- 1Urology Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, NY, United States
- 2Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, United States
- 3Department of Medicine, Weill Cornell Medical College, New York, NY, United States
- 4Department of Urology, Weill Cornell Medical College, New York, NY, United States
Muscle-invasive bladder cancer (MIBC) is often framed as a systemic disease given the risk of occult metastases and clinical under-staging at the time of radical cystectomy. The current standard of care for non-metastatic MIBC combines a cisplatin-based neoadjuvant chemotherapy regimen followed by radical cystectomy, pelvic lymph node dissection, and urinary reconstruction. Other systemic therapies initially developed for the metastatic setting are being explored in the neoadjuvant space with favorable clinical outcomes. Immune checkpoint inhibitors targeting the programmed cell death-1/ligand-1 (PD-1/PD-L1) axis have demonstrated promising outcomes for cisplatin-ineligible patients in the neoadjuvant setting. Other novel targeted therapies under investigation in the perioperative setting include fibroblast growth factor receptor or FGFR inhibitors and antibody drug conjugates (enfortumab vedotin targeting Nectin-4 and sacituzumab govitecan targeting Trop-2). Non-chemotherapy-based treatments have the potential to expand the application of neoadjuvant therapy for many patients, particularly those who are cisplatin-ineligible due to comorbidities or who harbor chemotherapy-resistant tumors. The expansion of neoadjuvant therapy options also provides an opportunity to characterize mechanisms of tumor resistance and elucidate tumor biology with ongoing correlative studies.
1 Introduction
While radical cystectomy remains a primary management strategy for muscle-invasive bladder cancer (MIBC), high rates of recurrence with surgery alone highlight the likelihood of occult micro-metastatic disease at the time of diagnosis. In this review, we discuss two decades of contemporary evidence for the benefit of neoadjuvant chemotherapy-based systemic therapy. We also review the results of ongoing trials evaluating neoadjuvant immunotherapy and other novel targeted therapies. These trials will likely expand neoadjuvant therapy options for cisplatin-ineligible patients. The growing insights into the molecular heterogeneity and biology of MIBC have paved the way for future biomarker-directed treatment selection as well as possible bladder-sparing approaches with systemic therapy.
Unique issues must be considered when selecting agents for use in the neoadjuvant setting. Neoadjuvant treatment should not delay time to radical cystectomy such that patients miss their window for cure. Additionally, side effects from treatment should not be so severe that they limit a patient’s surgical fitness. Adverse effects related to immunotherapy and newer targeted therapies must be recognized and treated expediently in the preoperative setting to reduce the risks of additional surgical and anesthetic complications.
2 Methods
An appraisal of the primary literature was performed to select clinical trials focused on neoadjuvant systemic therapies for bladder cancer. A preliminary search on clinicaltrials.gov using terms “bladder cancer” and “neoadjuvant” was performed, yielding 155 studies. Studies with status of suspended, terminated, unknown or withdrawn were excluded (n=37). Studies limited to non-muscle invasive disease were excluded (n=6), as well as adjuvant or upper tract only studies (n=4). Studies testing intravesical agents, behavioral interventions, surgical technique, imaging, or biomarkers were also beyond the scope of this review and excluded (n=28). In addition, bladder preservation regimens incorporating radiation therapy were excluded (n=14). These clinical trials were then cross-referenced with recently published abstracts or manuscripts with reportable results (preliminary or fully resulted). Select, multi-institutional trials whose trial designs have been presented at national meetings were also included. A list of 29 clinical trials were finalized for this review. Historical clinical trials were incorporated where relevant to provide context to existing studies.
3 Chemotherapy

Robust clinical trial data supports the use of neoadjuvant cisplatin-based chemotherapy for patients with nonmetastatic MIBC. Based on Level I evidence, use of preoperative cisplatin-based chemotherapy is now included in the guideline recommendations from the American Urologic Association (AUA), American Society of Clinical Oncology (ASCO), National Comprehensive Cancer Network (NCCN), and European Association of Urology (EAU) (1).
The pivotal SWOG 8710 randomized clinical trial published nearly two decades ago demonstrated a 33% reduced relative risk of death and improved median survival from 46 months to 77 months in patients receiving 3 cycles of preoperative methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) compared to immediate cystectomy (2). In this trial, pathologic complete response rate (pCR) at cystectomy was associated with an 85% 5-year survival. Overall, 38% of chemotherapy-treated patients demonstrated a pathologic complete response (pCR or pT0N0), compared to 12% in the cystectomy only arm. An additional randomized trial (BA06 30894) of 976 patients with MIBC evaluated neoadjuvant cisplatin, methotrexate, and vinblastine for a median follow up time of 8 years and demonstrated a statistically significant 16% reduction in mortality risk, corresponding to a 10-year survival increase from 30% to 36% in favor of neoadjuvant chemotherapy over local therapy alone (3).
Another commonly used and well-tolerated neoadjuvant regimen is the doublet of gemcitabine and cisplatin (GC). Although this combination has not been directly tested in a prospective randomized fashion, its efficacy is extrapolated from prior randomized trials in the locally advanced and metastatic disease setting showing no differences in survival, but improved toxicity and tolerability (4). A retrospective series of 154 patients with pT2a-T4aN0M0 MIBC demonstrated that neoadjuvant GC achieved a 21% complete pathologic response (ypT0N0) rate and 25% rate of downstaging to ypTa/Tis/T1N0, which in turn was associated with 5-year overall survival rates of 85% and 89% respectively (5). The ongoing French GETUG/AFU VESPER V05 trial (NCT 01812369), comparing 6 cycles of dose-dense (dd)MVAC to 4 cycles of standard dose GC in the perioperative setting (~90% neoadjuvant and ~10% adjuvant), demonstrated higher organ-confined disease (<ypT3N0) rates with ddMVAC (77% vs 63%, p=0.001), although progression free survival results are still pending (6). Dose-dense gemcitabine in combination with cisplatin (ddGC) was explored in a phase II multicenter trial evaluating 6 cycles of ddGC in 49 patients with MIBC and demonstrated pathologic downstaging (<ypT2) in 57% of patients, which was associated with improved recurrence-free survival and overall survival (7). While the primary endpoint was not to compare the pathologic down-staging rates of ddGC with studies of neoadjuvant ddMVAC, the reported pathologic response rates (≤pT2) were similar (57% vs 49-53%).
