- 1Clinical Psychology Section, Transplant Services, Fundacion Santa Fe de Bogotá, Bogotá, Colombia
- 2Transplant and Hepatobiliary Surgery Department, Fundación Santa Fe de Bogotá, Bogotá, Colombia
- 3Faculty of Psychology, Universidad de los Andes, Bogotá, Colombia
- 4Division of Anesthesiology Critical Care Medicine, and Pain Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 5Epidemiology and Biostatistics Group, Graduate School, Universidad CES, Medellín, Colombia
- 6Faculty of Medicine, Universidad de los Andes, Bogotá, Colombia
Background: Liver transplantation (LT) improves survival in end-stage liver disease. Several reports have addressed the impact of LT on patients’ lives, beyond purely medical outcomes. Although the quality of life and mental health have been demonstrated to improve with this procedure, such studies are still missing in Latin America.
Methods: Patients who received LT at the Fundación Santa Fe de Bogotá between 2017 and 2019 were assessed for quality of life (QoL), anxiety, and depression and they were followed up for one year after the procedure. Pre-transplant data were gathered at inclusion on the waiting list, while post-transplant data at 3- and 12 months after LT. European Quality of Life-5 Dimensions (EQ-5D) and European Quality of Life-Visual Analog Scale (EQ-VAS) instruments were used to evaluate QoL. The Hospital Anxiety and Depression Scale (HADS) was used for evaluating anxious and depressive symptoms.
Results: 115 recipients met the inclusion criteria. Mean pre-transplant EQ-VAS was 70.78, rising to 87.16 and 92.56 at 3- and 12-months, respectively. Improvements in all EQ-5D dimensions were found in response to LT. According to the HADS questionnaire, anxiety was reduced by 2.35 points and depression by 1.63 points after LT.
Conclusion: in the short term, LT is a successful strategy for enhancing QoL, anxiety, and depression in patients with liver disease. Long-term benefits must be assessed.
1 Background
Chronic liver disease causes significant physical and psychological impairments secondary to the reduction of the synthetic and detoxifying functions of the liver, as well as complications from portal hypertension and long-lasting cholestasis (1). The likelihood of surviving without a liver transplant (LT) for patients with cirrhosis depends on disease severity, which is assessed using two systems: the MELD score and the Child-Pugh score (2). For instance, 96.5% ± 0.3% of patients with a MELD score below 15 survive for 90 days, while only 15.6% ± 2.5% survive with a score of 40 (3). This contrasts with the overall survival rates of LT recipients, which reach up to 94.4% at one year and 84.1% at five years post-procedure (4). The significant trend toward excellent survival rates has prompted transplant teams to focus not only on medical outcomes but also on ensuring that transplantation enhances patients’ quality of life (QoL) (5–7).
QoL is defined as an individual's perception of his position in life, framed within the set of cultural values in which he is immersed, and that closely defines his goals, expectations, norms, and concerns. It is a broad concept that comprehensively includes the person's physical and psychological health, beliefs, social relationships, and environmental interaction (8). Health-related QoL is based on self-reported physical and mental health measures, including the ability to be socially active (social well-being) (9). The QoL of cirrhotic patients is significantly lower than that of the general population (10–13). Patients with liver disease experience a reduced QoL due to the emotional and physical burdens of the disease. Additionally, psychiatric disorders such as anxiety and depression, along with the perception of frailty, often further impact patients’ QoL (14).
The impact of the transplantation on QoL has raised recent interest in the transplant community (15). Beginning with the waiting list, patients listed for a LT typically experience worsening physical and psychological health, which is further aggravated by the uncertainty of their transplant status (16, 17). However, while Casanovas et al. identified that QoL improvement becomes evident as early as three months after transplantation (13), Girgenti et al. reported that 94% of patients who underwent liver transplant (LT) described their QoL as excellent when considering factors such as physical well-being, physical symptoms, psychological symptoms, existential well-being, and support (18). These benefits seem to also be explained by an amelioration of the anxious and depressive symptoms in these patients (19, 20). Furthermore, a systematic review indicates that the QoL five years after transplantation is comparable to that of the general population. However, it is noteworthy that this improvement primarily relies on emotional and functional benefits rather than on physical improvement (21). Supporting the long-term benefits of transplantation, a study by Domingos et al. evaluating the QoL in Brazilian patients over ten years post-LT found that, except for the mental health domain, their scores were comparable to or higher than those of the general population (22).
In this context, this study aims to evaluate the impact of LT on QoL, anxiety, and depression in patients with liver disease, comparing these outcomes to their pre-transplant baseline. It also examines how these changes interact with disease severity and psychosocial factors present at the time of surgery.
2 Methods
2.1 Design
All patients who underwent LT at the Fundación Santa Fe de Bogotá between 2017 and 2019 were screened for eligibility. A specialized psychology team followed the same operational standards to evaluate all LT candidates and their support networks at the time of their inclusion on the waiting list, and they conducted sequential follow-ups after the procedure. Only patients who were successfully followed up at three- and twelve-months post-transplantation were included. QoL along with symptoms of anxiety and depression, were retrospectively analyzed for changes. QoL surveys were conducted at 3- and 12-months post-procedure, while depression and anxiety symptoms were assessed only one year after the surgery.
2.2 Data collection instruments
Pre-transplant data were collected in person at the time of inclusion on the waiting list, while post-transplant data were obtained through in-person or telephone appointments. These evaluations aimed to assess patients’ understanding, beliefs, and commitment to the transplant process; identify psychosocial and emotional factors; evaluate adherence to medical treatments and healthy lifestyles; examine coping strategies; assess the quality of their support networks; and develop tailored action plans based on individual needs. The questionnaires used to assess patients’ QoL, as well as anxiety and depression, were delivered in a Spanish-validated version adapted to the Colombian context (23–27).
2.2.1 European Quality of Life-5 Dimensions (EQ-5D)
Developed in 1980, the EQ-5D is an instrument that assesses health-related QoL and has demonstrated validity for liver disease and post-LT period (28). From its conception, it was designed to be delivered both in person and via phone interviews (29). The EQ-5D measures the QoL by evaluating the following dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression (30–32).
2.2.2 European Quality of Life Visual Analog Scale (EQ-VAS)
The EQ-VAS is a component of the EQ-5D. It is a vertical scale ranging from 0 (worst imaginable health status) to 100 (best imaginable health status), allowing patients to quantitatively assess their overall health perception (23). Its validity for assessing the QoL in several conditions has been confirmed, making its application alongside the EQ-5D, appropriate for evaluating the QoL in cirrhotic patients and LT recipients (31, 32).
