- School of Agriculture, Food, and Wine, University of Adelaide, Adelaide, SA, Australia
The majority of the human population now lives in urban environments and that proportion is forecast to increase dramatically by 2050. As urbanization increases, the urban environment will increasingly play a role in biodiversity conservation. Floral visitors, often erroneously referred to as pollinators, are integral to the maintenance of ecosystem services and function. Several floral visitors are capable of adapting to urban environments, but for comprehensive protection, management practices must be tailored to specific groups. Urban biodiversity conservation is usually discussed from the northern hemisphere perspective, which has a very different ecology than its southern counterpart. Here we compare and contrast conservation strategies for urban flower visitors in Australia and New Zealand to the northern hemisphere, with a focus on birds and bees. The differences in flower visitors and floral characteristics mean that unique management strategies, which consider the local evolutionary context and integrate native flora, are required to support urban flower visitors. An additional important difference is that neither honey bees nor bumble bees, which reach high local densities in many areas, are native to the region, and thus should be excluded from urban biodiversity schemes.
The urban environment as focal areas for biodiversity conservation
As the human population increases, more stress is being placed on remaining wild habitats. While it is always preferable to maintain native ecosystems, urban environments are increasingly prevalent, and can play an increasingly important role in biodiversity conservation. These areas cannot replace wild habitat, but they can help to protect a subset of biodiversity. Targeted, local strategies should be developed to reach the full capability of urban areas for biodiversity conservation. Here we explore such strategies for flower visiting species in Australia and New Zealand.
The percentage of people living in urbanized areas is forecast to grow from the current 55% to nearly 70% by 2050 (UNDESA, 2018). The concept of flower visitor conservation in urban areas is just one component of the broader goal of urban biodiversity conservation. Urban areas, while generally detrimental to biodiversity overall, are able to support significant concentrations of native flowering plants and hence populations of insect and bird flower visitors, especially when managed effectively (Normandin et al., 2017). In addition, urban environments have been shown to host more abundant and diverse communities of insects than farmland (Baldock, 2020). Cities can provide adequate habitat for many insect species due to their relatively small functional requirements (i.e., habitat range, life cycle, and nesting behaviors) as compared to other types of biodiversity (New, 2018). Importantly, planning opportunities to support bee biodiversity in urban environments can easily be promoted as these can provide mutual benefits for bees and humans, in particular because the former provide pollination for backyard fruit crops (Iwasaki and Hogendoorn, 2021).
However, bees that do well in urban areas are often exotics or generalists, such as honey bees, and as such may be the least threatened species (Cane et al., 2006; Baldock et al., 2015; Fitch et al., 2019). While these generalist and introduced bees deliver pollination services in urban gardens, exotic species can have further negative effects on native flower visitors, and particularly on species that have specific dietary requirements (Geldmann and González-Varo, 2018; Iwasaki and Hogendoorn, 2022). Pollinator declines are largely driven by losses of specific plant resources and associated habitats, and is especially pronounced for specialist species, which can result in subsequent losses of rare or dependent flowering plant species leading to mutual extinctions (Waser et al., 1996; Wood et al., 2019). In the face of pollinator declines, public attention is often directed toward well-known species, potentially to the detriment of native species (Senapathi et al., 2015), which can also be charismatic for the public (Figure 1). Thus, to properly conserve the biodiversity of flower visiting species in urban areas, attention must be paid to the identity and relative needs of each species, and in as far as possible, support should be provided for every aspect of their life history.

Figure 1. Charismatic bees of Australia and New Zealand. Xylocopa aerata (Australia, Remko Leijs), Leioproctus fulvescens (New Zealand, Jay Iwasaki), and Amegilla sp. (Australia, David Marquina Reyes).
To maintain a diverse community of flower visiting bees and birds, year-round food availability is especially important as taxa have different seasonal patterns of emergence and resource requirements. Bees for example are completely reliant on pollen and nectar, and thus increasing floral resources (if nesting habitat is available) can increase local populations. Such an increase can be achieved not only by planting flowering plant species, but also by decreasing mowing frequency to allow weeds, crops, or ornamental species to flower (Wastian et al., 2016; Baldock, 2020). However, such measures will typically benefit generalist species the most (Baldock et al., 2015; Theodorou et al., 2017). For local native species, weeds and ornamentals may not provide the same food quality as the native plants that they have replaced and many species may not be used at all (Banaszak-Cibicka et al., 2016; Lowenstein et al., 2019). Therefore, it is key to evaluate the respective quality and quantity of useful floral resources available when managing urban green spaces. For example, specialist bees may be sustained by larger plantings of native plants in community gardens or collective efforts by neighboring gardeners.
