- Department of Urology, Yale School of Medicine, New Haven, CT, United States
Introduction: Genitourinary pathologies are increasingly common in low and lower-middle Q6 income countries (LMICs) however there is a disproportionate distribution of clinical trials in higher income countries as compared to LMICs. In order for evidence-based practice to be implemented in LMICs with regards to urologic conditions and malignancies, clinical trials need to be performed within LMICs such that the results can be analyzed based on the context of the local environment.
Methods: We queried ClinicalTrials.gov and the ICTRP for active clinical trials that were related to ‘prostatic neoplasms’, ‘urinary bladder neoplasms’, ‘kidney neoplasms’, ‘urolithiasis’, ‘urinary tract infections’ and ‘lower urinary tract symptoms’. The national incidence and DALYs was obtained from the Global Burden of Disease 2019 to analyze for a correlation between the numbers of clinical trials performed in a country with the burden of disease.
Results: A total of 4,169 clinical trials were identified based on the search query terms. Ninety percent of the clinical trials are being conducted in 32 countries. A majority of clinical trials are being performed in HICs. The proportion of non-oncologic urologic clinical trials performed in LMICs is greater than the proportion of urologic oncology clinical trials performed in LMICs (p <0.001). Linear regression models demonstrates a weak relationship between the global burden of disease and the number of clinical trials conducted in each country for the individual urologic conditions.
Discussion: A majority of urologic clinical trials are being conducted in high-income countries which does not coincide with the global burden of disease of urologic conditions.
Introduction
Urologic conditions have a significant impact on public health with regards to adverse quality of life and health outcomes for patients. Urologic diseases affect patients globally, with prostate and bladder cancer being the second and sixth most commonly diagnosed cancer in men globally in 2020 respectively (1). Other urologic conditions such as lower urinary tract symptoms, benign prostatic hyperplasia, nephrolithiasis and urinary tract infections have a high incidence among patients across the world (2–4). Clinical trials are paramount in promoting the growth of knowledge regarding the understanding and management of urologic conditions. These clinical trials help elucidate the efficacy, safety profile, and potential adverse effects of new therapies for these diseases (5).
Despite the global prevalence of all diseases, there is a significant disparity between the host sites of clinical trials, with approximately 83% of all clinical trials being performed in twenty-five countries (6). For countries classified by The World Bank as low and lower-middle income countries (LMICs), with a gross national income per capita of less than $4,096, less than 5% of all clinical trials were conducted in ninety-one LMICs at the time. Patients that are enrolled in clinical trials often are not representative of the population that the intervention is aimed at treating. The inconsistencies of conducting clinical trials in LMICs has previously been attributed to a lack of financial funding, migration of skilled personal to higher income countries, cultural apprehensions, and administrative regulations (7).
With regards to urologic diseases, there is a lack of knowledge regarding the specific disparity of the global distribution of ongoing clinical trials. A prior investigation found that the number of urologic clinical trials being conducted in the countries in the Middle East is lower than that as compared to studies being conducted in other countries (8). In this study, we compare the current distribution of ongoing clinical trials in LMICs with respect to urologic conditions.
Materials and methods
ClinicalTrials.gov is a registry database of public and privately funded clinical studies, with approximately 408,000 studies listed across 220 countries (9). The World Health Organization International Clinical Trials Registry Platform (ICTRP) is a publically available database that captures information regarding clinical trials from regional and national trial registries, and has been shown to include an increasing proportion of clinical trials that were not listed on ClinicalTrials.gov (10).
Using the filters available within ClinicalTrials.gov, search queries were performed to identify active clinical trials investigating urologic diseases. The search query on ClinicalTrials.gov included the terms “prostatic neoplasms, urinary bladder neoplasms, kidney neoplasms, urolithiasis, urinary tract infections, and lower urinary tract symptoms”. The search queries on the ICTRP included the terms “prostate cancer, bladder cancer, kidney cancer, kidney stones, urinary tract infections, and lower urinary tract symptoms” respectively.
On ClinicalTrials.gov, active interventional clinical trials which were in the “not yet recruiting, recruiting, enrolling by invitation, and active/not recruiting” were included. On the ICTRP, active international clinical trials that were listed in primary registries other than ClinicalTrials.gov were included. We did not exclude clinical trials on the basis of the trial’s recruitment of specific age groups or gender. All phases of clinical trials were included. From the search query, the country of the site of the clinical trial was obtained.
Geographic analysis was performed by stratifying countries based on the World Bank classification by gross national income per capita as either low income (less than $1,045), lower-middle income ($1,045 – $4,095), upper-middle ($4,096 - $12,695) or high income (greater than $12,695) (11). LMICs was defined as countries classified as low income or lower-middle income by the World Bank.
