
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Transplant. , 01 July 2024
Sec. Organ and Tissue Preservation
Volume 3 - 2024 | https://doi.org/10.3389/frtra.2024.1353124
 Paria Mahboub1,2,3
Paria Mahboub1,2,3 Mohamed Aburawi3,4
Mohamed Aburawi3,4 O. Sila Ozgur2,3*
O. Sila Ozgur2,3* Casie Pendexter2,3
Casie Pendexter2,3 Stephanie Cronin2,3
Stephanie Cronin2,3 Florence Min Lin3
Florence Min Lin3 Rohil Jain2,3
Rohil Jain2,3 Murat N. Karabacak2,3
Murat N. Karabacak2,3 Negin Karimian2,3
Negin Karimian2,3 Shannon N. Tessier2,3
Shannon N. Tessier2,3 James F. Markmann4
James F. Markmann4 Heidi Yeh4
Heidi Yeh4 Korkut Uygun2,3*
Korkut Uygun2,3*
Background: Donation after circulatory death (DCD) grafts are vital for increasing available donor organs. Gradual rewarming during machine perfusion has proven effective in mitigating reperfusion injury and enhancing graft quality. Limited data exist on artificial oxygen carriers as an effective solution to meet the increasing metabolic demand with temperature changes. The aim of the present study was to assess the efficacy and safety of utilizing a hemoglobin-based oxygen carrier (HBOC) during the gradual rewarming of DCD rat livers.
Methods: Liver grafts were procured after 30 min of warm ischemia. The effect of 90 min of oxygenated rewarming perfusion from ice cold temperatures (4 °C) to 37 °C with HBOC after cold storage was evaluated and the results were compared with cold storage alone. Reperfusion at 37 °C was performed to assess the post-preservation recovery.
Results: Gradual rewarming with HBOC significantly enhanced recovery, demonstrated by markedly lower lactate levels and reduced vascular resistance compared to cold-stored liver grafts. Increased bile production in the HBOC group was noted, indicating improved liver function and bile synthesis capacity. Histological examination showed reduced cellular damage and better tissue preservation in the HBOC-treated livers compared to those subjected to cold storage alone.
Conclusion: This study suggests the safety of using HBOC during rewarming perfusion of rat livers as no harmful effect was detected. Furthermore, the viability assessment indicated improvement in graft function.
Donation after circulatory death (DCD) grafts are a major resource when expanding the organ pool (1, 2). However, DCD grafts are associated with post-transplant challenges, such as primary non-function, ischemia reperfusion injury, and biliary complications (3–5). Therefore, the improvement of DCD graft quality before implantation is an essential step toward improving the outcome after transplant.
After the procurement of grafts, donor livers undergo a period of cold storage (CS), where they are kept on ice while being transported. During this period, hypoxic conditions lead to adenosine triphosphate (ATP) depletion and build-up of toxic metabolites, resulting in progressive organ deterioration (6). The combination of warm and cold ischemic injury is suggested to increase ischemia reperfusion injury, which explains the inferior quality of DCD grafts after transplantation (4). Machine perfusion (MP) is a new preservation method currently in clinical trials across the world and offers the likelihood of treating these DCD livers (7–11). Different MP protocols from hypothermic to sub-normothermic and normothermic have been studied before and the results have shown that machine perfusion is beneficial in reducing reperfusion injury (12–14). Recent clinical trials showed significant increase in DCD liver graft use after MP compared to CS, with notable reduction in early allograft dysfunction (EAD) and ischemic biliary complications (IBC) (9, 11). Among different perfusion protocols, normothermic machine perfusion with red blood cells (RBCs) is one of the most common methods in clinical trials as it offers viability assessment during the graft preservation period (8, 10, 11, 15).
Gradual rewarming during MP from hypothermia to normothermia has been described as a successful preservation method for eliminating reperfusion injury (16, 17). Meeting the high demand of oxygen with increasing temperatures in gradual rewarming requires the use of an oxygen carrier. In this study, the gradual rewarming with the supplementation of a hemoglobin-based oxygen carrier (HBOC) as the oxygen carrier was designed to assess the feasibility and safety of using an artificial oxygen carrier. The liver function was compared with CS alone as the baseline.
Male Lewis rats weighing 290–350 g were used in this study. Animals received care according to the National Research Council guidelines on animal experiments. The study protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at Massachusetts General Hospital. Inhalation anesthesia with 2%–3% isoflurane and oxygen was administered before and during the procurement. In anesthetized rats, anticoagulation was achieved by injecting 500 U of heparin in 1 ml of saline into the penile vein. The DCD model procedure and in situ warm ischemic time (30 min) have been described previously (18). During this time, the bile duct was cannulated, the portal vein was cannulated using an 18-gauge intravenous catheter, and at the end of warm ischemia period, the liver was flushed through the portal vein with 10 ml 0.9% NaCl at room temperature followed by 30 ml University of Wisconsin preservation solution (UW) at 4 °C. The liver was removed and stored in cold UW (4 °C) during CS preservation.
A total of 10 rat livers were divided into two experimental groups after DCD procurement (n = 5 per group). In the rewarming with HBOC group (rewarm&HBOC), after 270 min CS in UW media, the liver grafts underwent gradual rewarming perfusion from 8 °C to 37 °C for 90 min. Later, the grafts were flushed with 10 ml of cold saline and stored in a Petri dish covered with wet gauze at room temperature for 30 min to mimic the surgical implantation period. Subsequently, the grafts were reperfused at 37 °C for 120 min, with the protocol detailed below. In the CS group, the grafts were kept in ice cold UW for 360 min to align the total storage time of both groups. Subsequently, a 30-min storage period at room temperature was implemented to simulate implantation conditions, followed by reperfusion at 37 °C for 120 min, as in the rewarming group.
