
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Urol., 23 May 2022
Sec. Pediatric, Adolescent and Developmental Urology
Volume 2 - 2022 | https://doi.org/10.3389/fruro.2022.888400
Background: Hematuria is one of the common complaints of patients with kidney disease and often occurs concurrently with proteinuria. Hematuria caused by nutcracker syndrome (NCS) is relatively common, while hematuria caused by type I von Willebrand disease (VWD) is less common.
Case Presentation: A 12-year-old boy was admitted to our ward due to sudden gross hematuria that had lasted for 4 days. After admission, his gross hematuria lasted for 24 days without remission and was combined with nephrotic-range proteinuria. A series of blood biochemical and urine tests, as well as renal ultrasound, lower abdominal CT angiography, cystoscopy, kidney biopsy, and genetic testing, were completed, and he was eventually diagnosed with NCS combined with type 1 VWD. After nutritional support, oral angiotensin-converting enzyme inhibitor (ACEI), and plasma infusion treatment, gross hematuria and microhematuria disappeared, proteinuria turned negative, and there was no recurrence during the 10 months of follow-up.
Conclusions: NCS and/or VWD may exist in patients with hematuria, and bleeding disorders such as VWD should generally be on the list of suspected diagnoses in patients with hematuria.
Gross hematuria caused by nutcracker syndrome (NCS) is common, but hematuria caused by type 1 von Willebrand disease (VWD) is rare. Both type I VWD and NCS are challenging to clinically diagnose. A case of a pediatric patient whose NCS was combined with type 1 VWD was reported, a reminder to clinicians to pay attention to coexisting diseases when nutcracker syndrome was diagnosed.
A 12-year-old boy was initially referred to our ward due to sudden gross hematuria that had lasted for 4 days. Four days prior to referral, the child had new-onset gross hematuria without obvious inducement. This was accompanied by slight left lower abdominal pain, with no symptoms such as frequent urination, urgent urination, blood clot in the urine, fever, edema, rash, joint pain, or vomiting. He denied a history of hematological diseases, kidney diseases, or taking anticoagulant drugs. His grandfather had a history of kidney stones but denied a family history of hemorrhagic diseases. The patient’s body mass index was 19.4 kg/m2 with blood pressure (BP) 120/54 mmHg (the 90th percentile BP was 122/75 mmHg in children of the same age, sex and height (1)), and there were no other positive signs except for percussion pain in the left renal area. Laboratory investigations included the following: urine protein, 2+; urine red blood cells, > 200/high-power field (HPF); the ratio of dysmorphic red blood cells (RBCs), 70%; negative nitrite; absent casts; 24-hour urine protein quantification, 4994 mg/24 hr (126.9 mg/m2.h); serum albumin, 38 g/L (reference range 32–52 g/L); fibrinogen, 1.67 g/l (reference range 1.8-4 g/L); prothrombin time, activated partial thromboplastin time, thrombin time and D-dimer were normal; negative purified protein derivative (PPD) test and urine culture test. Other biochemical indexes were normal, including complete blood cell count, serum calcium, serum uric acid, C-reactive protein (CRP), C3, C4, anti-double-stranded DNA, anti-neutrophil cytoplasmic antibodies (ANCAs), anti-nuclear antibodies, anti-streptolysin O, urinary calcium/creatinine ratio, and 24 h urine calcium concentration. Dynamic changes of important clinical findings are presented in Supplementary Table 1. The results of a urine three-cup test showed that there were many RBCs in all three cups. Magnetic resonance urography (MRU) and abdominal computed tomography (CT) showed that the kidney was normal in shape and size, and hydronephrosis, masses, or stones were excluded. During hospitalization, the patient’s gross hematuria lasted for 24 days with no signs of remission. Moreover, the