
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Remote Sens. , 01 September 2022
Sec. Acoustic Remote Sensing
Volume 3 - 2022 | https://doi.org/10.3389/frsen.2022.940247
This article is part of the Research Topic Acoustic Remote Sensing of Cetacean and Pinniped Populations View all 13 articles
Prior to 1984, belugas (Delphinapterus leucas) were seen in large numbers during spring and summer in Kotzebue Sound, Alaska, and provided an important subsistence resource to coastal residents. Sightings and harvest declined sharply beginning in 1984: the average annual harvest dropped from 84/yr (1977–1983) to 16/yr (1984–2021). To examine the current seasonal and spatial occurrence of belugas in Kotzebue Sound, passive acoustic moorings were deployed in summer 2013 and year-round in 2014–2016. Three moorings were deployed off Cape Krusenstern, northwestern Kotzebue Sound, to monitor cetaceans traveling nearshore. A mooring was also deployed near Chamisso Island, southeastern Kotzebue Sound. We used automatic detectors to process the recordings for echolocation and tonal signals, and all detections were manually validated. Belugas, harbor porpoises (Phocoena), and transient killer whales (Orcinus orca) were detected in both areas, primarily from June to November. Detections extended into early winter for belugas, and sporadic detections were confirmed for porpoises from January to March. Belugas were detected on a total of 20 days, killer whales on 96 days, and porpoises on 179 days. All beluga detections were echolocation signals; the absence of social signals likely reflects an anti-predator response to transient killer whales and possibly to subsistence hunters. Killer whale detections were composed of echolocation signals, limited to very short click trains, double clicks, and single clicks, a known cryptic acoustic behavior used when targeting prey. Killer whales also emitted high frequency whistles (17–51 kHz) providing the first evidence of these types of signals for transients. Our results suggest transient killer whales in predation mode scouting harbor porpoise and beluga habitat, concurrent with belugas in silent anti-predation mode. This anti-predation acoustic behavior by belugas was also evident when killer whales were not present, conveying a continued perception of predation risk for this habitat. The combined natural and anthropogenic predation pressure in Kotzebue Sound could be playing an important role in the continued low occurrence of belugas.
Beluga whales (Delphinapterus leucas) in Alaska are an important subsistence resource for Alaska Natives in the Bering, Chukchi, and Beaufort seas. North of Bering Strait, they have been historically hunted in offshore leads during the bowhead whale (Balaena mysticetus) hunt (Nelson, 1969), or during cooperative hunting by Inuit and Iñupiat summering on the shores of shallow bays and estuaries (Fraker, 1980; Langdon, 1986; Frost and Lowry, 1990; McGhee, 1995).
Belugas were once abundant in Kotzebue Sound and comprised a separate stock from Eastern Chukchi Sea or Beaufort Sea belugas (O’Corry-Crowe et al., 2021). Historically, belugas entered Kotzebue Sound from the north, following the flaw lead formed in June parallel to shore near Cape Krusenstern (Figure 1). They first aggregated near Sisualik, a Qikiqtaġruŋmiut (Kotzebue people) and Nuataagmiut (Noatak people) beluga hunting area near Kotzebue, and later moved down the coast inside the sound, around Choris Peninsula, and, at high tide, into the shallow Eschscholtz Bay (Lowry et al., 1985; Seaman et al., 1988; Frost and Lowry, 1990; reviewed in O’Corry-Crowe et al., 2021). Belugas became less abundant beginning in the 1960s and 1970s but were hunted regularly by the communities in northern Kotzebue Sound and Eschscholtz Bay to the south (reviewed in Frost et al., 2021). In 1984, following years of high harvests (i.e., 1977–1983), the abundance of belugas in Kotzebue Sound declined precipitously and the traditional drive hunt in Eschscholtz Bay became no longer feasible. This decline is reflected in the greatly reduced annual harvest since 1983. The harvest averaged 84 (and up to 154) from 1977–1983, then declined to 16 during 1984–2021, and down to seven during 2011–2021 (Frost et al., 2021; O’Corry-Crowe et al., 2021; ABWC unpublished).
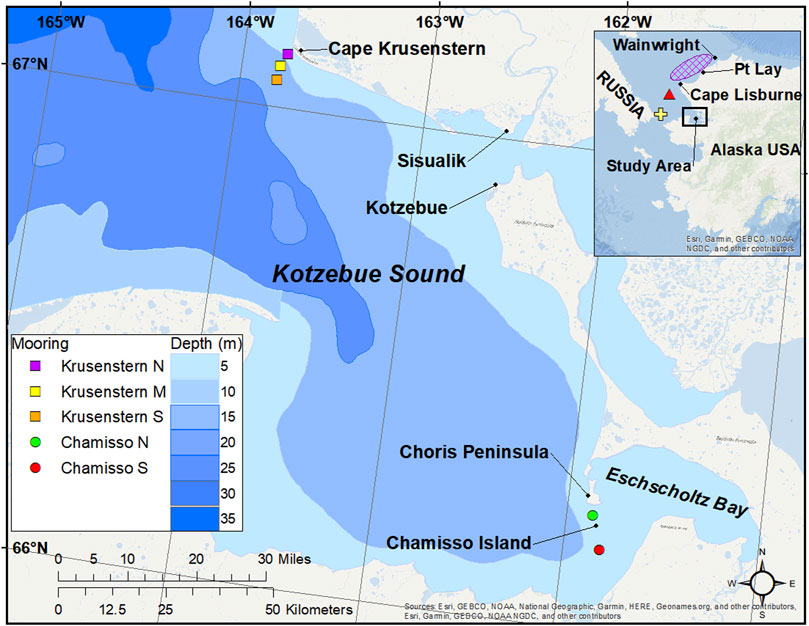
FIGURE 1. Map of the study area in Kotzebue Sound, Alaska. Passive acoustic mooring locations are indicated in colors as squares (Cape Krusenstern) or circles (Chamisso Island) for the summer 2013 pilot study, and the 2014–2016 study. Map insert: Previously published study areas by other authors, yellow cross for Stafford et al. (2019), red triangle for Madrigal et al. (2021), purple gridded area for Hannay et al. (2013).
Few belugas have been sighted in recent years, although occasional large groups are sometimes present in late July-September; they continue to be hunted when encountered (Frost and Lowry, 1990; Burch, 1994; ABWC unpublished). Hunters in Kotzebue Sound suspect that these larger groups may not be “Kotzebue Sound belugas”, because they do not follow the traditional movement patterns observed prior to 1984. Genetic analyses confirm that belugas from other stocks are sometimes harvested in Kotzebue Sound and suggest that Kotzebue Sound is currently used by belugas from the remnant Kotzebue Sound stock that, at times, co-occurs with whales from other stocks (O’Corry-Crowe et al., 2021).
Two exceptions to the reduced beluga harvests occurred in 1996 and 2007, when several hundred whales entered the sound and many were harvested. The genetic profile of these whales was distinct from those harvested prior to 1983, suggesting they belonged to the eastern Beaufort Sea population (O'Corry-Crowe et al., 2016; O’Corry-Crowe et al., 2021). In 2007, Alaska Native subsistence hunters reported seeing killer whales hunting and preying on belugas in Kotzebue Sound, along with observations of belugas moving into and remaining in shallow waters, even while being hunted.
Declining Arctic sea-ice, which drives shifts in prey and alters predation risk, has been suggested as the primary reason for changes in the movements and distribution of Arctic marine mammals (Kovacs, 2011; Matthews et al., 2020). However, the initial decrease in beluga presence in Kotzebue Sound occurred when the sound was largely ice-covered in winter and ice was regularly present until June. Alaska Native hunters from Kotzebue Sound suggest several factors in addition to hunting have contributed to decreased beluga presence and changed their movements and distribution, including disturbance from powerboat and aircraft traffic, deep-water hunting using powerboats, and improper human behavior while belugas are present. Additionally, ice entrapment off eastern Russia in 1984 may have contributed to decreased beluga presence in Kotzebue Sound (Frost et al., 2021; O’Corry-Crowe et al., 2021).
To examine the presence of belugas and their habitat use during this post-decline, reduced-ice era, a passive acoustic monitoring pilot study was implemented for ∼2 months in summer of 2013, followed by a 2-years study in 2014–2016, to describe the year-round presence of belugas and other cetaceans in Kotzebue Sound.
Low-profile compact mooring packages were used in this study to withstand the combination of shallow waters, ice, and high sedimentation rates of Kotzebue Sound. Mooring design followed that used in Cook Inlet, Alaska, for beluga monitoring (Lammers et al., 2013; Castellote et al., 2016). Mooring packages containing passive acoustic instruments were connected to 1.5 m of line and an expendable 50 kg anchor via an acoustic release (PORT LF, Edgetech, MA, USA). This configuration places the instruments at about 2 m above the seafloor depending on water current intensity. For the pilot study in 2013, packages contained two instruments: an acoustic recorder (EAR, Oceanwide Science Institute, HI, USA) that monitored the 10 Hz to 12.5 kHz frequency range to record cetacean social signals (whistles and calls), and an echolocation logger (C-POD v1, Chelonia Limited, Cornwall, United Kingdom) that monitored the 20–160 kHz frequency range to detect odontocete echolocation signals. The detection range for belugas in a shallow estuarine environment is estimated to be 2.2–3.3 km for EARs (Lammers et al., 2013), and 700–900 m for the C-POD (Castellote et al., 2016) because the latter instruments scan signals in very high frequencies where propagation loss is greater and because echolocation signals are more directional than whistles and other tonal vocalizations. Detection range for the C-POD for harbor porpoises (Phocoena) is estimated at 248–566 m in 13–20 m of water (Nuuttila et al., 2018). For our 2014–2016 study, these two instruments were replaced by a single wideband recorder (DSG-ST, Loggerhead Instruments, FL, USA) to monitor the frequency range of 10–144 kHz for both social and echolocation signals emitted by cetaceans. Detection ranges for these species are not available for DSG-ST, but they were assumed to be similar to those reported for EARs for social signals and for C-PODs for echolocation. Detection ranges for killer whales have not been quantified for any of these instruments, but we assumed they were similar to belugas since their signals’ source levels and frequency distribution overlap in range. Both EARs and DSG-STs were programmed on a duty cycle that varied from 2 min of every 4 min (50% cycle) to 2 min of every 27 min (7.4% cycle), depending on deployment duration, power, and memory capacity (see Table 1). C-PODs were programmed to monitor continuously for all deployments.
