- Department Life Science Systems, Plant Biodiversity Research, Technical University of Munich, Freising, Germany
Projects promoting bees in urban areas are initiated in cities around the world but evidence-based conservation concepts at a city-wide scale are scarce. We developed a holistic approach for assessment of bee and flowering plant diversity in a medium-sized city. In addition to standard mapping approaches in bee hotspots, we initiated citizen science projects for participative urban bee research to be able to collect comprehensive bee data across the entire city. We identified 22 hotspots of bee diversity, analyzed connectivity between those hotspots and evaluated the impact of flower patches planted in collaboration with the municipal gardens department as stepping stones for oligolectic bee species throughout the city. Participation by urban citizens in bee identification trainings was high (c. 630 persons) but their subsequent contribution through observation reports was relatively low (1,165 records by 140 observers). However, we identified a total of 139 bee taxa, seven of them only discovered by citizen scientists. Total species richness was higher in extensively managed orchards than in semi-natural and wasteland areas. Half of the stepping stone flower patches were occupied by the target oligolectic bee species in the year of planting. After 3 years, all but two species could be confirmed. We suggest a 5-step concept for bee management in cities: (1) identification of bee hotspots combined with standardized surveys, especially of rare species; (2) training of citizen scientists at two different levels for comprehensive surveys in all parts of the city: (a) half-day introductions to wild bee diversity, ecology and conservation in order to create more awareness and (b) 2-weeks workshops for in-depth training of a small number of dedicated citizen scientists; (3) extensive management of existing habitats and special conservation programs for very rare species; (4) creation of high-value habitats which take into account the varied resource needs of bees within flight ranges of only a few hundred meters; (5) creation of stepping stone habitats as floral and nesting resources, integrating educative and participative aspects.
1 Introduction
Global insect, pollinator and wild bee declines have received increased research attention in the last years (Hallmann et al., 2017; Powney et al., 2019; Zattara and Aizen, 2021). Habitat loss and changes in habitat quality were identified as main drivers for this decline (Potts et al., 2010; Sánchez-Bayo and Wyckhuys, 2019; Wagner et al., 2021). In light of global urban expansion (Seto et al., 2012; van Vliet, 2019), understanding the effects of urbanization on wild bee communities is highly relevant. The growing body of research on the topic indicates a loss of bee diversity with increased urbanization (Cardoso and Gonçalves, 2018). An increase of impervious surfaces (Geslin et al., 2016), fragmentation (Burdine and McCluney, 2019), and parasitism (Theodorou et al., 2016) have been identified as factors negatively impacting wild bees in urban environments. At the same time, cities have been shown to harbor diverse wild bee communities (Baldock, 2020; Theodorou et al., 2020) and many studies have identified hotspots and conservation potential in a range of urban habitat types and structures: community and residential gardens (Baldock et al., 2019; Felderhoff et al., 2022), urban parks (Banaszak-Cibicka et al., 2018; Daniels et al., 2020), urban grasslands (Buchholz et al., 2020), botanical gardens (Hofmann et al., 2018), gravel pits (Hofmann and Fleischmann, 2020), wastelands (Fischer et al., 2016; Twerd and Banaszak-Cibicka, 2019; Vereecken et al., 2021), green roofs (Kratschmer et al., 2018), flower strips (Blackmore and Goulson, 2014; Hofmann and Renner, 2020; Weweler et al., 2022), urban trees (Hausmann et al., 2016; Somme et al., 2016), roadsides, railway and power line corridors, and riparian corridors (Twerd et al., 2021; Villalta et al., 2021; Zhang et al., 2022).
The explanation for these seemingly contradictory findings might be that urbanization favors some functional groups of bees while others cannot survive in cities, leading to changes in patterns of functional diversity. There seems to be a general tendency for urbanization to favor cavity nesting, generalist and smaller sized species (e.g., Buchholz and Egerer, 2020; Ayers and Rehan, 2021; Fauviau et al., 2022). This would mean that although cities can be important strongholds for a large number of bee species, they might not be suitable habitats for the more specialized and larger bee species.
One of the biggest problems in our current biodiversity crisis is the lack of awareness and the increasing emotional distance of a large proportion of the urban population to nature and wild organisms. In order to slow down the loss of species, it is crucial to raise general awareness and knowledge about insects and other neglected groups (Wilson et al., 2017; Drossart and Gérard, 2020; Hall and Martins, 2020; Harvey et al., 2020; Wagner et al., 2021). In our current situation, citizen science projects, originally implemented mainly for conspicuous and easy to identify taxa like birds and mammals, could play an important role also in the conservation of smaller and less popular organisms. A number of community and citizen science projects for wild bees have been developed in recent years to carry out species inventories (Wilson et al., 2020; Flaminio et al., 2021; Vereecken et al., 2021), study bee-plant interactions (Bloom and Crowder, 2020) and nesting ecology (Lye et al., 2012; Graham et al., 2014; Noël et al., 2021). Overall, evaluation of data quality, educational impact and motivation of participants revealed that not only data of reasonable quality but also a strong educational impact can be achieved (Toomey and Domroese, 2013; van der Wal et al., 2015; Falk et al., 2019; Mason and Arathi, 2019; Christ et al., 2022). Many of these projects are carried out in cities, where outreach potential is particularly high.
Even though the current state of research suggests urban habitats could be important for bee conservation, evidence-based concepts at a city-wide scale are still rare (but see www.bienenstadt-braunschweig.de). We developed a holistic approach for the assessment of bee diversity and distribution and habitat management in Freising, a medium-sized German city. We (1) explored possibilities of a photography-based survey approach combining systematic specialist surveys with citizen scientist surveys; (2) analyzed wild bee taxon richness and community composition in sites representative of different habitat types and management intensities, and (3) analyzed connectivity between urban bee hotspots. We hypothesized that (a) the river dike, a semi-natural dry grassland corridor traversing the city, is an important connection to more distant nature reserves and source area for bees colonizing the city. This should be reflected in a higher species number and more oligolectic species compared to the rest of the city; (b) the city center with very few green patches and a high proportion of impervious surfaces constitutes a colonization barrier between the southern part of the city (including the river dike) and habitats in the rest of Freising. This should result in significantly reduced species numbers in the northern part of the city; (c) stepping stone flower patches allow oligolectic bees to cross unsuitable areas of the city and colonize the more isolated patches of suitable habitat.
2 Materials and methods
The study was carried out in the city of Freising in southern Bavaria, Germany, which has a total area of c. 89 km2 and a population of about 50 000 inhabitants (www.kreis-freising.de). The climate is temperate with annual rainfall of 806.21 mm and temperatures ranging from −14.13°C to +33.46°C (long-term average based on the values of the years 2012 to 2021, www.wetter-by.de). For additional information about the city and its location, see Supplementary Data 1, Supplementary Figures 1, 2. A total of 521 wild bee species have been recorded in Bavaria, and c. 300 species in the administrative district of Freising (Bayerisches Landesamt für Umwelt, 2001, 2021). The main semi-natural habitat types in the city are lawn-dominated public parks, rivers and smaller streams with lines of trees. Less common but more relevant for bees are meadow orchards, wastelands and the semi-natural river dikes.
We first identified the potentially most important habitat types for wild bees in the city: flower-rich meadow orchards and wastelands as well as a river dike mostly covered by dry grassland which traverses the city and forms a semi-natural corridor connecting Freising and several dry grassland nature reserves in the region. We performed systematic surveys of wild bee and flowering plant diversity in these habitats using standard mapping approaches. To be able to collect comprehensive bee data across the entire city, we initiated a citizen science project for participative urban bee research. Based on the results of the systematic surveys and the citizen science data, we analyzed the bee communities of each of the main habitat types as well as the effect of different management intensities or succession stages on bee diversity. We analyzed connectivity between wild bee hotspots and evaluated the impact of flower patches planted as stepping stones for oligolectic bee species in collaboration with the municipal gardens department.
2.1 Study site
2.1.1 Surveyed habitats
Systematic surveys of wild bees and flowering plants were performed in 22 sites representative of different management types or succession stages of the three habitat types “river dikes,” “meadow orchards” and “wastelands.” The total surface area of the surveyed river dikes is 16.25 ha (Figure 1, sites D01–D03; Figures 2A–C) with a total length of 5.6 km on both sides of a 4.3 km long section of the Isar river. We divided the dike area in three different patches with regards to the mowing time, the western section is mown in September, the south-eastern section is mown in August/September, and the north-eastern section is mown in July. For the meadow orchard habitat, we surveyed a total area of 8.14 ha divided between five orchards of 0.8–4.25 ha (Figure 1, sites M01–M05; Figures 2D–F), each with 50–216 fruit trees of up of ten different species ranging in age between 10 to >50 years. The meadow orchards have been subject to different management types and intensity ranging from fallow to grazing, mowing and multiple mulching per season. The total wasteland area surveyed was 9.37 ha, divided between 14 plots ranging from 0.09–2.08 ha (Figure 1, sites W01–W14; Figures 2G–I). We classified the wastelands into different succession stages according to the proportion of bare ground, herb layer, shrub layer and tree layer on each site (see Supplementary Table 1). Bare ground was most prominent on early succession stage wastelands but also occurred locally on the extensively managed meadow orchards (fallow, grazed, and mixed), and on the river dikes. In each of the studied sites, we mapped all insect-pollinated flowering plant species in the herb-, shrub, and tree layer. The surveys took place over the entire season and stopped when the sites had been mown.