2.2.3 Hospital Anxiety and Depression Scale (HADS)
The HADS comprises 14 items (7 for anxiety and 7 for depression); each item is scored from 0 to 3. Scores from 0 to 7 indicate the absence of symptoms, scores from 8 to 10 suggest that the disease is probably present, whereas scores from 11 to 21 indicate the presence of anxiety or depression (25–27).
2.3 Statistical analysis
Categorical variables are described in terms of their frequency and percentage, and they were compared using the Chi-squared test. Continuous variables were assessed for normality using the Shapiro-Wilk test. Data exhibiting a parametric distribution were presented as the mean and standard deviation (SD), while those with a non-parametric distribution were displayed as the median and interquartile range (IQR). Depending on their distribution and whether the groups were related or independent, comparisons were made using the Wilcoxon test, Kruskal-Wallis test, and Mann-Whitney U-test. The level of statistical significance was set at p < 0.05, and analyses were conducted using Real Statistics v7.9 and SPSS v27 (IBM Corp.).
2.4 Ethical principles statement
This study was approved by the Fundación Santa Fe de Bogotá Corporate Committee for Ethics in Research (CCEI-15246-2023). A waiver of consent was provided as these surveys are part of the standard of care for LT recipients. Clinical data were obtained from the institution's electronic medical records, adhering to the principles of anonymity, privacy, and confidentiality. Access to the information was restricted solely to the researchers. The investigators attested that their conduct was aligned with the ethical principles of the Helsinki Declaration.
3 Results
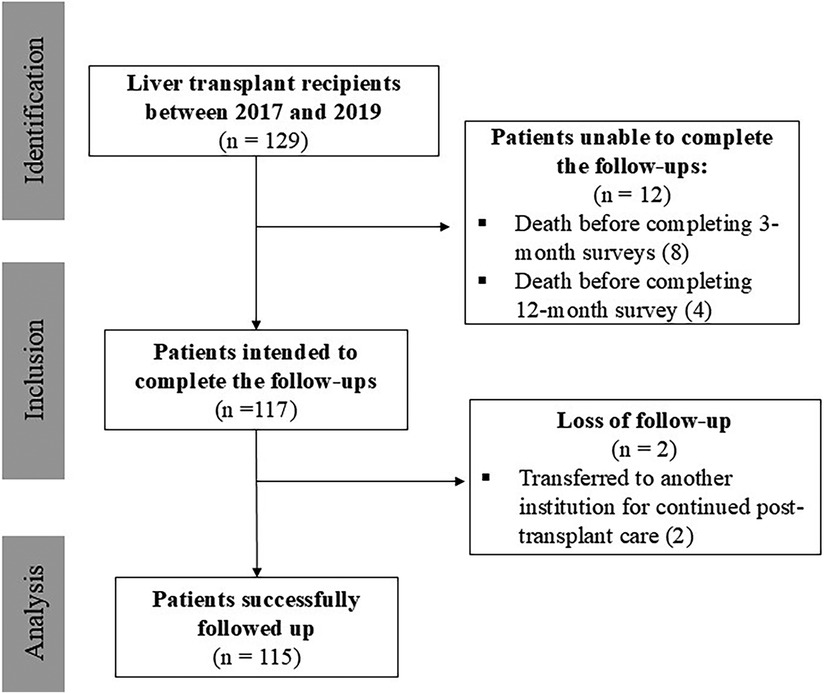
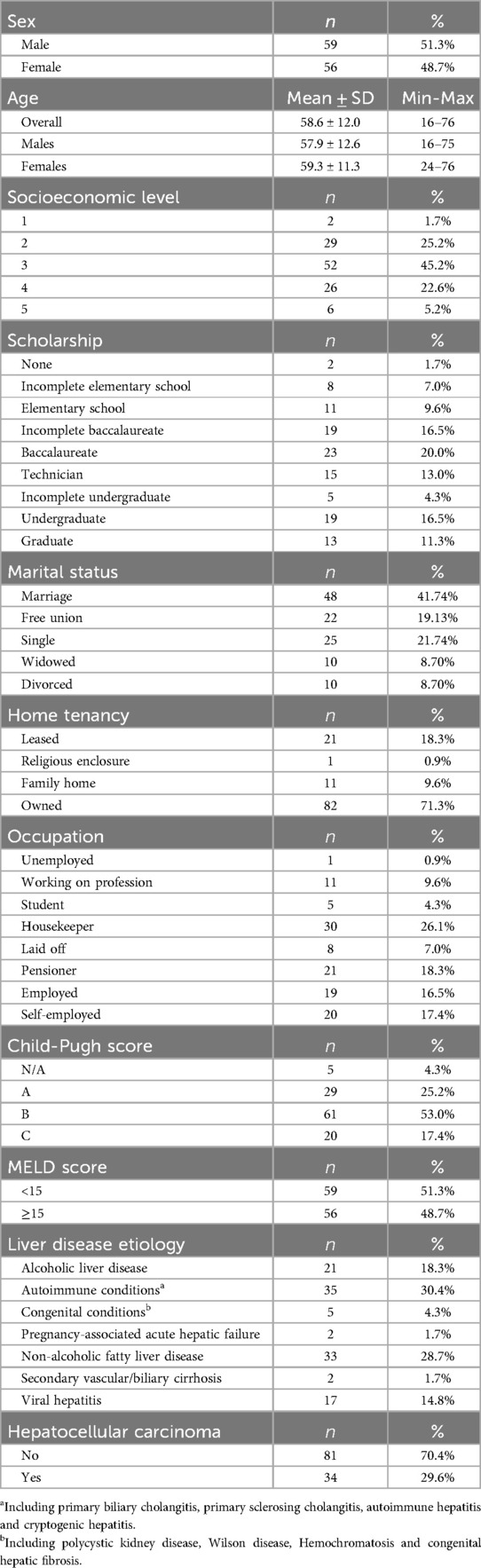
Between 2017 and 2019, 129 patients underwent LT at our institution. Of these, 115 patients met the study criteria (Figure 1). The mean age was 58.6 ± 12 years, fifty-nine were male (51.0%), and fifty-six female (49.0%). Regarding disease severity, the majority had Child-Pugh B cirrhosis (53.0%), followed by Child-Pugh A (25.0%), and Child-Pugh C (17.0%) and 49.0% of patients had a MELD score of 15 or higher. These scores were not applied to 5 patients, as their transplants were indicated for acute hepatic failure rather than the management of cirrhosis. Autoimmune diseases were the leading cause of liver transplantation (30.4%), followed by non-alcoholic fatty liver disease (28.7%) and alcoholic hepatitis (18.3%). At the time of transplantation, 34 patients (29.6%) had a diagnosis of hepatocellular carcinoma. A comprehensive description of patient's demographic and psychosocial characteristics is shown in Table 1.