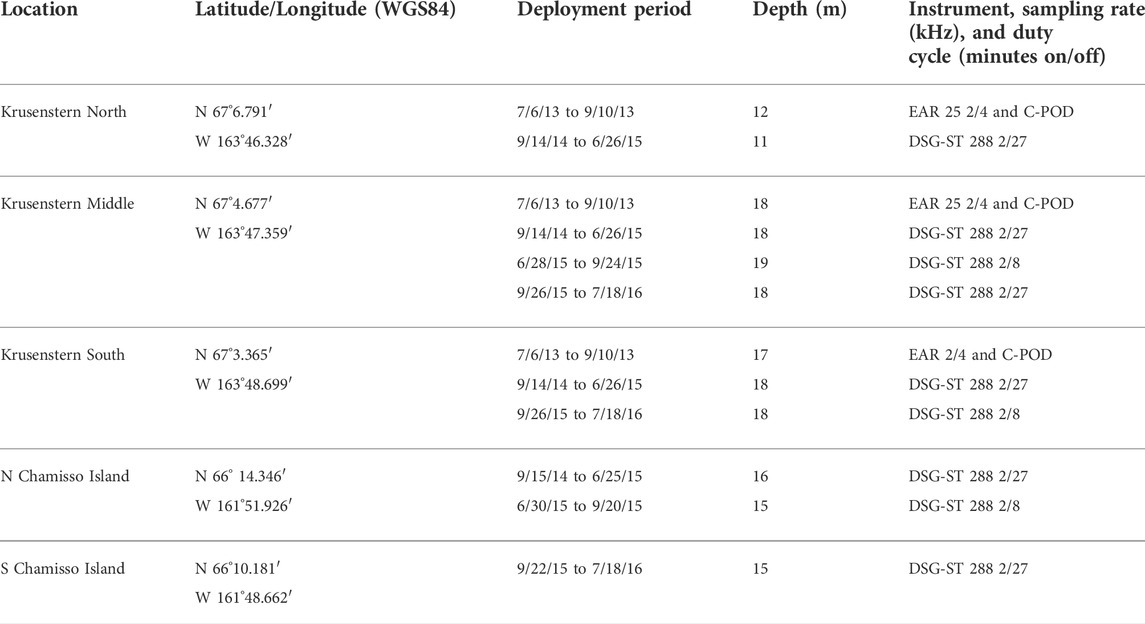
TABLE 1. Deployment details for the four locations in Kotzebue Sound, Alaska, acoustically monitored from 2013 to 2016 for cetacean presence.
Following discussions with local Kotzebue and Buckland beluga hunters and Elders, facilitated by the Alaska Beluga Whale Committee (ABWC), moorings were deployed in July 2013 off Cape Krusenstern in northwestern Kotzebue Sound (Figure 1). Three stations 3.6 km apart were selected in a line perpendicular to shore, providing an approximate detection range from shore to 12.6 km southwards. This enabled us to monitor cetaceans traveling in nearshore waters on their previously documented access route to the sound, determined by the primary spring ice lead. No belugas were detected at the south station during the pilot study (2013), therefore, we did not monitor this location for the 2014–2016 study. The mooring at the shallow (11 m deep) north station was lost during the 2014–2015 overwinter period, likely due to ice interaction, so we determined this location was too close to shore. Thus, from 2015 onwards, monitoring efforts at Cape Krusenstern took place at the middle station only. An additional mooring station was added off Chamisso Island in southeastern Kotzebue Sound for the 2014–2016 study (Figure 1). This mooring was relocated from north to south of the island after the 2014–2015 overwinter deployment, when it drifted nearly 5 km west of its original deployment location. This was likely due to the strong currents funneled by the channel between the island and Choris Peninsula. Deployment details for each station are described in Table 1.
Sound files recorded by both the EAR and DSG-ST instruments were processed using the acoustics analysis software Ishmael, version 1.4 (CIMRS Bioacoustics Lab, Oregon State University). Using the batch process tool in this program, a subsample of the 2014–2015 overwinter dataset, corresponding to 6% of the data (∼70 h of sound recordings), was visually and aurally reviewed to find signals from three species of interest: beluga, killer whale, and harbor porpoise. A full band (0–12.5 kHz for EAR data, 0–144 kHz for DSG-ST data) spectrogram of each sound file was examined for beluga and killer whale signals using a 10-s viewing frame. For DSG-ST data, the spectrogram frequency domain was depicted as a base five logarithmic scale to highlight the lower frequencies where social calls and whistles are emitted, while allowing to search for echolocation signals in the highest frequencies. The manually reviewed subsample was used to fine tune detector settings for each species. An energy summation detector was set up to search for echolocation signals for each of the three species individually, and a whistle and moan detector to search for calls and whistles of belugas or killer whales. Different detector settings were tested until false negative minutes no longer occurred in the data subsample (each minute of data with any calls, whistles, or echolocation clicks triggered at least one detection). This approach generated a relatively large amount of false detections, but prevented missing any minute where signals where present.
The EAR data were processed similarly: an energy summation detector targeted killer whale echolocation and the whistle and moan detector targeted both beluga and killer whale calls (Tables 2 and 3). The sample rate of the EARs (25 kHz) was too low to record the high frequency killer whale whistles and beluga and porpoise clicks. Each DSG-ST dataset was processed six times to run the energy summation detector targeting porpoise clicks, killer whale clicks, beluga clicks, killer whale high frequency whistles, the whistle and moan detector for killer whale calls and whistles, and beluga calls and whistles (Table 3).
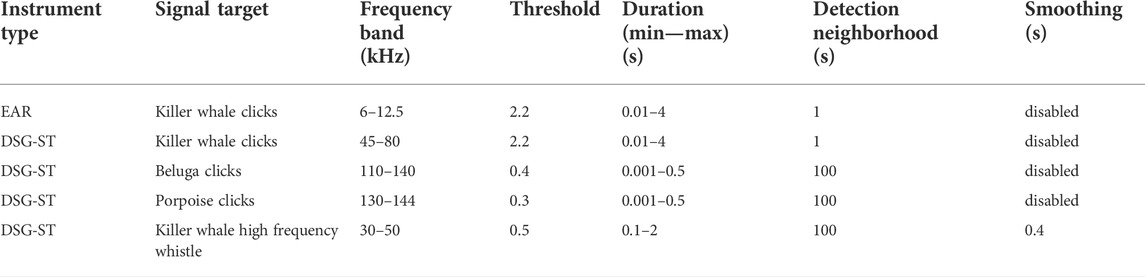
TABLE 2. Energy Summation detection algorithm and settings used in Ishmael 1.4 to search for beluga, porpoise, and killer whale echolocation signals (clicks), and killer whale high frequency whistles, in EAR and DSG-ST data.
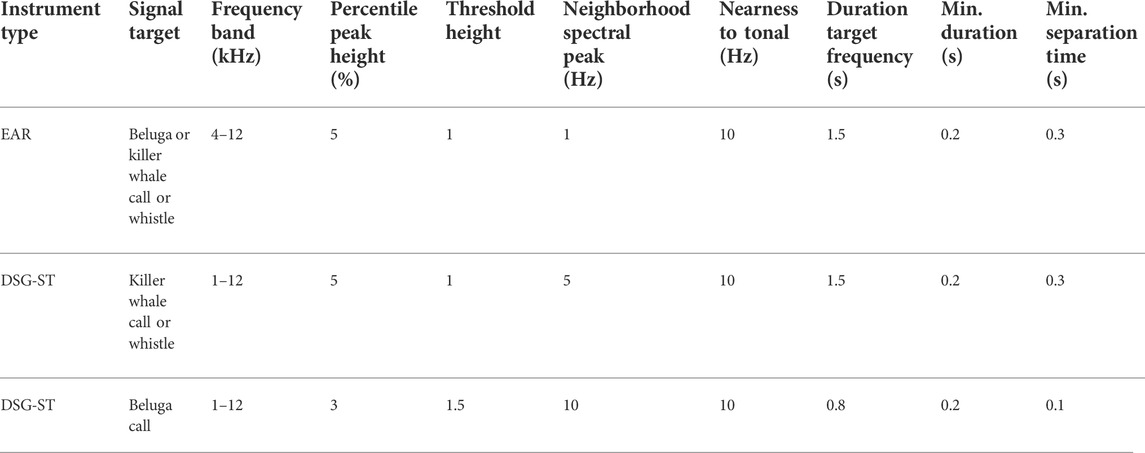
TABLE 3. Whistle and moan detection algorithm and its settings used in Ishmael 1.4 to search for beluga and killer whale social signals in EAR and DSG-ST data.
For each dataset batch process, a detection log was generated listing every file with at least one automatic detection. Each identified file was opened in Audition CS 5.5 (Adobe) to generate a spectrogram (Hanning window, 512 or 1024 FFT, 75% overlap, 10 s length) that was used to confirm or reject the automatic detection. False detections were discarded from further analysis.
C-POD data were analyzed using the default settings of the software C-POD. exe version 2.043; that is, “Hi and Mod train quality,” “all cetacean species,” unmodified “train values,” and “click filters.” Default settings only exclude doubtful and low quality click trains, which could include false detections, particularly in noisy conditions (e.g., breaking waves and ice noise). We manually validated all the “Hi” and “Mod” click train detections plotting the peak click frequency in the C-POD. exe analysis window with a time resolution of 100 ms (screen pixel width matching 100 ms in duration), which typically gives a screen window length of 2–3 min. Click train type classification (narrowband high frequency clicks, termed NBHF by CPOD. exe to refer to porpoise species, or other cetacean clicks) was also manually validated for each click train in the C-POD. exe analysis window based on considerable differences in peak frequency and click bandwidth among the echolocation clicks of beluga whales, killer whales, and porpoises (Au et al., 2004; Simon et al., 2007). See Castellote et al. (2016) for more details on this classification approach.