hematuria was complicated with nephrotic-range proteinuria (>40 mg/m2/h), but his serum albumin remained normal. Ultrasound (US) showed that the ratio of the inner diameter of the dilated part to the narrow part of the left renal vein (LRV) was 4.56 (0.73 cm vs 0.16 cm), and the ratio of the peak velocity (PV) of the LRV at the superior mesenteric artery (SMA) portion and at the renal hilar portion was 6.83 (112.7 cm/s vs 16.5 cm/s), suggesting that the LRV was compressed. CT angiography (CTA) examination revealed that the LRV was compressed between the abdominal aorta (AA) and SMA and presented a “beak sign” (Figure 1A). A sagittal view of the CTA showed that the angle between the AA and SMA was 19.2° (Figure 1B), which fulfilled the NCS diagnosis in CTA. Cystoscopy revealed left ureteral bleeding, which confirmed the diagnosis of NCS (Figure 1C). NCS was diagnosed on the 7th day of the illness. On the 16th day of the illness, due to persistent nephrotic-range proteinuria and the ratio of dysmorphic red blood cells in the morning urine routine reaching 70%, we performed a renal biopsy for the patient to rule out the possibility of coexisting glomerular disease. However, biopsy of the left kidney showed the following (Figure 2): (1) the pathological changes in renal tissue were slight; (2) there was no IgA deposition in the mesangial area; (3) the distribution of type IV collagens ɑ2 and ɑ5 was normal, as determined by immunofluorescence; and (4) there was a normal glomerular basement membrane (GBM) without abnormal splits and lamination, as shown by electron microscopy. The patient had been on bed rest since admission and given nutritional support since the diagnosis of NCS, gross hematuria persisted on the 21st day of illness (body mass index increased to 19.9 kg/m2 at the time), and the degree of hematuria did not change with body position. We have diagnosed NCS many times and have never seen such a patient with 24-hour indiscriminate excretion of hematuria, which prompted us to look for other possible causes of persistent hematuria. In unexplained persistent gross hematuria, tumors, vascular malformations, stones, infection and primary glomerular diseases are recognized as important differential diagnoses, as are hemorrhagic diseases (2). Although the patient denied a family history and previous history of hemorrhagic disease and the preliminary evaluation of coagulation function was normal, we also screened the related factors that could prolong the bleeding time. The platelet membrane glycoprotein IIb/IIIa was within the normal range. The von Willebrand factor (VWF) antigen level (VWF: Ag) was 87.10% (blood type O, normal range 42–140.8%). The ristocetin cofactor activity assay (VWF: RCo) was 27% (blood type O, normal range 40.3-125.9%). To confirm the diagnosis of VWD and further rule out inherited glomerular disease, genetic testing was performed on the 28th day of the illness. VWF heterozygous mutation was subsequently found in patients by whole-exome sequencing, and suspected pathogenic mutation was verified by Sanger sequencing (as shown in Figure 3). No suspicious or uncertain mutations in genes related to glomerular membrane collagen/proteinuria diseases were found. As a result, the patient was diagnosed with NCS combined with type 1 VWD (3, 4).
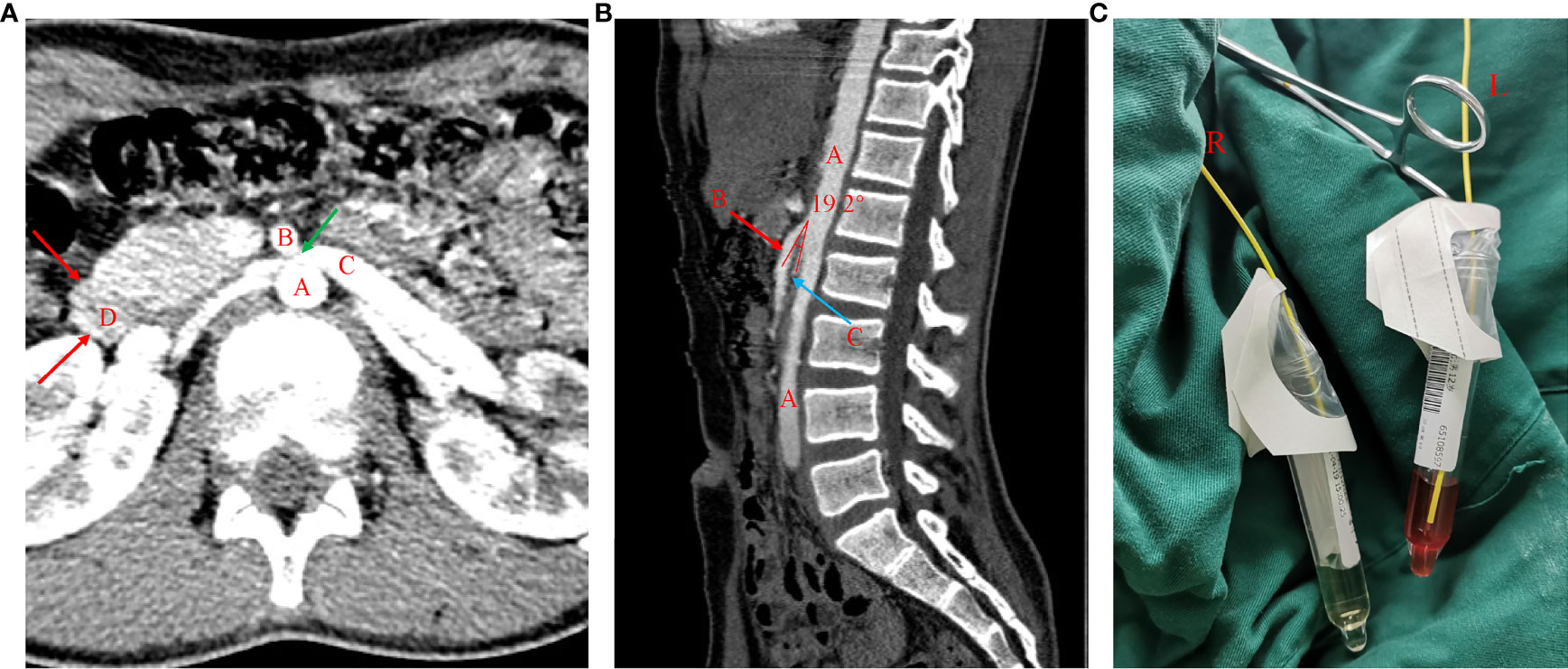
Figure 1 CTA imaging showing NCS and cystoscopy showed hematuria from the left ureter. (A) Axial view of the CTA chart: A= abdominal aorta (AA), B= superior mesenteric artery (SMA), C=left renal vein (LRV), D= duodenum. LRV was compressed between the AA and SMA and presented a “beak sign” (green arrow). (B) Sagittal view of the CTA chart: A= abdominal aorta (AA), B= superior mesenteric artery (SMA), C=left renal vein (LRV). The angle between the AA and SMA is less than 30° (measured at 19.2°), which fulfills the NCS diagnosis in CTA. (C) Cystoscopy showed hematuria from the left ureter.
