bladder cancer and tumor located within the distal intramural ureter. Overall, a single dose of MMC was found to have an absolute risk reduction of 11%, relative risk reduction of 40% with the number needed to treat to prevent one bladder tumor of nine (32). Sub-group analysis also revealed a decreased risk of BTR in well differentiated tumors (6%) compared to moderately differentiated (25%), and poorly differentiated (22%) tumors. No serious adverse events were associated with post-operative MMC instillations. Potential limitations of this trial include a lack of standardized timing of administration of MMC and no requirement for histologic proof of BTR.
The second randomized trial investigating adjuvant bladder instillations was performed by Ito et al. (33) A single intravesical instillation of 30mg of pirarubicin was administered within 48 hours of RNU. Surveillance cystoscopy and urine cytology were performed every 3 months for the first 2 years. Overall, a single dose of pirarubicin significantly reduced one-year BTR at 16.9% compared to 31.8% in the control group and two-year BTR of 16.9% compared to 42.2% in the control group (33). No serious adverse events were associated with pirarubicin instillations. On multivariable analysis, administration of post-operative intravesical pirarubicin and open surgery were noted to be independent predictors of decreased BTR (33).
These two randomized trials were included in a Cochrane systematic review investigating the effect of single-dose IVC (MMC or pirarubicin) after RNU for UTUC. A total of 361 patients were included. Variations in bladder cuff management, type of IVC, timing of administration and dwell time were present. This review concluded that a single dose of IVC may reduce the risk of BTR compared to no instillation (HR 0.51) (34). Despite the level 1 evidence demonstrating the benefit of a single dose of perioperative IVC with RNU, a national survey of urologic oncologists (n=158), performed by Lu et al. found that only 51% of urologic oncologists report using perioperative IVC in patients undergoing RNU (35). Unfortunately, 44% of urologists who do not administer IVC stated their reason for not administering IVC was due to lack of supporting data (35). Additionally, this study revealed a wide variation in timing of IVC administration with 33% administered intraoperatively, 7% at ≤3 days, 37% at 4-7 days, 20% at 8-14 days, and 3% at >14 days, consistent with the lack of evidence regarding optimal timing of treatment (35).
Both randomized trials demonstrating the efficacy of single dose IVC after RNU administered therapy in the post-operative period, ranging from 2-10 days. It has been hypothesized that intra-operative IVC may further decrease BTR. One of the main concerns of the investigators designing the randomized trials was the risk of IVC extravasation and resulting intraperitoneal leakage leading to pain and serious adverse events. However, the safety of intra-operative IVC at the time of RNU has been noted in a retrospective review by Moriarty et al. In this series patients received intraoperative MMC or adriamycin and no intra-operative or post-operative complications associated with IVC were noted (36).
The efficacy of intraoperative IVC and the time of RNU has been evaluated in two series. A single-institution retrospective study by Noennig et al. of 51 patients noted BTR in 16% of receiving intra-operative MMC vs 33% in those received MMC post-operatively (administered within 24-72hrs post-operatively) (37). Multivariate analysis noted significant associations of BTR within one year with the timing of MMC installation, surgical approach, surgical margin status, and presence of concomitant carcinoma in situ. Notably, patients who received intraoperative MMC had a significantly reduced risk of BTR in the 12 months following RNU (HR = 0.113, 95% CI = 0.028-0.63, p = 0.01). Results of this retrospective series led to an ongoing single-arm phase II study evaluating efficacy of intra-operative administration of MMC at time of RNU (NCT03658304).
A multi-center retrospective analysis of 137 patients who received intra-operative or post-operative MMC or gemcitabine were evaluated for BTR (38). The majority of their cohort, 81% received intra-operative IVC compared to 19% who received post-operative IVC (38). Similar BTR rates were noted in those who received intra-operative versus post-operative IVC instillations (18% 12 month BTR for intra-operative IVC group, 28% for the post-operative IVC group, P=0.365) (38). On multivariate analysis, tumor focality was the only independent predictor of BTR. It is also important to note that this is the first study evaluating use of gemcitabine as the IVC agent to reduce BTR. Based on the results, the authors concluded that intra-operative IVC was comparable to post-operative IVC in preventing BTR following RNU. Additionally, intra-operative IVC may be advantageous to increase dwell time, eliminate patient discomfort, and reduce the need for additional imaging prior IVC (38).