3.1 Quality of life
3.1.1 EQ-VAS
The mean pre-LT QoL was 70.78 (SD = 17.9). It increased to 87.16 (SD = 13.61) at 3 months and to 92.56 (SD = 10.72) at 12 months post-transplant. The results compared across different time points were significant in all cases (p < 0.001) (Figure 2). Table 2 summarized the impact of the clinical and psychosocial variables on patients’ perception of their QoL evaluated through the EQ-VAS. None of these characteristics seems to have differentially impacted on patient's reported QoL.
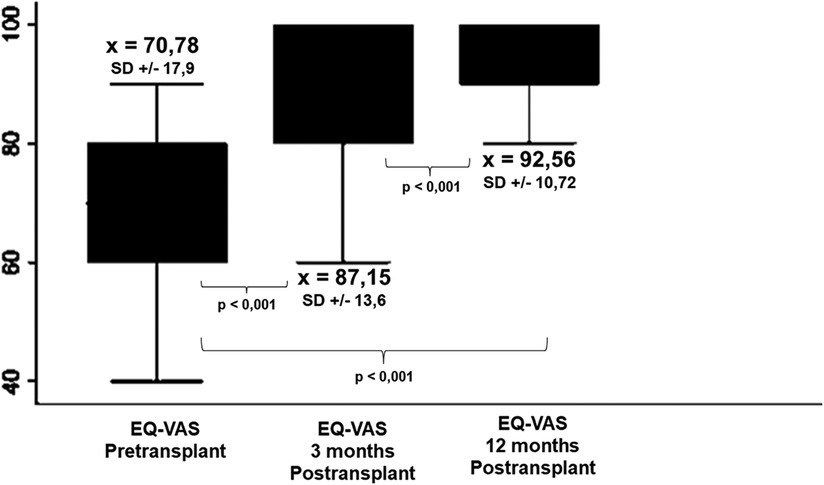
Figure 2. Comparison between Pre- and post-transplant EQ-VAS scores. Self-reported QoL improves as early as 3 months after transplantation and the benefit is maintained and even enhanced at 12 postoperative months.
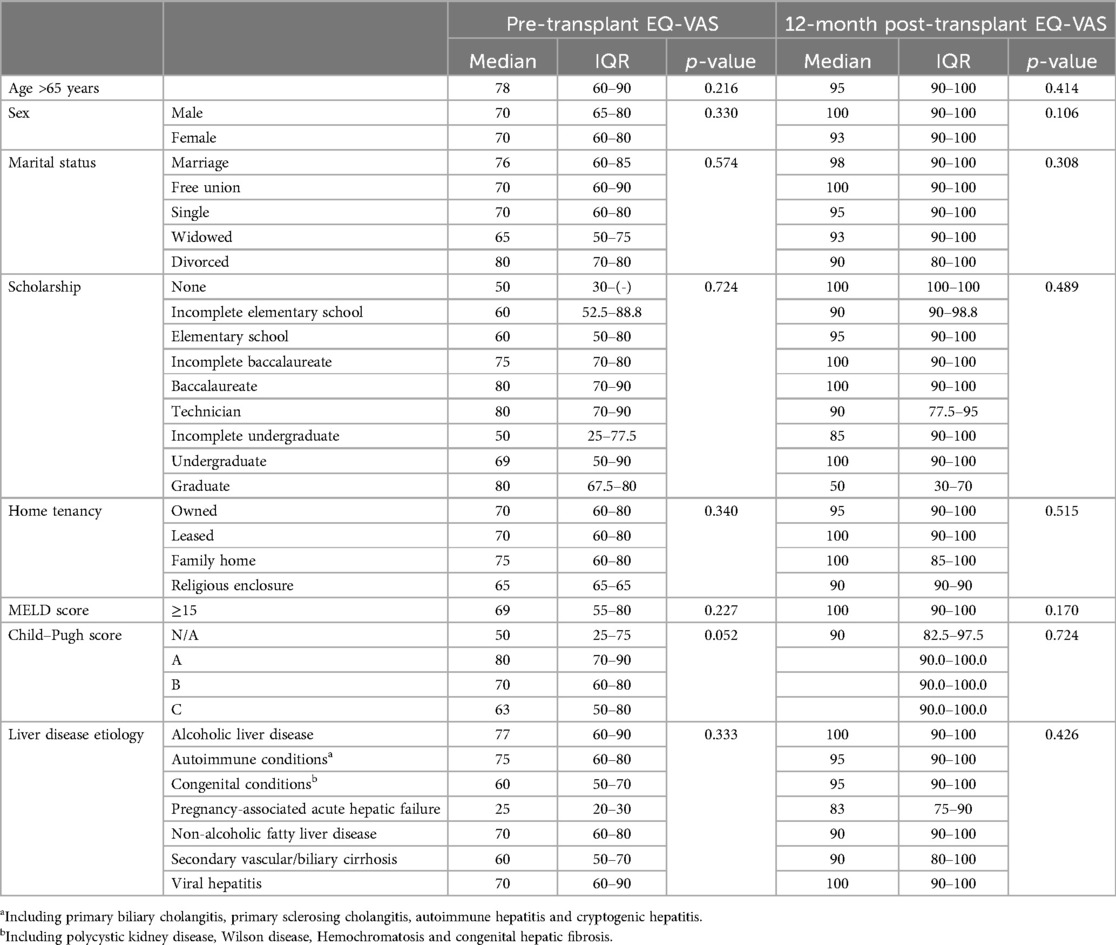
Table 2. Effect of psychosocial and clinical variables on overall pre-transplant and 12-month post-transplant EQ-VAS.
