
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Urol., 30 June 2022
Sec. Pediatric, Adolescent and Developmental Urology
Volume 2 - 2022 | https://doi.org/10.3389/fruro.2022.895057
This article is part of the Research TopicWomen in Pediatric, Adolescent, and Developmental Urology: Volume IView all 11 articles
 Marie K. Luff1*
Marie K. Luff1* David A. Zarrin1
David A. Zarrin1 Li Zhou1
Li Zhou1 Astha Sahoo1
Astha Sahoo1 Sophia Desai2
Sophia Desai2 Neha Iyer2
Neha Iyer2 Savannah L. Starr1
Savannah L. Starr1 Renea M. Sturm1,3,4*
Renea M. Sturm1,3,4*Introduction: Biodesign innovation processes provide a needs-driven approach to medical innovation, empowering both medical trainees and health care providers to take action in addressing the shortcomings of health care encountered in daily clinical practice. Our objective was to uncover the most pressing unmet clinical needs within a specific clinical setting, pediatric urology at UCLA.
Methods: The biodesign process involves a sequential process of identifying, validating, and prioritizing unmet needs, followed by solution landscaping and prototyping for the most promising needs. Opportunities for medical innovation were first identified through six weeks of clinical immersion, which involved both clinical observation and interview-based insight extraction. Interviews were conducted with 35 stakeholders, including patients, patient families, and health care staff by a medical student participant in Sling Health LA, a program which provides innovation training and incubation for ideas. Follow-up interviews with key stakeholders were performed to validate needs. Priority scores were then assigned to each validated need using a series of pre-determined and weighted criteria. Finally, genealogy maps were constructed and used to guide subsequent solution landscaping for the top three needs.
Results: 33 unmet clinical needs were identified throughout the clinical immersion phase, 27 of which were validated. Following coarse needs prioritization, five needs emerged as top contenders. After fine needs prioritization, three top needs were selected. The first top need arising from this ethnographic study was that “parents and children need a faster (<3 months to resolution) treatment option for resolving nocturnal enuresis that also prevents incontinence during the treatment phase”. Other discovered top needs included “parents and physicians need an accurate method to monitor retractile testes at-home and in the outpatient setting to reduce unnecessary surgical referrals and procedures”, and “a hospital system need to reduce complications and readmissions associated with post-operative catheter obstruction after urologic procedures”. A preliminary solution concept was generated for the top clinical need, nocturnal enuresis treatment.
Conclusion: Biodesign processes offer a standardized method for identifying pressing unmet clinical needs and informing solution development. The top three needs discovered within pediatric urology through this ethnographic investigation represent promising innovation targets for further solution prototyping and design.
The Biodesign innovation process was first developed at Stanford University to promote education in medical innovation and shape trainees into biomedical innovators. Using the three principles of “identification, invention, and implementation,” unmet clinical needs are identified and solutions are invented and implemented through a viable business strategy. The Biodesign process follows the sequential and iterative process of clinical immersion, needs identification, needs filtration, needs validation, and solution concept generation to produce tangible biomedical innovations (1, 2).
The Biodesign process is foundationally based on applying a rigorous process to innovation, starting with the development of a deep initial understanding of the unmet clinical needs within a specific field and environment. This process is built upon a thorough and rigorous method for clinical needs discovery and subsequent innovation development (1, 2). Importantly, needs driven innovation ensures that technologies are developed based on a legitimate clinical need and that potential markets exist for solutions before significant investment into innovation development. Since 2001, over 53 biomedical startups have arisen from projects initiated with the Biodesign innovation program at Stanford University alone. Nineteen percent of these companies have been acquired and 67% of companies remain currently active, demonstrating the success of this method for translation to meaningful clinical solutions (3). To date, this work has been less commonly implemented in medical education, though recently has become part of the offerings of UCLA with inclusion in the Sling Health program initiated in summer 2021. Within the context of a six-week summer elective course consisting of didactics, mentorship, and focused clinical immersion, students between their first and second week of medical school learn and apply these techniques to a single clinical area. Learning and applying biodesign-based processes empower students by providing a framework to assess and address observed unmet needs. These skills are readily transferable to any clinical environment and thus offer students a practical and effective means to guide future innovation throughout their careers.
