
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Cities, 11 January 2023
Sec. Urban Greening
Volume 4 - 2022 | https://doi.org/10.3389/frsc.2022.1099100
This article is part of the Research TopicWorld Bee Day 2022: Pollinators in Urban EnvironmentsView all 10 articles
Wild insect pollinators are essential to cultivated and natural ecosystems globally. Today, many pollinator species are declining. One reason is a general lack of flowering habitats at landscape scales. However, urban areas, including private gardens, may provide flowers, and constitute beneficial habitats for pollinators. Here, we evaluate the ecological outcomes of a citizen science campaign run by the Swedish Society for Nature Conservation (SSNC) (called “Operation: Save the bees”), encouraging citizens to incorporate interventions beneficial to wild pollinators (garden meadows, flower plantings, and bee hotels) in their gardens. Data on insect observations and flowering plants were collected through online questionnaires at the end of the growing season. In total, we received 3,758 responses for the three interventions. We found that participants were more likely to observe many pollinators (as opposed to few or none) in more species rich garden meadows, and in larger and older plantings. The surrounding environment also affected pollinator abundance: fewer pollinators were observed in plantings in dense urban areas. Direct counts of pollinators during 10-min surveys correlated strongly to the simplistic abundance assessment (none, few, or many insects seen over the summer season). Bee hotel occupancy was positively related to local flower availability and bee hotel age. Smaller nest holes (<10 mm) were more occupied than larger holes (11–15 mm) and hotels in rural gardens and natural/semi-natural sites were more occupied than those in urban gardens. This study demonstrates that flower-rich private gardens provide integral habitat for wild pollinators and that citizen science programs can provide a tool for implementing and evaluating conservation practices. However, longer lasting commitment resulting in older interventions are preferable and should be encouraged in future campaigns.
Wild insect pollinators are essential to both natural and managed ecosystems. Globally, around 90% of flowering plants (Ollerton et al., 2011) and 75% of crop species (Klein et al., 2007) are, to some degree, dependent on pollinators for seed or fruit set. Bees are the most well-documented insect pollinators but also, e.g., flies, beetles, moths, butterflies, wasps, and ants can act as pollinators (Rader et al., 2016). However, many wild pollinator species are declining (Biesmeijer et al., 2006; Zattara and Aizen, 2021), e.g., due to anthropogenic land use change, which has reduced the area of suitable habitat for foraging and nesting, mainly flower rich grasslands such as traditional meadows and pastures (Goulson et al., 2015; IPBES, 2016). One way to increase the availability of flower-rich habitats is to integrate them and promote their uptake into private gardens and green spaces. Gardens and backyards cover as much as 30% of urban areas (Goddard et al., 2010) and have the potential to act as a pollinator refuges, both in urban (Baldock, 2020) and rural (Samnegård et al., 2011) areas. Importantly however, this potential is moderated by pollinator ecological and life history traits. For example, hoverflies are more sensitive to urbanization than bees (Verboven et al., 2014; Persson et al., 2020), most likely because their larval stage is often connected to specific habitats largely lacking in urban areas, e.g., shaded wooded habitats with dead organic matter (Bartsch, 2009). For bees, above-ground (cavity) nesting, social, and generalist species tend to benefit from moderate urbanization (Fortel et al., 2014; Wenzel et al., 2019; Fauviau et al., 2022), and especially in comparison to land use dominated by agriculture (Wenzel et al., 2019). For bee body size, the results are so far inconclusive, and both large and small species have been shown to benefit from different aspects of urbanization (Wenzel et al., 2019; Gathof et al., 2022). Hence, the effects of increasing the cover of flower-rich habitats in urban gardens are expected to vary between taxa and trait groups.