The perspectives Down Under
Most insights into urban flower visitors are from the Northern Hemisphere, in particular Europe and North America (Baldock, 2020), and thus there is a gap in perspectives for the southern hemisphere, and particularly for Oceania. Due to their relative affluence, large population size, unique biodiversity, and high degree of urbanization (Cresswell and Murphy, 2017; UNDESA, 2018), Australia and New Zealand have the most relevance to urban pollinator conservation methods in Oceania. Therefore, and because of the large variation in geology, geography, and ecology within Oceania, we will restrict this review to these two countries.
North America and Europe, having had prehistoric geographic connectivity, share many plant and animal species with similar evolutionary lineages (80% of plant species from 15 families are shared; Rodriguez et al., 2006). Within Oceania, the Pacific Ocean has served as a significant barrier to colonization of bees in particular, but humans, prevailing winds, and currents have resulted in establishment of flora and fauna elements from Australasia on the relatively younger islands, which have largely been formed as a result of volcanic action (Groom and Schwarz, 2011; Dorey et al., 2021).
A prominent feature of the Australian environment is the prevalence of unpredictably but abundantly flowering nectar-rich shrubs and trees which feed a diverse range of vertebrates including honey eaters, parrots, bats, possums, as well as invertebrates (Armstrong, 1979; Ford et al., 1979; Woinarski et al., 2000; Gross, 2001; Cunningham et al., 2002; Abrol, 2012; Hermansen et al., 2014). Birds in the endemic family Meliphagidae (honeyeaters) are particularly significant flower visitors in Australia and New Zealand (Driskell and Christidis, 2004). In Australia, Gondwanan flora that is heavily utilized by bees and birds alike are representatives of the families Myrtaceae, Proteaceae, and Fabaceae (Acacia; Ford et al., 1979). Based on visitation records, Ford et al. (1979) suggest that about 100 plant species are bird pollinated, and many of these species belong to the group of Myrtaceae which have radiated throughout the continent in the last 35–60 million years (House, 1997). These species present large numbers of flowers that generally produce relatively weak nectar, which are thought to be adaptations to bird pollination (Ford et al., 1979). In addition, many species have either an unpredictable or an intermittent flowering phenology (House, 1997). New Zealand and most of the Pacific islands have no extant native Eucalyptus, but can have high abundances of other species in Myrtaceae (Metrosideros spp. in particular, which have been shown to be bird pollinated; Schmidt-Adam et al., 2009).
The areas also differ in their bee populations. Australia has a large and idiosyncratic bee fauna of over 1,700 bee species. Many Colletidae and Stenotritidae have a Gondwanan origin, while representatives of the families Halictidae, Megachilidae, and Apidae have colonized Australia from the north as the continent drifted closer to Eurasia (Houston, 2018). Most Australian bees are relative specialist and forage on Australian native plants in the family Myrtaceae (Michener, 1965). Many new species, possibly including endangered ones, are still being described (e.g., Leijs et al., 2018; Leijs and Hogendoorn, 2021). By contrast, New Zealand has a relatively poor bee fauna as a result of their recent origin and geographical isolation. The roughly 30 native bees in New Zealand are all closely related ground-nesting bees in the families Colletidae and Halictidae, and are likely derived from Australian progenitors relatively recently (i.e., ~23 mya; Donovan, 2007; Scott et al., 2014). The recent arrival and low number of bee species may have caused New Zealand pollination syndromes to be relatively more generalized (Godley, 1979; Newstrom and Robertson, 2005).
Australia and New Zealand also differ from Europe in that honey bees (Apis mellifera) and bumblebees (Bombus spp.) are introduced species. The introduction of both these species has resulted in large numbers of feral colonies. Honey bees are present throughout temperate and Mediterranean climates in Australia, where they may compete with hollow nesting birds and mammals for nesting hollows and with flower visitors for floral resources (Paini, 2004; Cunningham et al., 2022). Bumblebees are present throughout New Zealand and on Tasmania, where they are significant pollinators of weeds and fruit trees. In studies on competition in New Zealand, introduced bees had clear preferences for European plants over native species, suggesting niche partitioning by respective geographic origin (Iwasaki et al., 2018). Regardless, honey bee centric conservation goals (Iwasaki and Hogendoorn, 2021) proposed mostly in Europe and often mistakenly applied to North American urban areas are not applicable to Australia and New Zealand. The dominant focus on bumble bee conservation found in the northern hemisphere also has no place Down Under.
Thus, distinctions must be made between conservation of urban flower visitors, urban pollinators, urban bee conservation, and urban beekeeping as they are not synonymous. While many concepts may be similar, flower visitors are not necessarily pollinators, and, Down Under, they are a diverse group of animals, which include many bird species. In addition, as honey bees are not native to Oceania and consume large amounts of pollen and nectar (Cane and Tepedino, 2017), urban beekeeping is inconsistent with biodiversity conservation, despite the fact that bee conservation and urban beekeeping is often perceived as identical by the public (Geldmann and González-Varo, 2018). Many conservation actions may benefit all flower visitors, but when defining urban flower visitor conservation, it is important to make clear distinctions and to clarify the objectives.
