
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Transplant. , 12 September 2023
Sec. Abdominal Transplantation
Volume 2 - 2023 | https://doi.org/10.3389/frtra.2023.1240155
Introduction: The demographics of donor and recipient candidates for kidney transplantation (KT) have substantially changed. Recipients tend to be older and polymorbid and KT to suboptimal recipients is associated with delayed graft function (DGF), prolonged hospitalization, inferior long-term allograft function, and poorer patient survival. In parallel, donors are also older, suffer from several comorbidities, and donations coming from circulatory death (DCD) predominate, which in turn leads to early and late complications. However, it is unclear how donor and recipient risk factors interact.
Methods: In this retrospective cohort study, we assess the impact of a KT from suboptimal donors to suboptimal recipients. We focused on: 1) DGF; 2) hospital stay and number of dialysis days after KT and 3) allograft function at 12 months.
Results and discussion: Among the 369 KT included, the overall DGF rate was 25% (n = 92) and median time from reperfusion to DGF resolution was 7.8 days (IQR: 3.0–13.8 days). Overall, patients received four dialysis sessions (IQR: 2–8). The combination of pre-KT anuria (<200 ml/24 h, 32%) and DCD procurement (14%) was significantly associated with DGF, length of hospital stay, and severe perioperative complications, predominantly in recipients 50 years and older.
The prevalence of end-stage kidney disease (ESKD) has increased substantially in recent years (1). Kidney transplantation (KT) is the preferred renal replacement therapy for eligible patients. It improves survival, reduces morbidity and provides a quality of life benefit when compared to hemodialysis (2, 3, 4). However, due to the burden of ESKD, the shortage of living and deceased donor candidates, and stringent eligibility criteria, the availability of suitable organs is still the limiting factor for KT (5).
In KT, allograft procurement, preservation and transplantation are followed by highly standardized procedures to minimize cold and warm ischemia time and thereby reduce reperfusion injury and subsequent microvascular damage, thus preserving graft viability. Delayed graft function (DGF), a major clinical challenge, is mainly defined on serum creatinine values or the requirement of renal replacement therapy within the first week after KT. It's incidence reported worldwide varies between transplant centers, ranging approximately from 4%–10% in living donors KT to 19%–70% in deceased KT, also reflecting the heterogeneity of its definition (6). DGF has a negative connotation because it is associated with early acute rejection, peri-operative mortality, prolonged hospitalization, increased cost of care, and shortened allograft survival (7).
In recent years, the characteristics of recipient and donor candidates for KT have changed substantially in Switzerland, but also in other developed countries. Recipients tend to be older and polymorbid, often having spent several years on dialysis, and frequently are without residual urine production. KT to suboptimal recipients is associated with DGF, prolonged hospitalization, inferior long-term allograft function, and poorer patient survival (8, 9).
In parallel, donors nowadays are also older, suffer from several comorbidities (especially cardiovascular disease) and donation after cardiac death (DCD) predominate, which in turn leads to early and late complications (10–12).
For these reasons, the incidence of DGF is expected to increase in the coming years. To date, there are no FDA-approved treatment to minimize the ischemic injury that occursmaking the identification of potential risk factors an interesting prevention tool. Several risk factors for DGF (cold ischemia time, donor and recipient age, cause of donor death) have already been identified (6). Furthermore, several attempts have been made to predict the risk of DGF, such as nomograms based on donor and recipient risk factors (13), preimplantation biopsies (with (14) and without (15) transcriptomics), or urinary biomarkers measured early after transplantation (16, 17). However, these methods may not be very accurate, might show a high interobserver variability (preimplantation biopsies) or are not readily available at time of allocation (transcriptomics, urinary biomarkers). Additionnally, many prediction tools (e.g., Kidney Donor Profle Index [KDPI], UK Kidney Donor Risk Index [KDRI], UK Kidney Recipient Risk Index, US Estimated Post-Transplant Survival Score [REFS]) focus solely on recipient or donor parameters and estimate mid- and long-term survival, yet not perioperative DGF. A better understanding of donor- and recipient-derived variables influencing DGF that are already known at time of allocation would allow better use of resource, improve the KT outcomes, and thus promote widespread utilization of suboptimal kidney at risk for DGF.
In this study, we employed data from a single-center comprehensive KT cohort to assess the impact of KT from marginal donors to marginal recipients. We focused on 1) risk factors associated with DGF; 2) hospital stay duration and number of dialysis days after KT; 3) allograft function at 12 months; and 4) patient and allograft survival.
This study is an analysis of registry data from January 1st 2008 onwards and includes all patients who received a KT at the university hospital of Bern, Switzerland. The last patient was included on to April 7th 2022. Median follow-up time was 5.41 years (IQR: 2.06–8.31 years). Recipient and donor characteristics were collected from the clinical information platform and laboratory analyses extracted via Insel Data Science Center (IDSC). The IDSC is the IT organizational unit of Insel Gruppe AG for the collection, provision and use of digital data for scientific purposes. Patient data of deceased donors were extracted from the Swiss Organ Allocation System (SOAS). Pre- and post-KT dialysis sessions parameters in recipients were extracted from the dialysis therapy management database.