Urology is a technology-driven surgical field, where new innovations have been rapidly welcomed and implemented into common surgical practice. From pioneering the use of robotic surgery and minimally invasive surgical techniques, to new developments in regenerative medicine and tissue engineering, urology is an exciting field that is well-positioned to launch improvements in surgical and biomedical technology that will be transferable to numerous medical fields (4, 5). In contrast, pediatric innovation has historically been challenging as many barriers exist in the testing and regulation of technologies intended specifically for pediatric use. In pediatric surgery, many new technologies are first developed and approved for adult use, before eventual adaptation for pediatric use. This leaves a gap in solutions for problems that are uniquely pediatric. Thus, pediatric urology was identified as a particularly promising space in which to develop new innovations for unmet clinical needs and was selected for the described focused clinical immersion (6, 7).
Our goal was to apply a stepwise biodesign process in the field of pediatric urology to systematically identify opportunities for medical innovation in a single practice environment while demonstrating the feasibility and outcomes of this process when implemented by a medical trainee. The clinical immersion and needs discovery phases were conducted at pediatric urology clinics in Los Angeles, California as part of a summer medical student immersion program in biomedical innovation. To our knowledge, this is the first publication of the results of a biodesign-based clinical needs discovery process within the field of pediatric urology.
Clinical immersion was conducted over a six-week period from June 1 to July 13, 2021, at the UCLA Westwood and Santa Monica pediatric urology locations in Los Angeles, California. This study arose from a summer course for medical students at the David Geffen School of Medicine at UCLA. This curriculum was offered by a student organization called Sling Health LA, a chapter within the national organization Sling Health (8), which incubates medical technology ideas of students. The goal of clinical immersion was for medical students to act as a medical ethnographic researcher, directly observing and documenting current clinical practice and scenarios without interfering in the delivery of healthcare. During clinical immersion, observations where an existing clinical process, procedure, or patient encounter may be suboptimal were documented. For example, an ethnographer might shadow a pediatric urologist during regular clinic hours on a Tuesday morning and observe patients sitting in the waiting area and drinking water for over an hour. After observing the waiting area for several more minutes, the ethnographer could learn that children do this in order to supply two separate urine samples to the clinic: one for urodynamic testing, and another for urinalysis and culture. Seeing an opportunity to cut down on patient wait times, the ethnographer would then record their observation in a notebook, logging the date and time at which it occurred. In addition to direct observation, interviews were conducted with health care staff, patients, and family members to explore unmet clinical needs. There were several goals for stakeholder interviews in this study. The first goal was to interview as many individuals as possible over the six-week clinical immersion period, in order to obtain the greatest number of potential unmet clinical needs and to build student interview and observational skills. The second goal was to outline a broad range of unmet clinical needs by interviewing individuals with different roles on the healthcare team, with the aim for stakeholders to be distributed as evenly as possible across different healthcare staff and parents or patients. The medical student ethnographer requested interviews with stakeholders in-person while engaging in the six-week period of clinical immersion. In total, interviews were conducted with 35 stakeholders. 18 healthcare staff were interviewed, including three physicians (two pediatric urologists, one urology resident), five nurses, two medical assistants, two ultrasound technicians, and six administrative staff, in addition to 17 total family members and pediatric patients (Figure 1).
Needs validation was continuously performed through repeated clinical immersion and informal follow up interviews with healthcare staff and patients/patient families. This iterative approach was modeled after the Spiral Innovation Model developed at Johns Hopkins University (9). Each direct observation was translated into a “need statement,” a concise sentence that stated the desired outcome for each clinical need without specifying a particular solution. A need statement classically contains three components: the problem that the statement is addressing, the population that is affected by the problem, and the desired outcome. By design, the solution is initially unspecified to avoid restricting the genealogy mapping and solution landscaping steps implemented later in the process. This ensures that identified innovation targets avoid an early bias toward one particular mode of intervention based on a pre-specified solution embedded within the original needs statement. Need statements were continuously updated based on new insights gained throughout the six-week clinical immersion. Increasing the innovator’s knowledge base in pediatric urology conditions, procedures, and treatments was also emphasized to validate and further refine need statements.
