
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Urol. , 20 December 2022
Sec. Urologic Oncology
Volume 2 - 2022 | https://doi.org/10.3389/fruro.2022.1020215
This article is part of the Research Topic Genitourinary (GU) Oncology in Low-to-Middle Income Countries View all 5 articles
 Daniel Herchenhorn1,2,3*
Daniel Herchenhorn1,2,3* Vinicius Freire1,2
Vinicius Freire1,2The availability of new systemic therapies associated with better outcomes and survival for GU tumors is a major obstacle for most LMIC. Strategies to improve access are necessary and depend not only on drug availability, but from public health care system organization, discussion and priorities as well as strategies to decrease cost by rational treatment decision and individualize use of systemic therapies in limited resource countries. Efforts should be implemented to provide more real-world data coming from LMIC and studies focusing in strategies to decrease drug costs are urgently needed.
Globally, one in six deaths is due to cancer and about 70% of cancer deaths occur in low-middle income countries (LMICs) (1). These countries have less than 5% of the global resources for fighting cancer (2). A comparison of mortality-to-incidence ratio for these cancer sites between HICs and LMICs clearly shows poor survival outcomes in majority of patients diagnosed in LMICs for different types of cancer (1, 2).
In LMICs, large proportions of the population have limited access to medicines, either because of lack of availability or because patients have to pay for their prescriptions. In the absence of government reimbursements, insurance, or any exclusive access schemes in LMICs, many patients must bear the cost of treatment. Access to medicines for patients in LMIC is limited by government underfunding of medicines, and supply of medicines that contribute to poor inventory control (2).
Advances in systemic therapy have certainly led to improved survival in many cancers. However, there is a wide variation in the magnitude of the benefit associated with these new therapies. These gains should be balanced in the real world, specially in limited resource countries, where the availability of the new medicines should be of easy access and affordable for the population.
The bargaining power of small and low-income countries is limited, consequently affordability tends to be negatively correlated with market size and gross domestic product per capita (3). Although the overall incidence of cancer is lower in LMIC compared to high-income countries (HICs), total cancer-related mortality is significantly higher in LMIC, especially for people under 65 years of age, where the greater economic impact as a result of premature mortality and lost years of productivity is especially problematic (4–6). LMIC have experienced an increase in cancer-related mortality as a result of rising obesity rates; increasingly sedentary lifestyles; dietary factors; excessive use of tobacco and alcohol; and persistent cancer infections like Helicobacter pylori, hepatitis B virus, and human papilloma virus, not to mention other contributing factors that are less well understood like race or genetic factors (6, 7). Data from LMIC is limited when it comes to the current status of cancer care and infrastructure, primarily because health infrastructure in these settings has historically been built around treating communicable diseases and nutritional deficiencies (8).
There are four main priorities formulated to promote health services for cancer control and collecting data: capacitation in clinical research, policy and planning of oncology health services in LMICs (9); development of high-quality health data, such as population-based cancer registries, to identify cancer management process and outcomes to ensure they are iterative and achieve quality control (8, 10); more oncology-related economic and farmaco-economic evaluations in LMICs (11); and exploration of high-quality cancer control new models in LMICs note related to experiences coming from HICs. While the exact availability and types of anticancer therapies in different LMICs are unknown, a World Health Organization (WHO) survey has found that only 22% of African countries and 43% of Southeast Asian countries reported the availability of anticancer therapy, without specified details in therapies; this is in contrast to a reported availability exceeding 90% in Europe (12). Drug shortages are common and can also be a problem for patented as well as generic drugs (13). A WHO report found that 20% to 60% of health spending in developing countries is for medicines, which is significantly more than in developed countries (14, 15). Most LMICs have low allocation of gross domestic product (GDP) spending toward health care, despite the enormous economic losses of cancer mortality/morbidity.
The majority of the world’s population - more than 5 billion people - do not have access to accessible surgical services when needed, not to mention anesthetic care or narcotics/pain medications (16). The accessibility of radiotherapy is also inadequate to meet the needs of the large population in need in LMIC. One study estimated that the supply of radiotherapy machines in Africa was sufficient to meet only 18% of radiation needs, and 22 African and Asian countries did not have access to radiotherapy (17), in other countries like Brazil, the main problem is not the number of machines, but its inadequate distribution for the public health system in many states of the federation, specially in the poorer area of the north and northeast of the country (18).
Genito-urinary cancers, compromise a group of neoplastic diseases, that range from the most common prostatic cancer to urothelial lesions, renal and testicular cancer. Prostate cancer is the most common cancer in men, and accounts for 15% of all cancer cases (19). Mortality rates for prostate cancer and for other tumors, have decreased since the mid-1990s in many developed countries in North America and Europe reflecting advances in treatment and prevention strategies, in contrast to the increased rates in many countries in Asia, Africa and South America; probably a combination of rising incidence, longevity, inequity of the health system and limited access to more effective therapies (20).
Prostate cancer has been a focus of intense research with recent advances that changed the paradigm of the treatment in the last decades, with increasing rates of cure and longer disease survival. These advances ranged from more precise and less morbid robotic-prostatectomies, high precision radiotherapy, as well as an increase in the number of approved new systemic therapies for locally advanced and advanced disease, usually allowing more intense combinations upfront and new options for sequential therapies for patients with advanced disease (20, 21).
Genomic information is increasingly utilized for treatment of selected patients, as well as advances in imaging, particularly Prostate MRI and PSMA-PET/CT, from diagnoses to treatment (22).
Urothelial cancers (UC), in special bladder tumors, are highly prevalent, especially tabaco related and with an increase incidence in LMIC (23, 24). UC, frequently relapse even after potentially curable surgeries, and is known by its aggressive behavior. New therapies also have been incorporated. Ranging from non-muscle invasive disease (relapsed carcinoma in situ), adjuvant therapy as well as maintenance or sequential therapies in advanced disease stages. New targeted therapies with companion biomarkers like FGFR inhibitors, the increasing use of immunotherapy and new-antibody conjugates are the most evident changes in systemic therapy and now part of the most recent international guidelines (25).
Renal cell cancers are also increasingly diagnosed due to the appearance of incidental tumors in regular scans. Despite advances in surgery and imaging, relapses are still frequent and diagnoses with more advanced stages are frequent in patients from LMIC (26, 27). Approval of several new agents for advanced RCC are ongoing for over a decade, initially with oral anti-VEGF multikinase agents and later with the incorporation of immunotherapy (IO) (28). More recently, new IO-IO combinations and new anti-VEGF/IO combinations were incorporated becoming new standards for advanced disease and IO therapy coming also as an option for the first time as an adjuvant therapy (29).
Imaging and genetic technologies are relatively affordable in HIC, and sometimes can be available in selected LMICs, so the challenge is to identify optimal strategies that can lead to higher rates of cure and control and allow a higher number of patients in different places to have access to new technologies leaving to better outcomes. These changes came with significant cost for all health care systems, but present substantial opportunities to reshape and optimize care for our patients, with less morbid and effective therapies (30).
LMIC have huge limitations for adequate care of cancer patients, ranging from inadequate medical attention and untrained personnel, pathology or availability of pain and supportive care. The incorporation of new therapies with cost-effectiveness is a challenge faced by healthy economies and post an even higher wall for this heterogeneous group of LMIC.
When it comes to new drug incorporation, most of LMIC don’t have multidisciplinary discussion to allow health decisions that will guarantee cost effective therapies and prioritize its efficacy and impact for the local economy. Decisions for drug approval are usually not linked to the full incorporation and utilization of new therapies for the population. In many of LMIC, like Brazil, the availability of new therapies usually is adopted by those who can afford a private health insurance, rather for the majority of the population that depends on public health system exclusively (18).