3.1.2 EQ-5D
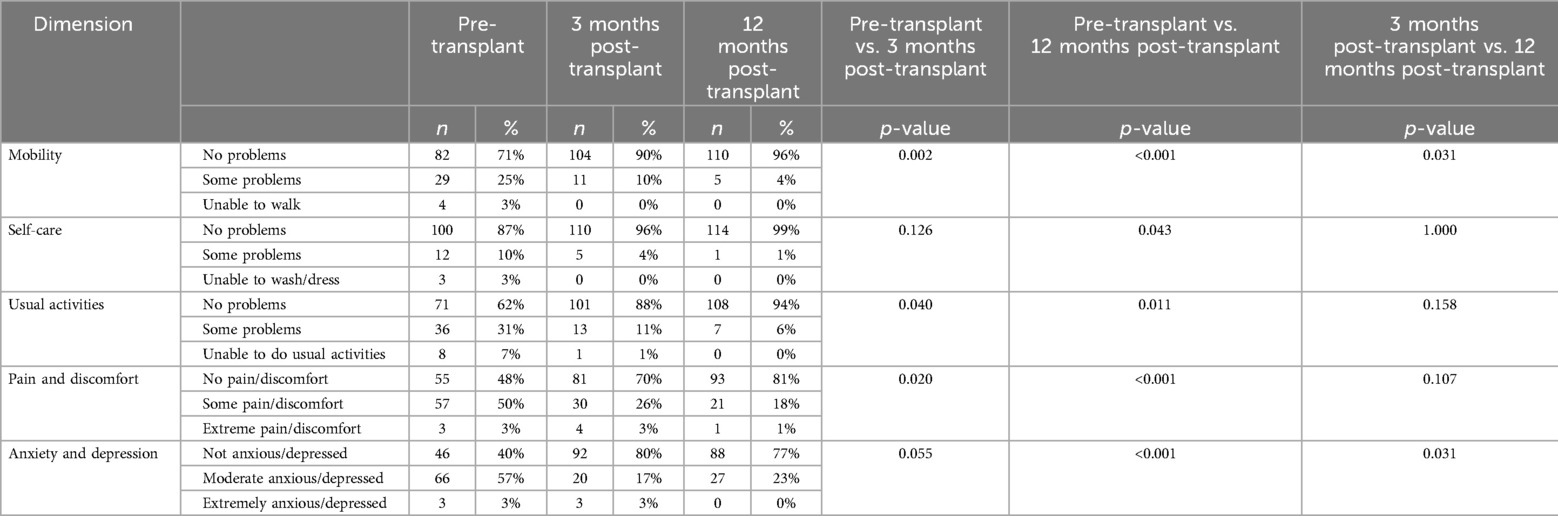
Table 3 illustrates patient progression in each component of the EQ-5D, from the pre-transplant evaluation to the assessments conducted at 3- and 12-months post-transplant.
Mobility: a significant improvement was observed in mobility between the pre-LT survey and 3- (p = 0.002) and 12 months post-transplant (p < 0.001) surveys; there was also a progressive improvement between the third and twelfth post-transplant months (p = 0.031).
Self-care: the capacity for self-care did not show significant improvement in the 3-month survey, but it did improve significantly in the 12-month post-transplant surveys compared to baseline (p = 0.126 and p = 0.043, respectively). Not significant improvement was seen between the third- and twelfth post-transplant surveys (p = 1.000).
Daily activities: patients reported an improvement when comparing pre-transplant surveys with 3- month and 12-month post-transplant surveys (p = 0.040 and p = 0.011, respectively), but no significant progression was seen between the two post-transplant questionnaires (p = 0.158).
Pain and discomfort: similarly, to the previous dimension, LT recipients reported an improvement when comparing pre-transplant surveys with both post-transplant surveys (p = 0.020 and p < 0.001, respectively), but no significant progression was seen between the two post-transplant timepoints (p = 0.107).
Anxiety and depression: the patients only demonstrated and improvement when comparing the pre-transplant surveys with the one-year post-transplant survey (p < 0.001). Improvement was also seen when compared the 3-month and the 12-month post-transplant reports (p = 0.031).
When analyzing each patient's progress across the dimensions (Supplementary Table S1), we found that out of the 29 patients who initially reported difficulties walking, 25 experienced symptom resolution by 12 months post-transplant (p = 0.031). Similarly, self-reported moderate anxiety and depression improved in 71.2% of cases, with extremely anxious or depressed patients showing significant improvement: 33.3% reported no problems, and 66.7% experienced at least moderate symptoms (p = 0.031). Furthermore, all 15 patients who reported self-care difficulties resolved their issues within a year post-transplant, although these differences were not statistically significant (p = 1.000). A comparable trend was observed in the dimension assessing usual activities, where only 11.4% of patients with reported issues and 12.5% of those unable to perform usual activities showed no change in their symptoms after one year (p = 0.158). Pain and discomfort also demonstrated improvement, with only 24.6% of patients reporting some pain and discomfort, and 33.3% experiencing extreme pain and discomfort, persisting at the 12-month mark. Supplementary Table S1 provides a more detailed analysis of each patient's progress across the various dimensions of the EQ-5D at different time points.
3.2 Anxiety and depression
3.2.1 HADS
Figure 3 depicts the overall anxiety and depression scores yielded by the HADS questionnaire. For anxiety, a mean difference of 2.35 ± 3.02 points was observed between the pre-transplant (5.10 ± 3.20) and post-transplant (2.75 ± 2.35) measurements. Regarding depressive symptoms, a mean difference of 1.63 ± 2.76 points was observed between the two periods (3.09 ± 2.72 and 1.46 ± 1.75, respectively). In all the scenarios the differences were statistically significant (p < 0.001). Table 4 displays how the clinical and psychosocial baseline characteristics interact with the overall HADS score during both pre-transplant and post-transplant stages. In this analysis, it was shown that females achieved up to three times more abnormal HADS score when compared to males during the pre-transplant stage (30.9% vs. 11.1%, p = 0.001), although this difference disappeared after receiving the transplant (1.8% vs. 0.9%, p = 0.612). Contrary to what it could be expected, patients who presented alcoholic liver disease did not have a markedly proportion of abnormal HADS scores compared to other etiologies like autoimmune conditions or the viral hepatitis (3.7% vs. 16.0% and 9.9%, respectively, p = 0.030). Moreover, a significant improvement from abnormal to normal scores after the transplant was seen in patients with decompensated Child-Pugh B/C liver disease.
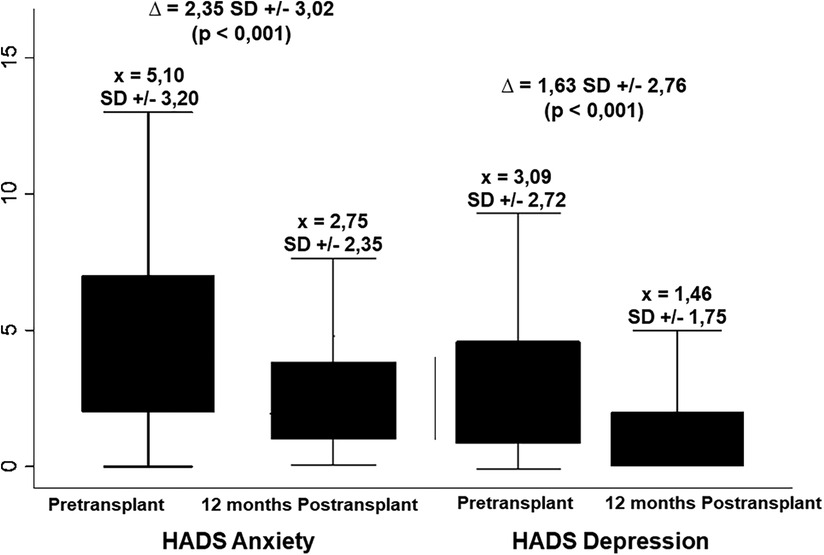
Figure 3. Changes in pre-transplant anxiety and depression according to the HADS tool at 12 months after LT. For both categories, a significant decrease was obtained with the LT.
