
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Urol. , 21 January 2022
Sec. Urologic Oncology
Volume 1 - 2021 | https://doi.org/10.3389/fruro.2021.796688
This article is part of the Research Topic Rising Stars in Urologic Oncology: 2021 View all 7 articles
 Simone Francavilla1,2,3*
Simone Francavilla1,2,3* Chiara Lonati1,2
Chiara Lonati1,2 Marco Sandri4
Marco Sandri4 Andrea Abate5
Andrea Abate5 Enrico De Marzo1,2
Enrico De Marzo1,2 Carlotta Palumbo1,2,6
Carlotta Palumbo1,2,6 Lucia Aretano1,2
Lucia Aretano1,2 Stefano Belotti1,2
Stefano Belotti1,2 Angelo Peroni2,3
Angelo Peroni2,3 Alessandro Peviani7
Alessandro Peviani7 Stefania Ferretti7
Stefania Ferretti7 Luigi Filippo Da Pozzo8
Luigi Filippo Da Pozzo8 Marco Roscigno8
Marco Roscigno8 Stefano Calza5
Stefano Calza5 Agostino Guerini9
Agostino Guerini9 Sandra Sigala5
Sandra Sigala5 Claudio Simeone1,2
Claudio Simeone1,2 Alessandro Antonelli10,11
Alessandro Antonelli10,11Introduction: The Immune compleX Predictive Index (iXip) is a predictive tool for prostate cancer (PCa) diagnosis that integrates PSA, PSA-IgM, prostate volume, and patient age. The aim of the study was to assess the correlation between iXip and clinically significant PCa in patients who underwent radical prostatectomy.
Material and Methods: A prospective multicenter study was conducted from February 2018 to August 2019 enrolling 235 patients. Stepwise-selected predictors were used to estimate multivariate regression models for each outcome, the reference model with only the set of predictors and the same model with the addition of iXip. The prediction accuracy of the two models was assessed calculating the partial area under the receiver operating characteristic curve.
Results: The ROC curve analysis showed significant differences in terms of partial area under the curve between iXip and pathological Gleason Score ≥ 7 and between iXip and tumor volume ≥ 2.5 mm3. The scatter plot analysis showed a positive linear correlation between iXip and tumor volume (considered as a continuous variable). The subpopulations with pT3–4 disease and cT3 disease and with positive surgical margins showed a significant linear relationship between iXip and tumor volume.
Conclusion: We found elements supporting a possible correlation between iXip and aggressive PCa in terms of Gleason Score ≥ 7 and tumor volume ≥ 2.5 mm3.
Prostate cancer (PCa) is the most commonly diagnosed cancer in men in Italy with 36,074 cases in 2019 (18.5% of the total tumor diagnosis in Italy) (1); the most frequent in males aged in the range between 50–69 and over 70 years (1).
Almost all PCa-related deaths are due to the development of metastatic condition, which substantially remains an incurable disease (2–4). Accordingly, many efforts have been done to achieve an early diagnosis, mainly through the widespread diffusion of prostate specific antigen (PSA) and inherent diagnostic framework (5). However, the indiscriminate adoption of PSA-based strategies has also determined a substantial rate of diagnosis of clinically insignificant diseases and consequently exposed patients to overtreatment (6). Thus, various risk-stratification tools have been suggested in order to distinguish clinically relevant from insignificant PCa at diagnosis (7–10).
The iXip (Immune CompleX Predictive Index, Xeptagen, Venice, Italy) is a diagnostic integrated tool designed to improve the predictive performance of the PSA-IgM immune complexes only merging these data with serum PSA value, prostate volume, and patient’s age (11, 12). The output generated by the algorithm is a numerical value ranging between 0% and 100% directly correlated with the risk of PCa at biopsy. A recent paper from our group (13) reviewed the actual clinical applications of this biomarker in biopsy naïve patients (11, 12, 14), in men with previous negative biopsy (15), and in patients with clinically significant cancers (12, 14) and during active surveillance (16). Based on the association of iXip with some biological features of PCa, we designed a prospective multicenter study (the Proxima trial) that aims to assess whether preoperative iXip can predict clinically relevance of PCa, as defined on pathological features available at radical prostatectomy specimen.