The care of men with prostate cancer in reference centers around the world is multi-disciplinary and has changed dramatically in the last 15 years with the approval, based on phase 3 randomized trials, of several new agents for hormone-sensitive and castration-resistant disease, like docetaxel, new androgen receptor inhibitors, radiopharmaceuticals, PARP inhibitors and even immunotherapy, as sequences or even in combination for more intensified therapies. Besides drugs, robotic-assisted surgeries and radiotherapy technologies helped to change the paradigm of treatment, from local disease to more advanced stages, and as a result, more patients are cured or living longer with better quality of life with the disease (31–41).
After many years being treated only with systemic chemotherapy, recently, the oncologic approach for localized, locally advanced and advanced stages has changed significantly (25).
For localized non-muscle invasive in situ carcinoma, the introduction of immunotherapy (IO) after BCG failure opened a new era of opportunities (42), as well as the recent incorporation of IO as an option for adjuvant therapy (overall survival data is still not available) in high-risk patients not candidates for cisplatin-based chemotherapy. In advanced stages, several new options are now approved with proved efficacy, from IO in first line, maintenance therapy after chemotherapy, to new agents like antibodies-conjugates and anti-FGFR inhibitors (43, 44).
The same phenomenon occurred worldwide in renal cell carcinomas. It started with the introduction of oral anti-VEGF tyrosine kinase inhibitors that were build up after the basic knowledge of the molecular and angiogenic background of this tumors, and more recently, the approval of several combinations of IO-IO therapy and anti-VEG-IO combinations. All of these new agents showed superior results compared to the “old” standards of anti-VEGF like Sunitinib (28, 29).
The advances pushed a more recent change with the incorporation of IO in the post-operative setting (45).
Optimal management of Genito-Urinary malignancies (GUm) requires the availability of imaging and pathology for diagnosis, surgery and radiation oncology for treatment of localized disease and drug therapy for management of metastatic disease, as well as access to pain medications, genetics and other supportive therapies. Although there may have been some improvements in delivery of surgery and radiotherapy in recent years, lack of trained staff and facilities remains a major barrier to optimize the treatment of patients with GUm. Very limited access to anticancer drugs has been highlighted in a recent survey (22, 46, 47).
Given the unbalanced investment in private (a minority of the population affording private health plans in LMICs) and public (governmental) sector, most of the advanced resources in LMICs are privately controlled. This creates a monetary barrier to access of modern technology, either radiotherapy machines or new drug incorporation.
Another barrier is geographical, due to urban concentration of cancer treatment centers in large countries like Brazil, India or South Africa, with concentration of most of the resources in limited areas of the country.
Any solution to cancer outcomes requires more than simple medical consensus or guidelines, but an approach that includes health care authorities (government), health providers, economic decisions, and patient advocacy.
Awareness of the barriers to technological access at all the levels of policy- and decision-making is required, along with clarity on understanding the value of high-quality technology incorporation. Active participation of clinical experts in policy- defining strategies regarding present and future technological needs is essential for achieving the desired access to quality of care in every country.
Most of the new therapies highlighted above are either approved or available for a minority of patients living in LMIC that depends mostly on public health systems (48).
Many patients in LMICs will present initially or subsequently with more advanced metastatic disease due to the lack of early diagnosis/prevention strategies and the difficulty in access to adequate early diagnosis and oncologic care (48).
Androgen deprivation therapy (ADT) remains the standard initial treatment and provides effective palliation of symptoms as well as disease control in most men with advanced prostate cancer. In HICs the usual option is chronic medical castration with GnRH agonists/antagonists. Orchiectomy (surgical castration), although equally effective and cheaper than medical castration, is performed in lower numbers around the world including in LMICs (49).
For patients with advanced disease, the more frequent use of orchiectomy (surgical castration) in LMIC, could potentially save money and decrease medical visiting and allow for resources directed to more intensive systemic therapies which hopefully will generate additional survival time and reduce disease complications related to disease progression (49).
The median survival of men with metastatic prostate cancer in HICs has improved from 2.5 to about 5 years with recent incorporation of additional hormonal agents (abiraterone, enzalutamide and apalutamide), chemotherapy (docetaxel and cabazitaxel), Radium-223, and more recently with olaparib, pembrolizumab and lutecium-PSMA, with gains also in symptom control, pain and quality of life (31–41).
Most of these new agents increased significantly the overall costs (with the exception of docetaxel and generic abiraterone), being of limited access for most patients in LMICs (50).
As the global access to effective new therapies is a major challenge, opportunities like the increase in use of surgical castration already mentioned, the choice of generic agents like abiraterone or docetaxel, and pharmacodynamic studies trying to reduce dosing (without compromising efficacy), should be explored as opportunities for limited resources countries (51).
In a phase II randomized study, the antitumor activity of abiraterone at low dose levels was evaluated. The dose of 250mg with breakfast was found to be an alternative to the dose standard dose of 1000 mg/d after an overnight fast, thereby reducing the cost by 75% (51). This study led the NCCN to recommend the lower dose with food as an alternative; this strategy leads in India to big costs savings in the healthcare system (51) and was also recommended in a recent consensus for countries with limited resources, although not clearly adopted in HICs.
Enzalutamide, another potent oral anti-androgen also can potentially be given at lower doses (half dose = half cost), as shown by a recent european phase 2 trial, also with the gain of reduced toxicities (52).
Below, we describe the availability of prostate cancer agents in some LMIC countries, as an example of disparities.
Most people in Brazil access healthcare through the public health system - SUS (covers 70% of the population), while the minority of wealthier citizens (<30%) can access private healthcare providers. The option for chemotherapy (e.g. docetaxel) has similar availability in these systems, but newer hormonal agents (abiraterone, apalutamide, enzalutamide or daralutamide) and cabazitaxel are not available outside clinical trials for patients in SUS, neither in the hormone sensitive or castration resistant status. There is a deficit of oncologists in almost half of Brazilian regions, mainly in the North, Midwest and Northeast and almost two-thirds of the health regions of Brazil do not have rooms for chemotherapy. The lack of oncologists and facilities results in displacement of patients, sometimes over long distances, so that they can receive appropriate treatment and follow-up, even without the new oncologic drugs approved in Brazil by ANVISA (the Brazilian equivalent to the FDA). In the private system patients can be treated according to international NCCN guidelines (18).
In India, another continental country, there has been low public sector budget allocation for healthcare and limited insurance coverage leading to heavy reliance on personal spendings. The low ratio of oncologists for the large number of cancer patients, often leads to delivery of systemic therapy by health care professionals without adequate training. The widespread availability of generics in India is an unique example, and had a tremendous positive impact on the access to new active agents for advanced prostate cancer while use of older drugs like less effective anti-androgens, estrogens and low-dose steroids although still prevalent, has decreased in the last years. For patients with metastatic prostate cancer, orchiectomy is offered routinely and leads to substantial cost-saving as well (53).
In most African countries, trained medical oncology specialists are very rare. Cancer patients sometimes pay out-of-pocket for cancer treatment while a very small proportion have partial National Health Insurance coverage for their medications. Those with higher income opt for treatment in private facilities either paying themselves or with private insurance coverage. Failure to complete treatment for financial reasons is quite common. Initiatives have established collaborations with pharmaceutical companies to improve access to cancer care by suppling medications at affordable prices, about 60% reduction in current price (54–56). The program aims to provide access to 20 cancer medicines, including docetaxel, bicalutamide and leuprolide, across many African countries.
