- 1Department of Medicine, Section of Human Anatomy, Clinical and Forensic, University of Perugia, School of Medicine, Perugia, Italy
- 2Andrology and Urogynecology Clinic, Santa Maria Terni Hospital, University of Perugia, Terni, Italy
- 3Department of Translational Research and New Technologies, Urology Unit, University of Pisa, Pisa, Italy
The aim of our study was to investigate the plasma NGF concentration and TrkA/p75NTR receptor expression on white blood cells (WBCs), in peripheral and corpus cavernosum blood isolated from patients with erectile dysfunction and metabolic syndrome (ED/MetS). This was a pilot case–control study. Inclusion criteria were as follows: men 18–65 years with ED and MetS and healthy subjects. The first sampling was performed at the level of the cubital vein (VC). Subsequently, 20 μg of intracavernous alprostadil was administered, and a second blood draw from the corpora cavernosa (CC) was performed once erection was achieved. Subsequently, the third blood sample was repeated at the level of the VC. We enrolled 8 cases with ED/MetS and 8 controls. There was no significant difference between the case and control group in terms of mean age (49.3 ± 5.9 and 53.13 ± 8.9, respectively). The case group had a lower IIEF score compared to the control group (14 ± 3.2 versus 27.3 ± 2.1; p < 0.05). Decreased NGF and TrKA expression on WBC and thiols were found in the plasma of ED/MetS patients compared to control. The study showed that patients with ED/MetS had a decrease in plasma NGF and thiol concentration, and they had a decrease in TrKA expression on WBCs.
Introduction
Erectile dysfunction (ED) is one of the most prevalent pathological conditions in adult male patients. It is defined as the “inability to achieve and/or maintain an erection sufficient to achieve satisfactory sexual intercourse” (1). ED is reported in 15% of men 40–50 years old, 45% of men in their 60s, and 70% of men over 70 years old (2). ED has many risk factors including increased body weight, hypertension, diabetes, insulin resistance, and dyslipidemia (3). The association of these risk factors determines a picture of metabolic syndrome (MetS).
MetS, in fact, is characterized by abdominal obesity, dyslipidemia [elevated triglycerides and low high-density lipoproteins (HDL)], elevation of arterial blood pressure, and dysregulated glucose homeostasis (4). The above conditions may cause endothelial dysfunction, arteriolosclerosis, and fibrous tissue, resulting in altered blood flow to the penis and therefore generate ED (5).
It is now known that diseases such as atherosclerosis, obesity, type 2 diabetes, and MetS are associated with an alteration in the levels of nerve growth factor (NGF) (6).
NGF belongs to the neurotrophin family, and it was first discovered for its effects on neuronal survival and differentiation (7). NGF exerts its biological functions by binding the high-affinity tyrosine kinase A receptor (TrKA) and the low-affinity pan-neurotrophin receptor p75 (p75NTR). Besides its classical role in neuronal growth and survival, NGF has shown pleiotropic effects on various populations of non-neuronal cells, including cancer and immune cells (8–10). NGF is well recognized to mediate multiple biological phenomena, ranging from neurotrophic through immunotrophic and epitheliotrophic to metabotropic effects (6).
Some studies explored the relationship between NGF and ED and are mostly done on animals (11–13). In fact, they are mostly dependent on rat models and lack large sample sizes, which has restricted further clinical application of cytokines such as NGF. However, it has been shown that the NGF/TrkA signaling pathway contributes to the erectile function and a downregulation in this signaling pathway may contribute to the pathogenesis of diabetic ED (11). Young and old rat penile tissues expressed mRNA transcripts for various NGFs (12). Furthermore, NGF treatment effectively improves type 2 diabetes-induced hypotestosterone and ED outcome through a mechanism that includes upregulation of key enzymes in testosterone biosynthesis. On the other hand, there are no studies on NGF and its receptors in patients with ED and MetS.
Materials and Methods
Study Participants
This is a case–control study. We included in the case group patients (age 18–65 years) with erectile dysfunction and metabolic syndrome (MetS/ED) evaluated at our Urology Clinic. ED was defined according to the terminology (1); the MetS was defined and diagnosed according to the Adult treatment Panel III criteria (14) [waist circumference (WC) >102 cm, elevated triglycerides (TGs) >150 mg/dl, reduced levels of high-density lipoprotein cholesterol (HDL) <40 mg/dl, high blood pressure (blood pressure >130/85 mmHg), and high fasting blood sugar (FBS) >110 mg/dl].
We excluded patients who received any treatment for ED in the last 6 months, patients with genital deformities, patients who have undergone pelvic surgery, patients with IIEF-5 score >21, and patients who did not adhere to the Adult treatment Panel III criteria. In the control group (CTRL), we included healthy subjects whose blood is present in our biobank.