Validated call, whistle, and echolocation detections were combined into a single detection time series with 1-min resolution. Any minute in which an echolocation click train, call, or whistle was detected by an EAR, DSG-ST, or C-POD was categorized as a detection positive minute (DPM). As such, a DPM may include one single type of cetacean signal, or up to all three types (echolocation, calls, and whistles), and signals at different rates (e.g., one single call or many calls). This DPM approach reduced behavioral effects when quantifying cetacean presence; e.g., a minute with many calls and whistles from socializing belugas would quantify the same amount of presence as a minute with just one click train from a quiet traveling beluga. Total DPMs per day (uncorrected for duty cycle) were grouped by species and mooring location to describe presence and absence of signals for the entire sampled periods from 2013 to 2016.
Sea ice concentration data for the Kotzebue Sound region were obtained from the NOAA National Snow and Ice Data Center (NSDIC) Climate Data Record of Passive Microwave Sea Ice Concentration, Version 3. This dataset contains daily sea ice concentration for grid cells 25 × 25 km. Monthly average ice concentration from the grid cells overlapping the mooring locations at Krusenstern Point and Chamisso Island were used in the visualization of acoustic detections.
Three odontocete species were detected annually on the recorders: belugas (echolocation), killer whales (echolocation and high frequency whistles), and harbor porpoises (echolocation) (Figure 2). Bearded seal (Erignathus barbatus) trills were abundant in most of the overwinter deployment periods, but we did not include them in our analysis.
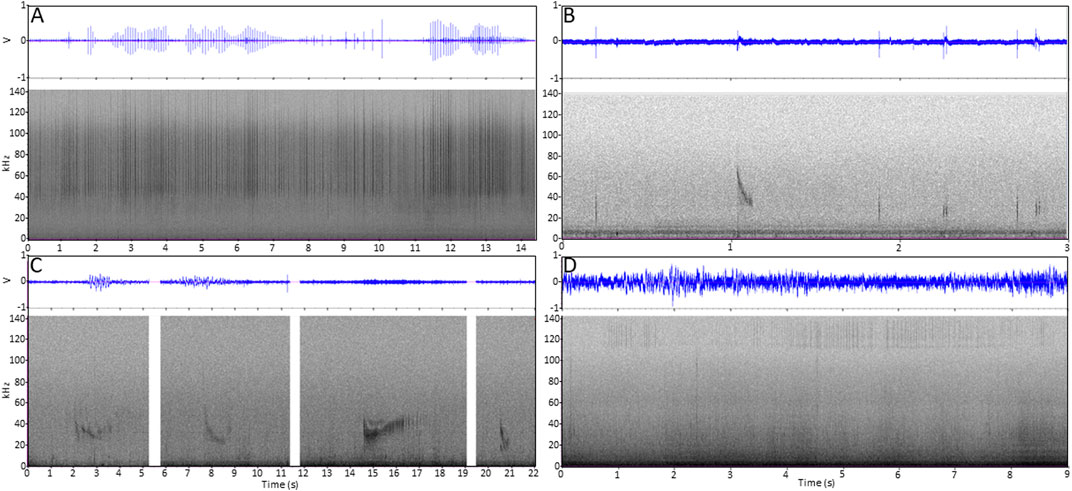
FIGURE 2. Waveform and spectrogram (linear 0–144 kHz, Hanning window, 1024 FFT, 75% overlap) of (A) 14 s of data with examples of beluga echolocation from Krusenstern Point on 20 November 2015 from at least three individuals (click trains show highest amplitude from approximately 40 kHz–130 KHz); (B) 3 s of data with examples of transient killer whale single clicks, double clicks, and a high frequency whistle from Krusenstern Point on 10 July 2015 (clicks show highest amplitude from approximately 24–34 kHz); (C) Four sound recording segments from Krusenstern Point on different dates during July 2015 showing transient killer whale high frequency whistles (whistles are modulated in the range 17–51 kHz); (D) Nine seconds of data with harbor porpoise echolocation from Chamisso Island on 12 January 2015 (click trains show highest amplitude from approximately 115 kHz–140 KHz).
Beluga signals detected throughout the study period consisted exclusively of short echolocation bursts. Detected killer whale signals included single clicks, double clicks, very short click trains, and high frequency whistles. Because killer whale single clicks can be easily confused with other common non-cetacean sources of noise, particularly if these clicks are received with a low signal-to-noise ratio (e.g., killer whales far from the recorder or off-axis), detections consisting exclusively of single clicks were considered valid only if these happened within 10 s of whistles or high frequency whistles, or if at least three single clicks were detected within a 10-s window.
Killer whale high frequency whistles were detected only at Krusenstern Point, on 29 occasions and always accompanied by single and double clicks (Figure 2). Whistles were frequency modulated in the range 17–51 kHz (mean of 35 kHz, stdv. 1.1 kHz) with a mean duration of 1.4 s (stdv. 0.7 s).
Most false detections were related to storm periods (breaking wave or rain noise), when background noise levels were elevated and exceeded the acoustic energy thresholds set for the energy summation detector, or, ice noise by pressure stress that triggered the tonal detector. On some occasions, skiff transits yielded false tonal detections due to outboard motor noise emissions with a rich tonal harmonic nature. Some false detections were also triggered by vegetative debris rubbing or hitting the mooring packages. The Chamisso Island 2014–2015 overwinter dataset was particularly noisy due to high currents in that deployment area, causing a large number of false detections for both detector types during periods of peak currents associated with the tidal cycle. These elevated flow noise conditions could have impacted the detectability of signals of interest in this dataset due to potential masking of beluga or killer whale vocalizations by the frequency overlapping flow noise of higher amplitude.
Results from the 2013 pilot study show the highest number of DPMs for all species combined at the north station (999 DPMs), closest to shore, followed by the middle station (936 DPMs), and then the south station (668 DPMs) (Figure 3). Both porpoise and killer whale detections decreased with increased distance to shore. All beluga detections occurred at the middle station except one DPM at the north station.
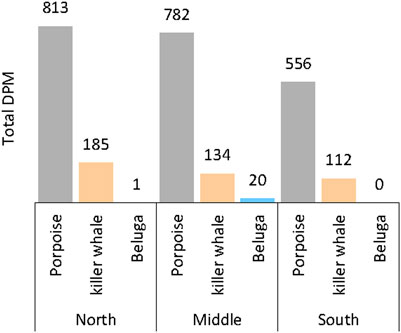
FIGURE 3. Total number of detection positive minutes (DPM) for each species detected at the three mooring stations in Krusenstern Point, Kotzebue Sound, Alaska, during the summer 2013 pilot study.
During the 2014–2016 study, harbor porpoise echolocation dominated the detections at all sites during the low ice or open water periods. There were also some unexpected detections near Chamisso Island in January-March 2015, when the area was heavily covered by ice (76–82% ice concentration) (see also Whiting et al. (2019)).
Killer whales were detected almost daily during the 2013 pilot study (59 of 67 days from July to September) at all three sites off Cape Krusenstern (Figure 4). In 2014, killer whales were detected on only 2 days in September. However, the moorings were not deployed until 14 September 2014, and a large period of expected killer whale presence (summer, based on 2013) was not sampled. In 2015, killer whale detections were recorded off Krusenstern on 22 days, from June to September 2015, and one detection near Chamisso Island on 23 July 2015. The 2015–2016 overwinter deployment period, which lasted until the moorings were recovered on 18 July 2016, documented killer whale presence on 3 days in October, on 23 November, and on 3 days in June off Cape Krusenstern. Killer whales were detected at Chamisso Island on 12 October 2015. Most of the 2014–2016 overwinter killer whale detections occurred in 0–16% ice concentration: a single detection on 12 October 2015 occurred when ice concentration over the moorings was 58%.
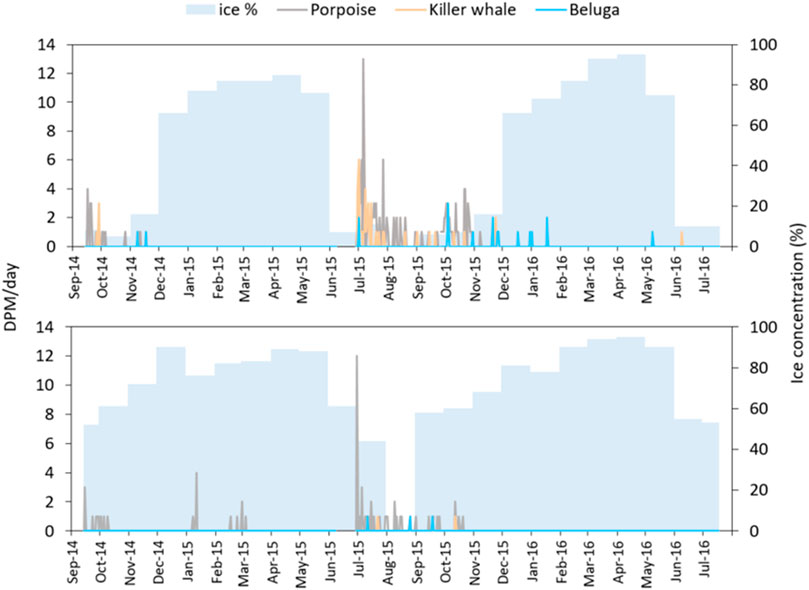
FIGURE 4. Acoustic detections as detection positive minutes per day (DPM/day) and 25 × 25 km monthly average ice concentration (0–100%) for the mooring sites at Krusenstern Point (upper panel) and Chamisso Island (lower panel), Kotzebue Sound, Alaska, for the 2014–2016 study.
In the 2013 pilot study, beluga detections occurred for a total of 20 min, on 2 days at the middle station (9 and 25 July 2013, with 10% ice concentration) and 1 day at the north station (19 August 2013, ice free). During 2014–2016, belugas were detected near Cape Krusenstern on 1 July 2015; 4 and 30 October 2015; 20, 25 and 26 November 2015; 17 and 30 December 2015, 1 and 17 January 2016; and 8 May 2016. All of these detections occurred with ice concentrations of 7–75%. Beluga detections at Chamisso Island occurred on 12 July, 26 August and 19 September 2015, under 0–58% ice concentration.