The case of newer agents like radioligand therapy, Lutecium-PSMA (Lu-PSMA) is likely to be restricted to HICs due to its high cost. Cost effectiveness studies comparing Lu-PSMA to cabazitaxel or other treatment options are awaited, but the other comparators are usually not available for most of the patients in LMIC, although, in countries like Brazil PET-PSMA and Lu-PSMA are already being given by those who can afford it, either with private insurances or out-of-pocket payments. Lu-PSMA, without property protection has also been used since 2013, it has the same PSMA binding peptide, but a different chelator. In some LMIC including South Africa, PSMA theragnostic has emerged as a cost-effective option when produced in hospital radiopharmacies, even used in preference to second-line androgen deprivation therapies such as enzalutamide or abiraterone, given the high costs of these agents (39). This highlights that investment in hospital radio-pharmacies is a longer-term solution that may enable greater access to this promising therapy, and other forms of nuclear medicine advances (57).
Regarding molecular targeted therapies. Although many pharmaceutical companies are now able to pay for companion genetic analysis. Incorporation of PARP-inhibitors or immunotherapy is not really planned in a short-term basis in most LMIC health systems due to the high cost of those medications and the restriction for a minority of patients with genetic abnormalities, not evaluated by public providers (58, 59).
Less frequent GU tumors like urothelial or renal cell are really not a priority for health care systems in LMIC, and although serious and deadly diseases that necessities specialized therapies and personal, don’t receive much attention when it comes for new drug incorporation. The availability of immunotherapy, widely used in HIC to treat both diseases as well as anti-VEGF agents are not available in countries like Brazil and most African counties. In India, the availability of generic oral anti-VEGF agents made possible its easier access to the population, but it includes only what is now considered old fashioned first line therapies for this disease.
Any proposed solution should be addressed as part of a complex group of ideas, as traditional health care systems in most LMICs have to be adapted or shifted towards a more standardized and quality-metric–based approach of modern cancer care, not only with emphasis in treatment, but in prevention strategies, access and results, with the final emphasis in reducing the burden of advanced cancer morbidity and mortality and also providing future rational for technological improvements.
It should be emphasized that for most oncologist in LMICs, the new medications discussed here, like new hormonal agents, targeted therapies or even immunotherapy are not part of the essential medicine list (EML) presented by the World Health Organization (WHO). In this list, the usual “old” chemotherapy agents, usually affordable as generic agents, are the majority (13).
The high priority drugs for LMICs oncologist differ a lot from the agents mentioned as high priority by HICs oncologists in a recent survey, these substantial differences only highlight the importance of analyzing the risk-benefit ratio for every new medicine in every country individually (60).
Fixed or flat doses of prescription drugs are familiar to patients but many of the most common cancer drugs are administered by weight, those medications have no standard dose. Instead, each patient receives a personalized dose based on his or her weight or body size. Because of safety considerations, the typical approach is to package these medications in single-dose vials that are intended for use by a single patient. This leads to a situation that has been seen by many as a major concern: discarded drugs. Single-dose vials come only in a limited number of specific sizes, so the amount of the drug contained within a vial may exceed the required weight-based dosage for a given patient, and whatever amount is left over will then be discarded. In some cases, that is a significant percentage of the vial, with costs implied.
The proportion of drug left over varies from 1% to 33% (from the list of the top 20 used drugs). Between these extremes are drugs such as bevacizumab, which comes in both 100 mg and 500 mg vials, and ipilimumab, which comes in both 40 mg and 100 mg. Small percentages can still lead to large dollar amounts. The October 2015 Medicare Average Sales Price files show that a dose of Ipilumumab might cost $29 000, meaning that the 7% left over would generate an additional $2000 in revenue for the company.
We estimate total US revenue from these drugs to be $18bn (£12.5bn; €16bn) in 2016, with 10% or $1.8bn from discarded drug.
Consider a 70 kg patient who requires Pembrolizumab with a dose of 140 mg (the drug is dosed at 2 mg/kg). When the drug was sold in 50 mg vials, reaching the desired dose would require three 50 mg vials and leave 10 mg unused. But with only 100 mg vials available, 60 mg is left over. According to the Medicare 2015 file, which lists Medicare’s reimbursement rates for these drugs, each milligram of pembrolizumab costs around $50. In this example the change in vial size alone increases the revenues for the company from leftover drug by sixfold, from $500 to $3000, for a single dose.
Policy makers should therefore explore approaches that would reduce or eliminate paying for leftover drug. Regulators could require manufacturers to provide drugs in a reasonable set of size options to ensure the amount of wasted drug is low, say 3% (61).
● Many infused cancer drugs are packaged in single dose vials but dosed based on body size, often resulting in leftover drug;
● All the drug in the vial has to be paid for, making wasted drug a source of unnecessary spending;
● Drug companies will earn around $1.8bn from leftover cancer drugs in the United States in 2016;
● Manufacturers should be required to package drugs in quantities that allow better matching with required doses or enable virtual return of leftover drug.
There are four broad determinants of medicine prices from the industry perspective: (a) costs of R&D; (b) costs of production and commercialization; (c) the “value” of medicine; and (d) sufficient returns on R&D.
Estimates of R&D costs, including for cancer medicines, are highly variable and not transparent. Reported estimates, after adjustments for the probability of trial failure and opportunity costs, range between US$ 100–150 million and US$ 4–6 billion, but the most commonly accepted estimates are between US$ 200 million and US$ 2.9 billion.
“Value-based pricing” has been proposed as a method of pricing new medicines. However, there are many uncertainties associated with estimating value, as a result of different technical approaches to assessment, incomplete evidence, comparison with inefficient practices, and different perceptions of value. This method may lead to unaffordable prices for cancer medicines.
Overall, the analysis suggests that the costs of R&D and production may bear little or no relationship to how pharmaceutical companies set prices of cancer medicines. Pharmaceutical companies set prices according to their commercial goals, with a focus on extracting the maximum amount that a buyer is willing to pay for a medicine. This pricing approach often makes cancer medicines unaffordable, preventing the full benefit of the medicines from being realized as well as being adopted for many high-income countries as well as those with limited resources.
Authorities in some countries have used strategies to achieve greater system efficiencies and improve access to cancer medicines Figure 1 (a) requiring clinicians to obtain approval from the payer before prescribing or dispensing a select set of high-cost and highly-specialized cancer medicines; (b) implementing policies to encourage prescribing and substitution of cancer medicines with generic or biologically similar products; (c) reduction or exemption of taxes on medicines; and (d) combining financial and non-financial resources across various purchasing authorities in order to create greater purchasing power and better negotiation.
