- The Department of Chemistry, College of Natural and Computational Sciences, Hawassa University, Hawassa, Ethiopia
Parthenium (Parthenium hysterophorus) weed is a noxious plant which inhabits many parts of the world. It is responsible for rapid environmental pollution causing a reduction in crop productivity by infesting farm lands and grazing lands. It also causes severe human and animal health problems. The aim of this study was assessing the potential of parthenium weed ash as a substitute for commercial alkali for the preparation of soap. Alkali solution was prepared from partheniun weed ash. Tests on its alkalinity properties revealed that the lye can be used for soap making. Similarly, the tests on physicochemical properties of the collected used cooking oil samples were found to be 141.0 ± 0.4–153.8 ± 0.33 mg KOH/g, 11.28 ± 0.5–14.1 ± 0.5 mg KOH/g, and 0.16 ± 0.2–0.19 ± 0.25 milieq/g for saponification value, acid value, and peroxide value, respectively. The data indicated that both the prepared lye solution and the collected used cooking oils would be suitable for soap preparation. Moreover, analyses of the moisture content, total alkali content, total fatty matter, the pH value, and chloride content of the prepared soap materials were found to be in the ranges of 10.5 ± 0.01–13 ± 0.01, 0.39 ± 0.01–1.63 ± 0.05%, 64.5 ± 0.16–76.4 ± 0.15%, 10.5 ± 0.03–10.63 ± 0.04, and 0.39 ± 0.2–0.45 ± 0.39%, respectively. The finding of the present study, is that it is possible to conclude that the Parthenium weed ash (or lye solution from this ash) and leftovers of used cooking oils can be used as cost-effective substitutes for commercial alkali solution and palm oil, respectively, for laundry soap preparation of acceptable grades. The finding also suggests that preparation of lye solution from parthenium weed ash can be used as an option for controlling the pollution of this invasive weed.
Introduction
Parthenium weed (Parthenium hysterophorus) is a noxious and invasive weed that is widely distributed in many parts of the world (Figure 1). It was native to North and South America and the West Indies but currently it aggressively has colonized many countries in the world (Fessehaie et al., 2005; Manpreet et al., 2014). It is known to be one of the worst weeds that can cause serious environmental pollution. The weed causes major negative impact on pasture and crops. The weed is also known to be responsible for severe human and animal health problems such as dermatitis, asthma, and bronchitis (Parsons and Cuthbertson, 1992; Lakshmi and Srinivas, 2007; Khan et al., 2012; Sushilkumar, 2012; Siddiqui et al., 2016). It is not palatable to livestock due to its irritating odor, taste, and presence of trichome hairs. If eaten by cattle, it will also cause clinical signs such as those of salivation, anorexia, pruritus, alopecia, and dermatitis. Gastrointestinal irritation may result in diarrhea, depigmentation of skin and also results in milk yield reduction if hungry cows eat it (Parsons and Cuthbertson, 1992; Fessehaie et al., 2005; Khan et al., 2012; Manpreet et al., 2014).

Figure 1. The Parthenium weed (Photo taken from Uffa Kebele, Kaffa zone, by Tesfaye G., May 2018GC.).
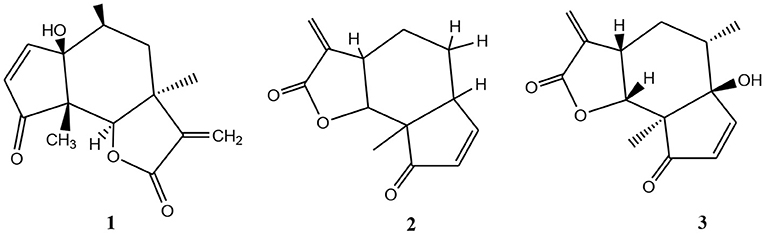
Parthenium weed inhibits the growth of other plants including crops as it exerts an allelopathic effect. This could be attributed to the presence of chemicals such as parthenin (1), hymenin (2), and ambrosin (3) (Figure 2) (Tefera, 2002; Devi et al., 2014). There are also reports from Ethiopia that revealed the threat of the weed in the reduction of the productivity of crops in different parts of the country (Mohammed, 2010; Taye et al., 2010; Gadisa et al., 2019).
Literature reports revealed different control methods to curb the invasion of parthenium in a human environment. The most common ones are (i) Physical control method: it involves the manual uprooting of parthenium before flowering and seed setting. This method is not effective if uprooting the weed after seed setting as it will increase the area of infestation, and it is also a time consuming and unpleasant option due to health-related problems to people that participate in weeding (Parsons and Cuthbertson, 1992; Lakshmi and Srinivas, 2007; Khan et al., 2012; Sushilkumar, 2012; Siddiqui et al., 2016); (ii) Chemical control method:- this is an effective method to control parthenium in the areas where its natural enemies are absent (Mishra and Bhan, 1994; Javaid, 2007; Gaikwad et al., 2008). Some of the limitations associated with the method are (a) it is effective only at the rosette stage of the weed (Khan et al., 2012), (b) toxicities of the chemicals to other useful plant species, and (c) resistance development against many of herbicides (Ware, 1986; Njoroge, 1991; Singh et al., 2004; Vila-Aiub et al., 2008; Javaid and Adrees, 2009); (iii) Biological control method: it is an environmentally sound and effective means of reducing the weed through the use of natural enemies such as microbial pathogens, insects, and botanicals (Watson and Wymore, 1990; Ray and Gour, 2012). Though this method has gained the highest acceptance (as compared to the rest two methods) in its safety and environmentally friendliness (Aneja, 2009), it is a very slow operation and currently used only in non-cropped areas. The limitations of the aforementioned methods suggest the need of a new and novel approach to prevent rapid infestation of the parthenium weed.
