- 1Grossman School of Medicine, New York University, New York, NY, United States
- 2Langone Medical Center, New York University, New York, NY, United States
Insomnia is the most commonly reported sleep disorder among children and adolescents, impacting their cognitive, emotional, behavioral, and physical development. The prevalence of insomnia generally increases with age, often persisting into adulthood if unaddressed. Insomnia is exceedingly common among those with developmental disabilities and is frequently comorbid with a great range of psychiatric diagnoses. The COVID-19 pandemic has only increased the prevalence of insomnia among children and adolescents. Health care providers are routinely called upon to treat insomnia in the pediatric population. Psychoeducation and behavioral interventions, especially cognitive behavioral therapy for insomnia (CBT-I), remain the first line treatments, given empirical evidence for their efficacy and success in relapse prevention. However, medications are frequently employed in clinical practice, despite the fact that no medications are approved by the Food and Drug Administration (FDA) for the treatment of pediatric insomnia. This review was designed to educate and support practitioners who are treating children and adolescents who struggle with insomnia. A thorough narrative review was completed to identify all published medication studies of pediatric insomnia; the identified studies are described and then graded into four categories according to the strength of the evidence supporting their use, side effect profiles, co-morbidities, and overall risk vs. benefit of each pharmacological treatment. This review will help practitioners in making clinical decisions for their pediatric patients who suffer with insomnia.
Introduction
Disordered sleep among children and adolescents is a public health concern, given its importance in the cognitive, emotional, and physical development and wellbeing of youth (Rolling et al., 2022). Insomnia is the most commonly reported sleep disorder within the pediatric population and is defined as difficulty falling and staying asleep, resulting in functional impairment (Badin et al., 2016). The prevalence generally increases with age (Ohayon, 2002), and if left untreated, insomnia often persists into adolescence and adulthood (Rolling et al., 2022).
Poor sleep has been repeatedly associated with an increased risk of psychiatric co-morbidities, including links between insomnia and suicide and self-harm behaviors (Winsler et al., 2015), disruption in the family environment (Cohen et al., 2018), poorer academic outcomes (Phillips et al., 2017), and threats to safety, such as motor vehicle accidents (Badin et al., 2016). Sleep difficulties have increased since COVID, and a recent meta-analysis observed a global prevalence of sleep difficulties among 46% of the pediatric population (Jahrami et al., 2022).
Psychoeducation and behavioral interventions, especially cognitive behavioral therapy for insomnia (CBT-I), remain the first line treatments, given empirical evidence for their efficacy and success in relapse prevention (Badin et al., 2016; Buckley et al., 2020). CBT-I is an evidence-based, multidimensional treatment that focuses on correcting the behavioral, environmental, psychological and physiological factors that both contribute to and perpetuate the symptoms of insomnia (Roane and Taylor, 2008). Improvements in sleep onset latency, sleep efficiency, total sleep time and wake after sleep onset have been well-established among adolescents and adults who receive CBT-I, and although some studies have found similar benefits for children, additional studies are needed (Schlarb et al., 2016; Bruni et al., 2018; Dewald-Kaufmann et al., 2019; Subotic-Kerry et al., 2023).
However, medications are frequently employed in clinical practice, despite the fact that no medications are approved by the Food and Drug Administration (FDA) for the treatment of pediatric insomnia. In a 2010 survey of 1,273 members of the American Academy of Child and Adolescent Psychiatry, 96% of physicians reported recommending at least one prescription medication, and 88% recommended at least one over-the-counter medication for insomnia in a typical month (Owens et al., 2010). Given the frequent use of medications for the treatment of insomnia in youth, this report provides graded recommendations for the pharmacological treatment of pediatric insomnia based upon a thorough review of the literature.
Method
Authors conducted this narrative review through the utilization of PubMed using key words including “pediatric sleep, insomnia, medications for pediatric insomnia/sleep, as well as alternative medicine for pediatric insomnia/sleep, and use of cannabis for insomnia/sleep.” Peer-reviewed articles, case control studies, clinical trials, meta-analyses and randomized controlled trials for the medications described to date were reviewed by the authors. There were no specified parameters regarding the date of publication of selected articles nor any exclusion criteria in order to conduct a comprehensive and up-to-date review. Based on this information, the authors developed a medication hierarchy for the treatment for pediatric insomnia. The four categories were built upon the currently available evidence, side effect profiles, co-morbidities, and overall risk vs. benefit of each treatment option. This hierarchy is designed to serve as a reference for providers when making clinical decisions for the treatment of pediatric insomnia. The categories were formed in order to summarize the information and to give a reference point for providers to consider for clinical decision-making in the treatment of pediatric insomnia.
OTC medications
Melatonin and melatonin agonists
Melatonin is a naturally occurring hormone produced from the amino acid tryptophan and secreted in darkness by the pineal gland that is largely responsible for regulation of circadian rhythm and sleep-wake cycles (Rolling et al., 2022). Melatonin is available for purchase in many countries in both immediate and extended-release forms and is currently the most frequently prescribed hypnotic for pediatric patients (Boafo et al., 2020; Delrosso et al., 2021; Wesselhoeft et al., 2021), with data from the Norwegian prescription database indicating a 3–5 fold increase in the use of off-label melatonin in the pediatric population between 2004 and 2011 (Hartz et al., 2012). Melatonin is the most thoroughly studied treatment of insomnia for youth with neurodevelopmental disorders, based, in part at least, upon the hypothesis that abnormalities in melatonin secretion may be responsible for the high rates of insomnia and circadian rhythm abnormalities among those with autism spectrum disorders (ASD; Buckley et al., 2020). Numerous randomized clinical trials have demonstrated the efficacy of melatonin among children and adolescents with autism spectrum disorder in increasing total sleep time and decreasing sleep latency (Gringras et al., 2017; Hayashi et al., 2022; Xiong et al., 2023). Melatonin appears to be generally safe with long-term use of up to 2 years (Maras et al., 2018; Malow et al., 2021).
While no specific formulations have received FDA approval for pediatric insomnia in the United States, one extended-release formulation (Slenyto) has been approved in the European Union for the treatment of insomnia in children (ages 2–18) with ASD and Smith Magenis syndrome. Tablets are available by prescription in 1 and 5 mg preparations, and the recommended dosing range is 2–10 mg taken 30 min before bedtime (Institute for Quality and Efficiency in Health Care, 2019).