Conservation efforts for flower visitors primarily entails maintaining or increasing native floral resources for nectar foragers (bees, bats, and birds) and nesting habitat within Australian cities and suburbs. As a result of the high relative nectar requirements of vertebrates, conservation of flower visiting birds, bats, and mammals in Australia involves nectar producing trees and shrubs rather than lower plants. Unlike Australian flowering trees and shrubs (namely Myrtaceae and Proteaceae), introduced tree species do not necessarily provide floral resources for native birds or insects. In addition, many of the introduced plants in urban gardens that are attractive to honey bees are not or hardly visited by native bees, presumably because they have not co-evolved with them (Michener, 1965; Houston, 2018; Brown and Cunningham, 2019). Most bees specialize on native plants, and several species are oligolectic on a subset (Michener, 1965; Houston, 2018).
Many bird species are similarly adapted to specific groups of plants. For example cockatoos and lorikeets have a bulbous scaly tongue to harvest nectar from Myrtaceae, while the thin, long, brush-tipped tongues of the honey eaters allows nectar collection from Proteaceae (Ford et al., 1979). Nectar and pollen from Australian trees are also significant components of the diets of arboreal marsupials and large fruit bats, both of which have been shown to be effective pollinators (Armstrong, 1979; House, 1997).
The plants that native urban flower visitors rely on are sometimes not preferred for urban gardens. For example, the Eucalyptus species that many native bees, bird, and bat species strongly depend on may not be chosen in urban gardens because of their size and tendency to drop limbs. Other useful flowering plants that support specific species may not be preferred because they are hazardous, slow growing, or only flower for a limited period of time.
Many species of bees in urban environments are ground nesting species, and ground cover has been shown to have a negative correlation with bee abundance (Banaszak-Cibicka et al., 2016). This is of particular importance in parks and gardens, where open soil is often covered with either lawn or a thick layer of mulch to prevent evaporation. This may partly explain why, in urban areas, a larger proportion of bees are cavity nesters as compared to suburban or natural habitats, but the mechanisms behind such biases are as yet unknown (Hernandez et al., 2009). The degree of uncertainty in the factors driving urban bee ecology highlight the importance of future research in precisely how to maintain robust and diverse bee populations in urban environments.
While supplemental nesting habitat (nest boxes) cannot completely compensate for a lack of nesting hollows for vertebrate flower visitors, they can support bat, marsupial, and bird biodiversity in Australian cities (Le Roux et al., 2016; Macak, 2020). However, nest boxes can also provide habitat for invasive species, in particular European honey bees (Cunningham et al., 2022), and their placement can therefore be counterproductive to the conservation of native flower visitors (Macak, 2020). For bees, placement of bee hotels is very popular worldwide. While they help to encourage and maintain public awareness of the existence of solitary native bees, it is questionable whether their placement is an adequate conservation action (MacIvor and Packer, 2015). Bee hotels can host many introduced species (MacIvor and Packer, 2015). This is particularly likely to be an issue in New Zealand for example, where six of the 12 species that would use bee hotels are adventive (Donovan, 2007). In Australia, there are many native hollow nesting bee species that would potentially use bee hotels (Houston, 2018). However, even without promoting introduced species, the potential to benefit bee conservation is uncertain, as they may enhance the populations of predators and parasites (e.g., MacIvor and Packer, 2015; Geslin et al., 2020).
Flower visitors in New Zealand include birds, possums (introduced), and bats and these vertebrates known to be, or have been, important pollinators (Lord, 1991; Anderson, 2003). Invertebrate pollinators are thought to be mostly generalist, with flies and butterflies reflecting the greatest diversity of species (Anderson, 2003; Newstrom and Robertson, 2005). The New Zealand bee taxa is has relatively low diversity, but they have been shown to be efficient pollinators of several native plants (Bischoff et al., 2013). Referencing key plant species that are particularly important for native pollinators (Donovan, 2007) and maintaining urban forest reserves likewise are integral for maintaining urban biodiversity in New Zealand.
In addition, invasive species have contributed to significant declines in plant, marsupial, and bird communities in Australia and New Zealand, and there is significant public support for conservation efforts to reverse these trends, including in urban environments (Wittmer et al., 2018). As birds can be highly vulnerable to cat predation, urban pollinator conservation of avian flower visitors may require extensive removal of feral cats, limiting outdoor cat ownership, and trapping within urban areas (Kikillus et al., 2017). Regionally concerted efforts to establish strict feline control policies have been attempted within cities, but support from the general public for such strict rules has been limited (Grayson et al., 2002; Kikillus et al., 2017).
