- 1Faculty of Science and Technology, University of Algarve, Faro, Portugal
- 2Hawaiian Islands Humpback Whale National Marine Sanctuary, Kīhei, HI, United States
- 3Cetacean Ecology and Acoustics Laboratories, School of Veterinary Science, The University of Queensland, Gatton, QLD, Australia
- 4Department of Biological Sciences, University of NB, Saint John, NB, Canada
- 5Departments of Psychology and Biology, University of Hawai’i at Hilo, Hilo, HI, United States
- 6The Dolphin Institute, Hilo, HI, United States
- 7CCMAR, Centre of Marine Sciences, University of Algarve, Faro, Portugal
Humpback whales (Megaptera novaeangliae) are exceptionally vocal among baleen whale species. While extensive research has been conducted on humpback whale songs, gaps remain in our understanding of other forms of communication, particularly non-song calls. Here, we compare the spectral features and temporal parameters of non-song calls recorded from AcousondeTM tagged humpback whales in three commonly observed group types in the breeding grounds: adult dyads (N = 3), singly escorted mother-calf pairs (N = 4), and competitive groups (N = 4). Recordings were collected off Maui, Hawai’i during the winter breeding seasons of 2019–2021. Individual calls were identified based on visual and aural inspection of spectrograms using Raven Pro 1.6 software, with a total of 842 calls isolated from 47.6 h of acoustic recordings. Competitive groups produced the most calls (N = 358); however, after adjusting for the differences in recording hours and the number of individuals, the call rate (calls/hour/whale) was not significantly different between group compositions. The temporal parameters and frequency measures of calls did not vary significantly across the groups. However, interesting patterns of calling behavior were observed (e.g., competitive groups had the shortest inter-call intervals and the highest frequency calls, and escorted mother-calf pairs had the longest inter-call intervals) and it is possible the lack of statistical significance could be attributed to the small sample size of tag deployments. This study provides new insights into humpback whale vocal communication behavior in the Hawaiian Islands breeding grounds.
Introduction
Cetaceans are well known for using acoustic signaling to mediate many aspects of their lives (Herman and Tavolga, 1980) such as reproductive behavior (Parsons et al., 2008; Smith et al., 2008; Herman, 2017), cooperative feeding (D’vincent et al., 1985; Cerchio and Dahlheim, 2001), and group contact (Clark, 1983; Wild and Gabriele, 2014). Among baleen whales, humpback whales (Megaptera novaeangliae), well known for their complex songs (Payne and McVay, 1971), are one of the most vocal. In addition to songs (produced by males throughout their ecological range), humpback whales of both sexes and all age classes produce a variety of non-song calls (Dunlop et al., 2008). Several studies have documented the repertoire of these calls in different locations (Cusano et al., 2020; Dunlop et al., 2007; Indeck et al., 2020; Stimpert et al., 2011; Epp et al., 2021a; Fournet et al., 2015; Rekdahl et al., 2017, unpublished dissertation Palanca, 2021), and it has been found that some calls show stability in their acoustic features over decades (Rekdahl et al., 2013; Fournet M. et al., 2018) and across allopatric populations (Fournet M. E. H. et al., 2018; Epp et al., 2021b). This suggests that calls serve an important function in social communication and that subtle variations in these calls may help to relay the motivational context and arousal of the signaler to conspecifics.
A recent study found that humpback whale calls recorded along an Australian migratory route (Dunlop, 2017) may convey motivational context, similarly to those produced by terrestrial mammalian species (Morton, 1977; August and Anderson, 1987). So-called aversive or appeasement humpback calls were high in frequency, whereas aggressive calls were lower in frequency with wider bandwidths (Dunlop, 2017), a trend noted previously in North American elk (Cervus elaphus; Feighny et al., 2006) and white-faced capuchin monkeys (Cebus capucinus; Gros-Louis et al., 2008). Furthermore, arousal (i.e., intensity of emotional states, e.g., Briefer, 2012) has also been linked to changes in signaling behavior, with high arousal situations linked to the production of higher frequency and longer duration signaling (Briefer, 2012; Fischer and Price, 2017). It has been suggested that both of these factors (motivational context and arousal) impact the social signaling of humpback whales (Cusano et al., 2020). For example, mother-calf pairs in the breeding grounds often segregate into shallow waters to avoid male harassment (Craig et al., 2014) or predation from orcas (Baird et al., 2006) and sharks (Pack et al., 2022). Either of these scenarios, presumptively, creates an aversive context characterized by increased arousal, which has been shown to cause changes in calling behavior within these groups (Cusano et al., 2020). Clearly, the context in which calls are produced can influence calling behavior, therefore, a factor such as group composition is important to consider when investigating the non-song calling behavior of humpback whales.
Other than mother-calf pairs, humpback whales on their breeding grounds have a social structure in which groups vary in composition, behavior, and the duration of association (Mobley and Herman, 1985). Groups include: singletons (which may be male or female); singleton singers (which are typically alone but sometimes with other whales); unescorted mother-calf pairs; dyads; singly escorted mother-calf pairs; all-male groups; and so-called “competitive groups'' consisting of either a mother-calf pair or a female without a calf, and two or more male escorts (summarized in Clapham, 2000; Herman et al., 2011). For this study, we focused on competitive groups containing a female without a calf, singly escorted mother-calf pairs, and dyads. Within a competitive group, the male escorts compete physically with each other for proximity to the single female, with the male defending the position closest to that female termed the “principal escort” and other males termed “secondary escorts” (Tyack and Whitehead, 1983; Baker and Herman, 1984). Escorted mother-calf pairs consist of a mother and her calf of the season accompanied by a single male escort presumed to be prospecting for a mating opportunity (Herman and Antinoja, 1977; Mobley and Herman, 1985). Dyads may be comprised of individuals of varying maturity level, although matures tend to pair together and immatures tend to pair together (Pack et al., 2012). The sex composition of most dyads is male-female, some are male-male and rarely are they two females (Pack et al., 2012).