Despite several clinical trials indicating a survival benefit with cisplatin-based NAC, uptake and utilization remain low nationally, both within academic centers and community-based practices (8, 9). The combination of advanced age, medical comorbidities, obstructive uropathy, and smoking history common among bladder cancer patients limit the use of cisplatin. Significant contraindications include renal, cardiac and neurologic co-morbidities, and studies suggest that only around 60% of patients are cisplatin-eligible (4, 10–12). A minority of cisplatin-eligible patients with MIBC undergo consultation with a medical oncologist to be counseled on the risks and benefits of NAC prior to surgery, and several retrospective studies demonstrated that less than 20% of eligible patients receive neoadjuvant chemotherapy (8, 13). Predictors of health care access including race, insurance status, geographic area and facility type are associated with marked differences in NAC use (13). While more contemporary studies suggest that uptake has been slowly increasing, (14, 15) a persistent need for non-cisplatin-based regimens remains.
Early efforts to develop predictive molecular signatures for chemosensitivity in bladder cancer laid the groundwork for a rapidly expanding interest in predictive biomarkers. (see Section 3) The co-expression extrapolation (COXEN) gene expression model was derived from pre-clinical models of bladder cancer, using cell lines tested against cisplatin to derive a molecular signature for chemosensitivity that was independent from clinical and pathologic features (16). In a subsequent prospective trial (SWOG 1314) evaluating the ability of COXEN to predict cisplatin sensitivity in 167 patients, however, there was no consistent association between the COXEN score and pCR rates in either the ddMVAC or GC arm in the neoadjuvant setting (17). Nevertheless, S1314 provided a platform to develop and validate additional biomarkers in the neoadjuvant setting.
4 Neoadjuvant Immunotherapy

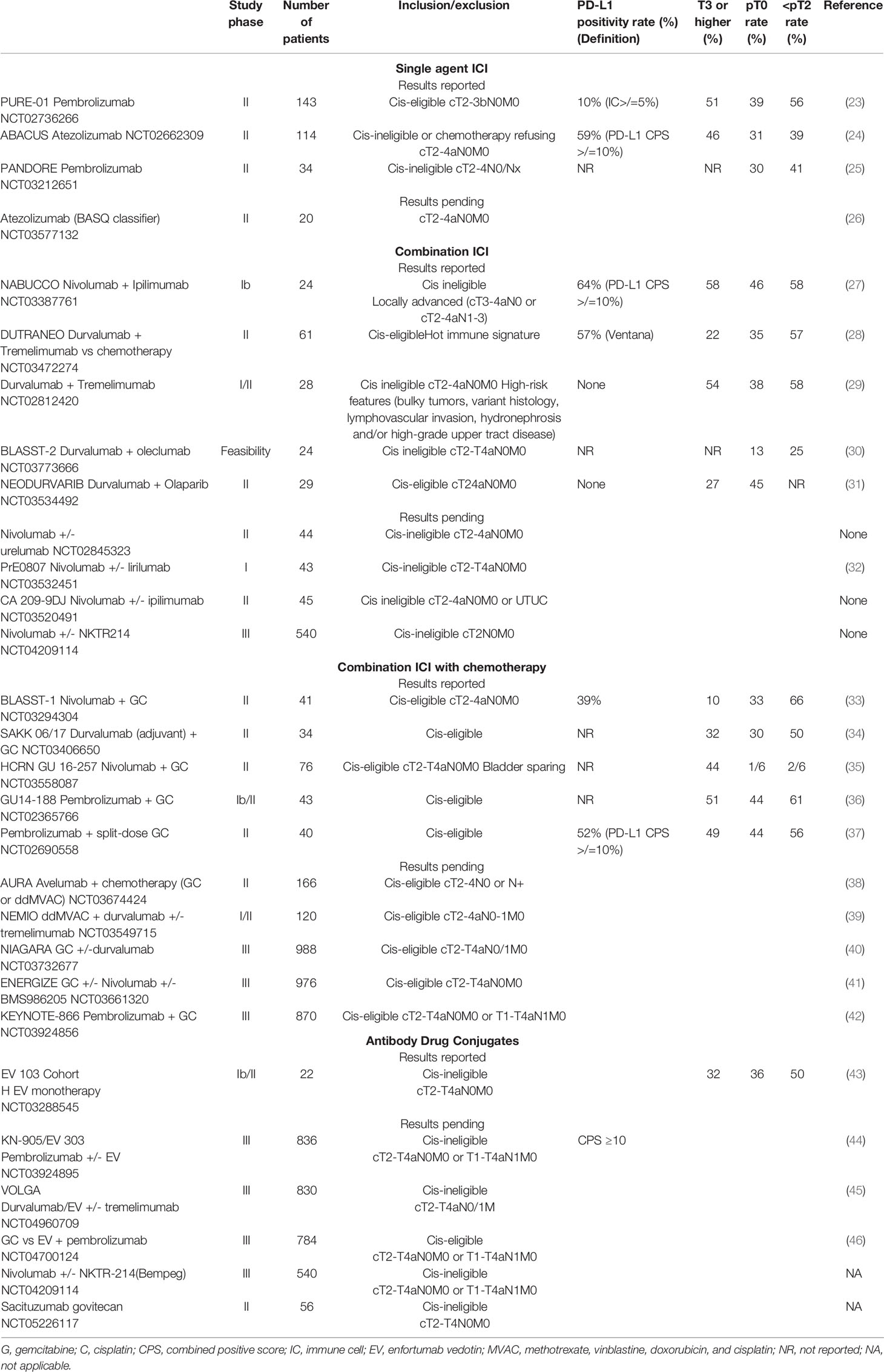
Immune checkpoint inhibitors (ICI) have emerged with broad application in urothelial cancers. These monoclonal antibodies (mAbs) target various immune checkpoint proteins (PD-L1, PD-1, CTLA-4) to inhibit local immune escape of cancer cells and enable T-cell priming in lymphoid tissue. Such agents include atezolizumab, durvalumab, and avelumab (anti-PD-L1); pembrolizumab and nivolumab (anti-PD-1); and ipilimumab and tremelimumab (anti-CTLA-4). Successful application of ICI in patients with metastatic or locally advanced urothelial cancer paved the way for their potential use in early stage disease (18–21 Phase Ib Study (22). Multiple ICIs are currently being evaluated in ongoing clinical trials in the neoadjuvant setting for patients with MIBC in both cisplatin eligible and ineligible populations (Table 1). Pooled complete response rates of published studies vary from 30-50% with 60-70% rates of pathological downstaging. Single agent and combination ICI trials are outlined below.