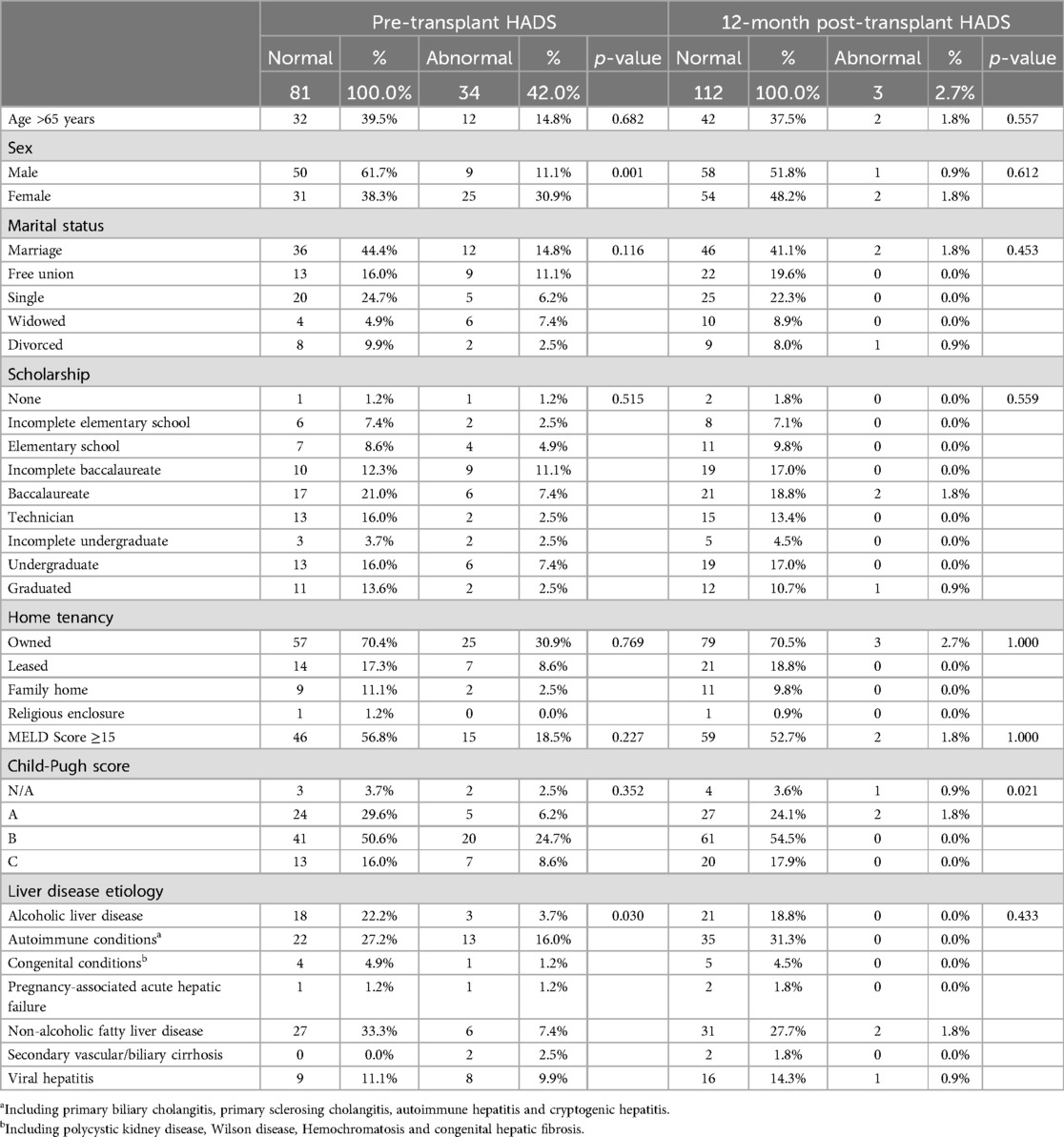
Table 4. Effect of psychosocial and clinical variables on overall pre- and post-transplant HADS score.
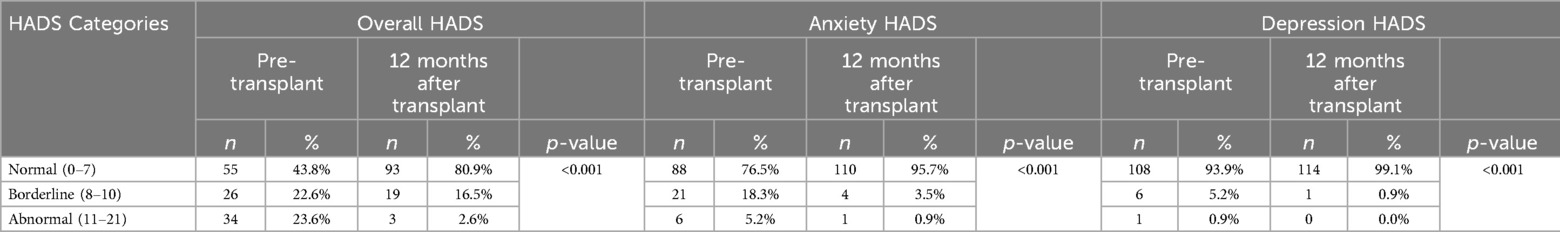
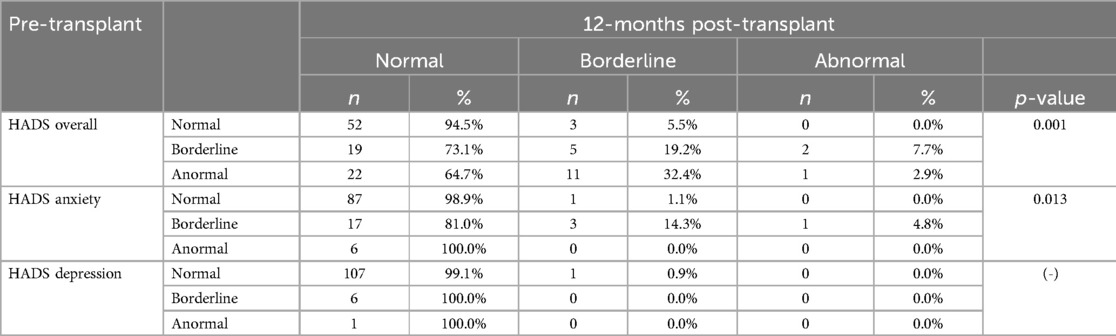
When the results are categorized as normal, borderline, and abnormal, it was observed that the percentage of patients scoring within the normal range on the HADS questionnaire increased from 43.8% in the pre-transplant stage to 80.9% in the post-transplant stage (p < 0.001). Notably, there was a significant improvement in the anxiety questionnaire, where the percentage of patients reporting normal levels raised from 76.5% before the liver transplantation to 95.7% afterward (p < 0.001) (Table 5).