DGF was defined as the need for at least one dialysis session within one week after KT (time between reperfusion and first dialysis start), regardless of indication (volume overload, hyperkalemia, azotemia). Time from reperfusion to first dialysis and to end of last dialysis was calculated, as well as number of dialysis sessions, cumulative dialysis time and cumulative ultrafiltration.
Anuria was defined as residual urine production of less than 200 ml/24 h at the time of KT. Following co-variables were also considered for the regression models: donor characteristics [age, acute kidney injury, resuscitation, cardiovascular comorbidities, type of organ procurement (donation after cardiac or brain death, DCD Mastricht III or DBD), KDPI, use of hypothermic machine perfusion]; recipient characteristics (age, primary kidney disease, dialysis history, residual urine volume, previous KT history,), cold and warm ischemia time, and first warm ischemia in the case of DCD. First warm ischemia was defined as timepoint of therapy withdrawal until start of procurement, i.e. time of aortic cross clamp (including a no-touch time of 5 min in case of DCD procurement). First warm ischemia was defined as 0 min in case of DBD procurement. The use of hypothermic machine perfusion was up to the decision of the allocating surgeon at the transplantation center. Finally, the type of induction therapy (depleting vs. non-depleting) was assessed. In-house complications were assessed according to the Clavien-Dindo classification (18). Post-operative biopsies of the kidney allografts and dialyses were excluded as relevant Clavien-Dindo complications for all patients in this study.
The primary outcome of the study was the risk for the dichotomous event of DGF after KT. Secondary outcomes were the risk for major complications, length of hospital stay, estimated glomerular filtration rate (eGFR) at indicated time points, and time to graft loss or patient death. eGFR, given in ml/min/1.73 m2 was calculated using the Chronic Kidney Disease Epidemiology Collaboration 2009 (CKD-EPI) creatinine equation (19) at 12 months after KT. For patients with graft loss before 12 month, eGFR was set to at 0 ml/min/1.73 m2. Further endpoints included the association of pre-KT dialysis (within 12 h before KT), depleting induction therapy, use of hypothermic machine perfusion, and cold ischemia time with DGF events.
Participants were excluded from the analysis if (1) they had a living donor transplantation (LDT); (2) were multi-organ recipient; (3) were paediatric recipients (age < 18 years old); or (4) had a primary graft non-function (absence of dialysis-independent graft function until terminal loss). The latest group was excluded due to heterogeneity of graft loss reasons [predominantly hyperacute rejection, surgical complications (arterial dissection, venous thrombosis)].
Neither patients nor the public were involved in the design, conduct, or analysis of this study.
Results were reported as number of participants (percentage) for categorical data and median (interquartile range) for continuous data.
Regression models were built from patients with complete data sets for the relevant factors: 304/369 (82%) for the full model and 343/369 (93%) for the reduced model. The odds ratio (OR) for a DGF event was calculated from a full model of donor-, recipient- and procedural-derived risk factors (donor: age, resuscitation status, DCD procurement [yes, no], comorbidities [any of hypertension, diabetes mellitus or cardiovascular disease]; recipient: age, dialysis vintage [years], anuria [yes, no]; procedural: cold and warm ischemia time). From here, the single most impacting factor from donor- and recipient side, along with donor- and recipient age, were retested in a reduced model. In a second approach, log-transformed first warm ischemia time (per 15 min) and residual urine volume (per 100 ml/24 h) were considered as quantitative parameters for model calculations. From these results, the risk for DGF was refitted for the KT cohort using the following parameters: donor and recipient age, DCD parameters (qualitative and quantitative), residual urine volume (qualitative and quantitative).
The discriminative powers of the reduced models were calculated by the area under the receiver operating characteristic curve (AUC-ROC) to predict DGF events. The models were further internally validated by calibration analysis and respective Brier scores calculated. The Brier score is the mean squared difference between the predicted probability and the actual outcome with a lower Brier score (scale: 0-1) reflecting a better calibrated model. The predictor variables residual urine volume, first warm ischemia time, donor and recipient age were used to build a nomogram predicting the DGF risk.
Time-to-event analyses were performed including length of hospital stay, time to first re-hospitalization, time to death-censored graft loss, patient death or the composite endpoint of patient death or graft loss, and plotted as Kaplan-Meier curves and cumulative incidence curves, respectively.
The incidence of perioperative complications (Clavien-Dindo grade IIIb or higher) was calculated for the respective groups, and further sub-analyzed for recipients younger or older than 50 years at the time of KT. Differences were measured using chi-square tests.