The GEMINI trial (NCT04398368) is expected to provided additional information on the safety and efficacy of intraoperative intravesical gemcitabine at the time of RNU. This single arm multi-center phase II study will evaluate 12-month BTR with an anticipated enrollment of 90 patients (39).
Current smoking is a well-known risk factor for urothelial carcinoma. However, former smoking history and continued smoking may also affect clinical outcomes in patients with UTUC. Hagiwara et al. performed a retrospective review of 245 patients treated with RNU to determine if an association exists between BTR rates and smoking status. They found that the 3-year BTR free survival was 32.6% in current smokers, 37.6% in former smokers and 61.7% in non-smokers (40). On multivariate analysis, both current smoking and former smoking status were independent risk factors for subsequent BTR (HR 1.58 and 1.77 respectively) (40). For those patients with greater than 50 pack-years or greater smoking history, incidence of BTR after RNU was twice as likely (p=0.003) (40). Therefore, the authors concluded that smoking —both current and former is associated with increased likelihood of BTR in those undergoing RNU for UTUC (40).
RNU with bladder cuff excision and regional lymph node dissection is the standard of care for the treatment of locoregional UTUC (41). Several techniques have been used to manage excision of the ureteral orifice, including intravesical versus extravesical excision, or via an endoscopic approach. Current guidelines by the National Comprehensive Cancer Network (NCCN) and the European Association of Urology (EAU) do not recommend a specific technique (42, 43). However, it has been suggested that the technique of bladder cuff excision impacts BTR. A retrospective review of 856 patients with no history of bladder cancer undergoing RNU evaluated the impact of extravesical ligation (EL) versus transvesical excision (TE) on BTR (44). TE was defined as visually confirming the ipsilateral ureteral orifice prior to excision of the bladder cuff and removal of the intramural portion of the ureter. EL was defined as ligation of the premural ureter close to the bladder (bladder was not incised and the ureteral orifice was not visually confirmed). BTR free survival for the TE group at 5 years was 59.9% compared to 49.3% in the EL group (p=0.008) (44). On multivariable analysis, EL was associated with BTR (HR 1.4, p=0.003) compared to TE (44). Other predictors of BTR included age, dURS, tumor location and pathologic N stage. In subgroup analysis based on tumor location, TE showed improved BTR in patients with renal pelvis tumors (p=0.003) but no improvement in those with ureteral tumors (44). TE also showed improved cancer specific survival at 5 years after RNU at 82% versus 73.8% in the EL group (p=0.0019). The authors report that incomplete distal ureteric resection is associated with poor oncological outcomes including BTR, and that intramural excision of the ureter should be the standard of care for UTUC patients undergoing RNU (44).
Another potential method to decrease tumor seeding during RNU is early ureteral ligation distal to the tumor. A prospective single-arm trial by Yamashita et al. included 74 patients who underwent early ureteral ligation were compared to historical controls for BTR. Early ureteral ligation was defined as ligation of the ureter as quickly as possible after entering the retroperitoneal space (45). In this series, 23% of patients had BTR at a median follow-up of 24 months. 12 and 24-month BTR rates in the early ureteral ligation group were 81% and 76% vs 75% and 63% in the control group (p=0.16) (45). Multivariate analysis of patients with renal pelvis cancer identified early ureteral ligation as an independent predictor of BTR (45). No improvement in BTR was noted with early ureteral ligation in patients with UTUC involving the ureter.
With the advent of laparoscopy and robotics, minimally invasive RNU has been widely adopted. A recent European Association of Urology (EAU) Guidelines systematic review including 42 studies of 7,554 patients comparing oncologic outcomes of open versus laparoscopic RNU found overall similar oncologic outcomes between open and laparoscopic RNU (46). Of the studies included, multiple series compared BTR in open versus laparoscopic RNUs. Of the 38 studies included, only one reported a significant difference in BTR between surgical approaches. An additional four studies compared BTR in patients undergoing laparoscopic RNU with endoscopic bladder cuff excision versus open RNU, none of which reported significant differences in observed BTR. While this systematic review did not report an impact of surgical technique on BTR, this finding is not consistent across all series.
The majority of series evaluating BTR after treatment of UTUC focus on patients undergoing RNU and there is little data evaluating BTR following RNU compared to nephron sparing techniques, especially distal ureterectomy. When comparing oncological outcomes of RNU versus distal ureterectomy for treatment of organ confined UTUC, Seisen et al. found no difference in BTR between the two groups on multivariable analysis (HR 0.9, p=0.52) (47). This is in agreement with another retrospective study performed by Dalpiaz et al. of 89 patients who underwent RNU or distal ureterectomy and found no statistical significant in BTR between the two groups (48).