4.1 Single Agent Immune Checkpoint Inhibition
Initial results from Phase II trials evaluating single-agent ICIs in the neoadjuvant setting have reported pCR rates ranging from 31% with atezolizumab (ABACUS) (24) to 37% with pembrolizumab (PURE 01) (47) with pathologic downstaging rates of 39% and 56%, respectively. More updated survival data from PURE-01 and ABACUS show a 12-month recurrence free survival (RFS) of 70% (PURE-01) and 79% (ABACUS) (24, 48). In the PURE 01 trial, 24-month RFS was 96% for patients with pCR and 75% for patients with pathologic downstaging, while node positive patients had a 24-month RFS rate of 40%. Of note, the ABACUS trial enrolled cisplatin ineligible patients, and PURE-01 included both cisplatin eligible and ineligible patients. Interestingly, neoadjuvant pembrolizumab was less effective in variant histology tumors (16% pCR) and may be more effective in basal subtypes of MIBC in retrospective analyses (23).
Safety results from ABACUS demonstrated that 17 (20%) of 87 patients experienced grades 3-5 adverse events (AE), including 13 (15%) with post-cystectomy atezolizumab-related AEs such as adrenal insufficiency and transaminitis. Of note, 3 deaths were reported, one of which was attributed to immune-related myocardial infarction (49). In the PURE-01 trial first reporting on 50 patients, the most frequent AE was thyroid dysfunction in 9 (18%) patients whereas only 3 (6%) patients experienced grade 3 AEs. Due to the long half-life of PD-1/PD-L1 inhibitors, AEs may occur late in the postoperative period and include critical endocrine abnormalities requiring early recognition and treatment (50).
Notably, patients with ypT2 disease had similar RFS outcomes compared to those with residual disease <ypT2 (79% vs 75% 24 month RFS), suggesting the presence of an immune-driven durable anti-cancer effect (48). The results of these single arm trials indicate a potential role for ICIs in the neoadjuvant space for patients who are cisplatin ineligible or refuse treatment, with long term survival outcomes pending. Single agent ICI trials are summarized in Table 1.
4.2 Combination ICI Therapy
Combination ICI regimens targeting both PD1/PD-L1 and anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) axes are thought to further potentiate the host immune response and may have a synergistic anti-tumor effect. The recently reported NABUCCO (nivolumab and ipilimumab) trial and durvalumab and tremelimumab trials demonstrated 38-46% pCR rates and 57-58% pathologic downstaging rates, respectively (Table 1) (27, 29). These response rates were balanced by a relatively high frequency of irAEs—nearly all patients in both studies experienced some form of irAEs, and 55% of patients in the NABUCCO trial and 21% in the durvalumab/tremelimumab trial experienced Grade 3 or higher side effects.
In the durvalumab/tremelimumab single arm study for cis-ineligible patients with high risk MIBC, six (21%) patients experienced grade 3 or higher iRAEs, among whom there were two delays to cystectomy (29). In this trial, irAEs were successfully managed with immunosuppressive therapy and only four patients required systemic immunosuppressive therapy in the form of steroids alone with or without mycophenolate and/or infliximab. Interestingly, three of these four patients were noted to be responders. It has been observed across multiple cancers that patients who experience irAE may also experience more profound anti-tumor responses (51). In NABUCCO, all patients had surgical resection and 23 (96%) patients underwent resection within 12 weeks of systemic therapy, while 1 patient had a delay of 4 weeks due to immune-related hemolysis and 6 patients received only two cycles due to irAEs. There were no treatment-related mortalities in either study.
These trials continue to establish a baseline for the safe administration of neoadjuvant therapy in cis-ineligible patients and upcoming results from other combination ICI studies will provide additional insight. It is important to emphasize that if ICIs are approved in the neoadjuvant setting, providers must maintain a high index of suspicion for irAEs, which must be quickly recognized and treated to mitigate perioperative complications. Additional combination ICI trials are listed in Table 1.
4.3 Combination ICI With Chemotherapy
A multi-institutional phase II trial (NCT02989584) evaluating combination neoadjuvant atezolizumab with GC for MIBC demonstrated that 69% of patients were downstaged to <ypT2N0 and 38% achieved ypT0 at cystectomy. All patients achieving <ypT2N0 were alive and disease free at a median follow-up of 16.7 months. Notably, AEs were due primarily to chemotherapy (neutropenia) and grade 3 irAEs were uncommon, with 2 patients requiring high-dose steroids for autoimmune hepatitis and nephritis (52). Another phase II trial (NCT02690558) evaluating pembrolizumab with split-dose GC demonstrated a 56% <ypT2N0 rate and 44% ypT0 rate (37). As with the prior study, no new safety signals were observed with combination therapy, with a single patient discontinuing therapy early for autoimmune diabetic ketoacidosis and 9 others due to AEs likely related to chemotherapy. A significant association between PD-L1 status and response was not observed in either study. Additional ongoing studies evaluating combined chemotherapy with ICI are outlined in Table 1.
5 Antibody Drug Conjugates
Antibody-drug conjugates are a novel class of biologic drugs that are likely to play a role the perioperative setting. Enfortumab vedotin (EV) is an ADC targeting Nectin-4, a surface antigen highly expressed in urothelial carcinoma, to deliver the microtubule destabilizing agent monomethyl auristatin E (MMAE). A phase II clinical trial of EV monotherapy (EV-201) demonstrated a high overall response rate in patients with heavily pretreated, metastatic, or locally advanced urothelial carcinoma, leading to expedited FDA approval (53). Due to its activity in advanced and metastatic disease, EV is now being tested in earlier disease states: EV-103 is a phase 1b/2 multi-cohort trial that is exploring enfortumab vedotin (EV) as monotherapy or in combination with various systemic therapies in both the metastatic and perioperative settings in patients with bladder cancer (54). At ASCO GU 2022, preliminary results reported from Cohort H included 22 cisplatin-ineligible patients with cT2-T4aN0M0 MIBC who received neoadjuvant EV followed by RC-PLND. The pCR rate, the primary endpoint of the trial, was 36%, while pathologic downstaging was observed in 50% of patients (43). No delays to cystectomy were seen, but three peri-operative deaths were reported. Although these deaths were not directly related to EV, the high mortality seen in this trial highlights the importance of both careful patient selection and the maintenance of perioperative surgical fitness with novel agents.
Sacituzumab govitecan (SG) is an ADC that targets TROP-2 to deliver SN-38, the active metabolite of the topoisomerase inhibitor irinotecan. SG is currently being tested in monotherapy in cis-ineligible patients (NCT05226117) as well as with or without pembrolizumab (55). Ongoing clinical trials exploring the role of ADCs with or without ICI in the perioperative setting are listed in Table 1.