Several randomized controlled trials suggest that melatonin is also safe and often effective for short-term use in typically developing children and adolescents (Smits et al., 2003; Jalilolghadr et al., 2022). Further, a consensus panel of pediatric sleep clinicians in the US has advised that 1–5 mg of melatonin taken 30–60 min before bedtime may be beneficial among youth who are refractory to behavioral interventions (Goldman et al., 2021). The American Academy of Sleep Medicine, however, has advised that there is weak evidence in favor of using strategically timed melatonin for the treatment of Delayed Sleep-Wake Phase Disorder in children and adolescents without psychiatric comorbidites to advance sleep onset or decrease sleep latency (Auger et al., 2015). Additionally, a 2023 meta-analysis published by the Danish Health Authority of eight randomized, controlled trials in typically developing youth with idiopathic insomnia found only a modest decrease in sleep latency and a similarly modest increase in total sleep time, but no significant effect on sleep quality or daytime functioning was observed. An increased prevalence [RR (relative risk) 3.44, 95% CI 1.25–9.24] of non-serious adverse events, such as headache, nausea, and mood changes among those taking melatonin (vs. placebo) was observed in this study, but the dropout rate was not significantly elevated (Edemann-Callesen et al., 2023). Recommendations for cautious use and short duration of treatment are given. Finally, although the International Pediatric Sleep Association's (IPSA) Melatonin Task Force has advised that developmentally disabled children 2 years-of-age and older can generally be prescribed 2–10 mg of melatonin safely and with some expectation of benefit (International Pediatric Sleep Association Melatonin Task Force, 2023) the IPSA has not issued a statement on the use of melatonin for adolescents with delayed sleep phase disorder or in typically developing children with insomnia.
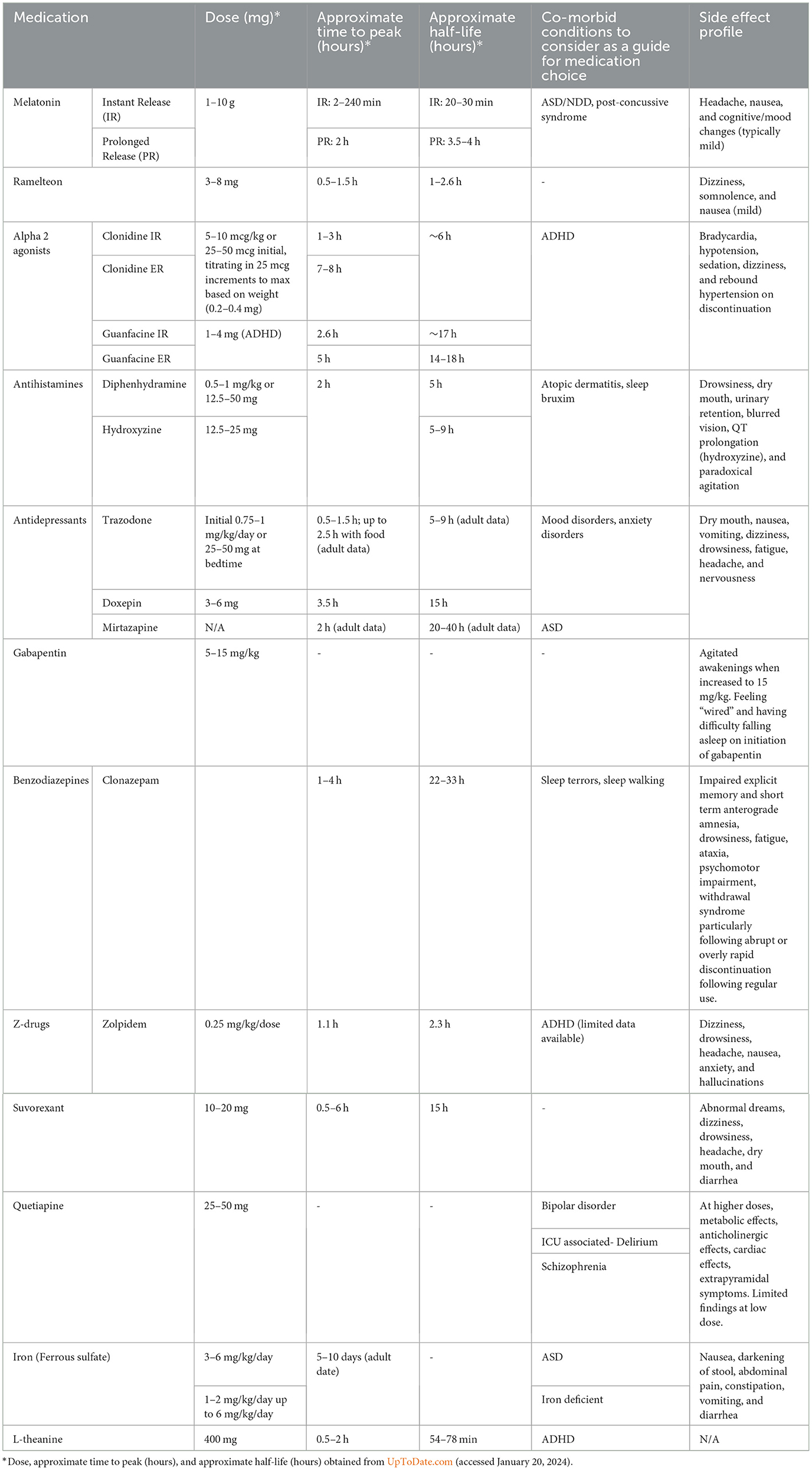
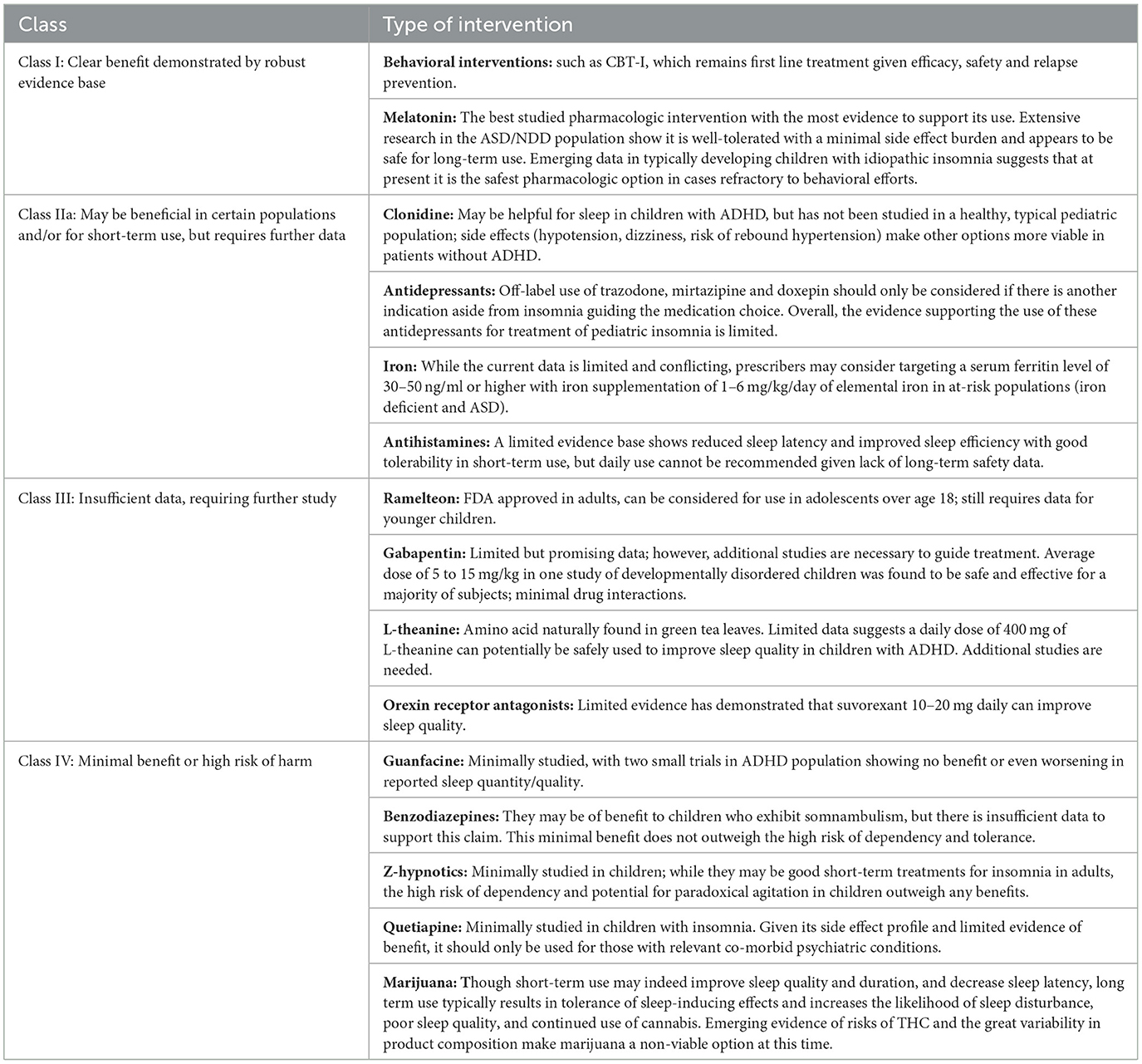
For patients who have not benefitted from behavioral strategies/CBT-I, we recommend melatonin as a first line medication for pediatric insomnia, given the relatively robust evidence for its safety and efficacy. Based on what is known about melatonin's efficacy in children with neurodevelopmental disorders, it deserves special consideration among this population, although it is a reasonable option in typically developing children as well. Physicians may refer to dosing guidelines in Table 1 for further information on using melatonin in practice. We recommend that patients prescribed melatonin for insomnia should continue to observe good sleep hygiene and utilize behavioral strategies while using the medication for optimal results. Prescribers are advised that because melatonin and other dietary supplements are not regulated by the FDA, the precise amount of melatonin within the tablets is not guaranteed. Numerous studies have found greatly varying amounts of active ingredient or its entire absence, along with the presence of other, unspecified ingredients such as serotonin, within tablets housed within the same bottle of melatonin (Erland and Saxena, 2017; Cohen et al., 2023). Because dietary supplements are regulated under the Dietary Supplement Health and Education Act and not by the FDA, consumers are advised to purchase only those products marketed as pharmaceutical grade.
Antihistamines
Antihistamines (H1 inverse agonists) induce sleep by suppressing the activity of histamine, a wake-promoting neurochemical, in the brain. Diphenhydramine has been included in the US Food and Drug Administration (FDA's) Final Drug Monograph for OTC use, and doxylamine (Unisom) is FDA approved for the treatment of insomnia in adults (Culpepper and Wingertzahn, 2015). Sedating antihistamines are routinely prescribed off-label to youth with insomnia (Bruni et al., 2018; Wesselhoeft et al., 2021), but the evidence in support of antihistamines for pediatric insomnia is severely limited. One double-blind, placebo-controlled crossover clinical trial of diphenhydramine at doses of 1 mg/kg in 50 children with sleep disorders found significantly reduced sleep latency and number of awakenings in those taking diphenhydramine as compared to placebo by parent report (Russo et al., 1976). Conversely, a randomized, double-blind clinical trial assessing the efficacy of diphenhydramine at 1 mg/kg in reducing nighttime awakenings in infants aged 6–15 months was terminated after showing no benefit over placebo (Merenstein et al., 2006).