Based upon the known the risk factors for BTR following RNU optimizing risk reduction is possible. Figure 1 demonstrates a potential pathway for risk reduction starting with the initial diagnosis. As proposed, future efforts in reducing BTR should incorporate multiple strategies throughout the treatment algorithm.
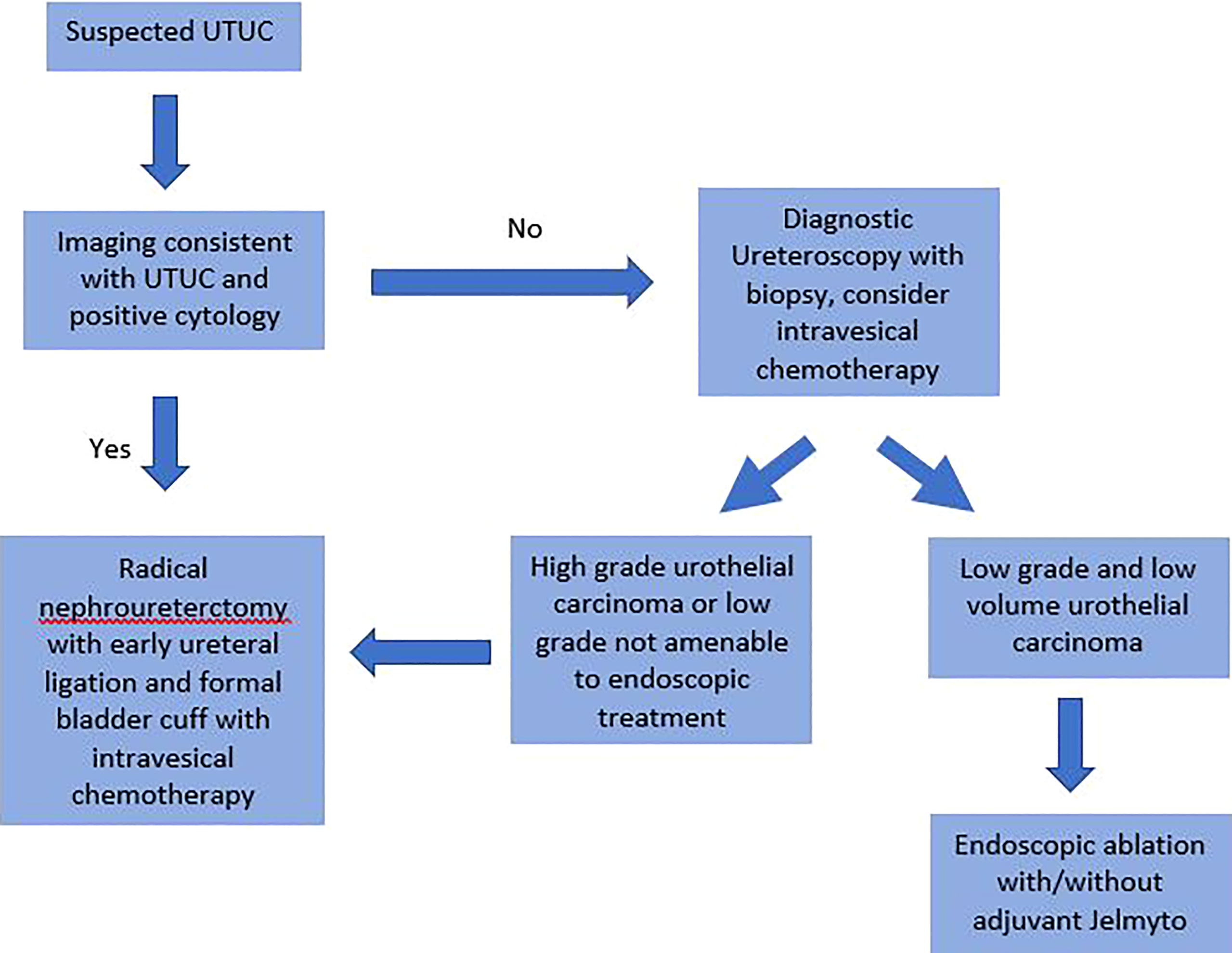
Figure 1 Proposed diagnosis and treatment algorithm of upper tract urothelial carcinoma to reduce bladder tumor recurrence following nephroureterectomy.