Controlling invasive species that have high public appeal also includes dissuading European honey beekeeping, which to the public is often the only bee they are familiar with. Urban beekeeping of honey bees is especially popular in Europe and in North America, where the public may be misled in thinking that honey bees are on the brink of extinction or otherwise imperiled (Egerer and Kowarik, 2020). In North America where honey bees are not native, urban beekeeping is more akin to maintaining livestock within cities and is not synonymous with maintaining or supporting local biodiversity (Colla and MacIvor, 2017). This is also the case in Oceania.
In Australia, native stingless bee can be kept in hives. Therefore, if hives are desired to be kept, native stingless bees, should be preferred for Australia. These species can thrive in urban areas (Kaluza et al., 2016) and have the potential to co-opt the focus on urban honey beekeeping. They also produce small quantities of unique honey, and can have greater foraging success in urban gardens than in forests or plantations (Kaluza et al., 2016). However, urban beekeeping of even a native species still may not align with the goals of urban pollinator conservation when it involves maintaining a single species at unnatural high densities. In those cases, the conservation benefits of artificially enhancing certain native bee species over others may be limited (Camps-Calvet et al., 2016).
The positive environmental effects of green spaces can also help to mitigate future threats from climate change, which will specifically increase harmful or catastrophic incidents from heat, fires, droughts, and flooding (Nicholson and Egan, 2020). The urban heat island effect is particularly exacerbated in urban environments, and in Australian capital cities the number of heatwave days are projected to triple within this century (Herold et al., 2018). Green spaces have the potential to mitigate some of the urban heat island effect, which may affect rarer specialist bees more negatively than generalists (Burdine and McCluney, 2019; Dew et al., 2019). Nevertheless, in the face of pollinator and biodiversity decline in general, conservation efforts in urban areas have great potential to protect biodiversity (Elmqvist et al., 2015). As urbanization increases, these efforts will become more important.
Conclusions
To summarize, habitat is the most important factor in supporting urban flower visitors. Habitat includes appropriate nutritional resources and nesting sites. In Australia and New Zealand in particular, unique flora and fauna means that northern hemisphere plant species may not provide the resources that native flower visitors require. Introduced mammalian predators also require control to protect predator-naïve species in urban areas (Table 1).
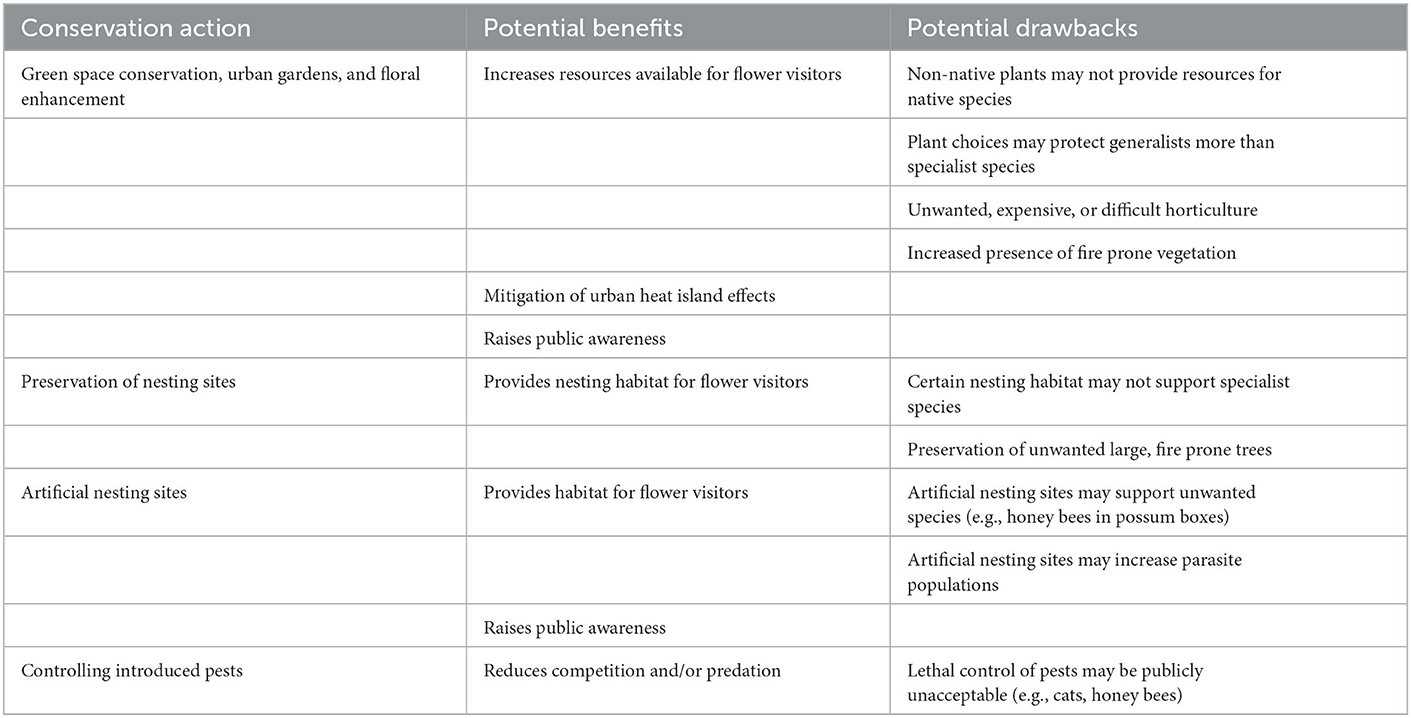
Table 1. List of key conservation actions for flower visitors in urban environments Down Under, including benefits and potential drawbacks.