Plant materials such as cassava peels, palm bunch, wood, cocoa pad, banana leaves, maize, sugar beet wastes, and many others give ashes that can be used for the preparation of lye (alkaline) solution for soap making. Most of the plant materials are readily and locally available. Moreover, preparation of lye solution from these materials and then soap making by treating them with animal fats/oils does not need expertise. So, farmers or anyone can prepare lye solution from their ashes and can prepare good grade soap with low cost as compared to those methods that involve expensive commercial inputs (e.g., NaOH or KOH) (Ambe, 2001). Onyegbado and Offor (2002) reported the preparation of solid odorless soap using alkali solution obtained from plantain peel ash. In another report, it has been mentioned that ashes from Mangrove products (e.g., leaves) were used to prepare alkali solution that resulted in good quality solid/hard soap (Lacerdo, 2004). Production of black soap has also been reported from palm bunch ashes (Mistral et al., 1993). Similar reports also showed that ashes from palm trees contain fundamental chemical components (e.g., sodium hydroxide and potash) needed for the production of soap (Godin and Spensley, 1971; Ashtor and Cevidails, 1983; Tite et al., 2006; Atiku et al., 2014; Undeiandeye et al., 2015).
Used cooking oils are other types of pollutants that cause environmental pollution. These are usually disposed to the environment as wastes after their repeated use for cooking or frying. When disposed to the environment, they cause of many environmental problems. These include the blockade of sanitary sewer systems in cities, and their degradation in pipes may also cause corrosion of metals and concrete elements. They also contaminate water and land resourses (Arju et al., 2008). Literature reports also revealed several human health-related problems of used cooking oils (Arju et al., 2008). Current trends show that with the increasing numbers of fast-food processing industries, hotels, and restaurants, it is expected that considerable amounts of used cooking oil wastes are to be discarded into human environment, and cause pollution. Therefore, proper collection and reusing them for other purposes such as biofuel (Khalisanni et al., 2008; Nor et al., 2013; Panadare and Rathod, 2015) and soap preparation (Kazuo and Kasukabe, 1989; Malaysia Batu Caves, 2015) are strongly recommended. There are reports that revealed the use of used cooking oil as raw materials for making good quality soap (Mistral et al., 1993; Legesse, 2020). However, there are no reports on the preparation of soap from lye solution obtained from ash of parthenium weed and used cooking oil. The aim of this study was to use the prepared lye solution and used cooking oil for soap making. This attempt or the findings of the study would serve as a means to minimize environmental pollutions caused both by parthenium weed and used cooking oil (wastes). Moreover, the benefit of making soap with low cost and with no need of expertise is also another motive that initiated this study.
Materials and Methods
Chemicals and Reagents
Different chemicals and reagents were used in the study. These include alcoholic potassium hydroxide, sodium carbonate, 36% hydrochloric acid, phenolphthalein, methyl orange, ethanol, potassium hydroxide solution, Iodine solution, glacial acetic acid, potassium iodide, 98% sulfuric acid, diethyl ether, sodium thiosulfate, calcium nitrite, chloroform, potassium dichromate, silver nitride, EDTA, and sodium chloride.
Plant Material Collections and Preparation
Parthenium weed was collected from Uffa Kebele, Gimbo Woreda, Kaffa Zone of South western Ethiopia in May 2018. The weed (leaves and stem) was dried for few days by direct sunlight. The dried weed was burned in an open air-rich locally constructed stove. The unburned black components (charcoal) were separated from the fine white ash using flour sifter. The ash was allowed to cool and then packed in polyethylene bags (Figure 3) until used for the preparation of lye as per the procedures reported in literature (Mak-Mensah and Firempong, 2011).
Preparation of Lye Solution
The lye solution was prepared by employing procedures reported in literature (Mak-Mensah and Firempong, 2011). It involved the mixing of 100 ml of distilled water and 5 g of ash in a 500 ml beaker. The mixture was boiled after mixing its contents properly with gentle stirring. The boiled solution was allowed to cool to room temperature followed by filtration using filter paper. The alkalinity of the filtrate (solution) was tested using indicators (methyl orange, phenolphthalein, and litmus paper) and pH meter. The concentration of the prepared lye was tested physically using a fresh whole egg. The floating of the egg was taken as an indicator for formation of lye that is strong enough to be used for soap preparation (Legesse et al., 2020).
Pre-treatment of Used Cooking Oil Samples
Used cooking oil samples were collected from hotels and street food venders found in Uffa kebele, Kaffa zone (Figure 4). The collected used cooking oil samples were pre-treated in order to remove solids, inorganic materials, and other contaminants. It was carried out first by heating the oils at 60°C. Then the hot oil samples were allowed to cool to room temperature followed by filtrations in order to get oil materials suitable for soap preparation (Legesse, 2020).
Analyses of Properties of Used Cooking Oil Samples
The properties of the pre-treated oil samples were tested by employing standard procedures reported in literature (Ibrahim and Yusuf, 2015) in order to assess their suitability for soap preparation. These include saponification value, acid value, and peroxide value (sections Saponification Value, Acid value, and Peroxide value). All the tests were carried out in triplicates.
Saponification Value
Two grams of oil sample was poured into 200 ml conical flask, and then 25 ml of 0.5 M of ethanoic potassium hydroxide solution was added in the flask. The mixture was heated on a water bath for 1 h with frequent shaking. The solution was allowed to cool to room temperature. The cold solution was titrated with warm 0.5 M hydrochloric acid. One % phenolphthalein was used as an indicator. The saponification values of the oil samples were determined according to the following relation (Ibrahim and Yusuf, 2015).
where,
A = 0.5 M of HCl used blank titration.
B = 0.5 M of HCl used sample titration.
Q = weight in grams of the oil sample.
28.05 = conversion factor.
Acid Value
Two point five grams of oil sample and 50 ml of freshly neutralized hot ethyl alcohol were mixed in a 250 ml conical flask. 1 ml of phenolphthalein indicator solution was added to the above mixture, and then the mixture was boiled for 5 min. The hot solution was titrated against 0.5 N standardized alkali solution shaken vigorously during the titration. The acid values of the oil samples were determined using the formula given below (Ibrahim and Yusuf, 2015).
where,
V = volume in ml of standard potassium hydroxide or sodium hydroxide solution.
N = Normality of the KOH or NaOH solution.
W = Weight grams of the oil sample.
56.1 = conversion factor.
Peroxide Value
Two grams of the oil sample was added into a 250 ml flask that initially contained 1 g of powdered potassium iodide (KI) and a solvent mixture (2:1 of glacial acetic and chloroform by volume). The solution was heated using a water bath for a few minutes for complete dissolution the solid components of the mixture. Twenty ml of 50% potassium iodide solution was introduced, and the mixture was titrated with 0.1 M Na2S2O3 solution. Starch solution was used as an indicator. The blank experiment was also carried out using a similar procedure to the one mentioned above. The peroxide values of the oil samples were determined using the formula given below (Ibrahim and Yusuf, 2015).
where, R and B stand for oil and blank samples in term of titrated values, respectively.
