- 1Department of Transplant Surgery, University of Wisconsin, Madison, WI, United States
- 2Ebling Library, University of Wisconsin, Madison, WI, United States
- 3Division of Abdominal Organ Transplantation, Oregon Health & Science University School of Medicine, Portland, OR, United States
- 4Department of Surgery, Division of Transplantation, Emory University, Atlanta, GA, United States
Nodular regenerative hyperplasia (NRH) is a primary disease of the liver that may cause noncirrhotic portal hypertension. Common causes include autoimmune, hematologic, immune deficiency, and myeloproliferative disorders. Given the limited data regarding the development of NRH in contemporary immunosuppressive protocols and the occurrence of NRH post-liver transplantation, we systematically reviewed NRH as it pertains to liver transplantation. We performed a comprehensive search for NRH and transplantation. Nineteen studies were identified with relevant data for NRH as an indication for a liver transplant. Thirteen studies were identified with relevant data pertaining to NRH development after liver transplant. Pooled analysis revealed 0.9% of liver transplant recipients had NRH. A total of 113 patients identified with NRH underwent liver transplantation. Most series report transplants done after the failure of endoscopic banding and TIPS management of portal hypertension. Reported 5-year graft and patient survival ranged from 73%–78% and 73%–90%. The pooled incidence of NRH after liver transplant for all indications was 2.9% and caused complications of portal hypertension. Complications related to portal hypertension secondary to NRH are a rare indication for a liver transplant. NRH can develop at any time after liver transplantation often without an identifiable cause, which may lead to portal hypertension requiring treatment or even re-transplantation.
Introduction
Nodular regenerative hyperplasia (NRH) is a pathology of the liver characterized by the development of nodules throughout the liver parenchyma without the presence of background fibrosis. While this may be an indolent finding, in some cases, it can cause noncirrhotic portal hypertension and the associated complications of variceal bleeding, thrombocytopenia, ascites, encephalopathy, and hepatopulmonary syndrome (HPS). NRH is a rare clinical diagnosis with much of the literature limited to small cohorts and case series. However, autopsy studies have found a prevalence of 2.1%–2.6% in the general population (1, 2). Although the majority of cases of NRH have no known underlying etiology, it is associated with autoimmune, hematologic, immune deficiency, and myeloproliferative disorders (3–8).
NRH is also a recognized entity post-transplantation, with a multitude of case reports and case series of transplant recipients developing NRH after renal, heart, and bone marrow transplantation (9–12). Many of these series are historical as they were reported in an era with high utilization of azathioprine (AZA)-based immunosuppressive therapy. AZA exposure is associated with NRH development in transplant recipients as well as other populations such as those with inflammatory bowel disease (13). However, these limited reports of AZA-related NRH lack modern clinical relevance as contemporary immunosuppression regimens have shifted away from AZA.
The challenge of managing NRH is understudied and can cause significant clinical challenges. In fact, the stigmata of portal hypertension secondary to NRH may become severe enough that a liver transplant is indicated. In addition, NRH may develop after liver transplantation in the donor liver. Much earlier literature is limited to case reports and a systematic review of these case reports (3). Since then, several larger retrospective cohorts of patients transplanted for NRH have been published. Given the limited data regarding the development of NRH in contemporary immunosuppressive protocols and the occurrence of NRH post-liver transplantation, we systematically reviewed the literature on NRH as it pertains to liver transplantation. Specifically, this review focuses on NRH as the indication for liver transplant and the development of NRH in the transplanted liver.
Methods
A systematic review of NRH and liver transplantation was conducted. Criteria for considering studies for this review included human case reports (1 case), case series (>1 case), randomized controlled trials, non-randomized controlled trials, case-control studies, and prospective and retrospective cohort series. The target population consisted of adult and pediatric male or female patients with a pathological diagnosis of NRH. All patients were diagnosed with NRH based on histology of a liver biopsy or final pathology of an explanted liver.
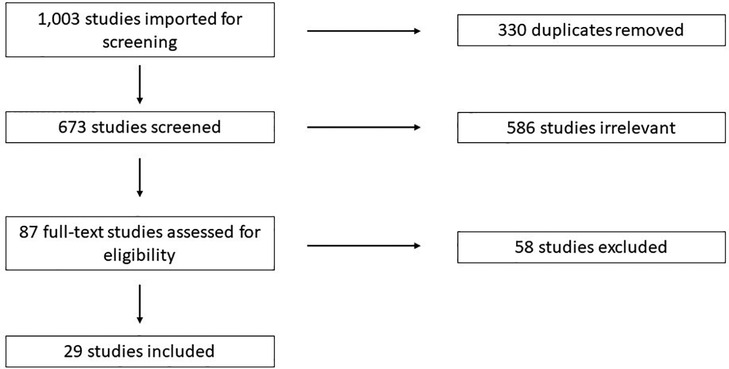
Search Terms: Studies were identified using a keyword search for relevant terms in PubMed, CINAHL, Scopus, Web of Science, and Cochrane. Search details are available in Supplementary File S1. References were imported into Covidence (https://www.covidence.org/) for screening. Studies were screened by three reviewers independently according to PRISMA guidelines (Figure 1).