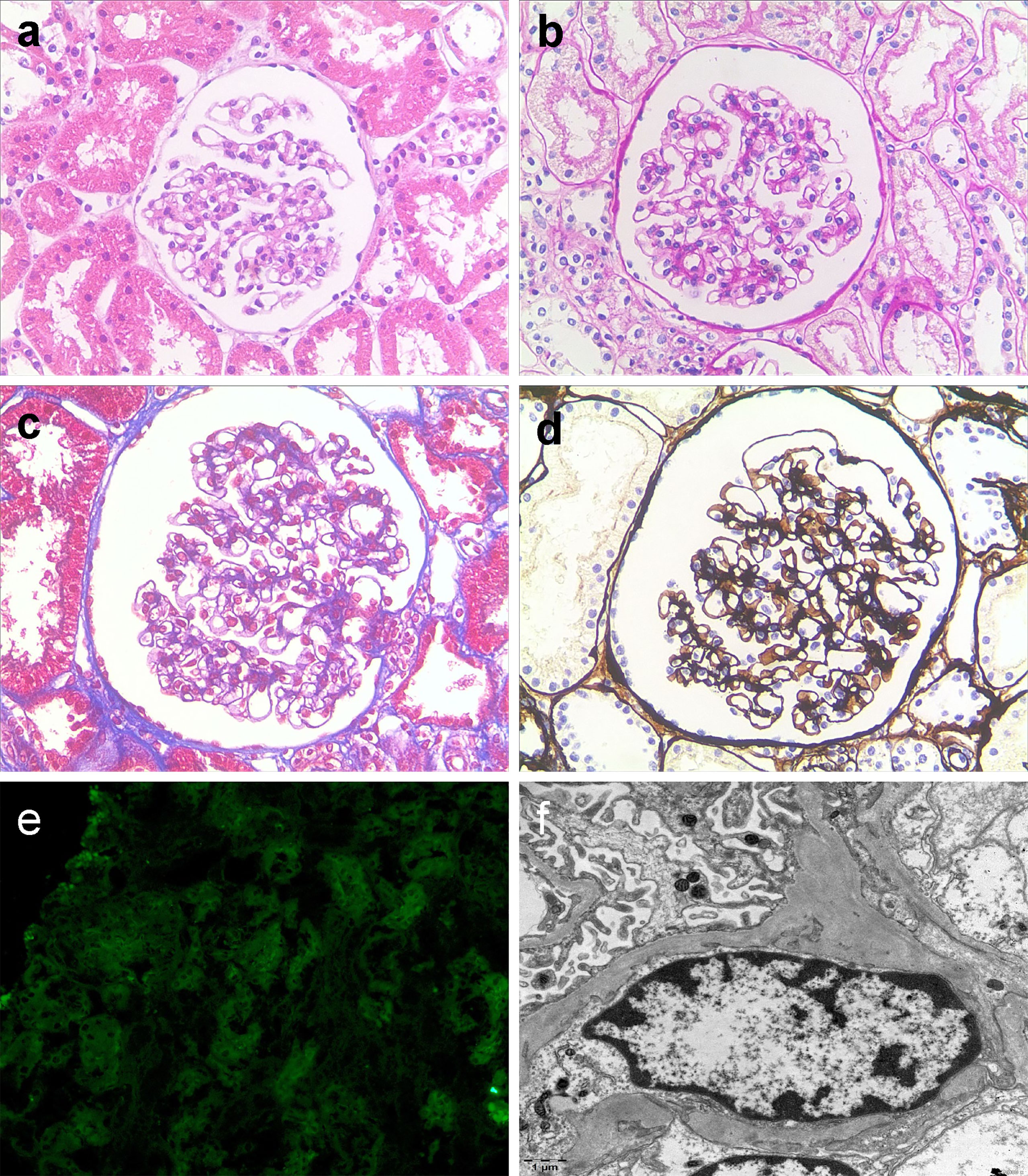
Figure 2 Light microscopic kidney biopsy of the patient (A–D, × 200) glomerular lesions were mild, some glomerular segmental mild mesangial hyperplasia, mild tubule degeneration with little interstitial inflammatory cell infiltration (a hematoxylin and eosin staining; b periodic acid-Schiff staining; c Masson staining; d periodic acid-silver methenamine. Fluorescent staining (E, × 200) no deposition of IgA. Electron microscopy (F) Normal GBM (the thickness of the basement membrane is 220-370 nm without abnormal splits and lamination), foot segmental fusion, and few electron dense deposits in the mesangial area.