Kidney function 12 months after KT was illustrated among the groups, and differences compared using Mann-Whitney U tests.
The association between DGF events and pre-KT interventions (dialysis, hypothermic machine perfusion, induction therapy, cold ischemia time) was analysed by logistic regression adjusted for residual urine volume and DCD procurement as important cofounders.
Results were expressed as multivariable-adjusted mean ± SD for categorical values and as adjusted regression coefficient for continuous variables. A two-tailed p < 0.05 was considered statistically significant.
Statistical analyses were performed using R (version 4.0.3) and R Studio (version 1.3.1093).
Of the initial 627 participants, 369 (58.8%) were included. The selection procedure is summarised in Supplementary Figure S1. The majority of participants were male, and donor and recipient age were 57 years (IQR: 44–67) and 58 years (IQR: 49–66), respectively. 22% of patients suffered from hypertensive and/or diabetic nephropathy. Median waiting time was 2.5 years (IQR: 1.44–3.99 years). Among donors, arterial hypertension was present in 31% of cases, 14% of KT came from DCD procurement, and the use of a hypothermic machine perfusion was reported only in few cases (14%). No patients with dual-kidney transplantation were included. Regarding the recipients, only a minority had a preemptive KT (8.5%), and the rest had already spent an average of 3.24 years (IQR 2.08–4.44) on hemodialysis or peritoneal dialysis, with 32% of participants already being anuric. 118 (32%) of patients were treated with a dialysis session within 24 h before KT. Warm and cold ischemia times were 29 min (IQR: 24–34) and 8.12 h (IQR: 6.40–10.40), respectively. (Tables 1A,B).
The overall frequency of DGF was 25% (n = 92). The absolute and relative frequencies of DGF between 2008 and 2022 are illustrated in Figures 1A,B. When renal replacement therapy was indicated post-operatively, patients received only haemodialysis (not peritoneal dialysis or continuous hemodiafiltration). Median time from reperfusion to end of last hemodialysis was 7.8 days (IQR: 3.0–13.8) (Figure 1C). Overall, patients required a median of 4 hemodialysis (IQR: 2–8) with a median total hemodialysis time per patient of 12.4 h (IQR 5.7–26.6). More details are provided in Table 2.
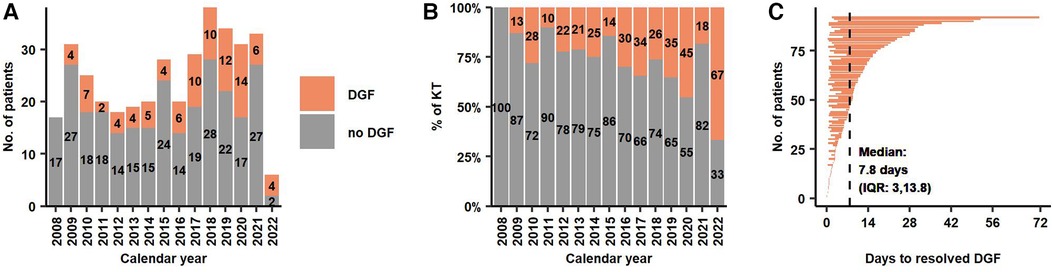
Figure 1. (A,B) Absolute and relative frequency of KT with and without DGF during the study period. (C) Time on dialysis after reperfusion for 92 patients with DGF. Median time from reperfusion to end of last dialysis was 7.8 days. DGF, Delayed Graft Function; KT, Kidney Transplantation.
In a regression model, we identified DCD procurement alongside with anuria as relevant risk factors for DGF. These factors remained statistically significant when analyzed in a reduced model including donor and recipient age, and independently predicted DGF events with an AUC of 70% (CI: 64%–77%, p < 0.05). Our model predicted DGF events from 15% in DBD KT for recipients with intact diuresis to 71% in DCD KT to anuric recipients (Table 3, Figures 2A,B). Calibration of observed and estimated DGF probabilities revealed that prediction was reliable over a wide range with a Brier Score of 0.16. The model overestimated the risk of DGF in patients with an intermediate predicted likelihood (Figure 2C). Similarly, quantitative values of first warm ischemia time and residual urine volume were significantly associated with DGF and gradually predicted events with an AUC of 69% (CI: 63%–76%) with an analogous calibration performance (Figures 2D–F). A nomogram predicting the DGF risk based residual urine volume, first warm ischemia time, donor and recipient age is shown in Supplementary Figure S2.