Results
Search results
A total of 1,003 studies were imported for screening, and 330 duplicates were removed. Reviewers screened the title and abstract of 673 studies and 87 were judged to be possibly relevant for final inclusion (Figure 1). Of the 87 full-text studies reviewed, 29 were included for data extraction. These included 17 studies pertaining to NRH in the native liver resulting in liver transplant or listing for liver transplant (6, 7, 14–29), and 11 studies pertaining to the development of NRH after liver transplantation (30–40).
NRH as an indication for liver transplant
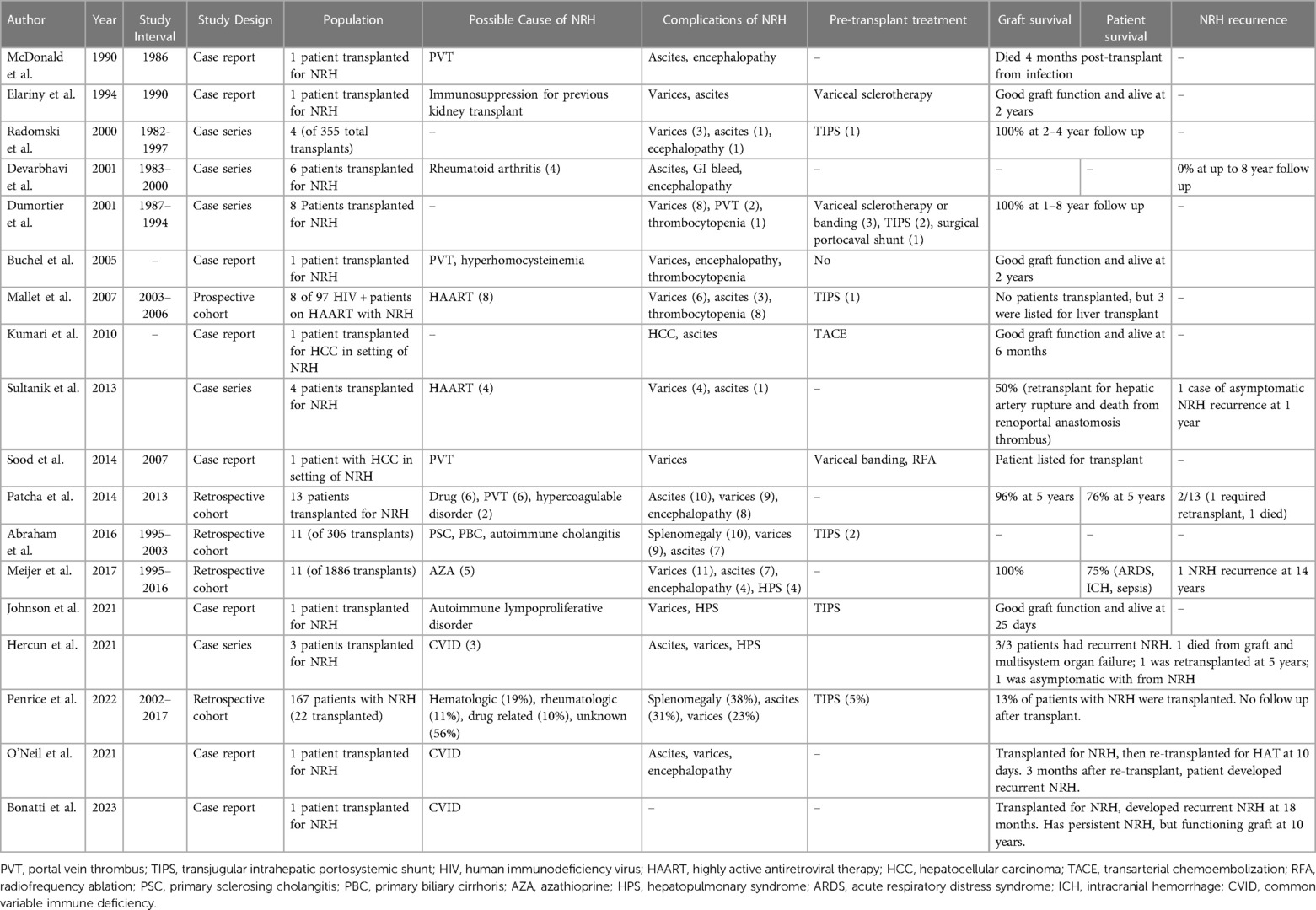
A summary of included studies is shown in Table 1. There were 113 liver transplants performed for patients with NRH. All patients had portal hypertension, and in 112, portal hypertension was the primary indication for transplant. In one patient, HCC was the primary indication for transplant (20). Of the studies that reported the incidence of NRH of the native liver amongst all liver transplant recipients, pooled incidence of NRH was 0.9% (16, 24, 33). The possible cause of NRH was highly variable, and a suggested cause of NRH was identified in 52% of cases. Five-year graft survival for studies reporting such data ranged from 73%–78% (3, 22, 24). with 5-year patient survival ranging from 73%–90% amongst NRH patients (3, 22, 24). We found reports of five patients being transplanted for