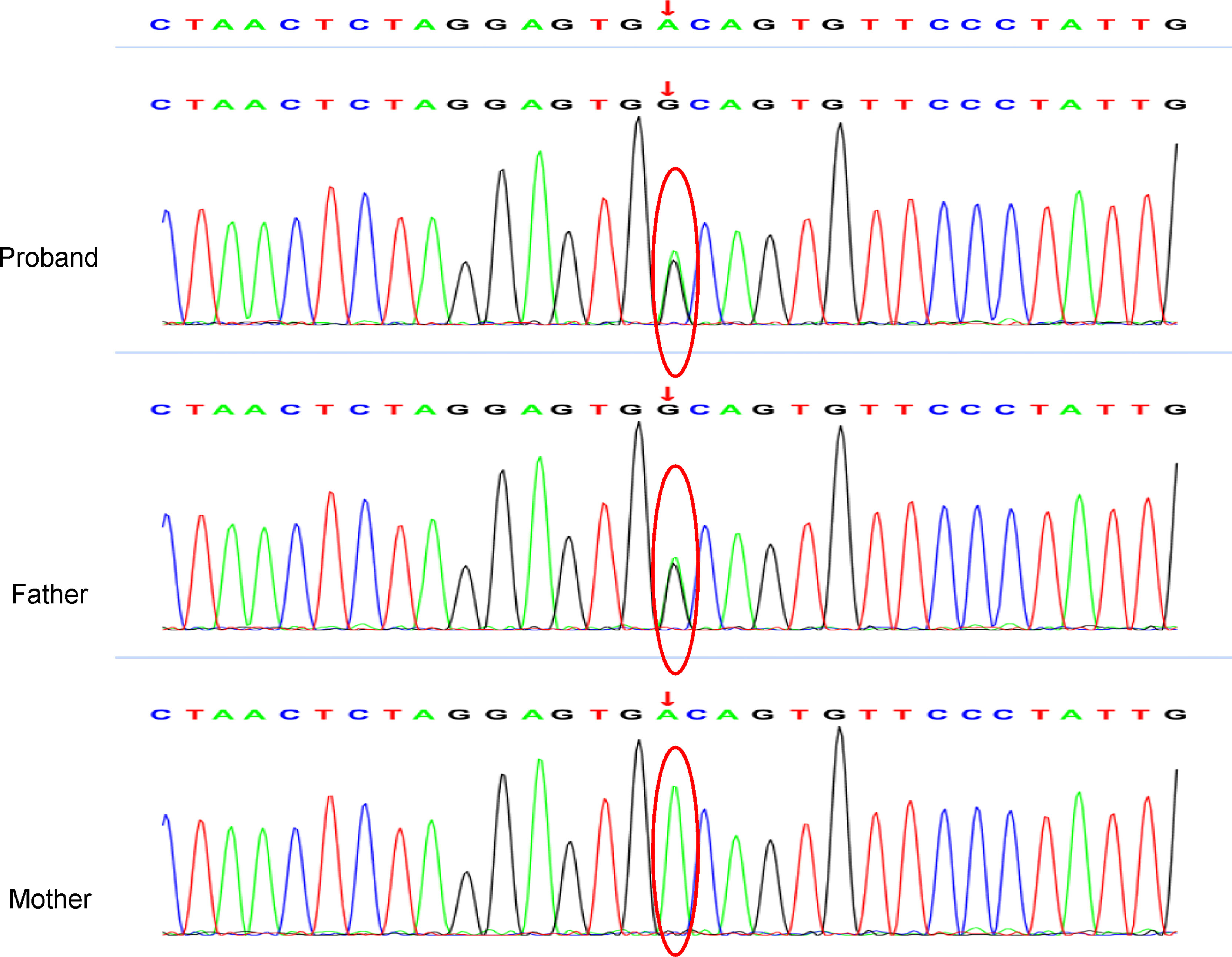
Figure 3 Whole-exome sequencing revealed a point mutation of VWF, and Sanger sequencing confirmed the variants chr12-6122806 and c.5461 exon 32 A > G. The variant was derived from his father.