Antihistamines such as diphenhydramine (Benadryl) have the benefit of being easily accessible, and their over-the-counter status may give parents the impression that they are benign; however, based on the paucity of information available in the pediatric population, we recommend that any use of this medication as a sleep aid be conservative and time-limited. Parents should be made aware of potential side effects including next-day sedation, anticholinergic effects, and risk of paradoxical agitation. This, and further dosing guidance, can be found in Table 1.
Prescription medications
Alpha agonists
Alpha agonists are often prescribed by US physicians for the treatment of pediatric insomnia (Schnoes et al., 2006), although there is limited evidence for this indication. The strongest support lies with clonidine, but even here the research is limited to retrospective chart reviews and case studies and only among patients with ADHD (Wilens et al., 1994; Prince et al., 1996; Ming et al., 2008). Dosages of 0.05–0.1 mg at bedtime have been reported to reduce sleep latency and improve sleep efficiency (Ming et al., 2008). Fewer studies have assessed guanfacine for the treatment of pediatric insomnia, but among this research, there is no evidence of benefit. One randomized, double-blind, placebo-controlled trial of extended release guanfacine administered in the morning in children with ADHD was terminated early after REM and total sleep time were found to be significantly reduced in the treatment group compared to placebo (Rugino, 2018). Similarly, a randomized, placebo-controlled trial of extended release guanfacine (1–4 mg) in children with comorbid ASD and ADHD that assessed change in sleep as a secondary outcome found no statistical separation between treatment and placebo groups (Politte et al., 2018).
In patients with ADHD who are struggling with insomnia (especially in the context of worsening or “rebound” symptoms in the evenings), clonidine may be preferentially used over guanfacine to maximize benefit for sleep. However, there is insufficient evidence to recommend its use in the healthy pediatric population, especially in light of potential side effects including hypotension, dizziness, and risk of rebound hypertension.
Antidepressants
Antidepressants are occasionally prescribed for the treatment of pediatric insomnia, given that some have sedative side effects. While the tricyclic antidepressant doxepin has been FDA approved in low doses for the treatment of insomnia in adults, there is no FDA approved antidepressant for pediatric insomnia (Shah et al., 2020). Nonetheless, trazodone is frequently utilized by clinicians for pediatric insomnia when comorbid with mood and anxiety disorders. One study, however, found that youth who were prescribed trazodone in addition to a selective serotonin re-uptake inhibitor were three times more likely to express thoughts of self-harm and six times less likely to experience benefit from the antidepressant (Brent, 2008); another study also observed that combining an antidepressant with trazodone reduced the likelihood of response to the antidepressant (Sultan and Courtney, 2017), an effect hypothesized to be due to the build-up of meta-chlorophenylpiperazine (mCPP) which may lead to worsening anxiety (Shamseddeen et al., 2012). These findings have not been observed among youth taking antidepressants with other medications commonly employed for sleep, such as antihistamines. Finally, there is limited evidence that mirtazapine may be successful in treating symptoms of insomnia, anxiety, and depression in patients with comorbid ASD (Posey et al., 2001; Gupta and Gupta, 2023). Thus, given the overall limited data available for use of antidepressants for pediatric insomnia, this class of drugs should not be used as first-line treatment for pediatric insomnia (as summarized in Table 2); however, prescribers can refer to Table 1 for dosing guidance for youth with co-morbid mood disorders and insomnia.