Of the non-modifiable risk factors for BTR, risk based upon tumor characteristics (stage, grade, location, and genomics) may prove valuable in developing risk based surveillance plans as suggested by Xylinas et al. (7). Furthermore, tumor stage is one of the principle determinants for considering adjuvant therapy after RNU. The POUT trial evaluated the benefit of adjuvant chemotherapy versus observation in patients with T2-4N0-3M0 or TanyN1-3M0 pathology at RNU (49). Three-year event free survival was significantly improved in patients receiving chemotherapy compared to observation, 71% versus 46% respectively (49). New non-muscle invasive bladder cancer was not considered an event when evaluating the primary endpoint, and BTR was not compared between treatment groups (49). CheckMate 274 evaluated the benefit of adjuvant nivolumab versus placebo in patients undergoing radical surgery urothelial carcinoma. While the majority of patients in the study underwent radical cystectomy for bladder cancer, 21% of participants underwent RNU for UTUC (50). In the entire study population a significant benefit in disease-free survival was noted in patients receiving nivolumab, 20.8 months, versus placebo, 10.8 months (50). However, the exploratory analysis focusing on patients with UTUC did not demonstrate a benefit in disease free survival. While the adjuvant strategies evaluated in POUT and CheckMate 274 focused on preventing systemic disease progression, no data is available on the impact of these treatments on BTR (49, 50).
Of the modifiable predictors of BTR there are several targets to improve patient outcomes. Decreasing the risk of BTR can begin when UTUC is first suspected. Limiting dURS is especially reasonable in patients with imaging consistent with UTUC and a positive urine cytology. Such patients do not require a dURS prior to RNU. Despite the risk of BTR associated with dURS and the option to offer patients RNU without a biopsy, dURS should not be completely abandoned. In cases where the diagnostic findings are equivocal, normal upper tract/suspicious upper tract imaging, and atypical/negative urine cytology dURS is recommended. Additionally, endoscopic ablation for low grade low volume tumors is appropriate based upon multiple series demonstrating disease control and low rates of progression (51). This approach to low grade upper tract tumors is reinforced with the approval of Jelmyto in which confirmation of low grade disease with a biopsy is required prior to treatment (52).
When RNU is indicated, it does not appear that open versus laparoscopic/robotic surgical approach has a significant impact on BTR. However, surgical techniques including early ureteral ligation distal to the tumor and complete excision of the ipsilateral ureteral orifice are certainly be of benefit. Increasing the use of IVC in the perioperative period is an obvious target for improvement based upon the disappointing results of the survey conducted in 2017 with only 51% of respondents utilizing IVC in the perioperative period. In addition to improvements that could be gained from increased utilization of IVC following RNU, further gains may come with optimizing the timing of IVC administration. The two ongoing trials evaluating intraoperative IVC during RNU will help to determine if the timing of treatment can help further reduce BTR.
BTR in UTUC patients is a relatively frequent event with considerable effect on patient quality of life. Several strategies have been suggested to reduce BTR in those undergoing RNU for UTUC including limiting dURS, early ureteral ligation, formal bladder cuff excision, and single dose of perioperative IVC. Current ongoing studies evaluating the choice and timing of IVC will further standardize management of UTUC and may increase usage rates of peri-operative IVC amongst urologists.