There are three main ways to treat VWD: (1) oral or intravenous injection of antifibrinolytic drugs (aminocaproic acid and tranexamic acid) to promote hemostasis; (2) intravenous desmopressin to stimulate endothelial cells to release endogenous VWF; and (3) transfusion therapy with blood products rich in VWF (4). For economic and social reasons, intravenous desmopressin, antifibrinolytic drugs (tranexamic acid and aminocaproic acid) and commercial VWF concentrate were not available in our hospital, and plasma infusion therapy was adopted after obtaining informed consent from the patient’s parents. The regimen involved infusion of 400 mL of fresh frozen plasma per day for 2 days. On the 25th day of the course of the disease (3 days after plasma infusion, 20 days after resting on bed and nutritional support), the gross hematuria of the patient was lessened. On the 28th day, gross hematuria disappeared; along with it, proteinuria decreased (813 mg/24 h, 20.7 mg/m2.h), and the left lower abdominal pain of the patient ceased. On the 63rd day, the 24-hour urine protein quantification decreased to 216 mg (5.49 mg/m2.h), and there was no gross hematuria or microhematuria. The patient started taking enalapril (5 mg/day) on the 63rd day of of the illness. On the 91st day, the proteinuria was further reduced to 124 mg/24 h (3.15 mg/m2.h). Enalapril was discontinued after being taken for a total of 2 months, and there was no recurrence during the 10 months of follow-up.
Here, we describe an unusual case of NCS. The uniqueness of this case is not only the coexistence of NCS and VWD but also many controversial topics in the diagnosis and treatment of patients with NCS: (1) What are the diagnostic process and gold standard of NCS? (2) What is the mechanism of hematuria in NCS? Do dysmorphic RBCs be seen in the urine of NCS? (3) What is the mechanism of proteinuria in NCS? Does nephrotic-range proteinuria be seen in NCS? (4) What is the appropriate time of renal biopsy in patients with persistent hematuria and proteinuria for uncertain reasons? (5) What are the indications of surgical treatment for NCS? (6) Can ACEIs be used to treat orthostatic proteinuria related to NCS? (7) Should the screening of bleeding diseases (especially bleeding time, VWF activity test, platelet function test) be used as a routine clinical examination for patients with hematuria?
Although the terminology nutcracker phenomenon can be used interchangeably with left renal vein compression syndrome, this anatomical structure does not always cause clinical superficial signs. Therefore, the terminology NCS should be restricted to patients with typical clinical symptoms and signs, especially hematuria, low back pain, proteinuria, male varicocele, and female pelvic congestion (3). NCS is an important diagnosis of unexplained hematuria due to the lack of clear diagnostic procedures and standards and the possibility of the coexistence of other glomerular diseases (5–8), and its diagnosis is often delayed. The diagnosis of NCS is mainly based on imaging examinations of patients with typical symptoms and signs, including US, CTA, magnetic resonance angiography (MRA), and intravascular ultrasound (IVUS) (9). US is often the preferred screening method (10), and when the PV of the LRV at the AM portion is 5 times higher than that at the hilar portion, NCS can be diagnosed (11). To clarify the anatomical relationship between the LRV, aorta, and SMA and exclude vascular malformations and heterogeneous infiltrates, CTA or MRA is often recommended as a noninvasive examination (12, 13). Cystoscopy confirms the diagnosis of NSC by intuitive hematuria positioning (14). IVUS was once considered the gold standard for the diagnosis of NCS (15), and NCS can be diagnosed when the pressure gradient between the LRV and inferior vena cava (IVC) is greater than 3 mmHg (16). However, because IVUS is invasive, an increasing number of researchers recommend CTA examination as the gold standard for diagnosis (9, 17).
The pathological and physiological mechanisms of hematuria and proteinuria in NCS are still unclear. The most mainstream theory is that hematuria may be related to the increase in venous pressure after the LRV is compressed, which leads to the rupture of thin-walled venules and blood entering the collecting system (18, 19); proteinuria may be related to hemoglobin leakage caused by hematuria and orthostatic proteinuria (20). Some scholars believe that subclinical immune damage (21, 22) and increased release of endogenous norepinephrine and angiotensin II caused by LRV hypertension (23) (24)are involved in the formation of proteinuria in NCS. Based on the above theoretical point of view, hematuria caused by NCS is considered nonglomerular, so the RBCs in the urine of NCS patients should be isomorphic under the microscope. However, similar to our cases, dysmorphic RBCs have also been observed in the urine of NCS patients, but the mechanism is unknown (25). It has been thought that a proportion of dysmorphic RBCs in the urine greater than 50% is a sensitive marker of glomerular hematuria, the specificity of which is poor (26). The patient in our case manifested nephrotic-range proteinuria and dysmorphic RBCs more than 50% at the onset of hematuria, without manifestations of hypoalbuminemia, hyperlipidemia, and anemia. On the 16th day of the illness, to rule out glomerular disease, we performed a renal biopsy for the patient after obtaining informed consent from the family members. Subsequent pathological findings suggested that the patient’s nephrotic-range proteinuria was not caused by glomerular disease, so we speculated which was associated with hemoglobin leakage and increased endogenous angiotensin II caused by nutcracker syndrome. There are also sporadic case reports of nephrotic-range proteinuria in NCS patients (27–29). In regard to NCS treatment, conservative treatment (bed rest, nutritional support, oral ACEI) is the first recommended treatment for adolescent patients under 18 years of age. Because spontaneous relief of the disease in growing individuals is common, surgery can be considered for adolescent patients who have failed conservative treatment for 24 months (3). Although gross hematuria was severe, the patient’s parents insisted on choosing conservative treatments. Therefore, we did not consider surgical treatment for NCS during the treatment process.