Opportunistic sightings and harvest data show scarce but consistent presence of belugas in the sound during the study period (A. Whiting unpubl. data; Frost et al., 2021). In 2013 15 belugas were sighted and five taken, in 2014 10 sighted and nine taken, in 2015 six sighted and one taken, in 2016 five sighted and four taken. Kotzebue area sighting dates generally correlate with the acoustic detections for both the 2013 pilot study and the 2014–2016 study. In 2013, sightings occurred from the middle of June until early August, with initial acoustic detections on 9 and 25 July. In 2014, belugas were sighted during summer but were not sighted or acoustically detected during the deployment period which did not begin until September. In 2015, first sightings occurred 5 days earlier than the first acoustic detections. In 2016, winter and spring acoustic detections were not correlated with any sightings. Killer whales were only reported twice within the study period, on 20 June 2014 outside Kotzebue Sound and during June 2016 in Eschscholtz Bay and to the west.
Our acoustic monitoring studies successfully collected data that help describe the seasonal occurrence of cetaceans in Kotzebue Sound. However, the overall shallow depth of Kotzebue Sound greatly limits the capacity to deploy overwintering moorings inside the sound, because sea ice can displace or destroy moorings in shallow water.
The very few beluga detections recorded during this study is consistent with the reduced abundance of belugas in Kotzebue Sound since the 1980s and the scarcity of reported sightings during the study period. Mooring deployment sites were based on the Indigenous Knowledge of local people who hunt and conduct many other marine and land-based activities in this region. People conducting boating and camping activities, and pilots of small aircrafts regularly report sightings of belugas. Even sightings of only a few belugas are widely known by the community. The absence of beluga detections near Chamisso Island, the main general hunting area inside the sound, during most of our study also supports local observations that belugas are no longer common inside Kotzebue Sound, not just off Cape Krusenstern. In contrast, harbor porpoises and killer whales were regularly detected in both northern and southern Kotzebue Sound, in particular from July to November.
Characteristics of our recorded killer whale signals indicate these whales were the transient ecotype, in accordance with all other killer whale reports in U.S. Arctic waters (Frost et al., 1992; George et al., 1994; George and Suydam, 1998; Hannay et al., 2013; Stafford, 2018; Willoughby et al., 2020; Madrigal et al., 2021; Stafford et al., 2022b; Willoughby et al., 2022). Detections were comprised almost exclusively of echolocation signals; however, most of the echolocation signals were limited to very short click trains, double clicks, and single clicks. Double and single click detections were initially discarded as false positives, but after observing the prevalence of these broadband and very short impulsive signals, a closer inspection revealed these were likely functional echolocation clicks; the same analysis process was described by Barrett-Lennard et al. (1996). In that study, the authors describe how they initially missed these sounds entirely, but later recognized them as isolated single or paired clicks produced by transient killer whales while predating on harbor seals (Phoca vitulina), Dall’s porpoises (Phocoenoides dalli), and harbor porpoises (Barrett-Lennard et al., 1996). This unique echolocation behavior has also been described for transient killer whales by Guinet (1992) in the Crozet Islands Archipelago, in the southern Indian Ocean. Guinet (1992) described how very few acoustic signals were observed in encounters with transient killer whales, with isolated clicks as the only acoustic signal produced while hunting southern elephant seals (Mirounga leonina). Both of these studies and our results suggest that the production of isolated and double clicks as non-sequenced echolocation signals emitted irregularly, rather than in rapid series, are an effectively cryptic but functional form of echolocation by killer whales that likely reduces the chances of alerting their targeted prey while searching for it. These signals were not detected in other studies that focused on the increasing presence of killer whales in the Pacific Arctic due to the lower sample rate of these studies (Stafford, 2018; Madrigal et al., 2021; Stafford et al., 2022b). We searched for sounds of fluke cavitation around the periods of killer whale detections but did not find them in the acoustic record. Fluke cavitation sound is caused by energetic fluking during a prey chase (Madrigal et al., 2021), which has been hypothesized as a means for killer whales to debilitate prey (Kenneth et al., 1988; Simon et al., 2005).
Despite belugas being a notoriously vocal species, no social calls or whistles were detected in any of the beluga presence periods during the 4 years of our studies. A decrease or even a cessation of acoustic activity by belugas in the presence of natural predators or human disturbance has been observed in both captive and free-ranging individuals, and it is interpreted as a survival strategy to avoid detection by predators (Schevill, 1964; Fish and Vania, 1971; Morgan, 1979; Lésage et al., 1999; Karlsen et al., 2002; Van Parijs et al., 2003; Castellote and Fossa, 2006; Castellote et al., 2012). This silent behavior, where the only acoustic detections were triggered by echolocation signals, has been described in Cook Inlet, Alaska, in an area of high human disturbance (Kendall et al., 2013) but social calls or whistles are the most common signal detected in the rest of their habitat range (Castellote et al., 2020). Thus, we suggest the silent behavior in Kotzebue Sound is likely related to the presence of transient killer whales, subsistence hunting boats, and the perceived risk of predation in this habitat.
Our study documented the presence of transient killer whales using an acoustically cryptic hunting mode in the same general region where belugas have occurred in the past. Killer whale detections occurred on 59 of 67 (88%) sampled days in 2013, 2 days in 2014, 26 days in 2015, and 1 day in 2016; but sampling during 2014–2016 was minimal during the ∼2-month period from mid-July to mid-September when detections occurred in 2013 (Table 1). The same acoustic behavior for belugas and killer whales was observed in both the 2013 pilot study and during 2014–2016.
Porpoises were by far the most abundant species in all sampled locations. Whiting et al. (2019) presented an analysis of 9 months of acoustic data (September 2014-June 2015) from our 2014–2016 study, and the porpoise results presented here expand on Whiting’s analysis. Harbor porpoises are the only small odontocetes that have been observed in Kotzebue Sound (Frost and Lowry, 1989). As expected, most detections occurred when little ice was present (≤ 16% ice concentration), during June to October. There were a few detections near Chamisso Island at the onset of ice formation in October 2014, with ice concentrations reaching 52%, and at the end of the ice season in July 2015 in periods of 44% concentration. Surprisingly, however, porpoise click trains were also detected throughout January to March 2015 at the Chamisso Island mooring. Ice concentrations ranged from 76 to 82% for that general area, consisting mainly of fast ice to the east and pack ice to the west. To our knowledge, this is the first time that harbor porpoises have been reported to occur in such heavy ice (Whiting et al., 2019).
Porpoise avoidance of the regions and times when transient killer whales were present in Kotzebue Sound was not evident: both species’ presence peaked during months of lowest ice concentration, and most days with killer whale detections also contained porpoise detections. However, we did not examine smaller time scales (e.g., hourly vs daily), so perhaps porpoises avoid killer whales on smaller spatial and temporal scales. Porpoise communication has been described as shaped by selection pressure from predation by killer whales and the ambient noise characteristics of coastal waters into a narrowband high frequency acoustic niche away from the main hearing range of killer whales (Andersen and Amundin, 1976; Madsen et al., 2005; Morisaka and Connor, 2007; Miller and Wahlberg, 2013). Porpoises might therefore have a higher tolerance of predation risk by transient killer whales than belugas. Porpoises are harder to detect acoustically, and are smaller and faster prey, and like belugas, able to use very shallow habitat that is inaccessible for killer whales. Our 2013 results show a decreasing rate of porpoise detections with increasing depth in the three moorings deployed in line off Cape Krusenstern, suggesting a preference for shallower waters in the sound.
High frequency killer whale whistles have been described for northeast Atlantic fish-eating populations (Samarra et al., 2010), for the eastern North Pacific offshore ecotype, and for an unknown ecotype in the western North Pacific (Filatova et al., 2012; Simonis et al., 2012). Andriolo et al. (2015) described these types of whistles in the western South Atlantic Ocean, and Trickey et al. (2014) reported high frequency whistles from type A killer whales in Antarctica. Our results suggest high frequency whistle production by transient killer whales in Arctic Alaskan waters. Two different explanations for the roles of this type of whistle emission have been suggested. Simonis et al. (2012) and Andriolo et al. (2015) suggested a potential role for these whistle types in functional echolocation, although the frequency modulation reported by these authors was more stereotypical than the more varied whistles detected in the current study. Filatova et al. (2012) however, proposed these whistles are used for close-range or ‘private’ communication, based on the need of an individual whale to communicate or share information among others in the group, while avoiding eavesdropping. Higher frequency signals would be beneficial because they attenuate more rapidly than low-frequency social calls and whistles. In our context, this function is more likely because killer whales were engaged in prey search in stealth mode (i.e., lack of longer click trains and other social signals).
Since ancient times, killer whales have been present in Kotzebue Sound, as has predation by killer whales on belugas. Killer whale–beluga interactions are referred to in traditional Iñupiaq stories. For example, Iñupiat who wanted good beluga hunting and a long life, left offerings to killer whale spirits in a cave on the Seward Peninsula where spirit people lived who were humans on land and killer whales in water (Lucier and VanStone, 1995). These offerings to killer whale spirits to promote good beluga hunting are understandable in view of known killer whale-beluga interactions and traditional Iñupiaq views of animal-human relationships. Another example in the Inuit folklore is the mythical composite animal Akhlut (Nelson, 1900), described as being similar in form to the killer whale and credited with the power of changing at will to a wolf to roam about over the land, and becoming a killer whale when returning back to the sea.