Preparation of Soap
Ten grams of oil sample was heated in a beaker at a temperature of 60°C. A 30 ml of ethanol and 60 ml of prepared alkali solution were added to the beaker containing the oil sample. The mixture was heated with continuous stirring using a wooden stirrer for 50 min or until the process of saponification was completed (Mak-Mensah and Firempong, 2011). The soap solution was poured into a beaker containing 50 ml saturated sodium chloride solution and stirred with a glass rod. Finally, the mixture was cooled and filtered with filter paper. The soap product was rinsed and was transferred to an aluminum-made dish for molding.
Physicochemical Properties Prepared Soap Materials
Some of the physicochemical properties of the prepared soap materials were determined using standard procedures reported in literature (Ambe, 2001). All the tests were carried out in triplicates. Similar tests were carried out for three commercial soap samples (Top, B-29, and peacock) purchased from a local market.
Moisture Content
Five grams of soap sample was dissolved in water. The solution was dried at 110°C in an oven. The dried material (residue) was weighed to determine its mass. The moisture content (MC) of the soap samples was determined using the following formula reported in literature (Ambe, 2001).
Where Wi = initial weight of the sample; Wf = weight after drying the sample.
Total Alkali Content
One hundred milliliter neutralized ethanol was added to a beaker containing 10 g of soap sample. Then, 5 ml of 1 N H2SO4 solution was added to the mixture, and it was heated until the soap sample was dissolved completely. The content of the flask was allowed to cool gradually to room temperature. The remaining sulfuric acid (excess sulfuric acid) was estimated by back titrating the test mixture with standard 1 N NaOH and also using phenolphthalein as an indicator. The total alkali calculated using the following formula (Ambe, 2001).
Where, VA = volume of acid; VB = volume of base, and W = weight of soap.
Total Fatty Matter
Five grams of soap sample was dissolved in 50 ml of distilled water. The volume of the solution was adjusted to 10 ml. The solution was made acidic by adding 10 ml of 0.1 M sulphuric acid. The solution was then subjected to extraction with 50 ml diethyl ether and subsequently with three 25 ml portions of diethyl ether. The combined ether extracts were filtered into a 250 ml flask. The solvent (ether) was vaporized to determine the total fatty matter. The amount of the fatty matter was calculated by subtracting the weight of the ether extract from the initial weight of the soap sample (Ambe, 2001).
pH Value
Ten grams of soap sample was dissolved in distilled water. The solution was made by adding excess water up to 100 ml in order to prepare 10% soap solution. The pH of the solution was measured using a meter that was calibrated using a buffer solution of pH 7 and 10. Then 2 g of the prepared soap sample was dissolved in 10 ml of distilled water. The pH of the solution was determined with the same pH meter used above (Ambe, 2001).
Chloride Content
Ten grams of soap sample was dissolved in 100 ml distilled water. The mixture was heated till the soap sample dissolved completely. The resulting solution was transferred into a 250 ml volumetric flask, and 20 ml of 15% Ca(NO3)2 solution was added to it. More distilled water was added to raise volume of the solution up to the 250 ml mark. The solution was filtered, and 100 ml of it was taken in a separate flask. Methyl red was added to this 100 ml of filtrated solution. The solution was then titrated against 10N H2SO4 until a pink color was obtained. The resulting solution was again titrated by 0.1N AgNO3 using K2CrO7 as an indicator until a brick red color was obtained. The % chloride was determined using the following formula reported in literature (Ambe, 2001).
where, W = weight of sample.
Lather Stability Test
Two grams of powder of the prepared soap sample was dissolved in 800 ml of distilled water to form uniform soap solution. Five hundred milliliters of the solution was poured into a conical flask and stirred vigorously for 2 min. Then it was allowed to settle until lather formation of the solution. Later, the solution lather was measured and recorded (Ambe, 2001).
Cleaning Ability Test
To determine cleaning ability (property) of the prepared soap material, pieces of cotton clothes that were stained with chicken sauce (“doro wot”) were used. The stained cotton cloth pieces were immersed into a test tube containing soap solution (that was prepared by dissolving 2 g of soap sample in distilled water). Then the test tube was shaken vigorously for 1 min. Finally, the cotton clothes were removed and rinsed with distilled water to test the cleaning ability of the prepared soap materials (Ambe, 2001).
Results and Discussion
Alkali (Lye) Solution Preparation
Plant ashes are used as sources of alkali (or lye) solutions that mainly consist of potassium hydroxide (KOH) or sodium hydroxide (NaOH) and can be used for soap making (Lacerdo, 2004; Mak-Mensah and Firempong, 2011). In this study, alkali solution was obtained from parthenium weed ash. It was prepared by boiling the ash in distilled water and subsequently filtered and then concentrating the filtrate by evaporation (Figure 5A). Alkalinity of the solution was tested using indicators such as methyl orange which changed to yellow color, phenolphthalein which changed to pink, and red litmus paper turned to blue. Three color changes indicated that the prepared lye solution is alkali in its nature. Its alkalinity was also determined using a pH meter. This test also confirmed the prepared solution to be basic as confirmed with its observed pH value (i.e., 11.9). In areas where indicators and pH meters are not available, physical tests can be used to test alkalinity of lye solutions before using them for soap making. This method involves the insertion of a fresh egg into the prepared lye solution (Figure 5A). In such a test, the egg was noticed to sink to the bottom (Figure 5B) whereas in the concentrated lye solution the egg was found to float (Figure 5C). Consistent with literature report (Legesse et al., 2020), the floating of the egg (Figure 5C) on the lye solution indicted that the prepared lye solution was concentrated and strong enough to be used for soap preparation.

Figure 5. The prepared lye solution (A), less concentrated lye (B), and concentrated lye solution (C).
Analyses of Properties of the Collected Oil Samples
The physicochemical analyses of the oil samples were carried out following methods reported in literature in order to test their quality or suitability for soap making (Ibrahim and Yusuf, 2015; Legesse, 2020). The properties considered were saponification values, acid values, and peroxide values (sections Saponification Value, Acid Value, and Peroxide Value).