Group size and membership can impact humpback whale calling behavior. Groups can vary widely in number depending on behavioral context, from a single individual (singleton or singer) to two individuals in mother-calf pairs or dyads, to 20 or more individuals in competitive groups in Hawaiian waters (e.g., Mobley and Herman, 1985; Herman et al., 2007) and as many as 200 individuals in feeding groups in South Africa (Findlay et al., 2017). Call rates (calls/hr/whale) have been shown to increase in conjunction with increasing group size, with small social groups like dyads and escorted mother-calf pairs typically producing fewer vocalizations than groups with one or multiple escorts (Silber, 1986; Cusano et al., 2020). However, the affiliation between group members may also be a factor that contributes to differences in call rates among groups. For example, solitary mother-calf pairs have likely evolved lower call rates as a strategy to avoid detection by predators and harassment from males (Craig et al., 2014; Dunlop, 2016; Videsen et al., 2017; Indeck et al., 2021; Indeck et al., 2022). Alternatively, several studies have shown that humpback whales increase their calling rate when unaffiliated whales join the group (Silber, 1986; Rekdahl et al., 2015; Cusano et al., 2020), and it has been proposed that variable call rates across escorted groups are attributable to the presence of males and differences in behavioral context (Seger, 2016, dissertation). Additionally, acoustic features of calls may relay physical attributes of the signaler to conspecifics, because the minimum frequency of calls in mysticete whales is somewhat limited by animal size (May-Collado et al., 2007; Martin et al., 2017).
Progress has been made in understanding how humpback whale group size, composition and behavior influence the use of non-song vocalizations. Yet, research in this area remains limited. This is especially true for comparisons of calling behavior across varying group compositions. Studies have varied historically in their scope, with some focused solely on a single group type, like mother-calf pairs (Zoidis et al., 2008; Indeck et al., 2020), and others that have drawn comparisons across groups (Palanca, 2021 dissertation, Seger, 2016 dissertation). Older studies that compared signaling across different groups lacked today’s technology (e.g., instrumented tags), which allows for more nuanced and robust long-term data collection on individual groups (Silber, 1986). In addition, most contemporary studies of humpback whale calling behavior within and across group types have been conducted along either migratory routes (Dunlop et al., 2007; Rekdahl et al., 2013; Rekdahl et al., 2015; Dunlop, 2016; Dunlop, 2017; Indeck et al., 2020; Recalde-Salas et al., 2020) or on feeding grounds (Fournet et al., 2015; Wild and Gabriele, 2014; Epp et al., 2021a) leaving differences in the calling behavior among groups on breeding grounds comparatively understudied. Therefore, this study used acoustic tags to determine the effects of group composition on the temporal pattern of calling behavior and variability in the spectral parameters of calls from humpback whales in the Hawaiian breeding grounds.
Materials and methods
Data collection
The data for this study were collected off west Maui, Hawai’i in the Hawaiian Islands Humpback Whale National Marine Sanctuary (Supplementary Appendix S1) during three winter breeding seasons (Jan-Mar) from 2019 to 2021. Acoustic recordings were made using suction cup AcousondeTM tags temporarily deployed on 11 adult humpback whales in three different group types: competitive groups, singly escorted mother-calf pairs, and dyads.
The two AcousondeTM tags used for this study (B010 and B046) were equipped with hydrophones recording 16-bit audio with the sampling rate set to 12,226 Hz, an 8th order elliptic anti-alias filter at 4646 Hz, a 4-stage cascaded high pass filter at 22 Hz, and a total path gain of +2.4 dB. The acoustic sensitivity of the B010 and B046 tags were -187.2 dB re 1 V/µPa and -187.9 dB re 1 V/µPa, respectively. Each AcousondeTM tag was equipped with suction cups and deployed on a whale’s dorsal surface just forward of the dorsal fin from a 9 m long hand-held carbon fiber pole by a tagger situated at the bow of an 11.5 m outboard vessel (see Stimpert et al., 2012 for best practices). Prior to tagging, the observers identified the group composition, recorded its behavioral state, and obtained identification images opportunistically of the ventral surface of each whale’s tail flukes (Katona et al., 1979). After a successful tagging event, the boat continued to follow the group at a distance of 200–300 m and the observers continued to opportunistically document whale behavior, group composition, and individual identities. These contextual notes continued while the research vessel remained with the animals, although this typically did not account for the entire tag deployment, which sometimes extended into evening and nighttime hours. To account for periods when context could not be recorded, recordings were only analyzed throughout the period of observation (starting from 10 minutes after tag deployment) and for the 2 hours following the end of observation. This 2-h grace period was chosen under the assumption that the group composition did not change immediately at the end of observation, but we acknowledge that over time the likelihood for group composition to change increases.
Call detection
Spectrograms were generated using Raven Pro 1.6 (The Cornell Laboratory of Ornithology, 2019) with a 4096-point DFT and 80% overlap. All recordings were visually and aurally inspected in their entirety, and all selections were made by one observer (JC). To account for any disturbances in vocal behavior that may have been caused by the tagging event (Williamson et al., 2016), calls from the initial 10 min of recording were eliminated from analyses. All calls were selected individually in Raven Pro, with boundaries set as tight as possible to the produced signal to ensure the highest accuracy for call measurements. For calls to be included in the selection process, it was necessary that there be a distinguishably clear start and endpoint, and little to no overlap with calls from conspecifics. Overlap did not occur often, and in total only 11 calls with overlap were included in analysis (all with overlap of <110 milliseconds). If any calls were produced in a repetitive pattern that resembled that of whale song from the current season (as per observations of background song recorded on the tags), they were not included in analyses. To be included in quantitative analysis, calls needed to have a signal-to-noise ratio (SNR) of at least 10 dB or higher. A SNR of greater than 10 dB is an accepted threshold established in previous studies (Dunlop et al., 2007; Dunlop et al., 2008; Stimpert et al., 2011; Rekdahl et al., 2013; Fournet et al., 2015) at which calls can be attributed to either the signaler or accompanying whales with relative confidence. However, without concurrent scan sampling to quantify the positions of nearby groups, it is difficult to determine with certainty if calls recorded with acoustic tags are produced by the tagged individual and group, or if they have been produced by other nearby conspecifics. It is important to note that, for this study, no call classification was performed. This was intentional as the naming schema of call types is still currently debated.
Call measurements
Due to the nature of acoustic tag recording, most files contained some flow noise in the 0–200 Hz range. With high rates of tourism in the study area, vessel noise was also occasionally disruptive in the recordings. To account for these disturbances, calls from all groups were measured using custom-written noise subtraction algorithms in MATLAB (Indeck et al., 2020). For every call selection, a corresponding noise selection (1–3 s long) was made in the time either immediately preceding or following the call selection to capture the ambient noise at the time of signaling. Occasionally, during a period of repetitive calls, one noise file was used for more than one signal file to ensure that noise selections corresponded as closely as possible to the respective signal. The spectrum from noise selections was subtracted from the corresponding signal file to remove most of the energy from flow noise, vessel traffic, and song from nearby conspecifics.