NRH secondary to common variable immunodeficiency (CVID) (6, 27, 28). The outcomes for these patients were poor. All patients developed recurrent NRH after transplant, albeit at variable duration. Two developed recurrent NRH very early after transplant leading to death or retransplant; another developed recurrence of NRH much later at 5 years and required a retransplant; and two others developed late NRH, which was asymptomatic. There were eight cases of reported recurrence of NRH (6, 21, 22, 24, 27, 29). Unfortunately, there were variable details on the severity or timing of recurrent NRH, with recurrence developing anywhere from 3 months to 14 years post-transplant and in severity ranging from asymptomatic (discovered on protocol biopsy) to requiring re-transplantation.
NRH development after liver transplant
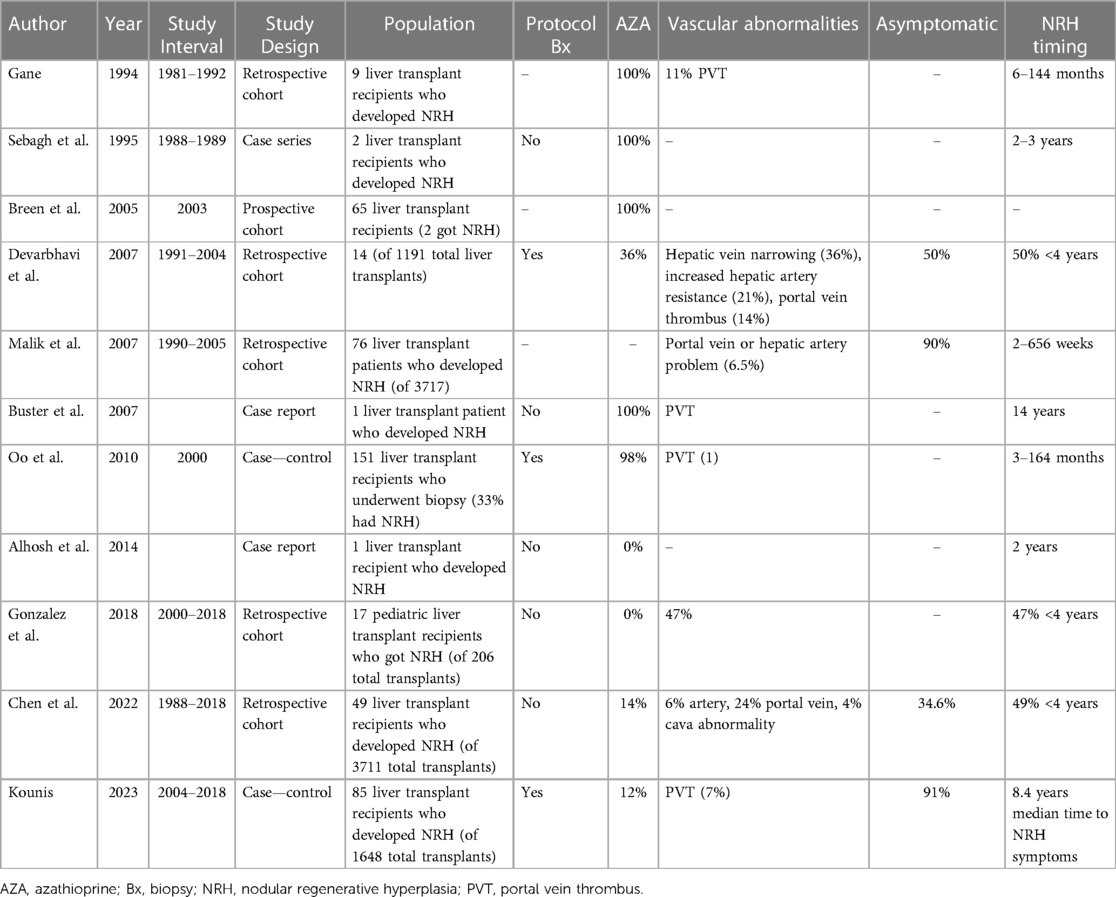
The mean incidence of NRH among liver transplant recipients was 2.9% (Tables 2, 3) (32, 34, 36, 38–40). Timing of NRH development was highly variable, and NRH was often discovered on protocol surveillance biopsies post-transplant in asymptomatic patients (33, 36, 40). For patients with NRH, those who were asymptomatic ranged anywhere from 34%–91%. Symptoms were largely those typically associated with portal hypertension. There was also significant heterogeneity among studies in rates of exposure to AZA and vascular abnormalities (known risk factors for NRH).