The perfusion device is a flow-controlled system for rodent organ perfusion (Figure 1) (19). In the rewarming group, the temperature of the perfusion solution was set at 8 °C at the beginning of rewarming, the minimum allowed by the equipment, and was gradually increased to 37 °C through 60 min. The temperature was kept stable at 37 °C for the final 30 min.
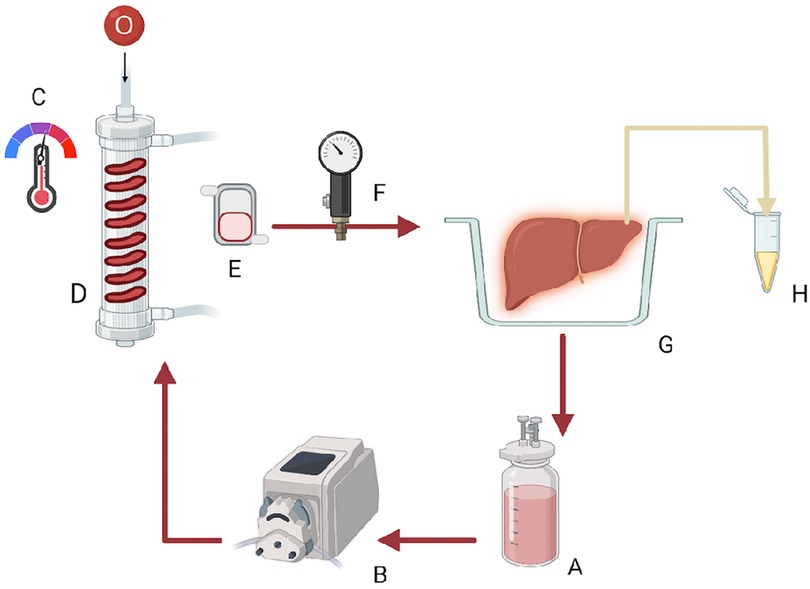
Figure 1 Graphic representation of the rodent liver perfusion system: (A) solution reservoir, (B) roller pump, (C,D) oxygenator containing silicon tubing and providing Carbogen and a heat exchanger with a thermoregulator, (E) bubble trap, (F) pressure probe, (G) organ chamber, and (H) bile Eppendorf.
To mimic the transplant process, a 30-min room temperature period was chosen to reflect the implantation in the recipient as described above followed by a 120 min normothermic perfusion duration, which was considered a simulated early post-transplantation period. In both the experiment and control groups, the perfusion temperature was set at 37 °C and perfusion was continued for a total of 120 min. The flow rate during this period was initially set at 8.0 ml/min and was adjusted according to the portal venous pressure, which was regulated at 50–140 mM H2O.
The perfusion solution consisted of Williams Medium E (Sigma-Aldrich, St. Louis, MO, USA) supplemented with insulin (2 U/L Humulin; Eli Lilly & Co., Indianapolis, IN, USA), L-glutamine (0.292 g/L; Gibco/Invitrogen, Waltham, MA, USA), heparin (1,000 U/L APP Pharmaceuticals, Schaumberg, IL, USA), Albumin 15% (Sigma-Aldrich), and 25% HBOC-201 v/v (provided by Hemoglobin Oxygen Therapeutics, Souderton, PA, USA). The total volume of perfusate was 300 ml for both the rewarming and reperfusion phases. Different HBOC concentrations were tested, and the final concentration was determined based on ensuring detectable hemoglobin in the perfusate, as well as a detectable change in the dissolved oxygen in the media. During the rewarming and reperfusion experiments, the solution was oxygenated using Carbogen, a mixture of 95% O2 and 5% CO2. Note that the same formula was used in both the gradual rewarming group and the reperfusion phase. In the rewarming group, during the 30-min simulated anastomosis time, the perfusion device was flushed clean and the perfusion solution was renewed. Bicarbonate supplementation is adjusted based on perfusate pH levels during rewarming but not during reperfusion to facilitate the clear monitoring of pH differences in both groups.
During rewarming, temperature, flow, and pressure were recorded at 30-min intervals and subsequently resistance was calculated. pH, bicarbonate, lactate, pO2, and pCO2 levels in the perfusate samples were analyzed using an I-Stat analyzer (Abbott, Chicago, IL, USA) every 30 min, and pH was corrected by adding 8.4% NaHCO3−. Bile production was observed and recorded at the end of the 90-min rewarming.
The same perfusion parameters noted in the rewarming procedure in addition to glucose were measured in both groups during 120 min of reperfusion.
Alanine aminotransferase (ALT) was measured in the perfusate samples using an ELISA kit (MBS041480 MyBioSource, Inc., San Diego, CA, USA) during reperfusion in both rewarming and CS groups.
Oxygen consumption during reperfusion was calculated in both groups using the following formula: . In which pO2 was measured in mmHg, sO2 in %, Hb in g/dl, portal vein flow in ml/min, and liver weight in g. c was the oxygen binding capacity of HBOC (1.26) and K was a constant (0.0225).
The tissue samples for ATP measurement were only taken at the end of reperfusion (t = 120 min) to prevent inducing injury to the liver grafts during rewarming and reperfusion. The method of extraction and measurement has been described previously (20, 21).
The produced bile was collected in Eppendorf tubes and measured in ml at the end of the 90-min rewarming period.
Bile production was recorded, and biliary epithelial cell function was assessed by measuring pH and bicarbonate concentration in bile (22). For this purpose, bile samples were collected under mineral oil and were analyzed immediately using the I-Stat analyzer.
Biopsies were obtained from the liver parenchyma at the end of reperfusion phase and were stored in 10% formalin for the histological evaluation. Paraffin-embedded slides of liver biopsies were prepared for hematoxylin and eosin (H&E).
Continuous data were presented as the median and interquartile range (IQR). The Mann–Whitney U-test was used to compare groups. A p-value <0.05 was considered significant. Analyses were performed using SPSS version 22.0 for Windows (IBM Corp., Armonk, NY, USA).