A prospective multicenter study was conducted from February 2018 to August 2019, the PROXIMA study (ClinicalTrials.gov identifier: NCT03413007). Three third-level urologic centers were involved after local ethical committee approval (protocol number 2969): the Spedali Civili Hospital of Brescia, the University Hospital of Parma, and the Papa Giovanni XXIII Hospital of Bergamo.
Between February 2018 and August 2019, all consecutive patients for radical prostatectomy to one of three urology departments located in North Italy (Brescia, Parma, and Bergamo) were screened for possible involvement in the present study; each patient signed an informed consent. The inclusion criteria were as follows: patients with histologically proven prostate cancer, patients scheduled for radical prostatectomy, and able to provide consent and aged between 18 and 80 years. The exclusion criteria included the following: patients treated with neoadjuvant hormone therapy, salvage radical prostatectomy, with concomitant solid or hematological tumors, autoimmune disorders, immunosuppressive therapies, acute bacterial, or viral infections.
Baseline, clinical, and pathological outcomes, including Gleason Score, ISUP grading group, TNM stage, prostate volume, and tumor volume, were collected anonymously.
We considered the correlation between iXip and the following outcomes as primary endpoints:
- the pathological Gleason Score ≥ 7 or the pathological ISUP GG ≥ 3
- the pathological tumor volume ≥ 2.5 mm3
- the pathological TNM pT stage ≥ 3 or pN > 0
As secondary endpoints, we evaluated the correlation between iXip and
- the clinically significant PCa (CS-PCa): tumor volume ≥ 2.5 mm3 and pISUP ≥ 3 and pT ≥3
- the tumor residual (R1): PSA ≥ 0.1 or positive surgical margins
The diagnosis of PCa was based on prostate biopsy done according to the protocol adopted by each institution, in general magnetic resonance imaging (MRI), while in a minority of cases the biopsy was done according to standard template (mapping). The indication to surgery was founded on international guidelines (17) and established by clinicians at each institution, generally after multidisciplinary discussion. Surgery was conducted through robotic, open, or laparoscopic approaches, according to the preference of the referring surgeon. Pathological examination was done at each institution by expert uro-pathologists, blinded of iXip evaluation.
Prostate volume was determined as a common procedure preceding the prostate biopsy by transrectal ultrasound using the ellipsoid formula (Volume = Length × Height × Width × π/6) (18). The tumor volume was calculated by the expert local uro-pathologist as the percentage of prostatic involvement by cancer using specific software that evaluated the area covered by the entire tumor foci manually marked on every microsection (19). To calculate the volume in cubic centimeters (cm3), the equation offered in the study by Humphrey et al. (20) was applied: tumor volume (cm3) = % of tumor volume × number of sections × 0.567 (conversion factor).
iXip was calculated for each patient using the online calculator http://iXip.xeptagen.com considering the initial PSA at biopsy, the PSA-IgM, the patient’s age, and the prostate volume. After patient enrollment, during the routine blood collection, an aliquot of blood was reserved for the PSA-IgM assay, according to the procedures below described. Briefly, serum was obtained from the sample, frozen at -20°C in aliquots of 500 µl each, and sent for the centralized measurement to the Pharmacology Section of the University of Brescia. The expression level of PSA-IgM was measured in duplicate (100 µl/sample), using the XEPTAGEN Prostate-IC Kit (Code XG007, Xeptagen SpA, Venice, Italy), following the manufacturer’s instructions. Absorbance was measured by an EnSight Multimode Plate Reader (PerkinElmer Italia, Milan, Italy) at 450 nm. The immunocomplex concentration of the patient samples was read directly on the x-axis by interpolating the absorbance on the standard curve. The PSA-IgM concentration was expressed in arbitrary units (AU/mL), within a linear range from 6.5 to 100 AU/mL). Sample size was calculated basing on data reported on iXip by Gallotta et al. (11) and considering appropriate to recruit at least 76 patients per center, for a total of at least 228 patients.
Categorical variables were summarized as the absolute and relative frequencies, and numerical variables are shown as the median and IQR. For each binary outcome, using stepwise selection the significant predictors were first selected from the following set of covariates: age, Age Adjusted Charlson Comorbidity Score, PSA at diagnosis, prostate volume at the preoperative transrectal ultrasound, number of positive specimens at the prostate biopsy, average % specimen involvement, max % specimen involvement, digital rectal examination, clinical Gleason Score, clinical ISUP, clinical stage T, and clinical Stage N. The selected predictors were then used to estimate two multivariable logistic regression models for each outcome, the reference model with only the set of predictors and the model using the same set, with the addition of iXip. The prediction accuracy of the two models was assessed calculating the partial area under the receiver operating characteristic (ROC) curve, or pAUC (21). The pAUCs were compared using the bootstrap test implemented in the pROC package for R (22). Differences with p-values < 0.05 were considered statistically significant.