HM, EDV, and PC contributed to conception and design of the study. HM, ED, and PC wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2018. CA Cancer J Clin (2018) 68(1):7–30. doi: 10.3322/caac.21442
2. Leow JJ, Liu Z, Tan TW, Lee YM, Yeo EK, Chong YL. Optimal Management of Upper Tract Urothelial Carcinoma: Current Perspectives. Onco Targets Ther (2020) 13:1–15. doi: 10.2147/OTT.S225301
3. Green DA, Rink M, Xylinas E, Matin SF, Stenzl A, Roupret M, et al. Urothelial Carcinoma of the Bladder and the Upper Tract: Disparate Twins. J Urol (2013) 189(4):1214–21. doi: 10.1016/j.juro.2012.05.079
4. Rouprêt M, Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2020 Update. Eur Urol (2020) 79:62–79. doi: 10.1016/j.eururo.2020.05.042
5. Raman JD, Messer J, Sielatycki JA, Hollenbeak CS. Incidence and Survival of Patients With Carcinoma of the Ureter and Renal Pelvis in the USA. BJU Int (2011) 107(7):1973–2005. doi: 10.1111/j.1464-410X.2010.09675.x
6. Yoldas M, Turk H, Yoldas TK. Clinical and Pathological Factors Predictive of Bladder Cancer Recurrence in Patients With Upper Tract Primary TCC. Niger J Clin Pract (2021) 24(5):774–7. doi: 10.4103/njcp.njcp_503_19
7. Xylinas E, Rink M, Margulis V, Karakiewicz P, Novara G, Shariat SF. Multifocal Carcinoma in Situ of the Upper Tract Is Associated With High Risk of Bladder Cancer Recurrence. Eur Urol (2012) 61(5):1069–70. doi: 10.1016/j.eururo.2012.02.042
8. Miyazaki J, Nishiyama H. Epidemiology of Urothelial Carcinoma. Int J Urol (2017) 24(10):730–4. doi: 10.1111/iju.13376
9. Colin P, Koenig P, Ouzzane A, Berthon N, Villers A, Biserte J, et al. Environmental Factors Involved in Carcinogenesis of Urothelial Cell Carcinomas of the Upper Urinary Tract. BJU Int (2009) 104(10):1436–40. doi: 10.1111/j.1464-410X.2009.08838.x
10. Stewart JH, Hobbs JB, McCredie MRE. Morphologic Evidence That Analgesic-Induced Kidney Pathology Contributes to the Progression of Tumors of the Renal Pelvis. Cancer (1999) 86(8):1576–82. doi: 10.1002/(SICI)1097-0142(19991015)86:8<1576::AID-CNCR27>3.0.CO;2-V
11. Refik Gökmen M, Cosyns JP, Arlt VM, Stiborová M, Phillips DH, Schmeiser HH, et al. The Epidemiology, Diagnosis, and Management of Aristolochic Acid Nephropathy: A Narrative Review. Ann Intern Med (2013) 158(6):469–77. doi: 10.7326/0003-4819-158-6-201303190-00006
12. Gylling AHS, Nieminen TT, Abdel-Rahman WM, Nuorva K, Juhola M, Joensuu EI, et al. Differential Cancer Predisposition in Lynch Syndrome: Insights From Molecular Analysis of Brain and Urinary Tract Tumors. Carcinogenesis (2008) 29(7):1351–9. doi: 10.1093/carcin/bgn133
13. Koornstra JJ, Mourits MJ, Sijmons RH, Leliveld AM, Hollema H, Kleibeuker JH. Management of Extracolonic Tumours in Patients With Lynch Syndrome. Lancet Oncol (2009) 10(4):400–8. doi: 10.1016/S1470-2045(09)70041-5
14. Reulen RC, Kellen E, Buntinx F, Brinkman M, Zeegers MP. A Meta-Analysis on the Association Between Bladder Cancer and Occupation. In: Scand J Urol Nephrol (2008) Vol 42:64–78. doi: 10.1080/03008880802325192
15. Campbell MT, Shah AY, Matin SF, Siefker-Radtke AO. Optimizing Management of Upper Tract Urothelial Carcinoma. Urol Oncol Semin Orig Investig (2017) 35(7):492–8. doi: 10.1016/j.urolonc.2017.05.009
16. Wu J, Xu PH, Luo WJ, Dai B, Shen YJ, Ye DW, et al. Intravesical Recurrence After Radical Nephroureterectomy of Upper Urinary Tract Urothelial Carcinoma: A Large Population-Based Investigation of Clinicopathologic Characteristics and Survival Outcomes. Front Surg (2021) 8:590448. doi: 10.3389/fsurg.2021.590448