Generally, concepts in urban biodiversity management schemes follow universal principles and can be applied in Oceania, but also must address the local contexts regarding introduced species, relatively high urbanization rates, and fire dependent ecologies. Critically, an assessment of target goals following design implementation is crucial for determining successful design implementation and ensuring future sustainability (Garrard et al., 2018; Rega-Brodsky et al., 2022). Taken together, these approaches are integral for protecting biodiversity in the face of human population growth.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JI wrote the article. JI and KH contributed to conceptualization and extensively edited the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abrol, D. P. (2012). Pollination Biology: Biodiversity Conservation and Agricultural Production. New York, NY: Springer.
Anderson, S. H. (2003). The relative importance of birds and insects as pollinators of the New Zealand flora. N. Zeal. J. Ecol. 27, 83–94.
Armstrong, J. A. (1979). Biotic pollination mechanisms in the Australian flora—A review. N. Zeal. J. Bot. 17, 467–508. doi: 10.1080/0028825X.1979.10432565
Baldock, K. C. (2020). Opportunities and threats for pollinator conservation in global towns and cities. Curr. Opin. Insect Sci. 38, 63–71. doi: 10.1016/j.cois.2020.01.006
Baldock, K. C. R., Goddard, M. A., Hicks, D. M., Kunin, W. E., Mitschunas, N., Osgathorpe, L. M., et al. (2015). Where is the UK's pollinator biodiversity? The importance of urban areas for flower-visiting insects. Proc. Royal Soc. B 282, 2849. doi: 10.1098/rspb.2014.2849
Banaszak-Cibicka, W., Ratyńska, H., and Dylewski, Ł. (2016). Features of urban green space favourable for large and diverse bee populations (Hymenoptera: Apoidea: Apiformes). Urb. For. Urb. Green. 20, 448–452. doi: 10.1016/j.ufug.2016.10.015
Bischoff, M., Campbell, D. R., Lord, J. M., and Robertson, A. W. (2013). The relative importance of solitary bees and syrphid flies as pollinators of two outcrossing plant species in the New Zealand alpine. Austral Ecol. 38, 169–176. doi: 10.1111/j.1442-9993.2012.02389.x
Brown, J., and Cunningham, S. A. (2019). Global-scale drivers of crop visitor diversity and the historical development of agriculture. Proc. Royal Soc. B 286, 20192096. doi: 10.1098/rspb.2019.2096
Burdine, J. D., and McCluney, K. E. (2019). Differential sensitivity of bees to urbanization-driven changes in body temperature and water content. Sci. Rep. 9, 1–10. doi: 10.1038/s41598-018-38338-0
Camps-Calvet, M., Langemeyer, J., Calvet-Mir, L., and Gómez-Baggethun, E. (2016). Ecosystem services provided by urban gardens in Barcelona, Spain: Insights for policy and planning. Environ. Sci. Pol. 62, 14–23. doi: 10.1016/j.envsci.2016.01.007
Cane, J. H., Minckley, R. L., Kervin, L. J., Roulston, T. H., and Neal, M. (2006). Complex responses within a desert bee guild (Hymenoptera : Apiformes) to urban habitat fragmentation. Ecol. Appl. 16, 632–644. doi: 10.1890/1051-0761(2006)016(0632:CRWADB)2.0.CO;2
Cane, J. H., and Tepedino, V. J. (2017). Gauging the effect of honey bee pollen collection on native bee communities. Conserv. Lett. 10, 205–210. doi: 10.1111/conl.12263
Colla, S. R., and MacIvor, J. S. (2017). Questioning public perception, conservation policy, and recovery actions for honeybees in North America. Conserv. Biol. 31, 1202–1204. doi: 10.1111/cobi.12839
Cresswell, I. D., and Murphy, H. T. (2017). Australia State of the Environment 2016: Biodiversity, Independent Report to the Australian Government Minister for the Environment and Energy. Canberra, ACT: Australian Government Department of the Environment and Energy. Available online at: https://www.researchgate.net/publication/315045059_Australia_state_of_the_environment_2016_biodiversity_independent_report_to_the_Australian_Government_Minister_for_the_Environment_and_Energy#fullTextFileContent (accessed January 31, 2023).