Temperature profile during rewarming is shown in Figure 2A. Portal vein flow was increased and resistance decreased and stabilized toward the end of rewarming perfusion (Figures 2B,C). Regarding other perfusion parameters, the bicarbonate level was slightly improved with the pH within the physiological range in all the liver grafts (Figures 2D,E). The lactate level was constant through the rewarming perfusion in all the liver grafts (Figure 2F). We also observed bile production (0.45–1.25 ml over 90 min) during rewarming, which is an indicator of liver function.
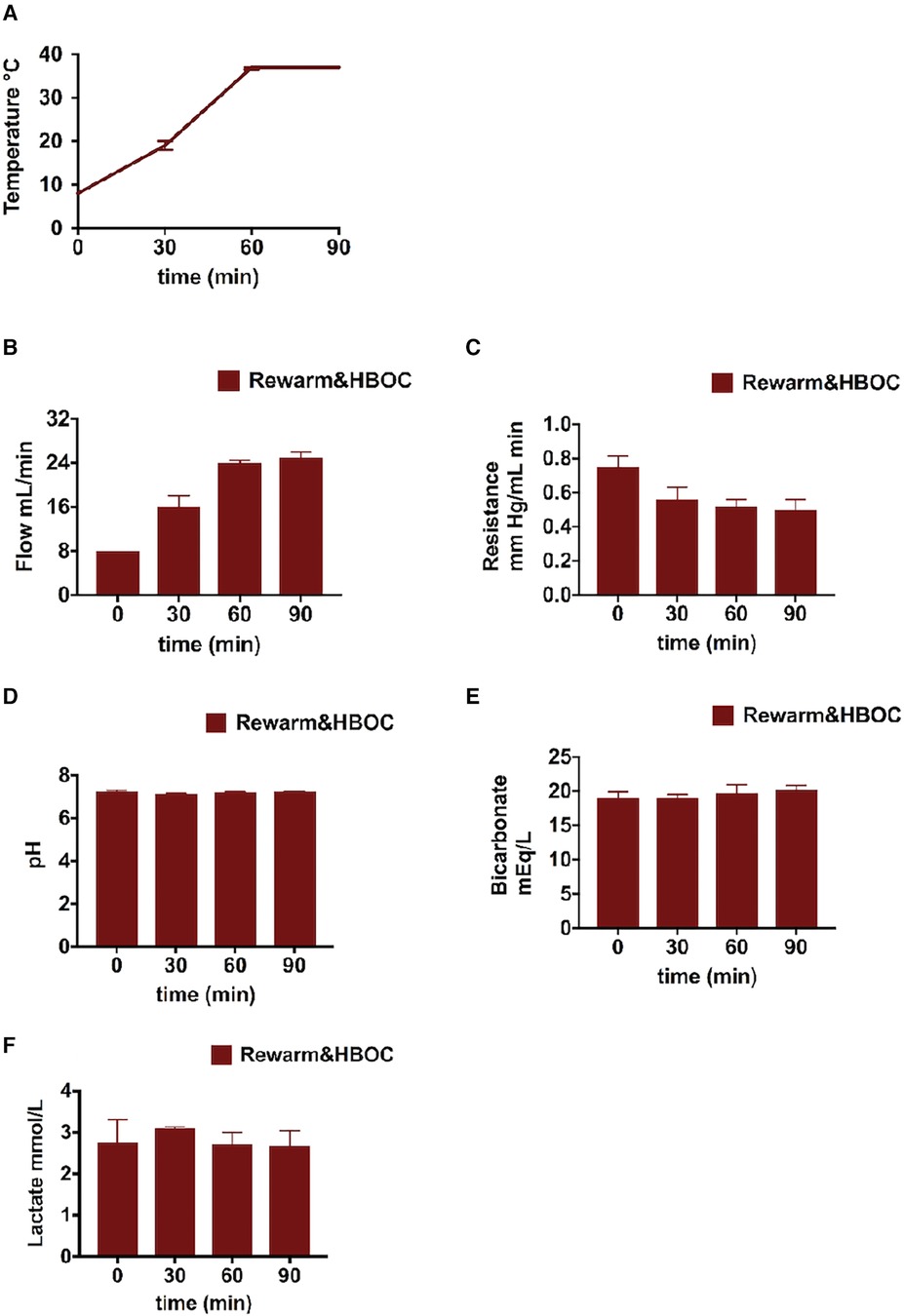
Figure 2 Liver profile during the 90-min gradual rewarming phase of the rewarm&HBOC group after CS: (A) temperature and (B,C) flow was increased and portal resistance slightly reduced during gradual rewarming. (D) pH normalized by the end of the gradual rewarming procedure. (E) Bicarbonate levels in perfusate. (F) Decrease in lactate levels.
pH was closer to the physiological range in the rewarm&HBOC group compared to the CS liver grafts during 120 min of reperfusion (Figure 3A). In the rewarm&HBOC group, the bicarbonate level was higher compared to the CS group in the first hour of perfusion (Figure 3B). Lactate concentrations displayed a slight initial increase, followed by a slight decrease in both groups, with concentration remaining lower in the rewarm&HBOC liver grafts compared to CS liver grafts throughout the rest of reperfusion procedure (between t = 30 and t = 120; p ≤ 0.05) (Figure 3C). The glucose concentration was measured in the perfusate samples during 120 min of reperfusion and was lower in the rewarm&HBOC from t = 30 until the end of reperfusion (p ≤ 0.05) (Figure 3D).