Saponification Value
The saponification value is a property that is expressed as the number of mg of potassium hydroxide required to saponify 1 g of oil. The oil sample is saponified by refluxing it with excess of alcoholic potassium hydroxide solution. The alkali solution required for saponification is determined by titration of the excess potassium hydroxide with standard hydrochloric acid [41]. The value gives information about the character of the fatty acid of the fat/oil. The lower the saponification value, the larger the molecular weight of fatty acid in the glycerides. The saponification value of the oil samples followed procedures reported in literature. The observed data showed that the saponification values to be 153.8 ± 0.33 mg KOH/g and 141±0.4 mg KOH/g of oil samples obtained from hotel and street food vendors, respectively (Table 1). These data are comparable to the data reported for oil obtained from sweet orange seed (106.30 mg KOH/g) but slightly lower than the reported saponification values of palm oil (199.1 mg KOH/g) and coconut oil (257.0 mg KOH/g) (Ibrahim and Yusuf, 2015). The finding of the present study indicated that the collected oil samples could be used as suitable inputs for soap making.
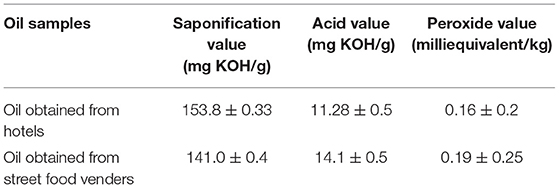
Table 1. Physicochemical properties (saponification values, acid values and peroxide values) of oil samples.
Acid Value
An acid value refers the number of milligrams of potassium hydroxide required to neutralize free fatty acid present in 1 gram of fat or oil (Debesh, 2013). It is a relative measurement of rancidity or free fatty acid formation during decomposition of oil glycerides. This value can be determined directly by titration oil in an alcoholic medium against standard sodium hydroxide solution. There are literature reports (Debesh, 2013; Ibrahim and Yusuf, 2015) that reveal that the lower the acid value of oil, the fewer fatty acid it contains. This makes it less susceptible to rancidity. In the present study, the acid values of the oil samples were found to be 11.28 ± 0.5 mg KOH/g and 14.1 ± 0.5 mg KOH/g for oil samples obtained from hotel and street food venders, respectively (Table 1). The finding also showed that the observed data are lower than the standard values (15 mg KOH/g) reported literature (Debesh, 2013; Ibrahim and Yusuf, 2015). Moreover, the data are also lower than that of olive oil (17.5 mg KOH/g) (Ibrahim and Yusuf, 2015). The finding also suggested that the collected used cooking oil samples used in the study are suitable for soap production.
Peroxide Value
The peroxide value refers to the amount of peroxide oxygen per kilograms of fats or oil. It is commonly expressed in unit of milli equivalent. This value can be determined by measuring the amount of iodine which is formed by the reaction of peroxide (formed in fat or oil) with iodide ion (Ibrahim and Yusuf, 2015). The peroxide values of the used cooking oil samples used in the present study were found to be 0.16 ± 0.2 millieq/kg and 0.19 ± 0.25 millieq/kg for the used cooking oil samples obtained from hotels and street food vendors, respectively (Table 1). Literature reports showed that peroxide values of fresh oils are usually <10 milliequivalent/kg. Moreover, a lower peroxide value indicates that a given oil sample is stable whereas a higher peroxide value is associated with a high rancidity rate (Ibrahim and Yusuf, 2015). The data observed in this study are lower than for pawpaw (3.12 meq/kg) and sweet orange seed oil (2.21 meq/Kg) as reported in literature (Ibrahim and Yusuf, 2015). This is an indication that the used cooking oil samples used in the study are suitable to be used for soap making.
Preparation of Soap
Laundry soap is prepared by treating vegetable oil/animal fats with strongly alkaline solution. In such a process of saponification (Figure 6), the fats/oils are first hydrolyzed to free fatty acid which then can be combined with alkali solution to form soap (Ambe, 2001; Dennis, 2011; Mak-Mensah and Firempong, 2011; Beetseh and Anzo, 2013). Palm oil has been widely used in the manufacture of soap due to the absence of highly unsaturated fatty acids in the oil (Ainie et al., 1996). In the present study, laundry soap was prepared using used cooking oils samples collected from hotels and also lye solution prepared from weed ash of parthenium. Five different types of laundry soap materials were prepared from the collected used cooking oil samples obtained from hotels (A), street food venders (B), blend of oils collected from hotel and street food venders (C), Blend of oils (with additive or in the presence of additive) (D), and a soap with colorant (E) (Figures 7A–E).