Statistical analyses were performed using Stata 16.1 (StataCorp. 2019, College Station, TX)) and R 4.0.3 (R Core Team 2020, Vienna, Austria).
A total of 235 patients fulfilling inclusion criteria were enrolled. Population baseline and clinical and pathological features are summarized in Table 1. The pathological Gleason Score (pGS) ≥7 was present in 179 (76%) patients; the pathological International Society of Urological Grading Group (pISUP GG) ≥ 3 was found in 82 (35%); and at pathological examination, the median tumor volume was 4.8 mm3 (2.5–8.0) and 61 patients (26%) had a TV < 2.5 mm3, 174 (74%) > 2.5 mm3.
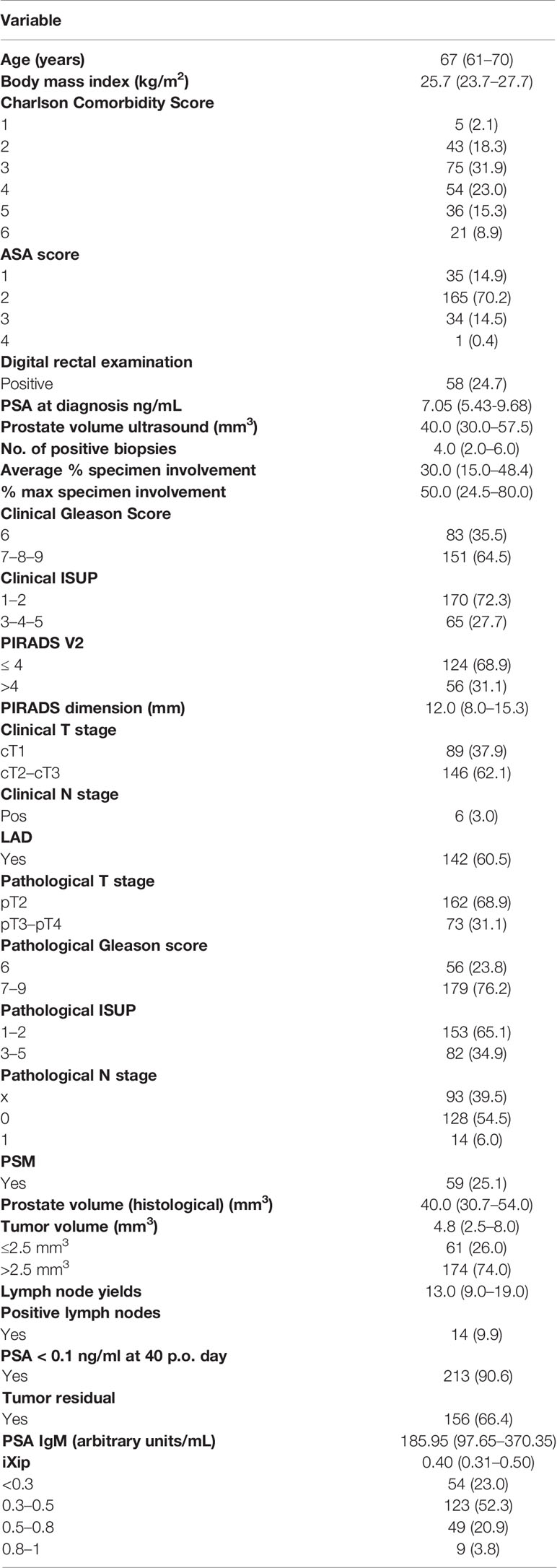
Table 1 Baseline patients’ characteristics: continuous variables were summarized as median and interquartile range, categorical variables as absolute and relative frequencies.
The pathological T2 (pT2) stage was found in 162 (69%) patients while the pT ≥ 3 in 73 (55%) patients; 14 patients (10%) had a lymph nodal invasion out of a total of 142 lymph node dissection performed. In clinically significant PCa (CS-PCa), a composite outcome including tumor volume ≥ 2.5 mm3 and pISUP GG ≥ 3 and pT ≥ 3 was present in 195 (83%) patients.