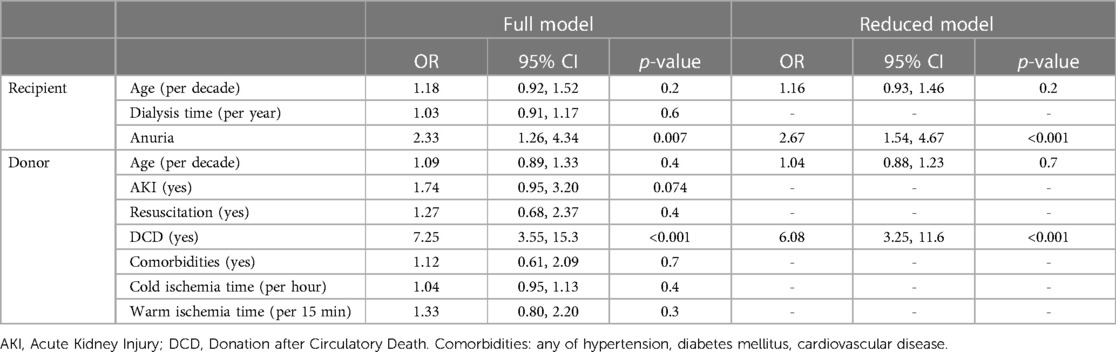
Table 3. Logistic regression model for DGF (yes/no) as dependent variable and donor-, recipient- and procedural factors as independent variables (full model). Donor and recipient age, as well as significant variables (DCD procurement, residual urine volume <200 ml/24 h) from the full model were used for a subsequent reduced model. DGF, Delayed Graft Function; DCD, Donation after Circulatory Death.
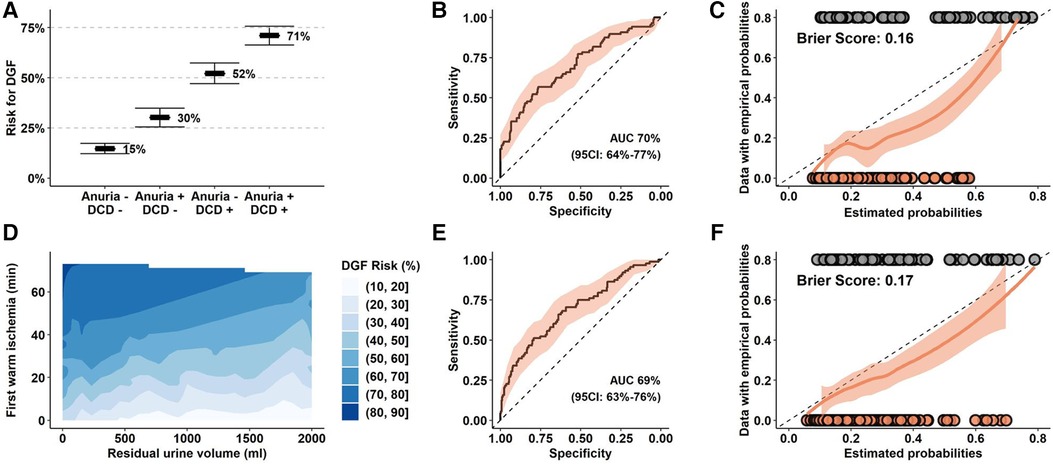
Figure 2. Prediction models for DGF in the Bernese cohort. (A–C) Prediction model for DGF with organ procurement (DBD versus DCD), residual urine volume (≥200 ml/24 h vs. <200 ml/24 h), donor and recipient age as exploratory parameters. (D–F) Prediction model for DGF with first warm ischemia time (per 15 min), residual urine volume (per 100 ml), donor and recipient age as quantitative exploratory variables. (B,E) ROC analysis and calculated AUC for the respective models. (C,F) Calibration analysis of empirical, estimated probabilities and Brier scores from the reduced models applied to the entire cohort. (D) Estimated risk for DGF based on the reduced model. DGF, Delayed Graft Function; DCD, Donation after Circulatory Death; DBD, Donation after Brain Death.
Severe perioperative complications (Clavien-Dindo grade IIIb or higher) were increased up to fivefold (4% in DBD KT to recipients without anuria vs. 20% in DCD KT to recipients with anuria). Strikingly, this ratio further increased in a subgroup of recipients aged 50 years and older at time of KT with an incidence of 33% in the highest risk group (Figures 3A,B). The majority of such complications was medical (11/23, 48%, mostly vascular events and respiratory failure), followed by surgical (8/23, 35%, mostly revisions and gastrointestinal interventions).