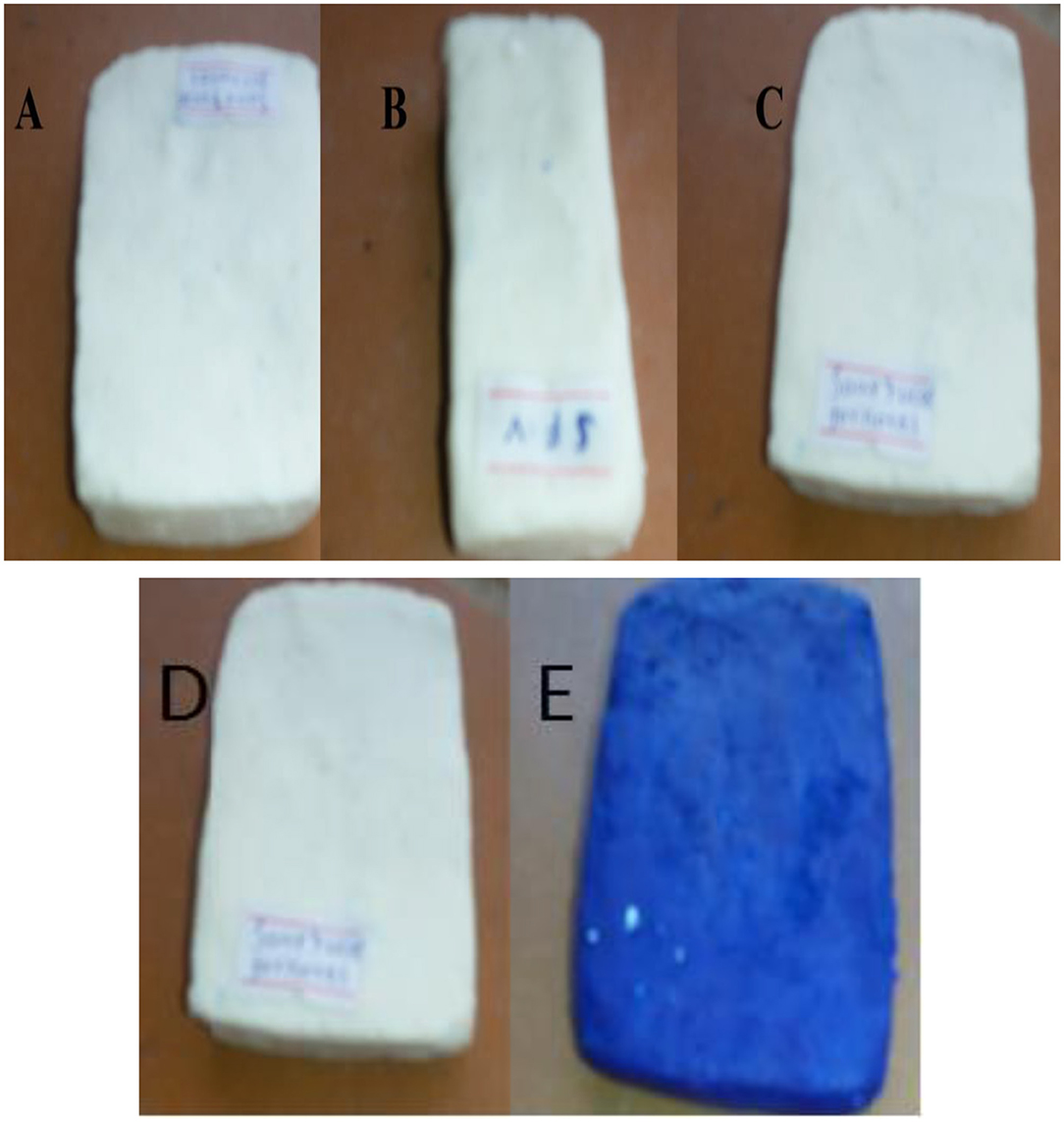
Figure 7. The laundry soaps from oil samples obtained from hotel (A), street food vendors (B), blend of hotel and a street food vendors (C), blend of hotel and a street food vendors with additive (D), and blend of hotel and a street food vendors (with colorant) (E).
Physicochemical Properties of Prepared Soap
Though soaps can be prepared through a simple process known as saponification, the prepared soap products cannot be used directly for human use for safety reasons. Any soap product is expected to fulfill some minimum requirements set by national and international bodies. In line with this fact, the following properties of the prepared soap materials were tested using standard procedures reported in the literature (Ambe, 2001). The tests were done in triplicates. Similar tests were also carried out for three commercial soap samples (Top, B-29, and Peacock) purchased from a local market, Hawassa city.
Moisture Content
Moisture content (MC) is a parameter that is used in assessing the shelf-life of a soap product (Onyango et al., 2014). The MC value was determined by drying soap samples in an oven to 110 ± 3°C. The MC values were found to be 13 ± 0.01%, 14.5 ± 0.01%, 12.5 ± 0.01%, and 10.5 ± 0.02% for soap materials prepared from used cooking oils obtained from hotel, street food vendors, a blend of both, and blend of the two oils (in the presence of additive), respectively (Table 2). The observed data indicated that the MC values of the prepared soap materials were comparable to that of the commercial soap samples used in the study. The soaps were Top, B-29, and Top with MC values of 9.5 ± 0.06%, 14 ± 0.01% and 13 ± 0.01, respectively (Table 2). Moreover, the data were comparable with literature-reported standard values (10.5–12.5%) (EAS, 2014).
Total Alkali Content
The total free alkali measures the percentage of either sodium hydroxide (for sodium soap) or potassium hydroxide (for potassium soap) (Ambe, 2001; Onyango et al., 2014). The total alkali contents of the soap materials prepared in the present study were found to be 1.4 ± 0.1%, 0.93 ± 0.05%, 1.43 ± 0.05%, and 1.63 ± 0.05% for soap prepared from oil samples obtained from hotel, street food vendors, blend of the two, and also blend of the two (with additive), respectively (Table 3). The observed data were also found to be comparable with data reported for commercial soaps namely Top (2.1 ± 0.1%), B-29 (1.76 ± 0.18%), and Peacock (1.3 ± 0.01%) (Table 3). Moreover, the observed data were less than the standard values reported by East African standard (EAS, 2014) (<5%) and International standard Organization (ISO) specification (<2%) (ISO 685, 2013; EAS, 2014). According EAS, good quality soap must have less than 5% alkali content (EAS, 2014) whereas according to ISO specification, soap should have alkali content below 2% (ISO 685, 2013). The finding of the present study suggests that the laundry soaps prepared by treating used cooking oil samples and lye solution prepared from parthenium weed ash could give good quality soap that can be prepared in large scale, but with low cost, for human use.
Total Fatty Matter
Total fatty matter (TFM) measured as total amount of fatty matter, is mostly fatty acid that can be separated from a sample after splitting with mineral acid. It is usually associated with hardness and lower quality of soaps. The higher TFM ensures that soaps are the least harmful to skin and do not cause dryness. The fatty matter most commonly present in soap are oleic, steric, and palmitic (Tetteh et al., 2020). The TMF values of the prepared soap materials in this study were 75.6 ± 0.1%, 64.4 ± 0.6%, 70 ± 0.16%, and 76.4 ± 0.15% for soaps prepared from oil materials obtained from hotel, street food vendors, oil blends, and oil blends (with additive), respectively (Table 4). Similar analyses revealed that the TFM values of commercial soaps were 77.3 ± 0.14%, 76.8 ± 0.15% and 73.4 ± 0.23% for Top, B-29, and Peacock, respectively (Table 4). The findings of the present study indicated that the TFM values of the prepared soap materials were higher than the minimum requirement reported in the literature (i.e., 50%) suggesting that they have acceptable quality (Lumley and Colwell, 1991). Reports also revealed the TFM values of laundry soaps to be in the range of 34-75% (Ambe, 2001). It has also been reported that the TFM value of soaps being <50% is usually associated with hardness and lower quality (Viorica et al., 2011). Some countries consider a soap product with a minimum TFM value of 75% as grade-1 and a minimum value of 65% as grade-2 (Viorica et al., 2011).