The tumor residual R1 intended PSA ≥ 0.1 ng/ml pT>3a or pathological N+(pN+) or positive surgical margins (PSM) was found in 79 (34%) patients.
We started considering the association between iXip (categorized into tertiles) and pGS. The number of patients with pGS ≥7 in the lowest tertile (iXip < 0.33) was 54/79 (68%), in the middle tertile (iXip between 0.33 and 0.45) was 67/78 (86%), and in the highest tertile was 58/78 (74%). Fisher’s exact test showed a significant difference between the three groups, p = 0.03.
The rates of patients with pISUP GG ≥3 were 22/79 (28%), 35/78 (45%), and 25/78 (32%), respectively. No statistically significant differences were found between the three groups, p = 0.07.
Considering the middle tertile as the reference group, patients in the lowest tertile showed a significant lower risk of pGS ≥7 (OR = 0.4, 95% CI 0.2–0.8, p = 0.01) and of pISUP GG ≥3 (OR = 0.5, 95% CI 0.2–0.9, p = 0.03). No significant differences were found in the highest tertile in terms of pGS ≥7 (OR = 0.5, 95% CI 0.2–1.1, p = 0.08) and pISUP GG ≥ 3 (OR = 0.6, 95% CI 0.3–1.1, p = 0.1).
Investigating the ability of iXip to identify patients with pGS ≥7, we found that the area under the ROC curve of the reference logistic model without iXip was 0.85; the addition of iXip to the model only slightly increased the AUC to 0.86 (p = 0.2). Comparing the partial AUCs of the two models in the interval with specificity between 0.6 and 1, we found a statistically significant difference (0.28 vs. 0.30; p = 0.02) (Figure 1).
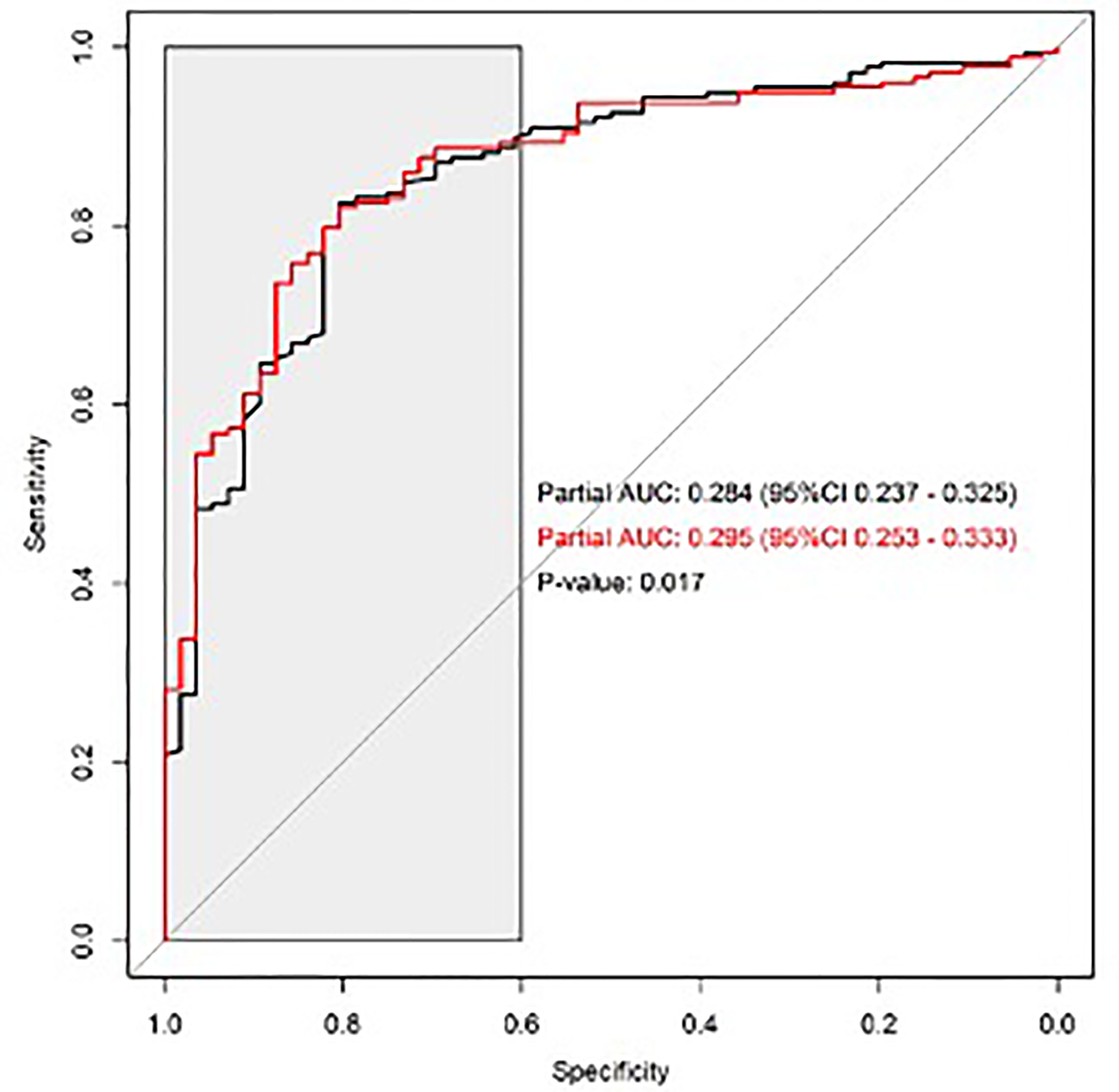