Need statements were prioritized via a Pugh decision-making matrix method with pre-determined and weighted scoring criteria (10). Scoring criteria and weighting factors were crafted by the medical student ethnographer with physician mentor input prior to needs prioritization. Overall scoring criteria involved five broad categories: feasibility and ability to solve, risk and investment, impact in pediatric urology or patient experience, market opportunity, and innovator preference (Table 1). Needs prioritization consisted of two phases: coarse and fine needs prioritization. For each unmet need, the medical student ethnographer assigned scores for each scoring criteria. The first phase, coarse needs prioritization, ranked needs by the first three scoring criteria: feasibility and ability to solve, risk and investment, and impact in pediatric urology or patient experience. The five top scoring needs then underwent fine needs prioritization, where needs were ranked by the two additional criteria: market potential and innovator preference. Market potential was assessed by conducting internet searches for existing solutions and analyzing publicly available data such as ease-of-use, device costs, and number of devices sold per year. Literature review was conducted for disease-specific incidence and prevalence information to assess the size of patient populations reached by a potential intervention.
Following needs prioritization, the three needs with the highest scores were selected for genealogy map construction. Genealogy maps illustrate a stepwise method of transforming needs into innovation targets by considering all potential underlying root causes for the problem at hand. Genealogy maps were utilized to assess root causes of each need and served as a scaffold for solution landscaping. In addition to information collected from interviews and observation, an Amazon product search was conducted utilizing the search term “bedwetting alarm” to identify existing devices on the market. Amazon reviews from 2016 - 2022 were extracted from the Amazon marketplace for all bedwetting products with more than 300 ratings. This filtered search resulted in 10 products which were each subsequently reviewed and analyzed for cost and common features. An additional internet search was conducted to assess alternative nocturnal enuresis products on the market, focusing in particular on novel technological devices that differ from classic bedwetting alarms.
Six weeks of clinical immersion identified a total of 33 unmet clinical needs that underwent subsequent needs validation. Twenty-seven needs were successfully validated and underwent coarse needs prioritization. The top five needs from coarse needs prioritization were then ranked via fine needs prioritization (Figure 2). After fine needs prioritization, the top three needs were determined (Table 2). The first top need arising from this study was that “parents and children need a faster (<3 months to resolution) treatment option for resolving nocturnal enuresis that also prevents incontinence during the treatment phase.” The second top need was that “physicians and parents need an accurate method to monitor retractile testes at-home and in the outpatient setting to reduce unnecessary surgical referrals and procedures.” The third top need revealed “a hospital system need to reduce complications and readmissions associated with post-operative catheter obstruction after urologic procedures.”