The pH Value
Basicity measurement (in terms of pH) is very important to determine the suitability of a soap product for human use. The pH values of the prepared soap materials were comparable to each other and to that of the three commercial soaps used in the study (Table 5). Moreover, the values were found to be in the recommended pH range (9–11) of common laundry soaps (Hui, 1996; Umor, 2002). These data can also indicate that the prepared soap materials have acceptable or permissible alkalinity to be used for common laundry purposes.
Chloride Content
Percentage of chloride in a soap product is a very important parameter (IUPAC International Union Pure Applied Chem., 1982). This is because excess chloride causes soap to crack. One factor that causes high chloride content in soaps could be use of chlorinated water to dissolve NaOH pellets for soap preparation (Taiwo et al., 2008)]. The observed data revealed that the percent of the chloride content of the prepared soap materials was slightly higher than the corresponding values of the three commercial soaps used for comparison (Table 6). But the values are either lower or comparable with values reported in literature (Ikhuoria et al., 2008; Taiwo et al., 2008; Mak-Mensah and Firempong, 2011; ISO 685, 2013). Ikhuoria et al. (2008) reported laundry soap with chloride content of 1-5% to be acceptable (permissible) to be used for human use (Ikhuoria et al., 2008). This value (1–5%) is also set as the Ghanian standard (Viorica et al., 2011). There are also reports of chloride contents of soap products in the range of 0.16–1.15% (Taiwo et al., 2008; Mak-Mensah and Firempong, 2011; Viorica et al., 2011). Therefore, lower chloride content values of the prepared soap materials and the commercial soaps used in the study could be used as indicator showing that the soaps can be used for cleaning purposes without risk of cracking.
Lather Stability Test
Lather formation of different soap products can be explained based on the fatty acids composition of oil used in soap formulation. It has been found out that lauric acid and myristic acids, which are all saturated fatty acids, produce soap with fluffy lather (Phansteil et al., 1998). The analyses of lather formation of the prepared soap materials (in this study) were found to show lather in the range of good to excellent (Table 7). As expected, adding additive enhanced lather formation. The data also showed the lather formation of the prepared soap materials to be generally comparable to that of the commercial laundry soap samples (Top, B-29 and Peacock) used in the study (Table 7).
Cleaning Ability Test
The cleaning ability of a soap product is a measure of quality of soap in cleaning dirt materials (Phansteil et al., 1998). The result showed all the prepared soap materials to have cleaning abilities comparable to each other and also to that of the commercial soaps (Top, B-29 and Peacock) used in the study (Table 8). All these data indicate that the oils collected from hotels and street food vendors can be used as laundry soaps by treating them with alkaline solution prepared from Parthenium weed.
Conclusions
In this study, soaps were prepared using lye solution prepared from parthenium weed ash and used cooking oils collected from hotels and street food vendors. Analyses of the prepared lye solution (pH) and physicochemical properties (saponification values, peroxide values, and acid values) of the collected used cooking oils were in acceptable ranges as compared to literature reports, suggesting that they can be used for soap making. Analyses of the properties (MC, TFM, TAC, pH, % chloride content, lather stability, and cleaning abilities) of the prepared soap materials revealed that the materials could be used for cleaning purposes. Moreover, the observed data from the analyses of the properties of the prepared soap materials were also found to be in acceptable (or permissible) ranges of reported standards of laundry soaps.
It is important to note that the aim of this study is not only an attempt to minimize environmental pollution but also to enable people to prepare laundry soap with low cost for household consumption using the abundantly available parthenium weed and any other weeds and also used cooking oils after food preparations.
Data Availability Statement
All the data generated in the study are included in the article. Further inquiries can be directed to the corresponding author.
Author Contributions
LA designed and supervised the experimental activities, and also prepared the manuscript. TG collected plant material and carried out the experimental activities. TT was co-supervisor of the experimental activities. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors kindly acknowledge The Department of Chemistry, College of Natural and Computational Sciences, Hawassa University. TG acknowledges Ministry of Education of Federal Republic of Ethiopia (FDRE) for financial support.
References
Ainie, K., Hamirin, K., and Peang-Kean, L. (1996). Chemical and physical characteristics of soap made from distilled fatty acids of palm oil and palm kernel oil. J. Am. Oil Chemist. Soc. 73, 105–108. doi: 10.1007/BF02523455
Ambe, G. A. (2001). Edible wild fruits in guinean savanes of cote d'ivoire state of knowledge by local population. J. Malinke Brotechnol. Ayron Soc. Environ. 5, 43–58. Available online at: https://agris.fao.org/agris-search/search.do?recordID=BE2001000707
Aneja, K. R. (2009). Biotechnology: An Alternative Novel Strategy in Agriculture to Control Weeds Resistant to Conventional Herbicides in Antimicrobial Resistance From Emerging Threats to Reality. New Delhi: Narosa Publishing House. p. 160–173.
Arju, B., Chhetri, K., Chris, W., and Rafiqul, I. (2008). Waste cooking oil as an alternate feedstock for biodisel production. Energies 1, 3–18. doi: 10.3390/en1010003
Ashtor, E., and Cevidails, G. (1983). Levantine alkali ashes and european industries. J. Eur. Ecol. Hist. 12, 475–522.
Atiku, F. A., Fakai, M., Wara, A. A., Birnin-Yauri, A. U., and Musa, M. A. (2014). Production of soap using locally available alkaline extract from millet stalk: a study on physical and chemical properties of soap. Int. J. Adv. Res. Chem. Sci. 1, 1–7. Available online at: https://www.arcjournals.org/pdfs/ijarcs/v1-i7/1.pdf
Beetseh, C. I., and Anzo, M. K. (2013). Chemical characterization of local black soap (chahul mtse) made by using cassava peels ashes (alkali base) and palmoil. J. Int. Inst. Sci. Technol.Educ. 3, 82–84. Available online at: http://www.iiste.org/; https://core.ac.uk/download/pdf/234677569.pdf
Debesh, M. (2013). Preparation of soap using different types of oils and exploring its properties (MSc thesis). Rourkela: National Institute of Technology Rourkela.