Cunningham, S. A., Crane, M. J., Evans, M. J., Hingee, K. L., and Lindenmayer, D. B. (2022). Density of invasive western honey bee (Apis mellifera) colonies in fragmented woodlands indicates potential for large impacts on native species. Sci. Rep. 12, 1–10. doi: 10.1038/s41598-022-07635-0
Cunningham, S. A., FitzGibbon, F., and Heard, T. (2002). The future of pollinators for Australian agriculture. Austr. J. Agri. Res. 53, 893–900. doi: 10.1071/AR01186
Dew, R. M., Silva, D. P., and Rehan, S. M. (2019). Range expansion of an already widespread bee under climate change. Glob. Ecol. Conserv. 17, e00584. doi: 10.1016/j.gecco.2019.e00584
Donovan, B. J. (2007). Apoidea (Insecta: Hymenoptera), Fauna of New Zealand 57. Lincoln: Landcare Research New Zealand. doi: 10.7931/J2/FNZ.57
Dorey, J. B., Groom, S. V. C., Velasco-Castrillón, A., Stevens, M. I., Lee, M. S. Y., and Schwarz, M. P. (2021). Holocene population expansion of a tropical bee coincides with early human colonization of Fiji rather than climate change. Mol. Ecol. 30, 4005–4022. doi: 10.1111/mec.16034
Driskell, A. C., and Christidis, L. (2004). Phylogeny and evolution of the Australo-Papuan honeyeaters (Passeriformes, Meliphagidae). Mol. Phylogenet. Evol. 31, 943–960. doi: 10.1016/j.ympev.2003.10.017
Egerer, M., and Kowarik, I. (2020). Confronting the modern Gordian Knot of urban beekeeping. Trends Ecol. Evol. 35, 956–959. doi: 10.1016/j.tree.2020.07.012
Elmqvist, T., Setälä, H., Handel, S. N., van der Ploeg, S., Aronson, J., Blignaut, J. N., et al. (2015). Benefits of restoring ecosystem services in urban areas. Curr. Opin. Environ. Sustainabil. 14, 101–108. doi: 10.1016/j.cosust.2015.05.001
Fitch, G., Wilson, C. J., Glaum, P., Vaidya, C., Simao, M. C., and Jamieson, M. A. (2019). Does urbanization favour exotic bee species? Implications for the conservation of native bees in cities. Biol. Lett. 15, 20190574. doi: 10.1098/rsbl.2019.0574
Ford, H. A., Paton, D. C., and Forde, N. (1979). Birds as pollinators of Australian plants. N. Zeal. J. Bot. 17, 509–519. doi: 10.1080/0028825X.1979.10432566
Garrard, G. E., Williams, N. S. G., Mata, L., Thomas, J., and Bekessy, S. A. (2018). Biodiversity sensitive urban design. Conserv. Lett. 11, 1–10. doi: 10.1111/conl.12411
Geldmann, J., and González-Varo, J. P. (2018). Conserving honey bees does not help wildlife. Science 359, 392–393. doi: 10.1126/science.aar2269
Geslin, B., Gachet, S., Deschamps-Cottin, M., Flacher, F., Ignace, B., Knoploch, C., et al. (2020). Bee hotels host a high abundance of exotic bees in an urban context. Acta Oecol. 105, 103556. doi: 10.1016/j.actao.2020.103556
Godley, E. J. (1979). Flower biology in New Zealand. N. Zeal. J. Bot. 17, 441–466. doi: 10.1080/0028825X.1979.10432564
Grayson, J., Calver, M., and Styles, I. (2002). Attitudes of suburban Western Australians to proposed cat control legislation. Austr. Vet. J. 80, 536–543. doi: 10.1111/j.1751-0813.2002.tb11030.x
Groom, S. V. C., and Schwarz, M. P. (2011). Bees in the southwest pacific: Origins, diversity and conservation. Apidologie 42, 759–770. doi: 10.1007/s13592-011-0079-8
Gross, C. L. (2001). The effect of introduced honeybees on native bee visitation and fruit-set in Dillwynia juniperina (Fabaceae) in a fragmented ecosystem. Biol. Conserv. 102, 89–95. doi: 10.1016/S0006-3207(01)00088-X
Hermansen, T. D., Britton, D. R., Ayre, D. J., and Minchinton, T. E. (2014). Identifying the real pollinators? Exotic honeybees are the dominant flower visitors and only effective pollinators of Avicennia marina in Australian temperate mangroves. Estuar. Coasts 37, 621–635. doi: 10.1007/s12237-013-9711-3
Hernandez, J. L., Frankie, G. W., and Thorp, R. W. (2009). Ecology of urban bees: A review of current knowledge and directions for future study. Cit. Environ. 2, 1–15. doi: 10.15365/cate.2132009
Herold, N., Ekström, M., Kala, J., Goldie, J., and Evans, J. P. (2018). Australian climate extremes in the 21st century according to a regional climate model ensemble: Implications for health and agriculture. Weather Climate Extr. 20, 54–68. doi: 10.1016/j.wace.2018.01.001
House, S. M. (1997). “Reproductive biology of eucalypts,” in Eucalypt Ecology: Individuals to Ecosystems, eds J. E. Williams and J. C. Z. Woinarski (Cambridge: Cambridge University Press), 30–55.