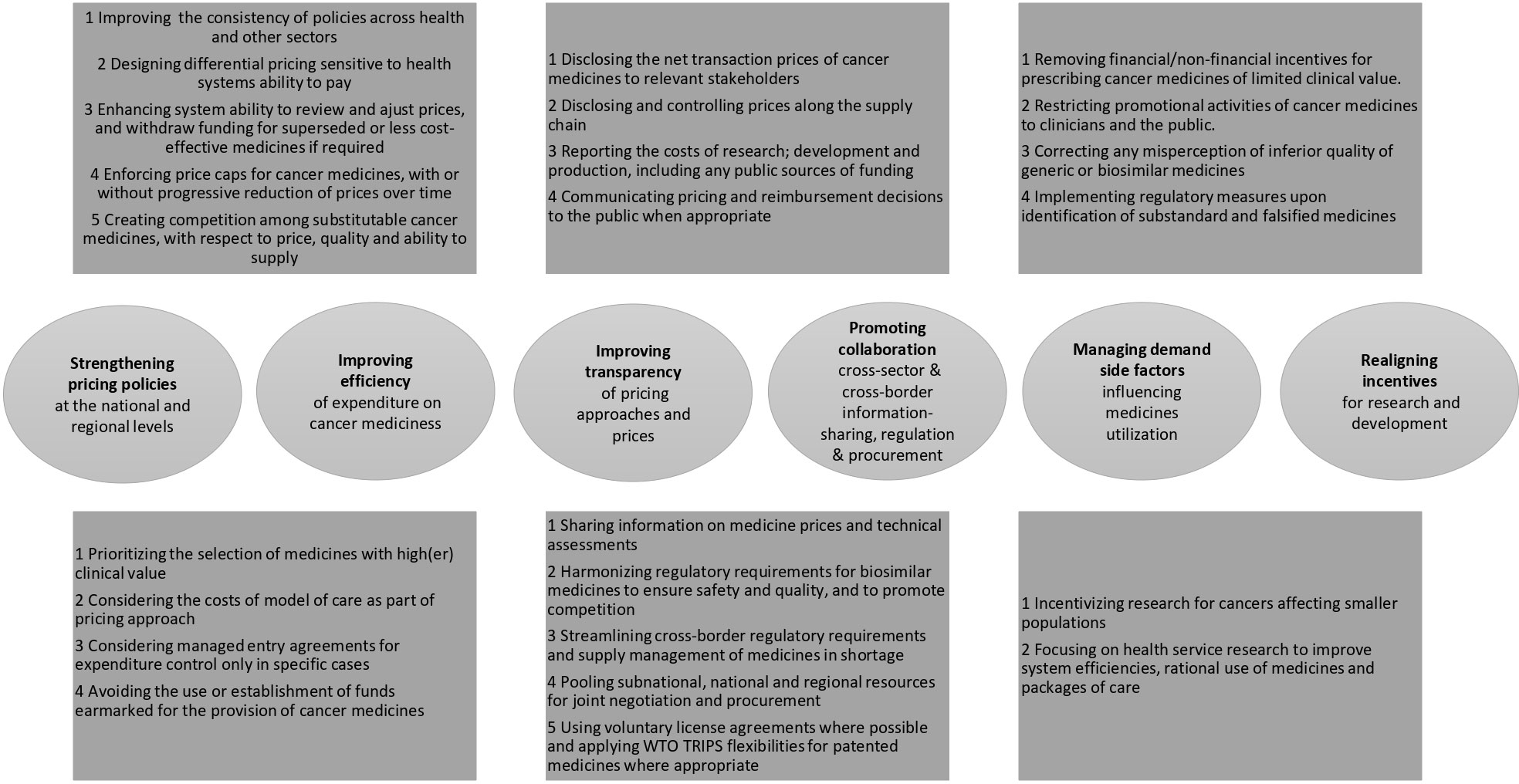
Figure 1 Sumary of options that might enhance the affordability and accessibility of cancer medicines.
Two large surveys examined the availability of medicines for solid tumours in the national formularies of 49 European countries in 2014 and 63 countries outside Europe in 2016. The study showed that countries with lower income had lower availability of cancer medicines, or availability only with higher out-of-pocket payments, especially for higher-cost medicines, including new targeted therapies. One survey found that 32.0% and 57.7% of essential medicine list cancer medicines were available in LMIC and LIC, respectively, only if patients were willing to incur their full costs.
Opportunities that might enhance the affordability and accessibility of cancer medicines have been identified through a review of policy and consultations with experts:
(a) strengthening pricing policies at the national and regional levels;
(b) improving the efficiency of expenditure on cancer medicines;
(c) improving the transparency of pricing approaches for high-cost cancer medicines;
(d) promoting cross-sector collaboration for information-sharing and regulation;
(e) managing factors that would influence the demand for cancer medicines;
(f) realignment of incentives for R&D.
Judicious selection of cancer medicines and rational application of access requirements with consideration of specific health system contexts can deliver better value for money without compromising population outcomes in cancer treatment. A policy of funding more new cancer medicines to achieve the same number of cancer medicines as in other countries would not result in substantive health improvement and would cost more. However, there is evidence that in some countries, cost-containment measures due to the high costs of cancer medicines have caused reduced, delayed and even cancellation of treatment, to the extent that it might have deleterious impacts on patient health outcomes.
A suite of approaches for setting medicine prices along the value chain: Payers have adopted a range of pricing approaches, individually or in combination, to set medicine prices. These include cost- based pricing, value-based pricing, reference pricing, and through tendering and negotiation, and regulating mark-up levels.
▪ Monitoring, evaluating and adjusting medicine prices throughout product life-cycle: Some government authorities have routinely monitored medicine prices, with a view to adjusting prices throughout the product life-cycle. These include reassessing prices when there is a change in market conditions (e.g. entry of generic and biosimilar products) or therapeutic landscape (e.g. extension of indications for the same medicine).
▪ Each to the system’s own context: The merits and disadvantages of individual pricing approaches must be interpreted with consideration to the countries’ population needs and system requirements.
▪ Pricing measures applied by government are necessary. There is evidence showing that (1) prices of cancer medicines grew significantly in the absence of regulations; (2) non-uniform pricing policies have led to differences in medicine prices, resulting in inefficient cost-shifting activities and potential inequity in access; and (3) greater level of price control can lower prices.
▪ There are approaches for promoting competition among medicines that are substitutable clinically. For example, me-too medicines, generic and biosimilar products have resulted in lower prices and expenditure savings. However, the magnitude of impact is variable because of contextual factors such as (1) existing pricing and non-price policies for branded medicines; (2) number of competing companies/products and market size; and (3) regulatory requirements and processes for generic and biosimilar medicines.
▪ The effectiveness of pricing policies would be enhanced by having robust competition policies and good governance to prevent anti-competitive and efficiency-impairing business practices, such as introducing pseudo-generics; engaging in tacit or actual collusion; product hopping; and wasteful non- value-added activities such as lobbying or creating patent clusters to delay generic/biosimilar entry.
The Working Group advised WHO to use an overall survival interval of at least 4 months for first-line treatments as a general guiding principle for considering medicines for inclusion in the essential medicine list (EML). For medicines with limited data on survival, evidence of disease-free or progression-free survival may be considered on a case-by-case basis, provided that the benefits are large, validated and consistent with other evidence.
In considering these recommendations, the Working Group noted the following points:
● Overall survival of less than 3 months would generally be considered as marginal and might not be relevant from both clinical and ethical perspectives to be accepted;
● Due to methodological biases, findings from clinical trials have the tendency to overestimate the likely benefits of cancer medicines when used in clinical practice. The usefulness of a medicine might also be impaired because of differences in the characteristics of patient populations and health care settings, including the capacity of health services in LMIC in delivering medicine according to best practice and managing drug-related toxicities.
● The Working Group considered the rating scales developed by the American Society of Clinical Oncology (ASCO’s Value Framework) and the European Society for Medical Oncology (ESMO’s Magnitude of Clinical Benefit Scale or MCBS) to facilitate the benefit assessment process. It agreed that both scales could be used but they expressed a preference for the scale by ESMO because:
● ESMO-MCBS allows assessment of benefits in relative and absolute terms. This is consistent with the requirements of the EML Expert Committee.
● ESMO is a nongovernmental organization (NGO) in official relationship with WHO;
● All newly approved cancer medicines since 2016 has been evaluated using ESMO-MCBS;
● ESMO plans to expand the MCBS to cover medicines for hematological malignancies in collaboration with the European Hematology Association.
Due to the high cost of medications to treat advanced squamous cell carcinoma of the head and neck (HNSCC), only 1-3% of the population has access to these medications in low- and middle-income countries. Retrospective data from low-dose chemotherapies of similar outcomes. Studies are evaluating low doses of Nivolumab would improve overall survival. An open-label randomized phase 3 superiority study that followed adult patients with advanced-recurrent HNSCC with palliative intent. They were randomized 1:1 to either methotrexate 15 mg/m2 orally weekly, celecoxib 200 mg orally daily, and erlotinib 150 mg orally daily (MC) or low-dose nivolumab 20 mg intravenously (MC-I) at full dose once every 3 weeks. Therapy was continued until progression or intolerable adverse events. Response assessment was performed every 2 months (RECIST). The primary endpoint was 1-year overall survival (OS). 151 patients were randomized, 75 to the novolumab 20 mg IV versus methotrexate 15 mg/m2 po weekly, celecoxib 200 mg po daily, and erlotinib 150 mg po daily arm. The addition of Nivolumab led to an improvement in 1-year overall survival from 16.3% to 33.4%. Median overall survival in the metronomic chemotherapy and metronomic chemotherapy and immunotherapy arms was 6.7 months and 10.1 months respectively. Median progression-free survival in the metronomic chemotherapy and metronomic chemotherapy and immunotherapy arms was 5.57 months and 6.57 months, respectively. Grade 3 and above adverse event rates were 50% and 46.1% in the MC and MCI arms respectively (62). In addition, low-dose nivolumab has improved overall survival and is a standard of care for those who can access full-dose nivolumab.