VWD is an autosomal inherited hemorrhagic disease involving mutations in the VWF gene that cause abnormalities or a deficiency of VWF. It has a prevalence of approximately 100/million inhabitants for clinically relevant cases (30). As a multimeric adhesion protein, VWF plays an active role in primary hemostasis by assisting platelet adhesion to endothelial cells at the site of vascular injury and platelet-platelet interactions under high shear rate conditions. Three types of VWD have been identified: type 1 (partial VWF deficiency), type 2 (qualitative VWF deficiency), and type 3 (severe VWF deficiency) (31), of which type 1 VWD is the most common and therefore the most common hereditary bleeding disease (32). The major symptoms of type 1 VWD include skin bleeding, epistaxis, bleeding due to dental extraction, menorrhagia, postoperative bleeding, and bleeding from slight wounds (33). Patients with acquired VWD may experience recurrent hematuria (34). Unlike other coagulation factor deficiencies, VWD, particularly type 1 VWD, is notoriously difficult to diagnose (35). Initial hemostasis laboratory evaluations usually include the following: (i) a platelet count and complete blood cell count; (ii) partial thromboplastin time (PTT); (iii) prothrombin time; and (iv) optionally either fibrinogen level or thrombin time. In addition, if there is an obvious history of mucocutaneous bleeding, VWF activity screening (VWF: Ag, VWF: RCo and FVIII) is suggested at the first visit (4). At present, there are two main assays for assessing VWF activity: testing for total protein (VWF: Ag) and platelet binding function either by GPIb binding assays (VWF: GPIbM or VWF: GPIbR) or ristocetin cofactor activity assays (VWF: RCo) (4). Although plasma VWF levels are affected by blood type, hormones, age, and other factors, patients with VWF levels less than 30%, obvious bleeding symptoms, and either a positive family history or VWF gene mutation can be diagnosed with type 1 VWD (36). Most cases of VWD do not suffer severe spontaneous bleeding and are relatively mild. Therefore, except for type 3 VWD, hemorrhage, severe epistaxis, menorrhagia, and VWD with persistent bleeding risk factors (such as vascular dysplasia), prophylaxis is rarely needed (37).
In conclusion, although the combination of NCS and type 1 VWD was accidental in our case, the two diseases may exist in patients with hematuria. We suggest that bleeding disorders such as VWD should generally be on the list of suspected diagnoses in patients with hematuria.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Ethics Committee of the Children’s Hospital of Zhejiang University School of Medicine. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
LL collected clinical data, drafted the manuscript, and reviewed the literature. CF, WG, and JM completed a kidney pathological analysis for the patient. LZ performed cystoscopy for the patient. ZC completed the CTA examination and analysis for the patient. HS, HF, and JM treated the patient and contributed to the follow-up of the patient. JM modified the manuscript. All authors issued final approval for the version to be submitted. All authors contributed to the article and approved the submitted version.
This article is funded by the National Natural Science Foundation of China (Grant No. U20A20351). The funder helped in the preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank the patients and their parents for their kind cooperation and their contribution to this report.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fruro.2022.888400/full#supplementary-material
NCS, nutcracker syndrome; VWF, von Willebrand Factor;ACEI, angiotensin-converting enzyme inhibitor; VWD, Willebrand diseases; PV, peak velocity; US, ultrasound; IVC, inferior vena cava; IVUS, intravascular ultrasound.
1. Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics (2017) 140(3):1–72. doi: 10.1542/peds.2017-1904
2. Vedula R, Iyengar AA. Approach to Diagnosis and Management of Hematuria. Indian J Pediatr (2020) 87(8):618–24. doi: 10.1007/s12098-020-03184-4
3. Ananthan K, Onida S, Davies AH. Nutcracker Syndrome: An Update on Current Diagnostic Criteria and Management Guidelines. Eur J Vasc Endovasc Surg (2017) 53(6):886–94. doi: 10.1016/j.ejvs.2017.02.015
4. Nichols WL, Hultin MB, James AH, Manco-Johnson MJ, Montgomery RR, Ortel TL, et al. Von Willebrand Disease (VWD): Evidence-Based Diagnosis and Management Guidelines, the National Heart, Lung, and Blood Institute (Nhlbi) Expert Panel Report (USA). Haemophilia (2008) 14(2):171–232. doi: 10.1111/j.1365-2516.2007.01643.x
5. Shin JI, Park JM, Shin YH, Lee JS, Kim MJ, Jeong HJ. Nutcracker Syndrome Combined With IgA Nephropathy in a Child With Recurrent Hematuria. Pediatr Int (2006) 48(3):324–6. doi: 10.1111/j.1442-200X.2006.02212.x
6. Ma Z, Liu X, Ning Y, Shao J, Liu W, He X. Nutcracker Phenomenon in Combination With Glomerular Nephritis in Isolated Hematuria Patients. Int Urol Nephrol (2013) 45(3):809–16. doi: 10.1007/s11255-012-0265-2
7. Imai N, Shirai S, Shibagaki Y, Kimura K. Nutcracker Phenomenon in IgA Nephropathy. Clin Kidney J (2014) 7(3):325–6. doi: 10.1093/ckj/sfu030
8. Wang C, Wang F, Zhao B, Xu L, Liu B, Guo Q, et al. Coexisting Nutcracker Phenomenon and Superior Mesenteric Artery Syndrome in a Patient With IgA Nephropathy: A Case Report. Med (Baltimore) (2021) 100(28):e26611. doi: 10.1097/MD.0000000000026611
9. Kim SH. Doppler US and CT Diagnosis of Nutcracker Syndrome. Korean J Radiol (2019) 20(12):1627–37. doi: 10.3348/kjr.2019.0084
10. Kurklinsky AK, Rooke TW. Nutcracker Phenomenon and Nutcracker Syndrome. Mayo Clin Proc (2010) 85(6):552–9. doi: 10.4065/mcp.2009.0586
11. Kim SH, Cho SW, Kim HD, Chung JW, Park JH, Han MC. Nutcracker Syndrome: Diagnosis With Doppler Us. Radiology (1996) 198(1):93–7. doi: 10.1148/radiology.198.1.8539413
12. Fu WJ, Hong BF, Gao JP, Xiao YY, Yang Y, Cai W, et al. Nutcracker Phenomenon: A New Diagnostic Method of Multislice Computed Tomography Angiography. Int J Urol (2006) 13(7):870–3. doi: 10.1111/j.1442-2042.2006.01430.x
13. He Y, Wu Z, Chen S, Tian L, Li D, Li M, et al. Nutcracker Syndrome–How Well Do We Know It? Urology (2014) 83(1):12–7. doi: 10.1016/j.urology.2013.08.033
14. Ali-El-Dein B, Osman Y, Shehab El-Din AB, El-Diasty T, Mansour O, Ghoneim MA. Anterior and Posterior Nutcracker Syndrome: A Report on 11 Cases. Transplant Proc (2003) 35(2):851–3. doi: 10.1016/s0041-1345(02)04026-5
15. Ahmed K, Sampath R, Khan MS. Current Trends in the Diagnosis and Management of Renal Nutcracker Syndrome: A Review. Eur J Vasc Endovasc Surg (2006) 31(4):410–6. doi: 10.1016/j.ejvs.2005.05.045
16. Beinart C, Sniderman KW, Tamura S, Vaughan ED Jr, Sos TA. Left Renal Vein to Inferior Vena Cava Pressure Relationship in Humans. J Urol (1982) 127(6):1070–1. doi: 10.1016/s0022-5347(17)54230-5
17. Mathews R, Smith PA, Fishman EK, Marshall FF. Anomalies of the Inferior Vena Cava and Renal Veins: Embryologic and Surgical Considerations. Urology (1999) 53(5):873–80. doi: 10.1016/s0090-4295(99)00007-2
18. Beinart C, Sniderman KW, Saddekni S, Weiner M, Vaughan ED Jr, Sos TA. Left Renal Vein Hypertension: A Cause of Occult Hematuria. Radiology (1982) 145(3):647–50. doi: 10.1148/radiology.145.3.7146391
19. Lopatkin NA, Morozov AV, Lopatkina LN. Essential Renal Haemorrhages. Eur Urol (1978) 4(2):115–9. doi: 10.1159/000473926
20. Oteki T, Nagase S, Hirayama A, Sugimoto H, Hirayama K, Hattori K, et al. Nutcracker Syndrome Associated With Severe Anemia and Mild Proteinuria. Clin Nephrol (2004) 62(1):62–5. doi: 10.5414/cnp62062
21. Ekim M, Ozcakar ZB, Fitoz S, Soygur T, Yuksel S, Acar B, et al. The “Nutcracker Phenomenon” With Orthostatic Proteinuria: Case Reports. Clin Nephrol (2006) 65(4):280–3. doi: 10.5414/cnp65280