Erectile function of patients was evaluated using the International Index of Erectile Function scoring system short form (IIEF-5) (15). All patients were evaluated with a detailed history, physical, and rectal examination. Height (cm), waist circumference (WC, cm), weight (kg), arterial blood pressure (ABP), and BMI of the patients were measured. WC was measured at the level of the umbilicus. In addition, patients had HDL cholesterol, triglycerides, and blood glucose measured within 48 h before study enrollment.
Their informed consent was obtained in accordance with the Declaration of Helsinki and as part of the protocol approved by our institutional ethical committee (CEAS Umbria 14373/18/AV).
Materials
In the description of the experimental phase if not otherwise specified, all chemicals were purchased from Sigma Aldrich (USA).
Blood Withdrawal and Plasma Collection
Three types of blood withdrawal were done: from the cubital vein (CV), from cubital vein after alprostadil (ALP) injection (CV+ALP), and from corpus cavernosum (CC) after ALP (CC+ALP).
The participants were placed in a supine position and blood sample from CV was withdrawn in vacuum tubes (Vacutainer) containing EDTA. Subsequently, an intracavernosal injection (ICI) with ALP 20 μg was performed using a 29-gauge, ½” needle on a 50-unit syringe. The clinician grasps the glans and gently stretches the penis away from the patient’s body so it is taut. The area to be injected is then cleansed with an alcohol pad. The prescribed dose is injected into the penile shaft at 2 o’clock or 10 o’clock with the needle injected up to the hub. Subsequently, there was a 10- to 15-min waiting period until there was >60% stiffness reported by the patient using the Erectile Hardness Scale (EHS) by making a score of 3 or 4. Patients who did not achieve a score of 3 or 4 were excluded from the study (16). Blood CV+ALP sampling was performed as previously described, and endocavernous venous sampling was performed using a 19-G × ¾” butterfly needle on a 10-ml sterile syringe.
Plasma was obtained after centrifugation at 4°C, 400 g, for 10 min and stored at −80°C until analysis.
In Vivo Study: Blood Cells Isolation
After lysis of erythrocytes in isotonic NH4Cl solution (155 mM NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA, pH 7.4) for 10 min on ice, blood samples obtained from different withdrawals were centrifuged at 400 g for 5 min. Supernatant was discarded and cells were suspended and washed three times in Dulbecco’s phosphate-buffered saline (PBS) (Gibco BRL) and similarly centrifuged. Finally, supernatant was discarded and cells were resuspended in PBS supplemented with 0.5% BSA (PBS/BSA).
In Vitro Study: PMBC Isolation and Alprostadil Treatments
Peripheral mononuclear blood cells (PBMCs) were isolated from buffy coats by density-gradient centrifugation. Briefly, 10 ml of blood was layered on 5 ml of Ficoll and centrifuged for 25 min at 400 g. PBMCs were collected, washed two times with RPMI (Invitrogen) supplemented with 5% fetal bovine serum (FBS) (Invitrogen), and resuspended in the medium at a final concentration of 1.5×106 cells/ml.
ALP (Cayman) stock solution was prepared by dissolving ALP in ethanol (EtOH) and diluting the solution in medium to a final concentration of 20 mg/ml and 20 pg/ml.
For all experiments, PMBCs were seeded in duplicates in 24-multiwells (Corning), at a density of 1.5×106 cells/ml. ALP, or its vehicle ethanol, was then added to the culture medium at the indicated concentrations, and cells were further incubated for 5, 15, 30, 45, and 60 min at 37°C in a humidified 5% CO2 atmosphere. The EtOH concentration in the culture medium never exceeded 0.1%.
TrKA and p75NTR Flow Cytometry Immunofluorescent Staining
Fresh PBMCs treated or not with ALP and cells isolated from blood of CV and CC before or after ALP administration were resuspended in PBS/BSA at a final concentration of 1×106 cells/ml. All procedures were performed at 4°C. The following fluorochrome-labeled mAbs were used to identify TrKA and p75NTR receptors on WBCs: FITC-labeled rabbit anti-human p75NTR extracellular domain (#ANT-007-F, Alomone Labs) and PE-labeled mouse anti-human TrKA (FAB1751P, R&D Systems). All FACS reagents were used at the concentration recommended by the manufacturers. Cells were then washed, resuspended in staining buffer (PBS +2% FBS +1% paraformaldehyde), and analyzed by FACS.
Flow Cytometry Analysis
A FACS Calibur (Becton Dickinson, Italy) equipped with a single 15-mW argon ion laser operating at 488 and 633 nm and interfaced with CellQuest Software (Becton Dickinson) was used. Samples were run using isotype controls or single fluorochrome-stained preparations for color compensation. The results were expressed as a percentage of positive cells/antibody used for staining (% positive cells) and as mean fluorescent intensity (MFI).
NGF ELISA Assay
Plasma and culture medium NGF levels were detected by a specific ELISA Kit (R&D Systems), according to the manufacturer’s instructions. The amount of NGF in each plasma and culture medium sample was determined from the standard curve supplied by the manufacturer. All samples were run in triplicate and the values were averaged. Plasma or culture medium-NGF concentration was expressed in pg/ml (detection limit, 7.8 pg/ml) or pg/106 cells, respectively.