High drug costs are one of the major factors for the disparities observed in GUm outcomes. Pricing of drugs is not related to its producing cost (as a higher amount of pharmas income will turn to research and development) and results in unsustainable cancer care for many countries with limited resources (as well as for high income countries that deal with a socialized health system). Non-availability of life-prolonging therapies is not often due to the cost of manufacture, but to protection imposed, and will be used to establish drug research and development costs. As a result, patients will receive suboptimal therapy, due at least in part to financial toxicity: as an example, new AR-targeted therapies are not currently funded by NICE in the UK (63, 64).
The objective of decreasing costs through development of new dosing regimens, while maintaining efficacy, was introduced a few years ago. Strategies include de-escalation of dosage, treatment duration and administration frequency, and substitution with therapeutic alternatives. Intravenous cytotoxic chemotherapies often have a dose response relationship and phase I studies are based on the “maximum tolerated dose” (MTD), moving to phase II and III studies. The MTD is less relevant to targeted therapies, but phase 1 trial design has remained the same with the assumption the higher the dosing, the more effective the treatment, not only for targeted but also for hormones and immunotherapy as well. Phase 1 trials for drugs like the targeted agents should be designed to determine the minimum effective dose. For treatment of prostate cancer, strategies for using lower doses of enzalutamide (52) and abiraterone (51) have been described, but it is important to emphasize that phase 1 studies for agents like enzalutamide or apalutamide were performed with a very limited number of patients and that they clearly were not meant to evaluate elderly and fragile patients with comorbidities, those who frequently will be using these drugs outside clinical trials in real world.
Few trials have assessed duration of androgen deprivation therapy. Intermittent medical hormonal therapy appeared equivalent to continuous treatment in randomized controlled trials of ADT largely in the era prior to the advent of multiple lines of life-prolonging therapies for relapsed disease. The role of intermittent ADT is less certain since the gains of drugs like abiraterone (51) or enzalutamide (52) have been demonstrated for men with hormone-sensitive prostate cancer, but intermittent ADT is certainly an option with less direct and indirect costs for men in LMICs, with low volume of disease, asymptomatic and especially those with co-morbidities. Even with the new hormone therapies, the question of whether indefinite vs time-limited dosing is needed is still an open one. There is a need not only to explore the minimum dose needed but also the minimum time: using too much drug for too long is financially damaging and can also leads to higher chronic toxicities not usually explored in advanced disease (65).
There is no clear interest from pharmaceutical companies to explore studies using reduced doses or duration schedules. This is an important mission that should be accomplished by governments, health care providers and academia, specially as an international collaboration. Near-equivalence studies have been proposed that combine various types of evidence to support the acceptability of an alternate treatment relative to standard of care (63). The approach of near equivalence aims to provide adequate efficacy for the greatest number of patients under real-world circunstances; with that, it can increase access to treatment and reduce financial toxicity, with minimal impact on efficacy and decreased toxicity. Evidence that could support the “near equivalence” of a new treatment include using an alternative drug in the same class as an approved standard of care; re-evaluation of “failed” non-inferiority studies which sometimes show similar outcomes even though they do not prove statistical non-inferiority, and combining pharmacodynamics and pharmacokinetic evidence of efficacy within small clinical trials (64). Lead by Ian Tannock, the randomized study comparing of abiraterone given at the “usual” dose of 1000mg/day (fasting) versus 250mg/day with food (51) is an example of a near-equivalence trial. In a recent consensus meeting with specialists focusing on limited resource countries, the use of low dose abiraterone was voted as a major strategy for most specialists (51).
Examples are also provided by the immunotherapy drugs nivolumab and pembrolizumab used in renal cell and urothelial cancers (28, 66). A large (N = 296) phase I trial of nivolumab demonstrated no trends to differences in target-binding, response rate, or survival at doses ranging from 0.1 mg/kg to 10 mg/kg every 2 weeks, and a phase II randomized trial for patients with advanced renal cell cancer showed no dose-response relationship for its primary end point (PFS) at 0.3, 2.0, and 10 mg/kg every 3 weeks. Eventhough, the dose and schedule in the registration trial for renal cell cancer was 3 mg/kg every 2 weeks (much higher than the minimal effective dose). Another important point, regarding the serum half-life of nivolumab, which is considered to be around 2-3 weeks, pharmacodynamic studies have indicated target occupancy on T-cells > 70% for at least 2 months (66). Pharmacodynamic analysis of pembrolizumab from early trials suggests maximum target at 1 mg/kg or greater, with no increased target inhibition up to 10 mg/kg. Other dosing trials found no trends to differences in antitumor activity (or toxicity) for pembrolizumab given at 2 or 10 mg/kg every 3 weeks for treatment of melanoma or non–small-cell lung cancer (67). Pembrolizumab is usually given at a fixed dose of 200 mg every 3 weeks, but its activity would likely be maintained at lower doses or with less frequent injections, with possible reduction in immune-related toxicities and costs for sure. For renal cell cancer, one of the discussions to better select patients for more affordable therapies has been the use of single agent anti-VEGF inhibitors for patients with low-risk advanced disease. The rational came from studies comparing sunitinib versus more modern and expensive combinations like ipilimumab/nivolumab, with better results in more favorable risk patients with the use of sunitinib alone (28, 29), in this specific scenario, the use of more “old” single oral agents can achieve excellent results, but even these agents are usually not available in many LMIC.
Results of all cancer RCTs published globally during 2014 to 2017. In that overview we found that only 8% of oncology RCTs (58 of 694) were led by UMICs or LMICs; China (42 of 58 [72%]) and India (6 of 58 [10%]) accounted for most of these trials.
First, only 8% of global oncology RCTs were led by investigators from LMICs and UMICs. Second, almost one-third of trials led by HICs enroll patients in LMICs. Third, HIC-led RCTs that enroll in LMICs do not match the burden of cancer in these countries. Fourth, HIC-led RCTs enrolling in LMICs systematically differ from trials that only enroll in HICs; LMICs are more likely to test new medicines in the palliative setting, have a larger sample size, and are more likely to be considered positive. Fifth, using the surrogate marker of cancer research ecosystem bibliometric output, the LMICs and UMICs most involved in HIC RCTs do not reflect strong endogenous cancer research ecosystems (68).
There is a strong relationship in both the cost-effectiveness and budgetary impact of individual drugs. Spending on cancer drugs has been increasing significantly in countries around the world. In addition to drug spending, the overall cost of cancer care is rising, in part due to spending on non-pharmacological treatments and diagnostic tests. Understanding costs is important, especially when working on a fixed budget. When considering healthcare costs, traditionally the area of greatest focus has been the perspective of the institutional payer, such as governments or insurers. The world of cancer treatment is entering an exciting era, with the arrival of immunotherapy and the development of treatments tailored specifically to the genetic makeup of an individual’s tumor. New targeted therapies are expected to be developed in the coming years in all fields of cancer. While advances in immunotherapy and targeted therapy are exciting, costs will pose a major challenge, particularly as combination therapies are introduced. Risk sharing is likely to be necessary to finance such interventions. Many new cancer drugs are biological agents and only biologically similar agents can be produced after the patent expires. Regulatory agencies have required that such biosimilar medicines undergo basic testing to demonstrate pharmacological equivalence, but rigorous clinical trials are not required. Despite this, however, the cost of developing such biosimilar medicines is considered to be higher than the development of generic medicines (69).