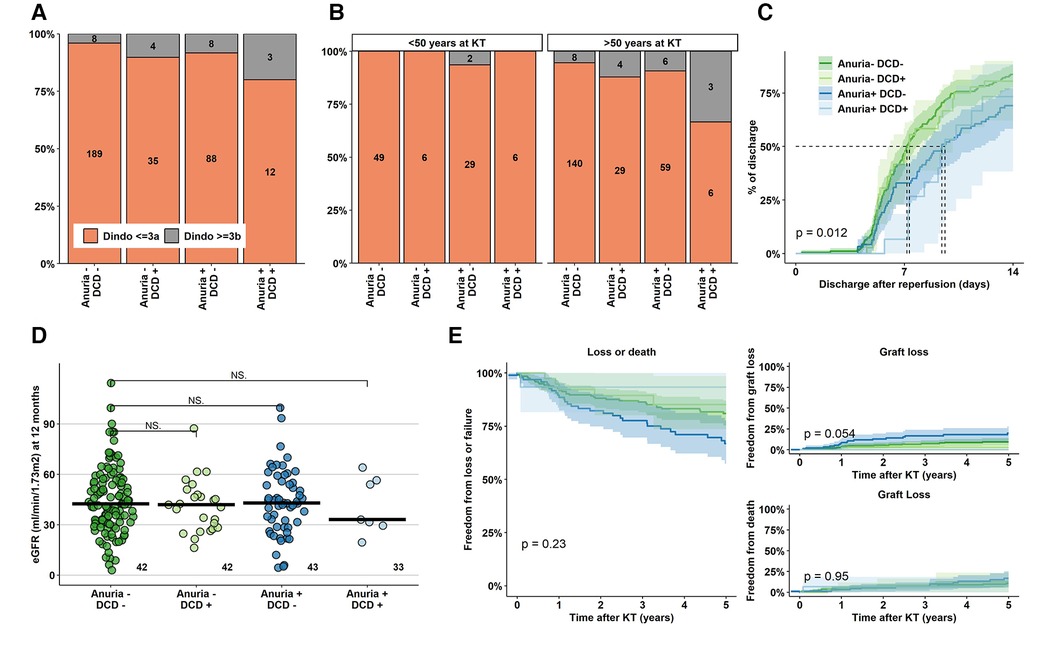
Figure 3. Perioperative complications, hospital stay duration and eGFR at 12 months after KT. (A) Absolute and relative frequencies of Clavien-Dindo complications ≥ IIIb (interventions under complete anaesthesia, intensive care unit treatment, death) in the various groups. (B) Absolute and relative rate of Clavien-Dindo grade ≥ IIIb complications stratified for recipients younger and older than 50 year at time of KT. (C) Duration until first hospital discharge after KT for the various groups, (D,E) Kidney function outcome. KT, Kidney Transplantation; eGFR, estimated Glomerular Filtration Rate; DCD, Donation after Circulatory Death.
Median hospital duration was overall short, but significantly longer in anuric recipients of both DBD and DCD KT (non-anuric recipients: 7.17–7.33 days, anuric recipients: 9.42–9.62 days, p < 0.05) (Figure 3C). Finally, KT function at 12 months and long-term patient and graft survival were comparable among the risk groups (Figures 3D,E).
Overall, hypothermic machine perfusion, depleting induction therapy, and hemodialysis treatment within 12 h before KT were infrequent and ranged from 15- to 34% of all KT among the groups. In a retrospective analysis and after correction for DCD procurement and residual urine volume, none of these interventions reduced DGF probability (Figure 4, Supplementary Table S1).
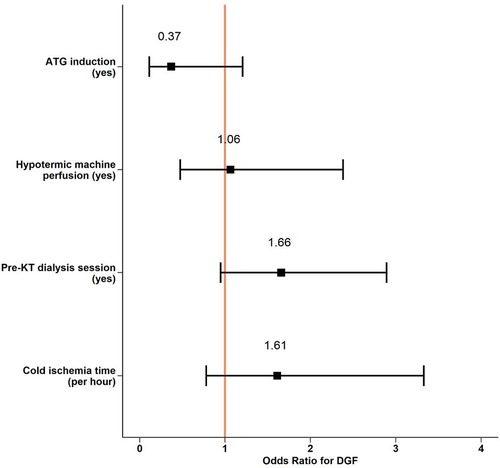
Figure 4. Perioperative interventions and DGF risk. Logistic regression model predicting DGF events from exploratory given variables and log-transformed cold ischemia time and residual urine volume as co-founding parameters. DGF, Delayed Graft Function; KT, Kidney Transplantation; ATG, Antithymocyte Globulin.
To our knowledge, this is the first study assessing the incidence, characteristics and outcome of DGF in a Swiss population. In a study sample of 369 participants, we found that DCD procurement and residual urine volume were strongly associated with DGF.
Considering the ESKD burden, the severe shortage of high-quality organs for KT and the use of nonstandard kidney from suboptimal donors, the incidence of DGF will remain high in the next years. For this reason, preventive measures in order to prevent DGF are under consideration.