22. Devarajan P. Mechanisms of Orthostatic Proteinuria: Lessons From a Transplant Donor. J Am Soc Nephrol (1993) 4(1):36–9. doi: 10.1681/ASN.V4136
23. Mazzoni MB, Kottanatu L, Simonetti GD, Ragazzi M, Bianchetti MG, Fossali EF, et al. Renal Vein Obstruction and Orthostatic Proteinuria: A Review. Nephrol Dial Transplant (2011) 26(2):562–5. doi: 10.1093/ndt/gfq444
24. Ha TS, Lee EJ. ACE Inhibition can Improve Orthostatic Proteinuria Associated With Nutcracker Syndrome. Pediatr Nephrol (2006) 21(11):1765–8. doi: 10.1007/s00467-006-0206-3
25. Suzuki T, Imai N, Hisamichi M, Ichikawa D, Koike J, Shibagaki Y. Can Nutcracker Phenomenon Cause Glomerular Hematuria? Nephrol (Carlton) (2018) 23(5):495. doi: 10.1111/nep.13096
26. Zaman Z, Proesmans W. Dysmorphic Erythrocytes and G1 Cells as Markers of Glomerular Hematuria. Pediatr Nephrol (2000) 14(10-11):980–4. doi: 10.1007/s004670050057
27. Ozcakar ZB, Yalcinkaya F, Fitoz S, Cipe G, Soygur T, Ozdemir H, et al. Nutcracker Syndrome Manifesting With Severe Proteinuria: A Challenging Scenario in a Single-Kidney Patient. Pediatr Nephrol (2011) 26(6):987–90. doi: 10.1007/s00467-011-1793-1
28. Velasquez-Jones L, Medeiros M, Patino-Ortega M, Guerrero-Kanan R, Valadez-Reyes MT, Valverde-Rosas S, et al. Nutcracker Syndrome: Cause of Non-Glomerular Hematuria and Massive Proteinuria. Bol Med Hosp Infant Mex (2014) 71(5):298–302. doi: 10.1016/j.bmhimx.2014.10.001
29. Schoffel N, Liehr RM, Bunger C, Kruger K, Rubin D. Nephrotic Syndrome and Microhematuria in a Patient With Nutcracker Syndrome: Report of a Case and Review of the Literature. Internist (Berl) (2018) 59(6):608–14. doi: 10.1007/s00108-017-0350-9
30. Castaman G, Federici AB, Rodeghiero F, Mannucci PM. Von Willebrand’s Disease in the Year 2003: Towards the Complete Identification of Gene Defects for Correct Diagnosis and Treatment. Haematologica (2003) 88(1):94–108. doi: 10.3324/%25x
31. Sadler JE, Budde U, Eikenboom JC, Favaloro EJ, Hill FG, Holmberg L, et al. Update on the Pathophysiology and Classification of Von Willebrand Disease: A Report of the Subcommittee on Von Willebrand Factor. J Thromb Haemost (2006) 4(10):2103–14. doi: 10.1111/j.1538-7836.2006.02146.x
32. Bowman M, Hopman WM, Rapson D, Lillicrap D, James P. The Prevalence of Symptomatic Von Willebrand Disease in Primary Care Practice. J Thromb Haemost (2010) 8(1):213–6. doi: 10.1111/j.1538-7836.2009.03661.x
33. Tosetto A, Rodeghiero F, Castaman G, Goodeve A, Federici AB, Batlle J, et al. A Quantitative Analysis of Bleeding Symptoms in Type 1 Von Willebrand Disease: Results From a Multicenter European Study (MCMDM-1 VWD). J Thromb Haemost (2006) 4(4):766–73. doi: 10.1111/j.1538-7836.2006.01847.x
34. Leebeek FW, Eikenboom JC. Von Willebrand’s Disease. N Engl J Med (2016) 375(21):2067–80. doi: 10.1056/NEJMra1601561
35. Flood VH, Garcia J, Haberichter SL. The Role of Genetics in the Pathogenesis and Diagnosis of Type 1 Von Willebrand Disease. Curr Opin Hematol (2019) 26(5):331–5. doi: 10.1097/MOH.0000000000000524
36. Sadler JE, Rodeghiero F, ISTH SSC Subcommittee on von Willebrand Factor. Provisional Criteria for the Diagnosis of VWD Type 1. J Thromb Haemost (2005) 3(4):775–7. doi: 10.1111/j.1538-7836.2005.01245.x
Keywords: case report, hematuria, proteinuria, nutcracker syndrome, Von Willebrand disease
Citation: Li L, Feng C, Shen H, Zhu L, Fu H, Chen Z, Gu W and Mao J (2022) Case Report: An Unusual Case of Nutcracker Syndrome and Literature Review. Front. Urol. 2:888400. doi: 10.3389/fruro.2022.888400
Received: 02 March 2022; Accepted: 25 April 2022;
Published: 23 May 2022.
Edited by:
Kiarash Taghavi, Monash Children’s Hospital, AustraliaReviewed by:
Hulya Nalcacioglu, Ondokuz Mayıs University, TurkeyCopyright © 2022 Li, Feng, Shen, Zhu, Fu, Chen, Gu and Mao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianhua Mao, bWFvamg4OEB6anUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.