Data from the Institute for Health Metrics and Evaluation Global Burden of Disease 2019 was obtained to compare the number of clinical trials performed in countries by the incidence of the disease and the disability-adjusted life years (DALYs). A linear regression model was fit to evaluate for a correlation between burden of disease and number of clinical trials being conducted in a country.
The search queries were performed on March 20, 2022. Descriptive analysis was performed for all the queried terms. Two-sample z-test was used for statistical analysis. P-values were reported as two sided and values less than 0.05 were considered statistically significant. Analyses were performed with StataMP 17 (StataCorp, College Station, TX) and Microsoft Excel (Redmond, WA).
Results
Using the aforementioned search queries, a total of 15,216 studies were identified between ClinicalTrials.gov and the ICTRP, of which 4,169 trials were included in the analysis. A total of 11,047 trials were excluded of which 2,334 trials were excluded due a non-interventional study design, 6,775 trials were excluded as they were already completed, and 1,938 duplicate trials were identified and removed as they were listed in both ClinicalTrials.gov and ICTRP (Figure 1).
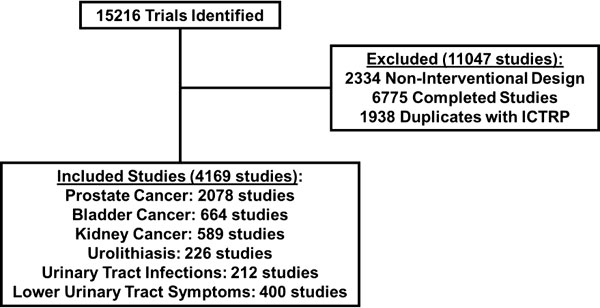
Figure 1 Selection of clinical trials for analysis obtained from search query on ClinicalTrials.gov and WHO ICTRP.
The included clinical trials obtained from the search queries are summarized in Table 1. The geographic distribution of clinical trials by country was visually represented using choropleth maps for each of the search query terms (Figure 2). Overall, the 4,169 clinical trials were conducted across 9,742 clinical trial sites in 92 different countries and 344 (3.53%) clinical trials were held in LMICs. The combined query for “prostatic neoplasms” and “prostate cancer” on ClinicalTrials.gov and ICTRP respectively was the most populated between the two registries and identified a total of 2,078 trials across 4,871 individual trial sites in 73 different countries. Of these trial sites, 942 (19.33%) are being performed in the United States while 89 (1.83%) are being performed in LMICs. In contrast, for clinical trials related to non-oncologic topics, the query for lower urinary tract symptoms between the two registries was the most populated, identifying 400 clinical trials across 564 clinical trials in 59 different countries. Of these trial sites, 119 (21.10%) are being performed in the United States while 47 (8.33%) are being performed in LMICs. Across the different search queries, LMICs represented a higher proportion of non-oncologic urologic clinical trials as compared to their representation of urologic oncology clinical trials (z = -16.05, p < 0.001). The number of countries needed to represent 33%, 50%, 67%, 75%, and 90% of the total number of clinical trials was reported in Table 2. Ninety percent of the clinical trials evaluated were conducted in 32 countries, with 85.58% of the clinical trials being conducted in 25 countries. Ninety percent of clinical trials related to prostate cancer were performed in 31 countries, related to bladder cancer were performed in 31 countries, related to kidney cancer were performed in 30 countries, related to urolithiasis were performed in 12 countries, related to urinary tract infections were performed in 40 countries, and related to lower urinary tract symptoms were performed in 31 countries. There are 109 countries that are not conducting a urologic clinical trial that is registered between the two registries.