17. Katims AB, Say R, Derweesh I, Uzzo R, Minervini A, Wu Z, et al. Risk Factors for Intravesical Recurrence After Minimally Invasive Nephroureterectomy for Upper Tract Urothelial Cancer (ROBUUST Collaboration). J Urol (2021) 206(3):568–76. doi: 10.1097/JU.0000000000001786
18. Ku JH, Choi WS, Kwak C, Kim HH. Bladder Cancer After Nephroureterectomy in Patients With Urothelial Carcinoma of the Upper Urinary Tract. Urol Oncol Semin Orig Investig (2011) 29(4):383–7. doi: 10.1016/j.urolonc.2009.04.007
19. Slaughter DP, Southwick HW, Smejkal W. “Field Cancerization” in Oral Stratified Squamous Epithelium. Clinical Implications of Multicentric Origin. Cancer (1953) 6(5):963–8. doi: 10.1002/1097-0142(195309)6:5<963::AID-CNCR2820060515>3.0.CO;2-Q
20. Kakizoe T. Development and Progression of Urothelial Carcinoma. Cancer Sci (2006) 97(9):821–8. doi: 10.1111/j.1349-7006.2006.00264.x
21. Xylinas E, Kluth L, Passoni N, Trinh QD, Rieken M, Lee RK, et al. Prediction of Intravesical Recurrence After Radical Nephroureterectomy: Development of a Clinical Decision-Making Tool. Eur Urol (2014) 65(3):650–8. doi: 10.1016/j.eururo.2013.09.003
22. Seisen T, Granger B, Colin P, Léon P, Utard G, Renard-Penna R, et al. A Systematic Review and Meta-Analysis of Clinicopathologic Factors Linked to Intravesical Recurrence After Radical Nephroureterectomy to Treat Upper Tract Urothelial Carcinoma. Eur Urol (2015) 67(6):1122–33. doi: 10.1016/j.eururo.2014.11.035
23. Moss TJ, Qi Y, Xi L, Peng B, Kim TB, Ezzedine NE, et al. Comprehensive Genomic Characterization of Upper Tract Urothelial Carcinoma. Eur Urol (2017) 72(4):641–9. doi: 10.1016/j.eururo.2017.05.048
24. Favaretto RL, Zequi SC, Oliveira RAR, Santana T, Costa WH, Cunha IW, et al. Tissue-Based Molecular Markers in Upper Tract Urothelial Carcinoma and Their Prognostic Implications. Int Braz J Urol (2018) 44(1):22–37. doi: 10.1590/S1677-5538.IBJU.2017.0204
25. Huang Y, Wei J, Fang Y, Chen Z, Cen J, Feng Z, et al. Prognostic Value of AIB1 and EIF5A2 in Intravesical Recurrence After Surgery for Upper Tract Urothelial Carcinoma. Cancer Manag Res (2018) 10:6997–7011. doi: 10.2147/CMAR.S185392
26. Liu J, Wu P, Lai S, Song X, Fu C, Wang X, et al. Preoperative Monocyte-To-Lymphocyte Ratio Predicts for Intravesical Recurrence in Patients With Urothelial Carcinoma of the Upper Urinary Tract After Radical Nephroureterectomy Without a History of Bladder Cancer. Clin Genitourin Cancer (2021) 19(3):e156–65. doi: 10.1016/j.clgc.2020.09.004
27. Tan P, Xie N, Yang L, Liu L, Tang Z, Wei Q. Diagnostic Ureteroscopy Prior to Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma Increased the Risk of Intravesical Recurrence. Urol Int (2018) 100(1):92–9. doi: 10.1159/000484417
28. Baboudjian M, Al-Balushi K, Michel F, Lannes F, Akiki A, Gaillet S, et al. Diagnostic Ureteroscopy Prior to Nephroureterectomy for Urothelial Carcinoma is Associated With a High Risk of Bladder Recurrence Despite Technical Precautions to Avoid Tumor Spillage. World J Urol (2020) 38(1):159–65. doi: 10.1007/s00345-019-02768-w
29. Chen H, Wang M, Weng T, Wei Y, Yang L, Ren K, et al. Prognostic Analysis of Diagnostic Ureteroscopic Biopsy for Intravesical Recurrence of Upper Urinary Tract Urothelial Carcinoma. Urol Int (2021) 106:86–194. doi: 10.1159/000517789
30. Li YR, Yu KJ, Chang YH, Lin PH, Shao IH, Kan HC, et al. Predictors of Intravesical Recurrence After Radical Nephroureterectomy and Prognosis in Patients With Upper Tract Urothelial Carcinoma. Cancer Manag Res (2020) 12:7439–50. doi: 10.2147/CMAR.S261087