Houston, T. (2018). A Guide to Native Bees of Australia. Clayton: CSIRO Publishing. doi: 10.1071/9781486304073
Iwasaki, J. M., Dickinson, K. J. M., Barratt, B. I. P., Mercer, A. R., Jowett, T. W. D., and Lord, J. M. (2018). Floral usage partitioning and competition between social (Apis mellifera, Bombus terrestris) and solitary bees in New Zealand: Niche partitioning via floral preferences? Austral Ecol. 2018, 1–12. doi: 10.1111/aec.12643
Iwasaki, J. M., and Hogendoorn, K. (2021). How protection of honey bees can help and hinder bee conservation. Curr. Opin. Insect Sci. 5, 5. doi: 10.1016/j.cois.2021.05.005
Iwasaki, J. M., and Hogendoorn, K. (2022). Mounting evidence that managed and introduced bees have negative impacts on wild bees : An updated review. Curr. Res. Insect Sci. 2, 100043. doi: 10.1016/j.cris.2022.100043
Kaluza, B. F., Wallace, H., Heard, T. A., Klein, A. M., and Leonhardt, S. D. (2016). Urban gardens promote bee foraging over natural habitats and plantations. Ecol. Evol. 6, 1304–1316. doi: 10.1002/ece3.1941
Kikillus, K. H., Chambers, G. K., Farnworth, M. J., and Hare, K. M. (2017). Research challenges and conservation implications for urban cat management in New Zealand. Pacific Conserv. Biol. 23, 15–24. doi: 10.1071/PC16022
Le Roux, D. S., Ikin, K., Lindenmayer, D. B., Bistricer, G., Manning, A. D., and Gibbons, P. (2016). Enriching small trees with artificial nest boxes cannot mimic the value of large trees for hollow-nesting birds. Restor. Ecol. 24, 252–258. doi: 10.1111/rec.12303
Leijs, R., Dorey, J., and Hogendoorn, K. (2018). Twenty six new species of Leioproctus (Colletellus): Australian Neopasiphaeinae, all but one with two submarginal cells (Hymenoptera, Colletidae, Leioproctus). ZooKeys 2018, 109–168. doi: 10.3897/zookeys.811.28924
Leijs, R., and Hogendoorn, K. (2021). New bee species from northern Queensland, Australia (Hymenoptera: Colletidae, Halictidae, Megachilidae). Austral Entomol. 60, 659–671. doi: 10.1111/aen.12572
Lord, J. M. (1991). Pollination and seed dispersal in Freycinetia baueriana, a dioecious liane that has lost its bat pollinator. N. Zeal. J. Bot. 29, 83–86. doi: 10.1080/0028825X.1991.10415545
Lowenstein, D. M., Matteson, K. C., and Minor, E. S. (2019). Evaluating the dependence of urban pollinators on ornamental, non-native, and ‘weedy’ floral resources. Urb. Ecosyst. 22, 293–302. doi: 10.1007/s11252-018-0817-z
Macak, P. V. (2020). Nest boxes for wildlife in victoria: An overview of nest box distribution and use. Victor. Naturalist 137, 4–14.
MacIvor, J. S., and Packer, L. (2015). “Bee hotels” as tools for native pollinator conservation: A premature verdict? PLoS ONE 10, 1–13. doi: 10.1371/journal.pone.0122126
Michener, C. D. (1965). A classification of the bees of the Australian and South Pacific regions. Bullet. Am. Museum Nat. Hist. 130, 1–362.