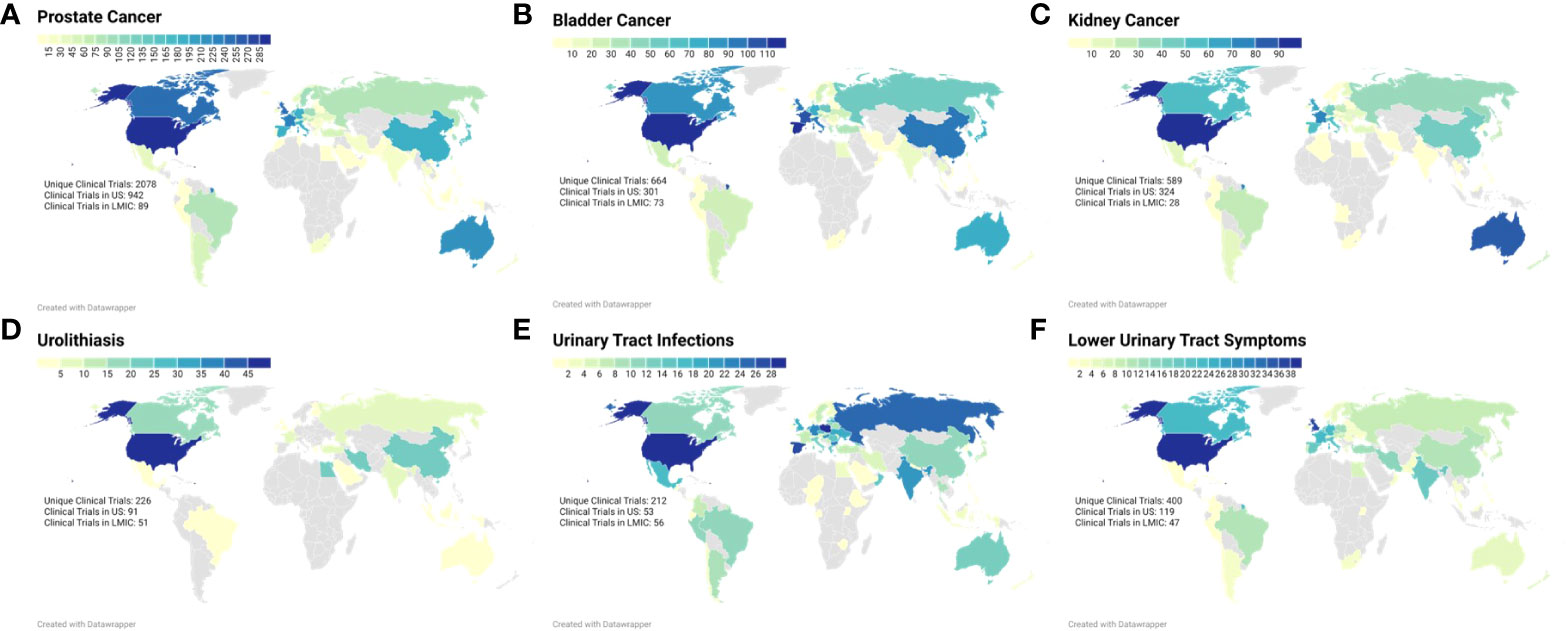
Figure 2 Choropleth maps demonstrating the active clinical trial sites within each country for each search query term: (A) Prostate Neoplasms (B) Urinary Bladder Neoplasms, (C) Kidney Neoplasms, (D) Urolithiasis, (E) Urinary Tract Infections, and (F) Lower Urinary Tract Symptoms.
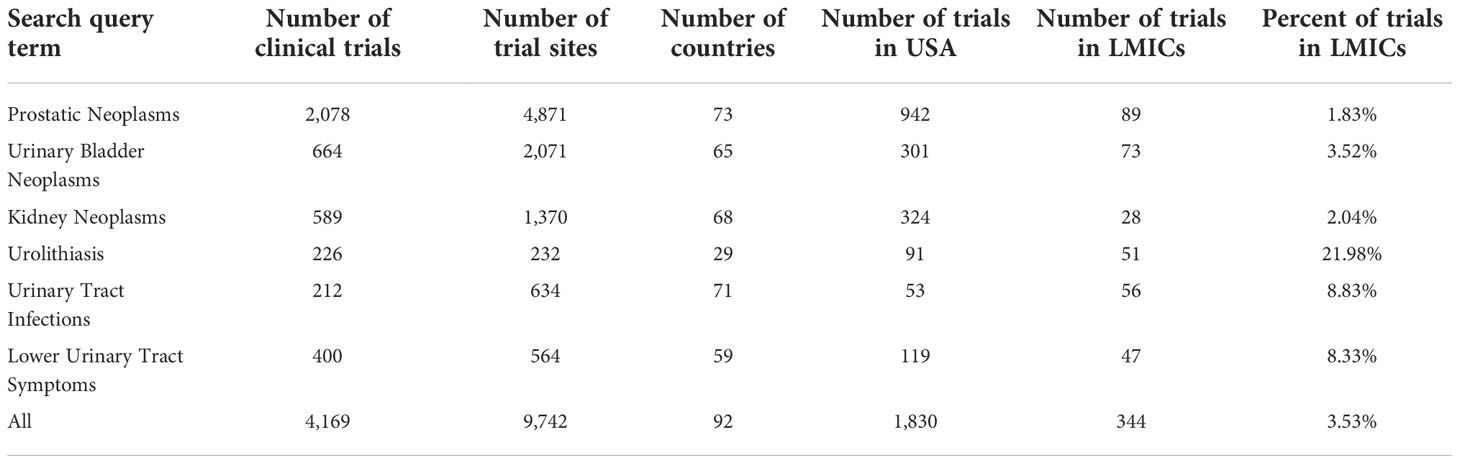
Table 1 Distribution of clinical trials being performed globally based on urologic search query terms.