Total Thiol Analysis
Colorimetric analysis of total thiol was carried out with Ellman’s assay; briefly, 0.5 mg of test sample proteins in triplicate was mixed with the Ellman reagent [5,5′-dithiobis (2-nitrobenzoic acid)] suspended in PBS, pH 7.5, to a final concentration of 200 μM. After incubation for 5 min at 37°C, the absorbance was measured at 412 nm and the final concentration of thiols was calculated against a calibration curve of glutathione.
Statistical Analysis
Results were expressed as means ± standard deviation (SD). The normality of the variables was checked by Kolmogorov–Smirnov tests. The comparison of the two experimental groups was done using Student’s t-test. Multiple group comparisons were performed using one-way analysis of variance (ANOVA) followed by Bonferroni multiple comparison tests. Statistical significance was set at p < 0.05. Statistical analysis was performed using GraphPad Prism 5 software.
Results
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
Subjects
In this study, the blood samples of 8 patients with ED have been analyzed and the samples of 8 healthy subjects were preserved in our biobank.
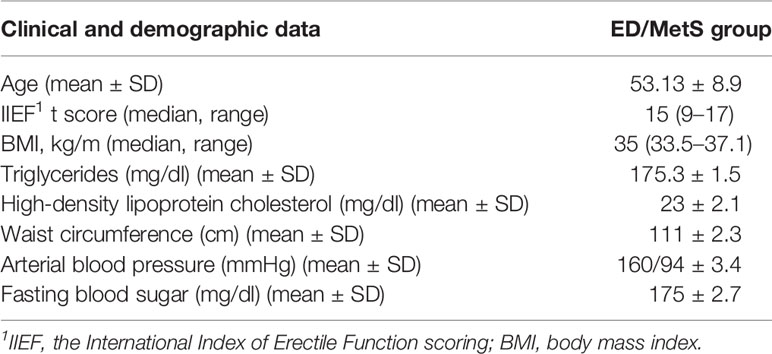
There was no significant difference between the control and ED/MetS groups in terms of mean age (49.3 ± 5.9 and 53.13 ± 8.9, p = 0.08). Table 1 shows the clinical and demographic data of the cases.
Alprostadil Did Not Modify NGF Release and Expression of Its Receptor in PBMC
ELISA and FACS analysis showed that the two ALP concentrations (20 μg/ml and 20 pg/ml) did not induce any significant differences in medium NGF levels and in the percentage of TrKA+- and p75NTR+-PBMC compared to untreated cells, at all times investigated (Figures 1A–C).
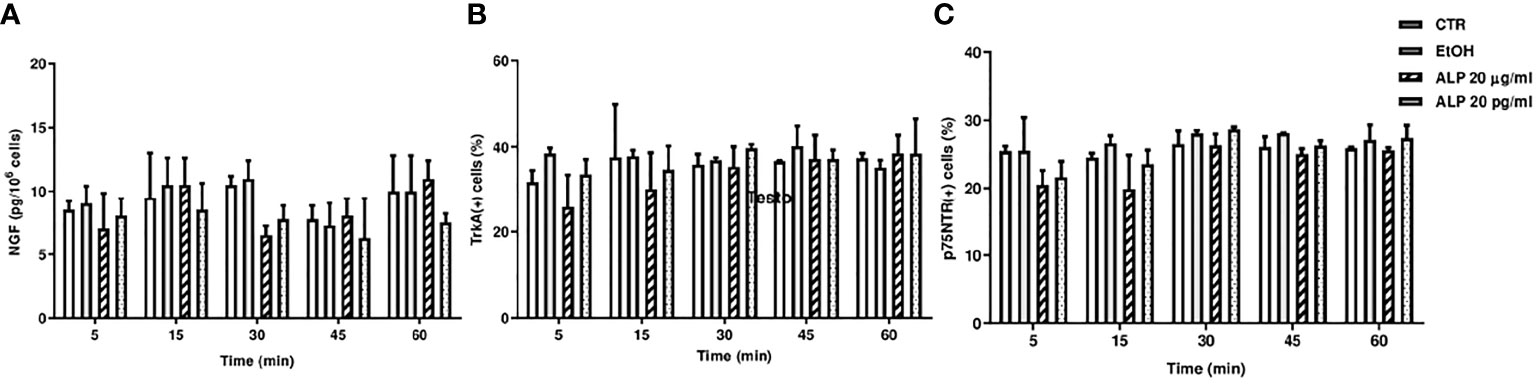
Figure 1 (A) Medium NGF levels in PBMC-treated (20 μg/ml or 20 pg/ml of ALP) or untreated cells. Description of what is contained in the first panel; (B) TrKA and (C) p75NTR Facs analysis of PBMC-treated (20 μg/ml or 20 pg/ml of ALP) or untreated cells. CTR, control; EtOH, alprostadil in ethanol; ALP, alprostadil.