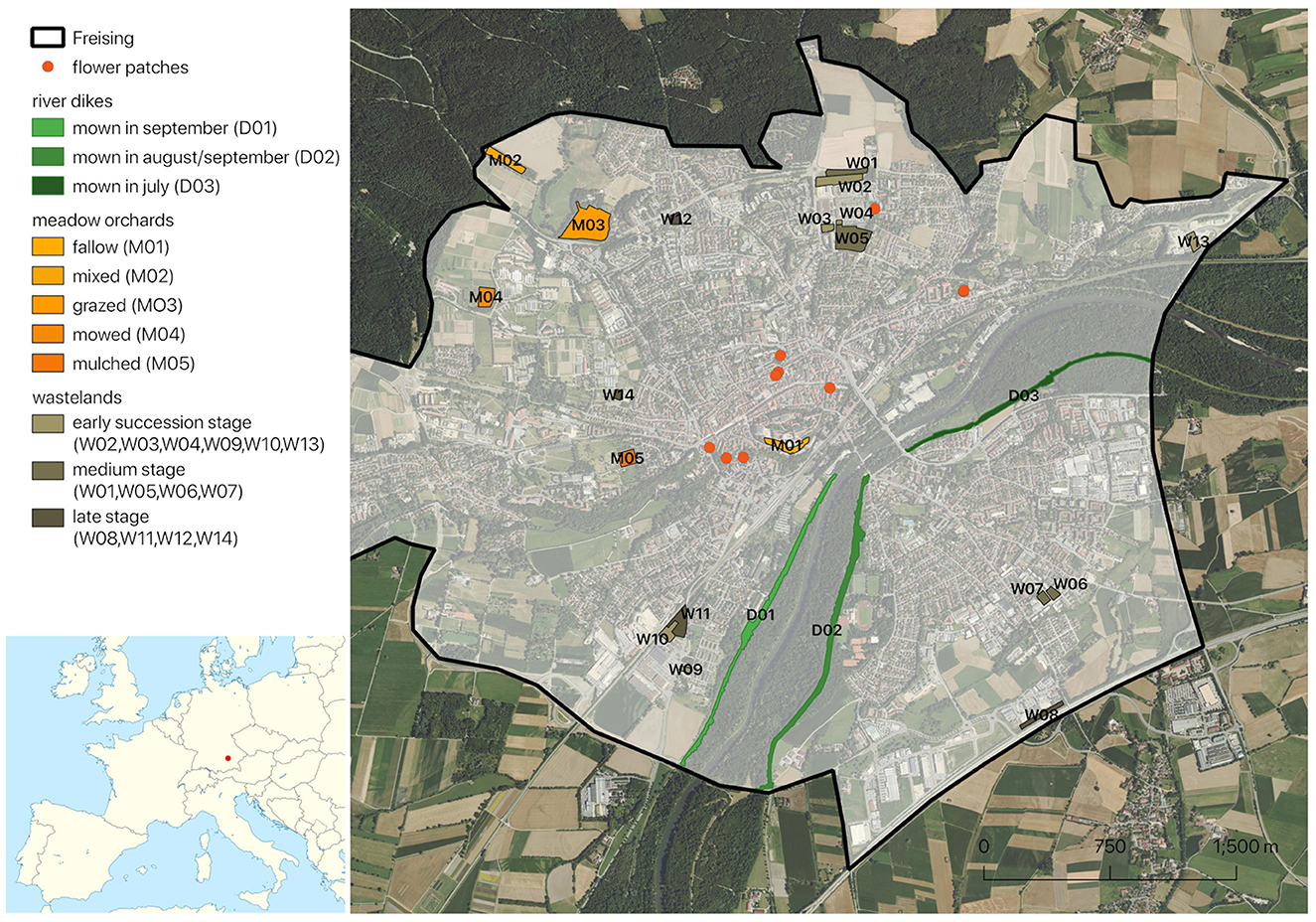
Figure 1. Location of Freising in Europe (inset) and distribution of bee rich habitats and stepping-stone flower patches in the city of Freising [basemaps: Bayerische Vermessungsverwaltung, 2023 (main map) & Wikimedia (inset)].

Figure 2. Bee habitats in Freising: (A) river dike mown in September; (B) river dike mown in August/September; (C) river dike mown in July; (D) meadow orchard fallow; (E) meadow orchard grazed; (F) meadow orchard mulched; (G) wasteland early succession stage; (H) wasteland medium succession stage; (I) wasteland late succession stage; (J) flower patch with Hesperis matronalis; (K) flower patch with Reseda lutea; (L) flower patch with Echium vulgare [(A–C) © RR, (D–F) © IW, (G–I) © SR, (J–L) © JW].
2.1.2 Stepping-stone flower patches
In 2019, we planted in collaboration with the municipal gardeners ten flower patches as stepping-stones between flower-rich parts of the city (Figure 1, orange dots; Figures 2J–L). In each patch, we planted a minimum of 30 individuals of a plant species chosen to provide pollen and nectar for specific oligolectic bee species: several bellflower species (Campanula persicifolia, C. rotundifolia, C. rapunculoides, C. trachelium, C. latifolia var. macrantha, and C. poscharskyana) for the rampion scissor bee, Chelostoma rapunculi; Hesperis matronalis to attract the threatened mason bee species Osmia brevicornis; Lysimachia punctata for the loosestrife oil bees, Macropis europaea and M. fulvipes; Reseda lutea for the large yellow-face bee, Hylaeus signatus; Lathyrus latifolius for the leaf cutter bee Megachile ericetorum; Stachys byzantina to attract the wool carder bee, Anthidium manicatum; Echium vulgare for the mason bee species Osmia (Hoplitis) adunca; Lythrum salicaria for the blunthorn bee, Melitta nigricans; Knautia arvensis for the sand bee Andrena hattorfiana; and Cichorium intybus for the pantaloon bee, Dasypoda hirtipes. The accompanying information boards provide photos and a few interesting details on each plant species and the respective target bee species.
2.2 Bee survey
2.2.1 Standardized surveys
Bee records were collected between 2017 and 2021 with most observations between April and August 2018. Systematic bee surveys were performed between 9 AM and 6 PM and only in dry, sunny weather with temperatures over 12°C. Additional observation time of 1 h in September was dedicated to the late flowering plant species Hedera helix to obtain occurrence data of the ivy bee, Colletes hederae, which is specialized on the flowers of ivy, which open in very late summer. Pollen and nectar offering plants in the herb and shrub layer were systematically observed for periods of 10–15 min to assess the visiting bees. We additionally identified potential nesting sites to assess the presence of nesting bees. In the meadow orchards, we dedicated an additional 101 h and 40 min of systematic observations on the following fruit tree species: plum (Prunus domestica), cherry plum (Prunus cerasifera), sweet cherry (Prunus avium), sour cherry (Prunus cerasus), pear (Pyrus communis, and apple (Malus domestica) (see Weissmann et al., 2021). Occurrence data for the ten selected oligolectic bee species was collected on existing and newly established flower patches throughout the city in 2019 and 2021.
Wild bees were photographed directly in the field or caught and cooled down on a cold pack to take high-resolution pictures for later identification (a specific permit to catch bees had been granted by the local conservation authorities at the Regierung von Oberbayern). In order to identify bees to species level from photographs, we developed a field identification guide (Weissmann and Schaefer, 2022). In this guide, species that are not distinguishable in the field (e.g., Colletes daviesanus, Colletes similis, and Colletes fodiens) are treated as species groups, a concept we also adopted for our surveys. For each recorded bee taxon and habitat type, at least one photograph has been uploaded on the iNaturalist platform, where we set up a specific project for wild bee observations in Freising (https://www.inaturalist.org/projects/wildbienen-in-freising-urban-pollinators-bees-in-freising). On this platform, the photographs are accessible by everybody and identifications can be checked and confirmed or updated.
2.2.2 Citizen scientist surveys
For the citizen science project, we set up a project webpage and directly contacted conservation NGOs, community garden groups, allotment gardens, beekeeper associations, schools and kindergartens. We offered public talks and guided walks advertised on the project website and through local media, as well as through personal visits in people's gardens to give an introduction to the most common bee genera and species, their morphology, behavior, nesting sites, and host plants, and identification methods. In 2018 and 2019, we offered eleven guided walks, seven talks and information events, as well as visits to six classes (sixth and eighth grade) in three schools and two elementary school children's groups (Supplementary Table 2). Eleven articles were published in local media about the project. Citizen scientists contributed observations through forms on the project website and via the iNaturalist project page. We did not perform specific surveys of citizen scientists' motivation or background.
2.3 Trait analyses
To characterize and compare the bee communities of the different parts of the city, we analyzed the following traits: threat level (Germany and Bavaria), life form, nesting type, nesting resources, lecty, female body size, preferred host plants (oligolectic bees), host species (parasitic bees). The trait information was compiled from Westrich (2018) for life form, nesting type, nesting resources, lecty, pollen sources of oligolectic species, hosts of parasitic species. Information on threat level was compiled from Bayerisches Landesamt für Umwelt (2021) for Bavaria and Westrich et al. (2011) for Germany. For species that are not distinguishable in the field, we chose a conservative approach and used the trait values of the most common, widespread and least threatened species in the species group based on Weissmann and Schaefer (2022) (e.g., Colletes daviesanus as representative of the species group C. daviesanus, C. similis, C. fodiens). In a few cases, frequency, distribution and threat level did not differ. Here, we chose the species based on the alphabetic order (e.g., Lasioglossum albipes for L. albipes/L. calceatum). To determine female body size, we calculated the average of the size range given in Dathe et al. (2016), Weissmann and Schaefer (2022) for Hylaeus, and Martin (2023) for Bombus (workers), and Psithyrus (queens). For parasitic species, we assigned the nesting type of the main host(s) according to Westrich (2018) (see Supplementary Data 2).
2.4 Statistical analyses
All statistical analyses were performed in R version 4.2.3 (R Core Team, 2023) and the packages vegan v.2.6-4 (Oksanen et al., 2022), VennDiagram v.1.7.3 (Chen, 2022), tidyverse v. 2.0.0 (Wickham et al., 2019), reshape 2 v.1.4.4 (Wickham, 2007) and patchwork v.1.1.2 (Pedersen, 2022) (see Supplementary Data 6 for the code for the analyses and the datasets).
2.4.1 Bee taxa richness
For each site, the cumulative wild bee taxa richness and the flowering plant species richness were summarized from all observation periods (22 sites, 586 h of total observation time). To study the effects of the site characteristics “species diversity of flowering plants,” “habitat type,” and “distance from the river dikes” on wild bee diversity, we performed a poisson regression model (function glm). To account for different mapping intensity on each site, we included total observation time in hours as offset (Zuur et al., 2009).
2.4.2 Bee community composition
We compiled the wild bee taxa list for each succession stage from the fourteen sites of the habitat type “wasteland” to obtain cumulative wild bee taxa lists for each habitat and management type (three management types for the habitat type “river dike,” five management types for the habitat type “meadow orchard,” three succession stages for the habitat type “wasteland;” 586 h of total observation time). We applied Non-metric Multi-dimensional Scaling (NMDS) to assess similarities in wild bee community composition between habitat type. To test whether there is a relationship between habitat type and wild bee community composition, we performed an Analysis of similarities (ANOSIM) and calculated the Sörensen index IA to evaluate the similarity between bee communities of the different habitat types with IA = (2g/(a+b)) * 100 (g = total number of bee taxa occurring in habitat type A and B; a = total number of bee taxa occurring in habitat type A; b = total number of bee taxa occurring in habitat type B).
2.4.3 Bee taxa traits
To test whether the number of bee species per trait (sociality, nesting, lecty) is similarly distributed across all habitat types, we performed Pearson's Chi-squared test (function chisq.test). Because some of the counts were less than five in the sociality and lecty tables, we confirmed that Fisher's Exact Test for Count Data (function fisher.test) gave similar results. To test significant differences in the sizes of bee species occurring in the different habitat types, we performed a Kruskal-Wallis rank sum test (function Kruskal.test) for each habitat type because the assumptions for an ANOVA were not met.
3 Results
3.1 Involvement of citizen scientists
Participation by citizen scientists in public talks, guided walks, and bee identification trainings was high (c. 630 persons, see Supplementary Table 2) but their subsequent contribution through observation reports was much lower (c. 1,165 records). A total of 140 observers (excluding the authors) contributed observations to our iNaturalist project but only six of them contributed more than thirty observations.