6 Biomarker Development

Growing insight into tumor heterogeneity and the role of the tumor microenvironment have spurred an explosion of research in the correlative analysis of pre- and post-treatment tissue in neoadjuvant studies. Emerging biomarkers include molecular subtype, DNA damage response (DDR) genes, tumor mutation burden (TMB), and gene expression signatures.
6.1 PD-L1
PD-L1 status, as determined by immunohistochemistry, has thus far been an imperfect predictive biomarker of response to ICI. Both PURE-01 and ABACUS correlated PD-L1 status with pathologic response at cystectomy. In PURE-01, pT0 responses were enriched in patients with a PD-L1 combined positivity score (CPS) ≥10% compared to <10% (54.3% vs 13.3%, p=0.011). Notably, 70% of patients had CPS≥10%, which is higher than reported in other studies. In the ABACUS trial, 40% of tumors were PD-L1 positive (SP142 antibody, ≥5% on immune cells) at baseline with a 37% rate of pCR in these patients. No significant correlation was found between PD-L1 expression and outcome, on either immune cells or tumor cells. Combination therapy trials, including NABUCCO (ipilimumab and nivolumab) and durvalumab and tremelimumab similarly did not identify a statistically significant correlation between PD-L1 positivity and pathologic response. Variation in the antibodies used and cutoffs to determine PD-L1 status, as well as differences in how PD-1/L1 are assessed (on tumor cells vs immune cells) may contribute to the interstudy variability and lack of correlation observed.
6.2 DNA Damage Response Gene Alterations
Alterations in DDR genes were found to be enriched in patients who responded to platinum chemotherapy in the neoadjuvant setting and immune checkpoint blockade in the metastatic setting (56–59). An initial retrospective extreme phenotype analysis identified mutations within the nucleotide excision repair DNA helicase ERCC2 enriched in patients who exhibited pT0 or CIS in the bladder at radical cystectomy following neoadjuvant cisplatin-based chemotherapy (58). Other studies implicated additional DDR genes as predictive for response, including ATM, FANCC, and RB1. In a prospective multicenter trial evaluating neoadjuvant ddGC, the presence of deleterious alterations in several DDR genes was associated with significant chemosensitivity and pathologic response, and no patient with a deleterious DDR gene alteration experienced recurrence at a median follow-up of 2 years (7).
While DDR alterations may be used to identify optimal candidates for preoperative platinum-based chemotherapy, four trials are actively testing the possibility of complete bladder preservation in select patients with DDR-altered tumors who achieve a clinical complete response to platinum-based chemotherapy with or without immunotherapy: the RETAIN trial (NCT02710734) testing MVAC, the RETAIN-2 trial (NCT04506554) testing MVAC with nivolumab, HCRN 16-257 trial (NCT03558087) testing 4 cycles of GC with nivolumab, and the Alliance A031701 trial testing 6 cycles of GC (NCT03609216).
In the PURE01 trial, alterations within DDR genes were associated with pathologic downstaging at cystectomy as was TMB. Additionally, residual invasive tumors showed a lower TMB than matched pre-treatment tumors, suggesting that pre-existing lower TMB tumor clones may have been resistant to checkpoint blockade (23). The NABUCCO trial of ipilimumab plus nivolumab found a correlation between DDR gene alterations and pCR (p=0.03), and a statistically non-significant trend towards response with high TMB (p=0.056) (27). In contrast, neither the durvalumab and tremelimumab nor ABACUS trials showed a correlation between DDR gene alterations or TMB and pathologic response (24, 29). The use of heterogeneous DDR gene panels, cohort sizes, and different inclusion criteria for the type of alteration (deleterious vs any) may underlie the variable results found to date.
6.3 Immune Gene Signatures
Several markers of immune-mediated inflammation have been examined in neoadjuvant IO trials. In the ABACUS trial, the presence of intraepithelial CD8+ T-cells was associated with response pCR (40% vs 20%, p<0.05) as was a cytotoxic T-cell 8-gene signature (tGE8, p<0.01) (24). While no correlation between intratumor CD8+ T-cell infiltrate (an inflamed tumor phenotype) and response was noted, the presence of both CD8+ T-cells and granzyme B, an immune effector molecule secreted by activated cytotoxic T-cells, was associated with response. Finally, TGFβ, which drives resistance to ICI therapy in metastatic bladder cancer by active T-cell exclusion (60), was also associated with resistance to neoadjuvant atezolizumab.
In the neoadjuvant durvalumab and tremelimumab study, the tGE8 signature was not associated with response, but the presence of tertiary lymphoid structures (TLS, ectopic lymphoid tissue that develops at sites of inflammation) were observed at higher density in pre-treatment tissue from responders compared to non-responders as well as improved survival outcomes (29). The NABUCCO trial showed a correlation between the presence of TLS within on-treatment tumor tissue and response, while B-cell infiltration of the stroma was associated with lack of response (27).
Upregulation of genes associated with inflammation may enhance response to ICI. In the DUTRENEO Phase II study, patients with an inflamed tumor immune score (TIS) based on an IFNγ gene expression signature are randomized to durvalumab and tremelimumab or cisplatin-based chemotherapy. Those without an inflamed immune score receive cisplatin-based chemotherapy (28). Early results have not shown significant differences in pCR rates in the patients with inflamed tumors who received ICI as compared to chemotherapy. Although these additional agents appeared to be well tolerated, it remains to be seen if the TIS can accurately prioritize patients for ICI over traditional chemotherapy.
6.4 Molecular Subtype
Consensus molecular subtypes have been described based on an RNA expression signature (61). These subtypes have been studied in a retrospective manner to determine an association with response rates after radical cystectomy. For example, basal and claudin-low subtypes were associated with more favorable pCR outcomes after neoadjuvant pembrolizumab (23). Another study of 26 residual tumors with ypT2-4 disease after neoadjuvant pembrolizumab showed that a scar-like subtype with higher luminal marker expression was associated with residual disease (62). In contrast, neoadjuvant cisplatin-based chemotherapy is associated with highest response rates in non-luminal subtypes (63). Differences in molecular classification systems make cross-trial and cross-cohort comparisons challenging and these subtypes have yet to be validated prospectively, although several ongoing and recently completed clinical trials of neoadjuvant systemic therapy have incorporated molecular subtypes analyses.
7 Future Directions
The landscape of neoadjuvant therapy in MIBC is rapidly evolving as novel agents previously approved in the metastatic setting are being used and tested in earlier disease states. While cisplatin based neoadjuvant chemotherapy remains an important backbone, either alone or in combination with other agents, ICI and ADCs have shown significant activity in patients who are cisplatin ineligible or intolerant. Indeed, cisplatin-ineligible patients with MIBC have the greatest unmet need for novel neoadjuvant regimens. Ongoing correlative studies enabled by pre- and post-treatment molecular analyses may one day give rise to predictive biomarkers than can not only personalize treatment for patients, but also identify patients for bladder-sparing strategies.