New, T. R. (2018). Promoting and developing insect conservation in Australia's urban environments. Austral Entomol. 57, 182–193. doi: 10.1111/aen.12332
Newstrom, L. E., and Robertson, A. W. (2005). Progress in understanding pollination systems in New Zealand. N. Zeal. J. Bot. 43, 1–59. doi: 10.1080/0028825X.2005.9512943
Nicholson, C. C., and Egan, P. A. (2020). Natural hazard threats to pollinators and pollination. Glob. Change Biol. 26, 380–391. doi: 10.1111/gcb.14840
Normandin, É., Vereecken, N. J., Buddle, C. M., and Fournier, V. (2017). Taxonomic and functional trait diversity of wild bees in different urban settings. PeerJ 2017, 1–35. doi: 10.7717/peerj.3051
Paini, D. R. (2004). Impact of the introduced honey bee (Apis mellifera) (Hymenoptera: Apidae) on native bees: A review. Austral Ecol. 29, 399–407. doi: 10.1111/j.1442-9993.2004.01376.x
Rega-Brodsky, C. C., Aronson, M. F. J., Piana, M. R., Carpenter, E. S., Hahs, A. K., Herrera-Montes, A., et al. (2022). Urban biodiversity: State of the science and future directions. Urb. Ecosyst. 1083–1096. doi: 10.1007/s11252-022-01207-w
Rodriguez, J., Hortal, J., and Nieto, M. (2006). An evaluation of the influence of environment and biogeography on community structure: The case of Holarctic mammals. J. Biogeogr. 33, 291–303. doi: 10.1111/j.1365-2699.2005.01397.x
Schmidt-Adam, G., Murray, B. G., and Young, A. G. (2009). The relative importance of birds and bees in the pollination of Metrosideros excelsa (Myrtaceae). Austral Ecol. 34, 490–498. doi: 10.1111/j.1442-9993.2009.01949.x
Scott, J. M., Lee, D. E., Fordyce, R. E., and Palin, J. M. (2014). A possible late oligocene-early miocene rocky shoreline on otago schist. N. Zeal. J. Geol. Geophys. 57, 185–194. doi: 10.1080/00288306.2013.814575
Senapathi, D., Biesmeijer, J. C., Breeze, T. D., Kleijn, D., Potts, S. G., Carvalheiro, L. G., et al. (2015). Pollinator conservation - The difference between managing for pollination services and preserving pollinator diversity. Curr. Opin. Insect Sci. 12, 1–9. doi: 10.1016/j.cois.2015.11.002
Theodorou, P., Albig, K., Radzevičiut,e, R., Settele, J., Schweiger, O., Murray, T. E., et al. (2017). The structure of flower visitor networks in relation to pollination across an agricultural to urban gradient. Funct. Ecol. 31, 838–847. doi: 10.1111/1365-2435.12803
UNDESA. (2018). World Urbanization Prospects. New York, NY: UNDESA. Available online at: https://population.un.org/wup/Publications/Files/WUP2018-KeyFacts.pdf
Waser, N. M., Chittka, L., Price, M., Williams, N., and Ollerton, J. (1996). Generalization in pollination systems, and why it matters. Ecology 77, 1043–1060. doi: 10.2307/2265575
Wastian, L., Unterweger, P. A., and Betz, O. (2016). Influence of the reduction of urban lawn mowing on wild bee diversity (Hymenoptera, Apoidea). J. Hymenoptera Res. 49, 51–63. doi: 10.3897/JHR.49.7929
Wittmer, H. U., Anton, V., Gruber, M. A. M., Ireland, L., Linklater, W., Russell, J. C., et al. (2018). Conservation and restoration in peopled landscapes in Oceania: Opportunities and challenges. Pacific Conserv. Biol. 24, 409–416. doi: 10.1071/PC18072
Woinarski, J. C. Z., Connors, G. C, and Franklin, D. (2000). Thinking honeyeater: Nectar maps for the Northern Territory, Australia. Pacific Conserv. Biol. 6, 61. doi: 10.1071/PC000061
Keywords: urbanization, beekeeping, pollinators, conservation, flower visitation
Citation: Iwasaki JM and Hogendoorn K (2023) The conservation of urban flower visitors Down Under. Front. Sustain. Cities 5:1103257. doi: 10.3389/frsc.2023.1103257
Received: 20 November 2022; Accepted: 25 January 2023;
Published: 10 February 2023.
Edited by:
Natacha P. Chacoff, Universidad Nacional de Tucumán, ArgentinaReviewed by:
Ray Wyatt, The University of Melbourne, AustraliaCopyright © 2023 Iwasaki and Hogendoorn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jay M. Iwasaki,  amF5Lml3YXNha2lAYWRlbGFpZGUuZWR1LmF1
amF5Lml3YXNha2lAYWRlbGFpZGUuZWR1LmF1
 Jay M. Iwasaki
Jay M. Iwasaki Katja Hogendoorn
Katja Hogendoorn