Table 6 displayed in a more detailed way how all the individuals progressed between the two timepoints. These improvements become evident when we examine each patient's progress. For instance, among the 26 patients with a borderline overall HADS score, 73.1% achieved normal scores one-year post-transplant, while 7.7% experienced a decline. A more significant improvement was noted in the 22 patients with abnormal pre-transplant HADS scores: 64.7% moved from an abnormal to a normal score, and 32.4% improved to a borderline score, with only one patient showing no improvement (p = 0.001). Analyzing the HADS anxiety questionnaire reveals that all patients with abnormal scores and 81.0% of those with borderline scores achieved normal results after the transplant (p = 0.013). Furthermore, all patients who initially scored as borderline or abnormal on the HADS depression questionnaire reported normal scores one-year post-transplant, with only one patient who was previously normal now showing a borderline score.
4 Discussion
As the number of LTs increases and the survival rate improves, recent studies are focusing on the evaluation of secondary outcomes beyond purely medical results. How this procedure affects all aspects of a patient's life is a topic of high interest to the transplantation community. This study retrospectively analyzed the QoL, and the anxious and depressive symptoms in a cohort of patients undergoing LT and compared their scores with their pre-transplant baselines.
Being part of an LT program has been shown to provide benefits beyond purely medical outcomes related to liver disease; it also improves the management of other chronic conditions by ensuring timely access to various medical specialties. In Brazil, Bastos et al. found that transplantation not only significantly improved liver-related symptoms, but also other social aspects, disease-related concerns, sexual function, and sleep quality (33). In Turkey, Tamer M. and Yava A. explored the QoL in 103 LT recipients; they concluded that LT had a positive impact on recipients’ QoL and functionality, despite the need for compliance for several medical appointments that this procedure induces and the LT-related complications (34).
Our study provides insights into how LT affects QoL in a Latin American context. We found that the QoL according to the EQ-5D improves when a patient with chronic liver disease receives a LT. This benefit is kept in all the dimensions that are evaluated by this instrument. However, we found that the improvement is not evenly distributed over time, and that LT affects each dimension differently. These findings are consistent with similar studies conducted globally over the past decade (13, 15, 21, 35).
In previous reports, several demographic factors were associated with the QoL in transplant recipients, including marital status, age, gender, and occupation (15, 35, 36). Like in the general population, older patients are expected to have a decline in their QoL (37). It has also been described that LT recipients who remained employed demonstrated better QoL compared to those who were not employed (37). In terms of gender, women often report a significantly lower QoL after LT compared to men (38), although this is not always supported in all the studies (15). Regarding marital status, Mendoza-Sanchez et al. concluded that this variable does not significantly affect the QoL in LT recipients (35). In our study, we found that males and females were homogenously represented, and most were between 50 and 70 years of age. Concerning the sociodemographic characteristics, many were part of the lower-middle class according to Colombian income classification. In our setting, this is typically associated with incomplete education. Most of the patients were married, the majority owned their homes, and most were engaged in household duties. We found no significant differences in QoL across these variable categories.
The EQ-VAS analysis quantified the impact of LT on QoL. In general, a significant improvement in response to transplantation was found. We did not find any differences in this measurement between men and women during the pre-transplant period. However, at three months post-transplant, while both genders reported an enhanced QoL, women had a lower improvement compared to men. These findings are similar to those found by Cowling et al. (38). Interestingly, these differences seem to disappear twelve months after the procedure, supporting Dąbrowska-Bender et al.'s findings (15).
Interestingly, a higher MELD score did not necessarily correlate with poorer QoL, but some were observed when using the Child-Pugh score. This effect was previously described by Cristin et al. and Rabiee et al. (14, 39). These authors explain that this discrepancy happens because the MELD score does not account for symptoms associated with poorer QoL such as encephalopathy and ascites, whereas the Child Pugh score does. Importantly, our population of transplanted patients predominantly suffered from autoimmune conditions, which collectively accounted for nearly one-third of the studied cohort. This was closely followed by cases of non-alcoholic steatohepatitis. Patients with alcoholic liver disease constituted less than one-fifth of the population. This distribution differs from the patterns typically observed in other studies. The potential impact of this pattern on patient-reported QoL should be explored in future studies.
We also found a significant improvement in anxious and depressive symptoms after LT supporting the results of Mejia et al. and Martin-Rodriguez et al. (20, 35). These findings demonstrate that LT has also a positive impact on the emotional health of patients with liver disease. Of note, females had more frequently abnormal HADS scores in the pre-transplant setting similarly to what was described by Dąbrowska-Bender et al. (15), although these differences were ameliorated at twelve months post-procedure. Moreover, an inverse relationship between anxiety and depression levels and QoL was found, both at three- and twelve months post-transplant, as measured by HADS and EQ-5D. This highlights the significant role of psychological health on overall health perception beyond medical issues in LT recipients, as stated by Rabiee et al. (14).
Finally, it's important to note that instruments like the EQ-5D and the HADS primarily serve as supplementary diagnostic tools rather than definitive assessments. They should not replace a thorough psychological or psychiatric evaluation by a skilled team. Moreover, these instruments differ in their performance at detecting symptoms of anxiety and depression as the EQ-5D relies on a single self-reported question, while the HADS provides a more comprehensive evaluation of anxious and depressive symptoms.
5 Future perspectives
Although this and several other studies have assessed short-term QoL in LT recipients, the impact of this intervention over more extended periods remains uncertain. More studies evaluating the QoL and mental health at 3, 5, and 10 years after LT might reveal the true impact of this procedure on patients’ lives and their reintegration into daily activities. These results may also guide transplant teams to intervene in these indicators. Finally, exploring other aspects of QoL beyond those evaluated by the EQ-5D could be worthwhile. Those aspects may include sexual function and other psychosocial factors (21).
6 Limitations
Although QoL questionnaires have been validated for liver disease and transplantation, they are not specific to this condition, and important factors affecting QoL in these patients may have been overlooked. While there are questionnaires specifically designed for cirrhotic patients, they have yet to be validated for use in the Latin American context. Despite efforts to address several variables impacting QoL, not all could be explored, including those related to post-transplant medical conditions and medications. Therefore, confounding variables should be examined more thoroughly in future studies. Lastly, data from patients who died, were lost to follow-up, or could not be evaluated due to their medical condition were not included in the analysis.