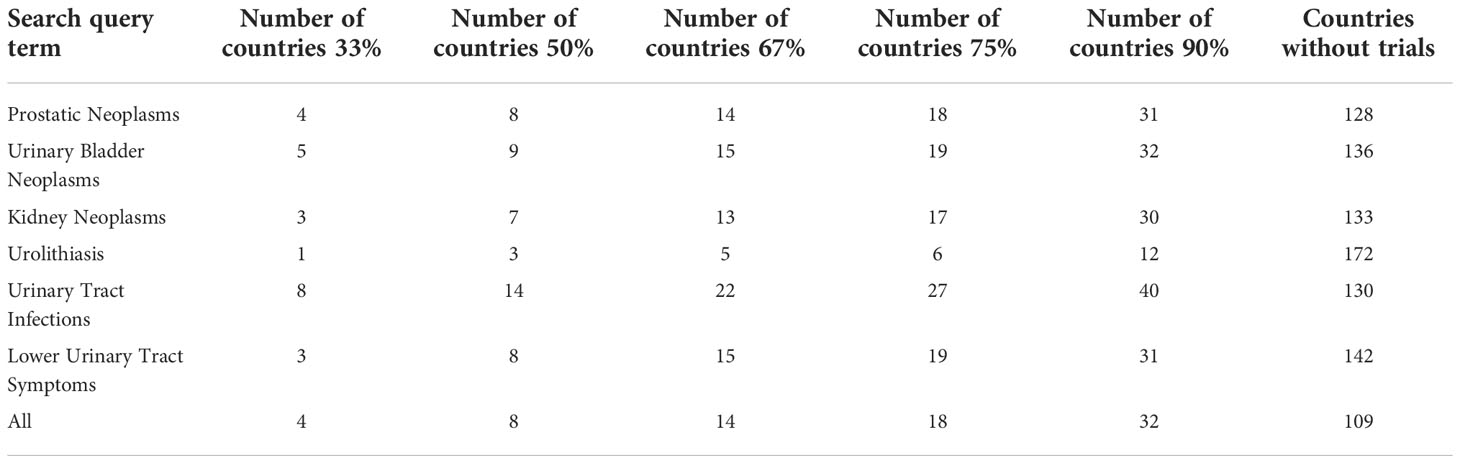
Table 2 Number of countries that contribute to a percentage of the total number of clinical trials being performed for a urologic disease.
The clinical trials sites were classified as either low, lower-middle, upper-middle or high income earning based on the World Bank Classification (Figure 3). Few low earning countries were trial sites for studies listed on ClinicalTrials.gov or ICTRP, with four trials listed for urinary tract infections, and one each for prostate cancer, kidney cancer and lower urinary tract symptoms. A majority of clinical trials were conducted in high income countries based on the World Bank Classification.
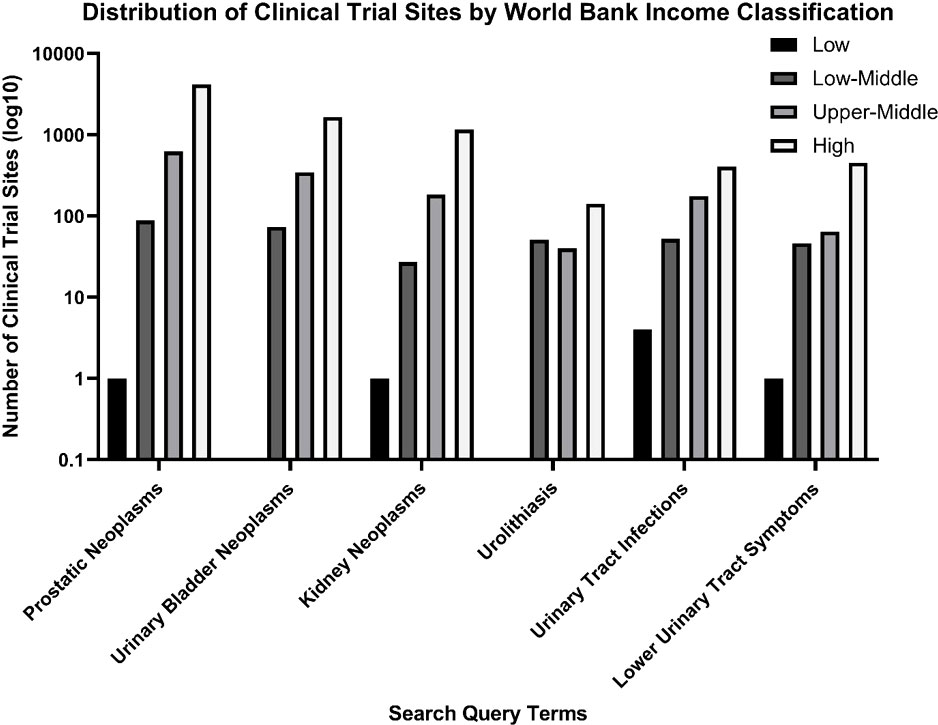
Figure 3 Distribution of clinical trial sites by World Bank Income classification for the search query terms on a logarithmic scale.