Generic drugs are the most common and easy form of therapeutic substitution (70). Probably, the best example came from India. It is responsible for the production of several generic medicines including those for treatment of prostate and renal cell cancer, and it also exports generic drugs to several countries including the United States, and countries in Europe, South America and Africa. Generics may provides up to 90% in saving costs comparing to reference drugs, being a great opportunity to maintain treatment options and decrease significantly the cost related to medication.
Research on generic drugs supports their safety but gives varying information about equivalence, as the salt used for drug manufacture can come from different sources and quality control can vary. In a study comparing the pharmaceutical quality of generic docetaxel versus Taxotere (Sanofi-Aventis), most generic formulations contained a lower-than-expected amount of docetaxel and/or a high level of impurities (56). Although generic drugs for treating common chronic illnesses had comparable clinical outcomes to original drugs, many physicians, pharmacists, and patients view them as inferior to original brand names due to real or perceived inferior quality and regulation of drugs (especially those produced in LMICs). Beyond chemotherapy, generic drugs are also extensively used in supportive care of pain medicines and even to diseases like acute leukemia (71).
In 2018, the patent for the original brand of abiraterone, Zytiga, was expired, and many generic brands are now available in LMICs for a price less than USD 200 per month (USD 25-50/month for 250mg/day given with food) (51). Similarly, generic enzalutamide (USD 300 per month) and cabazitaxel (USD 150 per cycle) are available in LMICs at a reduced cost comparing to the original brand (52), allowing more patients to be treated with a low cost drug.
As previously mentioned, there are several obstacles to provide adequate oncologic care in LMIC, from health care structures available and specialized staff. When the disease burden is of advanced late-stage diseases, there is usually no role for curative treatments. Hormone blockage can be cost-effectively if provided as surgical castration-orchidectomy, for men with metastases, this will not only reduce costs but also several hospital visits. Drug funding in LMICs should emphasize generics like abiraterone, docetaxel, gemcitabine and sunitinib (now available as a generic as well) (48), smart dose finding studies, and duration studies should be a priority for health care system objectives as well.
Cost control in LMIC as well as improvement in outcomes will not come only for drug availability but need emphasis on education. Health professionals working in oncology need to understand the unnecessary use of resources and futile therapies for non-fit candidates as an important goal as well. As an example, a study from Italy reported that about 45% of low/very low risk prostate cancer patients had unnecessary MRI or CT for staging when no guideline recommends it, at a cost of 5 millions euros per year. In this case, 25% of these patients also received ADT with all of the cost and adverse effects expected and no clear indication (22). The Congressional Budget Office estimated that up to 30% of care delivered in the United States goes toward unnecessary tests, procedures, physician visits, hospital stays, and other unnecessary services without patient`s benefit. Regional variations in health care costs have been documented; as physicians in limited resource regions order and provide evidence-based tests and treatments just as often as their colleagues in higher cost areas (65), but continuous education serves as an important tool to offer adequate care with better outcomes.
At the American Society of Clinical Oncology (ASCO) meeting in 2022, projects by navigator nurses for patient follow-up were presented. It was identified that the individualized assistance offered to these patients, family members and caregivers, accompanied by the nursing team, overcome the limitations of the health system. This process aims to expand access to cancer screening and monitoring for women residing in underdeveloped neighborhoods (72).
We also observed the discussion of disparities in the use of radiotherapy for cancer in the United States, where they are more highlighted by race, socioeconomic level, among others.
Health disparities were most prominently related by race, socioeconomic status, geographic location, insurance status, practice characteristics, and age.
Men were less likely to receive curative therapy or ladder-dose radiotherapy.
Men, blacks and Asians were less likely to receive proton therapy. Lower income was associated with reduced prostate-specific antibiotic testing and treatment with proton therapy or body stereotaxic ray (54).
To improve access to innovative cancer drugs, groups of oncologists interested in developing clinical research were formed not only in Brazil, but throughout Latin America. A great example is LACOG (Latin American Cooperative Oncology Group), dedicated exclusively to the development of clinical and translational research in cancer. Currently, the group has 400 research members in 16 Latin American countries.
We can cite as an example the study Alternative therapies to the scarcity of Bacillus Calmette-Guérin for non-muscle-invasive bladder cancer in Brazil and other underdeveloped countries: management considerations, which was published in 2019 in the Journal of Global Oncology, where it cites that review of literature aims to clarify alternatives to BCG during the shortage and propose measures to replace BCG, mainly in Brazil and probably in other low- and middle-income countries, where not all the treatments studied and commonly suggested are available (73).
• Establish adequate drug prices that allow pharma manufacturers to maintain production and continue innovation while also ensuring medicines that are affordable for the country
• Develop clear regulations for drug quality manufacturing and supply, favoring the use of generics whenever possible
• After careful discussion with specialist and researchers, establishment of high-priority cancer medicines for all the public and private health systems, following discussion of price reduction with the manufactors.
• Implement and continue monitor price policies paid by patients for cancer medicines in different health systems.
• Establish continued health technology assessment with multidisciplinary teams as well as in different levels in the community (country, states or cities) to provide improvement.
• Analysis trough integrate data of major outcomes, like hospitalization, mortality, costs per patient, to be able to provide continued better results and inform society.
The availability of new systemic therapies associated with better outcomes and survival for GU tumors is a major obstacle for most LMIC. Strategies to improve access are necessary and depend not only on drug availability, but from public health care system organization, discussion and priorities as well as strategies to decrease cost by rational treatment decision and individualize use of systemic therapies in limited resource countries. Efforts should be implemented to provide more real-world data coming from LMIC and studies focusing in strategies to decrease drug costs are urgently needed. We suggest increasing the portfolio of modern clinical trials in LIMC. This is a low-cost and easy way to enable cancer patients to have access to LMIC treatment (mainly targeted therapies and immunotherapy).
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
VF did: Introduction; Genito-urinary tumors in LMIC; Treatment pathways advances; Final recommendations for new drug incorporation in LMICs; Conclusions; References. DH did: Resources in LMIC; Drug Treatment; Possible ways to improve access to anticancer drugs; Pharmacoeconomic studies; Introduction of low-cost generic medications. All authors contributed to the article and approved the submitted version.
DH Honoraria: Janssen, Astellas, Merck Serono, Bristol-Myers Squibb, MSD Consulting or Advisory Role: Astellas Pharma, Janssen, Bayer, MSD, Merck Serono Research Funding: Pfizer, Janssen Travel, Accommodations, Expenses: Astellas, Janssen, MSD, Bayer.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. World Health Organization (WHO). Cancer: key facts sheet (2018). Available at: https://www.who.int/news-room/fact-sheets/detail/cancer (Accessed July 5, 2020).
2. World Health Organization. Technical report: Pricing of cancer medicines and its impacts: A comprehensive technical report for the world health assembly resolution 70.12: Operative paragraph 2.9 on pricing approaches and their impacts on availability and affordability of medicines. (Geneva: World Health Organization) (2018).