Once noise energy was removed from the call files, acoustic characteristics were measured from the observer-selected bounds of the call files. The temporal boundaries of call selections were made as close as possible to the visible start and endpoint of the call, and frequency boundaries were selected as close to the lowest and highest frequencies as possible. The measured acoustic characteristics of the calls included duration and several frequency measurements (i.e., peak frequency, center frequency, fifth percentile frequency, etc.) as used previously (Indeck et al., 2020; Supplementary Appendix S2).
Temporal patterns
To investigate the temporal differences in calling behavior, we looked at the time periods between calls. For this analysis, all calls were used, including those eliminated from other analyses due to poor SNR. This was done so as not to skew the period between calls, although it is inherently possible that some calls were not detected, due to ambient and flow noise. Every call selection made in Raven measured the beginning and end time of the call based on the time boundaries of the selection. These time measurements were then used to calculate the time between each social call by subtracting the end time of a call from the beginning time of the following call. This yielded a measurement of the inter-call interval (ICI). Call rates (calls/whale/hour) were also compared across the different group compositions. These were calculated for each tag deployment by dividing the total number of calls by the average number of whales in the group, by the number of recording hours.
Statistical analysis
Select frequency measurements (peak, center, first quartile, third quartile, fifth percentile, and ninety-fifth percentile frequencies) from high SNR (>10 dB) calls were compared across all group compositions. To analyze these measurements, generalized linear mixed models (GLMMs) were run using Tag ID as a random effect and a gamma distribution with a log link (suitable for non-normal data), using the glmmTMB package in R (Brooks et al., 2017). The emmeans package (Lenth, 2022) was then used post hoc to provide least squares means, which are adjusted to predict the effect of the factor variable on the response assuming equal sample sizes and, therefore, are more robust for unbalanced data than are observed averages (Harvey, 1960). Pairwise comparisons of call parameters across group composition were calculated using the “multivariate t" adjustment method, as it takes into consideration the correlation structure of the model (Lenth, 2022). Significance was set to p < 0.05. These statistical tests were also used to compare the temporal differences (duration and ICI) in calling across all groups. All means are presented as the least squares mean +/- standard deviation.
Results
A total of 47.6 h of acoustic recordings were analyzed from 11 deployments (Table 1) of AcousondeTM tags, with 18.4 h recorded in competitive groups (4 tag deployments), 14.0 h recorded in escorted mother-calf pairs (4 tag deployments), and 15.2 h recorded in dyads (3 tag deployments). In competitive groups, tags were deployed on two principal escorts, one secondary escort, and one presumed female, in groups ranging in size from three to eight individuals during observation. In singly escorted mother-calf pairs, tags were always deployed on the mother. For dyads, the gender of the tagged whale could not be determined based on behavioral role and therefore remained undetermined. Across all tag recordings and all group compositions, a total of 842 individual calls were selected, with 568 calls meeting the requirements to be included in the spectral analyses. Competitive groups produced the most calls (N = 358); however, after adjusting for the differences in recording hours of each group, the call rate (calls/hour/whale) was not significantly different between group compositions (Kruskal–Wallis, H = 0.727, df = 2, p = 0.695).
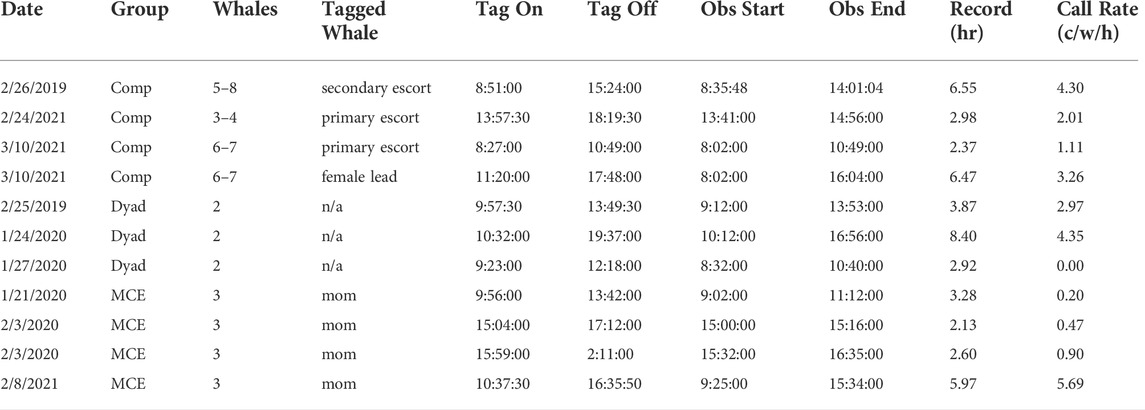
TABLE 1. Records of all eleven tags, including date of deployment, group composition, number of whales in the group, the role of the tagged whale, and tag on/off times, observation start and end times, recording hours that were included in data analyses, and the call rate calculated for each tag deployment. Call rate is a calculation of the total number of calls divided by the average number of whales divided by the recorded hours used. Names of group compositions have been abbreviated with comp representing competitive groups, and MCE representing escorted mother-calf pairs.
The period between social calls ranged from -0.105 s (escorted mother calf pairs) to 103 min (dyads). The range of these values are illustrated in Figure 1, where the shape of the violin plots represents the probability of distribution of values throughout the data set. The median inter-call interval was 0.527 (IQR ±10.6) seconds in competitive groups, 0.762 (IQR ±38.7) seconds in dyads, and 0.471 (IQR ±9.27) seconds in escorted mother-calf pairs. Competitive groups had shorter inter-call intervals (N = 571, mean = 121 ± 45.4 s) than dyads (N = 115, mean = 252 ± 138.6 s) and escorted mother-calf pairs (N = 156, mean = 275 ± 137.3 s).