3.2 Bee fauna of Freising
3.2.1 Bee taxa diversity
We identified 139 wild bee taxa in the city of Freising in 586 h of systematic observation plus an unknown amount of time for the non-standardized citizen scientist observations all over the city. The most diverse habitats were the meadow orchards with 98 taxa, followed by wastelands with 80 taxa, and then the river dikes with 77 taxa (see Figure 3, Supplementary Table 3, and Supplementary Data 2 for an extended taxon list). The two species Andrena clarkella and Osmia brevicornis have been reported but the photographs are not sufficient for unequivocal identification. Seven bee species have been observed and well-documented by citizen scientists only but not during the standardized surveys in the city (Andrena ventralis, Coelioxys afra, Epeoloides coecutiens, Melitta leporina, Nomada flavopicta, Pseudoanthidium nanum and Rophites quinquespinosus) plus two remarkable species in the administrative district of Freising outside the city (Melitta tricincta and Osmia spinulosa) (see Supplementary Data 3).

Figure 3. Some of the bee species observed in Freising: (A) Andrena agilissima on Sinapis arvensis; (B) Andrena hattorfiana on Knautia arvensis; (C) Melitta haemorrhoidalis on Campanula spec.; (D) Melitta nigricans on Lythrum salicaria; (E) Chelostoma campanularum/distinctum; (F) Megachile ericetorum on Lathyrus latifolius; (G) Dasypoda hirtipes on Cichorium intybus; (H) Colletes hederae on Hedera helix; (I) Megachile nigriventris on Baptisia australis; (J) Megachile pilidens; (K) Eucera nigrescens on Lathyrus pratensis; (L) Anthophora furcata on Nepeta grandiflora; (M) Halictus subauratus on Ranunculus spec.; (N) Anthidium punctatum on Lotus corniculatus; (O) Osmia caerulescens on Onobrychis viciifolia; (P) Anthidium oblongatum on Lotus corniculatus; (Q) Hylaeus nigritus on Leucanthemum vulgare; (R) Stelis punctulatissima on Calamintha nepeta; (S) Bombus subterraneus; (T) Bombus humilis on Calamintha nepeta; (U) Nomada sexfasciata; (V) Coelioxys cf. inermis; (W) Anthidium manicatum on Stachys byzantina; (X) Megachile cf. pilidens; (Y) Osmia leucomelana nesting in Rubus sect. Rubus [(B, N, V) © IW, (J) © SR, (S) © RR, (A, C–I, K–M, O–R, T, U, W–Y) © JW].
The overall bee community of Freising comprises at least 27 genera. The largest genera in the city are Andrena (24 taxa), Bombus/Psithyrus (18 taxa), Nomada (15 taxa), and Hylaeus (12 taxa). In the mid-range genera, Osmia/Hoplitis (8 taxa) is followed by Lasioglossum (7 taxa), Megachile (7 taxa), and Halictus (6 taxa). The remaining genera are only represented by five or fewer taxa. When compared to the other habitat types, the meadow orchards have the highest number of taxa of the genera Andrena, Nomada, Lasioglossum, Osmia, Anthophora, and Chelostoma. The genera Stelis, Panurgus, Anthidiellum, and Xylocopa were found in meadow orchards and other parts of the city (e.g., some private gardens) but not on the river dikes and in the wasteland patches. The river dikes have the highest richness of the genera Bombus and Sphecodes. The genus Megachile has the highest richness in wastelands and the parasitic genus Epeolus was found exclusively in wastelands (Supplementary Figure 3).
3.2.2 Bee trait diversity
With 59% of the taxa (82 taxa), the majority of Freising's bees is solitary. Much less common are social species (mostly Bombus) with 13.7% (19 taxa), and 2.2% (3 taxa) of the species have a communal life form (Figure 4A). No significant differences were detected across site types by Fisher's Exact Test for Count Data (P-value = 0.95).
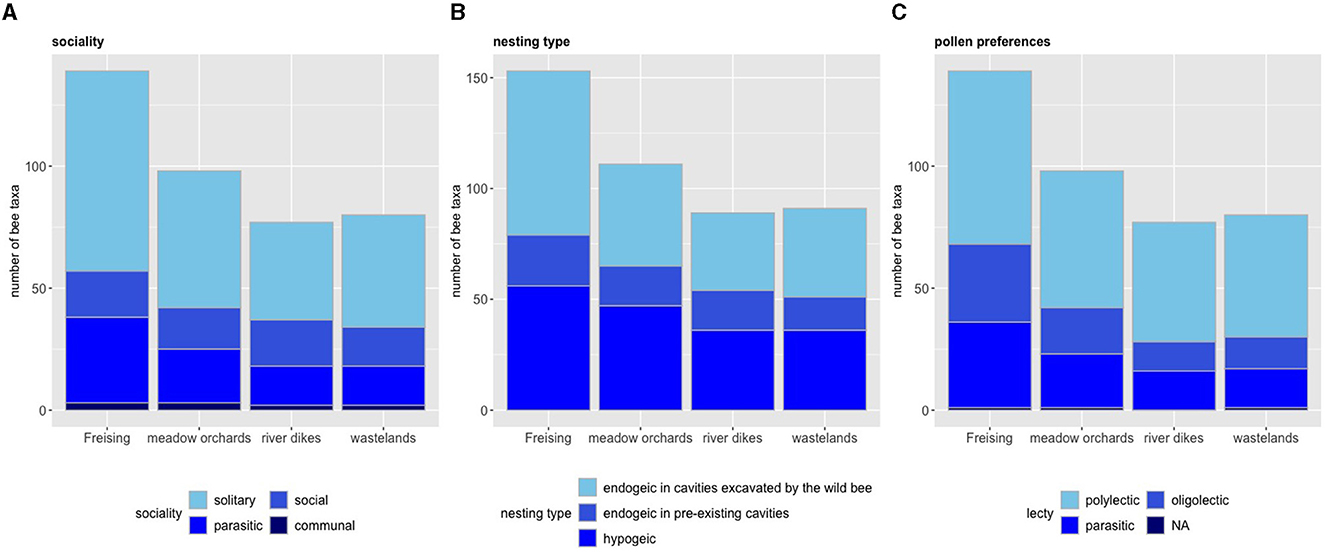
Figure 4. Sociality (A), nesting type (B), and pollen preferences (C) of bee taxa according to habitat type. Total bee taxon richness = 139 taxa, total observation time = 586 h of systematic observation plus additional non-standardized observations all over the city.
Regarding the nesting sites, 69.8% nest in the ground (97 taxa), while 40.3% have their nests above-ground (56 taxa) [for parasitic species, we assigned the nesting type of the main host(s)]. Some species are flexible in their nesting behavior and were counted as above- and below-ground taxon. The majority of the ground-nesting bees lives in self-excavated tunnel systems in the soil, usage of pre-existing cavities is relatively rare (Figure 4B). No significant differences were detected across site types by Pearsons's Chi-squared test (P-value = 0.93).
More than half of Freising's bee taxa are generalists (51.1%, 71 taxa), 23% (32 taxa) are oligolectic bees that rely on the pollen of a single or few plant species. Parasitic taxa represent 25.2% of the bee fauna of the city (35 taxa) (Figure 4C). No significant differences were detected across site types by Fisher's Exact Test for Count Data (P-value = 0.97).
The average size of female bees for all taxa recorded in Freising is 10.9 mm. When comparing the different habitats, there is no significant difference in body size (Supplementary Figure 4) (Kruskal-Wallis rank sum test: P-value = 0.57). On the river dike, however, bees are slightly larger (on average 11.52 mm compared to 10.98 mm and 10.91 mm on meadow orchards and wastelands). This difference disappears when the genus Bombus is excluded.
3.2.3 Threat level
Of the bee taxa recorded in Freising, 15 species are listed as “near threatened” in the Bavarian Red List (Bayerisches Landesamt für Umwelt, 2021), 12 are classified as “threatened,” one species, Bombus subterraneus from the river dikes, is classified as “highly threatened” and one species, Rophites quinquespinosus, is classified as “threatened with extinction.” We found the highest number of threatened bee species in the meadow orchards (8 near threatened, 5 threatened) and the lowest number of threatened species in the wastelands (4 near threatened, 4 threatened) (Table 1). In total, Freising harbors 11.3% of the red list species of Bavaria [categories V (near threatened), R (extremely rare), G (threat of unknown extent), 3 (threatened), 2 (highly threatened), 1 (threatened with extinction)] (Bayerisches Landesamt für Umwelt, 2021).
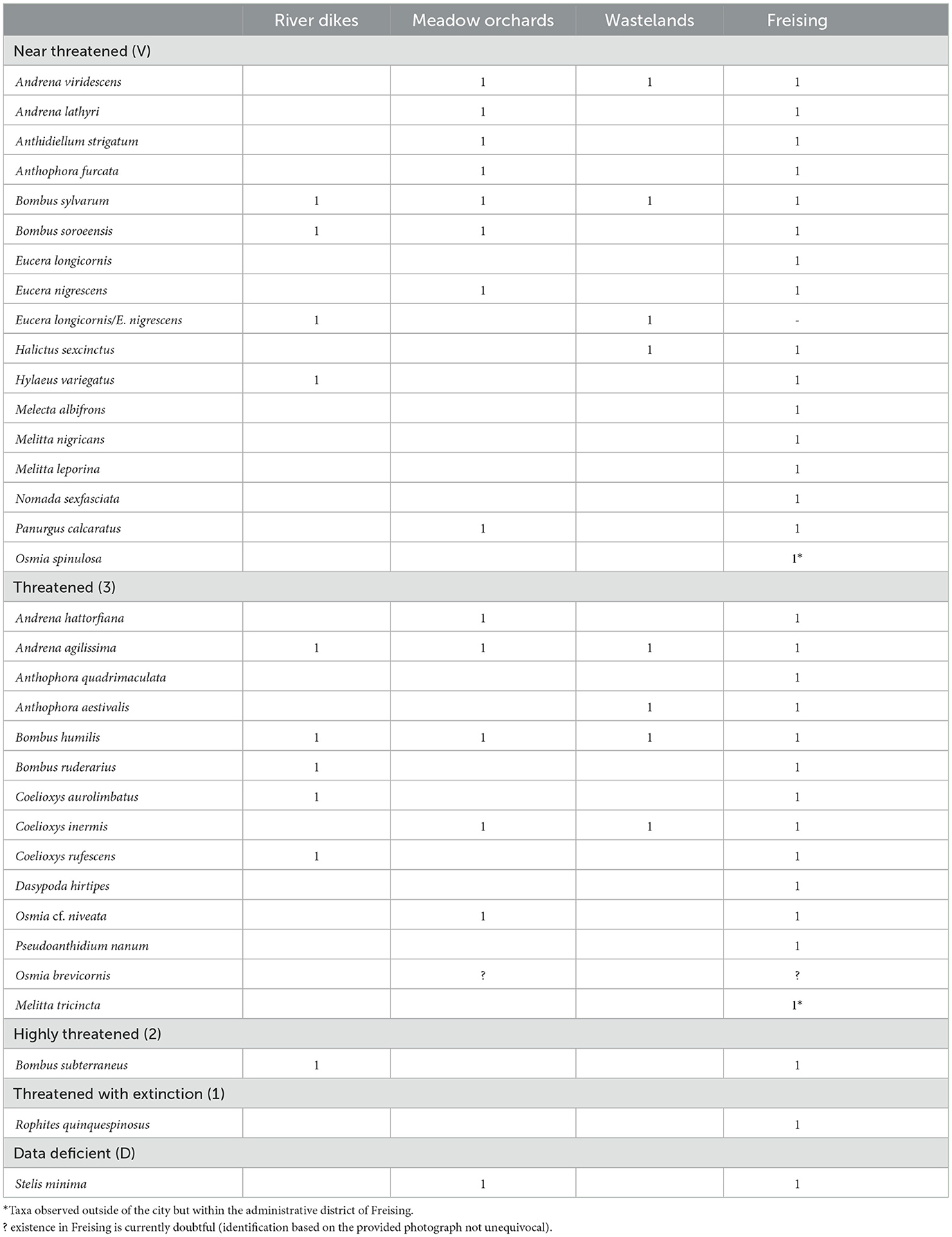
Table 1. Threatened and near threatened species found in Freising, according to the Bavarian Red List (Bayerisches Landesamt für Umwelt, 2021).