Plasma NGF Concentration Decreased in ED Patients
Decreased NGF levels were found in the plasma of ED patients compared to CTRL. Subgrouping the ED patients by blood withdrawal, we found a decreased NGF levels in CV-ED (36.77 ± 10.80 pg/ml) in CV-ED+ALP (36.58 ± 11.17 pg/ml), and in CC-ED (44.47 ± 19.07 pg/ml) compared to CV-CTRL (78.02 ± 9.54 pg/ml) (p > 0.001). No significant difference was found between the ED blood withdrawals (Figure 2).
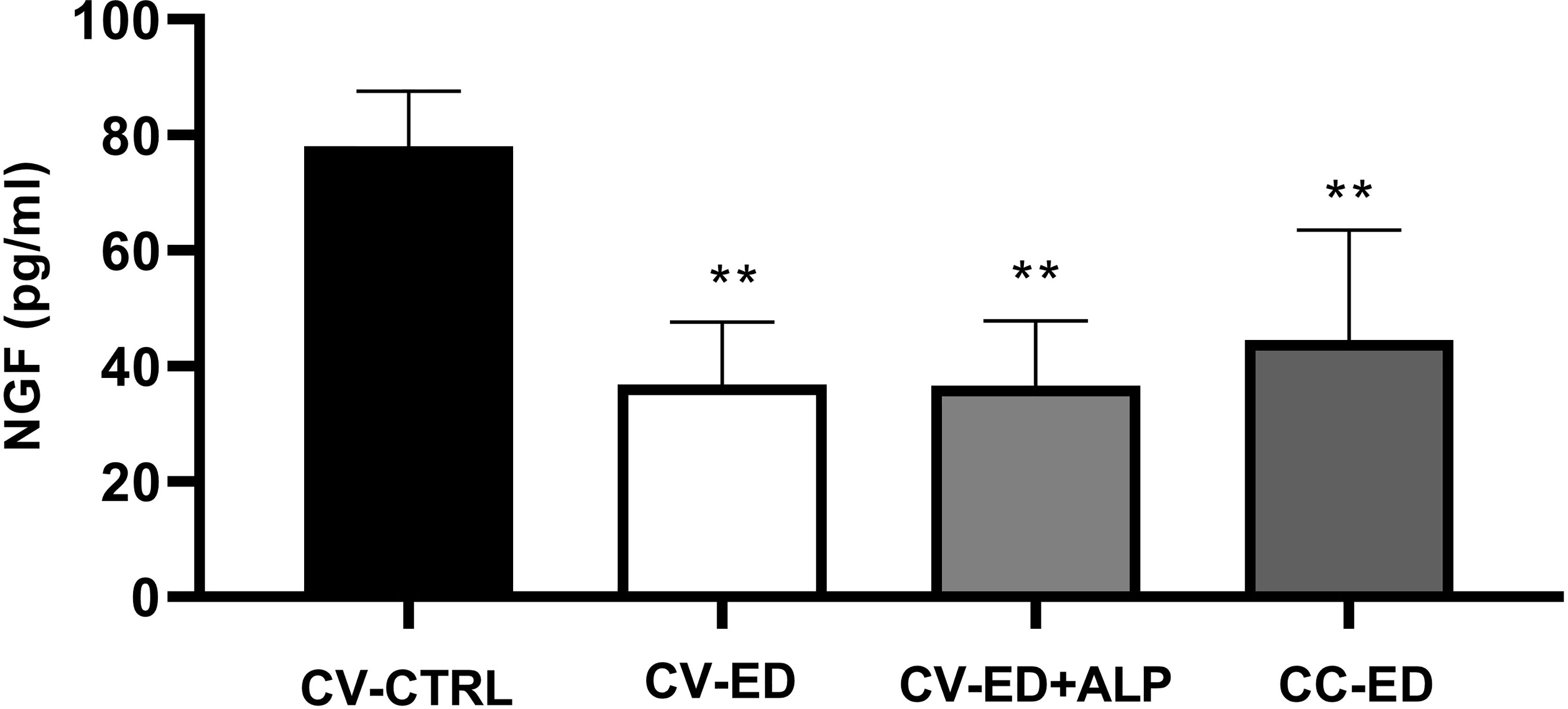
Figure 2 Plasma NGF levels in blood withdrawals of ED patients. Cubital vein of healthy subjects (CV-CTRL), cubital vein (CV) or corpus cavernosum (CC) of ED patients after Alprostadil (ALP) injection (CV+ALP), and CC after ALP (CC+ALP). Horizontal bars indicate mean values. ∗∗p < 0.001. Comparisons between the groups were made using one-way ANOVA followed by Bonferroni multiple comparison tests.