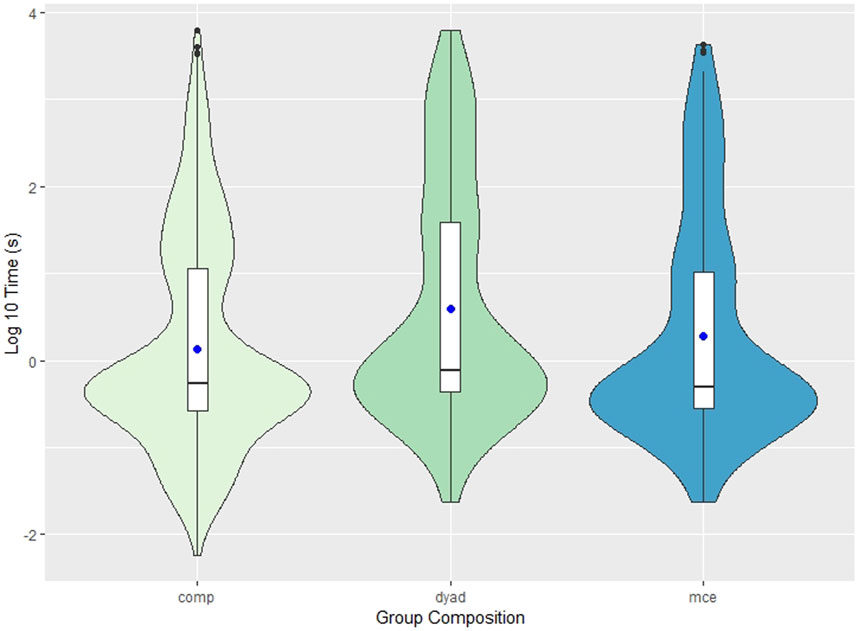
FIGURE 1. Violin plot depicting the inter-call interval of all three group compositions: competitive groups (comp), dyads, and escorted mother-calf pairs (mce). Due to outliers, the reported measurements were log transformed for better data visualization and easier interpretation. The y-axis in this figure represents the logarithm (base 10) of the measured inter-call intervals (seconds). The blue dot represents the mean values of each measurement. The internal box plot represents the median (centerline), interquartile range (box borders), and outliers (black dots). The surrounding kernel density plot shows the probability of distribution throughout the data set.
Spectral parameters
Table 2 shows the model-adjusted mean frequency values for all spectral parameters, and results of statistical comparisons across group compositions. All mean frequency values were highest in competitive groups. The fifth percentile frequency (a proxy for minimum frequency) had a mean of 384 Hz in dyads, 375 Hz in escorted mother-calf pairs, and 445 Hz in competitive groups (Figure 2). The mean peak frequency was 536 Hz, 544 Hz, and 657 Hz, while the mean ninety-fifth percentile frequency (a proxy for maximum frequency) was 1136 Hz, 1228, and 1276 Hz in dyads, escorted mother-calf pairs, and competitive groups, respectively.

TABLE 2. Emmeans results from the generalized linear mixed models, with the mean value of each acoustic parameter across group compositions presented in the first three columns and the pairwise comparisons in the last three columns. The odds ratios are the odds of parameter values being different between groups. A negative t-ratio indicates that parameter is more likely to have lower values in the first of the two group compositions listed. MCE: escorted mother-calf pairs; Comp: competitive groups.
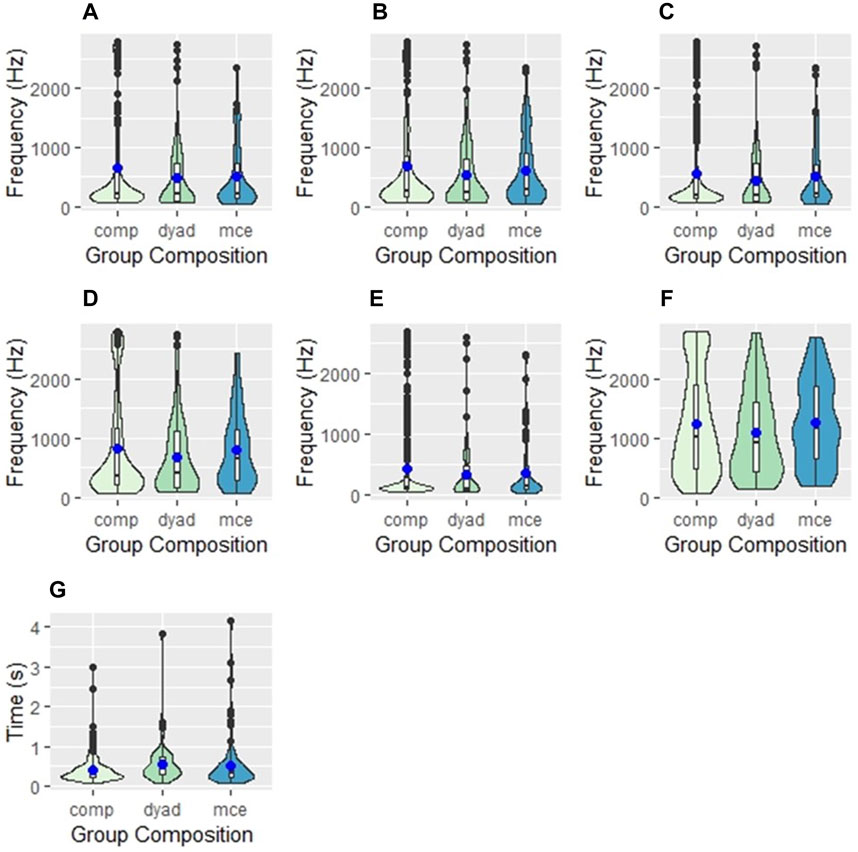
FIGURE 2. Violin plots showing the differences in (A) peak frequency, (B) center frequency, (C) first quartile frequency, (D) third quartile frequency, (E) fifth percentile frequency, (F) ninety-fifth percentile frequency, and (G) duration between three group compositions of humpback whales: competition groups (comp), dyads, and escorted mother-calf pairs (mce). The blue dot represents the mean values of each measurement. The internal box plot represents the median (centerline), interquartile range (box borders), and outliers (black dots). The surrounding kernel density plot shows the probability of distribution throughout the data set.
Additionally, the duration of calls was compared across all groups. Duration was longest in competitive groups, with calls averaging 0.501 s in length, followed by dyads (0.475 s) and escorted mother-calf pairs (0.461 s).