3.3 Bee hotspots
3.3.1 Bee communities in different habitat types
The bee communities of the different habitat types were similar across all sites. No significant inter-group and intra-group differences were detected by the ANOSIM (R-value = 0.191, P-value = 0.1184). Both the ANOSIM (Supplementary Figure 5) and the results of the Sörensen Index (Supplementary Table 4) indicate that the highest similarity was found between wastelands and meadow orchards, the lowest between wastelands and river dikes. According to the NMDS (Supplementary Figure 6), wasteland late succession stage, meadow orchard mowed and meadow orchard mixed were the sites most distinct in their composition from all other sites while all river dikes, the meadow orchards fallow, mixed and grazed, as well as the wasteland sites of early and mid-succession stages, respectively, were similar in wild bee community composition.
Twenty taxa were recorded only in the meadow orchards (Figure 5): six of those taxa are on the red list for Bavaria [Andrena hattorfiana (3), Osmia cf. niveata (3), Panurgus calcaratus (V), Anthophora furcata (V), Andrena lathyri (V), Stelis minima (data deficient)]; six are oligolectic (Andrena hattorfiana, A. proxima, Colletes hederae, Osmia cf. niveata, Panurgus calcaratus, and Andrena lathyri) and Osmia cornuta is a typical pollinator of fruit trees flowering early in the season; three have specific nesting requirements [Anthidiellum strigatum (builds nests attached to rocks and walls; resin), Anthophora furcata, Xylocopa violacea (rotten wood)]; seven are parasitic (Nomada fucata, Nomada lathburiana, Nomada signata, Stelis cf. ornatula, S. minima, S. punctulatissima, and S. cf. breviuscula) and three are unspecific (Halictus cf. eurygnathus, Lasioglossum zonulum, and Hylaeus difformis).
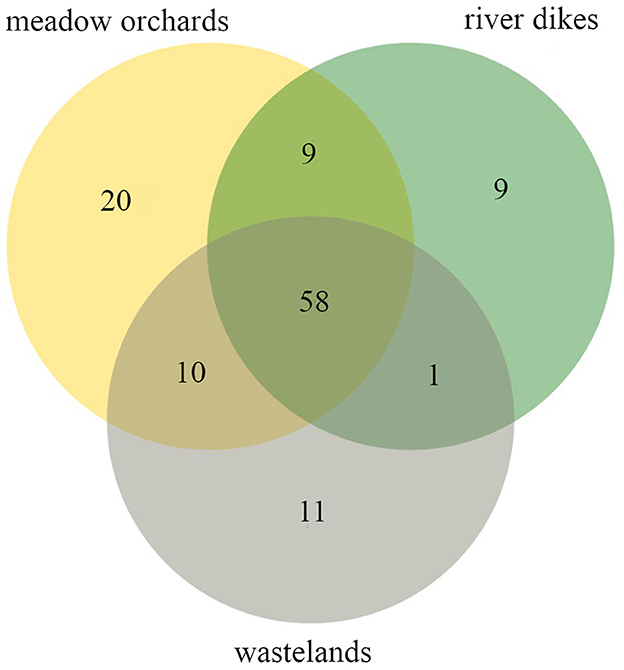
Figure 5. Bee taxa shared between the three habitat types (wild bee taxa found on meadow orchards in yellow, wild bee taxa found on the river dikes in green, wild bee taxa found on wastelands in gray). Total wild bee taxon richness = 118 taxa, total observation time = 586 h of systematic observation.
Eleven taxa were unique to the wastelands (Figure 5): two of those taxa are on the red list for Bavaria [Anthophora aestivalis (3), Halictus sexcinctus (V)], two are oligolectic (Andrena cf. praecox, Megachile lapponica), one has specific nesting requirements (Osmia aurulenta nests in empty snail shells); five are parasitic (Epeolus variegatus, Nomada fulvicornis, Nomada striata, Nomada cf. sheppardana, and Nomada fabriciana) and one is unspecific (Andrena nigroaenea).
Nine taxa were unique to the river dikes (Figure 5): five of those taxa are on the red list for Bavaria [Bombus subterraneus (2), Bombus ruderarius (3), Coelioxys aurolimbatus (3), Coelioxys rufescens (3), Hylaeus variegatus (V)]; two are oligolectic (Hylaeus signatus, Andrena vaga); three are parasitic (Coelioxys aurolimbatus, Coelioxys rufescens, and Sphecodes monilicornis) and one is unspecific (Colletes cunicularius).
Sixteen taxa were recorded outside the hotspot habitats: eight of those are on the red list for Bavaria [Rophites quinquespinosus (1), Pseudoanthidium nanum (2), Anthophora quadrimaculata (3), Dasypoda hirtipes (3), Melecta albifrons (V), Melitta leporina (V), Melitta nigricans (V), Nomada sexfasciata (V)]; seven are oligolectic (Andrena ventralis, Pseudoanthidium nanum, Dasypoda hirtipes, Melitta leporina, Melitta nigricans, Melitta haemorrhoidalis, and Rophites quinquespinosus); five have specific nesting requirements (Pseudoanthidium nanum requires pre-existing cavities and plant hair, Anthophora quadrimaculata requires vertical walls, Dasypoda hirtipes requires sandy soil, Megachile nigriventris requires dead wood and leaf cuttings, Megachile cf. pilidens requires leaf cuttings); five are parasitic (Coelioxys afra, Epeoloides coecutiens, Melecta albifrons, Nomada sexfasciata, and Nomada flavopicta); one is unspecific (Andrena tibialis), two were found on the flower patches and not on the study sites (Melitta nigricans and Melitta haemorrhoidalis); seven were solely recorded by citizen scientists (Andrena ventralis, Melitta leporina, Pseudoanthidium nanum, Coelioxys afra, Epeoloides coecutiens, Nomada flavopicta, and Rophites quinquespinosus). Two additional species were found outside of the city in the surroundings of Freising: Melitta tricincta [threatened, oligolectic on Odontites (Orobanchaceae)], Osmia spinulosa (near threatened, oligolectic on Asteraceae, nests in empty snail shells) (see Supplementary Data 3).
3.3.2 Influence of management-intensity and succession stage on bee taxon richness
Within a particular habitat type, bee taxon richness differed depending on succession stage or management type. The highest number of bee taxa occurred in fallow and grazed meadow orchards (67 and 68 bee taxa respectively), the river dike area with the latest mowing date (62 bee taxa), and in the wastelands of early succession stage (57 bee taxa) (Supplementary Data 2, Supplementary Table 5). This pattern is mirrored in flowering plant diversity, which was highest in fallow and grazed meadow orchards (85 and 82 species respectively), in the wastelands in early and mid-succession stage (137 and 122 species respectively—these are the cumulative species numbers of all wasteland sites with the respective succession stage) and on the river dike sections mown in September and August (117 and 114 species respectively) (Supplementary Data 4, Supplementary Table 5). In our poisson model, the number of flowering plant species on a site had a positive impact on wild bee taxa richness on site for our dataset (estimate: 1.358e-03), but the result cannot be generalized (P-value = 0.48636). According to the model, assuming a set observation time and a set number of plant species on site, one would expect on average 1.5 times more bee taxa on a wasteland site compared to a meadow orchard site (estimate: 3.946e-01, P-value = 9.98e-05 ***) and on average 0.6 times as many bee taxa on a river dike site compared to a meadow orchard site (estimate: −4.420e-01, P-value = 0.00293 **) (Supplementary Table 6).
3.3.3 Distribution of bee hotspots across the city
Two of the hotspots (meadow orchard M01, wasteland W09) are within 400 m distance of the river dikes; two are within 600 m (wastelands W10 and W11); seven within 1,000–2,000 m (meadow orchard M05 and wastelands W05 to W08, W13, and W14), five within 2,000–3,000 m (wastelands W01 to W04, W12, and W13) and three within 3,000–4,000 m (meadow orchards M02 to M04). The flower patches are within or close to the areas of continuous urban fabric according to the CORINE classification and within 800–2,100 m distance from the river dikes (Supplementary Figure 7). The number of bee taxa does not decrease with increasing distance from the dikes. Distance from the river dikes did not have a significant effect on the number of bee taxa in our poisson regression model (estimate: −7.540e-06; P-value = 0.84736) (Supplementary Table 6). The two most taxon rich patches are one of the closest and one of the most distant meadow orchards.
3.3.4 Colonization of stepping-stone patches
In six of ten flower patches we observed the target oligolectic bee species already in the first flowering season. Eight of ten were colonized in the third year after planting (Supplementary Table 7). We did not find Dasypoda hirtipes on the patch of Cichorium intybus planted to attract it but we found this bee species in two other locations at 2.6 km and 2.8 km distance of the flower patch. Similarly, Osmia brevicornis could not be observed in the planted patch of Hesperis matronalis but in another site nearby (unfortunately, the photograph is not detailed enough, so its existence in Freising remains somewhat doubtful). Other species were also found to profit from the patches, e.g., Melitta haemorrhoidalis, a bellflower specialist, and many generalist species.
4 Discussion
4.1 Survey method: challenges and opportunities of participative approaches
Our species identification approach based on photographs instead of collected bees leads to an underestimation of total species numbers, especially in species-rich difficult genera like Hylaeus, Lasioglossum, and Sphecodes. For those genera, pan trap or direct collecting allow more precise identification and therefore longer species lists. However, these lethal methods require collecting permits and citizen scientists are unlikely to be granted such permits even if they could be convinced to go through the trouble of applying. For citizen science projects, identification by photographs seems therefore the only realistic option. Since this approach is easier in some genera than in others (Weissmann and Schaefer, 2022), comparing diversity per genus or comparing diversity values between different studies gets more complicated. The real diversity and number of rare species in Freising can be expected to be higher than in our list but it seems unlikely that overall patterns would change dramatically when all our taxon groups were fully resolved into single species. Our approach delivers in-depth data for the bee communities systematically surveyed in urban bee hotspots, while the citizen scientist data helps to cover all the less accessible sites (e.g., private gardens and allotments).