Gabapentin
While Gabapentin is not a first-line treatment for pediatric insomnia, one retrospective case series of 23 children (average age of 7.2 years, a majority with neurodevelopmental disorders) reported gabapentin to be an effective treatment for both sleep-onset and sleep maintenance insomnia in 78% of subjects by parent report (Malow et al., 2021). Parents were simultaneously educated in behavioral interventions for sleep, which may have also contributed to the benefits observed. The average starting dose was 5 mg/kg at bedtime, advanced to a maximum dose of 15 mg/kg (as summarized in Table 1), which is significantly lower than typical dosages for epilepsy. Adverse effects included agitated awakenings, which generally resolved after lowering the dosage. While this one small report was promising, further studies of gabapentin have not been performed, and thus there is insufficient evidence to recommend its use for pediatric insomnia (as summarized in Table 2).
Ramelteon
Ramelteon is a melatonin agonist that is FDA approved for the treatment of insomnia in adults but with limited evidence in pediatric populations. A small number of case reports in children and young adults with ASD and severe developmental disability have shown potential benefit and good tolerability (Stigler et al., 2006; Miyamoto et al., 2013). As suggested in the grading recommendations in Table 2, there is currently insufficient data available to recommend its use in the general pediatric population.
Sedatives/hypnotics
Benzodiazepines
Benzodiazepines modulate the GABAa receptor, leading to decreased anxiety, muscle relaxation, and sedation (Griffin et al., 2013). Benzodiazepines can also decrease sleep latency and the frequency and duration of awakenings. Although statistically better than placebo in the treatment of insomnia among adults, their average effect size is small, resulting in only about 30 min of additional sleep per night (Glass et al., 2005). Unfortunately, benzodiazepines can also contribute to memory impairment, loss of coordination, and daytime sleepiness, and their chronic use is correlated with early onset dementia (He et al., 2019). Dependence and tolerance to benzodiazepines are considerable risks, and one study found that the discontinuation of benzodiazepines led to reduced sleep quality and onset, as well as increased suicide risk within 2 weeks of treatment cessation (Edinoff et al., 2021). Further, just as the dangers of benzodiazepine withdrawal in adults is well-documented, one study found that about 20% of children given benzodiazepines for sedation in an intensive care unit (typically midazolam) exhibited withdrawal effects, the symptoms of which varied and consisted of difficulty sleeping, agitation, tremors, and inconsolable crying (Edinoff et al., 2021). While benzodiazepines are FDA approved for the treatment of pediatric epilepsy, their benefit in treating pediatric insomnia has not been established. Clonazepam is the most frequently prescribed benzodiazepine for children and has been used to treat severe sleep terrors and sleepwalking (Simon and Byars, 2016). Despite the potential benefit of using benzodiazepines to treat somnambulism, there is insufficient evidence to support its use which, combined with the high likelihood of dependency and abuse potential, leads to the recommendation that benzodiazepines should not be used as a pharmacological treatment of pediatric insomnia (as summarized in Table 2).
Non-benzodiazepines (Z-hypnotics)
Like benzodiazepines, Z-hypnotics also bind to the GABAa receptor, though more selectively and thus target the sedative effect rather than the anxiolytic effect (Drover, 2004). They have been studied in adults less extensively than benzodiazepines but have been shown to cause significant impairment, lasting up to 11 h after dosing (Brandt and Leong, 2017). Two large randomized, double-blind studies of youth with insomnia comorbid with ADHD, one of zolpidem and the other of eszopiclone, found no benefits (Blumer et al., 2009; Singh and Loona, 2013). This, combined with the high likelihood of dependence, leads to the recommendation that Z-hypnotics should not be used as a pharmacological treatment for pediatric insomnia (further summarized in Table 2).
Orexin receptor antagonists
Orexin or hypocretin antagonists, three of which have been FDA approved for the treatment of insomnia in adults (suvorexant, lemborexant, and daridorexant), block hypothalamic orexin receptors that are important for arousal and wakefulness (Besterman and Jeste, 2023). There is limited data for their use in the pediatric population (Donskoy and Loghmanee, 2018). One small open-label study consisting of 30 patients (age range 10–20 years) reported significant improvement in the subjective sleep quality with suvorexant at a dose of 20 mg/day. Abnormal dreams were the most common side effect resulting in discontinuation (Kawabe et al., 2017). A case study of one 16-year-old male with bipolar 1 disorder given a trial of suvorexant at 10 mg nightly observed successful resolution of insomnia, along with improved sleep duration and quality (Prieto et al., 2019). Lastly, a case series of three youth and one adult with neurodevelopmental disorders reported benefit from suvorexant for both the 14-year-old adolescent and the 28-year-old young adult, who experienced significant improvements in sleep initiation and maintenance (Besterman and Jeste, 2023). The 9 and 13-year-old girls, however, experienced no benefit nor significant side effects, and their medication was discontinued. Thus, at this time, there is limited evidence that suvorexant 10-20 mg daily can improve sleep quality (as outlined in Tables 1, 2).
Antipsychotics
Some atypical antipsychotics are being increasingly used off-label for the treatment of insomnia (Hermes et al., 2013; Thompson et al., 2016), largely due to their affinity for H1 receptors. In a cross-sectional review of 2,613 providers at a single Veteran's Administration Medical Center over a 20-month period, quetiapine was found to be the most frequently prescribed antipsychotic for sleep in adults (Hermes et al., 2013). Notably, prescriptions of quetiapine written by family physicians for sleep disturbances increased by 300% in Canada between 2005 and 2012 (Thompson et al., 2016); and a similar pattern was observed in the US where 70% of antipsychotic prescriptions written between 1996 and 2003 were for indications other than psychosis (Sankaranarayanan and Puumala, 2007). However, a 2008 US guideline for the management of chronic insomnia found insufficient evidence for atypical antipsychotics in treating insomnia, advising against their use as a first-line therapy (Thompson et al., 2016). There is even more limited data on the use of quetiapine for pediatric insomnia. Despite this fact, a 5-year observational study (2012–2016) of prescription claims for Medicaid-insured ADHD youth (ages 3–18) found that quetiapine was one of the most frequently prescribed medications for sleep (Klein et al., 2019). One case report of a 15-year-old observed that low dose quetiapine effectively treated somnambulism, possibly due to its effect in decreasing delta wave density during stage 3 sleep (Gill et al., 2011).
Some studies have shown promising results for quetiapine at 25–50 mg for the treatment of insomnia through a range of ages (Dujardin et al., 2018; Frase et al., 2018). This has also been outlined in Table 1 for dosing guidance. However, the effects of antipsychotic medications on sleep have mostly been investigated in psychiatric patients with comorbid conditions. Few small, randomized, controlled trials of adults using quetiapine at dosages of 25–100 mg at bedtime for the treatment of insomnia without co-morbid psychiatric disorders have been published, and among these studies, the data has demonstrated only non-significant trends for prolonged sleep and decreased sleep latency at best (Modesto-Lowe et al., 2021). Within the pediatric population, antipsychotic use should be restricted to only those with co-morbid psychiatric disorders (Frase et al., 2018). Quetiapine is associated with adverse metabolic events and serious adverse events include fatal hepatotoxicity, akathisia, weight gain, and restless legs syndrome (Coe and Hong, 2012; Dujardin et al., 2018). Given this side effect profile and limited evidence, its use should be limited only for those with relevant co-morbid psychiatric conditions, as summarized in Table 2.