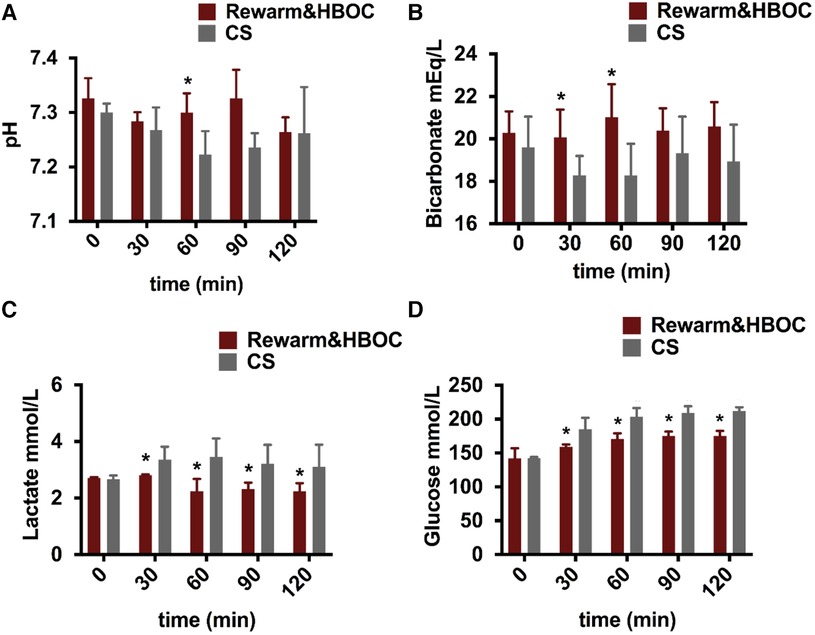
Figure 3 Livers undergoing gradual rewarming showed improved function and recovery compared to CS controls during the 2-h reperfusion with HBOC. (A) pH was better in the rewarm&HBOC group compared to the CS group, with a significant difference at t = 60 (p = 0.016). (B) Bicarbonate levels were better in the rewarm&HBOC group with a significant difference at t = 30 (p = 0.05) and t = 60 (p = 0.032). (C) In contrast to the CS group, the lactate level was significantly lower in the rewarm&HBOC group between t = 30 and t = 120 (p ≤ 0.05). (D) Glucose concentration remained significantly lower in the rewarm&HBOC group compared to the CS group between t = 30 and t = 120 (p = ≤0.05). * represents individual time point significances of the experimental group.
ALT, an indicator of hepatic injury, was measured in the perfusate samples during reperfusion. ALT in the rewarm&HBOC group was somewhat lower compared to that in the CS group, although it did not reach significance, with p = 0.056 (Figure 4A).
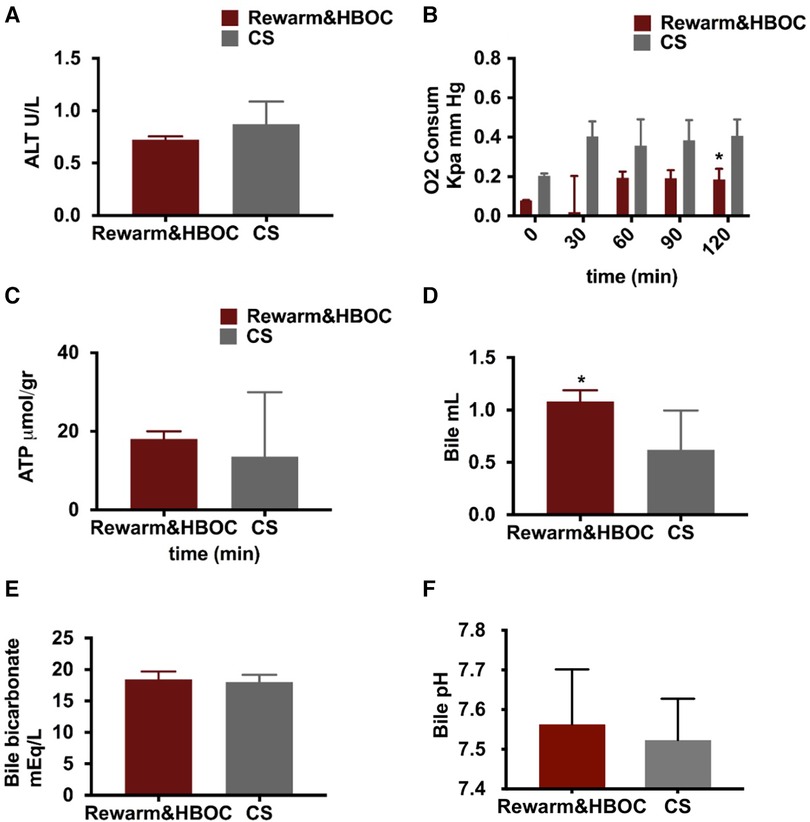
Figure 4 Graphical presentation of oxygen concentration, ATP level, and bile fluid in both the rewarming and CS groups. (A) There was a lower trend of ALT in the rewarm&HBOC group compared to the CS group during 120 min of reperfusion (p = 0.056). (B) Oxygen consumption remained higher in the CS group in comparison with the rewarming group, and this difference was significant at t = 90 (p = 0.024). (C) There was no significant difference in ATP levels between both groups (p = 0.55). (D) The total volume of bile production measured at the end of 120 min of reperfusion was meaningfully higher in the rewarm&HBOC group compared to the CS group. (E) There was no difference in the level of bicarbonate and (F) bile pH in bile samples of the rewarm&HBOC and CS groups during 120 min of reperfusion. * represents individual time point significances.
Oxygen consumption was measured during 120 min of reperfusion and was slightly higher in the CS group in comparison to that in the rewarm&HBOC group, with a significant difference at t = 120 (p = 0.01) (Figure 4B). After 120 min of reperfusion, no statistical differences were found in the ATP production between the rewarm&HBOC and CS groups (p = 0.55) (Figure 4C), although this appeared to be more a result of the high variability in the CS livers and values trended higher with less variability in the rewarm&HBOC group.
Cumulative bile production and biliary cholangiocyte function were measured, the median bile production was higher in the rewarm&HBOC group in comparison with the CS group (p = 0.03) (Figure 4D). There was no difference in the level of bicarbonate (p = 0.6) (Figure 4E) and pH (p = 0.71) (Figure 4F) and in the bile samples between the rewarm&HBOC and CS groups.