TrKA+ Cells, But Not p75NTR+ Cells Decreased in ED Patients
In CV and CC blood of ED patients, we found a significant decrease in the percentage of TrKA+ cells compared to CTRL. In particular, subgrouping the ED patients by blood withdrawal, we found a significant decrease in the percentage of TrKA+ cells and MFI in CV-ED (36.56 ± 4.46%, MFI 13.94 ± 6.8) in CV-ED+ALP (33.60 ± 7.17%; MFI 12.07 ± 1.22), and in CC-ED (30.19 ± 8.47%; MFI 11.27 ± 3.27) compared to CV-CTRL (53.57 ± 2.14%; MFI 21.54 ± 6.82 pg/ml) (p > 0.001) (Figures 3A, B). No significant difference was found between the ED blood withdrawals.

Figure 3 FACS analysis of TrKA in blood withdrawals of ED patients. (A) Percentage of TrKA+ cells; (B) MFI: mean fluorescent intensity. Cubital vein of healthy subjects (CV-CTRL), cubital vein (CV) or corpus cavernosum (CC) of ED patients after Alprostadil (ALP) injection (CV+ALP), and CC after ALP (CC+ALP). Horizontal bars indicate mean values. ***p < 0.0001; **p < 0.001; *p < 0.01. Comparisons between the groups were made using one-way ANOVA followed by Bonferroni multiple comparison tests. CV-CTRL.
Furthermore, no significant difference was found in the percentage and in MFI p75NTR in all blood withdrawals examined (data not shown).
Thiol Levels Decreased in ED Patients
Decreased thiol levels were found in the plasma of ED patients compared to CTRL. Subgrouping the ED patients by blood withdrawal, we found decreased thiol levels in CV-ED (54.58 ± 21.67 mM/mg protein), in CV-ED+ALP (45.99 ± 27.61 mM/mg protein), and in CC-ED (49.73 ± 21.68 mM/mg protein) compared to CV-CTRL (178.40 ± 24.16 mM/mg protein) (p > 0.001). No significant difference was found between the ED blood withdrawals (Figure 4A).
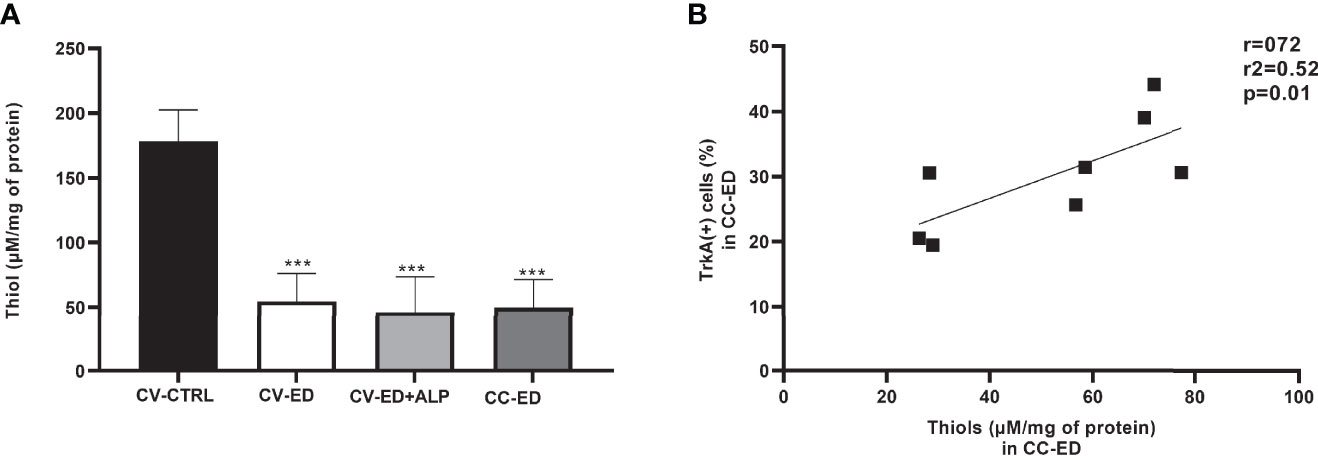
Figure 4 (A) Colorimetric analysis of total thiol in plasma of ED patients. Cubital vein of healthy subjects (CV-CTRL), cubital vein (CV) or corpus cavernosum (CC) of ED patients after alprostadil (ALP) injection (CV+ALP), and CC after ALP (CC+ALP). Horizontal bars indicate mean values. ***p < 0.0001. Comparisons between the groups were made using one-way ANOVA followed by Bonferroni multiple comparison tests. (B) Correlation between TrKA+ cells and thiol levels in CC-ED. r, Pearson index; r2, coefficient of determination.
Linear regression analysis showed a positive correlation between plasma thiol concentrations and the percentage of TrKA+ cells in CC blood (r = 0.72, r2 = 0.52; p = 0.01) (Figure 4B).
Discussion
This study showed that in men with ED, there was a decrease in plasma of NGF concentration, a decrease of TrKA expression on CV and CC blood cells, and a decrease in plasma of thiol levels, compared to healthy subjects. Furthermore, for the first time, we report an important correlation in ED patients between thiol levels and an increase in the percentage of TrKA+ CC cells. No differences were found in p75NTR expression in all blood withdrawal examined. To exclude possible interference by alprostadil injection during withdrawal from corpus cavernosum blood, we treated, in an in vitro study, PMBCs with different concentrations of alprostadil: 20 mg/ml was the injection concentration and 20 pg/ml was the maximum concentration revealed in the blood of corpus cavernosum after the administration of 20 mg/ml (17). At all times investigated, no differences were found in NGF medium level and in TrKA and p75NTR expression in PBMCs at all concentrations used.