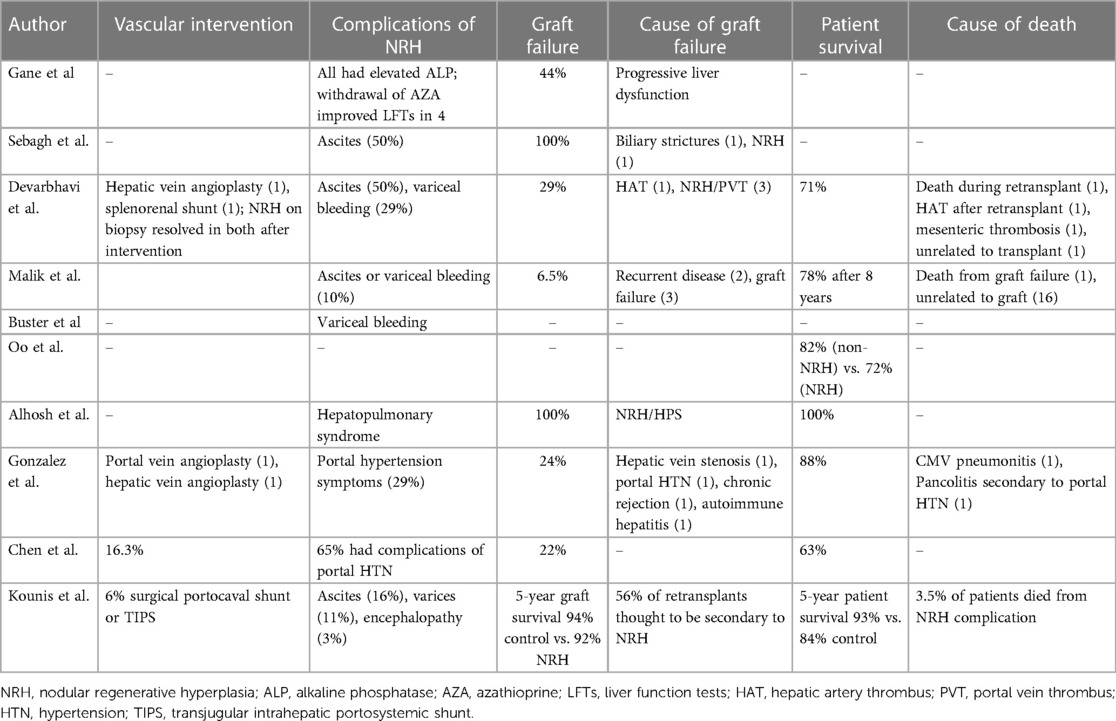
Complications of NRH development after liver transplantation ranged in severity from changes in liver function to the development of portal hypertension. There are reports of intervening on vascular abnormalities, with variable follow-up as to the persistence or resolution of NRH (33, 38, 39). Some patients required endovascular or surgical portocaval shunts for the treatment of portal hypertension (40). Of studies with larger cohorts of patients, graft failure ranged anywhere from 6%–44% and patient survival ranged from 63%–93% (30, 33, 34, 36, 39, 40).
Discussion
Autopsy studies suggest NRH is a relatively common phenomenon (incidence of up to 2%) that remains indolent in the majority of patients; however, NRH is a rare indication for liver transplantation to treat the symptoms of associated portal hypertension. Given the rarity of NRH, in this study, we systematically reviewed the literature on NRH as it relates to liver transplantation. Although NRH is rare as an indication for liver transplantation, studies that followed patients long-term demonstrated that graft and patient survival were acceptable and comparable to outcomes for liver transplantation for other indications. Most graft losses and death were for reasons unrelated to NRH, and recurrence was uncommon.