Discussion
Interest in the study of humpback whale calls has grown globally in recent years with research conducted in regions such as Australia (Dunlop et al., 2007; Rekdahl et al., 2013; Rekdahl et al., 2015; Dunlop, 2016; Dunlop, 2017; Cusano et al., 2020; Indeck et al., 2020; Recalde-Salas et al., 2020; Cusano et al., 2021), North America (Zoidis et al., 2008; Stimpert et al., 2011; Epp et al., 2021a; Fournet et al., 2015; Fournet M. et al., 2018), South America (Simão and Moreira, 2005; Oña et al., 2019), and Africa (Rekdahl et al., 2017). However, comparatively little work has been conducted to compare calling behavior across different group compositions using long-term datasets. This study is one of few from the Hawaiian breeding grounds to examine variations of humpback whale social calling behavior between competitive groups, dyads, and escorted mother-calf pairs using data from suction cup recording tags.
Temporal patterns
There are many possible factors that can affect the temporal patterns of humpback whale social calls. Two such factors we considered strongly when interpreting these results were the effect of arousal and motivational context of the signaler. These factors have previously been shown to affect calling behavior (August and Anderson, 1987; Morton, 1977; Cusano et al., 2020), with call rates increasing in high arousal contexts, such as those associated with mating behavior (e.g., red deer, Cervus elaphus, Clutton-Brock and Albon, 1979). The calling patterns we observed in competitive groups reflected the nature of these social interactions. Competitions can last for many hours (Tyack and Whitehead, 1983; Clapham et al., 1992; Herman et al., 2007) and can be energetically costly for the whales involved. Intense periods of male displays are often observed (e.g., head lunging, jaw claps, linear bubble trails, blocking), as well as more aggressive actions such as chases and body strikes (Baker and Herman, 1984; Herman et al., 2007). On the Hawaiian breeding grounds, these competitions have been observed to also include short periods of rest between aggressive displays (Herman et al., 2007). The inter-call intervals in competitive groups were shortest out of all compositions, with only a few periods of extended silence between calls. It is possible that short inter-call intervals occurred during periods of aggression, while periods of silence coincided with rest, although further observation would be necessary to confirm this. The pattern of frequent calling in competitive groups observed in this study may indicate that calling plays an important function in humpback whale competitions.
In contrast to competitive groups, escorted mother-calf pairs produced calls less frequently with longer inter-call intervals. The longer ICIs observed in escorted mother-calf pairs were somewhat expected and are likely explained by known mother-calf calling behavior (Indeck et al., 2022) and the group interactions observed during the tag deployments. Although escorts show a preference for females without a calf, as they typically have a greater reproductive potential than maternal females (Craig et al., 2003), they will also prospect among mothers for those that may be in post-partum estrus (Chittleborough, 1958; Pallin et al., 2018). The addition of one or more escorts to a mother-calf pair increases their energy expenditure (Craig et al., 2014) and could result in injuries to the calf or its separation from its mother (Smultea, 1994). As such, these pairs must balance communication with each other with the potential of being joined by escorts, which is believed to be reflected by lower call rates and levels in these groups (Videsen et al., 2017; Indeck et al., 2022). Nevertheless, these pairs have been found to increase calling rates during periods of increased separation as a means of maintaining acoustic contact while continuing to limit detectability by nearby whales (Indeck et al., 2022). Similar calling behavior is likely also useful during non-agonistic interactions with a single escort, as a means of minimizing the risk of mother-calf separation, as well as reducing the possibility of attracting additional escorts. This could explain the lower calling rates observed in escorted mother-calf pairs here. With the exception of one tag deployment during which increased calling was associated with aggressive behaviors between the mother and escort, MCE groups in this study predominantly displayed low intensity interactions accompanied by low call rates and longer inter-call intervals.
Of all the groups, dyads were expected to produce the fewest social calls, consistent with observations made in previous studies (Silber, 1986; Cusano et al., 2020). While these groups did produce the fewest calls out of the three groups, we did not find call rates (calls/hour/whale) to vary significantly between groups. The temporal pattern of call production in dyads was similar to escorted mother-calf pairs with longer inter-call intervals observed in these groups. On the Hawaiian breeding grounds, dyads are most often comprised of a male and female (Pack et al., 2012) that often spend extended periods resting below the surface with no signs of agonism (Jones, 2010). The results observed here may be partly due to a lack of agonism associated with these groups (Jones, 2010) or may reflect a reduced need for these whales to communicate acoustically when in close proximity (Cusano et al., 2020). Furthermore, the simple fact that there were fewer whales in these groups may have contributed to the longer inter-call intervals observed.
Call measurements
Competitive groups, which are the most aggressive in nature, exhibited the highest frequency calls on average. This may be due in part to an increased level of intensity expected from the agonistic context of these groups, which has previously been linked with higher frequency calls (Lemasson et al., 2012). Additionally, the contribution of aversive calls in competitive groups may contribute to the higher frequencies observed, considering that aversive calls are typically associated with higher frequencies (Morton, 1977, August and Anderson, 1987; Dunlop, 2017).
Escorted mother-calf pairs produced calls with frequencies between that of dyads and competitive groups for all parameters, except fifth percentile. The contribution of calf calls could potentially contribute to increasing the frequency levels of calls in these groups. To an extent, the minimum frequency of calls in mysticete whales is limited by animal size (May-Collado et al., 2007; Martin et al., 2017), with smaller animals possibly being incapable of producing the lowest frequency calls. It is possible that a balance between calf calls, which are generally produced at higher frequencies than those of adults (Zoidis et al., 2008; Indeck et al., 2020), and calls from the adults in these groups, contributed to the frequency values observed
Frequency measurements were relatively consistent with expected patterns, which was also true for observed call durations. High arousal during high intensity contexts (i.e., competitive groups) is frequently associated with longer duration calls (Lemasson et al., 2012). This pattern was observed here, with competitive groups producing the longest duration calls. However, dyads, which were not observed interacting aggressively, were expected to have the shortest call durations, which were instead exhibited by escorted mother-calf pairs. It is possible, however, that the duration of calls could have been affected by the physical attributes of the signaler (i.e., body size, age class, vocal ontogeny/calling experience; Martin et al., 2017), rather than the dynamics of the group.
Conclusion
Our study provides better understanding of social communication within humpback whale groups while also raising further questions that warrant investigation. For example, it would be useful to investigate how the position of a whale in a competitive group affects social signaling behavior. Furthermore, we still know little about how the sex of the signaler affects calling behavior. While the analysis of acoustic signaling in humpback whales remains difficult, studies like this one continue to increase our understanding of the social signaling behavior of this species and provide guidance for future studies.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study research protocols were reviewed and approved by the University of Hawai’i Institutional Animal Care and Use Committee (IACUC Protocol #11–1109–8,9,10).