The role of NGF in patients with MetS is well known. Chaldakov et al. found that the circulating plasma levels of NGF were lower in patients with MetS compared to the levels found in the controls (p < 0.05). This result could be explained by the metabotrophic role of the NGF and by the reduction of synthesis/accelerated degradation of NGF vascular tissue (6).
In literature, many studies investigated the role of the TrKA/NGF pathway in diabetic ED, above all in ED diabetic rats and not always with plasma investigations; the results of which, however, can be taken into consideration given the common pathophysiology between ED/MetS and diabetic ED (11, 18–20).
Bleustein et al. reported that in diabetic patients, the dysfunction of the penile nerves was observed earlier than other peripheral nerves (20). Dai et al. investigated, instead, the content of NGF in vitro, in penis of rats with diabetic ED (18). They report that in the advanced stage of diabetes, the injury of pelvic splanchnic nerves could raise the levels of NGF and NGF receptors. However, these levels were not sufficient to regenerate the nerve fibers, and the ability of combination of NGF with NGF receptor may also be compromised (12). Wu et al., in a randomized clinical study and in vitro cell line experiments, confirmed these results, proving that the treatment by NGF could significantly improve the erectile function and the IIEF-5 score in diabetic male patients with sensorimotor polyneuropathy and ED (21). Probably the improvement of ED following intramuscular administration of NGF could indirectly testify to a decrease in plasma NGF levels. This may explain why our ED/MetS patients have lower plasma NGF levels compared to the control group.
Yi Hou et al. also evaluated also the role of TrkA in rat penile corpus cavernosum with diabetic ED, demonstrating that the TrkA and NGF levels were low and the activation of the NGF/TrkA signaling pathway becomes a key role to improve the erectile function (11).
NGF can influence the nitric oxide synthase (NOS) expression and the nitric oxide (NO) production, the principal mediator of penile erection; in turn, the NO can modulate NGF-mediated neurotrophic action (11, 22, 23). NOS induces the relaxation of cavernous and vascular tissue, while the endothelial nitric oxide synthase (eNOS) promotes the blood flow into erectile tissue and maintains erection (24). The lack of NO causes the constriction of the corpus cavernosum smooth muscle. The balance between relaxation and constriction is crucial to maintain erectile function.
Some previous studies found decreased total thiol levels in patients with ED, which indicates that thiols may play a role in ED (25). However, in the literature, there were no studies on thiol levels in blood of corpus cavernosum in patients with ED and MetS.
Oxidative stress is induced by imbalance between antioxidant molecules and reactive oxygen species (ROS) in favor of ROS (26). ROS causes a redox reaction oxidation, and it transforms a sulfur atom into disulfide (27). The increase in disulfides compared to thiols is the first stage of the oxidative damage. Thiol/disulfide homeostasis is important for antioxidant defense, apoptosis, and stabilization of protein chemical structures. The excess of disulfide is related with diabetes, cardiovascular disease (28), and MetS (29–32). Micoogullari et al. reported that thiol/disulfide homeostasis is altered also in ED, and this may be a factor in its pathophysiology. Our study, in accordance with Micoogullari, shows that the thiol levels decreased in patients with ED (25).
The strengths of our work were that the evaluations were made both on penile and peripheral blood; our patients were compared with a control group; the research was divided into two phases: an in vitro and an in vivo study. Furthermore, to our knowledge, this is the only study that evaluates NGF/TrkA, p75NTR and thiol levels in patients with ED and MetS.
The main limitations of our study were the reduced size of the sample and the lack of an evaluation of NGF/TrkA levels based on the severity of ED. Furthermore, another limitation of the study is the lack of blood sampling from the corpora cavernosa in the control group. It may be the subject of further studies in the future. Unfortunately, the limited sample is attributable to the difficulty of recruiting subjects willing to perform a cavernous sampling.
Conclusions
In conclusion, the patients with ED and MetS had low NGF/TrkA and thiol levels, as it happens in other diseases such as diabetes. These results suggest a promising perspective in the future urological practice, in particular by creating target therapies that could activate the deficient pathways.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) CEAS Umbria 14373/18/AV. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conceptualization: EC and MR. Methodology: AS, AP, and AZ. Software: FT. Validation: EI and FT. Formal analysis: FT. Investigation: FT and AZ. Data curation: EI. Writing—original draft preparation: AS, AP, FT, and EI. Writing—review and editing: AS, AP, AZ, and EI. Supervision: EC. Funding acquisition: EC. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by Medicine Department, University of Perugia. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Consensus N. Development Conference Statement. NIH Consensus Development Panel on Impotence. Int J Impot Res (1993) 4:181.