All reports of NRH were diagnosed by histology. However, many did not report which histologic criteria were required for NRH diagnosis. Similar to past reports, there was a diverse array of hypothesized causes of NRH that were an indication for patients to undergo liver transplantation (3). As has been demonstrated previously, portal vein thrombus (PVT) was sometimes found in NRH patients though studies did not report on the temporal relationship of PVT and NRH, making it challenging to draw conclusions about the causative relationship of PVT to NRH. We did not identify any trends between patients with and without an identifiable cause of NRH. One specific cause, however, stood out. The outcomes after transplant for CVID were exceptionally poor, as outlined above with a 100% NRH recurrence rate and 3/5 having either a recipient death or graft failure. Although this is a limited sample size, this scenario should be approached with caution given the high NRH recurrence rates in this specific patient population.
Another unique scenario identified involves the development of hepatocellular carcinoma (HCC) in the setting of NRH, as NRH is not traditionally thought to be a premalignant lesion. We found one report of a liver transplant performed for HCC, and the explanted liver showed NRH, without cirrhosis (20). This HCC was initially treated with transarterial chemoembolization (TACE), and the patient had a satisfactory outcome. In an autopsy series including five patients with NRH and HCC, all patients had TACE therapy (41). It is unclear in any of these cases if HCC developed in a background of NRH or if TACE treatment of HCC in a non-cirrhotic liver led to NRH development. This is plausible given the known risk of vascular abnormalities causing NRH. However, there has been a report of a patient being followed up for NRH having an HCC discovered on a surveillance ultrasound (29).
It is not well described in the literature when to transplant patients with NRH. Some studies report attempts of managing portal hypertension and its stigmata with usual best-practice medical modalities—endoscopic treatment of varices, ascites management with diuretics, and pharmacologic management of encephalopathy. Meanwhile, others report the use of transjugular intrahepatic portosystemic shunt (TIPS) for managing NRH-associated portal hypertension. It is interesting that therapies such as TIPS were not used more aggressively pre-transplant in this patient population. However, our search may not have encompassed all uses of TIPS in NRH, as we focused on liver transplantation and NRH. For example, successful long-term treatment of patients with portal hypertension from NRH is described for ascites and variceal bleeding management, and these patients may successfully avoid liver transplantation (42).
After a liver transplant, NRH occurrence is more common than we expect. It is difficult to predict who will get NRH after transplant, and there is no consensus about the causes of NRH after transplantation. One study identifies older donors as a risk factor for NRH (40), while other studies are not able to identify any independent risk factors. The timing of when NRH develops is also unpredictable and the prognosis of early vs. late NRH development is not well known. There have been conflicting reports, with some studies suggesting early NRH is more likely to lead to a negative outcome of graft loss or patient death, and others have found the opposite to be true, with late NRH (>4 years) being a predictor of a poor outcome (33, 39). Additionally, it is difficult to know what to do with the information if a recipient has NRH discovered on biopsy. Much of the larger previous series were done at centers that were standardly performing protocol biopsies at various intervals after transplant. The incidence of clinically meaningful NRH is likely much lower than what was reported in these studies. This series of protocol biopsies discovering asymptomatic NRH suggests that likely many cases of NRH never go on to develop portal hypertension or have a meaningful impact on graft function.
Many studies included patients with exposure to AZA as a part of their immunosuppression. This is much less relevant for modern immunosuppression regimens; in fact, it has been documented that withdrawal of AZA can reverse some cases of NRH in the transplanted liver (30). However, in a population of pediatric liver transplant recipients with no AZA exposure, an NRH incidence of 8% was observed, suggesting NRH can develop in immunosuppressed patients in the absence of AZA. However, this was a pediatric population with a high rate of vascular abnormalities being discovered post-transplant which could explain why NRH was common in this series (38). Another important point is that NRH was discovered on protocol biopsy of the transplanted liver in many of these studies. This calls into question whether the incidence of clinically relevant NRH is overestimated in these series. This point is further highlighted by the wide range of severity of NRH from asymptomatic to severe portal hypertension requiring liver re-transplantation.
It is challenging to know what the best management strategy is when NRH is discovered. If there is an identified medical or vascular cause thought to be contributing to the NRH, this should obviously be immediately discontinued or reversed (e.g., stopping any offending medications or evaluating for any vasculature anomalies that can be intervened on). For more severe cases, we cannot draw any conclusions from the available literature regarding standard management. However, there have been cases of successfully managing portal hypertension with TIPS, and patients have successfully been retransplanted. Not surprisingly, patients with NRH and symptomatic portal hypertension have a higher mortality rate than those without portal hypertension (39, 40).