Author Contributions
CC, GI, and BB contributed to conception, design, writing, revision, and approval of this manuscript.
Conflict of Interest
GI, Consulting/Advisory Board, Janssen, Mirati Therapeutics, Basilea, Flare Therapeutics, LOXO. Speaker’s fees, Gilead Sciences, The Lynx Group Research. Funding, Mirati Therapeutics, Novartis, Debiopharm Group, Bayer, Janssen, Seagen.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chang SS, Bochner BH, Chou R, Dreicer R, Kamat AM, Lerner SP, et al. Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA/ASCO/ASTRO/SUO Guideline. J Urol (2017) 198:552–59. doi: 10.1016/j.juro.2017.04.086
2. Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. Neoadjuvant Chemotherapy Plus Cystectomy Compared With Cystectomy Alone for Locally Advanced Bladder Cancer. N Engl J Med (2003) 349:859–66. doi: 10.1056/NEJMoa022148
3. International Collaboration of Trialists, International Collaboration of, the European Organisation for Research Medical Research Council Advanced Bladder Cancer Working Party, Cancer Treatment of Cancer Genito-Urinary Tract, Research European Organisation for, the Australian Bladder Cancer Study Treatment of Cancer Genito-Urinary Tract Cancer Group, the National Cancer Institute of Canada Clinical Trials Australian Bladder Cancer Study Group, Group National Cancer Institute of Canada Clinical Trials, Norwegian Bladder Cancer Study Finnbladder, et al. International Phase III Trial Assessing Neoadjuvant Cisplatin, Methotrexate, and Vinblastine Chemotherapy for Muscle-Invasive Bladder Cancer: Long-Term Results of the BA06 30894 Trial. J Clin Oncol (2011) 29:2171–7. doi: 10.1200/JCO.2010.32.3139
4. von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and Cisplatin Versus Methotrexate, Vinblastine, Doxorubicin, and Cisplatin in Advanced or Metastatic Bladder Cancer: Results of a Large, Randomized, Multinational, Multicenter, Phase III Study. J Clin Oncol (2000) 18:3068–77. doi: 10.1200/JCO.2000.18.17.3068
5. Iyer G, Tully CM, Zabor EC, Bochner BH, Dalbagni G, Herr HW, et al. Neoadjuvant Gemcitabine-Cisplatin Plus Radical Cystectomy-Pelvic Lymph Node Dissection for Muscle-Invasive Bladder Cancer: A 12-Year Experience. Clin Genitourinary Cancer (2020) 18:387–94. doi: 10.1016/j.clgc.2020.02.014
6. Pfister C, Gravis G, Fléchon A, Soulié M, Guy L, Laguerre B, et al. Randomized Phase III Trial of Dose-Dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin, or Gemcitabine and Cisplatin as Perioperative Chemotherapy for Patients With Muscle-Invasive Bladder Cancer. Analysis of the GETUG/AFU V05 VESPER Trial Secondary Endpoints: Chemotherapy Toxicity and Pathological Responses. Eur Urol (2021) 79:214–21. doi: 10.1016/j.eururo.2020.08.024
7. Iyer G, Balar AV, Milowsky MI, Bochner BH, Dalbagni G, Donat SM, et al. Multicenter Prospective Phase II Trial of Neoadjuvant Dose-Dense Gemcitabine Plus Cisplatin in Patients With Muscle-Invasive Bladder Cancer. J Clin Oncol (2018) 36:1949–56. doi: 10.1200/JCO.2017.75.0158
8. David KA, Milowsky MI, Ritchey J, Carroll PR, Nanus DM. Low Incidence of Perioperative Chemotherapy for Stage III Bladder Cancer 1998 to 2003: A Report From the National Cancer Data Base. J Urol (2007) 178:451–4. doi: 10.1016/j.juro.2007.03.101
9. Raj GV, Karavadia S, Schlomer B, Arriaga Y, Lotan Y, Sagalowsky A, et al. Contemporary Use of Perioperative Cisplatin-Based Chemotherapy in Patients With Muscle-Invasive Bladder Cancer. Cancer (2011) 117:276–82. doi: 10.1002/cncr.25429
10. Galsky MD, Hahn NM, Rosenberg J, Sonpavde G, Hutson T, Oh WK, et al. A Consensus Definition of Patients With Metastatic Urothelial Carcinoma Who Are Unfit for Cisplatin-Based Chemotherapy. Lancet Oncol (2011) 12:211–4. doi: 10.1016/S1470-2045(10)70275-8
11. Galsky MD, Ma E, Shah-Manek B, Mills R, Ha L, Krebsbach C, et al. Cisplatin Ineligibility for Patients With Metastatic Urothelial Carcinoma: A Survey of Clinical Practice Perspectives Among US Oncologists. Bladder Cancer (2019) 5:281–88. doi: 10.3233/BLC-190235
12. Jiang DIM, Gupta S, Kitchlu A, Meraz-Munoz A, North SA, Alimohamed NS, et al. Defining Cisplatin Eligibility in Patients With Muscle-Invasive Bladder Cancer. Nat Rev Urol (2021) 18:104–14. doi: 10.1038/s41585-020-00404-6
13. Fedeli U, Fedewa SA, Ward EM. Treatment of Muscle Invasive Bladder Cancer: Evidence From the National Cancer Databasto 2007. J Urol (2011) 185:72–8. doi: 10.1016/j.juro.2010.09.015
14. Booth CM, Karim S, Brennan K, Siemens DR, Peng Y, Mackillop WJ. Perioperative Chemotherapy for Bladder Cancer in the General Population: Are Practice Patterns Finally Changing? Urol Oncol (2018) 36:89.e13–20. doi: 10.1016/j.urolonc.2017.11.015
15. Macleod LC, Yabes JG, Yu M, Fam MM, Hale NE, Turner RM, et al. Trends and Appropriateness of Perioperative Chemotherapy for Muscle-Invasive Bladder Cancer. Urol Oncol (2019) 37:462–69. doi: 10.1016/j.urolonc.2019.04.006