It is well-established that more local flower resources will attract pollinators and potentially benefit populations, both in urban (e.g., Quistberg et al., 2016; Baldock et al., 2019) and rural agricultural (e.g., Jönsson et al., 2015) settings. So called “urban meadows,” can be created either by reducing the intensity of mowing, or by sowing or planting seedlings of native herbaceous plants. They have been shown to benefit invertebrates in general (Garbuzov et al., 2015; Norton et al., 2019) and insect pollinators in particular (Blackmore and Goulson, 2014; Fischer et al., 2016), and to increase local insect pollinator diversity (Griffiths-Lee et al., 2022). Promoting meadow-like vegetation in private gardens and green spaces may thus benefit pollinators across urban residential areas. Traditional flowerbeds dominated by ornamental and non-native plants will mainly benefit generalist pollinator species (Hanley et al., 2014; Wenzel et al., 2019). As generalists are particularly common in urban areas, such resources can be expected to benefit a large proportion of urban pollinator communities (Wenzel et al., 2019). For example, small-scale additions of an exotic ornamental plant species in urban sites resulted in increased abundance and species density of small sized Halictid bees, with species density further increasing the following year (Simao et al., 2018). The rationale behind promoting bee hotels (often made from cut bamboo sticks or drilled holes in blocks of wood) is to benefit solitary cavity nesting bee populations through increased availability of nest sites. There is evidence from rural settings that man-made nests can led to increased populations (Steffan-Dewenter and Schiele, 2008), although the actual benefits of bee hotels are contested (MacIvor and Packer, 2015).
Given that urban areas are human-dominated landscapes, citizen science initiatives provide an outlet for engaging the public in pollinator conservation efforts, as well as to assess the effects of such efforts on pollinator communities. Residents invest both their time and money in gardens, allotments, and other private green spaces in order to provide, e.g., space for recreation (Barnes et al., 2020), and gardening of pollinator dependent crops (Lin and Egerer, 2017). There is thus great potential to introduce biodiversity friendly interventions and management of gardens (Goddard et al., 2013). To engage residents in local biodiversity conservation may also be important in the transition toward a more sustainable society, e.g., through the so called Pigeon paradox, hypothesizing that encounters with biodiversity where people live and work may lead to an increased understanding and engagement in biodiversity conservation (Dunn et al., 2006). Previous research has shown that people's perceived behavioral control (feeling able to help pollinators) is an important predictor of pro-pollinator actions (Knapp et al., 2021). Hence, it is important to evaluate to what degree people draw conclusions about the level of success of interventions based on the ecological outcomes, in this case pollinator activity and abundance.
Citizen science is a way for researchers to collect amounts of data that would not otherwise be possible by including society and individual voluntary citizens in the process (Bonney et al., 2009). Research is thus facilitated while the public is engaged and made aware of important issues. Internationally, there are several examples of successful citizen science projects focusing on pollinators [e.g., Bumble Bee Watch (North America), the Bumblebee Conservation trust's “Bee walk” (UK), and Spipoll (France)]. Such projects have the potential to generate data and knowledge relevant to pollinator conservation (e.g., Deguines et al., 2012; Bates et al., 2015; Griffiths-Lee et al., 2022).
The campaign “Operation: Rädda bina” (“Operation: Save the bees” in English), was run by The Swedish Society for Nature Conservation (SSNC) during 2018–2021. The aim was to benefit wild pollinators and especially bees by encouraging the public to increase the flower density in private gardens and green spaces, either through establishment of meadows or plantings, and to put up bee hotels (SSNC., 2022). Here, we aim to evaluate the citizen science project carried out in connection with the above-mentioned campaign. To this end, we use data from 2020 on three pollinator friendly interventions collected through online questionnaires administered by the SSNC. In addition, in order to verify the robustness of the simple pollinator assessment, we use data on direct pollinator counts from a follow-up survey done in 2021. We subsequently compare standardized counts with the simple assessment method.
We evaluate whether the campaign has given the desired result, that is, to what extent garden meadows, flower plantings, and bee hotels have attracted wild pollinators, and how the surrounding environment may have affected the outcome. The following ecological questions are examined:
(i) How is the abundance of pollinators in flower interventions (garden meadows and plantings) affected by the local quality of interventions in terms of size, age, and flower species richness?
(ii) How is the occupancy of bee hotels related to size of nesting cavities, bee hotel age, and surrounding flower availability?
(iii) How is the presence of pollinators in interventions moderated by the surrounding environment?
(iv) (How) does the abundance of pollinators observed affect how successful participants judge their flower intervention to be?
We expected that more pollinators would be observed in interventions that were larger, older and more flower-rich, and that bee hotels in moderately urbanized areas would be more occupied than in either highly urbanized or rural areas. We further expected that observing more insects would lead to a higher score for intervention success.