Killer whales were regularly reported in Eschscholtz Bay and near Kotzebue in the 1970s, 1980s, and early 1990s, even when ice was present in the sound (ABWC, 1992, 1993, 1997). Killer whales visit the deeper waters in summer just off the western and southern shores of Choris Peninsula, near our Chamisso Island mooring site, likely because they are in pursuit of belugas or other marine mammal prey. The shallows in Eschscholtz Bay are important for beluga calving and feeding and may provide refuge from attacks by killer whales (Frost and Lowry, 1990; Lucier and VanStone, 1995). Iñupiat hunters report that belugas being pursued by killer whales sometimes beach themselves or “hide” by entering shallow bays or lagoons where killer whales cannot follow (Frost and Lowry, 1990). According to beluga hunters in Kotzebue Sound, the beluga escape response is so strong when killer whales are nearby that belugas will even move into and stay in shallow areas while being hunted by people (Lucier and VanStone, 1995). This behavior contributed to one of the last large beluga hunts in Kotzebue Sound in 2007, when 151 belugas were harvested over a period of 2 days in late July by over 20 hunting boats, while multiple killer whales sightings were also documented from Kotzebue to Chamisso Island, including beluga kills by killer whales (Frost et al., 2021, Whiting pers. comm.). In contrast, only five belugas were harvested in Eschscholtz Bay in 1979. This was variously attributed to disturbance by boats in the constricted entrance to Eschscholtz Bay, or to the presence of killer whales at the mouth of the bay (Langdon, 1986). Beluga hunting was more successful in 1980–83 when hunting activities were organized to reduce disturbance. The Kaηigmiut, a group of Kotzebue Sound Iñupiat that have historically hunted belugas in Eschscholtz Bay, reported that no one seems to remember belugas being consistently absent from the bay before the mid-1980s, except for times when killer whales chased belugas out of the sound (Morseth, 1997).
Other studies in the Chukchi Sea report irregular presence of transient killer whales. Hannay et al. (2013) collected data from large passive acoustic recorder arrays in the northeastern Chukchi Sea from 2007 to 2011, and described 4–6 days of transient killer whale detections per open-water season from late July to early October. In the southern Chukchi Sea near Bering Strait, Stafford (2018) reported transient killer whale detections on 10–28% of the days from June-November 2009–2015, from a location just north of Bering Strait, a month longer than Hannay et al. (2013) reported and similar to our results. Near Point Hope, between the study areas of Stafford (2018) and Hannay et al. (2013), Madrigal et al. (2021) sampled year-round and recorded killer whale detections on 7–16 days per year during June to August 2013–2015. During a 2009–2020 study of the Chukchi Plateau, Stafford et al. (2022b) detected no killer whales prior to 2016, on just 1 day per year in July-August 2016–2018 and 2020, and during three consecutive weeks in August 2020.
Comparing the results of our study with other acoustic studies reporting transient killer whale presence in the U. S. Arctic waters is difficult due to differences in methodologies and habitat disparities. Hannay et al. (2013), Stafford (2018), Stafford et al. (2022b), and Madrigal et al. (2021) used a much lower sampling rate than the instruments in our study, which impedes the detection of high frequency echolocation signals or high frequency whistles. They sampled different proportions of time (other studies sampled longer recording periods but had longer stand-by intervals between recordings): only 5% of data were processed for Hannay et al. (2013), compared to 100% in Stafford (2018), Stafford et al. (2022b), Madrigal et al. (2021), and our study. Deployment depths were greater in the other four studies, yielding larger detection ranges.
In general, the amount of time that we detected transient killer whales in Kotzebue Sound was greater than reported elsewhere. Our results show higher presence of transient killer whales per open-water season (59 days in 2013, 26 days in 2015, 3 days in 2016 but only sampled until 18 July) as per Hannay et al. (2013) and Stafford et al. (2022b), per overall % of the days from June to November (88%, 32%, 6% of the days for 2013, 2015 and 2016, respectively) as per Stafford (2018), or per year in July-September (59 days 2013, 22 days in 2015, 3 days in 2016 but only sampled until 18 July) as per Madrigal et al. (2021). This may be related to the migratory behavior of prey and longer residence times in Kotzebue Sound: harbor porpoises are regularly present and widespread during the open water season, belugas return annually to calve and feed (albeit now in reduced numbers), and phocid seals are abundant in late summer and fall. In contrast, cetacean prey in the Chukchi Sea is often migrating through on their way north.
All four published studies cited above report acoustic detections that correspond to vocally active transient killer whales. Such vocal activity has been attributed to social interactions when pursuing large prey or after a successful kill (Deecke et al., 2005; Riesch and Deecke, 2011). In our study, we did not detect vocally active killer whales. Their very different vocal behavior renders killer whales in Kotzebue Sound much more difficult to detect acoustically. Therefore, the acoustic presence reported in our study, even if much larger than in other studies, is likely just a small fraction of the actual presence of transient killer whales in Kotzebue Sound. For example, although we did not detect these whales near Chamisso Island during June-July 2016, they were observed by subsistence hunters.
Ongoing changes in ice cover and prey availability in the Chukchi Sea due to the current climate warming have been linked to changes in beluga foraging and migratory behavior (Hauser et al., 2017; Hauser et al., 2018). Despite these changes and despite their seasonal association with sea ice, belugas still show philopatry to coastal migratory destinations (O'Corry-Crowe et al., 2016; Hauser et al., 2018; O'Corry-Crowe et al., 2018). In contrast, changes in predation pressure can profoundly affect prey species’ habitat selection, including Arctic marine mammals (Kovacs, 2011). Predation is arguably the most influential top-down forcing in an ecosystem, regulating prey populations and even shaping entire communities (Paine, 1966; Hixon et al., 2002). Relatively few studies have evaluated the effect of transient killer whale presence on cetaceans, and in general, these have been focused on short-term effects (i.e., Fish and Vania, 1971; Laidre et al., 2006; Breed et al., 2017; Matthews et al., 2020). In contrast, studies of terrestrial carnivores clearly show how longer-term changes in predation pressure, or in the prey perception of predation risk, deeply affect prey species’ habitat use (Laundre et al., 2001; Peacor and Werner, 2001; Fortin et al., 2005; Preisser et al., 2005; Verdolin, 2006; Laundre, 2010; Gaynor et al., 2019). Terrestrial prey is driven towards less risky habitats by fear of predation, through movement and habitat selection (Lima and Dill, 1990; Creel et al., 2005; Thaker et al., 2011). Thus, the ecological consequences of predation, via top-down forcing, can be transmitted not only by the killing and consumption of prey, but also by changes in prey behavior induced by non-consumptive effects (Werner and Peacor, 2003; Preisser et al., 2007; Preisser, 2009). To our knowledge, only two cetacean studies to date have shown the magnitude of predator mediated non-consumptive effects by transient killer whales: on narwhals, a close relative to belugas (Breed et al., 2017), and bowhead whales (Matthews et al., 2020). Breed et al. (2017) describe highly significant differences in the spatial use and behavioral state of narwhals in the presence and absence of transient killer whales, for a period of 10 days, and often at distances well beyond acoustic range of each other in the Eastern Canadian Arctic. Matthews et al. (2020) provide evidence of changes in habitat use and behavior of bowhead whales in the presence of killer whales for a period of 3 weeks. Our passive acoustic results reflect a prevalent seasonal presence of transient killer whales in the Kotzebue Sound area, and an awareness of the risk of predation by belugas, reflected by acoustically cryptic behavior during both the open water season when killer whales were present, and outside that season when killer whales were not detected.
The presence of transient killer whales elicits a strong change in the acoustic behavior of beluga, but also a strong spatial response. Sergeant and Brodie (1969) proposed that beluga summer distribution is restricted to the Arctic or to subarctic estuaries (i.e., very shallow water) by predation from killer whales. The use of very shallow waters as a defense for killer whale predation has been observed in Alaskan estuaries (Frost et al., 1992), and in other Arctic regions (e.g., Lydersen et al., 2001), as well as spatial displacement away from predator presence (Fish and Vania, 1971; Westdal, 2016). These behavioral responses to predation are well-known and well-described in Indigenous Knowledge from different Arctic cultures, including from Kotzebue and Noatak people, as described in previous sections of this paper.
Killer whales are not the only predators of belugas in Kotzebue Sound. For millennia, Alaska Native people have hunted belugas for subsistence. Throughout the 20th century, with the advent of increasingly powerful outboard motors and better weapons, hunts became more efficient. Beluga response to hunters’ boats has been described as being very similar to their response to transient killer whales: acoustic signaling is minimized, combined with avoidance behaviors such as longer diving, snorkeling (breathing with only the blowhole exposed with no body roll, Howe et al. (2015)), and swimming away towards shallower waters (Frost et al., 1992; Huntington, 2000; Karlsen et al., 2002; Van Parijs et al., 2003). Although hunting pressure has decreased, belugas continue to be hunted during May-October over a wide section of the Sound. Thus, beluga hunting represents an additional predation pressure in the Kotzebue Sound ecosystem, and an important and often ignored element of the natural predator-prey landscape.
The prevalence of killer whales has likely increased during the open water season in recent years, as suggested by several studies in U.S. Arctic waters (Clarke et al., 2013; George et al., 2017; Stafford, 2018; Willoughby et al., 2020; Stafford et al., 2022a; Stafford et al., 2022b; Willoughby et al., 2022), as well as in other regions of the Arctic Ocean (Lennert and Richard, 2017; Filatova et al., 2019; Lefort et al., 2020). This increase has been linked to the climate change induced reduction in seasonal sea ice coverage, allowing killer whales to penetrate farther into the Arctic environment and stay for longer periods of time (Higdon and Ferguson, 2009).
Regional land, vessel, and aerial visual surveys indicate that transient killer whales are widely present but occur sporadically throughout the Chukchi Sea, and increasingly the Beaufort Sea, during the open water season (Ljungblad, 1983; Lowry et al., 1987; Aerts et al., 2013; Clarke et al., 2013; VateBrattstrom et al., 2019; Clarke et al., 2020; Willoughby et al., 2020; Willoughby et al., 2022). An increase in killer whale predation on bowhead whales has been described throughout the eastern Chukchi and western Beaufort (George et al., 2017; Willoughby et al., 2020). However, there is no empirical evidence of an increase of transient killer whale presence in Kotzebue Sound, and our data cannot test this hypothesis. Our results do show a surprisingly extensive acoustic presence of transient killer whales compared to other studies. However, our study is the first of its kind for Kotzebue Sound, so a previous baseline on killer whale acoustic presence is not available, and, we cannot estimate an annual trend because sampling was not continuous over the 2013–2016 study period. Thus, the general increase in killer whale prevalence described in Arctic waters remains to be tested for Kotzebue Sound.