Data on the burden of urologic diseases on a country was obtained from the Institute for Health Metrics and Evaluation in the form of incidence and DALYs. This was analyzed with respect to the number of clinical trials performed in the country and further stratified by LMICs and high-income countries (Figure 4). Simple linear regression was performed and generally demonstrated a weak correlation between the numbers of clinical trials being performed with respect to the incidence of DALYs within a country for that disease (Table 3). Within this limitation, there was a stronger correlation for an increased number of clinical trials in countries with an increased incidence and DALYs of oncologic conditions as compared to non-oncologic conditions. A subgroup analysis was performed and generally demonstrated a weaker correlation for low-middle income countries between number of clinical trials and burden of disease as compared to high income countries. However for urinary tract infections and lower urinary tract symptoms, a stronger correlation was seen between the number of clinical trials and burden of disease for low-middle income countries as compared to high income countries. The top ten most burdensome conditions and the number of clinical trials conducted in the country was summarized in Supplementary Table 1.
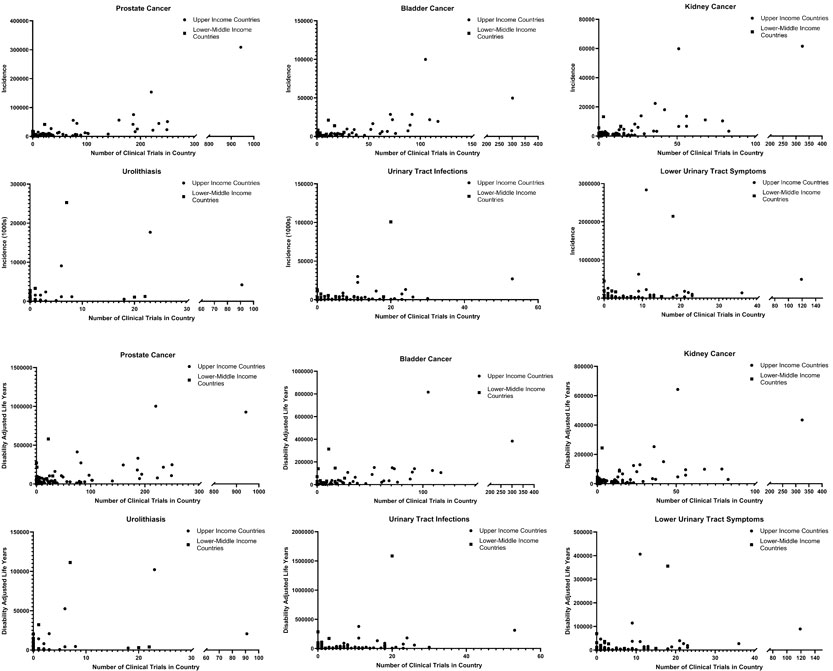
Figure 4 Global burden of disease for urologic conditions stratified by upper and lower-middle income countries with respect to the incidence (top) and DALYs (bottom) as compared to the number of clinical trials performed in the country.