DGF was a rather frequent event and occurred in 25% of patients receiving a KT. Similar results were recently published by Jadlowiec et al. (20). As described in a previous cohort (21), we identified DCD procurement as an important predictor of DGF. This procurement approach is linked to a variable first warm ischemia time, during which relevant hypoxic damage occurs in the kidney with subsequent reperfusion injury and development of acute tubular necrosis. To prevent DGF, one of the best-known preventive measures is to decrease warm and cold ischemia time. If on the one hand surgeons have a concrete way to act and reduce the risk of DGF on the other hand also hypothermic preservation machines provide an additional benefit. Several studies have proved the superiority of hypothermic machine perfusion over standard cold storage in terms of DGF (22, 23, 24), yet its impact on long-term outcome is less clear. In our study, only a minority of KT underwent a hypothermic machine perfusion, and this intervention was not independently associated with a relevant reduction of DGF events (OR 1.06, CI: 0.46–2.33, p = 0.9). It's not excluded that with more widespread use in our institution of hypothermic machine perfusion, our results, currently not significant, could positively change the results.
The duration of kidney replacement therapy before KT is an important risk factor associated with DGF and transplant outcome (25, 26). It is not fully understood whether the time spent on dialysis alters the recipient's environment such that the incidence of ischemia-reperfusion injury increases and consequently influences the risk of DGF, or whether this is directly related to the progressive loss of residual urine volume. We found that the recipient's residual urine volume, rather than the dialysis history, was significantly associated with the risk of DGF. This is intuitive, since decreasing residual diuresis is associated with minimal residual kidney function and, more important, with consequently poor volume control. Interestingly, although other factors (including cold- and warm ischemia time, donor and recipient age, or comorbidities) were (insignificantly) associated with DGF risk in our study, their influence was much smaller compared to DCD procurement and residual urine volume.
We demonstrate overall stable and sufficient graft function in suboptimal-to-suboptimal KT, although 12-month eGFR tends to be lower and is likely associated with accelerated graft deterioration. We further show that suboptimal-to-suboptimal KT is associated with longer primary hospitalization duration, which compromises rehabilitation and significantly increases the burden on the health care system. Finally, we found that early complications are uncommon but increased yet some up to fivefold in KT settings from DCD donors to anuric recipients. This risk is notably highest in recipients aged 50 years and older. This is important since perioperative complications after KT are associated with significant mid- and long-term morbidity. As reported by Jadlowiec et al., the readmission rate as well as the complications in this group of patients may explain the less favorable outcomes at 6-months and the lower graft survival (20). This would suggest that others factors, independent of DGF, could have a considerable impact on KT outcomes.
Identifying factors contributing to KT outcome is essential. For example, minimizing perioperative stress on the recipient and the transplanted organ would likely increase the chance of immediate graft function without DGF. Calcineurin inhibitors (CNI), the mainstay of standard immunosuppression in transplant recipients, are potent vasoconstrictors that may contribute to additional injury leading to long-term development of interstitial fibrosis and tubular atrophy. In this study, the majority of recipients received non-depleting induction and had CNI started the day after KT, irrespective of DGF evolution. Delayed initiation of CNI treatment in conjunction with depleting induction therapy may reduce the risk of prolonged DGF (27, 28), and our results trended in this direction. Novel therapeutic options, such as early conversion to belatacept-based immunosuppression (29, 30) or peri-transplantation administration of eculizumab (31) could contribute to DGF minimization in this context. Among the recently published studies, two randomised controlled trials show that the administration of eculizumab was safe but does not reduce the rate of DGF (32). These therapeutic interventions should be studied prospectively at a later date. Surprisingly, the cold ischemia time was not associated with a reduced risk for DGF, as reported in other studies. In our study, median cold ischemia time was short with 8h07min and thereby likely had minimal impact on DGF rate.
Our study was conducted on a population-based sample, allowing generalisation of the results to similar Caucasian populations. The sample size of our study population together with the completeness of the data collected, allowed us to explore new associations between donor and recipient risk factors and DGF.
Our study also has some limitations. First, although complete and extensive in terms of parameters evaluated, it represents a retrospective and single centre study.
Second, the definition of DGF (hemodialysis requirement in the first week after KT) may have biased the results by overestimating DGF events without directly reflecting a marker of ischemic reperfusion injury.
Third, KT from DCD donors to anuric KT recipients clearly increased during the study period (data not shown), so that the groups were not ideally balanced. Therefore, our results are possibly biased by a small percentage of DCD procurement included in our study, and the mid- and long-term outcome results are consequently limited. This is for instance reflected in the very low rate of graft loss in DCD KT to anuric recipients, which is likely the result of selection bias at time of KT.
Finally, inactive waiting time would clearly be an important determinant to measure DGF, since is associated with inferior transplant outcome. Unfortunately, this parameter is not uniformly available for our cohort and this correlation has not been investigated.
In conclusion, DCD procurement and recipient residual urine volume independently predict DGF rate after KT. These factors are associated with inferior short- and midterm outcome, including increased risk of postoperative complications, notably in recipients aged 50 years and more. DCD procurement and residual diuresis must be included in allocation considerations, not as independent factors, but as an interacting network. Likely, suboptimal-to-suboptimal KT should be restricted to recipients with sufficient stamina for early perioperative complication.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The study was approved by the Cantonal Ethics Commitee of Bern. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
FB conducted the literature search, interpreted the results and wrote the manuscript. DS performed the statistical analyses, interpreted the results and thoroughly revised the manuscript. CK, GB, FS, and NM participated to conceiving the study. All authors have read and approved this version of the manuscript.