31. Gallioli A, Boissier R, Territo A, Vila Reyes H, Sanguedolce F, Gaya JM, et al. Adjuvant Single-Dose Upper Urinary Tract Instillation of Mitomycin C After Therapeutic Ureteroscopy for Upper Tract Urothelial Carcinoma: A Single-Centre Prospective Non-Randomized Trial. J Endourol (2020) 34(5):573–80. doi: 10.1089/end.2019.0750
32. O’Brien T, Ray E, Singh R, Coker B, Beard R. Prevention of Bladder Tumours After Nephroureterectomy for Primary Upper Urinary Tract Urothelial Carcinoma: A Prospective, Multicentre, Randomised Clinical Trial of a Single Postoperative Intravesical Dose of Mitomycin C (the ODMIT-C Trial). Eur Urol (2011) 60(4):703–10. doi: 10.1016/j.eururo.2011.05.064
33. Ito A, Shintaku I, Satoh M, Ioritani N, Aizawa M, Tochigi T, et al. Prospective Randomized Phase II Trial of a Single Early Intravesical Instillation of Pirarubicin (THP) in the Prevention of Bladder Recurrence After Nephroureterectomy for Upper Urinary Tract Urothelial Carcinoma: The THP Monotherapy Study Group Trial. J Clin Oncol (2013) 31(11):1422–7. doi: 10.1200/JCO.2012.45.2128
34. Hwang EC, Sathianathen NJ, Jung JH, Kim MH, Dahm P, Risk MC. Single-Dose Intravesical Chemotherapy After Nephroureterectomy for Upper Tract Urothelial Carcinoma. Cochrane Database Syst Rev (2019) 2019(5). doi: 10.1002/14651858.CD013160.pub2
35. Lu DD, Boorjian SA, Raman JD. Intravesical Chemotherapy Use After Radical Nephroureterectomy: A National Survey of Urologic Oncologists. Urol Oncol Semin Orig Investig (2017) 35(3):113.e1–7. doi: 10.1016/j.urolonc.2016.10.016
36. Moriarty MA, Uhlman MA, Bing MT, O’Donnell MA, Brown JA, Tracy CR, et al. Evaluating the Safety of Intraoperative Instillation of Intravesical Chemotherapy at the Time of Nephroureterectomy Urological Oncology. BMC Urol (2015) 15(1):45. doi: 10.1186/s12894-015-0039-0
37. Noennig B, Bozorgmehri S, Terry R, Otto B, Su LM, Crispen PL. Evaluation of Intraoperative Versus Postoperative Adjuvant Mitomycin C With Nephroureterectomy for Urothelial Carcinoma of the Upper Urinary Tract. Bl Cancer (2018) 4(4):389–94. doi: 10.3233/BLC-180174
38. Freifeld Y, Ghandour R, Singla N, Woldu S, Bagrodia A, Lotan Y, et al. Intraoperative Prophylactic Intravesical Chemotherapy to Reduce Bladder Recurrence Following Radical Nephroureterectomy. Urol Oncol Semin Orig Investig (2020) 38(9):737.e11–e16. doi: 10.1016/j.urolonc.2020.05.002
39. Packiam VT, Leibovich BC, Thompson RH, Potretzke AM, Chow GK, Tollefson MK, et al. GEMINI: An Open-Label, Single-Arm, Phase II Trial of Intraoperative Gemcitabine Intravesical Instillation in Patients Undergoing Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma. J Clin Oncol (2020) 38(6_suppl). doi: 10.1200/jco.2020.38.6_suppl.tps594
40. Hagiwara M, Kikuchi E, Tanaka N, Matsumoto K, Ide H, Miyajima A, et al. Impact of Smoking Status on Bladder Tumor Recurrence After Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma. J Urol (2013) 189(6):2062–8. doi: 10.1016/j.juro.2013.01.024
41. Azémar MD, Comperat E, Richard F, Cussenot O, Rouprêt M. Bladder Recurrence After Surgery for Upper Urinary Tract Urothelial Cell Carcinoma: Frequency, Risk Factors, and Surveillance. Urol Oncol Semin Orig Investig (2011) 29(2):130–6. doi: 10.1016/j.urolonc.2009.06.003
42. Rouprêt M, Babjuk M, Compérat E, Zigeuner R, Sylvester RJ, Burger M, et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Cell Carcinoma: 2015 Update. Eur Urol (2015) 68(5):868–79. doi: 10.1016/j.eururo.2015.06.044
43. Nazzani S, Preisser F, Mazzone E, Tian Z, Mistretta FA, Soulières D, et al. Nephroureterectomy With or Without Bladder Cuff Excision for Localized Urothelial Carcinoma of the Renal Pelvis. Eur Urol Focus (2020) 6(2):298–304. doi: 10.1016/j.euf.2018.09.007