Author contributions
JSC and MOL contributed to the conception and design of the study. AAP provided the permit under which the data collection was carried out and contributed ideas for statistical analysis. KLI assisted with data analysis in MATLAB. JSC wrote the initial draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Acknowledgments
We would like to thank the National Marine Sanctuary Foundation for partial funding that supported the purchase of one of the acoustic tags utilized for this project and we would like to acknowledge Eden Zang, Jessie Kittel, Anke Kügler, Dani Kleinhenz, Sara Wood, Ted Gruppenhof, Kiki Mann and Jason More (Hawaiian Islands Humpback Whale National Marine Sanctuary), whose field support provided the necessary data to execute this project. We would also like to acknowledge Elisa Girola and Maëlle Torterotot, who helped create, refine, and troubleshoot the MATLAB script for acoustic measurements. Data were collected under NOAA Scientific Research Permit #19655 and University of Hawai’i IACUC protocol 11–1109–8,9,10 to AAP.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frsen.2022.910455/full#supplementary-material
References
August, P. V., and Anderson, J. G. T. (1987). Mammal sounds and motivation-structural rules: A test of the hypothesis. J. Mammal. 68, 1. doi:10.2307/1381039
Baird, R. W., Mcsweeney, D. J.d, Bane, C., Barlow, J., Saldan, D. R., Antoine, L. R. K., et al. (2006). Killer whales in Hawaiian waters: Information on population identity and feeding habits. Pac. Sci. 60, 523–530. doi:10.1353/psc.2006.0024
Baker, C. S., and Herman, L. M. (1984). Aggressive behavior between humpback whales (Megaptera novaeangliae) wintering in Hawaiian waters. Can. J. Zool. 62, 1922–1937. doi:10.1139/z84-282
Briefer, E. F. (2012). Vocal expression of emotions in mammals: Mechanisms of production and evidence. J. Zool. 288, 1–20. doi:10.1111/j.1469-7998.2012.00920.x
Brooks, M. E., Kristensen, K., van Benthem, K. J., Magnusson, A., Berg, C. W., Nielsen, A., et al. (2017). Modeling zero-inflated count data with glmmTMB. BioRxiv, 132753. doi:10.1101/132753
Cerchio, S., and Dahlheim, M. (2001). Variation in feeding vocalizations of humpback whales Megaptera novaeangliae from southeast Alaska. Bioacoustics 11, 277–295. doi:10.1080/09524622.2001.9753468
Chittleborough, R. (1958). The breeding cycle of the female humpback whale, Megaptera nodosa (Bonnaterre). Mar. Freshw. Res. 9, 1–18. doi:10.1071/mf9580001
Clapham, P. J. (2000). “The humpback whale,” in Cetacean Societies: Field studies of dolphins and whales (Chicago: University of Chicago), 173–196.
Clapham, P. J., Palsbøll, P. J., Mattila, D. K., and Oswaldo, V. (1992). Composition and dynamics of humpback whale competitive groups in the West Indies. Behav. 122, 182–194. doi:10.1163/156853992x00507
Clark, C. W. (1983). “Acoustic communication and behavior of the southern right whale,” in Communication and behavior of whales (Boulder, CO).
Clutton-Brock, T. H., and Albon, S. D. (1979). The roaring of red deer and the evolution of honest advertisement. Behav. 69, 145–170. doi:10.1163/156853979x00449
Craig, A. S., Herman, L. M., Gabriele, C. M., and Pack, A. A. (2003). Migratory timing of humpback whales (Megaptera novaeangliae) in the central North Pacific varies with age, sex and reproductive status. Behav. 140, 981–1001. doi:10.1163/156853903322589605
Craig, A. S., Herman, L. M., Pack, A. A., and Waterman, J. O. (2014). Habitat segregation by female humpback whales in Hawaiian waters: Avoidance of males? Behav. 151, 613–631. doi:10.1163/1568539x-00003151
Cusano, D. A., Indeck, K. L., Noad, M. J., and Dunlop, R. A. (2020). Humpback whale (Megaptera novaeangliae) social call production reflects both motivational state and arousal. Bioacoustics 31, 1–24. doi:10.1080/09524622.2020.1858450
Cusano, D. A., Paton, D., Noad, M. J., and Dunlop, R. A. (2021). Socially complex breeding interactions in humpback whales are mediated using a complex acoustic repertoire. Front. Mar. Sci. 8. doi:10.3389/fmars.2021.665186
D'vincent, C. G., Nilson, R. M., and Hanna, R. E. (1985). Vocalization and coordinated feeding behavior of the humpback whale in southeastern Alaska. Sci. Rep. Whales Res. Inst. 36, 41–47.