2. Illiano E, Trama F, Ruffo A, Romeo G, Riccardo F, Iacono F, et al. Shear Wave Elastography as a New, non-Invasive Diagnostic Modality for the Diagnosis of Penile Elasticity: A Prospective Multicenter Study. Ther Adv Urol (2021) 13:17562872211007978. doi: 10.1177/17562872211007978
3. Feldman HA, Johannes CB, Derby CA, Kleinman KP, Mohr BA, Araujo AB, et al. Erectile Dysfunction and Coronary Risk Factors: Prospective Results From the Massachusetts Male Aging Study. Prev Med (2000) 30:328–38. doi: 10.1006/pmed.2000.0643
4. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation (2009) 120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644
5. Leisegang K, Henkel R, Agarwal A. Obesity and Metabolic Syndrome Associated With Systemic Inflammation and the Impact on the Male Reproductive System. Am J Reprod Immunol (2019) 82:e13178. doi: 10.1111/aji.13178
6. Chaldakov G. The Metabotrophic NGF and BDNF: An Emerging Concept. Arch Ital Biol (2011) 149:257–63.
7. Sofroniew MV, Howe CL, Mobley WC. Nerve Growth Factor Signaling, Neuroprotection, and Neural Repair. Ann Rev Neurosci (2001) 24:1217–81. doi: 10.1146/annurev.neuro.24.1.1217
8. Arcidiacono P, Stabile AM, Ragonese A, Pistilli S, Calvieri U, Bottoni A, et al. Anticarcinogenic Activities of Sulforaphane Are Influenced by Nerve Growth Factor in Human Melanoma A375 Cells. Food Chem Toxicol (2018) 113:154–61. doi: 10.1016/j.fct.2018.01.051
9. Stabile AM, Marinucci L, Balloni S, Giuliani A, Pistilli A, Bodo M, et al. Long Term Effects of Cigarette Smoke Extract or Nicotine on Nerve Growth Factor and its Receptors in a Bronchial Epithelial Cell Line. Toxicol In Vitro (2018) 53:29–36. doi: 10.1016/j.tiv.2018.07.020
10. Stabile A, Pistilli A, Crispoltoni L, Montagnoli C, Tiribuzi R, Casali L, et al. A Role for NGF and Its Receptors Trka and P75ntr in the Progression of COPD. Biol Chem (2016) 397:157–63. doi: 10.1515/hsz-2015-0208
11. Hou Y, Jia L, Zhang Y, Ji W, Li H. Activation of the Ngf/Trka Signaling Pathway Attenuates Diabetic Erectile Dysfunction. Oncotarget (2017) 8:105692. doi: 10.18632/oncotarget.22389
12. Dahiya R, Chui R, Perinchery G, Nakajima K, Oh B, Lue T. Differential Gene Expression of Growth Factors in Young and Old Rat Penile Tissues is Associated With Erectile Dysfunction. Int J Impot Res (1999) 11:201–6. doi: 10.1038/sj.ijir.3900405
13. Wang C, Liao C, Liu H, Lin J, Kuo H. Association of Urinary Nerve Growth Factor Levels With Erectile Function in Young Men With Type 2 Diabetes Mellitus. Int J Impot Res (2017) 29:101–4. doi: 10.1038/ijir.2017.2
14. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA (2001) 285:2486–97. doi: 10.1001/jama.285.19.2486
15. Rosen RC, Cappelleri J, Smith M, Lipsky J, Pena B. Development and Evaluation of an Abridged, 5-Item Version of the International Index of Erectile Function (IIEF-5) as a Diagnostic Tool for Erectile Dysfunction. Int J Impot Res (1999) 11:319–26. doi: 10.1038/sj.ijir.3900472
16. Mulhall JP, Goldstein I, Bushmakin A, Cappelleri J, Hvidsten K. Validation of the Erection Hardness Score. J Sex Med (2007) 4:1626–34. doi: 10.1111/j.1743-6109.2007.00600.x
17. Cawello W, Leonhardt A, Schweer H, Seyberth H, Bonn R, Lomeli AL. Dose Proportional Pharmacokinetics of Alprostadil (Prostaglandin E1) in Healthy Volunteers Following Intravenous Infusion. Br J Clin Pharmacol (1995) 40:273–6. doi: 10.1111/j.1365-2125.1995.tb05784.x
18. Dai Y, Chen Y, Yao L, Yang R, Sun Z, Wen D. Expression of Nerve Growth Factor in Cavernous Tissue and its Effects on the Treatment of Rats With Diabetic Erectile Dysfunction. Zhonghua Nan Ke Xue (2005) 11:748–751, 54.