Data on the three interventions were collected in 2020 within the citizen-science SSNC campaign “Operation: Save the bees” using an online questionnaire prepared by the lead author (AP) in collaboration with officers at the SSNC. The campaign started in 2018 when volunteers in Sweden could register pollinator-friendly interventions that they had carried out in their gardens and other green spaces. The campaign encouraged three different interventions: (i) flowering “garden meadows,” (ii) bee-friendly flower plantings, and (iii) bee hotels. Those who registered that they had undertaken an intervention received an email with a link to questionnaires with queries regarding their intervention(s) at the end of the growing season in September 2020 (Table 1 and Supplementary material). Separate surveys were provided for each type of intervention. Hence, if a participant registered more than one type of intervention they received, and potentially answered, two or three separate surveys. The surveys were sent to all who had registered interventions between 2018 and 2020. Note that respondents could register interventions that had been established before the start of the campaign in 2018.
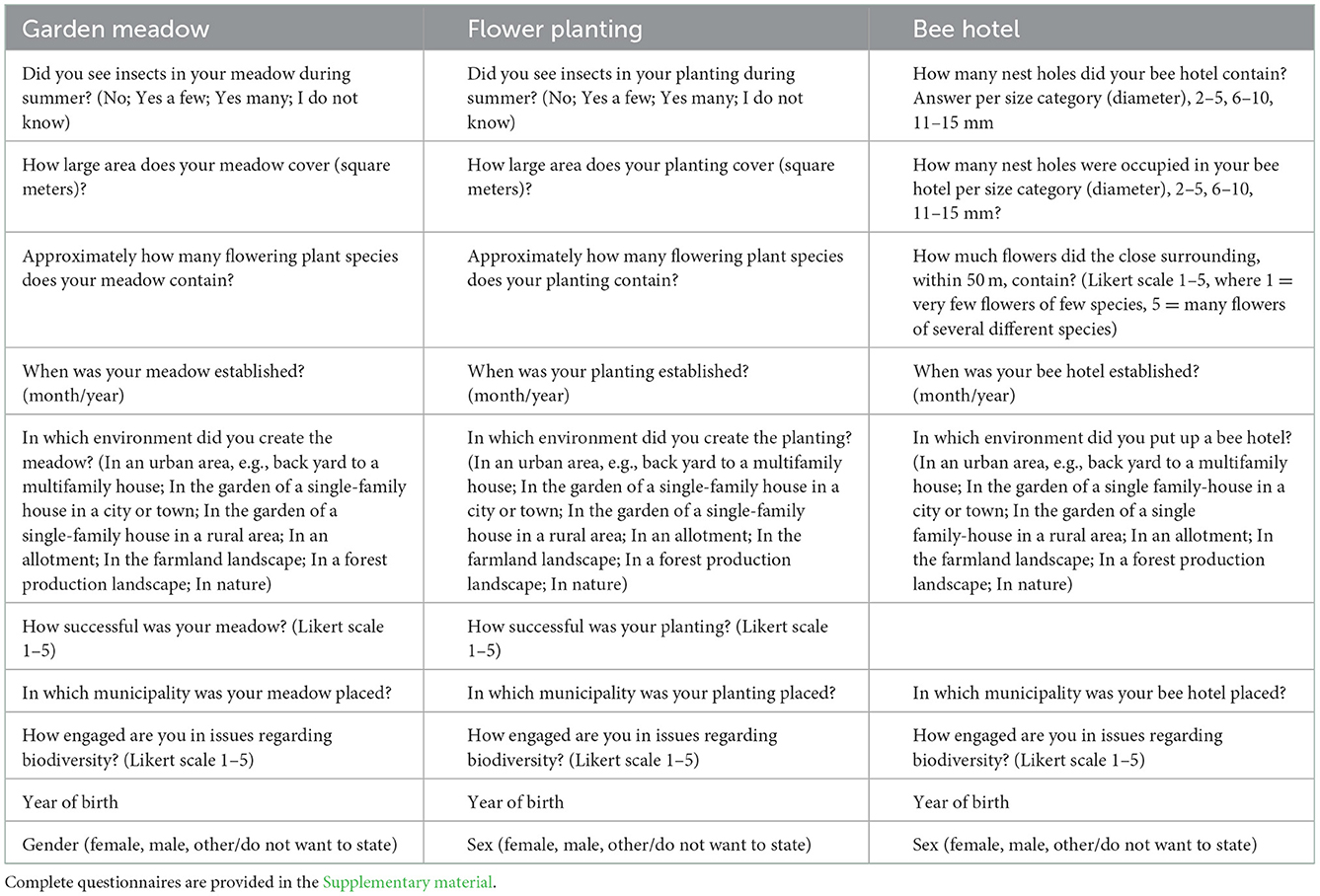
Table 1. The questions used to evaluate the success of interventions to benefit pollinators, with answer alternatives stated in parenthesis.