Dunlop, R. A. (2016). Changes in vocal parameters with social context in humpback whales: Considering the effect of bystanders. Behav. Ecol. Sociobiol. 70, 857–870. doi:10.1007/s00265-016-2108-0
Dunlop, R. A. (2017). Potential motivational information encoded within humpback whale non-song vocal sounds. J. Acoust. Soc. Am. 141, 2204–2213. doi:10.1121/1.4978615
Dunlop, R. A., Cato, D. H., and Noad, M. J. (2008). Non-song acoustic communication in migrating humpback whales (Megaptera novaeangliae). Mar. Mammal Sci. 24, 613–629. doi:10.1111/j.1748-7692.2008.00208.x
Dunlop, R. A., Noad, M. J., Cato, D. H., and Stokes, D. (2007). The social vocalization repertoire of east Australian migrating humpback whales (Megaptera novaeangliae). J. Acoust. Soc. Am. 122, 2893–2905. doi:10.1121/1.2783115
Epp, M. V., Fournet, M. E. H., and Davoren, G. K. (2021a). Humpback whale call repertoire on a northeastern Newfoundland foraging ground. Mar. Mammal Sci. 38, 256–273. doi:10.1111/mms.12859
Epp, M. V., Fournet, M. E. H., Silber, G. K., and Davoren, G. K. (2021b). Allopatric humpback whales of differing generations share call types between foraging and wintering grounds. Sci. Rep. 11, 16297. doi:10.1038/s41598-021-95601-7
Feighny, J. A., Williamson, K. E., and Clarke, J. A. (2006). North American elk bugle vocalizations: Male and female bugle call structure and context. J. Mammal. 87, 1072–1077. doi:10.1644/06-mamm-a-079r2.1
Findlay, K. P., Seakamela, S. M., Meyer, M. A., Kirkman, S. P., Barendse, J., Cade, D. E., et al. (2017). Humpback whale "super-groups" - a novel low-latitude feeding behaviour of Southern Hemisphere humpback whales (Megaptera novaeangliae) in the Benguela Upwelling System. PLoS One 12, e0172002. doi:10.1371/journal.pone.0172002
Fischer, J., and Price, T. (2017). Meaning, intention, and inference in primate vocal communication. Neurosci. Biobehav. Rev. 82, 22–31. doi:10.1016/j.neubiorev.2016.10.014
Fournet, M. E. H., Gabriele, C. M., Culp, D. C., Sharpe, F., Mellinger, D. K., and Klinck, H. (2018a). Some things never change: Multi-decadal stability in humpback whale calling repertoire on southeast alaskan foraging grounds. Sci. Rep. 8, 13186. doi:10.1038/s41598-018-31527-x
Fournet, M. E. H., Jacobsen, L., Gabriele, C. M., Mellinger, D. K., and Klinck, H. (2018b). More of the same: Allopatric humpback whale populations share acoustic repertoire. PeerJ 6, e5365. doi:10.7717/peerj.5365
Fournet, M. E., Szabo, A., and Mellinger, D. K. (2015). Repertoire and classification of non-song calls in Southeast Alaskan humpback whales (Megaptera novaeangliae). J. Acoust. Soc. Am. 137, 1–10. doi:10.1121/1.4904504
Gros-Louis, J. J., Perry, S. E., Fichtel, C., Wikberg, E., Gilkenson, H., Wofsy, S., et al. (2008). Vocal repertoire of Cebus capucinus: Acoustic structure, context, and usage. Int. J. Primatol. 29, 641–670. doi:10.1007/s10764-008-9263-8
Harvey, W. R. (1960). Least-squares analysis of data with unequal subclass numbers. Washington, DC: Agricultural Research Service, United States Department of Agriculture.
Herman, E. Y. K., Herman, L. M., Pack, A. A., Marshall, G., Shepard, M. C., and Bakhtiari, M. (2007). When whales collide: Crittercam offers insight into the competitive behavior of humpback whales on their Hawaiian wintering grounds. Mar. Technol. Soc. J. 41, 35–43. doi:10.4031/002533207787441971
Herman, L. M. (2017). The multiple functions of male song within the humpback whale (Megaptera novaeangliae) mating system: Review, evaluation, and synthesis. Biol. Rev. 92, 1795–1818. doi:10.1111/brv.12309
Herman, L. M., and Antinoja, R. C. (1977). Humpback whales in the Hawaiian breeding waters: Population and pod characteristics. The Scientific Reports of the Whales Research Institute 29, 59–85.
Herman, L. M., Pack, A. A., Rose, K., Craig, A., Herman, E. Y., Hakala, S., et al. (2011). Resightings of humpback whales in Hawaiian waters over spans of 10–32 years: Site fidelity, sex ratios, calving rates, female demographics, and the dynamics of social and behavioral roles of individuals. Mar. Mammal Sci. 27, 736–768. doi:10.1111/j.1748-7692.2010.00441.x
Herman, L. M., and Tavolga, W. N. (1980). “The communication systems of cetaceans,” in Cetacean behavior: Mechanisms and functions (New York: Wiley Interscience), 149–209.
Indeck, K. L., Girola, E., Torterotot, M., Noad, M. J., and Dunlop, R. A. (2020). Adult female-calf acoustic communication signals in migrating east Australian humpback whales. Bioacoustics 30, 341–365. doi:10.1080/09524622.2020.1742204
Indeck, K. L., Noad, M. J., and Dunlop, R. A. (2022). Humpback whale adult females and calves balance acoustic contact with vocal crypsis during periods of increased separation. Ecol. Evol. 12, e8604. doi:10.1002/ece3.8604
Indeck, K. L., Noad, M. J., and Dunlop, R. A. (2021). The conspecific avoidance strategies of adult female-calf humpback whales. Behav. Ecol. 32, 845–855. doi:10.1093/beheco/arab031
Jones, M. E. (2010). Female humpback whale (Megaptera novaeangliae) reproductive class and male-female interactions during the breeding season PhD thesis. Keene (NH): Antioch University New England.
Katona, S., Baxter, B., Brazier, O., Kraus, S., Perkins, J., and Whitehead, H. (1979). “Identification of humpback whales by fluke photographs,” in Behavior of marine animals: Current Perspectives in Research Editor H. E. Winn, and B. L. Olla (New York: Plenumr) Vol. 3, 33–44.
Lemasson, A., Remeuf, K., Rossard, A., and Zimmermann, E. (2012). Cross-taxa similarities in affect-induced changes of vocal behavior and voice in arboreal monkeys. PLoS One 7, e45106. doi:10.1371/journal.pone.0045106
Lenth, R. (2022). emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.8.0. Available at: https://CRAN.R-project.org/package=emmeans.
May-Collado, L. J., Agnarsson, I., and Wartzok, D. (2007). Reexamining the relationship between body size and tonal signals frequency in whales: A comparative approach using a novel phylogeny. Mar. Mamm. Sci. 23, 524–552. doi:10.1111/j.1748-7692.2007.02250.x
Martin, K., Tucker, M. A., and Rogers, T. L. (2017). Does size matter? Examining the drivers of mammalian vocalizations. Evolution 71, 249–260. doi:10.1111/evo.13128
Mobley, J. R., and Herman, L. M. (1985). Transience of social affiliations among humpback whales (Megaptera novaeangliae) on the Hawaiian wintering grounds. Can. J. Zool. 63, 762–772. doi:10.1139/z85-111
Morton, E. S. (1977). On the occurrence and significance of motivation-structural rules in some bird and mammal sounds. Am. Nat. 111, 855–869. doi:10.1086/283219
Oña, J., Duque, E., Garland, E. C., Seger, K., Narváez, M., Maldonado, J., et al. (2019). A giant’s dance: Underwater social and vocal behavior of humpback whales (megaptera novaeangliae) recorded on the northern coast of Ecuador. Aquat. Mamm. 45, 456–464. doi:10.1578/am.45.4.2019.456
Pack, A. A., Herman, L. M., Spitz, S. S., Craig, A. S., Hakala, S., Deakos, M. H., et al. (2012). Size-assortative pairing and discrimination of potential mates by humpback whales in the Hawaiian breeding grounds. Anim. Behav. 84, 983–993. doi:10.1016/j.anbehav.2012.07.024
Pack, A. A., Waterman, J. O., and Craig, A. S. (2022). Diurnal increases in depths of humpback whale (Megaptera novaeangliae) mother‐calf pods off West Maui, Hawaiʻi: A response to vessels? Mar. Mammal Sci., 1–17. doi:10.1111/mms.12926
Palanca, M. (2021). Humpback whale (Megaptera novaeangliae) social calls in the Southeast Pacific population: Context, diversity, and call bouts analysis. Valencia, Spain: Master of Science, University of Bonn.