Our review has several limitations. There is significant heterogeneity of patient populations and endpoints measured. This heterogeneity makes it difficult to directly compare different studies. There are also a wide range of time periods included. Older studies may be less relevant as practice patterns have changed, with less reliance on AZA-based immunosuppression and protocol biopsies after liver transplant. Specifically for the patient population that developed NRH in a transplanted liver, the larger series have very limited donor data which may be an important variable in NRH development. A challenge in interpreting the current data is that little is known about the natural history of NRH. It seems, especially based on incidentally discovered, asymptomatic NRH that at least some cases of NRH do not cause significant problems. Still, other cases cause severe portal hypertension. How to best navigate this spectrum of disease severity without knowing the natural history will continue to be a challenge for clinicians. Finally, as described above, the rarity of the pathology, variable severity, and treatments make it difficult to draw conclusions regarding optimal treatment plans.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
BB, PS, MH, AB, SK, and DA contributed to the literature search, analysis, drafting of the manuscript, and final editing. All author contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors PS and DA declared that they were editorial board members of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frtra.2023.1221765/full#supplementary-material
References
1. Nakanuma Y. Nodular regenerative hyperplasia of the liver: retrospective survey in autopsy series. J Clin Gastroenterol. (1990) 12(4):460–5. doi: 10.1097/00004836-199008000-00023
2. Wanless IR. Micronodular transformation (nodular regenerative hyperplasia) of the liver: a report of 64 cases among 2,500 autopsies and a new classification of benign hepatocellular nodules. Hepatology. (1990) 11(5):787–97. doi: 10.1002/hep.1840110512
3. Manzia TM, Gravante G, Di Paolo D, Orlando G, Toti L, Bellini MI, et al. Liver transplantation for the treatment of nodular regenerative hyperplasia. Dig Liver Dis. (2011) 43(12):929–34. doi: 10.1016/j.dld.2011.04.004
4. Jain P, Patel S, Simpson HN, Silver RM, Lewin DN, Campbell RC, et al. Nodular regenerative hyperplasia of the liver in rheumatic disease: cases and review of the literature. J Investig Med High Impact Case Rep. (2021) 9:23247096211044616. doi: 10.1177/23247096211044617
5. Al-Mukhaizeem KA, Rosenberg A, Sherker AH. Nodular regenerative hyperplasia of the liver: an under-recognized cause of portal hypertension in hematological disorders. Am J Hematol. (2004) 75(4):225–30. doi: 10.1002/ajh.20024
6. Hercun J, Parikh E, Kleiner DE, Fuss I, Uzel G, Strober W, et al. Recurrent nodular regenerative hyperplasia following liver transplantation in common variable immunodeficiency. Hepatology. (2021) 74(3):1698–701. doi: 10.1002/hep.31775
7. Mallet V, Blanchard P, Verkarre V, Vallet-Pichard A, Fontaine H, Lascoux-Combe C, et al. Nodular regenerative hyperplasia is a new cause of chronic liver disease in HIV-infected patients. AIDS. (2007) 21(2):187–92. doi: 10.1097/QAD.0b013e3280119e47
8. Stromeyer FW, Ishak KG. Nodular transformation (nodular “regenerative” hyperplasia) of the liver. A clinicopathologic study of 30 cases. Hum Pathol. (1981) 12(1):60–71. doi: 10.1016/S0046-8177(81)80242-0
9. Snover DC, Weisdorf S, Bloomer J, McGlave P, Weisdorf D. Nodular regenerative hyperplasia of the liver following bone marrow transplantation. Hepatology. (1989) 9(3):443–8. doi: 10.1002/hep.1840090317
10. Malik S, Sadd D, Behari J, Chadalavada R, Demetris AJ, Ahmad J. Nodular regenerative hyperplasia post solid organ (non-liver) transplant. Hepatology. (2007) 46(4):503A–4A.
11. Rushakoff J, Kransdorf E, Patel J, Kobashingawa J, Guindi M. Impact of fibrosis and nodular regenerative hyperplasia in liver biopsies on survival following heart transplant. Lab Invest. (2021) 101:986.