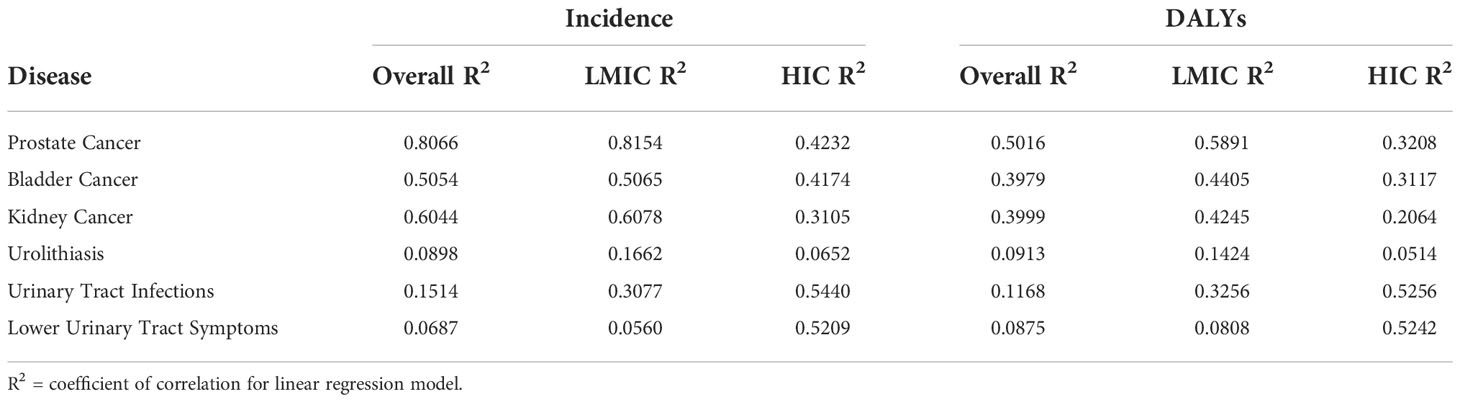
Table 3 Coefficient of Correlations for linear regression model for number of clinical trials based on the incidence and DALYs within the country for that condition, with sub-group analysis for low-middle income and high income countries.
Discussion
This study analyzes the geographic distribution of urologic clinical trials registered on ClinicalTrials.gov and the ICTRP for urologic diseases by country with respect to the World Bank classification of gross national income per capita. The findings of this study are notable for a disproportionate number of clinical trials being performed in countries with high gross national income per capita and the distribution of clinical trials is more disproportionate for oncologic conditions such as prostate, bladder and kidney cancer. A majority of these urologic clinical trials, approximately 90%, are being performed in 32 countries. Although overall weak, there is a stronger correlation between the number of clinical trials being performed and the burden of disease in countries for oncologic conditions as compared to non-oncologic conditions. When stratifying this analysis into high income countries and LMICS, the correlation between burden of disease and number of clinical trials is stronger for high income countries except for trials related to urinary tract infections and lower urinary tract symptoms.
The findings of this study also highlights the disconnect between the burden of disease within a country and the number of clinical trials being conducted within the country, suggesting that clinical trials are being conducted on the basis of available wealth and resources as compared to population needs. For example, although India has the tenth highest incidence of prostate cancer diagnoses and the third highest DALYs, only 22 of 2078 prostate cancer clinical trials are being conducted here. Nigeria has the seventh highest DALYs from prostate cancer yet no prostate cancer clinical trials are being conducted in this country. Likewise, Pakistan, Japan, the Philippines and Ukraine had the sixth through ninth highest incidence of urolithiasis respectively, however, no clinical trials related to urolithiasis are being conducted in these countries. It is also likely that the true disparity is greater, as the actual incidence of these urologic diseases is likely higher than what is reported due to challenges in diagnostic capabilities and access to care in LMICs (12).
Prior studies within other fields of medicine have identified similar disparities in clinical trial distributions in LMICs. In a retrospective cohort study, investigators found that the number of phase III randomized clinical trials related to oncologic treatments was disproportionately higher in HICs relative to the burden of disease. This study notes that findings from clinical trials conducted in LMICs tended to be published in academic journals with lower impact factor (13).
The disproportionate global distribution of clinical trials being conducted in LMICs has previously been attributed to availability of resources, migration of skilled personnel to higher income countries and administrative regulations. A prior systematic review that has evaluated the challenges to conducting clinical trials in LMICs has cited access to financial resources as the most common barrier (7). The monthly cost of conducting an oncologic clinical trials has often been appraised to be greater that the annual gross national income per capita of LMICs (14). For clinical trials conducted in LMICs with industry sponsors, the eventual approved treatment may not be financially accessible or offered within the LMIC populations that contributed to the trial study (15). The increased cost of oncologic clinical trials likely plays a role as to why LMICs have a greater focus in the non-oncologic areas of research where the cost of conducting and treatment of those diseases is more affordable. The source of funding for sponsoring oncologic clinical trials is predominantly from academic institutions within high income countries followed by pharmaceutical countries. In addition to financial capital, skilled human capital is required to conduct a clinical trial and there has been a migration of skilled clinical and scientific personally within LMICs to higher income countries (16). The challenge of retaining urologists within LMICs has been identified, as a study has expressed the concern for an increasing demand of urologists in sub-Saharan Africa (17, 18). Likewise, the disparate global distribution of pediatric urologists in LMICs has been identified secondary to a lack of skilled training centers (19). Lastly, in response to prior instances of exploitation of the disadvantaged populations in LMICs, stringer governmental review processes have been established to protect the rights of study participants (20).