Dietary supplements
Iron
Sleep disturbances in children with Attention-Deficit/Hyperactivity Disorder (ADHD) and adults with periodic limb movement disorders are correlated with low serum ferritin levels (Blackmer and Feinstein, 2016). This observation may be the result of iron's role as a co-factor in the dopamine-opiate system, which influences the sleep-wake cycle. Two parallel clinical trials conducted in a large sample of infants at high risk for iron deficiency and those with anemia observed that micronutrient iron and iron-folic supplementation increased sleep duration and reduced night waking (Kordas et al., 2009). Iron supplementation may be particularly relevant for autistic youth at high-risk for iron deficiency secondary to narrow food preferences. A retrospective, cross-sectional review of 53 pediatric ASD patients found that a median ferritin level of 24 ng/ml or lower was associated with sleep fragmentation and poor sleep efficiency (Youssef et al., 2013). Another study demonstrated improvement in restless sleep in 33 autistic youth (2–6 years of age) with oral supplementation of iron for 8 weeks (Dosman et al., 2007). In contrast, a controlled clinical trial of 20 children with ASD and low ferritin levels did not demonstrate improvement in insomnia among those treated with ferrous sulfate vs. placebo (Reynolds et al., 2020). Thus, while the available data is incomplete and conflicting, prescribers may consider treating low ferritin levels among their pediatric patients with insomnia, targeting 30–50 ng/ml with iron supplementation of 1–6 mg/kg/day of elemental iron in at-risk populations (Blackmer and Feinstein, 2016).
L-theanine
L-theanine is an amino acid found in green tea leaves, Camellia sinensis, and is thought to alleviate stress and improve sleep through a glutamate receptor-mediated mechanism (Lyon et al., 2011; Innocenti et al., 2023). One randomized, 10-week, double-blind, placebo-controlled trial conducted with 98 boys, ages 8–12 years, with a diagnosis of ADHD observed improved sleep efficiency as measured by actigraphy but no change in sleep latency or duration at doses of 200 mg twice daily. The treatment was well-tolerated without observable side effects (Lyon et al., 2011). Thus, providers can consider a daily dose of L-theanine 400 mg to potentially improve sleep quality in youth with ADHD, although further studies are required for this to become a part of practice (as outlined in Tables 1, 2).
Marijuana
Irregular sleep schedules have been found to correlate with frequency of substance use, and numerous studies have found that sleep problems often precede, and can be predictive of, future adolescent substance use (Babson et al., 2017). One study found that young adult cannabis users with greater past-year use had significantly worse sleep quality. Higher dosage and/or more frequent use was predictive of poorer sleep (Maple et al., 2016). Occasional endocannabinoid use generally promotes sleep by reducing sleep latency and increasing slow wave and REM sleep.
Chronic cannabis use, however, likely decreases endocannabinoid signaling and, subsequently, decreases endogenous cannabinoid effects, which results in a reduction in slow wave and REM sleep and an increase in excessive daytime sleepiness. Because slow wave and REM sleep are necessary for the efficient consolidation of long-term memory, interrupting these sleep stages can lead to significant memory difficulties (Hirvonen et al., 2011). Thus, the minimal benefit does not outweigh the high risk of harm of using marijuana and it, therefore, should not be recommended as a potential treatment for pediatric insomnia. Table 1 summarizes the aforementioned pharmacological interventions.
Conclusion and recommendations
Limited evidence supports the use of psychopharmacological interventions for the treatment of pediatric insomnia. Psychoeducation and behavioral interventions, especially cognitive behavioral therapy for insomnia (CBT-I), remain the first line treatments, given empirical evidence for their efficacy and success in relapse prevention (Badin et al., 2016; Buckley et al., 2020). CBT-I is an evidence-based, multidimensional treatment that focuses on correcting the behavioral, environmental, psychological and physiological factors that both contribute to and perpetuate the symptoms of insomnia (Roane and Taylor, 2008). Sleep education, stimulus control, sleep restriction therapy, sleep hygiene, cognitive therapy and relaxation training are the mainstays of CBT-I treatment and can be taught to patients over a small number of sessions (Badin et al., 2016). Improvements in sleep onset latency, sleep efficiency, total sleep time and wake after sleep onset have been well-established among adolescents and adults who receive CBT-I, and although some studies have found similar benefits for children, additional studies are needed (Schlarb et al., 2016; Bruni et al., 2018; Dewald-Kaufmann et al., 2019; Subotic-Kerry et al., 2023).
A thorough risk benefit analysis should be employed when considering pharmacological interventions for pediatric insomnia. The severe detriments of sleep deprivation on the developing brain in children and the increase in risk-taking behavior in under-slept adolescents should factor into this decision, and in some cases using agents with less established evidence may be warranted (Shatkin, 2017). Practitioners are advised to involve patients and their families in the decision-making process as much as possible and to start with psychoeducation and behavioral interventions and to continue them even while using psychopharmacology as an adjunct. The limited data in this report can be used as a guide to help practitioners make rational clinical decisions.
The evidence for each of the agents has been assigned to a graded recommendation scale described in Table 2.