16. Havaleshko DM, Cho H, Conaway M, Owens CR, Hampton G, Lee JK, et al. Prediction of Drug Combination Chemosensitivity in Human Bladder Cancer. Mol Cancer Ther (2007) 6:578–86. doi: 10.1158/1535-7163.MCT-06-0497
17. Flaig TW, Tangen CM, Daneshmand S, Alva A, Lerner SP, Lucia MS, et al. A Randomized Phase II Study of Coexpression Extrapolation (COXEN) With Neoadjuvant Chemotherapy for Bladder Cancer (SWOG S1314; Nct02177695). Clin Cancer Res (2021) 27:2435–41. doi: 10.1158/1078-0432.CCR-20-2409
18. Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in Patients With Locally Advanced and Metastatic Urothelial Carcinoma Who Have Progressed Following Treatment With Platinum-Based Chemotherapy: A Single-Arm, Multicentre, Phase 2 Trial. Lancet (London England) (2016) 387:1909–20. doi: 10.1016/S0140-6736(16)00561-4
19. Massard C, Gordon MS, Sharma S, Rafii S, Wainberg ZA, Luke J, et al. Safety and Efficacy of Durvalumab (MEDI4736), an Anti-Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients With Advanced Urothelial Bladder Cancer. J Clin Oncol (2016) 34:3119–25. doi: 10.1200/JCO.2016.67.9761
20. Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in Metastatic Urothelial Carcinoma After Platinum Therapy (CheckMate 275): A Multicentre, Single-Arm, Phase 2 Trial. Lancet Oncol (2017) 18:312–22. doi: 10.1016/S1470-2045(17)30065-7
21. Apolo AB, Infante JR, Balmanoukian A, Patel MR, Wang D, Kelly K, et al. Avelumab, an Anti-Programmed Death-Ligand 1 Antibody, In Patients With Refractory Metastatic Urothelial Carcinoma: Results From a Multicenter, Phase Ib Study. J Clin Oncol (2017) 35:2117–24. doi: 10.1200/JCO.2016.71.6795
22. Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, et al. Atezolizumab as First-Line Treatment in Cisplatin-Ineligible Patients With Locally Advanced and Metastatic Urothelial Carcinoma: A Single-Arm, Multicentre, Phase 2 Trial. Lancet (London England) (2017) 389:67–76. doi: 10.1016/S0140-6736(16)32455-2
23. Necchi A, Raggi D, Gallina A, Madison R, Colecchia M, Lucianò R, et al. Updated Results of PURE-01 With Preliminary Activity of Neoadjuvant Pembrolizumab in Patients With Muscle-Invasive Bladder Carcinoma With Variant Histologies. Eur Urol (2020) 77:439–46. doi: 10.1016/j.eururo.2019.10.026
24. Powles T, Kockx M, Rodriguez-Vida A, Duran I, Crabb SJ, van der Heijden MS, et al. Clinical Efficacy and Biomarker Analysis of Neoadjuvant Atezolizumab in Operable Urothelial Carcinoma in the ABACUS Trial. Nat Med (2019) 25:1706–14. doi: 10.1038/s41591-019-0628-7
25. Goubet A-G, Silva CAC, De Melo LL, Gazzano M, Lebacle C, Thibault C, et al. ‘Bacteria-Specific CXCL13-Producing Follicular Helper T Cells are Putative Prognostic Markers to Neoadjuvant PD-1 Blockade in Muscle-Invasive Urothelial Carcinoma’. J Clin Oncol (2022) 40:535–35. doi: 10.1200/JCO.2022.40.6_suppl.535
26. Yuk HD, Jeong CW, Kwak C, Kim H, Moon KC, Ku >JH. ‘Efficacy of Neoadjuvant Atezolizumab Treatment in Patients With Advanced Urothelial Bladder Cancer According to the BASQ Classification: A Study Protocol for an Open-Label, Two-Cohort, Phase II Trial’. BMJ Open (2020) 10:e035530–e30. doi: 10.1136/bmjopen-2019-035530
27. van Dijk N, Gil-Jimenez A, Silina K, Hendricksen K, Smit LA, de Feijter JM, et al. Preoperative Ipilimumab Plus Nivolumab in Locoregionally Advanced Urothelial Cancer: The NABUCCO Trial. Nat Med (2020) 26:1839–44. doi: 10.1038/s41591-020-1085-z
28. Grande E, Guerrero F, Puente J, Galante I, Duran I, Dominguez M, et al. DUTRENEO Trial: A Randomized Phase II Trial of DUrvalumab and TREmelimumab Versus Chemotherapy as a NEOadjuvant Approach to Muscle-Invasive Urothelial Bladder Cancer (MIBC) Patients (Pts) Prospectively Selected by an Interferon (INF)-Gamma Immune Signature. J Clin Oncol (2020) 38:5012–12. doi: 10.1200/JCO.2020.38.15_suppl.5012
29. Gao J, Navai N, Alhalabi O, Siefker-Radtke A, Campbell MT, Slack Tidwell R, et al. Neoadjuvant PD-L1 Plus CTLA-4 Blockade in Patients With Cisplatin-Ineligible Operable High-Risk Urothelial Carcinoma. Nat Med (2020) 26:1845–51. doi: 10.1038/s41591-020-1086-y
30. Wei XX, McGregor BA, Lee RJ, Gao X, Kilbridge KL, Preston MA, et al. ‘Durvalumab as Neoadjuvant Therapy for Muscle-Invasive Bladder Cancer: Preliminary Results From the Bladder Cancer Signal Seeking Trial (BLASST)-2’. J Clin Oncol (2020) 38:507–07. doi: 10.1200/JCO.2020.38.6_suppl.507
31. Rodriguez-Moreno JF, de Velasco G, Fernandez IB, Alvarez-Fernandez C, Fernandez R, Vazquez-Estevez S, et al. Impact of the Combination of Durvalumab (MEDI4736) Plus Olaparib (AZD2281) Administered Prior to Surgery in the Molecular Profile of Resectable Urothelial Bladder Cancer: NEODURVARIB Trial’. J Clin Oncol (2020) 38:542–42. doi: 10.1200/JCO.2020.38.6_suppl.542
32. Grivas P, Puligandla M, Cole S, Courtney KD, Dreicer R, Gartrell BA, et al. ‘PrE0807 Phase Ib Feasibility Trial of Neoadjuvant Nivolumab (N)/lirilumab (L) in Cisplatin-Ineligible Muscle-Invasive Bladder Cancer (BC)’. J Clin Oncol (2019) 37:TPS4594–TPS94. doi: 10.1200/JCO.2019.37.15_suppl.TPS4594