7 Conclusions
The medical advancements of the last two decades have rendered LT a safe procedure for the management of chronic liver disease. Consequently, outcomes beyond mere survival must be measured to gauge the impact of transplantation on these individuals. In this context, this study demonstrated that, at least in the short term, LT is a successful strategy for enhancing the QoL of LT recipients. Our results support that in Latin America LT improves the QoL and mental health of the patients, as described in other regions. Special attention and follow-up by the medical, paramedical, and psychological teams must be ensured for women and patients displaying anxious or depressive symptoms, as these subgroups demonstrated less significant improvements in terms of QoL. Timely interventions might enhance this indicator.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Mieth, Klaus; Moreno, Bernardo; Quijano, Diana; Rojas, Jorge; Perdomo, Carlos; Gonzales, Margarita; Palacio, Ana; Santamaría, Eugenia; Caro, Angela; Galvis, Katherine; Garcia, Gloria; Camacho, Maria; Holguin, Lope. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Due to the retrospective nature of the study. All the data was collected per clinical protocol as part of the liver transplantation process.
Author contributions
RC-A: Conceptualization, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. VR-F: Conceptualization, Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. NAC-M: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. DFB-R: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Project administration, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. VM-H: Conceptualization, Validation, Writing – original draft, Writing – review & editing. SS-G: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. AV-T: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Fundación Santa Fe de Bogotá Transplant Department through internal resources.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frtra.2024.1476952/full#supplementary-material
Abbreviations
EQ-5D, European Quality of Life-5 Dimensions; EQ-VAS, European Quality of Life Visual Analog Scale; HADS, Hospital Anxiety and Depression Scale; LT, liver transplantation; QoL, quality of life.
References
1. Pérez-San-Gregorio MÁ, Martín-Rodríguez A, Domínguez-Cabello E, Fernández-Jiménez E, Bernardos-Rodríguez Á. Quality of life and mental health comparisons among liver transplant recipients and cirrhotic patients with different self-perceptions of health. J Clin Psychol Med Settings. (2013) 20(1):97–106. doi: 10.1007/s10880-012-9309-0
2. Peng Y, Qi X, Guo X. Child-Pugh versus MELD score for the assessment of prognosis in liver cirrhosis: a systematic review and meta-analysis of observational studies. Medicine (Baltimore). (2016) 95(8):e2877. doi: 10.1097/MD.0000000000002877
3. VanDerwerken DN, Wood NL, Segev DL, Gentry SE. The precise relationship between model for end-stage liver disease and survival without a liver transplant. Hepatology. (2021) 74(2):950–60. doi: 10.1002/hep.31781
4. Service NH. Annual Report on Liver Transplantation. Available online at: https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/24593/nhsbt-liver-transplant-report-2021-final.pdf2021 (accessed January 23, 2024).
5. Burra P, Germani G. Long-term quality of life for transplant recipients. Liver Transpl. (2013) 19(S2):S40–3. doi: 10.1002/lt.23725
6. Bravata DM, Keeffe EB. Quality of life and employment after liver transplantation. Liver Transpl. (2001) 7(11B):s119–23. doi: 10.1053/jlts.2001.28520
7. Tome S, Wells JT, Said A, Lucey MR. Quality of life after liver transplantation. A systematic review. J Hepatol. (2008) 48(4):567–77. doi: 10.1016/j.jhep.2007.12.013
8. Haraldstad K, Wahl A, Andenaes R, Andersen JR, Andersen MH, Beisland E, et al. A systematic review of quality of life research in medicine and health sciences. Qual Life Res. (2019) 28(10):2641–50. doi: 10.1007/s11136-019-02214-9
9. Kondo Y, Yoshida H, Tateishi R, Shiina S, Mine N, Yamashiki N, et al. Health-related quality of life of chronic liver disease patients with and without hepatocellular carcinoma. J Gastroenterol Hepatol. (2007) 22(2):197–203. doi: 10.1111/j.1440-1746.2006.04456.x
10. Saab S. Evaluation of the impact of rehospitalization in the management of hepatic encephalopathy. Int J Gen Med. (2015) 8:165–73. doi: 10.2147/IJGM.S81878
11. Bownik H, Saab S. Health-related quality of life after liver transplantation for adult recipients. Liver Transpl. (2009) 15(S2):S42–9. doi: 10.1002/lt.21911
12. Stine JG, Stukenborg GJ, Wang J, Adkins A, Niccum B, Zimmet A, et al. Liver transplant candidates have impaired quality of life across health domains as assessed by computerized testing. Ann Hepatol. (2020) 19(1):62–8. doi: 10.1016/j.aohep.2019.06.018
13. Casanovas T, Herdman M, Chandia A, Pena MC, Fabregat J, Vilallonga JS. Identifying improved and non-improved aspects of health-related quality of life after liver transplantation based on the assessment of the specific questionnaire liver disease quality of life. Transplant Proc. (2016) 48(1):132–7. doi: 10.1016/j.transproceed.2015.11.009
14. Rabiee A, Ximenes RO, Nikayin S, Hickner A, Juthani P, Rosen RH, et al. Factors associated with health-related quality of life in patients with cirrhosis: a systematic review. Liver Int. (2021) 41(1):6–15. doi: 10.1111/liv.14680
15. Dąbrowska-Bender M, Kozaczuk A, Pączek L, Milkiewicz P, Słoniewski R, Staniszewska A. Patient quality of life after liver transplantation in terms of emotional problems and the impact of sociodemographic factors. Transplant Proc. (2018) 50(7):2031–8. doi: 10.1016/j.transproceed.2018.03.113
16. Sinclair M, Poltavskiy E, Dodge JL, Lai JC. Frailty is independently associated with increased hospitalisation days in patients on the liver transplant waitlist. World J Gastroenterol. (2017) 23(5):899–905. doi: 10.3748/wjg.v23.i5.899
17. Stewart KE, Hart RP, Gibson DP, Fisher RA. Illness apprehension, depression, anxiety, and quality of life in liver transplant candidates: implications for psychosocial interventions. Psychosomatics. (2014) 55(6):650–8. doi: 10.1016/j.psym.2013.10.002