Author contributions
JS: Conceptualization, Methodology, Project administration, Writing – original draft, Writing – review & editing. SD: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. NK: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. HB: Conceptualization, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Auger, R. R., Burgess, H. J., Emens, J. S., Deriy, L. V., Thomas, S. M., and Sharkey, K. M. (2015). Clinical practice guideline for the treatment of intrinsic circadian rhythm sleep-wake disorders: advanced sleep-wake phase disorder (ASWPD), delayed sleep- wake phase disorder (DSWPD), non-24-hour sleep-wake rhythm disorder (N24SWD), and irregular sleep-wake rhythm disorder (ISWRD). An update for 2015: an American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 11, 1199–1236. doi: 10.5664/jcsm.5100
Babson, K. A., Sottile, J., and Morabito, D. (2017). Cannabis, cannabinoids, and sleep: a review of the literature. Curr. Psychiatr. Rep. 19, 2–3. doi: 10.1007/s11920-017-0775-9
Badin, E., Haddad, C., and Shatkin, J. P. (2016). Insomnia: the sleeping giant of pediatric public health. Curr. Psychiatr. Rep. 18:47. doi: 10.1007/s11920-016-0687-0
Besterman, A. D., and Jeste, S. S. (2023). Dual orexin receptor antagonists for insomnia in youth with neurodevelopmental disorders: a case series and review. Eur. Child Adolesc. Psychiatr. 32, 527–531. doi: 10.1007/s00787-021-01883-7
Blackmer, A. B., and Feinstein, J. A. (2016). Management of sleep disorders in children with neurodevelopmental disorders: a review. Pharmacotherapy 36, 84–98. doi: 10.1002/phar.1686
Blumer, J. L., Findling, R. L., Shih, W. J., Soubrane, C., and Reed, M. D. (2009). Controlled clinical trial of zolpidem for the treatment of insomnia associated with attention- deficit/hyperactivity disorder in children 6 to 17 years of age. Pediatrics 123, e770–e776. doi: 10.1542/peds.2008-2945
Boafo, A., Greenham, S., Sullivan, M., Bazaid, K., Suntharalingam, S., Silbernagel, L., et al. (2020). Medications for sleep disturbance in children and adolescents with depression: a survey of Canadian child and adolescent psychiatrists. Child Adolesc. Psychiatr. Mental Health 14:10. doi: 10.1186/s13034-020-00316-8
Brandt, J., and Leong, C. (2017). Benzodiazepines and z-drugs: an updated review of major adverse outcomes reported on in epidemiologic research. Drugs R&D 17, 493–507. doi: 10.1007/s40268-017-0207-7
Brent, D. (2008). Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with ssri-resistant depression. J. Am. Med. Assoc. 299:901. doi: 10.1001/jama.299.8.901
Bruni, O., Angriman, M., Calisti, F., Comandini, A., Esposito, G., Cortese, S., et al. (2018). Practitioner review: treatment of chronic insomnia in children and adolescents with neurodevelopmental disabilities. J. Child Psychol. Psychiatr. 59, 489–508. doi: 10.1111/jcpp.12812
Buckley, A. W., Hirtz, D., Oskoui, M., Armstrong, M. J., Batra, A., Bridgemohan, C., et al. (2020). Practice guideline: treatment for insomnia and disrupted sleep behavior in children and adolescents with autism spectrum disorder: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 94, 392–404. doi: 10.1212/WNL.0000000000009033
Coe, H. V., and Hong, I. S. (2012). Safety of low doses of quetiapine when used for insomnia. Ann. Pharmacother. 46, 718–22. doi: 10.1345/aph.1Q697
Cohen, E. M., Dossett, M. L., Mehta, D. H., Davis, R. B., and Lee, Y. C. (2018). Factors associated with insomnia and complementary medicine use in children: results of a national survey. Sleep Med. 44, 82–88. doi: 10.1016/j.sleep.2018.01.007
Cohen, P. A., Avula, B., Wang, Y. H., Katragunta, K., and Khan, I. (2023). Quantity of melatonin and CBD in melatonin gummies sold in the US. J. Am. Med. Assoc. 329:1401. doi: 10.1001/jama.2023.2296
Culpepper, L., and Wingertzahn, M. A. (2015). Over-the-counter agents for the treatment of occasional disturbed sleep or transient insomnia: a systematic review of efficacy and safety. Prim. Care Companion CNS Disord. 17:10.4088/PCC.15r01798. doi: 10.4088/PCC.15r01798
Delrosso, L. M., Mogavero, M. P., Ferri, R., Bruni, O., and Chen, M. L. (2021). Update and progress in pediatric sleep disorders. J. Pediatr. 239, 16–23. doi: 10.1016/j.jpeds.2021.08.030
Dewald-Kaufmann, J., de Bruin, E., and Michael, G. (2019). Cognitive behavioral therapy for insomnia (CBT-i) in school-aged children and adolescents. Sleep Med. Clin. 14, 155–165. doi: 10.1016/j.jsmc.2019.02.002
Donskoy, I., and Loghmanee, D. (2018). Insomnia in adolescence. Med. Sci. 6:30072. doi: 10.3390/medsci6030072
Dosman, C. F., Brian, J. A., Drmic, I. E., Senthilselvan, A., Harford, M. M., Smith, R. W., et al. (2007). Children with autism: effect of iron supplementation on sleep and ferritin. Pediatr. Neurol. 36, 152–158. doi: 10.1016/j.pediatrneurol.2006.11.004
Drover, D. R. (2004). Comparative pharmacokinetics and pharmacodynamics of short- acting hypnosedatives. Clin. Pharmacokinet. 43, 227–238. doi: 10.2165/00003088-200443040-00002
Dujardin, S., Pijpers, A., and Pevernagie, D. (2018). Prescription drugs used in insomnia. Sleep Med. Clin. 13, 169–182. doi: 10.1016/j.jsmc.2018.03.001
Edemann-Callesen, H., Andersen, H. K., Ussing, A., Virring, A., Jennum, P., Debes, N. M., et al. (2023). Use of melatonin in children and adolescents with idiopathic chronic insomnia: a systematic review, meta-analysis, and clinical recommendation. Eclinicalmedicine 61:102048. doi: 10.1016/j.eclinm.2023.102048
Edinoff, A. N., Nix, C. A., Hollier, J., Sagrera, C. E., Delacroix, B. M., Abubakar, T., et al. (2021). Benzodiazepines: uses, dangers, and clinical considerations. Neurol. Int. 13, 594–607. doi: 10.3390/neurolint13040059
Erland, L. A., and Saxena, P. K. (2017). Melatonin natural health products and supplements: presence of serotonin and significant variability of melatonin content. J. Clin. Sleep Med. 13, 275–281. doi: 10.5664/jcsm.6462
Frase, L., Nissen, C., Riemann, D., and Spiegelhalder, K. (2018). Making sleep easier: pharmacological interventions for insomnia. Expert Opin. Pharmacother. 19, 1465–1473. doi: 10.1080/14656566.2018.1511705
Gill, J. S., Pillai, S. K., Koh, O. H., and Jambunathan, S. T. (2011). Low dose quetiapine in the treatment of an adolescent with somnambulism: a case report. Acta Neurol. Belg. 111, 155–156.
Glass, J., Lanctôt, K. L., Herrmann, N., Sproule, B. A., and busto, U.E. (2005). Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. Br. Med. J. 331:1169. doi: 10.1136/bmj.38623.768588.47
Goldman, R. D., Bongiorno, P. B., Olcese, J. M., Witt-enderby, P. A., and Shatkin, J. P. (2021). Myths and evidence regarding melatonin supplementation for occasional sleeplessness in the pediatric population. Pediatr. Ann. 50, e391–e395. doi: 10.3928/19382359-20210823-01
Griffin, C. E. 3rd., Kaye, A. M., Bueno, F. R., and Kaye, A. D. (2013). Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner. J. 13, 214–223.
Gringras, P., Nir, T., Breddy, J., Frydman-Marom, A., and Findling, R. L. (2017). Efficacy and safety of pediatric prolonged-release melatonin for insomnia in children with autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatr. 56, 948–957. doi: 10.1016/j.jaac.2017.09.414
Gupta, N., and Gupta, M. (2023). Diagnostic overshadowing in high-functioning autism: mirtazapine, buspirone, and modified cognitive behavioral therapy (CBT) as treatment options. Cureus 15:39446. doi: 10.7759/cureus.39446
Hartz, I., Furu, K., Bratlid, T., Handal, M., and Skurtveit, S. (2012). Hypnotic drug use among 0–17 year olds during 2004–2011: a nationwide prescription database study. Scand. J. Publ. Health 40, 704–711. doi: 10.1177/1403494812464446
Hayashi, M., Mishima, K., Fukumizu, M., Takahashi, H., Ishikawa, Y., Hamada, I., et al. (2022). Melatonin treatment and adequate sleep hygiene interventions in children with autism spectrum disorder: a randomized controlled trial. J. Aut. Dev. Disord. 52, 2784–2793. doi: 10.1007/s10803-021-05139-w
He, Q., Chen, X., Wu, T., Li, L., and Fei, X. (2019). Risk of dementia in long-term benzodiazepine users: evidence from a meta-analysis of observational studies. J. Clin. Neurol. 15, 9–19. doi: 10.3988/jcn.2019.15.1.9
Hermes, E. D., Sernyak, M., and Rosenheck, R. (2013). Use of second-generation antipsychotic agents for sleep and sedation: a provider survey. Sleep 36, 597–600. doi: 10.5665/sleep.2554
Hirvonen, J., Goodwin, R. S., Li, C. T., Terry, G. E., Zoghbi, S. S., Morse, C., et al. (2011). Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol. Psychiatr. 17, 642–649. doi: 10.1038/mp.2011.82
Innocenti, A., Lentini, G., Rapacchietta, S., Cinnirella, P., Elia, M., Ferri, R., et al. (2023). The role of supplements and over-the-counter products to improve sleep in children: a systematic review. Int. J. Mol. Sci. 24:97821. doi: 10.3390/ijms24097821
Institute for Quality and Efficiency in Health Care (2019). Melatonin (Slenyto) for the Treatment of Insomnia: Overview. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK541969/ (accessed May 9, 2019). Cologne: Institute for Quality and Efficiency in Health Care (IQWiG).