Interest in bee talks and identification trainings was high, which resulted in a large number of people getting some basic understanding of bee diversity, bee ecology and the problems that bees are facing in our cities. Some participants of these basic introductions became really interested in the topic, continued to attend our program, and contributed large numbers of observations to our webpage and the iNaturalist portal. This, in combination with self-studies allowed them to reach advanced levels of identification knowledge in a few years' time. In the end, these people have not only contributed a large percentage of the total records but even discovered some rare species we had not found in our systematic surveys. While this is a fantastic result, we encountered two main challenges to reach larger numbers of dedicated participants. First, wild bees are often difficult to identify to species level even for specialists. Since they are often small and fast, taking high-quality photographs is a challenge and needs patience and persistence. Without such high-quality pictures, even experienced specialists or the best artificial intelligence algorithm will not be able to reliably identify the species. Second, reporting of bee observations and species identifications should be as easy as possible, ideally with one or two clicks on the smart phone. To tackle the first challenge, we developed a field guide focusing only on the pool of bee species occurring in the region (here: Bavaria) to make the identification based on photographs more accessible (Weissmann and Schaefer, 2022). In this field guide, we acknowledge that some species cannot reliably be distinguished with photographs alone and propose a system to group those species into consistent taxon groups. This enables comparisons of diversity between projects following the same system. The approach could be easily expanded to other regions of Europe. Regarding the second challenge, we realized that iNaturalist (www.inaturalist.org) is the perfect platform for quick and easy reporting of wild bee observations and species identification by observers of different levels of experience. A strength of this approach lies in its potential to provide continuous data in space, including private gardens, and time: wild bee observations are continuously added to our iNaturalist project while the funding period for in-depth surveys is limited in time. Moreover, iNaturalist includes an artificial intelligence species identification algorithm, which gets better the more correctly identified photographs of a particular species are uploaded for a particular geographic region. So, over time, the need for specialists to provide and review identifications should go down. It is, however, clear that regular trainings by bee specialists would be very helpful, for example as part of targeted communal survey events (e.g., Bioblitz; Roger and Klistorner, 2016) to provide additional in-depth snapshots. Ideally, these projects should be combined with university courses and specialist surveys (Paradise and Bartkovich, 2021; Vereecken et al., 2021). This would allow to maximize data availability, minimize bee capture, and focus the very limited specialist capacities on the most relevant (endangered) species and habitats. The fact that some of the most endangered species in our study were not discovered during our (or other) specialist surveys but through chance observations of citizen scientists shows that a large community of trained citizens can be more efficient than few specialists.
4.2 Is the city a hotspot or a refugium for wild bees?
Overall, with 139 wild bee taxa recorded in 2017–2022 (29% of the 472 bee species currently known in Bavaria), the total wild bee taxon richness in Freising (c. 90 km2) is comparable to results from other central European cities: 331 species have been recorded in Munich (c. 310 km2) since 1841 (Schuberth and Bräu, 2022) and 232 species have been re-observed or newly observed in 1997–2017 (Hofmann and Renner, 2020); 104 species have been recorded in Poznan (Poland, c. 260 km2, 2006–2008) (Banaszak-Cibicka and Zmihorski, 2012), 87 species in Paris (France, c. 100 km2, 2011–2016) (Ropars et al., 2018); 291 in the Lyon Metropolis (France, c. 530 km2, 2012–2014) (Fortel et al., 2015); 210 in the Brussels-Capital Region (Belgium, c. 160 km2, 1999–2020) (Vereecken et al., 2021), 170 in Zurich (Switzerland, c. 9 km2) (Casanelles-Abella et al., 2021). In the nearby area protected under the Habitat's directive (Fauna Flora Area) “Isarauen von Unterföhring bis Landshut” and the protected area “Isarauen zwischen Hangenham und Moosburg,” both natural riverine forest and gravel bank habitats, a total of 118 wild bee species was recorded during specialist surveys in 2015 (Mandery, 2016; Bayerisches Landesamt für Umwelt, 2021).
Freising harbors 11.3% of the red list species of Bavaria (categories 1, 2, 3, G, R, V) (Bayerisches Landesamt für Umwelt, 2021). The share of oligolectic taxa in Freising (23%) is comparable to that of Bavaria (23.3%). This is in contrast to other studies that tend to show that generalist species are more prevalent in urban areas (Buchholz and Egerer, 2020) but it is possible that generalist diversity is underrepresented due to our identification approach. The share of parasitic taxa in Freising (25.2%) is comparable to that in Bavaria (25.6%), which is remarkable because in these genera, species-level identification based on photographs is often impossible. The share of hypogeic species (excluding parasitic species) was higher in Freising (31.1%) than in Bavaria (23.5%), a pattern that has been related to urbanization (Wilson and Jamieson, 2019), although Gathof et al. (2022) have shown that urban dry grassland can be a favorable habitat for ground-nesting species. Overall, although common species are predominant, we found that Freising harbors a relatively species-rich wild bee community including some rare and specialized taxa regarding pollen/nectar requirements, but also regarding nesting requirements: resin (Anthidium strigatum and Megachile ericetorum), dead/rotting wood (Anthophora furcata, Megachile nigriventris, and Xylocopa violacea), empty snail shells (Osmia aurulenta, O. bicolor, and O. spinulosa).
The following taxa were recorded by us in addition to the 230 species recorded for the administrative district of Freising since 1856 (Bayerisches Landesamt für Umwelt, 2023): Andrena agilissima, Anthophora quadrimaculata, Coelioxys afra, Coelioxys aurolimbatus, Coelioxys inermis, Coelioxys rufescens, Colletes hederae, Epeoloides coecutiens, Hylaeus dilatatus, Hylaeus variegatus, Megachile lapponica, Megachile rotundata, Melitta tricincta, Nomada flavopicta, Nomada sexfasciata, Nomada cf. sheppardana, Osmia cf. brevicornis, Osmia cornuta, Rophites quinquespinosus, Sphecodes albilabris, Stelis minima, Stelis cf. ornatula, and Xylocopa violacea. The taxa Dasypoda hirtipes, Coelioxys afra, Rophites quinquespinosus, Megachile lapponica, and Nomada cf. sheppardana were recorded during our study but not yet in Munich, where 331 wild bee species have been recorded since 1841 (Schuberth and Bräu, 2022). For the taxa that could not unequivocally be identified, verification through capture and barcoding would be useful. Considering that more than twice as many bee taxa have been recorded in Munich in 150 years of surveys compared to our findings in Freising, it is evident that bee surveys should ideally be performed over long time scales. Although some of the species recorded for the administrative district of Freising and for Munich are difficult to detect with our method, they also include numerous taxa that we would have identified to species level from photographs. This shows that there is potential for additional species including rare ones to be found in Freising in the coming years.
4.3 Urban hotspots and their specific contribution to a diverse wild bee community
Overall, the river dike hosts a large part of the wild bee communities of the city, which is comparable to studies on river dikes along the Rhine (Westrich, 1985) and Loire (Villalta et al., 2021). The dikes had ten Red-List-species, and were particularly attractive for bumblebees, including the species with the highest threat level in the city (Bombus subterraneus). This might be related to the high availability of abandoned rodent holes in the dike (see also McFrederick and LeBuhn, 2006). The high share of Sphecodes bees on the dikes, a parasitic genus specialized mainly on Andrena and halictid bee hosts, indicates availability of nesting sites for its ground nesting host bee species. Two species only found on the dikes, the Reseda specialist Hylaeus signatus and the willow specialist Andrena vaga probably did not find enough host plants in the other habitat types.
We found the highest number of bee taxa in the meadow orchards and also the highest total number of near threatened and threatened species, and the highest share of oligolectic species. Two specialists of rotten wood (Anthophora furcata and Xylocopa violacea) and the resin specialist Anthidiellum strigatum were only found here. Although, we know of only one other study from urban orchard meadows (Rada et al., 2023), meadow orchards in general (usually located in the surroundings of small villages far from cities) have been found to be important wild bee habitats in other parts of Germany and Europe (Steffan-Dewenter, 2003; Steffan-Dewenter and Leschke, 2003; Schwenninger and Wolf-Schwenninger, 2012; Horak et al., 2013; Saure, 2016).
Although the wastelands had the lowest species number among our main study sites and the lowest number of threatened species, they were still hotspots within the city with 80 taxa and particularly important for Megachile and Epeolus. The willow specialist Andrena praecox, Megachile lapponica which needs Epilobium, and Osmia aurulenta which needs empty snail shells were only found on wastelands. Studies focusing on wasteland bee communities in other European cities revealed even higher numbers: 112 species in Freiburg (Germany) (Klatt, 1989), 127 species in Brussels (Belgium) (Vereecken et al., 2021), and 201 species of bees in Bydgoszcz (Poland), which is 42% of all bee species reported from Poland (Twerd and Banaszak-Cibicka, 2019). This demonstrates the huge importance of these ephemeral and often overlooked habitats, which often have large proportions of bare ground and favorable microclimatic conditions resulting in good nesting and foraging conditions for bees until succession or development projects put an end to the bee community in this site and new wastelands nearby are needed.
As river valleys can be important corridors for wild bees (see e.g., Braun-Reichert et al., 2021), we hypothesized that the river dike in Freising would be the main bee hotspot of the city. Contrary to our expectations, meadow orchards and not the river dikes had the highest species richness. This might be partly explained by the fact that the studied river dike sections were relatively uniform overall while there was more structural diversity within the different meadow orchard (and wasteland) sites we studied. Furthermore, since we were unable to locate nesting sites of most species, we do not know if the observed foraging habitats are also suitable for nesting. In fact, many studies highlight the importance of the availability of non-floral resources (Potts et al., 2005; Appenfeller et al., 2020; Requier and Leonhardt, 2020). Providing bare soil for ground nesting bee species is a relatively easy measure in in urban environments that can have a large impact (Noël et al., 2021).
4.4 Management of hotspots
We recorded the highest bee diversity on two patches of extensively managed meadow orchards, on the river dike area with the latest date of mowing (September), and on wastelands in early succession stages. Those were also the sites with the highest flowering plant species diversity. Our findings support extensive management of green spaces with grazing animals or late summer/autumn mowing (1–2 times per year), always leaving some stripes or patches unmown. Besides protecting natural areas and fostering flower- und structure-rich parks and gardens, the importance of wasteland in early succession stages should not be overlooked. These habitats tend to be short-lived in cities but will reappear whenever new demolition or construction sites appear.
4.5 Promoting connectivity—and at which scale?
Overall, the bee community composition in the hotspots was relatively similar. Bee species richness on a site is not related to the distance from the river dikes, and the city center does not seem to be a barrier. This might partly be explained by the relatively small size of Freising and of its highly urbanized areas. The particular topography of the city might also play a role, as bees might be more easily displaced by wind from elevated hill sites. Body size of female bees was overall very similar in the different habitats. A slightly larger size found on the river dikes is an effect of the higher number of bumblebee species on the dikes. The lack in size patterns is in contrast to other studies (e.g., Greenleaf et al., 2007) suggesting higher dispersal potential for larger-sized bees.