3. Van Harten WH, Wind A, de Paoli P, Saghatchian M, Oberst S. Actual costs of cancer medicines in 15 European countries. Lancet Oncol (2016) 17:18–20. doi: 10.1016/S1470-2045(15)00486-6
4. Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends - an update. Cancer Epidemiol Biomarkers Previous (2016) 2516-27. doi: 10.1158/1055-9965.EPI-15-0578
5. Sankaranarayanan A. Screening for cancer in low- and middle-income countries. Ann Glob Health (2014) 80:412–7. doi: 10.1016/j.aogh.2014.09.014
6. Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob Health (2016) 4:e609–16. doi: 10.1016/S2214-109X(16)30143-7
7. de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, et al. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol (2012) 13:607–15. doi: 10.1016/S1470-2045(12)70137-7
8. Hanna TP, Kangolle ACT. Cancer control in developing countries: Using health data and health services research to measure and improve access, quality, and efficiency. BMC Int Health Hum Rights (2010) 10:24. doi: 10.1186/1472-698X-10-24
9. Chan M, Kazatchkine M, Lob-Levyt J, Obaid T, Schweizer J, Sidibe M, et al. Meeting the demand for results and accountability: A call to action on health data from eight global health agencies. PloS Med (2010) 7:e1000223. doi: 10.1371/journal.pmed.1000223
10. Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, et al. Cancer incidence on five continents. volume IX. IARC Sci Publ (2008) 160:1–837.
11. Gonzalez Block MA, Mills A. Assessing health policy and systems research capacity in low- and middle-income countries. Health Res Policy System (2003) 1:1. doi: 10.1186/1478-4505-1-1
12. Alwan A, Maclean D, Mandil A. Assessment of national capacity for noncommunicable disease prevention and control: the report of a global survey/prepared. WHO/MNC/01.2 69 p. WHO/MNC/01.2. (2001).
13. Robertson J, Barr R, Shulman LN, Forte GB, Magrini N. Essential medicines for cancer: WHO recommendations and national priorities. Bull World Health Organ (2016) 94:735–42. doi: 10.2471/BLT.15.163998
14. Organization for Economic Co-operation and Development. Spending on medicines in OECD countries has increased by almost a third since 1998, according to new OECD data. Available at: http://www.oecd.org/health/drugspendinginoecdcountriesupbyquaseathirddesde1998.
15. WHO. State of the world medicines report. Available at: http://www.who.int/medicines/areas/policy/world_medicines_situation/en.
16. Meara JG, Leather AJM, Hagander L, Alkire BC, Alonso N, Ameh EA, et al. Global surgery 2030: Evidence and solutions for achieving health, well-being and economic development. Lancet (2015) 386:569–624. doi: 10.1016/S0140-6736(15)60160-X
17. Barton MB, Frommer M, Shafiq J. Role of radiotherapy in cancer control in low- and middle-income countries. Lancet Oncol (2006) 7:584–95. doi: 10.1016/S1470-2045(06)70759-8
18. Mendez LC, Moraes FY, Fernandes GDS, Weltman E. Cancer deaths due to lack of universal access to radiotherapy in the Brazilian public health system. Clin Oncol (R Coll Radiol) (2018) 30(1):e29–36. doi: 10.1016/j.clon.2017.09.003
19. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021). doi: 10.3322/caac.21660
20. Ferlay J, Ervik M, Lam F. Global cancer observatory: Cancer today (2020). Available at: https://gco.iarc.fr/today (Accessed 16th April 2021).
21. Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, et al. Cancer statistics for the year 2020: An overview. Int J Cancer (2021). doi: 10.1002/ijc.33588
22. Sokhi HK, Padhani AR, Patel S, Pope A. Diagnostic yields in patients with suspected prostate cancer undergoing MRI as the first-line investigation in routine practice. Clin Radiol (2020) 75(12):950–6. doi: 10.1016/j.crad.2020.08.011
24. Shafey O, Dolwick S, Guindon GE. (2003). Tobacco COntrol Country Profiles, Second Edition 2003. American Cancer Society.
25. Black PC, Alimohamed NS, Berman D, Blais N. Optimizing management of advanced urothelial carcinoma: A review of emerging therapies and biomarker-driven patient selection. Can Urol Assoc J (2020) 14(8):E373–82. doi: 10.5489/cuaj.6458
26. Bray F, Colombet M, Mery L. Cancer incidence in five continents (2017). Available at: https://ci5.iarc.fr/Default.aspx (Accessed 31/1/22).
27. Ferlay J, Colombet M, Bray F. Cancer Incidence in Five Continents. Volume XI; IARC Scientific Publication No.166. (2018).
28. Calvo E, Porta C, Grünwald V, Escudier B. The current and evolving landscape of first-line treatments for advanced renal cell carcinoma. Oncologist (2019) 24(3):338–48. doi: 10.1634/theoncologist.2018-0267
29. Vano Y-A, Ladoire S, Elaidi Réza, Dermeche S. First-line treatment of metastatic clear cell renal cell carcinoma: What are the most appropriate combination therapies? Cancers (Basel) (2021) 13(21):5548. doi: 10.3390/cancers13215548
30. A guide to good practice for digital and data-driven health technologies (2021). Available at: https://www.gov.uk/government/publications/code-of-conduct-for-data-driven-health-and-care-technology/initial-code-of-conduct-for-data-driven-health-and-care-technology (Accessed 31/01/2021).
31. European Urology association guidelines on prostate cancer . Available at: https://uroweb.org/guideline/prostate-cancer/ (Accessed 27/7/2020).
32. Maluf FC, Pereira FMT, Silva AGonçalves, Dettino ALourençoA. Consensus on the treatment and follow-up for metastatic castration-resistant prostate cancer: A report from the first global prostate cancer consensus conference for developing countries (PCCCDC). JCO Glob Oncol (2021) 7:559–71. doi: 10.1200/GO.20.00511
33. Sweeney CJ, Chen Y-H, Carducci M, Liu G. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med (2015) 373:737–46. doi: 10.1056/NEJMoa1503747
34. Tannock IF, Wit Rde, Berry WR, Horti J. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med (2004) 351:1502–12. doi: 10.1056/NEJMoa040720
35. Ryan CJ, Smith MR, Bono JSde, Molina A. Randomized phase 3 trial of abiraterone acetate in men with metastatic castration-resistant prostate cancer and no prior chemotherapy. N Engl J Med (2013) 368(2):138–48. doi: 10.1056/NEJMoa1209096
36. Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med (2014) 371(5):424–33. doi: 10.1056/NEJMoa1405095
37. Chi KN, Agarwal N, Bjartell A, Chung BHa. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med (2019) 381:13–24. doi: 10.1056/NEJMoa1903307
38. Fizazi K, Shore N, Tammela TL, Ulys A. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med (2019) 380:1235–46. doi: 10.1056/NEJMoa1815671
39. Hofman MS, Emmett L, Sandhu SK, Iravani A. 177Lu-PSMA-617 (LuPSMA) versus cabazitaxel in metastatic castration-resistant prostate cancer (mCRPC) progressing after docetaxel: Updated results including progression-free survival (PFS) and patient-reported outcomes (PROs) (TheraP ANZUP 1603). J Clin Oncol (2022) 39(6_suppl):6–6. doi: 10.1200/JCO.2021.39.6_suppl.6
40. Hofman MS, Emmett L, Sandhu SK, Iravani A, Joshua AM, Goh JC, et al. TheraP: A randomised phase II trial of 177Lu-PSMA-617 (LuPSMA) theranostic versus cabazitaxel in metastatic castration resistant prostate cancer (mCRPC) progressing after docetaxel: Initial results (ANZUP protocol 1603). J Clin Oncol (2020) 38(15_suppl):5500. doi: 10.1200/JCO.2020.38.15_suppl.5500
41. Bono Jde, Mateo J, Fizazi K, Saad F. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med (2020) 382(22):2091–102. doi: 10.1056/NEJMoa1911440