International Pediatric Sleep Association Melatonin Task Force (2023). Interim Report. IPSA. Available online at: https://www.pedsleep.org/Melatonin-TaskForce (accessed January 20, 2024).
Jahrami, H. A., Alhaj, O. A., Humood, A. M., Alenezi, A. F., Fekih-romdhane, F., Alrasheed, M. M., et al. (2022). Sleep disturbances during the COVID-19 pandemic: a systematic review, meta-analysis, and meta-regression. Sleep Med. Rev. 62:101591. doi: 10.1016/j.smrv.2022.101591
Jalilolghadr, S., Roozmehr, S., Yazdi, Z., and Soltanabadi, M. (2022). The effect of treatment with melatonin on primary school aged children with difficulty in initiation and maintenance of sleep. Turk. J. Pediatr. 64, 993–1000. doi: 10.24953/turkjped.2018.1381
Kawabe, K., Horiuchi, F., Ochi, M., Nishimoto, K., Ueno, S. I., and Oka, Y. (2017). Suvorexant for the treatment of insomnia in adolescents. J. Child Adolesc. Psychopharmacol. 27, 792–795. doi: 10.1089/cap.2016.0206
Klein, T., Woo, T. M., Panther, S., Odom-Maryon, T., and Daratha, K. (2019). Somnolence-producing agents: a 5-year study of prescribing for medicaid-insured children with attention deficit hyperactivity disorder. J. Pediatr. Health Care 33, e1–e8. doi: 10.1016/j.pedhc.2018.10.002
Kordas, K., Siegel, E. H., Olney, D. K., Katz, J., Tielsch, J. M., Kariger, P. K., et al. (2009). The effects of iron and/or zinc supplementation on maternal reports of sleep in infants from Nepal and Zanzibar. J. Dev. Behav. Pediatr. 30, 131–139. doi: 10.1097/DBP.0b013e31819e6a48
Lyon, M. R., Kapoor, M. P., and Juneja, L. R. (2011). The effects of l-theanine (suntheanine(r)) on objective sleep quality in boys with attention deficit hyperactivity disorder (ADHD): a randomized, double-blind, placebo-controlled clinical trial. Altern. Med. Rev. 16, 348–354.
Malow, B. A., Findling, R. L., Schroder, C. M., Maras, A., Breddy, J., Nir, T., et al. (2021). Sleep, growth, and puberty after 2 years of prolonged-release melatonin in children with autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatr. 60, 252–261. doi: 10.1016/j.jaac.2019.12.007
Maple, K. E., McDaniel, K. A., Shollenbarger, S. G., and Lisdahl, K. M. (2016). Dose-dependent cannabis use, depressive symptoms, and faahgenotype predict sleep quality in emerging adults: a pilot study. Am. J. Drug Alcohol Abuse 42, 431–440. doi: 10.3109/00952990.2016.1141913
Maras, A., Schroder, C. M., Malow, B. A., Findling, R. L., Breddy, J., Nir, T., et al. (2018). Long-term efficacy and safety of pediatric prolonged- release melatonin for insomnia in children with autism spectrum disorder. J. Child Adolesc. Psychopharmacol. 28, 699–710. doi: 10.1089/cap.2018.0020
Merenstein, D., Diener-West, M., Halbower, A. C., Krist, A., and Rubin, H. R. (2006). The trial of infant response to diphenhydramine: the TIRED study–a randomized, controlled, patient-oriented trial. Arch. Pediatr. Adolesc. Med. 160, 707–712. doi: 10.1001/archpedi.160.7.707
Ming, X., Gordon, E., Kang, N., and Wagner, G. C. (2008). Use of clonidine in children with autism spectrum disorders. Brain Dev. 30, 454–460. doi: 10.1016/j.braindev.2007.12.007
Miyamoto, A., Fukuda, I., Tanaka, H., Oka, R., Araki, A., and Cho, K. (2013). Treatment with ramelteon for sleep disturbance in severely disabled children and young adults. No To Hattatsu 45, 440–444.
Modesto-Lowe, V., Harabasz, A. K., and Walker, S. A. (2021). Quetiapine for primary insomnia: consider the risks. Cleveland Clin. J. Med. 88, 286–294. doi: 10.3949/ccjm.88a.20031
Ohayon, M. M. (2002). Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med. Rev. 6, 97–111. doi: 10.1053/smrv.2002.0186
Owens, J. A., Rosen, C. L., Mindell, J. A., and Kirchner, H. L. (2010). Use of pharmacotherapy for insomnia in child psychiatry practice: a national survey. Sleep Med. 11, 692–700. doi: 10.1016/j.sleep.2009.11.015
Phillips, A. J. K., Clerx, W. M., O'Brien, C. S., Sano, A., Barger, L. K., Picard, R. W., et al. (2017). Irregular sleep/wake patterns are associated with poorer academic performance and delayed circadian and sleep/wake timing. Sci. Rep. 7:3216. doi: 10.1038/s41598-017-03171-4
Politte, L. C., Scahill, L., Figueroa, J., Mccracken, J. T., King, B., and Mcdougle, C. J. (2018). A randomized, placebo-controlled trial of extended-release guanfacine in children with autism spectrum disorder and ADHD symptoms: an analysis of secondary outcome measures. Neuropsychopharmacology 43, 1772–1778. doi: 10.1038/s41386-018-0039-3
Posey, D. J., Guenin, K., Kohn, A. E., Swiezy, N. B., and McDougle, C. J. (2001). A naturalistic open-label study of mirtazapine in autistic and other pervasive developmental disorders. J. Child Adolesc. Psychopharmacol. 11, 267–277. doi: 10.1089/10445460152595586
Prieto, D. I., Zehgeer, A. A., and connor, D. F. (2019). Use of suvorexant for sleep regulation in an adolescent with early-onset bipolar disorder. J. Child Adolesc. Psychopharmacol. 29:395. doi: 10.1089/cap.2019.0029
Prince, J. B., Wilens, T. E., Biederman, J., Spencer, T. J., and Wozniak, J. R. (1996). Clonidine for sleep disturbances associated with attention-deficit hyperactivity disorder: a systematic chart review of 62 cases. J. Am. Acad. Child Adolesc. Psychiatr. 35, 599–605. doi: 10.1097/00004583-199605000-00014
Reynolds, A. M., Connolly, H. V., Katz, T., Goldman, S. E., Weiss, S. K., Halbower, A. C., et al. (2020). Randomized, placebo-controlled trial of ferrous sulfate to treat insomnia in children with autism spectrum disorders. Pediatr. Neurol. 104, 30–39. doi: 10.1016/j.pediatrneurol.2019.07.015
Roane, B. M., and Taylor, D. J. (2008). Adolescent insomnia as a risk factor for early adult depression and substance abuse. Sleep 31, 1351–1356.