Figure 1 Predictive ability of iXip for the identification of patients with pathological Gleason Score ≥7; ROC curves of the reference model without iXip (black line) and of the reference model with iXip (red line); the gray area indicates the region where the two partial AUCs were calculated and compared.
Considering the tumor volume as a continuous variable, we found a significant linear relationship between iXip and the tumor volume in the subpopulation with pT3-4 disease, with a slope of 14.1 (95% CI 3.1–25.2, p < 0.001) and a correlation of 0.4 (p < 0.001), while for pT2 patients the slope was 0.6 (95% CI -3.3–4.6, p = 0.8) with a correlation of 0.03 (p = 0.7) (Figure 2). The slopes of pT2 and pT3-4 groups were significantly different (p = 0.004).
A significant association between iXip and tumor volume was also found in the clinical T3 (cT3) subpopulation (OR 16.4, 95% CI 3.2–30, p = 0.02).
The subpopulation with positive surgical margins showed a significant relationship between iXip and tumor volume with a slope of 13.2 (95% CI 3.2–23.2, p = 0.01) and a correlation of 0.4 (p = 0.004), while in the group with negative surgical margins the slope was 2.2 (95% CI 2.3–6.7, p = 0.3) with a correlation of 0.06 (p = 0.4) (Figure 3). The two slopes were significantly different (p = 0.03).
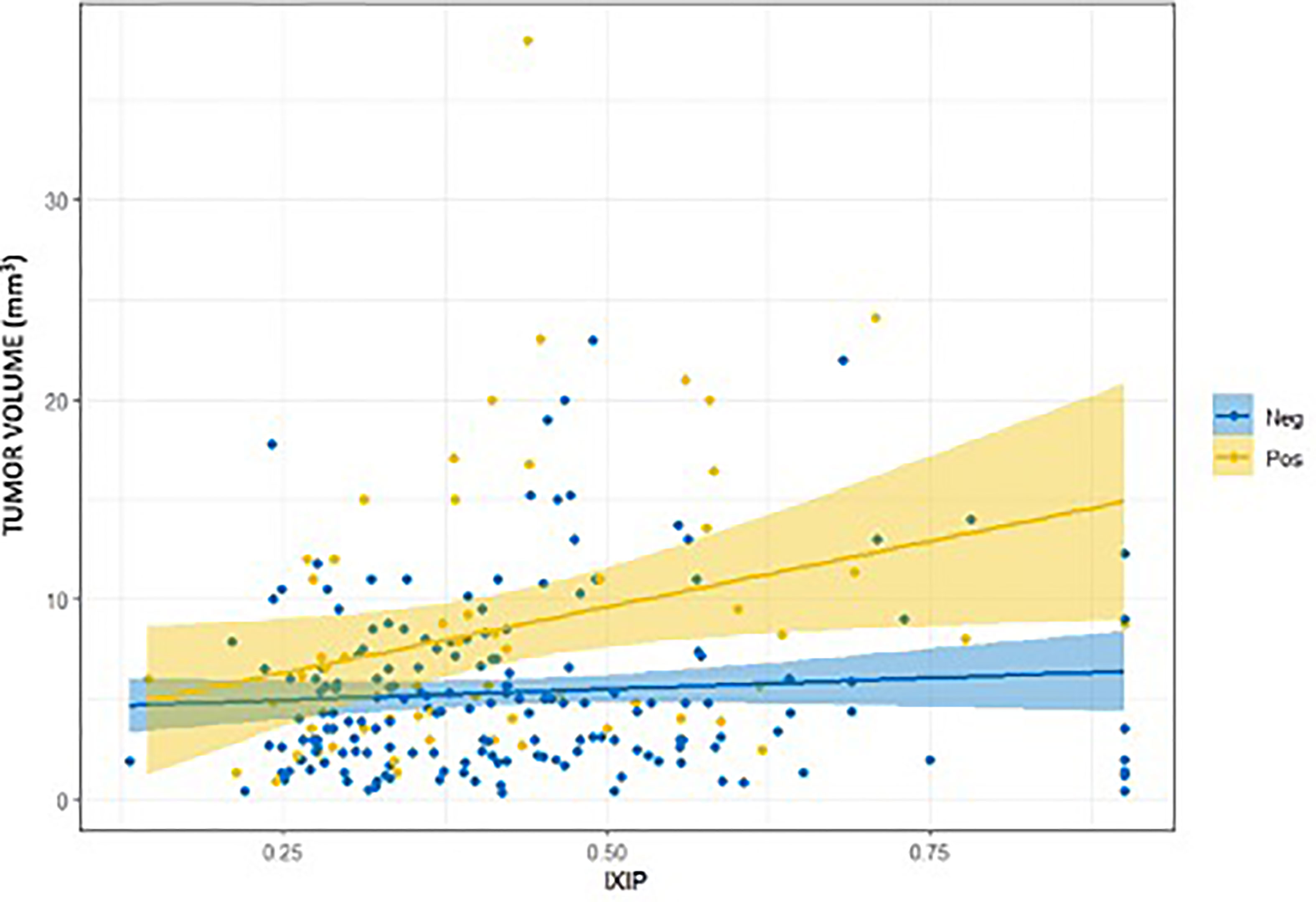
Figure 3 Relationship between iXip and tumor volume splitting in the subgroups of patients with positive (Pos) and negative (Neg) surgical margins.
In this study, we noticed a relationship between iXip and the PCa biological aggressiveness; patients with iXip < 0.33 had a lower risk of pGS ≥ 7 and pISUP GG ≥ 3 considering, as reference tertile, iXip between 0.33 and 0.45.