12. Allison MC, Mowat A, McCruden EA, McGregor E, Burt AD, Briggs JD, et al. The spectrum of chronic liver disease in renal transplant recipients. Q J Med. (1992) 83(301):355–67.1438671
13. Vernier-Massouille G, Cosnes J, Lemann M, Marteau P, Reinisch W, Laharie D, et al. Nodular regenerative hyperplasia in patients with inflammatory bowel disease treated with azathioprine. Gut. (2007) 56(10):1404–9. doi: 10.1136/gut.2006.114363
14. McDonald JA, Painter DM, Gallagher ND, McCaughan GW. Nodular regenerative hyperplasia mimicking cirrhosis of the liver. Gut. (1990) 31(6):725–7. doi: 10.1136/gut.31.6.725
15. Elariny HA, Mizrahi SS, Hayes DH, Boudreaux JP, Hussey JL, Farr GH. Nodular regenerative hyperplasia: a controversial indication for orthotopic liver transplantation. Transpl Int. (1994) 7(4):309–13. doi: 10.1111/j.1432-2277.1994.tb01580.x
16. Radomski JS, Chojnacki KA, Moritz MJ, Rubin R, Armenti VT, Wilson GA, et al. Results of liver transplantation for nodular regenerative hyperplasia. Am Surg. (2000) 66(11):1067–70. doi: 10.1177/000313480006601119
17. Devarbhavi H, Nzeako U, Hassoun Z, Shah V, Petrovic L, Wiesner R, et al. Liver transplantation and nodular regenerative hyperplasia: risk factors and outcomes. Hepatology. (2001) 34(4):197A. doi: 10.1002/hep.24527
18. Dumortier J, Bizollon T, Scoazec JY, Chevallier M, Bancel B, Berger F, et al. Orthotopic liver transplantation for idiopathic portal hypertension: indications and outcome. Scand J Gastroenterol. (2001) 36(4):417–22. doi: 10.1080/003655201300051298
19. Buchel O, Roskams T, Van Damme B, Nevens F, Pirenne J, Fevery J. Nodular regenerative hyperplasia, portal vein thrombosis, and avascular hip necrosis due to hyperhomocysteinaemia. Gut. (2005) 54(7):1021–3. doi: 10.1136/gut.2004.055921
20. Kumari R, Bouneva I, Schadde E, Oshima K, Bacon B, Tuttle-Newhall J. Liver transplantation for hepatocellular carcinoma in the setting of nodular regenerative hyperplasia. Am J Gastroenterol. (2010) 105(1):S279–80.
21. Sultanik P, Coilly A, Sebagh M, Antonini TM, Teicher E, Roche B, et al. Lengthy follow-up after liver transplantation for nodular regenerative hyperplasia in human immunodeficiency virus-infected patients: does the disease recur? Transplantation. (2013) 96(11):e79–81. doi: 10.1097/01.TP.0000436930.53768.45
22. Patcha VR, Kontis E, Jabri Y, Srinivasan P, Prachalias A, Menon K, et al. An experience of liver transplantation in patients with nodular regenerative hyperplasia. Liver Transpl. (2014) 20:S345.
23. Abraham SC, Kamath PS, Eghtesad B, Demetris AJ, Krasinskas AM. Liver transplantation in precirrhotic biliary tract disease: portal hypertension is frequently associated with nodular regenerative hyperplasia and obliterative portal venopathy. Am J Surg Pathol. (2006) 30(11):1454–61. doi: 10.1097/01.pas.0000213286.65907.ea
24. Meijer B, Simsek M, Blokzijl H, de Man RA, Coenraad MJ, Dijkstra G, et al. Nodular regenerative hyperplasia rarely leads to liver transplantation: a 20-year cohort study in all dutch liver transplant units. United Eur Gastroenterol J. (2017) 5(5):658–67. doi: 10.1177/2050640616680550
25. Johnson G, Huber A, Levstik M, Laryea M. A case of hepatopulmonary syndrome requiring living donor liver transplantation in a patient with nodular regenerative hyperplasia and autoimmune lymphoproliferative syndrome-associated with granulomatous hepatitis without cirrhosis. Hepatology. (2021) 74:S2660. doi: 10.1002/hep.31764
26. Penrice DD, Thakral N, Kezer CA, Lennon R, Moreira RK, Graham RP, et al. Outcomes of idiopathic versus secondary nodular regenerative hyperplasia of the liver: a longitudinal study of 167 cases. Liver Int. (2022) 42(6):1379–85. doi: 10.1111/liv.15202
27. O’Neil M, Kumer S, Schmitt T, Heronemus M, Gilroy R. Severe common variable immunodeficiency and aggressive recurrence of nodular regenerative hyperplasia post liver transplant resulting in bone marrow transplant: a case study. Liver Transpl. (2014) 20:S866–287.