Determining whether killer whale predation pressure is increasing in Kotzebue Sound should be considered a research priority, since characterizing the processes by which climate change can affect marine mammals has been flagged as a conservation imperative (Gulland et al., 2022). Killer whales are major predators that may reshape marine ecosystems, fisheries, and cultural practices (Higdon and Ferguson, 2009; Lennert and Richard, 2017). The increase in duration of ice-free habitat is altering the presence of subarctic species in Arctic ecosystems (Moore and Huntington, 2008), including killer whales, in particular the transient ecotype. An increasing trend in transient killer whale presence in Kotzebue Sound could have profound ecological implications. An increase in predation pressure by killer whales as well as by humans (and its consequent increase in fear of predation) could trigger behavioral and ecological adjustments in habitat use, as documented in terrestrial carnivore studies. Both the pattern of return by the remnants of the original Kotzebue beluga population, and the dispersal by other beluga populations into this key beluga aggregation habitat could be reduced and discouraged by killer whale presence.
Overall, our study demonstrates that cetaceans can be successfully monitored year-round in Kotzebue Sound using passive acoustic moorings deployed at ice-resistant depths. Because we have no historical data with which to compare our findings, we cannot conclude whether the high prevalence of acoustic detections for killer whales and harbor porpoises reflects historical abundance or a more recent increase due to a warming climate and reduced ice coverage. The types of acoustic signals we identified for belugas and transient killer whales suggest intense predation pressure by killer whales and humans, and a residual effect of the risk of predation outside the period of killer whale presence. Further research should be focused on this Arctic ecosystem because longer-term changes in predation pressure, or in the prey perception of predation risk, deeply affect prey species’ habitat use.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by the AFSC Institutional Animal Care and Use Committee.
MC, RS, KS, AW, and KF contributed to conception and design of the study. MC led data collection, performed the data analysis, and wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding for the project was provided by the North Slope Borough Department of Wildlife Management (NSB) and the Native Village of Kotzebue (NVOK), along with in-kind support from the ADF&G and NOAA Fisheries. This publication was partially funded by the Cooperative Institute for Climate, Ocean, and Ecosystem Studies (CIOCES) under NOAA Cooperative Agreement NA20OAR4320271, Contribution No. 2022–1203. All work was conducted under National Marine Fisheries Service (NMFS) permit no. 14245.
We thank the Alaska Beluga Whale Committee for developing interest in passive acoustics studies of Kotzebue Sound and, in partnership with the Native Village of Kotzebue, helping to make these studies happen. Lloyd Lowry and Robert Suydam contributed to the design and development of this project. The Native Village of Kotzebue and the U.S. Fish and Wildlife Service provided logistical and operational support. David Mann, Loggerhead Instruments, provided valuable help in trying to determine the problems we encountered when using 512 GB cards in DSG-ST recorders and kindly replaced instruments and memory cards at no cost to the project. We sincerely thank Kotzebue residents Henry Goodwin, John Goodwin, Willie Goodwin, Jr and Frank Garfield of the Alaska Department of Fish and Game (ADF&G) for their support and assistance in field operations. The City of Kotzebue Public Works kindly provided the material and labor to make all the anchors used in our deployments. The ADF&G Kotzebue Office provided warehouse space for refurbishing moorings and Jeff Mondragon of the ADF&G provided assistance with logistics. Kim Shelden (NOAA-NMFS-AFSC) prepared Figure 1. Kim Shelden, Catherine Berchok (NOAA-NMFS-AFSC) and Gregory O’Corry-Crowe (Harbor Branch Oceanographic Institute) reviewed and helped improve an earlier version of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor SP declared a past co-authorship with the author KS.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aerts, L. A. M., McFarland, A. E., Watts, B. H., Lomac-MacNair, K. S., Seiser, P. E., Wisdom, S. S., et al. (2013). Marine mammal distribution and abundance in an offshore sub-region of the northeastern Chukchi Sea during the open-water season. Cont. Shelf Res. 67, 116–126. doi:10.1016/j.csr.2013.04.020
Andersen, S. H., and Amundin, M. (1976). Possible predator-related adaption of sound production and hearing in the harbour poirpoise (phocoena phocoena). Aquat. Mamm. 4, 56–57.
Andriolo, A., Reis, S. S., Amorim, T. O., Sucunza, F., de Castro, F. R., Maia, Y. G., et al. (2015). Killer whale (Orcinus orca) whistles from the western South Atlantic Ocean include high frequency signals. J. Acoust. Soc. Am. 138 (3), 1696–1701. doi:10.1121/1.4928308
Barrett-Lennard, L. G., Ford, J. K. B., and Heise, K. A. (1996). The mixed blessing of echolocation: Differences in sonar use by fish-eating and mammal-eating killer whales. Anim. Behav. 51 (3), 553–565. doi:10.1006/anbe.1996.0059
Breed, G. A., Matthews, C. J., Marcoux, M., Higdon, J. W., LeBlanc, B., Petersen, S. D., et al. (2017). Sustained disruption of narwhal habitat use and behavior in the presence of Arctic killer whales. Proc. Natl. Acad. Sci. U. S. A. 114 (10), 2628–2633. doi:10.1073/pnas.1611707114
Burch, E. S. (1994). The cultural and natural heritage of Northwest Alaska. the Inupiaq nations of Northwest Alaska. Fairbanks: University of Alaska Press.
Castellote, M., and Fossa, F. (2006). Measuring acoustic activity as a method to evaluate welfare in captive belugas (Delphinapterus leucas). Aquat. Mamm. 32 (3), 325–333. doi:10.1578/am.32.3.2006.325
Castellote, M., Leeney, R. H., O’Corry-Crowe, G., Lauhakangas, R., Kovacs, K. M., Lucey, W., et al. (2012). Monitoring white whales (Delphinapterus leucas) with echolocation loggers. Polar Biol. 36 (4), 493–509. doi:10.1007/s00300-012-1276-2
Castellote, M., Small, R. J., Lammers, M. O., Jenniges, J. J., Mondragon, J., and Atkinson, S. (2016). Dual instrument passive acoustic monitoring of belugas in Cook Inlet, Alaska. J. Acoust. Soc. Am. 139 (5), 2697–2707. doi:10.1121/1.4947427
Castellote, M., Small, R. J., Lammers, M. O., Jenniges, J., Mondragon, J., Garner, C. D., et al. (2020). Seasonal distribution and foraging occurrence of Cook Inlet beluga whales based on passive acoustic monitoring. Endanger. Species Res. 41, 225–243. doi:10.3354/esr01023
Clarke, J., Stafford, K., Moore, S., Rone, B., Aerts, L., and Crance, J. (2013). Subarctic cetaceans in the southern Chukchi Sea: Evidence of recovery or response to a changing ecosystem. Oceanogr. Wash. D. C). 26 (4), 136–149. doi:10.5670/oceanog.2013.81
Clarke, J. T., Brower, A. A., Ferguson, M. C., Willoughby, A. L., and Rotrock, A. D. (2020). “Distribution and relative abundance of marine mammals in the eastern Chukchi Sea, eastern and western Beaufort seas, and Amundsen Gulf, 2019,” in OCS study (Washington, D.C., USA: BOEM).
Creel, S., Winnie, J., Maxwell, B., Hamlin, K., and Creel, M. (2005). Elk alter habitat selection as an antipredator response to wolves. Ecology 86 (12), 3387–3397. doi:10.1890/05-0032
Deecke, V. B., Ford, J. K. B., and Slater, P. J. B. (2005). The vocal behaviour of mammal-eating killer whales: Communicating with costly calls. Anim. Behav. 69 (2), 395–405. doi:10.1016/j.anbehav.2004.04.014
Filatova, O. A., Ford, J. K., Matkin, C. O., Barrett-Lennard, L. G., Burdin, A. M., and Hoyt, E. (2012). Ultrasonic whistles of killer whales (Orcinus orca) recorded in the North Pacific (L). J. Acoust. Soc. Am. 132 (6), 3618–3621. doi:10.1121/1.4764874
Filatova, O. A., Shpak, O. V., Ivkovich, T. V., Volkova, E. V., Fedutin, I. D., Ovsyanikova, E. N., et al. (2019). Large-scale habitat segregation of fish-eating and mammal-eating killer whales (Orcinus orca) in the western North Pacific. Polar Biol. 42 (5), 931–941. doi:10.1007/s00300-019-02484-6
Fish, J. F., and Vania, J. S. (1971). Killer whale, Orcinus orca, sounds repel white whales, Delphinapterus leucas. Fish. Bull. Natl. Ocean. Atmos. Adm. 69 (3), 531–535.
Fortin, D., Beyer, H. L., Boyce, M. S., Smith, D. W., Duchesne, T., and Mao, J. S. (2005). Wolves influence elk movements: Behavior shapes a trophic cascade in Yellowstone National Park. Ecology 86 (5), 1320–1330. doi:10.1890/04-0953
Fraker, M. A. (1980). Status and harvest of the Mackenzie stock of white whales (Delphinapterus leucas). Cambridge, UK: International Whallng Commission.
Frost, K., Gray, T. J., Sr, W. G., Schaeffer, R., and Suydam, R. (2021). Alaska Beluga Whale Committeea unique model of co-management. Polar Res. 40. doi:10.33265/polar.v40.5611
Frost, K. J., and Lowry, L. F. (1990). “Distribution, abundance and movements of beluga whales, Delphinapterus leucas, in coastal waters of western Alaska,” in Advances in research on the beluga whale, Delphinapterus leucas. Editors T. G. D. Smith, J. S. Aubin, and J. R. Geraci (Ottawa: Department of Fisheries and Oceans), 39–57.
Frost, K. J., and Lowry, L. F. (1989). “Marine mammals of Kotzebue sound and southeastern Hope basin,” in Bering Strait-hope basin : Habitat utilization and ecological characterizationCooperative program (RU 690). Editors H. M. Feder, A. S. Naidu, M. Baskaran, K. Frost, J. M. Hameedi, S. C. Jewettet al. (Fairbanks, AK: NOAA, Ocean Assessment Division).