As flow rate was increased, resistance in the portal vein gradually decreased in both the rewarming and CS groups, with lower values attained in the rewarm&HBOC group (Figures 5A,B). Overall, it was possible to obtain higher flow rates in livers in the rewarm&HBOC group consistently with pressures that were statistically the same or lower compared to those of CS controls. In parallel with the observed differences, the livers in the CS group showed more signs of venous congestion compared to those in the rewarm&HBOC group (Figures 5C,D).
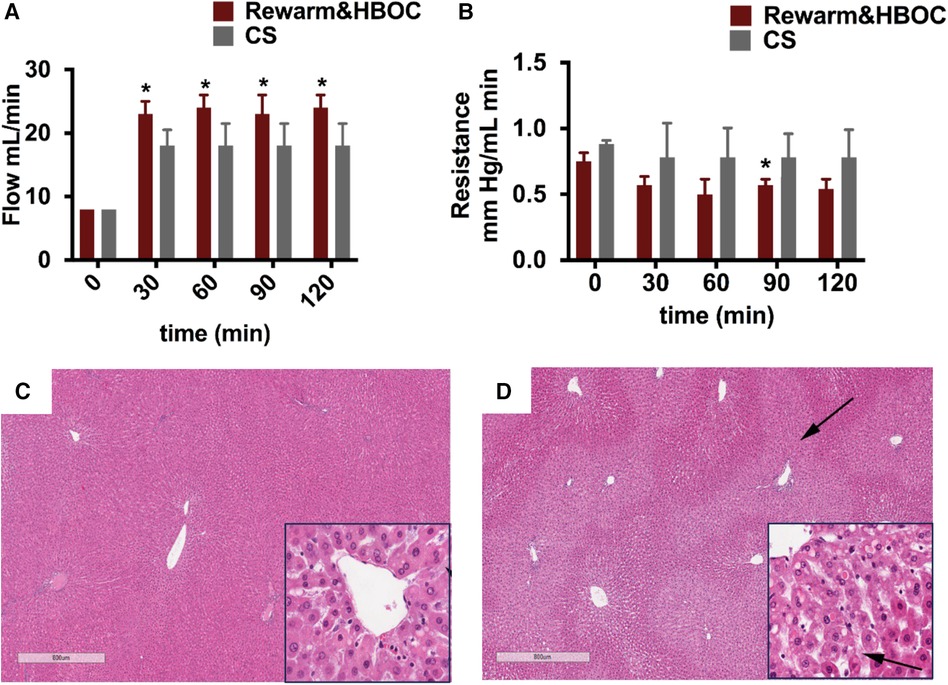
Figure 5 Vascular injury parameters and H&E staining from the rewarming and CS groups at the end of reperfusion. (A) Flow was significantly higher in the rewarm&HBOC group compared to the CS group from t = 30 to t = 120 (p ≤ 0.05). (B) Resistance was lower in the rewarm&HBOC group compared to the CS group during 120 min of reperfusion with a significant difference at t = 90 (p = 0.02). CS liver tissues (D) demonstrated higher venous congestion (stasis of fluid in parenchyma shown by arrows) compared to the rewarm&HBOC liver grafts (C).
There are two key methods used in organ preservation: traditional CS and MP. CS remains the clinical standard owing to its simplicity and low costs. However, CS can exacerbate organ deterioration, particularly in DCD grafts that have already suffered from a hypoxic period. MP is a groundbreaking approach in this field, enabling supplementation of nutrients combined with continuous oxygenation. Previous studies showed significant improvements of post-transplantation outcomes in DCD grafts after normothermic MP compared to CS (11). Although normothermic MP sustains physiological conditions and supplies vital substrates, it triggers a rapid surge in the demand for nutrients and oxygen subsequent to CS that may exacerbate reperfusion injury. The gradual and slow rewarming process, coupled with metabolic support, could potentially alleviate reperfusion injury after cold storage. A single-center clinical trial demonstrated improved early allograft function in livers subjected to rewarming after CS compared to CS alone (23). Comparing both reconditioning protocols, gradual rewarming and normothermic MP, gradual rewarming of liver grafts demonstrated superior results in terms of better energetic recovery and improved function (24). Markedly, gradual rewarming appears to mitigate rewarming injury by adapting to enhance metabolic upregulation.
Effective oxygen delivery during machine perfusion is essential for preserving organ viability and function. A major challenge with increasing temperatures during gradual rewarming is maintaining adequate oxygenation, as the oxygen content in water-based solutions decreases with rising temperatures. Therefore, an additional oxygen carrier is necessary, particularly at temperatures above 20 °C (25). Blood-based products used in machine perfusion provide effective oxygenation and have been shown to remain stable across various temperatures. The utilization of RBCs during gradual rewarming has been demonstrated by van Leeuwen et al. when compared to HBOC, revealing no significant difference between outcomes (26). Despite their efficacy, blood products come with several logistical challenges, including limited availability, high costs, and short shelf life. Moreover, blood-based perfusates may increase the risk of microvascular failure and bacterial growth (27, 28).
Artificial oxygen carriers enhance the rate of oxygenation, while avoiding these adverse events (29, 30). A number of novel oxygen carriers, such as perfluorocarbons (PFCs) and Hemarina (M101), have been developed and used in different organ preservation protocols, such as hypothermic perfusion and cold storage (31), and could be other alternatives. However, the early outcome of adding PFC in kidney machine perfusion showed instability and an adverse effect of PFC on renal function, which limits further use of this oxygen carrier in the perfusion experiment (32, 33). M101 is another novel oxygen carrier introduced recently and has a very high affinity for oxygen. Thuillier et al. have shown that adding Hemarina during cold storage to the preservation saluting improves renal function; however, there is no evidence of using M101 in liver preservation (34). HBOC has been utilized in numerous liver perfusion protocols and has previously demonstrated stability with no adverse effects at different temperatures (24, 25, 35), a crucial factor for the gradual rewarming protocol.