The design of the current study goes above and beyond the previous research, investigating in depth the predictive ability of the PCa biological aggressiveness from a different perspective. iXip has been evaluated to account for comprehensive clinical data (including MRI) and bioptic features, referring to the description of PCa as a final pathology of radical prostatectomy.
Describing the proportion of patients with significant PCa in the different iXip tertiles, we observed a bell distribution for both the rates of pGS ≥7 and pISUPGG ≥3 which were higher in the middle tertile of iXip (0.33–0.45) than the lowest tertile (iXip < 0.33) and the highest tertile (iXip > 0.45). A possible explanation to this distribution could be found in how iXip was designed: the algorithm was developed in order to optimize the ROC curve at its ends rather than to obtain the best curve based on the highest value of AUC (11).
We acknowledge that the definition of tumor aggressiveness by pGS ≥7 is prone to criticism, but the ability of iXip resulted to be significant in terms of pAUC in the ROC curve only in the pGS > 7 cohort. These results supplement previous study results, based on prostate biopsy, which demonstrated the cutoff 0.3 for GS ≥ 7 disease (12, 14).
Tumor volume represents a key role by defining significant prostate cancer (19, 20), and EAU guidelines recommend to incorporate multivariable clinical risk-prediction tools into the decision-making process (17). The role of iXip as a predictive factor of tumor volume in cT3 tumors, confirmed after surgery in the pT3–4 and PSM subgroup, opens a possibility to integrate MRI variables in a new algorithm in order to better define the clinical tumor volume, further optimizing the diagnostic performance of iXip.