In order to help mitigate the disproportionate distribution of clinical trials, the World Health Organization has recommended the development of international collaborations. The current state of global health has been described by Abimbola et al. as a remnant of prior colonial practices. The collaborations of institutions and individuals between HICs and LMICs is recommended to proceed as a shared enterprise rather than a top-down approach (21). An association was created to promote international research endeavors, the Prostate Cancer Transatlantic Consortium, including countries in Africa, the Caribbean Islands, North America and Europe (22). A similar initiative, the Asian Prostate Cancer Study, includes countries across Asia to promote clinical research collaborations. The migration of skilled personnel from LMICs can be countered by increasing incentives for retention, in the form of training grants, professional development incentives, and access to educational material. With regards to institutional research regulations, there appears the need for a balance between an efficient review process and the protection of participants of clinical trials. Specific to the field of urology, clinical trials need to be developed specific to the burden of pathologies affecting the population. Several influential clinical trials have been conducted in LMICs. For example, the POP-RT clinical trial conducted by investigators from the Homi Bhabha National Institute in India identified the role for prophylactic pelvic nodal radiotherapy for high risk, locally advanced prostate cancer (23).
The findings that are presented need to be interpreted with the understanding of the limitations of this study. The data collected from ClinicalTrials.gov and the ICTRP is assumed to be reliable from the manual input of information into the registries. Regarding the methodology, the information that is retrieved from the registries is the countries of the clinical trial sites, however we do not differentiate between the sizes of the recruitment population from within these countries. Hence, we are not assessing for the impact of the recruitment of the participants from a specific country on the eventual data obtained from the clinical trial. We acknowledge that grouping an entire population of a country as one does not account for regional and ethnic differences. Lastly, this study evaluates for the quantity of clinical trials performed in countries but does not examine the quality and potential impact of the research topics being studied.
Conclusion
With regards to the urologic diseases examined, there is a disproportionately greater number of urologic clinical trials being performed in high-income countries. There is a weak correlation between the burden of disease and the number of clinical trials being conducted in a country for these urologic conditions. If urologic clinical trials are to be performed in a more proportionate manner to the number of patients affected from the disease within a country, the findings of this study suggests the need for a greater number of clinical trials to be performed in LMICs.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
AC and IK were responsible for conception of the project. AC, CH, SR and SL were responsible for data collection. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fruro.2022.1069265/full#supplementary-material
Abbreviations
- LMICs, low and middle-income countries; HICs, high-income countries; ICTRP, International Clinical Trial Registry Platform; DALYs, disability-adjusted life years.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Launer BM, McVary KT, Ricke WA, Lloyd GL. The rising worldwide impact of benign prostatic hyperplasia. BJU Int (2020) 127:722–8. doi: 10.1111/bju.15286
3. Lang J, Narendrula A, El-Zawahry A, Sindhwani P, Ekwenna O. Global trends in incidence and burden of urolithiasis from 1990 to 2019: An analysis of global burden of disease study data. Eur Urol Open Science. (2022) 35:37–46. doi: 10.1016/j.euros.2021.10.008
4. Öztürk R, Murt A. Epidemiology of urological infections: A global burden. World J Urol (2020) 38:2669–79. doi: 10.1007/s00345-019-03071-4
5. Roberts TG. Trends in the risks and benefits to patients with cancer participating in phase 1 clinical trials. JAMA. (2004) 292:2130. doi: 10.1001/jama.292.17.2130