Our stepping-stone flower patches were very successful, similar to the results of Hofmann and Renner (2020), who found that flower strips in Munich already supported a quarter of Munich's bee species in the first year with oligolectic species not being underrepresented compared to the city's overall species pool. This suggests that some oligolectic bee species are relatively well established in cities, where they find their specific host plants in gardens (e.g., Campanula spp. as ornamental plants) or on wasteland and roadsides (e.g., Echium vulgare or Reseda lutea). In a way, this might be misleading since they do not rely on additional stepping-stones. Maybe more attention should be given to those species with more specific needs who will take some time to colonize the new patches (e.g., Osmia brevicornis or Dasypoda hirtipes).
Overall, our results indicate that, for cities to harbor diverse bee communities including rare species, it might be more important to provide small-scale connectivity between foraging and nesting resources than to provide continuous connectivity between floral resources throughout the entire city. While foraging ranges are estimated to reach only a few hundred meters especially in the smaller bee species (Hofmann et al., 2020), flight ranges for colonization of new habitats are probably larger and bees might occasionally be able to cross local physical barriers. For species nesting in above-ground structures, dispersal by human transport of rocks, wood, or building materials might be common in cities. And even ground-nesting species could be transported with soil or sand for construction and landscaping. Providing continuous corridors e.g., in areas mainly covered with impervious surfaces or along road axes might come with the price of creating partial habitats (Westrich, 1996) and in the worst case could form sinks/traps e.g., by attracting bees to sites with heavy traffic (Martin et al., 2018; Dániel-Ferreira et al., 2022a,b). Nevertheless, flower patches in densely populated areas have an important potential to raise awareness for the very specific habitat needs of wild bees when accompanied e.g., by information boards. They might be more important in larger cities with larger areas of impervious surface if the goal is high bee diversity throughout the city. However, if the goal is to provide hotspots and refugial areas for rare and threatened species, we argue the better approach is to create structurally diverse habitats taking into account the resources within a few hundred meters radius (Hofmann et al., 2020) of the three-fold needs of wild bees by providing: (1) pollen and nectar sources: ideally flowering plants species of different plant families flowering throughout the season and with a special focus on the host plants of oligolectic bees; (2) nesting sites: shifting the focus from the very popular provision of nesting aids for cavity nesting bees [which are usually colonized only by very common species (Geslin et al., 2022)] toward the needs of ground-nesting species. Also, dead wood specialists (Eckerter et al., 2021) and bees nesting in pithy plant stems suffer from the lack of “wild” places in parks and private gardens and need special help [e.g., unmown grass patches during winter (Unterweger et al., 2018) and leaving dry Verbascum or Rubus stems for at least two winters]; (3) nesting materials: e.g., moist clay for mason bees, and hairy plant species for wool carder bees.
4.6 Implications for bee conservation
We suggest the following concept for bee-friendly management of urban spaces: (1) identification of bee hotspots and systematic surveys for rare species (also considering habitat corridors at a larger scale); (2) training of citizen scientists at two levels for comprehensive surveys across the city: half-day introductions to bee diversity, bee ecology and bee conservation to create general awareness, and 2-weeks workshops for in-depth training in bee identification of a small number of dedicated citizen scientists; (3) extensive management of existing habitats and targeted conservation of rare species; (4) creation of high-value habitats to account for all resource needs of bees within flight ranges of only a few hundred meters; (5) creation of stepping stone habitats (with particular attention to rare oligolectic species) as floral and nesting resources, integrating educative and participative aspects. When integrated into the general green space management of a city and with the support of local NGOs, schools, and universities, this approach can be very cheap. Even though it is a time-consuming task to map and identify bees, using the suggested citizen science approach will not only make this more efficient than a traditional scientific study, it will also produce as a side-effect a lot of new bee-enthusiasts and even some future bee specialists, which are desperately needed for long-term conservation work of this fascinating group of insects.
Data availability statement
The datasets presented in this study can be found in online repositories. The observation data for this study can be found in our project on the iNaturalist platform at https://www.inaturalist.org/projects/wildbienen-in-freising-urban-pollinators-bees-in-freising and in the Supplementary Data 5 of this publication.
Author contributions
JW and HS designed the study and wrote the first draft of the manuscript. JW, SR, RR, IW, JP, and KS collected data. JW organized the database and performed the statistical analysis. All authors contributed to manuscript revision, read, and approved the submitted version.
Acknowledgments
We are especially grateful to all citizen scientists in Freising who participated in the project, and to the iNaturalist community for feedback on species identification. We thank the Regierung von Oberbayern for permission to collect wild bees in the city of Freising (permit numbers 55.1-8646-4-2018 and ROB-55.1-8646.NAT_02-8-23-5). We thank A. Aigner (Municipal Department for Parks and Gardens) for help with flower patches, C. Honert, D. Rzehaczek, and K. Tartler for wild bee observations and involvement with school classes and A. Fleischmann, C. Margraf, and M. Bräu for bee data from Munich and the Isar region. We thank S. Haug (TUM|Stat) and E. M. Ortiz for Statistics advice and the editor C. Baldock and reviewers for comments that helped to strengthen the manuscript considerably. The following individuals granted permission to perform pollinator observations in their orchards/gardens or those of their institution: E. Hobelsberger, M. Maino, R. Lackermaier, P. Jungbeck, S. Kilian, S. Grünwald, B. Hertle, K. Kell, P. Besgen, M. Meidinger, C. Novak, M. Lange, I. Steidl, P. Schiferli, C. Flinker, S. Vanderhaeghen, T. Heinze, C. Margraf, M. Drobny, and M. Hofmann.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frsc.2023.1155714/full#supplementary-material
References
Appenfeller, L. R., Lloyd, S., and Szendrei, Z. (2020). Citizen science improves our understanding of the impact of soil management on wild pollinator abundance in agroecosystems. PLoS ONE 15, e0230007. doi: 10.1371/journal.pone.0230007
Ayers, A. C., and Rehan, S. M. (2021). Supporting bees in cities: how bees are influenced by local and landscape features. Insects 12, 128. doi: 10.3390/insects12020128
Baldock, K. C. (2020). Opportunities and threats for pollinator conservation in global towns and cities. Curr. Opin. Insect Sci. 38, 63–71. doi: 10.1016/j.cois.2020.01.006
Baldock, K. C. R., Goddard, M. A., Hicks, D. M., Kunin, W. E., Mitschunas, N., Morse, H., et al. (2019). A systems approach reveals urban pollinator hotspots and conservation opportunities. Nat. Ecol. Evol. 3, 363–373. doi: 10.1038/s41559-018-0769-y
Banaszak-Cibicka, W., Twerd, L., Fliszkiewicz, M., Giejdasz, K., and Langowska, A. (2018). City parks vs. natural areas - is it possible to preserve a natural level of bee richness and abundance in a city park? Urban Ecosyst. 21, 599–613. doi: 10.1007/s11252-018-0756-8
Banaszak-Cibicka, W., and Zmihorski, M. (2012). Wild bees along an urban gradient: winners and losers. J. Insect Conserv. 16, 331–343. doi: 10.1007/s10841-011-9419-2
Bayerische Vermessungsverwaltung (2023). Digitales Orthophoto DOP80 WMS. Available online at: https://geodatenonline.bayern.de/geodatenonline/seiten/wms_dop80cm (accessed January 30, 2023).
Bayerisches Landesamt für Umwelt (2001). Arten- und Biotopschutzprogramm Bayern. Landkreis Freising, aktualisierter Textband. Available online at: https://www.lfu.bayern.de/natur/bayaz/absp/programm_daten/index.htm#kreisfrei (accessed January 30, 2023).
Bayerisches Landesamt für Umwelt (2021). Rote Liste und Gesamtartenliste Bayern – Bienen – Hymenoptera, Anthophila. Augsburg: Bayerisches Landesamt für Umwelt.
Bayerisches Landesamt für Umwelt (2023). Daten aus dem Bayerischen Fachinformationssystem Naturschutz (FIS-Natur). Artenschutzkartierung Bayern. Artenstatistik für den Landkreis 178 und die Ordnung 5IQ, Stand 05.01.2023 (Unpublished Document).
Blackmore, L. M., and Goulson, D. (2014). Evaluating the effectiveness of wildflower seed mixes for boosting floral diversity and bumblebee and hoverfly abundance in urban areas. Insect. Conserv. Divers. 7, 480–484. doi: 10.1111/icad.12071
Bloom, E. H., and Crowder, D. W. (2020). Promoting data collection in pollinator citizen science projects. Citiz. Sci. Theory Pract. 5, 3. doi: 10.5334/cstp.217
Braun-Reichert, R., Scheuchl, E., Wickl, K.-H., Doczkal, D., and Poschlod, P. (2021). Stechimmen im Landkreis Passau - Wanderkorridor Donau und Waldlichtungen als kleinflächige Habitate. Der Bayerische Wald 34(1+2 NF), 26–49. Available online at: https://www.zobodat.at/pdf/DerBayerischeWald_34_1-2_0026-0049.pdf
Buchholz, S., and Egerer, M. H. (2020). Functional ecology of wild bees in cities: towards a better understanding of trait-urbanization relationships. Biodivers. Conserv. 29, 2779–2801. doi: 10.1007/s10531-020-02003-8
Buchholz, S., Gathof, A. K., Grossmann, A. J., Kowarik, I., and Fischer, L. K. (2020). Wild bees in urban grasslands: urbanisation, functional diversity and species traits. Landsc. Urban Plan. 196, 103731. doi: 10.1016/j.landurbplan.2019.103731
Burdine, J. D., and McCluney, K. E. (2019). Differential sensitivity of bees to urbanization-driven changes in body temperature and water content. Sci. Rep. 9, 1643. doi: 10.1038/s41598-018-38338-0
Cardoso, M. C., and Gonçalves, R. B. (2018). Reduction by half: the impact on bees of 34 years of urbanization. Urban Ecosyst. 21, 943–949. doi: 10.1007/s11252-018-0773-7
Casanelles-Abella, J., Chauvier, Y., Zellweger, F., Villiger, P., Frey, D., Ginzler, C., et al. (2021). Applying predictive models to study the ecological properties of urban ecosystems: a case study in Zürich, Switzerland. Landsc. Urban Plan. 214, 104137. doi: 10.1016/j.landurbplan.2021.104137
Chen, H. (2022). VennDiagram: Generate High-Resolution Venn and Euler Plots. R Package Version 1.7.3. Available online at: https://cran.r-project.org/web/packages/VennDiagram/index.html (accessed January 30, 2023).