As a limitation of our analysis, this marker was used in a for-cause cohort of men already selected for radical prostatectomy and may perform differently in a screening setting where the prevalence of prostate cancer is lower.
The strengths of our study include the multicenter, prospective study design in which all participants underwent radical prostatectomy for histological evaluation.
We provided evidence supporting a possible correlation between iXip and aggressive PCa at final pathology even in terms of Gleason Score ≥ 7 and ISUP ≥ 3, according to the previous studies, and in addition, we demonstrated an interesting proportional relationship with tumor volume, in particular in the cT3, pT3–4, and PSM subgroups.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Brescia ethical committee, Brescia, Italy. The patients/participants provided their written informed consent to participate in this study.
Conception: SFr, AAn, CS, SS, SFe, LdP. Interpretation or analysis of data: SFr, AAn, CS, SS, MS, SC, CL, AAb, EDM, CP, LA, SB, AlP, MR. Preparation of the manuscript: SFr, CL, AAn, MS. Revision for important intellectual content: AAn, MS, SS, AAb. Supervision: AAn, AnP, CS, SS, AG, SFe, LdP, MR. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Associazione Italiana Di Oncologia Medica. Linee Guida 2020 – Carcinoma Della Prostata (2020). Available at: https://www.registri-tumori.it/cms/sites/default/files/pubblicazioni/2020Numeri_Cancro-pazienti.pdf.
2. Liang Dong L, Zieren RC, Xue W. Metastatic Prostate Cancer Remains Incurable, Why? Asian J Urol (2019) 6(1):26–41. doi: 10.1016/j.ajur.2018.11.005
3. Arnold M, Karim-Kos HE, Coebergh JW, Byrnes G, Antilla A, Ferlay J, et al. Recent Trends in Incidence of Five Common Cancers in 26 European Countries Since 1988: Analysis of the European Cancer Observatory. Eur J Cancer (2015) 51:1164. doi: 10.1016/j.ejca.2013.09.002
4. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2018. CACancer J Clin (2018) 68:7–30. doi: 10.3322/caac.21442
5. Drazer MW, Huo D, Eggener SE. National Prostate Cancer Screening Rates After the 2012 US Preventive Services Task Force Recommendation Discouraging Prostate-Specific Antigen-Based Screening. J Clin Oncol (2015) 33(22):2416–23. doi: 10.1200/JCO.2015.61.6532
6. Li J, Berkowitz Z, Hall IJ. Decrease in Prostate Cancer Testing Following the US Preventive Services Task Force (USPSTF) Recommendations. J Am Board Fam Med (2015) 28(4):491–3. doi: 10.3122/jabfm.2015.04.150062
7. Loeb S, Sanda MG, Broyles DL, Shin SS, Bangma CH, Wei JT, et al. The Prostate Health Index Selectively Identifies Clinically Significant Prostate Cancer. J Urol (2015) 193(4):1163–9. doi: 10.1016/j.juro.2014.10.121
8. Nordström T, Vickers A, Assel M, Lilja H, Grönberg H, Eklund M. Comparison Between the Four-Kallikrein Panel and Prostate Health Index for Predicting Prostate Cancer. Euro Urol (2015) 68(1):139–46. doi: 10.1016/j.eururo.2014.08.010
9. Chevli KK, Duff M, Walter P, Yu C, Capuder B, Elshafei A, et al. Urinary PCA3 as a Predictor of Prostate Cancer in a Chort of 3,073 Men Undergoing Initial Prostate Biopsy. J Urol (2014) 191:1743–8. doi: 10.1016/j.juro.2013.12.005
10. McKiernan J, Donovan MJ, O’Neill V, Bentink S, Noerholm M, Belzer S, et al. A Novel Urine Exosome Gene Expression Assay to Predict High-Grade Prostate Cancer at Initial Biopsy. JAMA Oncol (2016) 2:882–9. doi: 10.1001/jamaoncol.2016.0097
11. Gallotta A, Ziglioli F, Ferretti S, Maestroni U, Moretti M, Aloe R, et al. A Novel Algorithm for the Prediction of Prostate Cancer in Clinically Suspected Patients. Cancer Biomarkers (2013) 13(4):227–34. doi: 10.3233/CBM-130357
12. Gallotta A, Giannarini G, Laurini L, Zani D, Garbeglio A, Guazzieri S, et al. Clinical Validation of the Ixip in Avoiding Unnecessary Prostate Biopsy: Results From a Prospective Multicenter Study Involving 426 Patients. Cancer Treat Res Commun (2017) 10:40–5.