Frost, K. J., Russell, R. B., and Lowry, L. F. (1992). Killer whales, Orcinus-orca, in the southeastern Bering sea - recent sightings and predation on other marine mammals. Mar. Mamm. Sci. 8 (2), 110–119. doi:10.1111/j.1748-7692.1992.tb00370.x
Gaynor, K. M., Brown, J. S., Middleton, A. D., Power, M. E., and Brashares, J. S. (2019). Landscapes of fear: Spatial patterns of risk perception and response. Trends Ecol. Evol. 34 (4), 355–368. doi:10.1016/j.tree.2019.01.004
George, J. C., Philo, M., Hazard, K. L., Withrow, D., Carroll, G. M., and Suydam, R. (1994). Frequency of killer whale (Orcinus orca) attacks and ship collisions based on scarring on bowhead whales (Balaena mysticetus) of the bering-chukchi-beaufort seas stock. Arctic 47 (3), 247. doi:10.14430/arctic1295
George, J. C., Sheffield, G., Reed, D. J., Tudor, B., Stimmelmayr, R., Person, B. T., et al. (2017). Frequency of injuries from line entanglements, killer whales, and ship strikes on bering-chukchi-beaufort seas bowhead whales. Arctic 70 (1). doi:10.14430/arctic4631
George, John C., and Suydam, R. (1998). Observations of killer whale (Orcinus orca) predation in the northeastern Chukchi and western Beaufort seas. Mar. Mamm. Sci. 14 (2), 330–332. doi:10.1111/j.1748-7692.1998.tb00722.x
Guinet, C. (1992). Comportement de chasse des orques (Orcinus orca) autour des Iles Crozet. Can. J. Zool. 70 (9), 1656–1667. doi:10.1139/z92-231
Gulland, F. M. D., Baker, J. D., Howe, M., LaBrecque, E., Leach, L., Moore, S. E., et al. (2022). A review of climate change effects on marine mammals in United States waters: Past predictions, observed impacts, current research and conservation imperatives. Clim. Change Ecol. 3, 100054. doi:10.1016/j.ecochg.2022.100054
Hannay, D. E., Delarue, J., Mouy, X., Martin, B. S., Leary, D., Oswald, J. N., et al. (2013). Marine mammal acoustic detections in the northeastern Chukchi Sea, september 2007–july 2011. Cont. Shelf Res. 67, 127–146. doi:10.1016/j.csr.2013.07.009
Hauser, D. D. W., Laidre, K. L., Stafford, K. M., Stern, H. L., Suydam, R. S., and Richard, P. R. (2017). Decadal shifts in autumn migration timing by Pacific Arctic beluga whales are related to delayed annual sea ice formation. Glob. Chang. Biol. 23 (6), 2206–2217. doi:10.1111/gcb.13564
Hauser, D. D. W., Laidre, K. L., Stern, H. L., Suydam, R. S., Richard, P. R., and Wiersma, Y. (2018). Indirect effects of sea ice loss on summer-fall habitat and behaviour for sympatric populations of an Arctic marine predator. Divers. Distributions 24 (6), 791–799. doi:10.1111/ddi.12722
Higdon, J. W., and Ferguson, S. H. (2009). Loss of Arctic sea ice causing punctuated change in sightings of killer whales (Orcinus orca) over the past century. Ecol. Appl. 19 (5), 1365–1375. doi:10.1890/07-1941.1
Hixon, M. A., Pacala, S. W., and Sandin, S. A. (2002). Population regulation: Historical context and contemporary challenges of open vs. closed systems. Ecology 83 (6), 1490–1508. doi:10.1890/0012-9658(2002)083[1490:prhcac]2.0.co;2
Howe, M., Castellote, M., Garner, C., McKee, P., Small, R. J., and Hobbs, R. (2015). Beluga, Delphinapterus leucas, ethogram: A tool for cook inlet beluga conservation? Mar. Fish. Rev. 77 (1), 32–40. doi:10.7755/mfr.77.1.3
Huntington, H. P. (2000). Traditional knowledge of the ecology of belugas, Delphinapterus leucas, in cook inlet, Alaska. Mar. Fish. Rev. 62, 134–140.
Karlsen, J., Bisther, A., Lydersen, C., Haug, T., and Kovacs, K. (2002). Summer vocalisations of adult male white whales (Delphinapterus leucas) in Svalbard, Norway. Polar Biol. 25 (11), 808–817. doi:10.1007/s00300-002-0415-6
Kendall, L. S., Širović, A., and Roth, E. H. (2013). Effects of construction noise on the cook inlet beluga whale (Delphinapterus leucas) vocal behavior. Can. Acoust. Can. Acoust. - Acoust. Can. 41 (3), 3–14.
Kenneth, M., Norris, K. S., Moore, P. W. B., and Englund, K. A. (1988). “Loud impulse sounds in odontocete predation and social behavior,” in Animal sonar: Processes and performance. Editors P. E. Nachtigall, and P. W. Moore (New York: Springer Science and Business Media), 567–579.
Kovacs, K. M., Lydersen, C., Overland, J. E., and Moore, S. E. (2011). Impacts of changing sea-ice conditions on Arctic marine mammals. Mar. Biodivers. 41 (1), 181–194. doi:10.1007/s12526-010-0061-0
Laidre, K. L., Heide-Jorgensen, M. P., and Orr, J. R. (2006). Reactions of narwhals, Monodon monoceros, to killer whale, Orcinus orca, attacks in the eastern Canadian arctic. Can. Field. Nat. 120 (4), 457–465. doi:10.22621/cfn.v120i4.355
Lammers, M. O., Castellote, M., Small, R. J., Atkinson, S., Jenniges, J., Rosinski, A., et al. (2013). Passive acoustic monitoring of Cook Inlet beluga whales (Delphinapterus leucas). J. Acoust. Soc. Am. 134 (3), 2497–2504. doi:10.1121/1.4816575
Langdon, S. J. (1986). Contemporary Alaskan native economies. Lanham, Md: University Press of America.
Laundre, J. W., Hernandez, L., and Ripple, W. J. (2010). The landscape of fear: Ecological implications of being afraid∼!2009-09-09∼!2009-11-16∼!2010-02-02∼!. Open Ecol. J. Open Ecol. J. 3 (2), 1–7. doi:10.2174/1874213001003030001
Laundre, J. W., Lucina, H., and Altendorf, K. R. (2001). Wolves, elk, and bison: reestablishing the "landscape of fear" in Yellowstone National Park, U.S.A. Can. J. Zool. 79 (8), 1401–1409. doi:10.1139/z01-094
Lefort, K. J., Matthews, C. J. D., Higdon, J. W., Petersen, S. D., Westdal, K. H., Garroway, C. J., et al. (2020). A review of Canadian Arctic killer whale (Orcinus orca) ecology. Can. J. Zool. 98 (4), 245–253. doi:10.1139/cjz-2019-0207
Lennert, A. E., and Richard, G. (2017). At the cutting edge of the future: Unravelling depredation, behaviour and movement of killer whales in the act of flexible management regimes in Arctic Greenland. Ocean Coast. Manag. 148, 272–281. doi:10.1016/j.ocecoaman.2017.08.016
Lésage, V., Barrette, C., Kingsley, M. C. S., and Sjare, B. (1999). The effect of vessel noise on the vocal behavior of belugas in the St. Lawrence River estuary, Canada. Mar. Mamm. Sci. 15 (1), 65–84. doi:10.1111/j.1748-7692.1999.tb00782.x
Lima, S. L., and Dill, L. M. (1990). Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68 (4), 619–640. doi:10.1139/z90-092
Ljungblad, D. K., and Moore, S. E. (1983). Killer Whales (Orcinus orca) Chasing Gray Whales (Eschrichtius robustus) in the Northern Bering Sea. Arct. Arct. 36 (4), 361–364. doi:10.14430/arctic2291
Lowry, L. F., Nelson, R. R., and Frost, K. J. (1985). Investigations of belukha whales in coastal waters of western and northern Alaska. Fairbanks, Alaska: Alaska Dept. of Fish and Game.
Lowry, L. F., Nelson, R. R., and Frost, K. J. (1987). Observations of killer whales, Orcinus orca, in western Alaska - sightings, strandings, and predation on other marine mammals. Can. Field-Naturalist 101 (1), 6–12.
Lucier, C. V., and VanStone, J. W. (1995). Traditional beluga drives of the Iñupiat of Kotzebue sound, Alaska. Chicago: Field Museum of Natural History.
Lydersen, C., Martin, A. R., Kovacs, K. K., and Gjertz, I. (2001). Summer and autumn movements of white whales Delphinapterus leucas in Svalbard, Norway. Mar. Ecol. Prog. Ser. 219, 265–274. doi:10.3354/meps219265
Madrigal, B. C., Crance, J. L., Berchok, C. L., and Stimpert, A. K. (2021). Call repertoire and inferred ecotype presence of killer whales (Orcinus orca) recorded in the southeastern Chukchi Sea. J. Acoust. Soc. Am. 150 (1), 145–158. doi:10.1121/10.0005405
Madsen, P. T., Carder, D. A., Bedholm, K., and Ridgway, S. H. (2005). Porpoise clicks from a sperm whale nose—convergent evolution of 130 kHz pulses in toothed whale sonars? Bioacoustics 15 (2), 195–206. doi:10.1080/09524622.2005.9753547
Matthews, C. J. D., Breed, G. A., LeBlanc, B., and Ferguson, S. H. (2020). Killer whale presence drives bowhead whale selection for sea ice in Arctic seascapes of fear. Proc. Natl. Acad. Sci. U. S. A. 117(12), 6590–6598. doi:10.1073/pnas.1911761117
McGhee, R. (1995). Beluga hunters : An archaeological reconstruction of the history and culture of the mackenzie delta kittegaryumiut. Hull, Quebec: Canadian Museum of Civilization.