42. Balar AV, Kamat AM, Kulkarni GS, Uchio EM. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol (2021) 22:919–30. doi: 10.1016/S1470-2045(21)00147-9
43. Vuky J, Balar AV, Castellano D, O'Donnell PH, Grivas P, Bellmunt J, et al. Long-term outcomes in KEYNOTE-052: Phase II study investigating first-line pembrolizumab in cisplatin-ineligible patients with locally advanced or metastatic urothelial cancer. J Clin Oncol (2020) 38(23):2658–66. doi: 10.1200/JCO.19.01213
44. O'Donnell PH, Balar AV, Vuky J, Castellano D. First-line pembrolizumab (pembro) in cisplatin-ineligible patients with advanced urothelial cancer (UC): Response and survival results up to five years from the KEYNOTE-052 phase 2 study. J Clin Oncol (2017) 39(15_suppl):4508–8. doi: 10.1200/JCO.2021.39.15_suppl.4508
45. Botta GP, Granowicz E, Costantini C. Advances on immunotherapy in genitourinary and renal cell carcinoma. Transl Cancer Res (2017) 6(1):17–29. doi: 10.21037/tcr.2017.02.09
46. Gardner U Jr, McClelland S, Deville C. Disparities in the utilization of radiation therapy for prostate cancer in the united states: A comprehensive review. Adv Radiat Oncol (2022) 7(4):100943. doi: 10.1016/j.adro.2022.100943
47. Schroder FH, Hugosson J, Roobol MJ, Tammela TLJ, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med (2009) 360(13):1320–8. doi: 10.1056/NEJMoa0810084
48. Brand NR, Qu LG, Chao A, Ilbawi AM. Delays and barriers to cancer care in low- and middle-income countries: A systematic review. Oncologist (2019) 24(12):e1371–e80. doi: 10.1634/theoncologist.2019-0057
49. Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med (2016) 375(15):1415–24. doi: 10.1056/NEJMoa1606220
50. Gillessen S, Attard G, Beer TM, Beltran H, Bjartell A, Bossi A, et al. Management of patients with advanced prostate cancer: Report of the advanced prostate cancer consensus conference 2019. Eur Urol (2020) 77(4):508–47. doi: 10.1016/j.eururo.2020.01.012
51. Patel A, Tannock IF, Srivastava P, Biswas B. Low-dose abiraterone in metastatic prostate cancer: Is it practice changing? facts and facets. JCO Glob Oncol (2020) 6:382–6. doi: 10.1200/JGO.19.00341
52. Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med (2019) 381(2):121–31. doi: 10.1056/NEJMoa1903835
53. Collaborators IS-LDBIC. The burden of cancers and their variations across the states of India: The global burden of disease study 1990-2016. Lancet Oncol (2018) 19:1289–306. doi: 10.1016/S1470-2045(18)30447-9
54. Gueye SM, Zeigler-Johnson CM, Friebel T, Spangler E, Jalloh M, MacBride S, et al. Clinical characteristics of prostate cancer in African americans, American whites, and Senegalese men. Urology (2003) 61(5):987–92. doi: 10.1016/S0090-4295(02)02588-8
55. Seraphin TP, Joko-Fru WY, Kamaté B, Chokunonga E, Wabinga H. Rising prostate cancer incidence in Sub-Saharan Africa: A trend analysis of data from the African cancer registry network. Cancer Epidemiol Biomarkers Prev (2021) 30(1):158–65. doi: 10.1158/1055-9965.EPI-20-1005
56. Vial J, Cohen M, Sassiat P, Thiébaut D. Pharmaceutical quality of docetaxel generics versus originator drug product: a comparative analysis. Curr Med Res Opin (2008) 24(7):2019–33. doi: 10.1185/03007990802207874
57. Sathekge M, Bruchertseifer F, Knoesen O, Reyneke F. Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: a pilot study. (2018). doi: 10.1007/s00259-018-4167-0
58. Kantoff PW, Higano CS, Shore ND, Berger R. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med (2010) 363:411–22. doi: 10.1056/NEJMoa1001294
59. Hussain M, Mateo J, Fizazi K, Saad F. Survival with olaparib in metastatic castration-resistant prostate cancer. N Engl J Med (2020) 383:24. doi: 10.1056/NEJMoa2022485
61. Bach PB, Conti RM, Muller RJ, Schnorr GC, Saltz LB. Overspending driven by oversized single dose vials of cancer drugs. BMJ (2016) 352:i788. doi: 10.1136/bmj.i788
62. Patil VM, Noronha V, Menon NS, Bhattacharjee A, Kumar S, Purandare N, et al. Phase 3 randomised study evaluating the addition of low-dose nivolumab to palliative chemotherapy in head and neck cancer. J Clin Oncol 40(17_sippl). doi: 10.1200/JCO.2022.40.17_sippl.LBA6016
63. Serritella AV, Strohbehn GW, Goldstein DA, Lichter AS. Interventional pharmacoeconomics: A novel mechanism for unlocking value. Clin Pharmacol Ther (2020) 108(3):487–93. doi: 10.1002/cpt.1853.
64. Tannock IF, Ratain MJ, Goldstein DA, Lichter AS, Rosner GL, Saltz LB. Near-equivalence: Generating evidence to support alternative cost-effective treatments. J Clin Oncol (2021) 39(9):950–5. doi: 10.1200/JCO.20.02768
65. Vale CL, Fisher DJ, White IR, Carpenter JR, Burdett S, Clarke NW, et al. What is the optimal systemic treatment of men with metastatic, hormone-naive prostate cancer? a STOPCAP systematic review and network meta-analysis. Ann Oncol (2018) 29(5):1249–57. doi: 10.1093/annonc/mdy071
66. Motzer RJ, Rini BI, McDermott DF, Redman BG. Nivolumab for metastatic renal cell carcinoma: Results of a randomized phase II trial. J Clin Oncol (2015) 33(13):1430–7. doi: 10.1200/JCO.2014.59.0703
67. Tu H-Y, Zhang Qi, Wu Y-L. Optimal pembrolizumab dosing for non-small cell lung cancer: further studies still needed. doi: 10.21037/jtd.2017.10.152
68. Rubagumya F, Hopman WM, Gyawali B, Mukherji D, Hammad N, Pramesh CS, et al. Participation of lower and upper middle–income countries in oncology clinical trials led by high-income countries. JAMA Netw Open (2022) 5(8):e2227252. doi: 10.1001/jamanetworkopen.2022.27252
69. Goldstein DA, Stemmer SM, Gordon N. The cost and value of cancer drugs – are new innovations outpacing our ability to pay? Isr J Health Policy Res (2016) 5(40):1–4. doi: 10.1186/s13584-016-0097-0
70. Desai RJ, Sarpatwari A, Dejene S, Khan NF, Lii J, Desai JRRR, et al. Comparative effectiveness of generic and brand-name medication use: A database study of US health insurance claims. PloS Med (2019) 16(3):e1002763. doi: 10.1371/journal.pmed.1002763
71. Meara JG, Leather AJ, Hagander L, Alkire BC, Alonso PN, Ameh PEA, et al. Global surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Lancet (2015) 386(9993):569–624. doi: 10.1016/S0140-6736(15)60160-X
72. Oncology Nursing Socuety, Association of Oncology Social Work, National Association of Social Workers. Oncology nursing society, the association of oncology social work, and the national association of social workers joint position on the role of oncology nursing and oncology social work in patient navigation. Oncol Nurs Forum (2010) 37:251–2. doi: 10.1002/cncr.21214
Keywords: low-middle income countries (LMIC), access of new systemic therapies for Genito-urinary (GU) cancers, treatment pathways advances, resources in LMIC, possible ways to improve access to anticancer drugs
Citation: Herchenhorn D and Freire V (2022) Access of new systemic therapies for Genito-urinary cancers in low-middle income countries. Front. Urol. 2:1020215. doi: 10.3389/fruro.2022.1020215
Received: 16 August 2022; Accepted: 28 November 2022;
Published: 20 December 2022.
Edited by:
Fernando Maluf, Albert Einstein Israelite Hospital, BrazilReviewed by:
Maria Jiang, University Health Network, CanadaCopyright © 2022 Herchenhorn and Freire. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel Herchenhorn, aGVyY2hlbmhvcm5AaG90bWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.