Christ, L., Hahn, M., Sieg, A.-K., and Dreesmann, D. C. (2022). Be(e) engaged! how students benefit from an educational citizen science project on biodiversity in their biology classes. Sustainability 14, 14524. doi: 10.3390/su142114524
Dániel-Ferreira, J., Berggren, Å., Bommarco, R., Wissman, J., and Öckinger, E. (2022a). Bumblebee queen mortality along roads increase with traffic. Biol. Conserv. 272, 109643. doi: 10.1016/j.biocon.2022.109643
Dániel-Ferreira, J., Berggren, Å., Wissman, J., and Öckinger, E. (2022b). Road verges are corridors and roads barriers for the movement of flower-visiting insects. Ecography 2022, e05847. doi: 10.1111/ecog.05847
Daniels, B., Jedamski, J., Ottermanns, R., and Ross-Nickoll, M. (2020). A “plan bee” for cities: Pollinator diversity and plant-pollinator interactions in urban green spaces. PLoS ONE 15, e0235492. doi: 10.1371/journal.pone.0235492
Dathe, H. H., Scheuchl, E., and Ockermüller, E. (2016). Illustrierte Bestimmungstabelle für die Arten der Gattung Hylaeus F. (Maskenbienen) in Deutschland, Österreich und der Schweiz. Wien: Österreichische Entomologische Gesellschaft.
Drossart, M., and Gérard, M. (2020). Beyond the decline of wild bees: optimizing conservation measures and bringing together the actors. Insects 11, 649. doi: 10.3390/insects11090649
Eckerter, T., Buse, J., Bauhus, J., Förschler, M. I., and Klein, A. M. (2021). Wild bees benefit from structural complexity enhancement in a forest restoration experiment. For. Ecol. Manag. 496, 119412. doi: 10.1016/j.foreco.2021.119412
Falk, S., Foster, G., Comont, R., Conroy, J., Bostock, H., Salisbury, A., et al. (2019). Evaluating the ability of citizen scientists to identify bumblebee (Bombus) species. PLoS ONE 14, e0218614. doi: 10.1371/journal.pone.0218614
Fauviau, A., Baude, M., Bazin, N., Fiordaliso, W., Fisogni, A., Fortel, L., et al. (2022). A large-scale dataset reveals taxonomic and functional specificities of wild bee communities in urban habitats of Western Europe. Sci. Rep. 12, 18866. doi: 10.1038/s41598-022-21512-w
Felderhoff, J., Gathof, A. K., Buchholz, S., and Egerer, M. (2022). Vegetation complexity and nesting resource availability predict bee diversity and functional traits in community gardens. Ecol. Appl. 33, e2759. doi: 10.1002/eap.2759
Fischer, L. K., Eichfeld, J., Kowarik, I., and Buchholz, S. (2016). Disentangling urban habitat and matrix effects on wild bee species. PeerJ. 4, e2729. doi: 10.7717/peerj.2729
Flaminio, S., Ranalli, R., Zavatta, L., Galloni, M., and Bortolotti, L. (2021). Beewatching: a project for monitoring bees through photos. Insects 12, 841. doi: 10.3390/insects12090841
Fortel, L., Vaissière, B., Mouret, H., and Morison, N. (2015). “Des abeilles plus citadines qu'on ne le croit…” in Le programme Urbanbees dans le Grand Lyon Le courrier de la nature 293, 27–33.
Gathof, A. K., Grossmann, A. J., Herrmann, J., and Buchholz, S. (2022). Who can pass the urban filter? A multi-taxon approach to disentangle pollinator trait–environmental relationships. Oecologia 199, 165–179. doi: 10.1007/s00442-022-05174-z
Geslin, B., Le Féon, V., Folschweiller, M., Flacher, F., Carmignac, D., Motard, E., et al. (2016). The proportion of impervious surfaces at the landscape scale structures wild bee assemblages in a densely populated region. Ecol. Evol. 6, 6599–6615. doi: 10.1002/ece3.2374
Geslin, B., Ropars, L., Zakardjian, M., and Flacher, F. (2022). The misplaced management of bees. Preprints. doi: 10.22541/au.164319695.57033003/v1
Graham, J. R., Tan, Q., Jones, L. C., and Ellis, J. D. (2014). Native Buzz: citizen scientists creating nesting habitat for solitary bees and wasps. Florida Scientist. 77, 204–218. Available online at: https://www.jstor.org/stable/24321925
Greenleaf, S. S., Williams, N. M., Winfree, R., and Kremen, C. (2007). Bee foraging ranges and their relationship to body size. Oecologia 153, 589–596. doi: 10.1007/s00442-007-0752-9
Hall, D. M., and Martins, D. J. (2020). Human dimensions of insect pollinator conservation. Curr. Opin. Insect Sci. 38, 107–114. doi: 10.1016/j.cois.2020.04.001
Hallmann, C. A., Sorg, M., Jongejans, E., Siepel, H., Hofland, N., Schwan, H., et al. (2017). More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 12, e0185809. doi: 10.1371/journal.pone.0185809
Harvey, J. A., Heinen, R., Armbrecht, I., Basset, Y., Baxter-Gilbert, J. H., Bezemer, T. M., et al. (2020). International scientists formulate a roadmap for insect conservation and recovery. Nat. Ecol. Evol. 4, 174–176. doi: 10.1038/s41559-019-1079-8
Hausmann, S. L., Petermann, J. S., and Rolff, J. (2016). Wild bees as pollinators of city trees. Insect Conserv. Divers. 9, 97–107. doi: 10.1111/icad.12145
Hofmann, M. M., and Fleischmann, A. (2020). A photo-based assessment of wild bees in a filled-up gravel pit in Riem, Munich with a species list of bees found in Central European gravel pits (Hymenoptera, Apiformes). SPIXIANA 43, 161–174. Available online at: https://www.zobodat.at/pdf/Spixiana_043_0161-0174.pdf
Hofmann, M. M., Fleischmann, A., and Renner, S. S. (2018). Changes in the bee fauna of a German botanical garden between 1997 and 2017, attributable to climate warming, not other parameters. Oecologia 187, 701–706. doi: 10.1007/s00442-018-4110-x
Hofmann, M. M., Fleischmann, A., and Renner, S. S. (2020). Foraging distances in six species of solitary bees with body lengths of 6 to 15 mm, inferred from individual tagging, suggest 150 m-rule-of-thumb for flower strip distances. J. Hymenopt. Res. 77, 105–117. doi: 10.3897/jhr.77.51182
Hofmann, M. M., and Renner, S. S. (2020). One-year-old flower strips already support a quarter of a city's bee species. J. Hymenopt. Res. 75, 87–95. doi: 10.3897/jhr.75.47507
Horak, J., Peltanova, A., Podavkova, A., Safarova, L., Bogusch, P., Romportl, D., et al. (2013). Biodiversity responses to land use in traditional fruit orchards of a rural agricultural landscape. Agric. Ecosyst. Environ. 178, 71–77. doi: 10.1016/j.agee.2013.06.020
Klatt, M. (1989). Insektengemeinschaften an Ruderalvegetation der Stadt Freiburg im Breisgau (Hymenoptera: Apoidea; Diptera: Syrphidae; Lepidoptera: Rhopalocera, Hesperiidae, Zygaenidae). Mitt. bad. Landesver. Naturkunde u. Naturschutz 14, 869–890. Available online at: https://www.zobodat.at/pdf/Mitt-Bad-Landesver-Natkde-Natschutz-Freiburg_NF_14_0869-0890.pdf
Kratschmer, S., Kriechbaum, M., and Pachinger, B. (2018). Buzzing on top: Linking wild bee diversity, abundance and traits with green roof qualities. Urban Ecosyst. 21, 429–446. doi: 10.1007/s11252-017-0726-6
Lye, G. C., Osborne, J. L., Park, K. J., and Goulson, D. (2012). Using citizen science to monitor Bombus populations in the UK: nesting ecology and relative abundance in the urban environment. J. Insect Conserv. 16, 697–707. doi: 10.1007/s10841-011-9450-3
Mandery, K. (2016). “Fachbeitrag Wildbienen, Wespen,” in Renaturierung der Mittleren Isar zwischen Freising und Moosburg. Dokumentation und Erfolgskontrolle der Entwicklung von natürlich neu geschaffenen dynamischen Fluss-Lebensräumen. 41 S. + 5 ausführliche Einzelberichte der Kartierungen + 3 Anhänge zu Hydrologie und Morphologie, eds. Chr. Margraf, M. Drobny, K. Mandery, Chr. Magerl, and W. Willner (Freising: BUND Naturschutz) (Unpublished Document).
Martin, A. E., Graham, S. L., Henry, M., Pervin, E., and Fahrig, L. (2018). Flying insect abundance declines with increasing road traffic. Insect Conserv. Divers. 11, 608–613. doi: 10.1111/icad.12300
Martin, H.-J. (2023). Hummel-Arten: Bombus spec. Available online at: https://www.wildbienen.de/huarten.htm (accessed January 30, 2023).
Mason, L., and Arathi, H. S. (2019). Assessing the efficacy of citizen scientists monitoring native bees in urban areas. Glob. Ecol. Conserv. 17, e00561. doi: 10.1016/j.gecco.2019.e00561
McFrederick, Q. S., and LeBuhn, G. (2006). Are urban parks refuges for bumble bees Bombus spp. (Hymenoptera: Apidae)? Biol. Conserv. 129, 372–382. doi: 10.1016/j.biocon.2005.11.004
Noël, G., Van Keymeulen, V., Barbier, Y., Smets, S., Van Damme, O., Colinet, G., et al. (2021). Nest aggregations of wild bees and apoid wasps in urban pavements: a “street life” to be promoted in urban planning. BioRxiv, 2021-12. doi: 10.1101/2021.12.15.472743
Oksanen, J., Simpson, G. L., Blanchet, F. G., Kindt, R., Legendre, P., Minchin, P. R., et al (2022). vegan: Community Ecology Package. R package version 2.6-4. Available online at: https://CRAN.R-project.org/package=vegan (accessed October 23, 2023).
Paradise, C., and Bartkovich, L. (2021). Integrating citizen science with online biological collections to promote species and biodiversity literacy in an entomology course. Citiz. Sci. Theory Pract. 6, 28. doi: 10.5334/cstp.405
Pedersen, T. L. (2022). Patchwork: The Composer of Plots. R package version 1.1.2. Available online at: https://CRAN.R-project.org/package=patchwork (accessed October 23, 2023).
Potts, S. G., Biesmeijer, J. C., Kremen, C., Neumann, P., Schweiger, O., and Kunin, W. E. (2010). Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353. doi: 10.1016/j.tree.2010.01.007
Potts, S. G., Vulliamy, B., Roberts, S., O'Toole, C., Dafni, A., Ne'eman, G., et al. (2005). Role of nesting resources in organising diverse bee communities in a Mediterranean landscape. Ecol. Entomol. 30, 78–85. doi: 10.1111/j.0307-6946.2005.00662.x
Powney, G. D., Carvell, C., Edwards, M., Morris, R. K. A., Roy, H. E., Woodcock, B. A., et al. (2019). Widespread losses of pollinating insects in Britain. Nat. Commun. 10, 1018. doi: 10.1038/s41467-019-08974-9
R Core Team (2023). R: A language and environment for statistical computing. Available online at: https://www.R-project.org/ (accessed October 23, 2023).