This study shows the feasibility of using HBOC in gradual rewarming of the liver and some end points may suggest improved graft function in rewarming using HBOC compared to the clinical standard, CS preservation. We opted for a 90-min rewarming duration, aligning with temperature stabilization within this timeframe (23), while a 120-min duration for reperfusion has been identified as optimal for ex vivo organ assessment (36). The outcomes of reperfusion are indicated by better flow rate and physiologically balanced perfusion pH and bicarbonate during reperfusion. Better bicarbonate concentration is a result of the liver using up the lactate; therefore, better lactate clearance results in more balanced bicarbonate and pH. The absence of an initial lactate peak in both groups is likely a result of metabolite dilution in the perfusion solution. Regardless, these findings also explain the significantly reduced lactate in the rewarm&HBOC group during reperfusion.
Increased bile production in the rewarm&HBOC group suggests improved liver function as bile production is an early indicator of liver function. Nonetheless, there was no notable difference in bile bicarbonate and bile pH levels between the groups.
The use of HBOC in patients has been reported to induce vasoconstriction and lead to systemic hypertension in some cases (37). In contrast to these earlier studies, however, no evidence of hypertension was detected in our rewarming model and we even experienced positive effects highlighted by significantly higher flow rate and lower trend of vascular resistance in the rewarm&HBOC group. This outcome is in line with the use of HBOC in sub-normothermic and normothermic liver perfusion studies in which no negative effect of HBOC on perfusion pressure and resistance was reported (30, 35). In the rewarm&HBOC group, lactate concentration declined and glucose level in the perfusate remained significantly lower, which demonstrates that the livers in this group had superior lactate and glucose metabolism and better liver function. Low ALT concentration (p = 0.056) in the perfusate samples in the rewarm&HBOC group may indicate lower liver parenchyma injury. Our findings are in concordance with previous rewarming studies showing improved liver transaminase levels with gradual rewarming after CS compared to CS alone (23). The histological examination showed higher liver congestion in the CS group. It has been shown in the literature that hepatic congestion could increase liver enzymes and lead to liver injury (38).
Oxygen consumption was found to be higher toward the end of reperfusion in the CS group compared to the rewarming group with no significant difference in ATP production. This finding is in line with previous results in which the investigators found higher oxygen consumption during reperfusion after prolonged CS preservation, compared to well-preserved liver grafts (39, 40). This difference in oxygen consumption was explained previously by referring to respiratory burst and oxygen debt in severely injured post-ischemic livers with no meaningful increase in ATP production (40), and our results are in concurrence.
The present study has some limitations. In future studies, the function of liver sinosoidal endothelial cells (LSECs) will be evaluated for further assessment of the endothelial structure, and research endeavors involving trials of gradual rewarming with other oxygen carriers will provide better understanding in isolated effects of HBOC. The potential dilution effect during rewarming will be further evaluated with the inclusion of an additional hypothermic machine perfusion group. Investigating reactive oxygen species (ROS) and cytokine profile will also provide insights on the oxygenation benefit of HBOC. While these studies yield informative data, transplantation procedures are crucial for understanding the practical implications. Importantly, the expected cost of incorporating an oxygen carrier into MP is a crucial consideration. To date, this remains uncertain since artificial carriers are not commercially available. However, the presence of multiple competing products in this domain increases the likelihood of reasonable pricing in the future.
In conclusion, this study shows the feasibility of gradual rewarming with the use of HBOC, and increased efficacy in the recovery of DCD liver grafts compared to CS controls in a DCD rat model.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The animal study was approved by the Institutional Animal Care and Use Committee (IACUC), Massachusetts General Hospital. The study was conducted in accordance with the local legislation and institutional requirements.
PM: Writing – original draft, Conceptualization, Data curation, Investigation. MA: Writing – review & editing, Investigation. OO: Writing – review & editing, Data curation. CP: Writing – review & editing, Data curation. SC: Writing – review & editing, Data curation. FL: Writing – review & editing, Data curation. RJ: Writing – review & editing, Data curation. MK: Writing – review & editing, Data curation. NK: Writing – review & editing, Conceptualization, Data curation. ST: Writing – review & editing, Conceptualization. JM: Writing – review & editing, Conceptualization. HY: Writing – review & editing, Conceptualization. KU: Writing – review & editing, Conceptualization.
The authors declare that financial support was received for the research, authorship, and/or publication of this article.
This material is partially based upon work supported by the National Science Foundation under Grant No. EEC 1941543. Support from the US National Institutes of Health (grants R01DK096075, R01DK114506, R01DK134590 and R01DK131543) and the Shriners Hospitals for Children is gratefully acknowledged.
We would like to acknowledge the Mass Spectrometry Core Facility at Shriners Hospital for Children for processing our samples and performing the ATP analysis. We acknowledge HBO2 Therapeutics LLC for providing the hemoglobin-based oxygen carrier used in this study (Hemopure).