Genealogy mapping of the top three needs was performed. Genealogy mapping of the top need revealed several shortcomings of bedwetting alarms, which are the current first-line intervention for nocturnal enuresis (Table 3). Shortcomings include: alarms are ineffective at arousing children directly with children who experience nocturnal enuresis considered difficult to arouse by their parents alarms may unintentionally awaken other household members, children often require assistance to use the alarm correctly, the alarm does not prevent enuresis directly while being used, and prolonged treatment times (>3 months) that are typically required before achieving dry nights.
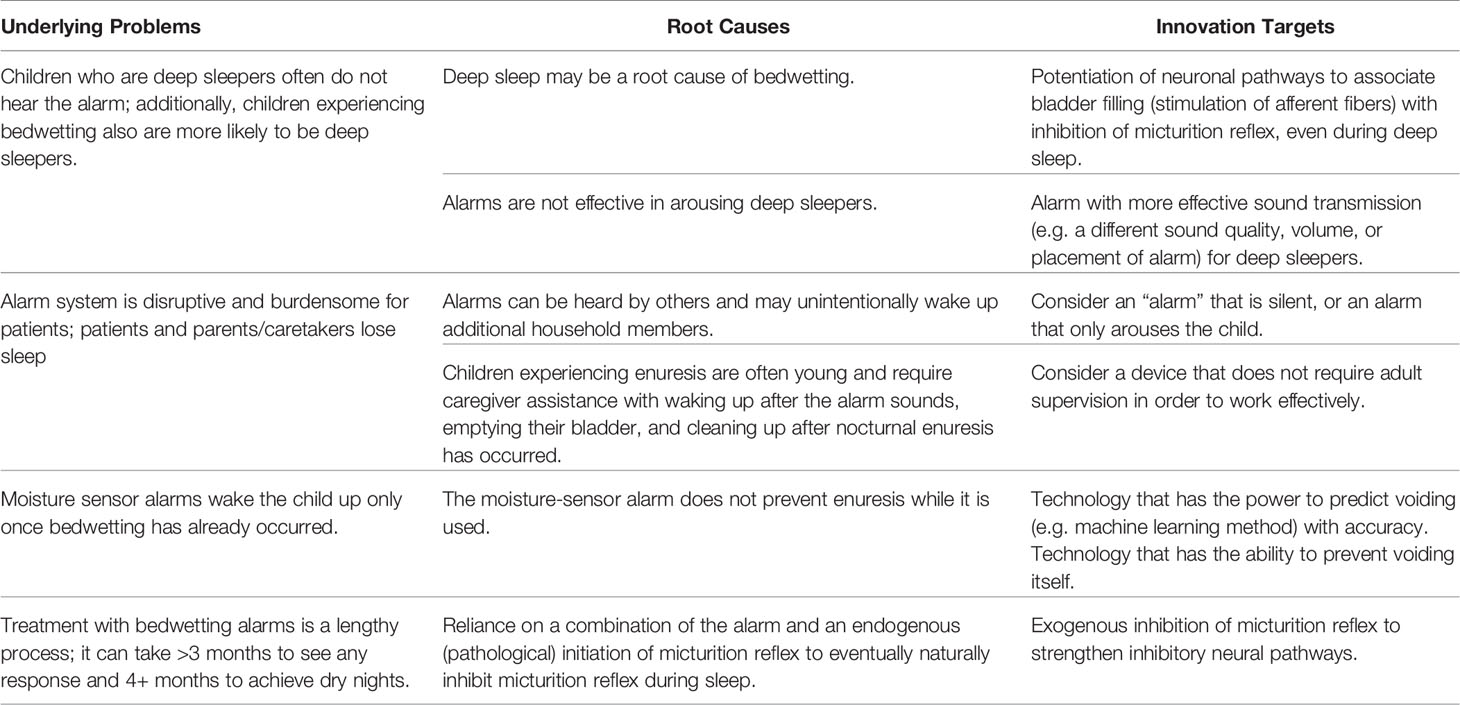
Table 3 Genealogy map example demonstrating a root cause analysis of the top discovered clinical need.
Root cause analysis of the shortcomings of existing bedwetting alarms was performed to identify preliminary innovation targets. Potential targets for innovation included devising a more effective enuresis signal that would arouse deep sleepers without disturbing additional household members. Other innovation targets included the creation of a device that would prevent voiding while minimally interfering with patient sleep, a device that reinforces neural pathways that promote nighttime continence, and a device that could predict when voiding will occur each night (Table 3).
Market analysis of ten total bedwetting alarms revealed a cost range of $30-$300. The most common features of current bedwetting alarms were those to improve child awakening including loud volume, vibration, and lights. Analysis of nocturnal enuresis devices in development or with recent entry to the market additionally identified several novel technologies, including a device that leverages predictive learning of biometric data to sound an alarm before enuresis occurs (11).
A preliminary solution concept for nocturnal enuresis treatment was developed utilizing the innovation targets that were brainstormed. Based on informal follow-up discussions with clinicians and external research, the most important characteristics to include in our solution concept were to offer a faster (<3 month) treatment option for resolving nocturnal enuresis coupled with prevention of incontinence during the treatment phase. Our solution concept consists of a dual detection-response system with two components: a sensor component and a stimulator component. The sensor system would provide both an in vivo non-invasive bladder pressure and/or volume monitoring while patients sleep and would also provide an alarm in the setting of voiding. A direct bladder volumetric individualized machine learning system could additionally be developed to provide pre-void detection. The stimulator system would consist of a neuromodulation-based response system that leverages autonomic control of bladder and sphincter muscles to prevent urination. The goals for the response system would be to temporally stimulate muscle contraction of the pelvic floor or decrease contractility of the bladder in order to prevent urination.