33. Gupta S, Sonpavde G, . Weight CJ, McGregor BA, Gupta S, Maughan BL, et al. ‘Results From BLASST-1 (Bladder Cancer Signal Seeking Trial) of Nivolumab, Gemcitabine, and Cisplatin in Muscle Invasive Bladder Cancer (MIBC) Undergoing Cystectomy’. J Clin Oncol (2020) 38:439–39. doi: 10.1200/JCO.2020.38.6_suppl.439
34. Cathomas R, Petrausch U, Hayoz S, Schneider M, Schardt JA, Seiler R, et al. ‘Perioperative Chemoimmunotherapy With Durvalumab (Durva) in Combination With Cisplatin/Gemcitabine (Cis/Gem) for Operable Muscle-Invasive Urothelial Carcinoma (MIUC): Preplanned Interim Analysis of a Single-Arm Phase II Trial (SAKK 06/17)’. J Clin Oncol (2020) 38:499–99. doi: 10.1200/JCO.2020.38.6_suppl.499
35. Galsky MD, Daneshmand S, Chan KG, Dorff TB, Cetnar JP, O Neil B, et al. ‘Phase 2 Trial of Gemcitabine, Cisplatin, Plus Nivolumab With Selective Bladder Sparing in Patients With Muscle- Invasive Bladder Cancer (MIBC): HCRN GU 16-257’. J Clin Oncol (2021) 39:4503–03. doi: 10.1200/JCO.2021.39.15_suppl.4503
36. Hoimes CJ, Albany C, Hoffman-Censits J, Fleming MT, Trabulsi E, Picus J, et al. ‘A Phase Ib/II Study of Neoadjuvant Pembrolizumab (Pembro) and Chemotherapy for Locally Advanced Urothelial Cancer (UC)’. Ann Oncol (2018) 29:viii726–viii26. doi: 10.1093/annonc/mdy424.039
37. Rose TL, Harrison MR, Deal AM, Osterman CK, Ramalingam S, Whang YE, et al. ‘Phase II Study of Gemcitabine and Split-Dose Cisplatin Plus Pembrolizumab as Neoadjuvant Therapy Prior to Radical Cystectomy (RC) in Patients With Muscle-Invasive Bladder Cancer (MIBC)’. J Clin Oncol (2021) 39:396–96. doi: 10.1200/JCO.2021.39.6_suppl.396
38. Martinez Chanza N, Soukane L, Barthelemy P, Carnot A, Gil T, Casert V, et al. ‘Avelumab as Neoadjuvant Therapy in Patients With Urothelial non-Metastatic Muscle Invasive Bladder Cancer: A Multicenter, Randomized, non-Comparative, Phase II Study (Oncodistinct 004 - AURA Trial)’. BMC Cancer (2021) 21:1292–92. doi: 10.1186/s12885-021-08990-3
39. Thibault C, Elaidi R, Vano Y-A, Rouabah M, Braychenko E, Helali I, et al. ‘Open-Label Phase II to Evaluate the Efficacy of NEoadjuvant Dose-Dense MVAC In Combination With Durvalumab and Tremelimumab in Muscle-Invasive Urothelial Carcinoma: NEMIO’. Bull du Cancer (2020) 107:eS8–eS15. doi: 10.1016/S0007-4551(20)30281-2
40. Powles T, Meeks JJ, Galsky MD, van der Heijden MS, Nishiyama H, Al-Ahmadie HA, et al. ‘A Phase III, Randomized, Open-Label, Multicenter, Global Study of Efficacy and Safety of Durvalumab in Combination With Gemcitabine Plus Cisplatin for Neoadjuvant Treatment Followed by Durvalumab Alone for Adjuvant Treatment in Muscle-Invasive Bladder Cancer (NIAGARA)’. J Clin Oncol (2021) 39:TPS505–TPS05. doi: 10.1200/JCO.2021.39.6_suppl.TPS505
41. Sonpavde G, Necchi A, Gupta S, Steinberg GD, Gschwend JE, van der Heijden MS, et al. ‘ENERGIZE: A Phase III Study of Neoadjuvant Chemotherapy Alone or With Nivolumab With/Without Linrodostat Mesylate for Muscle-Invasive Bladder Cancer’. Future Oncol (London England) (2020) 16:4359–68. doi: 10.2217/fon-2019-0611
42. Siefker-Radtke AO, Steinberg GD, Bedke J, Nishiyama H, Fang X, Kataria R, et al. ‘Phase III Study of Perioperative Pembrolizumab (Pembro) Plus Neoadjuvant Chemotherapy (Chemo) Versus Placebo Plus Neoadjuvant Chemo in Cisplatin-Eligible Patients (Pts) With Muscle-Invasive Bladder Cancer (MIBC): KEYNOTE-866’. J Clin Oncol (2020) 38:TPS599–TPS99. doi: 10.1200/JCO.2020.38.6_suppl.TPS599
43. Petrylak DP, Flaig TW, Mar N, Gourdin TS, Srinivas S, Rosenberg JE, et al. Study EV-103 Cohort H: Antitumor Activity of Neoadjuvant Treatment With Enfortumab Vedotin Monotherapy in Patients (Pts) With Muscle Invasive Bladder Cancer (MIBC) Who are Cisplatin-Ineligible. J Clin Oncol (2022) 40:435–35. doi: 10.1200/JCO.2022.40.6_suppl.435
44. Galsky MD, Necchi A, Shore ND, Plimack ER, Jia C, Sbar E, et al. ‘KEYNOTE-905/EV-303: Perioperative Pembrolizumab or Pembrolizumab Plus Enfortumab Vedotin (EV) and Cystectomy Compared to Cystectomy Alone in Cisplatin-Ineligible Patients With Muscle-Invasive Bladder Cancer (MIBC)’. J Clin Oncol (2021) 39:TPS507–TPS07. doi: 10.1200/JCO.2021.39.6_suppl.TPS507
45. Powles T, Drakaki A, Teoh JY-C, Grande E, Fontes-Sousa M, Porta C, et al. ‘A Phase 3, Randomized, Open-Label, Multicenter, Global Study of the Efficacy and Safety of Durvalumab (D) + Tremelimumab (T) + Enfortumab Vedotin (EV) or D + EV for Neoadjuvant Treatment in Cisplatin-Ineligible Muscle-Invasive Bladder Cancer (MIBC) (VOLGA)’. J Clin Oncol (2022) 40:TPS579–TPS79. doi: 10.1200/JCO.2022.40.6_suppl.TPS579
46. Hoimes CJ, Bedke J, Loriot Y, Nishiyama H, Fang X, Kataria RS, et al. ‘KEYNOTE-B15/EV-304: Randomized Phase 3 Study of Perioperative Enfortumab Vedotin Plus Pembrolizumab Versus Chemotherapy in Cisplatin-Eligible Patients With Muscle-Invasive Bladder Cancer (MIBC)’. J Clin Oncol (2021) 39:TPS4587–TPS87. doi: 10.1200/JCO.2021.39.15_suppl.TPS4587