KU is inventor on pending patents relevant to this study, has a financial interest in Sylvatica Biotech, a company focused on developing organ preservation technology, whose interests are managed by the MGH and Mass General Brigham in accordance with their conflict of interest policies. ST and KU have several patent applications disclosures on extended organ preservation that may be relevant to this study. The HOBC-201 used in this study was provided by HBO2 Therapeutics LLC.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Mahboub P, Bozorgzadeh A, Martins PN. Potential approaches to improve the outcomes of donation after cardiac death liver grafts. World J Transplant. (2016) 6(2):314–20. doi: 10.5500/wjt.v6.i2.314
2. Haque O, Yuan Q, Uygun K, Markmann JF. Evolving utilization of donation after circulatory death livers in liver transplantation: the day of DCD has come. Clin Transplant. (2021) 35(3):e14211. doi: 10.1111/ctr.14211
3. Pine JK, Aldouri A, Young AL, Davies MH, Attia M, Toogood GJ, et al. Liver transplantation following donation after cardiac death: an analysis using matched pairs. Liver Transpl. (2009) 15(9):1072–82. doi: 10.1002/lt.21853
4. Monbaliu D, Liu Q, Vekemans K, Roskams T, Pirenne J. Potentiation of adverse effects of cold by warm ischemia in circulatory death donors for porcine liver transplantation. Transplant Proc. (2012) 44(9):2874–9. doi: 10.1016/j.transproceed.2012.09.078
5. Quintini C, Muiesan P, Detry O, Gastaca M, de Jonge J, Clavien PA, et al. Early allograft dysfunction and complications in DCD liver transplantation: expert consensus statements from the international liver transplantation society. Transplantation. (2021) 105(8):1643–52. doi: 10.1097/TP.0000000000003877
6. Hirao H, Nakamura K, Kupiec-Weglinski JW. Liver ischaemia-reperfusion injury: a new understanding of the role of innate immunity. Nat Rev Gastroenterol Hepatol. (2022) 19(4):239–56. doi: 10.1038/s41575-021-00549-8
7. Sutherland AI, Oniscu GC. Challenges and advances in optimizing liver allografts from donation after circulatory death donors. J Nat Sci Biol Med. (2016) 7(1):10–5. doi: 10.4103/0976-9668.175017
8. Ravikumar R, Jassem W, Mergental H, Heaton N, Mirza D, Perera MT, et al. Liver transplantation after ex vivo normothermic machine preservation: a phase 1 (first-in-man) clinical trial. Am J Transplant. (2016) 16(6):1779–87. doi: 10.1111/ajt.13708
9. van Rijn R, Schurink IJ, de Vries Y, van den Berg AP, Cortes Cerisuelo M, Darwish Murad S, et al. Hypothermic machine perfusion in liver transplantation—a randomized trial. N Engl J Med. (2021) 384(15):1391–401. doi: 10.1056/NEJMoa2031532
10. Nasralla D, Coussios CC, Mergental H, Akhtar MZ, Butler AJ, Ceresa CDL, et al. A randomized trial of normothermic preservation in liver transplantation. Nature. (2018) 557(7703):50–6. doi: 10.1038/s41586-018-0047-9
11. Markmann JF, Abouljoud MS, Ghobrial RM, Bhati CS, Pelletier SJ, Lu AD, et al. Impact of portable normothermic blood-based machine perfusion on outcomes of liver transplant: the OCS liver PROTECT randomized clinical trial. JAMA Surg. (2022) 157(3):189–98. doi: 10.1001/jamasurg.2021.6781
12. Westerkamp AC, Karimian N, Matton AP, Mahboub P, van Rijn R, Wiersema-Buist J, et al. Oxygenated hypothermic machine perfusion after static cold storage improves hepatobiliary function of extended criteria donor livers. Transplantation. (2016) 100(4):825–35. doi: 10.1097/TP.0000000000001081
13. Bruinsma BG, Avruch JH, Weeder PD, Sridharan GV, Uygun BE, Karimian NG, et al. Functional human liver preservation and recovery by means of subnormothermic machine perfusion. J Vis Exp. (2015) (98). doi: 10.3791/52777
14. Vogel T, Brockmann JG, Quaglia A, Morovat A, Jassem W, Heaton ND, et al. The 24-hour normothermic machine perfusion of discarded human liver grafts. Liver Transpl. (2017) 23(2):207–20. doi: 10.1002/lt.24672
15. Bral M, Gala-Lopez B, Bigam D, Kneteman N, Malcolm A, Livingstone S, et al. Preliminary single-center Canadian experience of human normothermic ex vivo liver perfusion: results of a clinical trial. Am J Transplant. (2017) 17(4):1071–80. doi: 10.1111/ajt.14049
16. von Horn C, Baba HA, Hannaert P, Hauet T, Leuvenink H, Paul A, et al. Controlled oxygenated rewarming up to normothermia for pretransplant reconditioning of liver grafts. Clin Transplant. (2017) 31(11). doi: 10.1111/ctr.13101
17. Minor T, Efferz P, Fox M, Wohlschlaeger J, Luer B. Controlled oxygenated rewarming of cold stored liver grafts by thermally graduated machine perfusion prior to reperfusion. Am J Transplant. (2013) 13(6):1450–60. doi: 10.1111/ajt.12235
18. van den Eijnden MM, Leuvenink HG, Ottens PJ, ‘t Hart NA, van Oeveren W, Morariu AM, et al. Effect of brain death and non-heart-beating kidney donation on renal function and injury: an assessment in the isolated perfused rat kidney. Exp Clin Transplant. (2003) 1(2):85–95.15859914
19. Izamis ML, Calhoun C, Uygun BE, Guzzardi MA, Price G, Luitje M, et al. Simple machine perfusion significantly enhances hepatocyte yields of ischemic and fresh rat livers. Cell Med. (2013) 4(3):109–23. doi: 10.3727/215517912X658927
20. Lorenz MA, El Azzouny MA, Kennedy RT, Burant CF. Metabolome response to glucose in the beta-cell line INS-1 832/13. J Biol Chem. (2013) 288(15):10923–35. doi: 10.1074/jbc.M112.414961