6. Drain PK, Parker RA, Robine M, Holmes KK, Bassett IV. Correction: Global migration of clinical research during the era of trial registration. PloS One (2018) 13:1–13. doi: 10.1371/journal.pone.0199952
7. Alemayehu C, Mitchell G, Nikles J. Barriers for conducting clinical trials in developing countries- a systematic review. Int J Equity Health (2018) 17:1–11. doi: 10.1186/s12939-018-0748-6
8. Özdener F, Sursal A, Narter F. Current status of urological surgery clinical trials in the middle East and its analysis in comparison to global. J Urological Surg (2019) 6:266–72. doi: 10.4274/jus.galenos.2019.2688
9. Zarin DA, Tse T, Williams RJ, Califf RM, Ide NC. The ClinicalTrials.gov results database — update and key issues. N Engl J Med (2011) 364:852–60. doi: 10.1056/NEJMsa1012065
10. Banno M, Tsujimoto Y, Kataoka Y. Studies registered in non-ClinicalTrials.gov accounted for an increasing proportion of protocol registrations in medical research. J Clin Epidemiol (2019) 116:106–13. doi: 10.1016/j.jclinepi.2019.09.005
11. Shepherd H. Classification, cognition and context: The case of the world bank. Poetics (2010) 38:134–50. doi: 10.1016/j.poetic.2009.11.006
12. Metzler I, Bayne D, Chang H, Jalloh M, Sharlip I. Challenges facing the urologist in low- and middle-income countries. World J Urol (2020) 38:2987–94. doi: 10.1007/s00345-020-03101-6
13. Wells JC, Sharma S, Del Paggio JC, Hopman WM, Gyawali B, Mukerji D, et al. An analysis of contemporary oncology randomized clinical trials from Low/Middle-income vs high-income countries. JAMA Oncol (2021) 7:379–85. doi: 10.1001/jamaoncol.2020.7478
14. Rubagumya F, Hopman WM, Gyawali B. Participation of lower and upper middle-income countries in clinical trials led by high-income countries. JAMA Network Open (2022) 5:1–25. doi: 10.1001/jamanetworkopen.2022.27252
15. Grover S, Xu M, Jhingran A, Mahantshetty U, Chuang L, Small W Jr, et al. Clinical trials in low and middle-income countries — successes and challenges. Gynecologic Oncol Rep (2017) 19:5–9. doi: 10.1016/j.gore.2016.11.007
16. Beine M, Docquier F, Rapoport H. Brain drain and human capital formation in developing countries: Winners and losers. Economic J (2008) 118:631–52. doi: 10.1111/j.1468-0297.2008.02135.x
17. Odedina FT, Shamley D, Okoye I, Ezeani A, Ndlovu N, Dei-Adomakoh Y, et al. Landscape of oncology clinical trials in Africa. JCO Global Oncol (2020), 6:932–41. doi: 10.1200/JGO.19.00189
18. Olapade-Olaopa EO, Onawola KA. Challenges for urology in sub-Saharan Africa in 2006. J Men’s Health Gender (2006) 3:109–16. doi: 10.1016/j.jmhg.2006.01.004
19. deVries CR. A global view of pediatric urology. J Pediatr Urology (2022) 18:271–9. doi: 10.1016/j.jpurol.2022.02.002
20. Chawan VS, Gawand KV, Phatak AM. Impact of new regulations on clinical trials in India. Int J Clin Trials (2015) 2:56. doi: 10.18203/2349-3259.ijct20150592
21. Abimbola S, Pai M. Will global health survive its decolonisation? Lancet; (2015) 396:1627–8. doi: 10.1016/S0140-6736(20)32417-X
22. Oladoyinbo CA, Akinbule OO, Sobo AA, Bolajoko OO, Bassey IE. Behavioural risk factors associated with prostate cancer: The prostate cancer transatlantic consortium (CaPTC) cohort study. J Global Oncol (2018) 4:5s–s. doi: 10.1200/jgo.18.93000
23. Murthy V, Maitre P, Kannan S, Panigrahi G, Krishnatry R, Bakshi G, et al. Prostate-only versus whole-pelvic radiation therapy in high-risk and very high-risk prostate cancer (POP-RT): Outcomes from phase III randomized controlled trial. J Clin Oncol (2016) 39:1234–1242. doi: 10.1200/JCO.20.03282
Keywords: clinical trials, disparities, urology, representation, health research
Citation: Choksi AU, Hayden CS, Rahman SN, Lokeshwar SD and Kim IY (2023) The disparities in clinical trials addressing urologic conditions among lower-income countries. Front. Urol. 2:1069265. doi: 10.3389/fruro.2022.1069265
Received: 13 October 2022; Accepted: 28 November 2022;
Published: 05 January 2023.
Edited by:
Mohammed Shahait, King Hussein Medical Center, JordanReviewed by:
Haydee Verduzco-Aguirre, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ), MexicoEdward Christopher Dee, Memorial Sloan Kettering Cancer Center, United States
Copyright © 2023 Choksi, Hayden, Rahman, Lokeshwar and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Isaac Y. Kim, aXNhYWMua2ltQHlhbGUuZWR1
 Ankur U. Choksi
Ankur U. Choksi Christopher S. Hayden
Christopher S. Hayden