Biodesign-based innovation processes offer trainees, clinicians, and other stakeholders a unique, needs driven approach for developing meaningful clinical solutions. Participating in need assessment and validation early in the innovation process allows academic researchers to focus their research early on topics that are clinically impactful. The ability for researchers to quickly screen and select research topics based on clinical relevance and impact opens the doors for increased efficiency and productivity, which may be particularly valuable when adapting to the rapid pace of technological developments worldwide. Similarly, approaching medical innovation using a biodesign-based process may greatly benefit patients when the innovation is developed quickly or is driven by tangible, validated patient needs. For students, structured innovation processes teach those early in their training how to thoughtfully question the norm in clinical settings. All initial observations in the clinical immersion phase are documented with the purpose of promoting big-idea thinking and creative brainstorming without limits, opening opportunities for novel innovations that defy the status quo.
Pediatric urology is a promising field for developing new innovations for unmet clinical needs. Innovation in pediatrics has lagged behind that of adult urology due to low market incentivization for companies to develop new solutions in a population with a low disease prevalence, considerable regulatory burden and ethical considerations for randomized trials, and limited funding sources for developing medical devices (6, 7, 12, 13). In 2016, the Food and Drug Administration (FDA) acknowledged the need for medical devices designed for children and cited differences in pediatric anatomy and lack of commercial incentives as barriers to development (14). Specific to pediatric urology, randomized trials comprise only a small fraction of published literature in the field in part due to the relative scarcity and diversity of conditions represented (15). In particular, few randomized trials exist for assessing robotic and minimally invasive procedures (16, 17). Utilizing a stepwise, defined innovations process in pediatric urology and other pediatric fields offers the opportunity for an impactful unmet clinical need with validated market potential to present itself early in the development process, opening the door for innovation acceleration within this field.
We discovered three top needs that represent promising innovation targets for further solution prototyping and design. Based on the categorizations and weighting criteria provided, our highest ranked unmet clinical need was to improve treatment options for nocturnal enuresis. Nocturnal enuresis (bedwetting) is the most common urologic complaint in pediatric patients. Five to 7 million children from ages 5-15 are affected by nocturnal enuresis each year, with an estimated 15% of US children affected at age 5. Moisture-sensing bedwetting alarms provide a 70% success rate for achieving long-term dry nights. However, it can take >3 months for families to see any response to these devices, and up to four months for children to achieve dry nights (18, 19). Additionally, alarms require significant cooperation and effort from the family, who often must wake up with their child to ensure their compliance with the alarm system. Lastly, moisture-sensing bedwetting alarms do not prevent urination at night while the alarm is being used. Detecting nocturnal enuresis before urination occurs, without awakening the patient or their family, with dryness occurring in less than 3 months will reduce burden and improve sleep quality for children and families, while also decreasing clinician burden and clinic office visits. Our preliminary solution concept addresses these concerns and targets all stakeholder needs in this area with an innovation that addresses the shortcomings of current first-line intervention for nocturnal enuresis, bedwetting alarms. To note, there are several high-tech recent innovative devices on the market that offer alternative solutions to the classic bedwetting alarm; most notably there exists a sophisticated alarm device (GOGO Band System) that utilizes predictive learning of biometric data to predict enuresis episodes, thereby decreasing time to continence (11). Our solution concept validates the need informing this device and builds on the concept of the importance of monitoring and active management during the treatment phase, thereby avoiding the previously described negative effects of most current alarm-based systems. In recent years, randomized control trials for transcutaneous tibial nerve stimulation and percutaneous tibial nerve stimulation as treatment options for nocturnal enuresis have emerged, yet to our knowledge, a nocturnal enuresis device that uses electrical stimulation to prevent urination in combination with active bladder monitoring during the treatment phase has not yet entered the market (20, 21).
There are several limitations of this study. The present findings require multi-institutional validation, as this investigation was conducted at a single institution. This additional external validation will account for the wide variation in healthcare delivery and patient populations across different institutions. Additionally, the Pugh decision-making matrix relies on innovators to score each unmet need based on their own perceptions of factors such as technical complexity and impact in the field. While these considerations were informed by experts in the field and external research, further stakeholder evaluation across a wider network can assist in mitigating the impact of innovator bias and expertise that may affect ranking of unmet needs. Additionally, although not utilized for this particular study, an additional weighting factor to account for an uneven distribution of interviewed individuals according to their role (e.g. healthcare team stakeholders versus patient/patient family stakeholders) may be implemented in coarse and fine needs prioritization. This additional weighting factor can account for a potential bias toward either healthcare-oriented or patient-oriented perspectives, respectively. It came as little surprise that the top unmet clinical need was patient-centric, given nearly half (49%) of all interviewees were patients and their family members. Indeed, other subgroups of interviewees such as physicians (9%) were relatively less represented, and accordingly represent a population for further study.