Rada, P., Bogusch, P., Pech, P., Pavlíček, J., Rom, J., and Horák, J. (2023). Active management of urban fruit orchard meadows is important for insect diversity. Ecol. Eng. 186, 106833. doi: 10.1016/j.ecoleng.2022.106833
Requier, F., and Leonhardt, S. D. (2020). Beyond flowers: including non-floral resources in bee conservation schemes. J. Insect Conserv. 24, 5–16. doi: 10.1007/s10841-019-00206-1
Roger, E., and Klistorner, S. (2016). BioBlitzes help science communicators engage local communities in environmental research. J. Sci. Commun. 15, A06. doi: 10.22323/2.15030206
Ropars, L., Dajoz, I., and Geslin, B. (2018). La diversité des abeilles parisiennes. Osmia 7, 14–19. doi: 10.47446/OSMIA7.3
Sánchez-Bayo, F., and Wyckhuys, K. A. G. (2019). Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 232, 8–27. doi: 10.1016/j.biocon.2019.01.020
Saure, C. (2016). Streuobstwiesen in Sachsen-Anhalt und ihre Bedeutung für Bienen, Wespen und Schwebfliegen (Hymenoptera part.; Diptera: Syrphidae). Naturschutz im Land Sachs.-Anhalt 53, 3–54. Available online at: https://lau.sachsen-anhalt.de/fileadmin/Bibliothek/Politik_und_Verwaltung/MLU/LAU/Wir_ueber_uns/Publikationen/Zeitschrift_fuer_Naturschutz_im_LSA/Dateien/53_Jg_2016_N-LSA_JH.pdf
Schuberth, J., and Bräu, M. (2022). Entwurf einer Checkliste für die in München nachgewiesenen Wildbienen-Arten (Unpublished document).
Schwenninger, H. R., and Wolf-Schwenninger, K. (2012). Ermittlung der Wildbienenarten als Bestäuberpotenzial von Streuobstwiesen und Entwicklung eines speziellen Maßnahmenkonzepts zu ihrer dauerhaften Förderung. Available online at: https://pudi.lubw.de/detailseite/-/publication/10019 (accessed January 3, 2021).
Seto, K. C., Guneralp, B., and Hutyra, L. R. (2012). Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc. Natl. Acad. Sci. 109, 16083–16088. doi: 10.1073/pnas.1211658109
Somme, L., Moquet, L., Quinet, M., Vanderplanck, M., Michez, D., Lognay, G., et al. (2016). Food in a row: urban trees offer valuable floral resources to pollinating insects. Urban Ecosyst. 19, 1149–1161. doi: 10.1007/s11252-016-0555-z
Steffan-Dewenter, I. (2003). Importance of habitat area and landscape context for species richness of bees and wasps in fragmented orchard meadows. Conserv. Biol. 17, 1036–1044. doi: 10.1046/j.1523-1739.2003.01575.x
Steffan-Dewenter, I., and Leschke, K. (2003). Effects of habitat management on vegetation and above-ground nesting bees and wasps of orchard meadows in Central Europe. Biodivers. Conserv. 12, 1953–1968. doi: 10.1023/A:1024199513365
Theodorou, P., Radzevičiut,e, R., Lentendu, G., Kahnt, B., Husemann, M., Bleidorn, C., et al. (2020). Urban areas as hotspots for bees and pollination but not a panacea for all insects. Nat. Commun. 11, 576. doi: 10.1038/s41467-020-14496-6
Theodorou, P., Radzevičiut,e, R., Settele, J., Schweiger, O., Murray, T. E., and Paxton, R. J. (2016). Pollination services enhanced with urbanization despite increasing pollinator parasitism. Proc. R. Soc. B Biol. Sci. 283, 20160561. doi: 10.1098/rspb.2016.0561
Toomey, A. H., and Domroese, M. C. (2013). Can citizen science lead to positive conservation attitudes and behaviors? Hum. Ecol. Rev. 20, 50–62. Available online at: https://www.jstor.org/stable/24707571#:~:text=We%20also%20felt%20that%20as,approach?to%20affect%20conservation%2Drelated
Twerd, L., and Banaszak-Cibicka, W. (2019). Wastelands: their attractiveness and importance for preserving the diversity of wild bees in urban areas. J. Insect Conserv. 23, 573–588. doi: 10.1007/s10841-019-00148-8
Twerd, L., Sobieraj-Betlińska, A., and Szefer, P. (2021). Roads, railways, and power lines: are they crucial for bees in urban woodlands? Urban For. Urban Green. 61, 127120. doi: 10.1016/j.ufug.2021.127120
Unterweger, P. A., Klammer, J., Unger, M., and Betz, O. (2018). Insect hibernation on urban green land: a winter-adapted mowing regime as a management tool for insect conservation. BioRisk 13, 1–29. doi: 10.3897/biorisk.13.22316
van der Wal, R., Anderson, H., Robinson, A., Sharma, N., Mellish, C., Roberts, S., et al. (2015). Mapping species distributions: A comparison of skilled naturalist and lay citizen science recording. Ambio 44, 584–600. doi: 10.1007/s13280-015-0709-x
van Vliet, J. (2019). Direct and indirect loss of natural area from urban expansion. Nat. Sustain. 2, 755–763. doi: 10.1038/s41893-019-0340-0
Vereecken, N. J., Weekers, T., Marshall, L., D'Haeseleer, J., Cuypers, M., Pauly, A., et al. (2021). Five years of citizen science and standardised field surveys in an informal urban green space reveal a threatened Eden for wild bees in Brussels, Belgium. Insect Conserv. Divers. 14, 868–876. doi: 10.1111/icad.12514
Villalta, I., Ledet, R., Baude, M., Genoud, D., Bouget, C., Cornillon, M., et al. (2021). A DNA barcode-based survey of wild urban bees in the Loire Valley, France. Sci. Rep. 11, 4770. doi: 10.1038/s41598-021-83631-0
Wagner, D. L., Grames, E. M., Forister, M. L., Berenbaum, M. R., and Stopak, D. (2021). Insect decline in the Anthropocene: death by a thousand cuts. Proc. Natl. Acad. Sci. 118, e2023989118. doi: 10.1073/pnas.2023989118
Weissmann, J. A., and Schaefer, H. (2022). Feld-Bestimmungshilfe für die Wildbienen Bayerns. Nachrichtenblatt Bayer. Entomol. 69, 1–64. Available online at: https://meg-bayern.de/feld-bestimmungshilfe-fuer-die-wildbienen-bayerns/
Weissmann, J. A., Walldorf, I. R. M., and Schaefer, H. (2021). The importance of wild bee communities as urban pollinators and the influence of honeybee hive density on wild bee crop visitation rates. J. Pollinat. Ecol. 29, 204–230. doi: 10.26786/1920-7603(2021)641
Westrich, P. (1985). Zur Bedeutung der Hochwasserdämme in der Oberrheinebene als Refugien für Wildbienen (Hymenoptera, Apoidea). Natur u. Landschaft 60, 92–97. Available online at: https://www.wildbienen.info/downloads/westrich_21.pdf
Westrich, P. (1996). “Habitat requirements of central European bees and the problems of partial habitats,” in The Conservation of Bees. Linnean Society Symposium Series, eds. S. Matheson, S. L. Buchmann, C. O'Toole, P. Westrich, and I. H. Williams (London: Academic Press) 1–16. Available online at: https://www.wildbienen.info/downloads/westrich_40.pdf
Westrich, P., Frommer, U., Mandery, K., Riemann, H., Ruhnke, H., Saure, C., et al. (2011). “Rote Liste und Gesamtartenliste der Bienen (Hymenoptera: Apidae) Deutschlands,” in Rote Liste gefährdeter Tiere, Pflanzen und Pilze Deutschlands, Band 3: Wirbellose Tiere (Teil 1). – Münster (Landwirtschaftsverlag), eds M. Binot-Hafke, S. Balzer, N. Becker, H. Gruttke, H. Haupt, N. Hofbauer, G. Ludwig, G. Matzke-Hajek, and M. Strauch, (Münster: Landwirtschaftsverlag), Naturschutz und biologische Vielfalt 70, 373–416. Available online at: https://www.rote-liste-zentrum.de/de/Bienen-Hymenoptera-Apidae-1733.html (accessed January 30, 2023).
Weweler, S., Bluth, T., and Schmid-Egger, C. (2022). Urbane Biodiversität - Der Blüherfolg von mehrjährigen Blühflächen in Berlin und ihre Bedeutung für Wildbienen. Naturschutz und Landschaftsplanung 54, 20–27. doi: 10.1399/NuL.2022.03.02
Wickham, H. (2007). Reshaping data with the reshape package. J. Stat. Softw. 21, 1–20. doi: 10.18637/jss.v021.i12
Wickham, H., Averick, M., Bryan, J., Chang, W., McGowan, L., François, R., et al. (2019). Welcome to the tidyverse. J. Open Source Softw. 4, 1686. doi: 10.21105/joss.01686
Wilson, C. J., and Jamieson, M. A. (2019). The effects of urbanization on bee communities depends on floral resource availability and bee functional traits. PLoS ONE 14, e0225852. doi: 10.1371/journal.pone.0225852
Wilson, J. S., Forister, M. L., and Carril, O. M. (2017). Interest exceeds understanding in public support of bee conservation. Front. Ecol. Environ. 15, 460–466. doi: 10.1002/fee.1531
Wilson, J. S., Pan, A. D., General, D. E. M., and Koch, J. B. (2020). More eyes on the prize: an observation of a very rare, threatened species of Philippine Bumble bee, Bombus irisanensis, on iNaturalist and the importance of citizen science in conservation biology. J. Insect Conserv. 24, 727–729. doi: 10.1007/s10841-020-00233-3
Zattara, E. E., and Aizen, M. A. (2021). Worldwide occurrence records suggest a global decline in bee species richness. One Earth 4, 114–123. doi: 10.1016/j.oneear.2020.12.005
Zhang, X., Zhang, L., Wang, Y., Shao, Y., Daniels, B., Roß-Nickoll, M., et al. (2022). Pollinators and urban riparian vegetation: important contributors to urban diversity conservation. Environ. Sci. Eur. 34, 78. doi: 10.1186/s12302-022-00661-9
Keywords: biodiversity, bee conservation, citizen science, meadow orchard, river dike corridor, urban ecology, wasteland
Citation: Weissmann JA, Rader S, Ritz R, Walldorf IRM, Probst J, Szydlik KR and Schaefer H (2023) Holistic wild bee management in urban spaces. Front. Sustain. Cities 5:1155714. doi: 10.3389/frsc.2023.1155714
Received: 31 January 2023; Accepted: 20 October 2023;
Published: 23 November 2023.
Edited by:
Feni Agostinho, Paulista University, BrazilReviewed by:
Joan Casanelles Abella, Snow and Landscape Research (WSL), SwitzerlandKris Braman, University of Georgia, United States
Copyright © 2023 Weissmann, Rader, Ritz, Walldorf, Probst, Szydlik and Schaefer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julie A. Weissmann, anVsaWUud2Vpc3NtYW5uQHR1bS5kZQ==
 Julie A. Weissmann
Julie A. Weissmann Sandra Rader
Sandra Rader Hanno Schaefer
Hanno Schaefer