13. Antonelli A, Francavilla S, Gallotta A, Da Pozzo LF, Ferretti S, Sigala S, et al. Current Evidence and Future Perspectives About the Role of Ixip® in the Diagnosis of Prostate Cancer. Minerva Urol Nefrol (2019) 71(3):201–4. doi: 10.23736/S0393-2249.19.03329-0
14. Francavilla S, Ferretti S, Gnocchi C, Dipalo M, Aloe R, Gallotta A, et al. Clinical Evaluation of Immune Complex Predictive Index (Ixip) to Avoid Unnecessary Negative Prostate Biopsies for Prostate Cancer. Abstract Book 89th National Congress of the Italian Society of Urology (SIU). Venice, Italy (2016).
15. Galosi AB, Dell’Atti L, Bertaccini A, Gion M, Francavilla S, Ferretti S, et al. Clinical Evaluation of the Ixip Index to Reduce Prostate Re-Biopsies. Cancer Treat Res Commun (2018) 16:59–63. doi: 10.1016/j.ctarc.2018.07.001
16. Milanese G, Francesco Garofalo F, Mengoni P, Cervelli B, Morecellini R, Betrici V, et al. PSA-IgM Based Algorithm (Ixip Score) During Follow Up of Active Surveillance. Anticancer Res (2017) 37:2051–158.
17. Mottet N, Cornford P, Van den Bergh RC, Briers E, De Santis M, Fanti S, et al. EAU – ESTRO – ESUR – SIOG Guidelines on Prostate Cancer. Edn. Presented at the EAU Annual Congress Amsterdam. Arnhem, The Netherlands: EAU Guidelines Office (2020) p. 978–94-92671-07-3. Available at: https://uroweb.org/guideline/prostate-cancer/.
18. Harvey CJ, Pilcher J, Richenberg J, Patel U, Frauscher F. Applications of Transrectal Ultrasound in Prostate Cancer. Br J Radiol (2012) 85(1):3–17. doi: 10.1259/bjr/56357549
19. Antonelli A, Vismara Fugini A, Tardanico R, Giovanessi L, Zambolin T, Simeone C. The Percentage of Core Involved by Cancer is the Best Predictor of Insignificant Prostate Cancer, According to an Updated Definition (Tumor Volume Up to 2.5 Cm3): Analysis of a Cohort of 210 Consecutive Patients With Low-Risk Disease. Urology (2014) 83(1):28–32. doi: 10.1016/j.urology.2013.07.056
20. Humphrey PA, Vollmer RT. Percentage Carcinoma as a Measure of Prostatic Tumor Size in Radical Prostatectomy Tissues. Modern Pathol (1997) 10(4):326–33.
21. McClish DK. Analyzing a Portion of the ROC Curve. Med Decis Making (1989) 9(3):190–5. doi: 10.1177/0272989X8900900307
Keywords: iXip, biomarkers, PSA, PSA-IgM, prostate cancer
Citation: Francavilla S, Lonati C, Sandri M, Abate A, De Marzo E, Palumbo C, Aretano L, Belotti S, Peroni A, Peviani A, Ferretti S, Da Pozzo LF, Roscigno M, Calza S, Guerini A, Sigala S, Simeone C and Antonelli A (2022) Correlation Between iXip and Final Pathology in Patients Affected by Prostate Cancer Undergoing Radical Prostatectomy: A Multicenter Prospective Trial (PROXIMA—PROstate iXip Index Multicenter Analysis). Front. Urol. 1:796688. doi: 10.3389/fruro.2021.796688
Received: 17 October 2021; Accepted: 03 December 2021;
Published: 21 January 2022.
Edited by:
Juan Gomez Rivas, Hospital Clínico San Carlos, SpainReviewed by:
Ignacio Puche-Sanz, Virgen de las Nieves University Hospital, SpainCopyright © 2022 Francavilla, Lonati, Sandri, Abate, De Marzo, Palumbo, Aretano, Belotti, Peroni, Peviani, Ferretti, Da Pozzo, Roscigno, Calza, Guerini, Sigala, Simeone and Antonelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Simone Francavilla, c2ltb25lLmZyYW5jYXZpbGxhQHBvbGlhbWJ1bGFuemEuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.