Dennis, V. J. (2011). Palm Ash a Humble Product of Many Uses. Cincinnati, OH: Middle Book nve. p. 117–121.
Devi, Y. N., Dutta,1, B. K., and Romesh S, Singh, N. I. (2014). Allelopathic effect of Parthenium hysterophorus L. on growth and productivity of Zea mays L. and its phytochemical screening. Int. J. Curr. Microbiol. App. Sci. 3. 837–846. Available online at: https://www.ijcmas.com/vol-3-7/Y.Nganthoi%20Devi,%20et%20al.pdf
EAS (2014). East African Standared Laundery Soap- Specification. Arusha: East Affrication Community; EAS.
Fessehaie, R., Chichayibelu, M., and H/Giorgis, M. (2005). Spread and ecological consequences of Parthenium hysterophorus in Ethiopia. Appl. Environ. Microbio. 6, 11–21. Available online at: https://agris.fao.org/agris-earch/search.do?recordID=ET2006000003
Gadisa, D., Daniel, F., and Firew, K. (2019). Socioeconomic and ecological consequences of parthenium weed (Parthenium hysterophorus l.) in boset woreda, Ethiopia. African J. Agri. Res. 14, 1921–1942. doi: 10.5897/AJAR2019.14247
Gaikwad, C. B., Kasture, M. C., and Lambade, B. M. (2008). Evaluation of herbicides for control of partheniumin waste land. Indian J. Weed Sci. 40, 79–81. Available online at: https://www.isws.org.in/IJWSn/File/2008_40_Issue-1&2%20Supplymentary_79-81.pdf
Hui, H. Y. (1996). “Soap,” in Bailey's Industrial Oil and Fat Products. New York, NY: A Wiley International Scientific Publishing John Wiley and Sons Inc. p. 93–98.
Ibrahim, I. A., and Yusuf, A. J. (2015). Extraction and physic analysis of citrus sinesis seed oil (sweat orange). Eur. J. Exp. Bio. 5, 77–81. Available online at: http://www.pelagiaresearchlibrary.com/
Ikhuoria, E. U., Aiwonegbe, A. E., Okoli, P., and Idu, M. (2008). Characteristics and composition of african oil bean seed. J. Appl. Sci. 8, 1337–1339. doi: 10.3923/jas.2008.1337.1339
IUPAC International Union Pure and Applied Chem. (1982). Standard Method for the Analysis of Oils, Fats and Derivative, Alkaline Soap Prepared for Publication. p. 1257–1295. doi: 10.1351/pac198254061257
Javaid, A. (2007). Efficacy of some chemical herbicides against Parthenium hysterophorus L. Pakistan J. Weed Sci. Res. 13, 93–98. Available online at: https://www.researchgate.net/publication/258210859
Javaid, A., and Adrees, H. (2009). Parthenium management by cultural filtrates of phytopathogenic fungi. Nat. Prod. Res. 23, 1541–1551. doi: 10.1080/14786410902726167
Khalisanni, K., Khalizani, M. S., and Rohani, K. (2008). Analysis of waste cooking oil as raw material for biofuel production. Global J. Environ. Res. 2, 81–83. Available online at: http://idosi.org/gjer/gjer2(2)08/4.pdf
Khan, H., Khan, B. M., Hassan, G., and Khan, M. A. (2012). Chemical control of Parthenium hysterophorus L at different growth stages in non-cropped area. Pakistan J. Botan. 44, 1721–1726. Available online at: https://www.pakbs.org/pjbot/PDFs/44(5)/35.pdf
Lacerdo, L. D. (2004). Public awareness on the environmental important of mangrove. ISMC gLOMIS Electron. J. 4, 185–196. Available online at: https://agris.fao.org/agris-search/search.do?recordID=BE2001000707
Lakshmi, C., and Srinivas, C. R. (2007). Type i hypersensitivity to Parthenium hysterophorus in patients with parthenium dermatitis. Indian J. Dermatol. Venereol. Leprol. 73, 103–105. doi: 10.4103/0378-6323.31895
Legesse, A. (2020). Preparation of laundry soap from used cooking oils: getting value out waste. Sci. Res. Essays. 15, 1–10. doi: 10.5897/SRE2019.6649
Legesse, A., Habtamu, A., and Tegene, T. (2020). Use of Jatropha seed oil and alkali solution obtained from its ash for soap making. An environmentally friendly and cost effective approach. J. Appl. Sci. Environ. Manage. 24: 2005–2015
Lumley, I. D., and Colwell, R. K. (1991). Fats From Fatty Foods and Determination of Fat Content in Analysis of Fat and Fatty Foods. London: Elsevier Applied Science, p. 238–247.
Mak-Mensah, O. E., and Firempong, C. K. (2011). Chemical characteristic of toilet soap preparation from neem (Azadirachia indica a Juss) seed oil. Asian. J. Plant. Sci. Res. 1:1–7. Available online at: https://www.imedpub.com/articles/chemical-characteristics-of-toilet-soap-prepared-from-neemazadirachta-indica-a-juss-seed-oil.pdf
Malaysia Batu Caves (2015). Malaysia recycle used cooking oil into eco-soaps and biodisesel. Brazilian Oper. Pro. Mangt. 12, 66–72.
Manpreet, K., Neeraj, K., Aggarwal, V., and Kumar, R. D. (2014). Effects management of Parthenium hysterophorus: a weed of global significance. J. Int. School. Res. Notices. 1, 2–12. doi: 10.1155/2014/368647
Mishra, J. S., and Bhan, V. M. (1994). Efficacy of sulfonyl urea herbicides against Parthenium hysterophorus. Weed News 1.