Pallin, L. J., Baker, C. S., Steel, D., Kellar, N. M., Robbins, J., Johnston, D. W., et al. (2018). High pregnancy rates in humpback whales ( Megaptera novaeangliae ) around the Western Antarctic Peninsula, evidence of a rapidly growing population. R. Soc. Open Sci. 5, 180017. doi:10.1098/rsos.180017
Parsons, E. C. M., Wright, A. J., and Gore, M. A. (2008). The nature of humpback whale (Megaptera novaeangliae) song. J. Mar. Animals Their Ecol. 1.
Payne, R. S., and McVay, S. (1971). Songs of humpback whales. Science 173, 585–597. doi:10.1126/science.173.3997.585
Recalde-Salas, A., Erbe, C., Salgado Kent, C., and Parsons, M. (2020). Non-song vocalizations of humpback whales in Western Australia. Front. Mar. Sci. 7. doi:10.3389/fmars.2020.00141
Rekdahl, M. L., Dunlop, R. A., Goldizen, A. W., Garland, E. C., Biassoni, N., Miller, P., et al. (2015). Non-song social call bouts of migrating humpback whales. J. Acoust. Soc. Am. 137, 3042–3053. doi:10.1121/1.4921280
Rekdahl, M. L., Dunlop, R. A., Noad, M. J., and Goldizen, A. W. (2013). Temporal stability and change in the social call repertoire of migrating humpback whales. J. Acoust. Soc. Am. 133, 1785–1795. doi:10.1121/1.4789941
Rekdahl, M., Tisch, C., Cerchio, S., and Rosenbaum, H. (2017). Common nonsong social calls of humpback whales (Megaptera novaeangliae) recorded off northern Angola, southern Africa. Mar. Mamm. Sci. 33, 365–375. doi:10.1111/mms.12355
Seger, K. (2016). Ambient acoustic environments and cetacean signals: Baseline studies from humpback whale and gray whale breeding grounds. Doctorate of philosophy in oceanography dissertation. San Diego: University of California.
Silber, G. K. (1986). The relationship of social vocalizations to surface behavior and aggression in the Hawaiian humpback whale (Megaptera-novaeangliae). Can. J. Zool. 64, 2075–2080. doi:10.1139/z86-316
Simão, S. M., and Moreira, S. (2005). Vocalizations of a female humpback whale in Arraial do Cabo (RJ, Brazil). Mar. Mamm. Sci. 21, 150–153. doi:10.1111/j.1748-7692.2005.tb01215.x
Smith, J. N., Goldizen, A. W., Dunlop, R. A., and Noad, M. J. (2008). Songs of male humpback whales, Megaptera novaeangliae, are involved in intersexual interactions. Anim. Behav. 76, 467–477. doi:10.1016/j.anbehav.2008.02.013
Smultea, M. A. (1994). Segregation by humpback whale (Megaptera novaeangliae) cows with a calf in coastal habitat near the island of Hawaii. Can. J. Zool. 72, 805–811. doi:10.1139/z94-109
Stimpert, A. K., Au, W. W., Parks, S. E., Hurst, T., and Wiley, D. N. (2011). Common humpback whale (Megaptera novaeangliae) sound types for passive acoustic monitoring. J. Acoust. Soc. Am. 129, 476–482. doi:10.1121/1.3504708
Stimpert, A. K., Mattila, D., Nosal, E. M., and Au, W. W. L. (2012). Tagging young humpback whale calves: Methodology and diving behavior. Endanger. Species Res. 19, 11–17. doi:10.3354/esr00456
The Cornell Laboratory of Ornithology (2019). Raven Pro: Interactive sound analysis software 1.6. 1 ed. Ithaca, New York: The Cornell Lab of Ornithology.
Tyack, P., and Whitehead, H. (1983). Male competition in large groups of wintering humpback whales. Behav. 83, 132–154. doi:10.1163/156853982x00067
Videsen, S. K. A., Bejder, L., Johnson, M., Madsen, P. T., and Goldbogen, J. (2017). High suckling rates and acoustic crypsis of humpback whale neonates maximise potential for mother–calf energy transfer. Funct. Ecol. 31, 1561–1573. doi:10.1111/1365-2435.12871
Wild, L. A., and Gabriele, C. M. (2014). Putative contact calls made by humpback whales (Megaptera novaeangliae) in Southeastern Alaska. Can. Acoust. 42, 10.
Williamson, M. J., Kavanagh, A. S., Noad, M. J., Kniest, E., and Dunlop, R. A. (2016). The effect of close approaches for tagging activities by small research vessels on the behavior of humpback whales (Megaptera novaeangliae). Mar. Mamm. Sci. 32, 1234–1253. doi:10.1111/mms.12324
Keywords: humpback whale, social calls, vocal communication, group composition, acoustic tag, Hawaiian islands
Citation: Carvalho J, Lammers MO, Indeck KL, Pack AA and Castilho R (2022) Comparing the social signaling behavior of humpback whales in three group types on the Hawaiian breeding grounds using acoustic tags. Front. Remote Sens. 3:910455. doi: 10.3389/frsen.2022.910455
Received: 01 April 2022; Accepted: 01 August 2022;
Published: 30 August 2022.
Edited by:
DelWayne Roger Bohnenstiehl, North Carolina State University, United StatesReviewed by:
Kerri D. Seger, Applied Ocean Sciences, United StatesGail K. Davoren, University of Manitoba, Canada
Copyright © 2022 Carvalho, Lammers, Indeck, Pack and Castilho. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jessica Carvalho, amNhcnZhbGhvLmRoc0BnbWFpbC5jb20=
 Jessica Carvalho
Jessica Carvalho Marc O. Lammers
Marc O. Lammers Katherine L. Indeck
Katherine L. Indeck Adam A. Pack
Adam A. Pack Rita Castilho
Rita Castilho