44. Ryoo H, Kim J, Kim T, Kang M, Jeon HG, Jeong BC, et al. Effects of Complete Bladder Cuff Removal on Oncological Outcomes Following Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma. Cancer Res Treat (2021) 53(3):795–802. doi: 10.4143/crt.2020.919
45. Yamashita S, Ito A, Mitsuzuka K, Ioritani N, Ishidoya S, Ikeda Y, et al. Efficacy of Early Ureteral Ligation on Prevention of Intravesical Recurrence After Radical Nephroureterectomy for Upper Urinary Tract Urothelial Carcinoma: A Prospective Single-Arm Multicenter Clinical Trial. Jpn J Clin Oncol (2017) 47(9):870–5. doi: 10.1093/jjco/hyx085
46. Peyronnet B, Seisen T, Dominguez-Escrig JL, Bruins HM, Yuan CY, Lam T, et al. Oncological Outcomes of Laparoscopic Nephroureterectomy Versus Open Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: An European Association of Urology Guidelines Systematic Review. Eur Urol Focus (2019) 5(2):205–23. doi: 10.1016/j.euf.2017.10.003
47. Seisen T, Nison L, Remzi M, Klatte T, Mathieu R, Lucca I, et al. Oncologic Outcomes of Kidney Sparing Surgery Versus Radical Nephroureterectomy for the Elective Treatment of Clinically Organ Confined Upper Tract Urothelial Carcinoma of the Distal Ureter. J Urol (2016) 195(5):1354–61. doi: 10.1016/j.juro.2015.11.036
48. Dalpiaz O, Ehrlich G, Quehenberger F, Pummer K, Zigeuner R. Distal Ureterectomy is a Safe Surgical Option in Patients With Urothelial Carcinoma of the Distal Ureter. Urol Oncol Semin Orig Investig (2014) 32(1):34.e1–8. doi: 10.1016/j.urolonc.2013.01.001
49. Birtle A, Johnson M, Chester J, Jones R, Dolling D, Bryan RT, et al. Adjuvant Chemotherapy in Upper Tract Urothelial Carcinoma (the POUT Trial): A Phase 3, Open-Label, Randomised Controlled Trial. Lancet (2020) 395(10232):1268–77. doi: 10.1016/S0140-6736(20)30415-3
50. Bajorin DF, Witjes JA, Gschwend JE, Schenker M, Valderrama BP, Tomita Y, et al. Adjuvant Nivolumab Versus Placebo in Muscle-Invasive Urothelial Carcinoma. N Engl J Med (2021) 384(22):2102–14. doi: 10.1056/nejmoa2034442
51. Seisen T, Peyronnet B, Dominguez-Escrig JL, Bruins HM, Yuan CY, Babjuk M, et al. Oncologic Outcomes of Kidney-Sparing Surgery Versus Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: A Systematic Review by the EAU Non-Muscle Invasive Bladder Cancer Guidelines Panel. Eur Urol (2016) 70(6):1354–61. doi: 10.1016/j.eururo.2016.07.014
52. Kleinmann N, Matin SF, Pierorazio PM, Gore JL, Shabsigh A, Hu B, et al. Primary Chemoablation of Low-Grade Upper Tract Urothelial Carcinoma Using UGN-101, a Mitomycin-Containing Reverse Thermal Gel (OLYMPUS): An Open-Label, Single-Arm, Phase 3 Trial. Lancet Oncol (2020) 21(6):776–85. doi: 10.1016/S1470-2045(20)30147-9
Keywords: nephroureterectomy, bladder tumor recurrence, upper tract urothelial carcinoma, ureteroscopy (URS), intravesical chemotherapy
Citation: Miyagi H, Di Valerio EA, O’Malley P, Brisbane WG, Su L-M and Crispen PL (2022) Predicting and Decreasing Bladder Tumor Recurrence Following Nephroureterectomy. Front. Urol. 2:903693. doi: 10.3389/fruro.2022.903693
Received: 24 March 2022; Accepted: 19 April 2022;
Published: 12 May 2022.
Edited by:
Trushar Patel, University of South Florida, United StatesReviewed by:
Mohammed Shahait, King Hussein Medical Center, JordanCopyright © 2022 Miyagi, Di Valerio, O’Malley, Brisbane, Su and Crispen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paul L. Crispen, cGF1bC5jcmlzcGVuQHVyb2xvZ3kudWZsLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.