Rolling, J., Rabot, J., and Schroder, C. M. (2022). Melatonin treatment for pediatric patients with insomnia: is there a place for it? Nat. Sci. Sleep 14, 1927–1944. doi: 10.2147/NSS.S340944
Rugino, T. A. (2018). Effect on primary sleep disorders when children with ADHD are administered guanfacine extended release. J. Atten. Disord. 22, 14–24. doi: 10.1177/1087054714554932
Russo, R. M., Gururaj, V. J., and Allen, J. E. (1976). The effectiveness of diphenhydramine HCI in pediatric sleep disorders. J. Clin. Pharmacol. 16, 284–288. doi: 10.1002/j.1552-4604.1976.tb02406.x
Sankaranarayanan, J., and Puumala, S. E. (2007). Antipsychotic use at adult ambulatory care visits by patients with mental health disorders in the United States, 1996–2003: national estimates and associated factors. Clin. Therapeut. 29, 723–741. doi: 10.1016/j.clinthera.2007.04.017
Schlarb, A. A., Bihlmaier, I., Velten-Schurian, K., Poets, C. F., and Hautzinger, M. (2016). Short- and long-term effects of CBT-I in groups for school-age children suffering from chronic insomnia: the KiSS-program. Behav. Sleep Med. 16, 380–397. doi: 10.1080/15402002.2016.1228642
Schnoes, C. J., Kuhn, B. R., Workman, E. F., and Ellis, C. R. (2006). Pediatric prescribing practices for clonidine and other pharmacologic agents for children with sleep disturbance. Clin. Pediatr. 45, 229–238. doi: 10.1177/000992280604500304
Shah, Y., Stringel, V., Pavkovic, I. M., and Kothare, S. V. (2020). Doxepin in children and adolescents with symptoms of insomnia: a single-center experience. J. Clin. Sleep Med. 16, 743–747. doi: 10.5664/jcsm.8338
Shamseddeen, W., Clarke, G., Keller, M. B., Wagner, K. D., Birmaher, B., Emslie, G. J., et al. (2012). Adjunctive sleep medications and depression outcome in the treatment of serotonin-selective reuptake inhibitor resistant depression in adolescents study. J. Child Adolesc. Psychopharmacol. 22, 29–36. doi: 10.1089/cap.2011.0027
Shatkin, J. P. (2017). Born to Be Wild—Why Teens Take Risks and How We Can Help Keep Them Safe. New York, NY: TarcherPerigee.
Simon, S. L., and Byars, K. C. (2016). Behavioral treatments for non-rapid eye movement parasomnias in children. Curr. Sleep Med. Rep. 2, 152–157. doi: 10.1007/s40675-016-0049-9
Singh, G., and Loona, N. (2013). Zolpidem-induced hallucinations: a brief case report from the indian subcontinent. Ind. J. Psychol. Med. 35:212. doi: 10.4103/0253-7176.116260
Smits, M. G., Van Stel, H. F., Van Der Heijden, K., Meijer, A. M., Coenen, A. M. L., and Kerkhof, G. A. (2003). Melatonin improves health status and sleep in children with idiopathic chronic sleep-onset insomnia: a randomized placebo-controlled trial. J. Am. Acad. Child Adolesc. Psychiatr. 42, 1286–1293. doi: 10.1097/01.chi.0000085756.71002.86
Stigler, K. A., Posey, D. J., and Mcdougle, C. J. (2006). Ramelteon for insomnia in two youths with autistic disorder. J. Child Adolesc. Psychopharmacol. 16, 631–636. doi: 10.1089/cap.2006.16.631
Subotic-Kerry, M., Werner-Seidler, A., Corkish, B., Batterham, P. J., Sicouri, G., Hudson, J. L., et al. (2023). Protocol for a randomised controlled trial evaluating the effect of a CBT-I smartphone application (Sleep Ninja®) on insomnia symptoms in children. BMC Psychiatr. 23:5185. doi: 10.1186/s12888-023-05185-x
Sultan, M. A., and Courtney, D. B. (2017). Adjunctive trazodone and depression outcome in adolescents treated with serotonin re-uptake inhibitors. PubMed 26, 233–240.
Thompson, W., Quay, T. A. W., Rojas-Fernandez, C., Farrell, B., and Bjerre, L. M. (2016). Atypical antipsychotics for insomnia: a systematic review. Sleep Med. 22, 13–17. doi: 10.1016/j.sleep.2016.04.003
Wesselhoeft, R., Rasmussen, L., Jensen, P. B., Jennum, P. J., Skurtveit, S., Hartz, I., et al. (2021). Use of hypnotic drugs among children, adolescents, and young adults in Scandinavia. Acta Psychiatr. Scand. 144, 100–112. doi: 10.1111/acps.13329
Wilens, T. E., Biederman, J., and Spencer, T. (1994). Clonidine for sleep disturbances associated with attention-deficit hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatr. 33, 424–426. doi: 10.1097/00004583-199403000-00018
Winsler, A., Deutsch, A., Vorona, R. D., Payne, P. A., and Szklo-Coxe, M. (2015). Aleepless in fairfax: the difference one more hour of sleep can make for teen hopelessness, suicidal ideation, and substance use. J. Youth Adolesc. 44, 362–378. doi: 10.1007/s10964-014-0170-3
Xiong, M., Li, F., Liu, Z., Xie, X., Shen, H., Li, W., et al. (2023). Efficacy of melatonin for insomnia in children with autism spectrum disorder: a meta-analysis. Neuropediatrics 54, 167–173. doi: 10.1055/s-0043-1761437
Keywords: pediatric sleep, pediatric insomnia, medications for pediatric insomnia, alternative medicine for pediatric insomnia, use of cannabis for insomnia
Citation: Dhir S, Karim N, Berka H and Shatkin J (2024) Pharmacological management of pediatric insomnia. Front. Sleep 3:1389052. doi: 10.3389/frsle.2024.1389052
Received: 20 February 2024; Accepted: 20 May 2024;
Published: 19 June 2024.
Edited by:
Stephen Sheldon, Northwestern University, United StatesReviewed by:
Oliviero Bruni, Sapienza University of Rome, ItalyPaul Yeh, University of Texas Health Science Center at Houston, United States
Copyright © 2024 Dhir, Karim, Berka and Shatkin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jess Shatkin, jess.shatkin@nyumc.org
 Sakshi Dhir
Sakshi Dhir Nicolette Karim
Nicolette Karim Haley Berka
Haley Berka Jess Shatkin
Jess Shatkin