We are currently proceeding with laboratory validation of a developed prototype through partnership with a senior capstone bioengineering team on the UCLA campus, which is anticipated to be followed by a future clinical study to further ensure customer validation and effectiveness of each component of our solution concept. Device efficacy will be evaluated via urodynamics and pelvic floor neurologic responses in healthy adults, followed by evaluation in children diagnosed with nocturnal enuresis. The ultimate goal of the solution development phase is to develop a device that will provide a faster and more effective cure for children experiencing nocturnal enuresis and, in so doing, will transform the landscape of devices currently used for disorders involving bladder control and incontinence.
This is the first description of the application of a stepwise innovations process modeled on both the Stanford Biodesign curriculum and the Spiral Innovation Model developed at Johns Hopkins, as administered by Sling Health LA, within the novel clinical setting of pediatric urology. The described process consisted of clinical immersion, needs validation, needs prioritization, needs selection, and solution landscaping within the setting of pediatric urology. Biodesign-based processes provide students early in their clinical training with the skills to identify unmet needs within their environment. These innovation skills can empower students to continue identifying and solving unmet clinical needs throughout their career in any clinical specialty or practice environment, thereby positively impacting both healthcare providers and the communities they serve. Applying this process not only provided a key facet of medical education, but it also led to discovery and description of impactful unmet clinical needs within a focused pediatric surgical field. This has been followed by development of a preliminary solution concept for the top-ranked unmet need: a better treatment option for nocturnal enuresis that will decrease patient awakening, improve urine volume and continence parameters, and minimize the time required to achieve continence. This work demonstrates that a needs-driven approach to medical innovation in pediatric urology not only benefits patients, students, and researchers, but also uniquely facilitates the development of innovative solutions that will be readily translated into clinical practice.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethical review and approval was not required for the study on human participants, in accordance with the local legislation and institutional requirements.
ML, DZ, LZ, and RS contributed to the design and conception of the study. AS, NI, SD, and SS contributed to the market analysis. All authors participated in review and revision of the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Process, in: Stanford Byers Center for Biodesign. Available at: https://biodesign.stanford.edu/about-us/process.html (Accessed February 16, 2022).
2. Brinton TJ, Kurihara CQ, Camarillo DB, Pietzsch JB, Gorodsky J, Zenios SA, et al. Outcomes From a Postgraduate Biomedical Technology Innovation Training Program: The First 12 Years of Stanford Biodesign. Ann BioMed Eng (2013) 41(9):1803–10. doi: 10.1007/s10439-013-0761-2
3. Trainee Outcomes, in: Stanford Byers Center for Biodesign. Available at: https://biodesign.stanford.edu/our-impact/trainee-outcomes.html (Accessed April 19, 202).
4. Venkatramani V. Urovision 2020: The Future of Urology. Indian J Urol IJU J Urol Soc India (2015) 31(2):150–5. doi: 10.4103/0970-1591.152918
5. Bernstein DE, Bernstein BS. Urological Technology: Where Will We Be in 20 Years’ Time? Ther Adv Urol (2018) 10(8):235–42. doi: 10.1177/1756287218782666
6. Wall J, Wynne E, Krummel T. Biodesign Process and Culture to Enable Pediatric Medical Technology Innovation. Semin Pediatr Surg (2015) 24(3):102–6. doi: 10.1053/j.sempedsurg.2015.02.005
7. Krummel TM, Gertner M, Makower J, Milroy C, Gurtner G, Woo R, et al. Inventing Our Future: Training the Next Generation of Surgeon Innovators. Semin Pediatr Surg (2006) 15(4):309–18. doi: 10.1053/j.sempedsurg.2006.07.011
8. Linderman SW, Appukutty AJ, Russo MV, Shah AP, Javaherian K. Advancing Healthcare Technology Education and Innovation in Academia. Nat Biotechnol (2020) 38(10):1213–7. doi: 10.1038/s41587-020-0689-7
9. Spiral Innovation Model, Biomedical Engineering JHU CBID, in: The Johns Hopkins Center for Bioengineering Innovation & Design . Available at: https://cbid.bme.jhu.edu/innovations/spiral-innovation-model/ (Accessed March 12, 2022).