The author DS declared that he was an editorial board member of Frontiers at the time of submission. This had no impact on the peer review process and the final decision.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frtra.2023.1240155/full#supplementary-material
1. Thurlow JS, Joshi M, Yan G, Norris KC, Agodoa LY, Yuan CM, et al. Global epidemiology of end-stage kidney disease and disparities in kidney replacement therapy. Am J Nephrol. (2021) 52:98–107. doi: 10.1159/000514550
2. Purnell TS, Auguste P, Crews DC, Lamprea-Montealegre J, Olufade T, Greer R, et al. Comparison of life participation activities among adults treated by hemodialysis, peritoneal dialysis, and kidney transplantation: a systematic review. Am J Kidney Dis. (2013) 62:953–73. doi: 10.1053/j.ajkd.2013.03.022
3. Ojo AO, Hanson JA, Wolfe RA, Leichtman AB, Agodoa LY, Port FK. Long-term survival in renal transplant recipients with graft function. Kidney Int. (2000) 57:307–13. doi: 10.1046/j.1523-1755.2000.00816.x
4. Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. (1999) 341:1725–30. doi: 10.1056/NEJM199912023412303
5. Kuhn C, Lang BM, Lörcher S, Karolin A, Binet I, Beldi G, et al. Outcome of kidney transplantation from very senior donors in Switzerland—a national cohort study. Transpl Int. (2021) 34:689–99. doi: 10.1111/tri.13836
6. Mezzolla V, Pontrelli P, Fiorentino M, Stasi A, Pesce F, Franzin R, et al. Emerging biomarkers of delayed graft function in kidney transplantation. Transplant Rev (Orlando). (2021) 35:100629. doi: 10.1016/j.trre.2021.100629
7. Yarlagadda SG, Coca SG, Formica RN, Poggio ED, Parikh CR. Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol Dial Transplant. (2009) 24:1039–47. doi: 10.1093/ndt/gfn667
8. Geddes CC, Woo YM, Jardine AG. The impact of delayed graft function on the long-term outcome of renal transplantation. J Nephrol. (2002) 15:17–21.11936421
9. Heuer M, Zeiger A, Kaiser GM, Mathé Z, Goldenberg A, Sauerland S, et al. Use of marginal organs in kidney transplantation for marginal recipients: too close to the margins of safety. Eur J Med Res. (2010) 15:31–4. doi: 10.1186/2047-783X-15-1-31
10. Danovitch GM, Cecka JM. Allocation of deceased donor kidneys: past, present, and future. Am J Kidney Dis. (2003) 42:882–90. doi: 10.1016/j.ajkd.2003.07.017
11. Bae S, Massie AB, Thomas AG, Bahn G, Luo X, Jackson KR, et al. Who can tolerate a marginal kidney? Predicting survival after deceased donor kidney transplant by donor-recipient combination. Am J Transplant. (2019) 19:425–33. doi: 10.1111/ajt.14978
12. Ojo AO, Hanson JA, Meier-Kriesche HU, Okechukwu CN, Wolfe RA, Leichtman AB, et al. Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J Am Soc Nephrol. (2001) 12:589–97. doi: 10.1681/ASN.V123589
13. Irish WD, Ilsley JN, Schnitzler MA, Feng S, Brennan DC. A risk prediction model for delayed graft function in the current era of deceased donor renal transplantation. Am J Transplant. (2010) 10:2279–86. doi: 10.1111/j.1600-6143.2010.03179.x
14. Lopes JA, Moreso F, Riera L, Carrera M, Ibernon M, Fulladosa X, et al. Evaluation of pre-implantation kidney biopsies: comparison of Banff criteria to a morphometric approach. Kidney Int. (2005) 67:1595–600. doi: 10.1111/j.1523-1755.2005.00241.x
15. Mueller TF, Reeve J, Jhangri GS, Mengel M, Jacaj Z, Cairo L, et al. The transcriptome of the implant biopsy identifies donor kidneys at increased risk of delayed graft function. Am J Transplant. (2008) 8:78–85. doi: 10.1111/j.1600-6143.2007.02032.x
16. Parikh CR, Jani A, Mishra J, Ma Q, Kelly C, Barasch J, et al. Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am J Transplant. (2006) 6:1639–45. doi: 10.1111/j.1600-6143.2006.01352.x
17. Maier HT, Ashraf MI, Denecke C, Weiss S, Augustin F, Messner F, et al. Prediction of delayed graft function and long-term graft survival by serum and urinary neutrophil gelatinase-associated lipocalin during the early postoperative phase after kidney transplantation. PLoS One. (2018) 13:e0189932. doi: 10.1371/journal.pone.0189932
18. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae
19. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
20. Jadlowiec CC, Frasco P, Macdonough E, Wagler J, Das D, Budhiraja P, et al. Association of DGF and early readmissions on outcomes following kidney transplantation. Transpl Int. (2022) 35:10849. doi: 10.3389/ti.2022.10849
21. Rijkse E, Ceuppens S, Qi H, IJzermans JNM, Hesselink DA, Minnee RC. Implementation of donation after circulatory death kidney transplantation can safely enlarge the donor pool: a systematic review and meta-analysis. Int J Surg. (2021) 92:106021. doi: 10.1016/j.ijsu.2021.106021
22. Malinoski D, Saunders C, Swain S, Groat T, Wood PR, Reese J, et al. Hypothermia or machine perfusion in kidney donors. N Engl J Med. (2023) 388:418–26. doi: 10.1056/NEJMoa2118265
23. Moers C, Smits JM, Maathuis MH, Treckmann J, Van Gelder F, Bogdan P, et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. (2009) 360:7–19. doi: 10.1056/NEJMoa0802289
24. Tingle SJ, Figueiredo RS, Moir JA, Goodfellow M, Talbot D, Wilson CH. Machine perfusion preservation versus static cold storage for deceased donor kidney transplantation. Cochrane Database Syst Rev. (2019) 3:CD011671. doi: 10.1002/14651858.CD011671.pub2
25. Keith DS, Cantarovich M, Paraskevas S, Tchervenkov J. Duration of dialysis pretransplantation is an important risk factor for delayed recovery of renal function following deceased donor kidney transplantation. Transpl Int. (2008) 21:126–32. doi: 10.1111/j.1432-2277.2007.00571.x
26. Aufhauser DD, Peng AW, Murken DR, Concors SJ, Abt PL, Sawinski D, et al. Impact of prolonged dialysis prior to renal transplantation. Clin Transplant. (2018) 32:e13260. doi: 10.1111/ctr.13260
27. Butler T, Hayde N. Impact of induction therapy on delayed graft function following kidney transplantation in mated kidneys. Transplant Proc. (2017) 49:1739–42. doi: 10.1016/j.transproceed.2017.06.032
28. Chapal M, Foucher Y, Marguerite M, Neau K, Papuchon E, Daguin P, et al. PREventing delayed graft function by driving immunosuppressive induction treatment (PREDICT-DGF): study protocol for a randomized controlled trial. Trials. (2015) 16:282. doi: 10.1186/s13063-015-0807-x
29. Patel A, Shertel T, Wynd M, Wadhera V, Serur D, Schleich B, et al. Outcomes of de novo belatacept-based immunosuppression regimen and avoidance of calcineurin inhibitors in recipients of kidney allografts at higher risk for underutilization. Nephrology (Carlton). (2022) 27:901–5. doi: 10.1111/nep.14106
30. Hennawy HE, Safar O, Al Faifi AS, El Nazer W, Kamal A, Mahedy A, et al. Belatacept rescue therapy of cni-induced nephrotoxicity, meta-analysis. Transplant Rev (Orlando). (2021) 35(4):100653. doi: 10.1016/j.trre.2021.100653
31. Grenda R, Durlik M. Eculizumab in renal transplantation: a 2017 update. Ann Transplant. (2017) 22:550–4. doi: 10.12659/AOT.905917
32. Schröppel B, Akalin E, Baweja M, Bloom RD, Florman S, Goldstein M, et al. Peritransplant eculizumab does not prevent delayed graft function in deceased donor kidney transplant recipients: results of two randomized controlled pilot trials. Am J Transplant. (2020) 20(2):564–72. doi: 10.1111/ajt.15580
Keywords: kidney transplant, delayed graft function, recipient anuria, donor after circulatory death, hospital stay duration
Citation: Bocchi F, Beldi G, Kuhn C, Storni F, Müller N and Sidler D (2023) Impact of suboptimal donor to suboptimal recipient kidney transplant on delayed graft function and outcome. Front. Transplant. 2:1240155. doi: 10.3389/frtra.2023.1240155
Received: 14 June 2023; Accepted: 17 August 2023;
Published: 12 September 2023.
Edited by:
Marc Melcher, Stanford University, United StatesReviewed by:
Brian Shaw, Duke University, United States© 2023 Bocchi, Beldi, Kuhn, Storni, Müller and Sidler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Federica Bocchi ZmVkZXJpY2EuYm9jY2hpQGluc2VsLmNo
†ORCID Federica Bocchi orcid.org/0000-0003-4198-1645 Christian Kuhn orcid.org/0000-0003-1346-3429 Daniel Sidler orcid.org/0000-0002-2435-5936
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.