Miller, L. A., and Wahlberg, M. (2013). Echolocation by the harbour porpoise: life in coastal waters. Front. Physiol. 4, 52. doi:10.3389/fphys.2013.00052
Moore, S. E., and Huntington, H. P. (2008). Arctic marine mammals and climate change: impacts and resilience. Ecol. Appl. 18 (2), S157–S165. doi:10.1890/06-0571.1
Morgan, D. W. (1979). “The vocal and behavioural reactions of the beluga whale, Delphinapterus leucas, to playback of its sounds,” in Behaviour of marine animals: Current perspectives in research. Editors H. E. Winn, and B. L. Olla (New York: Plenum Press), 311–343.
Morisaka, T., and Connor, R. C. (2007). Predation by killer whales (Orcinus orca) and the evolution of whistle loss and narrow-band high frequency clicks in odontocetes. J. Evol. Biol. 20 (4), 1439–1458. doi:10.1111/j.1420-9101.2007.01336.x
Morseth, C. M. (1997). Twentieth-Century Changes in Beluga Whale Hunting and Butchering by the Kaηiġmiut of Buckland, Alaska. Arctic 50 (3), 241–255.
Nuuttila, H. K., Brundiers, K., Dähne, M., Koblitz, J. C., Thomas, L., Courtene‐Jones, W., et al. (2018). Estimating effective detection area of static passive acoustic data loggers from playback experiments with cetacean vocalisations. Methods Ecol. Evol. 9 (12), 2362–2371. doi:10.1111/2041-210x.13097
O'Corry-Crowe, G., Mahoney, A. R., Suydam, R., Quakenbush, L., Whiting, A., Lowry, L., et al. (2016). Genetic profiling links changing sea-ice to shifting beluga whale migration patterns. Biol. Lett. 12 (11), 20160404. doi:10.1098/rsbl.2016.0404
O'Corry-Crowe, G., Suydam, R., Quakenbush, L., Potgieter, B., Harwood, L., Litovka, D., et al. (2018). Migratory culture, population structure and stock identity in North Pacific beluga whales (Delphinapterus leucas). PLoS One 13 (3), e0194201. doi:10.1371/journal.pone.0194201
O’Corry-Crowe, G., Ferrer, T., Citta, J. J., Suydam, R., Quakenbush, L., Burns, J. J., et al. (2021). Genetic history and stock identity of beluga whales in Kotzebue Sound. Polar Res. 40. doi:10.33265/polar.v40.7623
Paine, R. T. (1966). Food Web Complexity and Species Diversity. Am. Nat. 100 (910), 65–75. doi:10.1086/282400
Peacor, S. D., and Werner, E. E. (2001). The contribution of trait-mediated indirect effects to the net effects of a predator. Proc. Natl. Acad. Sci. U. S. A. 98(7), 3904–3908. doi:10.1073/pnas.071061998
Preisser, E. L., Bolnick, D. I., and Benard, M. F. (2005). Scared to death? The effects of intimidation and consumption in predator–prey interactions. Ecology 86 (2), 501–509. doi:10.1890/04-0719
Preisser, E. L., Orrock, J. L., and Schmitz, O. J. (2007). Predator hunting mode and habitat domain alter nonconsumptive effects in predator-prey interactions. Ecology 88 (11), 2744–2751. doi:10.1890/07-0260.1
Preisser, E. L. (2009). The physiology of predator stress in free-ranging prey. J. Anim. Ecol. 78 (6), 1103–1105. doi:10.1111/j.1365-2656.2009.01602.x
Riesch, R., and Deecke, V. B. (2011). Whistle communication in mammal-eating killer whales (Orcinus orca): further evidence for acoustic divergence between ecotypes. Behav. Ecol. Sociobiol. 65 (7), 1377–1387. doi:10.1007/s00265-011-1148-8
Samarra, F. I., Deecke, V. B., Vinding, K., Rasmussen, M. H., Swift, R. J., and Miller, P. J. (2010). Killer whales (Orcinus orca) produce ultrasonic whistles. J. Acoust. Soc. Am. 128 (5), EL205–210. doi:10.1121/1.3462235
Schevill, W. E. (1964). Underwater sounds of Cetaceans, Mar. Bio-Acoustics, Tech. Rpt. No. MMC-77/13, 307–316.
Seaman, G. A., Frost, K. J., and Lowry, L. F. (1988). Investigations of belukha whales in coastal waters of western and northern Alaska - 1 - distribution, abundance, and movements. Anchorage, Alaska: U.S. National Ocean Service. Office of Oceanography and Marine Assessment.
Sergeant, D. E., and Brodie, P. F. (1969). Body size of white whales (Delphinapterus leucas). J. Fish. Res. Bd. Can. 26, 2561–2580. doi:10.1139/f69-251
Simon, M., Magnus, W., Fernando, U., and Miller, L. A. (2005). Acoustic characteristics of underwater tail slaps used by Norwegian and Icelandic killer whales (Orcinus orca) to debilitate herring (Clupea harengus). J. Exp. Biol. 208 (12), 2459–2466. doi:10.1242/jeb.01619
Simonis, A. E., Baumann-Pickering, S., Oleson, E., Melcon, M. L., Gassmann, M., Wiggins, S. M., et al. (2012). High-frequency modulated signals of killer whales (Orcinus orca) in the North Pacific. J. Acoust. Soc. Am. 131 (4), EL295–301. doi:10.1121/1.3690963
Stafford, K., Farley, E., Ferguson, M., Kuletz, K., and Levine, R. (2022a). Northward Range Expansion of Subarctic Upper Trophic Level Animals into the Pacific Arctic Region. Oceanogr. Wash. D. C). 35 (1). doi:10.5670/oceanog.2022.101
Stafford, K. M. (2018). Increasing detections of killer whales (Orcinus orca), in the Pacific Arctic. Mar. Mamm. Sci. 35 (2), 696–706. doi:10.1111/mms.12551
Stafford, K. M., Melling, H., Moore, S. E., Berchok, C. L., Braen, E. K., Brewer, A. M., et al. (2022b). Marine mammal detections on the Chukchi Plateau 2009-2020. J. Acoust. Soc. Am. 151 (4), 2521–2529. doi:10.1121/10.0010208
Thaker, M., Vanak, A. T., Owen, C. R., Ogden, M. B., Niemann, S. M., and Slotow, R. (2011). Minimizing predation risk in a landscape of multiple predators: effects on the spatial distribution of African ungulates. Ecology 92 (2), 398–407. doi:10.1890/10-0126.1
Trickey, J. S., Reyes, M. V. R., Baumann-PickerinG, S., Melcón, M. L., Hildebrand, J. A., and Iñíguez, M. A. (2014). Acoustic encounters of killer and beaked whales during the 2014 SORP cruise. Cambridge, UK: International Whaling Commission.
Van Parijs, S. M., Lydersen, C., and Kovacs, K. M. (2003). Sounds produced by individual white whales, Delphinapterus leucas, from Svalbard during capture. J. Acoust. Soc. Am. 113 (1), 57–60. doi:10.1121/1.1528931
VateBrattstrom, L., Mocklin, J. A., Crance, J. L., and Friday, N. A. (2019). “Arctic Whale Ecology Study (ARCWEST): Use of the Chukchi Sea by endangered Baleen and other whales (westward extension of the BOWFEST),” in OCS study BOEM 2018-022 (Seattle, WA: Marine Mammal Laboratory, Alaska Fisheries Science Center, NMFS, NOAA).
Verdolin, J. L. (2006). Meta-analysis of foraging and predation risk trade-offs in terrestrial systems. Behav. Ecol. Sociobiol. 60 (4), 457–464. doi:10.1007/s00265-006-0172-6
Werner, E. E., and Peacor, S. D. (2003). A Review of Trait-Mediated Indirect Interactions in Ecological Communities. Ecology 84 (5), 1083–1100. doi:10.1890/0012-9658(2003)084[1083:arotii]2.0.co;2
Westdal, K. H., Davies, J., McPherson, A., Orr, J., and Ferguson, S. H. (2016). Behavioural changes in Belugas (Delphinapterus leucas) during a Killer Whale (Orcinus orca) attack in southwest Hudson Bay. Can. Field. Nat. 130 (4), 315–319. doi:10.22621/cfn.v130i4.1925
Whiting, A., Castellote, M., Small, R. J., Frost, K. J., and Suydam, R. (2019). Unexpected mid‐winter presence of harbor porpoises (Phocoena phocoena) in Kotzebue Sound, Alaska. Mar. Mamm. Sci. 36 (1), 354–358. doi:10.1111/mms.12641
Willoughby, A. L., Ferguson, M. C., Stimmelmayr, R., Clarke, J. T., and Brower, A. A. (2020). Bowhead whale (Balaena mysticetus) and killer whale (Orcinus orca) co-occurrence in the U.S. Pacific Arctic, 2009–2018: evidence from bowhead whale carcasses. Polar Biol. 43 (11), 1669–1679. doi:10.1007/s00300-020-02734-y
Willoughby, A. L., Stimmelmayr, R., Brower, A. A., Clarke, J. T., and Ferguson, M. C. (2022). Gray whale (Eschrichtius robustus) and killer whale (Orcinus orca) co-occurrence in the eastern Chukchi Sea, 2009–2019: evidence from gray whale carcasses observed during aerial surveys. Polar Biol. 45, 737–748. doi:10.1007/s00300-022-03015-6
Keywords: white whales, transient killer whales, cetacean, top-down forcing, arctic, climate change, passive acoustic monitoring, nonconsumptive effects
Citation: Castellote M, Small RJ, Stafford KM, Whiting A and Frost KJ (2022) Beluga (D. leucas), harbor porpoise (P. phocoena), and killer whale (O. orca) acoustic presence in kotzebue sound, alaska: Silence speaks volumes. Front. Remote Sens. 3:940247. doi: 10.3389/frsen.2022.940247
Received: 10 May 2022; Accepted: 01 August 2022;
Published: 01 September 2022.
Edited by:
Susan E. Parks, Syracuse University, United StatesReviewed by:
William Halliday, Wildlife Conservation Society, CanadaCopyright © 2022 Castellote, Small, Stafford, Whiting and Frost. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manuel Castellote, TWFudWVsLmNhc3RlbGxvdGVAbm9hYS5nb3Y=
†Present address: Kathryn J. Frost, Alaska Beluga Whale Committee, Utqiaġvik, AK, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.