10. Pugh S. Concept Selection - A Method That Works In: Hubka, V. (ed.) Review of Design Methodology. Proceedings International Conference on Engineering Design (1981), 497–506.
11. Welcome to My GOGOBand, in: GOGO Band. Available at: https://mygogoband.com/ (Accessed March 10, 2022).
12. Sun RC, Kamat I, Byju AG, Wettergreen M, Heffernan MJ, Willson R, et al. Advancing Pediatric Medical Device Development via Non-Dilutive NIH SBIR/STTR Grant Funding. J Pediatr Surg (2021) 56(11):2118–23. doi: 10.1016/j.jpedsurg.2021.01.025
13. Pediatric Medical Device Development by Surgeons via Capstone Engineering Design Programs. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5545169 (Accessed March 10, 2022).
14. Food and Drug Administration Fiscal Year 2016 Justification of Estimates for Appropriations CommitteesAvailable at: www.fda.gov (2016).
15. Welk B, Afshar K, MacNeily AE. Randomized Controlled Trials in Pediatric Urology: Room for Improvement. J Urol (2006) 176(1):306–9. doi: 10.1016/S0022-5347(06)00560-X
16. da Cruz JA, Passerotti CC. Reconstructive Laparoscopy in Pediatric Urology. Curr Opin Urol (2010) 20(4):330–5. doi: 10.1097/MOU.0b013e32833aa3ed
17. Andolfi C, Kumar R, Boysen WR, Gundeti MS. Current Status of Robotic Surgery in Pediatric Urology. J Laparoendosc Adv Surg Tech (2019) 29(2):159–66. doi: 10.1089/lap.2018.0745
18. Gomez Rincon M, Leslie SW, Lotfollahzadeh S. Nocturnal Enuresis, in: StatPearls (2022). StatPearls Publishing. Available at: http://www.ncbi.nlm.nih.gov/books/NBK545181 (Accessed March 10, 2022).
19. Nocturnal Enuresis in Children: Management - Uptodat . Available at: https://www.uptodate.com/contents/nocturnal-enuresis-in-children-management?search=nocturnal-enuresis-in-children-management.&source=search_result&selectedTitle=1~46&usage_type=default&display_rank=1 (Accessed March 10, 2022).
20. Abdelhalim NM, Ibrahim MM. A Comparative Study of Transcutaneous Interferential Electrical Stimulation and Transcutaneous Electrical Nerve Stimulation on Children With Primary Nocturnal Enuresis: A Randomized Clinical Trial. Int Urol Nephrol (2020) 52(3):409–15. doi: 10.1007/s11255-019-02340-w
21. Ghijselings L, Renson C, Van de Walle J, Everaert K, Spinoit AF. Clinical Efficacy of Transcutaneous Tibial Nerve Stimulation (TTNS) Versus Sham Therapy (Part I) and TTNS Versus Percutaneous Tibial Nerve Stimulation (PTNS) (Part II) on the Short Term in Children With the Idiopathic Overactive Bladder Syndrome: Protocol for Part I of the Twofold Double-Blinded Randomized Controlled TaPaS Trial. Trials (2021) 22(1):247. doi: 10.1186/s13063-021-05117-8
Keywords: innovation, BIODESIGN, pediatric urology, Medtech, urology, needs-based, nocturnal enuresis, incontinence
Citation: Luff MK, Zarrin DA, Zhou L, Sahoo A, Desai S, Iyer N, Starr SL and Sturm RM (2022) Clinical Needs Discovery in Pediatric Urology: Utilizing the Biodesign Process. Front. Urol. 2:895057. doi: 10.3389/fruro.2022.895057
Received: 12 March 2022; Accepted: 16 May 2022;
Published: 30 June 2022.
Edited by:
Emilie K Johnson, Ann & Robert H. Lurie Children’s Hospital of Chicago, United StatesReviewed by:
Stephen Canon, Arkansas Children’s Hospital, United StatesCopyright © 2022 Luff, Zarrin, Zhou, Sahoo, Desai, Iyer, Starr and Sturm. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marie K Luff, bWx1ZmZAbWVkbmV0LnVjbGEuZWR1; Renea M. Sturm, cnN0dXJtQG1lZG5ldC51Y2xhLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.