47. Necchi A, Anichini A, Raggi D, Briganti A, Massa S, Lucianò R, et al. Pembrolizumab as Neoadjuvant Therapy Before Radical Cystectomy in Patients With Muscle-Invasive Urothelial Bladder Carcinoma (PURE-01): An Open-Label, Single-Arm, Phase II Study. J Clin Oncol (2018) 36:3353–60. doi: 10.1200/JCO.18.01148
48. Bandini M, Gibb EA, Gallina A, Raggi D, Marandino L, Bianchi M, et al. Does the Administration of Preoperative Pembrolizumab Lead to Sustained Remission Post-Cystectomy? First Survival Outcomes From the PURE-01 Study☆. Ann Oncol (2020) 31:1755–63. doi: 10.1016/j.annonc.2020.09.011
49. Szabados B, Rodriguez-Vida A, Durán I, Crabb SJ, van der Heijden MS, Pous AF, et al. Toxicity and Surgical Complication Rates of Neoadjuvant Atezolizumab in Patients With Muscle-Invasive Bladder Cancer Undergoing Radical Cystectomy: Updated Safety Results From the ABACUS Trial. Eur Urol Oncol (2021) 4:456–63. doi: 10.1016/j.euo.2020.11.010
50. Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated With Immune Checkpoint Blockade. N Engl J Med (2018) 378:158–68. doi: 10.1056/NEJMra1703481
51. Das S, Johnson DB. Immune-Related Adverse Events and Anti-Tumor Efficacy of Immune Checkpoint Inhibitors. J ImmunoTher Cancer (2019) 7. doi: 10.1186/s40425-019-0805-8
52. Funt SA, Lattanzi M, Whiting K, Al-Ahmadie H, Quinlan C, Teo MY, et al. Neoadjuvant Atezolizumab With Gemcitabine and Cisplatin in Patients With Muscle-InvasiveBladder Cancer: A Multicenter, Single-Arm, Phase II Trial. J Clin Oncol (2022) 40(12):1312–22. doi: 10.1200/JCO.21.01485
53. Rosenberg JE, ODonnell PH, Balar AV, McGregor BA, Heath EI, Yu EY, et al. Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. J Clin Oncol (2019) 37:2592–600. doi: 10.1200/JCO.19.01140
54. Friedlander TW, Milowsky MI, Bilen MA, Srinivas S, McKay RR, Flaig TW, et al. Study EV-103: Update on Durability Results and Long Term Outcome of Enfortumab Vedotin + Pembrolizumab in First Line Locally Advanced or Metastatic Urothelial Carcinoma (La/mUC). J Clin Oncol (2021) 39:4528–28. doi: 10.1200/JCO.2021.39.15_suppl.4528
55. Necchi A, Raggi D, Bandini M, Gallina A, Capitanio U, Gandaglia G, et al. SURE: An Open Label, Sequential-Arm, Phase II Study of Neoadjuvant Sacituzumab Govitecan (SG), and SG Plus Pembrolizumab (Pembro) Before Radical Cystectomy, for Patients With Muscle-Invasive Bladder Cancer (MIBC) Who Cannot Receive or Refuse Cisplatin-Based Chemotherapy. J Clin Oncol (2021) 39:TPS506–TPS06. doi: 10.1200/JCO.2021.39.6_suppl.TPS506
56. Plimack ER, Dunbrack RL, Brennan TA, Andrake MD, Zhou Y, Serebriiskii IG, et al. Defects in DNA Repair Genes Predict Response to Neoadjuvant Cisplatin-Based Chemotherapy in Muscle-Invasive Bladder Cancer. Eur Urol (2015) 68:959–67. doi: 10.1016/j.eururo.2015.07.009
57. Teo MY, Seier K, Ostrovnaya I, Regazzi AM, Kania BE, Moran MM, et al. Alterations in DNA Damage Response and Repair Genes as Potential Marker of Clinical Benefit From PD-1/PD-L1 Blockade in Advanced Urothelial Cancers. J Clin Oncol Off J Am Soc Clin Oncol (2018) 36:1685–94. doi: 10.1200/JCO.2017.75.7740
58. Van Allen EM, Mouw KW, Kim P, Iyer G, Wagle N, Al-Ahmadie H, et al. Somatic ERCC2 Mutations Correlate With Cisplatin Sensitivity in Muscle-Invasive Urothelial Carcinoma. Cancer Discov (2014) 4:1140–53. doi: 10.1158/2159-8290.CD-14-0623
59. Miron B, Hoffman-Censits JH, Anari F, ONeill J, Geynisman DM, Zibelman MR, et al. Defects in DNA Repair Genes Confer Improved Long-Term Survival After Cisplatin-Based Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer. Eur Urol Oncol (2020) 3:544–47. doi: 10.1016/j.euo.2020.02.003
60. Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. Tgfβ Attenuates Tumour Response to PD-L1 Blockade by Contributing to Exclusion of T Cells. Nature (2018) 554:544–48. doi: 10.1038/nature25501
61. Kamoun A, de Reyniès A, Allory Y, Sjödahl G, Robertson AG, Seiler R, et al. A Consensus Molecular Classification of Muscle-Invasive Bladder Cancer. Eur Urol (2020) 77:420–33. doi: 10.1016/j.eururo.2019.09.006
62. Necchi A, de Jong JJ, Raggi D, Briganti A, Marandino L, Gallina A, et al. Molecular Characterization of Residual Bladder Cancer After Neoadjuvant Pembrolizumab. Eur Urol (2021) 80:149–59. doi: 10.1016/j.eururo.2021.03.014
Keywords: neoadjuvant, radical cystectomy (RC), immunotherapy, cisplatin-based chemotherapy, muscle invasive bladder cancer (MIBC)
Citation: Chu CE, Iyer G and Bochner BH (2022) Neoadjuvant Systemic Therapies in Bladder Cancer. Front. Urol. 2:890761. doi: 10.3389/fruro.2022.890761
Received: 06 March 2022; Accepted: 27 April 2022;
Published: 02 September 2022.
Edited by:
Stephen Boorjian, Mayo Clinic, United StatesReviewed by:
Alexandre Zlotta, University of Toronto, CanadaMohamad Moussa, Lebanese University, Lebanon
Copyright © 2022 Chu, Iyer and Bochner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bernard H. Bochner, Ym9jaG5lcmJAbXNrY2Mub3Jn
 Carissa E. Chu
Carissa E. Chu Gopa Iyer
Gopa Iyer Bernard H. Bochner1,4*
Bernard H. Bochner1,4*