21. Bruinsma BG, Avruch JH, Sridharan GV, Weeder PD, Jacobs ML, Crisalli K, et al. Peritransplant energy changes and their correlation to outcome after human liver transplantation. Transplantation. (2017) 101(7):1637–44. doi: 10.1097/TP.0000000000001699
22. Op den Dries S, Karimian N, Westerkamp AC, Sutton ME, Kuipers M, Wiersema-Buist J, et al. Normothermic machine perfusion reduces bile duct injury and improves biliary epithelial function in rat donor livers. Liver Transpl. (2016) 22(7):994–1005. doi: 10.1002/lt.24436
23. Minor T, von Horn C, Zlatev H, Saner F, Grawe M, Luer B, et al. Controlled oxygenated rewarming as novel end-ischemic therapy for cold stored liver grafts. A randomized controlled trial. Clin Transl Sci. (2022) 15(12):2918–27. doi: 10.1111/cts.13409
24. Hoyer DP, Paul A, Luer S, Reis H, Efferz P, Minor T. End-ischemic reconditioning of liver allografts: controlling the rewarming. Liver Transpl. (2016) 22(9):1223–30. doi: 10.1002/lt.24515
25. Bodewes SB, van Leeuwen OB, Thorne AM, Lascaris B, Ubbink R, Lisman T, et al. Oxygen transport during ex situ machine perfusion of donor livers using red blood cells or artificial oxygen carriers. Int J Mol Sci. (2020) 22(1):235. doi: 10.3390/ijms22010235
26. van Leeuwen OB, Bodewes SB, Lantinga VA, Haring MPD, Thorne AM, Bruggenwirth IMA, et al. Sequential hypothermic and normothermic machine perfusion enables safe transplantation of high-risk donor livers. Am J Transplant. (2022) 22(6):1658–70. doi: 10.1111/ajt.17022
27. Moritz ED, Winton CS, Tonnetti L, Townsend RL, Berardi VP, Hewins ME, et al. Screening for Babesia microti in the U.S. blood supply. N Engl J Med. (2016) 375(23):2236–45. doi: 10.1056/NEJMoa1600897
28. Senay S, Toraman F, Gunaydin S, Kilercik M, Karabulut H, Alhan C. The impact of allogenic red cell transfusion and coated bypass circuit on the inflammatory response during cardiopulmonary bypass: a randomized study. Interact Cardiovasc Thorac Surg. (2009) 8(1):93–9. doi: 10.1510/icvts.2008.183608
29. Sadowsky D, Zamora R, Barclay D, Yin J, Fontes P, Vodovotz Y. Machine perfusion of porcine livers with oxygen-carrying solution results in reprogramming of dynamic inflammation networks. Front Pharmacol. (2016) 7:413. doi: 10.3389/fphar.2016.00413
30. Fontes P, Lopez R, van der Plaats A, Vodovotz Y, Minervini M, Scott V, et al. Liver preservation with machine perfusion and a newly developed cell-free oxygen carrier solution under subnormothermic conditions. Am J Transplant. (2015) 15(2):381–94. doi: 10.1111/ajt.12991
31. Hosgood SA, Nicholson HF, Nicholson ML. Oxygenated kidney preservation techniques. Transplantation. (2012) 93(5):455–9. doi: 10.1097/TP.0b013e3182412b34
32. Hosgood SA, Mohamed IH, Nicholson ML. The two layer method does not improve the preservation of porcine kidneys. Med Sci Monit. (2011) 17(1):BR27–33. doi: 10.12659/MSM.881326
33. Martins PN, Berendsen TA, Yeh H, Bruinsma BG, Izamis ML, Op den Dries S, et al. Oxygenated UW solution decreases ATP decay and improves survival after transplantation of DCD liver grafts. Transplantation. (2019) 103(2):363–70. doi: 10.1097/TP.0000000000002530
34. Thuillier R, Dutheil D, Trieu MT, Mallet V, Allain G, Rousselot M, et al. Supplementation with a new therapeutic oxygen carrier reduces chronic fibrosis and organ dysfunction in kidney static preservation. Am J Transplant. (2011) 11(9):1845–60. doi: 10.1111/j.1600-6143.2011.03614.x
35. Matton APM, Burlage LC, van Rijn R, de Vries Y, Karangwa SA, Nijsten MW, et al. Normothermic machine perfusion of donor livers without the need for human blood products. Liver Transpl. (2018) 24(4):528–38. doi: 10.1002/lt.25005
36. Tessier SN, de Vries RJ, Pendexter CA, Cronin SEJ, Ozer S, Hafiz EOA, et al. Partial freezing of rat livers extends preservation time by 5-fold. Nat Commun. (2022) 13(1):4008. doi: 10.1038/s41467-022-31490-2
37. Taverne YJ, de Wijs-Meijler D, Te Lintel Hekkert M, Moon-Massat PF, Dube GP, Duncker DJ, et al. Normalization of hemoglobin-based oxygen carrier-201 induced vasoconstriction: targeting nitric oxide and endothelin. J Appl Physiol (1985). (2017) 122(5):1227–37. doi: 10.1152/japplphysiol.00677.2016
38. Alvarez AM, Mukherjee D. Liver abnormalities in cardiac diseases and heart failure. Int J Angiol. (2011) 20(3):135–42. doi: 10.1055/s-0031-1284434
39. Sutton ME, Op den Dries S, Karimian N, Weeder PD, de Boer MT, Wiersema-Buist J, et al. Criteria for viability assessment of discarded human donor livers during ex vivo normothermic machine perfusion. PLoS One. (2014) 9(11):e110642. doi: 10.1371/journal.pone.0110642
Keywords: cold storage, gradual rewarming, HBOC, machine perfusion, donation after cardiac death (DCD)
Citation: Mahboub P, Aburawi M, Ozgur O.S, Pendexter C, Cronin S, Lin FM, Jain R, Karabacak MN, Karimian N, Tessier SN, Markmann JF, Yeh H and Uygun K (2024) Gradual rewarming with a hemoglobin-based oxygen carrier improves viability of donation after circulatory death in rat livers. Front. Transplant. 3:1353124. doi: 10.3389/frtra.2024.1353124
Received: 9 December 2023; Accepted: 10 June 2024;
Published: 1 July 2024.
Edited by:
Kojiro Nakamura, Kyoto University, JapanReviewed by:
Rebecca A. Sosa, UCLA Health System, United States© 2024 Mahboub, Aburawi, Ozgur, Pendexter, Cronin, Lin, Jain, Karabacak, Karimian, Tessier, Markmann, Yeh and Uygun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: O. Sila Ozgur, b296Z3VyQG1naC5oYXJ2YXJkLmVkdQ==; Korkut Uygun, a3V5Z3VuQG1naC5oYXJ2YXJkLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.