Mistral, M. K., Ragland, W., and Baker, A. J. (1993). Wood ash composition as a function of furnace temperature. J. Biomass Bioenergy 3, 103–116. doi: 10.1016/0961-9534(93)90032-Y
Mohammed, W. (2010). Prevalence and distribution survey of an invasive alien weed (Parthenium hysterophorus L.) in sheka zone, southwestern ethiopia. African J. Agri. Res. 5, 922–927. doi: 10.5897/AJAR09.048
Njoroge, J. M. (1991). Tolerance of Biden spilosa L. and Parthenium hysterophorus L. to arquet (Gramooxone) in Kenya. J. Kenya Coffee 56, 999–1001. Available online at: https://www.cabi.org/isc/abstract/19912307806
Nor, H., Abdullah, S., Haji, H., and Nurrul, R. M. (2013). Biodiesel production based on waste cooking oil (WCO). Int. J. Material Sci. Eng. 1, 95–99. doi: 10.12720/ijmse.1.2.94-99
Onyango, P., Vivian, O., Nathan, A., Osano, L., Mesopirr, W., and Nyaigoti, O. (2014). Assessment of the physicochemical properties of selected commercial soaps manufactured and sold in kenya. J. Appl Sci. 4, 433–440 doi: 10.4236/ojapps.2014.48040
Onyegbado, I. E. T., and Offor, O. J. (2002). Solid soap production using plantain peel ash as source of alkali. J. App.Sci. Environ. Manage. 6, 73–77. doi: 10.4314/jasem.v6i1.17200
Panadare, D. C., and Rathod, V. K. (2015). Application of waste cooking oil other than biodiesel. Iranian J. Chem. Eng. 11, 71–721. Available online at: https://iranjournals.nlai.ir/handle/123456789/83395
Parsons, W. T., and Cuthbertson, E. G. (1992). Noxious Weeds of Australia. Melbourne, VIC: In Kata Press.
Phansteil, O. N., Dueno, E., and Xianghong, W. Q. (1998). Synthesis of exotic soap in the chemistry laboratory. J. Chem. Educ. 75, 612–614. doi: 10.1021/ed075p612
Ray, P., and Gour, H. N. (2012). Integrated management of Parthenium hysterophorus L. (Asteraceae): a weed of worldwide significance. Indian Soc. Mycol. Plant Pathol. 5, 605–632. https://www.researchgate.net/publication/236343171
Siddiqui, M. H., Khalid, S., Shehzad, M., Shah, Z. A., and Ahmad, A. (2016). Parthenium hysterophorus herbage mulching: a potential source of weed control in soybean. J. Sci. ELO Anal. 36, 2–9. doi: 10.1590/s0100-83582018360100035
Singh, S., Yadav, R., Balyan, S., Malik, R. K., and Singh, M. (2004). Control of ragweed parthenium (Parthenium hysterophorus) and associated weeds. J. Weed Technol. 18, 658–664. doi: 10.1614/WT-03-128R2
Sushilkumar, K. (2012). Current spread, impact and management of parthenium weed in India. J. Int. Parthenium News 5, 1–13. Available online at: https://www.ijcmas.com/vol-3-7/Y.Nganthoi%20Devi,%20et%20al.pdf
Taiwo, A., Oluwadare, I., Shobo, A., and Amolegbs, S. (2008). Physical and chemical characteristics of soap. Sci. Res. Essay 3, 515–517. doi: 10.5897/SRE.9000496
Taye, T., Rupschus, C., Wiesner, M., Rezene, F., Firehun, T., Ulrichs, C., et al. (2010). Parthenium weed (Parthenium hysterophorus L.) research in ethiopia: impacts on food production, plant biodiversity and human health. Ethiop. J. Agric. Sci. 20, 128–150. Available online at: https://www.ajol.info/index.php/ejas/article/view/142890
Tefera, T. (2002). Allelopathic effects of parthenium hysterophorus extracts on seed germination and seedling growth of Eragrostis tef . J. Agronomy Crop Sci. 188, 306–310. doi: 10.1046/j.1439-037X.2002.00564.x
Tetteh, R., Philip, E., and Carter, J. (2020). Total Fatty Matter Content In Selected Soaps. Available online at: https://www.academia.edu/29821723/TOTAL_FATTY_MATTER_CONTENT_IN_SELECTED_SOAPS (accessed 9 September, 2020).
Tite, M. S., Shortland, A., Y-Maniati, D., Kovouss, S., and Harris, A. (2006). The composition of the soda-rich and mixed alkali plant ashes used in the production of glass. J. Archaeol. Sci. 35, 1284–1293. doi: 10.1016/j.jas.2006.01.004
Umor, M. (2002). “Cosmetics soap, detergent and nAFDAC regulatory requirement,” in A Paper Presented of a Training Workshop for Small and Medium Scale Enterprise Organized by UNDP/JCSI Ministry of Commerce and Idustry (Masduguri), 3–5.
Undeiandeye, J., Vopnu, B. S., Eybuomowan, B. O., and Amkiri, K. H. (2015). Assessment of alkali levels in palm bunch ash, for black soap productions. Eur. Int. J. Sci. Technol. 8, 138–139. Available online at: https://www.eijst.org.uk/images/frontImages/gallery/Vol._4_No._8/15._138-142.pdf
Vila-Aiub, M. M., Vidal, R. A., Balbi, M. C., Gundel, P. E., Trucco, F., Ghersa, M., et al. (2008). Glyphosate-resistant weeds of south american cropping systems: an overview. Pest Manage. Sci. 64, 366–371. doi: 10.1002/ps.1488
Viorica, P., Alina, S., and Simona, D. (2011). Quality control and evaluation of certain properties for soap made in Romania. J. Sci. Stud. Res. Chem. Eng. Biotechnol. Food Ind. 12, 257–261. Available online at: https://core.ac.uk/reader/26208750
Ware, G. W. (1986). Fundamentals of Pesticides: A Self-Instruction Guide. 2nd Edn. Fresno, CA: Thomson Publications.
Keywords: lye, used cooking oil, parthenium, soap, saponification, glycerol
Citation: Adane L, Gelaye T and Tesfaye T (2021) Exploring of the Potential of Parthenium Weed Ash as Substitute for Commercial Alkali for Preparation of Laundry Soap: As a Means to Control Invasion of Parthenium. Front. Sustain. 2:607125. doi: 10.3389/frsus.2021.607125
Received: 16 September 2020; Accepted: 06 April 2021;
Published: 20 May 2021.
Edited by:
Ruchi Agrawal, TERI Gram, IndiaCopyright © 2021 Adane, Gelaye and Tesfaye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Legesse Adane, YWRhbmVsZWdlc3NlQGdtYWlsLmNvbQ==
 Legesse Adane
Legesse Adane Tesfaye Gelaye
Tesfaye Gelaye