19. Hecht MJB, Neundörfer B, Kiesewetter F, Hilz MJ. Neuropathy Is a Major Contributing Factor to Diabetic Erectile Dysfunction. Neurol Res (2001) 23:651–4. doi: 10.1179/016164101101198965
20. Bleustein C, Arezzo J, Eckholdt H, Melman A. The Neuropathy of Erectile Dysfunction. Int J Impot Res (2002) 14:433–9. doi: 10.1038/sj.ijir.3900907
21. Wu Y, Yang C, Meng F, Que F, Xiao W, Rao H, et al. Nerve Growth Factor Improves the Outcome of Type 2 Diabetes—Induced Hypotestosteronemia and Erectile Dysfunction. Reprod Sci (2019) 26:386–93. doi: 10.1177/1933719118773421
22. Sweni S, Meenakshisundaram R, Senthilkumaran S, Thirumalaikolundusubramanian P. Propofol’s Derivative: A Potential Drug for Erectile Dysfunction? Med Hypotheses (2011) 77:668–70. doi: 10.1016/j.mehy.2011.07.011
23. Binnington JC, Kalisch BE. Nitric Oxide Synthase Inhibitors Modulate Nerve Growth Factor-Mediated Regulation of Amyloid Precursor Protein Expression in PC12 Cells. J Neurochem (2007) 101:422–33. doi: 10.1111/j.1471-4159.2006.04378.x
24. Kim IG, Piao S, Lee JY, Hong SH, Hwang TK, Kim SW, et al. Effect of an Adipose-Derived Stem Cell and Nerve Growth Factor-Incorporated Hydrogel on Recovery of Erectile Function in a Rat Model of Cavernous Nerve Injury. Tissue Eng Part A (2013) 19:14–23. doi: 10.1089/ten.tea.2011.0654
25. Micoogullari U, Karatas OF, Kisa E, Keskin MZ, Atmaca AF, Neselioglu S, et al. Thiol/Disulfide Homeostasis in Patients With Erectile Dysfunction. J Sex Med (2020) 17:1934–41. doi: 10.1016/j.jsxm.2020.07.011
26. Ates I, Ozkayar N, Topcuoglu C, Dede F. Relationship Between Oxidative Stress Parameters and Asymptomatic Organ Damage in Hypertensive Patients Without Diabetes Mellitus. Scand Cardiovasc J (2015) 49:249–56. doi: 10.3109/14017431.2015.1060355
27. Cremers CM, Jakob U. Oxidant Sensing by Reversible Disulfide Bond Formation. J Biol Chem (2013) 288:26489–2696. doi: 10.1074/jbc.R113.462929
28. Erel O, Neselioglu S. A Novel and Automated Assay for Thiol/Disulphide Homeostasis. Clin Biochem (2014) 47:326–32. doi: 10.1016/j.clinbiochem.2014.09.026
29. Elmas B, Karacan M, Dervişoğlu P, Kösecik M, İşgüven ŞP, Bal C. Dynamic Thiol/Disulphide Homeostasis as a Novel Indicator of Oxidative Stress in Obese Children and Its Relationship With Inflammatory-Cardiovascular Markers. Anatol J Cardiol (2017) 18:361. doi: 10.14744/AnatolJCardiol.2017.7740
30. Qiao M, Zhao Q, Lee CF, Tannock LR, Smart EJ, LeBaron RG, et al. Thiol Oxidative Stress Induced by Metabolic Disorders Amplifies Macrophage Chemotactic Responses and Accelerates Atherogenesis and Kidney Injury in LDL Receptor-Deficient Mice. Arterioscler Thromb Vasc Biol (2009) 29:1779–86. doi: 10.1161/ATVBAHA.109.191759
31. Ustundag-Budak Y, Sambel M, Alisik M, Aydos M, Erel O, Oner S, et al. Thiol/Disulphide Homeostasis Levels in Erectile Dysfunction Patients. Andrologia (2017) 49:e12695. doi: 10.1111/and.12695
Keywords: erectile dysfunction, metabolic syndrome, NGF, TrKA, thiols
Citation: Stabile AM, Illiano E, Pistilli A, Rende M, Trama F, Bartolini D, Zucchi A and Costantini E (2022) The Role of NGF and Its Receptor TrKA in Patients With Erectile Dysfunction. Front. Urol. 2:860612. doi: 10.3389/fruro.2022.860612
Received: 23 January 2022; Accepted: 21 March 2022;
Published: 13 May 2022.
Edited by:
Giorgio Ivan Russo, University of Catania, ItalyReviewed by:
Celeste Manfredi, University of Campania Luigi Vanvitelli, ItalyCarla Braga Mano Gallo, Rio de Janeiro State University, Brazil
Copyright © 2022 Stabile, Illiano, Pistilli, Rende, Trama, Bartolini, Zucchi and Costantini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Trama, ZnJhbmNlc2NvdHJhbWFAZ21haWwuY29t
†These authors have contributed equally to this work
 Anna Maria Stabile
Anna Maria Stabile Ester Illiano
Ester Illiano Alessandra Pistilli1
Alessandra Pistilli1 Mario Rende
Mario Rende Francesco Trama
Francesco Trama Alessandro Zucchi
Alessandro Zucchi Elisabetta Costantini
Elisabetta Costantini