18. Girgenti R, Tropea A, Buttafarro MA, Ragusa R, Ammirata M. Quality of life in liver transplant recipients: a retrospective study. Int J Environ Res Public Health. (2020) 17(11):3809. doi: 10.3390/ijerph17113809
19. Martín-Rodríguez A, Pérez-San-Gregorio MA, Domínguez-Cabello E, Fernández-Jiménez E, Bernardos-Rodríguez Á. Funcionamiento biopsicosocial entre pacientes cirróticos en distin-tas etapas del proceso de trasplante y su comparación con trasplantados hepáticos. An Psicol. (2014) 30(1):83–92. doi: 10.6018/analesps.30.1.148241
20. Moreno-Medina K, Gómez MT, Mejía G. Evaluación de la calidad de vida relacionada con la salud pre y post trasplante hepático, en pacientes de un hospital de alta complejidad. Psychologia. (2019) 13(1):65–72. doi: 10.21500/19002386.3721
21. Yang LS, Shan LL, Saxena A, Morris DL. Liver transplantation: a systematic review of long-term quality of life. Liver Int. (2014) 34(9):1298–313. doi: 10.1111/liv.12553
22. Domingos MF, Coelho JCU, Nogueira IR, Parolin MB, Matias JEF, De Freitas ACT, et al. Quality of life after 10 years of liver transplantation. J Gastrointestin Liver Dis. (2020) 29(4):611–6. doi: 10.15403/jgld-2829
23. Herdman M, Badia X, Berra S. EuroQol-5D: a simple alternative for measuring health-related quality of life in primary car. Aten Primaria. (2001) 28(6):425–30. doi: 10.1016/S0212-6567(01)70406-4
24. Bailey HH, Janssen MF, Varela RO, Moreno JA. EQ-5D-5l population norms and health inequality in Colombia. Value Health Reg Issues. (2021) 26:24–32. doi: 10.1016/j.vhri.2020.12.002
25. Cassiani-Miranda CA, Scoppetta O, Cabanzo-Arenas DF. Validity of the hospital anxiety and depression scale (HADS) in primary care patients in Colombia. Gen Hosp Psychiatry. (2022) 74:102–9. doi: 10.1016/j.genhosppsych.2021.01.014
26. Hinz A, Finck C, Gómez Y, Daig I, Glaesmer H, Singer S. Anxiety and depression in the general population in Colombia: reference values of the hospital anxiety and depression scale (HADS). Soc Psychiatry Psychiatr Epidemiol. (2014) 49(1):41–9. doi: 10.1007/s00127-013-0714-y
27. Javier L, Rico MR, Molina M. Adapatacion y validacion de la escala hospitalaria de ansiedad y depresion (HAD) en una muestra de pacientes con cancer del instituto nacional de cancerologia de Colombia. Av Med. (2005) 3:73–86.
28. Cano Gutiérrez C, Arciniegas Rubio A, Germán Borda M, Samper-Ternent R, Gil Laverde F, Londoño Trujillo D. Perception of health-related quality of life using the EURO-QOL in older adults in Bogotá, Colombia. Eur Geriatr Med. (2016) 7(4):340–5. doi: 10.1016/j.eurger.2016.01.008
29. McPhail S, Lane P, Russell T, Brauer SG, Urry S, Jasiewicz J, et al. Telephone reliability of the Frenchay activity Index and EQ-5D amongst older adults. Health Qual Life Outcomes. (2009) 7:48. doi: 10.1186/1477-7525-7-48
30. Devlin NJ, Brooks R. EQ-5D and the EuroQol group: past, present and future. Appl Health Econ Health Policy. (2017) 15(2):127–37. doi: 10.1007/s40258-017-0310-5
31. Janssen MF, Lubetkin EI, Sekhobo JP, Pickard AS. The use of the EQ-5D preference-based health status measure in adults with type 2 diabetes mellitus. Diabetic Med. (2011) 28(4):395–413. doi: 10.1111/j.1464-5491.2010.03136.x
32. Johnson JA, Coons SJ. Comparison of the EQ-5D and SF-12 in an adult US sample. Qual Life Res. (1998) 7(2):155–66. doi: 10.1023/A:1008809610703
33. Bastos KX, Freire de Aguiar MI, Garcia JHP, Dourado Arrais PS. Quality of life and liver transplant: a comparative evaluation between different post-transplant phases. J Young Pharm. (2023) 15(1):167–73. doi: 10.5530/097515050491
34. Tamer M, Yava A. The quality of life of patients after liver transplantation. Ann Med Res. (2022) 29(2):139–43. doi: 10.5455/annalsmedres.2021.04.366
35. Mendoza-Sánchez F, Ramírez-González LR, Reyes-Cruz AA, González-Ojeda A, Hernández-Machuca JS, Fuentes-Orozco C. Evaluacion de la calidad de vida en pacientes en pacientes con trasplante hepatico. Rev Med Inst Mex Seguro Soc. (2016) 54(2):176–81.
36. Aguiar MI, Braga VA, Garcia JH, Lima CA, Almeida PC, Souza AM, et al. Quality of life in liver transplant recipients and the influence of sociodemographic factors. Rev Esc Enferm USP. (2016) 50(3):411–8. doi: 10.1590/S0080-623420160000400006
37. Kotarska K, Wunsch E, Kempinska-Podhorodecka A, Raszeja-Wyszomirska J, Bogdanos DP, Wojcicki M, et al. Factors affecting health-related quality of life and physical activity after liver transplantation for autoimmune and nonautoimmune liver diseases: a prospective, single centre study. J Immunol Res. (2014) 2014:738297. doi: 10.1155/2014/738297
38. Cowling T, Jennings LW, Goldstein RM, Sanchez EQ, Chinnakotla S, Klintmalm GB, et al. Liver transplantation and health-related quality of life: scoring differences between men and women. Liver Transpl. (2004) 10(1):88–96. doi: 10.1002/lt.20013
Keywords: quality of life, European Quality of Life-5 Dimensions (EQ-5D), European Quality of Life Visual Analog Scale (EQ-VAS), Hospital Anxiety and Depression Scale (HADS), liver transplantation (LT)
Citation: Cordoba-Alvarado R, Romero-Fonnegra V, Cortes-Mejia N, Bejarano-Ramirez DF, Maldonado-Hoyos V, Sanchez-Garcia SJ and Vera-Torres A (2024) Quality of life, anxiety, and depression improve at one-year after liver transplantation in patients with advanced liver disease. Front. Transplant. 3:1476952. doi: 10.3389/frtra.2024.1476952
Received: 6 August 2024; Accepted: 28 October 2024;
Published: 21 November 2024.
Edited by:
Linda Sher, University of Southern California, United StatesReviewed by:
Luca Saverio Belli, Niguarda Ca' Granda Hospital, ItalyArchita Desai, Indiana University, United States
Copyright: © 2024 Cordoba-Alvarado, Romero-Fonnegra, Cortes-Mejia, Bejarano-Ramirez, Maldonado-Hoyos, Sanchez-Garcia and Vera-Torres. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alonso Vera-Torres, YWxvbnNvLnZlcmFAZnNmYi5vcmcuY28=; cnVyYWwudHJhc3BsYW50ZXNAZnNmYi5vcmcuY28=
 Rosana Cordoba-Alvarado
Rosana Cordoba-Alvarado Valentina Romero-Fonnegra3
Valentina Romero-Fonnegra3 Nicolas Cortes-Mejia
Nicolas Cortes-Mejia