28. Bonatti HJR, Roman AL, Krebs E, Sifri CD, Hagspiel KD, Sawyer RG, et al. Good long-term outcome following liver transplant in a patient with common variable immunodeficiency syndrome despite multiple infections and recurrent nodular regenerative hyperplasia. Exp Clin Transplant. (2023) 21(1):66–9. doi: 10.6002/ect.2022.0067
29. Sood A, Cox GA, McWilliams JP, Wang HL, Saab S. Patients with nodular regenerative hyperplasia should be considered for hepatocellular carcinoma screening. Hepatol Res. (2014) 44(6):689–93. doi: 10.1111/hepr.12136
30. Gane E, Portmann B, Saxena R, Wong P, Ramage J, Williams R. Nodular regenerative hyperplasia of the liver graft after liver transplantation. Hepatology. (1994) 20(1 Pt 1):88–94. doi: 10.1002/hep.1840200114
31. Sebagh M, Farges O, Samuel D, Bismuth H, Reynès M. Nodular regenerative hyperplasia of the liver following orthotopic liver transplantation. Transplant Proc. (1995) 27(4):2510–1.7652907
32. Breen DP, Marinaki AM, Arenas M, Hayes PC. Pharmacogenetic association with adverse drug reactions to azathioprine immunosuppressive therapy following liver transplantation. Liver Transpl. (2005) 11(7):826–33. doi: 10.1002/lt.20377
33. Devarbhavi H, Abraham S, Kamath PS. Significance of nodular regenerative hyperplasia occurring de novo following liver transplantation. Liver Transpl. (2007) 13(11):1552–6. doi: 10.1002/lt.21142
34. Malik S, Sass D, Behari J, Chadalavada R, DeVera M, Fontes P, et al. Outcome of nodular regenerative hyperplasia after liver transplantation. Am J Transplant. (2007) 2AD:7:474.
35. Buster EHCJ, van Vuuren HJ, Zondervan PE, Metselaar HJ, Tilanus HW, de Man RA. Thiopurine-methyltransferase and inosine triphosphate pyrophosphatase polymorphism in a liver transplant recipient developing nodular regenerative hyperplasia on low-dose azathioprine. Eur J Gastroenterol Hepatol. (2008) 20(1):68–72. doi: 10.1097/MEG.0b013e32825a6a8a
36. Oo Y, Quaas A, Gunson B, Hubscher S, Neil D, Thorburn D. Prevalence and clinical outcome of nodular regenerative hyperplasia in post liver transplant recipients in a single centre. J Hepatol. (2010) 52:S183. doi: 10.1016/S0168-8278(10)60488-6
37. Alhosh R, Genyk Y, Alexopoulos S, Thomas D, Zhou S, Yanni G, et al. Hepatopulmonary syndrome associated with nodular regenerative hyperplasia after liver transplantation in a child. Pediatr Transplant. (2014) 18(5):E157–60. doi: 10.1111/petr.12281
38. González I, Lu HC, Ritter JH, Maluf HM, Dehner LP, He M. Clinicopathologic characteristics of de novo nodular regenerative hyperplasia in pediatric liver transplant. Pediatr Transplant. (2019) 23(5):e13471. doi: 10.1111/petr.13471
39. Chen AK, Lunow-Luke T, Yamaguchi S, Praglin C, Agudelo E, Mehta N, et al. Nodular regenerative hyperplasia after liver transplant; It's All in the presentation. Front Surg. (2022) 9:876818. doi: 10.3389/fsurg.2022.876818
40. Kounis I, Sebagh M, Evain M, Cailliez V, Roche B, De Martin E, et al. Nodular regenerative hyperplasia is not a rare condition after liver transplantation: incidence, predictive factors, and impact on survival. Transplantation. (2023) 107(2):410–9. doi: 10.1097/TP.0000000000004303
41. Kobayashi S, Saito K, Nakanuma Y. Nodular regenerative hyperplasia of the liver in hepatocellular carcinoma. An autopsy study. J Clin Gastroenterol. (1993) 16(2):155–9. doi: 10.1097/00004836-199303000-00016
Keywords: nodular regenerative hyperplasia, portal hypertension, ascites, encephalopathy, varices, biopsy
Citation: Biesterveld BE, Schroder PM, Hitchcock ME, Bolognese A, Kim SC and Al-Adra DP (2023) Nodular regenerative hyperplasia and liver transplantation: a systematic review. Front. Transplant. 2:1221765. doi: 10.3389/frtra.2023.1221765
Received: 12 May 2023; Accepted: 17 August 2023;
Published: 6 September 2023.
Edited by:
Andrew S. Barbas, Duke University, United StatesReviewed by:
Jane Hartley, Birmingham Women's and Children's Hospital, United KingdomDamiano Patrono, Azienda Ospedaliero Universitaria Città della Salute e della Scienza di Torino, Italy
Owen Lawrence Cain, University Hospitals Birmingham NHS Foundation Trust, United Kingdom
© 2023 Biesterveld, Schroder, Hitchcock, Bolognese, Kim and Al-Adra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David P. Al-Adra YWxhZHJhQHdpc2MuZWR1
 Ben E. Biesterveld
Ben E. Biesterveld Paul M. Schroder
Paul M. Schroder Mary E. Hitchcock2
Mary E. Hitchcock2 Alexandra Bolognese
Alexandra Bolognese Steven C. Kim
Steven C. Kim David P. Al-Adra
David P. Al-Adra