In the questionnaires, the participants were asked about flower-visiting insects in general, i.e., potential pollinators. Hereafter, we refer to them as pollinators. Participants were not asked to distinguish between different insect taxa. We used the questions related to the abundance of pollinators observed, the size, age, and floweriness or flowering plant species richness of the intervention, and the type of surrounding habitat for further analyses, Table 1. The assessment of abundance of insects in flower interventions was answered as either: “no insects,” “a few,” “many,” or “I do not know,” Table 1. Note that participants were not given any instruction on the definitions of “a few” and “many” insect pollinators.
Responses to the number of insects seen stating “I do not know” were removed from further analyses, as were interventions accidently stated to have been established before year 1900 or after September 2020, responses with an incorrect number of digits for year of establishment, and responses stating zero or >50 flowering plants species in flower interventions. One bee hotel, listed with a 1 million nest holes (a straw roof) was removed prior to analysis, resulting in a range of bee hotels with 1–2,500 nest holes. We also excluded responses where the number of occupied nest holes exceeded the total number of nest holes listed for the bee hotel, or where the number of nest holes per size category did not match the total number of nest holes listed. Collectively, this resulted in 370 responses being removed prior to analyses.
The number of insects seen in meadows and plantings was transformed into a binomial variable for further analyses, where 0 = No, or few, insects seen, and 1 = Many insects seen. We used year of establishment to infer age of intervention as a numerical factor 1–5, where interventions established before 2016 were merged into the oldest category (5) and years 2017–2020 were kept as four separate categories (4, 3, 2, 1). For the number of flowering plant species, responses of zero species were removed and the remaining responses were categorized in to five-step intervals: 1–5, 6–10, 11–15, 16–20, and >20 species. To assess potential effects of surrounding environment, the three non-garden categories (agricultural landscape, forestry landscape, and nature, Table 1) were merged into category rural. Gardens of urban single-family houses and allotment gardens were merged into category urban garden. Balconies and yards of multi-family houses were merged into category dense urban. Single-family rural gardens were kept as a single category, hereafter called rural garden. Thus, in total four environment categories were used for further analyses.
For the evaluation of how successful the respondents perceived their intervention to be (1–5, Likert scale), answers were grouped into three categories: low success (1–2), medium success (3), and highly successful (4–5).
To evaluate the accuracy of the simple assessment of pollinator abundance (none, few, or many insects seen in flower interventions), we used data from 2021 collected through another online questionnaire. Similar to 2020, this questionnaire was sent to all participants in the campaign 2018–2021, asking them to assess the abundance of insects in their intervention(s). In 2021, however, participants were also asked to complete a 10-min survey of 50 m2 of their garden, which included their flower intervention, and to count all flower visiting insects into five groups (bees and wasps, hoverflies, butterflies, beetles, and other insects). The survey was to be performed sometime between 11.00 and 16.00 on a calm, sunny, and warm day (>16°C) in July.
We modeled to what extent flowering interventions (meadows and plantings) were visited by pollinators using generalized linear models (GLM), with binomial error distribution. We specified separate models for meadows and plantings. The proportion of participants who stated they observed “many insects” (as opposed to “none, or few, insects”), were modeled as a function of intervention area (categorical), intervention age (numeric: 1–5), species richness of flowers (categorical), and the type of surrounding environment (categorical). We assessed if flower intervention age and plant species richness was correlated using Spearman rank correlations, for meadows and plantings separately.
We modeled bee hotel occupancy using a GLM, specified with a beta binomial distribution. Occupancy was modeled as a function of environment (categorical), nest size category (categorical: 2–5, 6–10, and 11–15 mm wide), degree of flowering (numeric: 1–5), and bee hotel age (numeric: 1–5). We accounted for zero-inflation in the response. We assessed the interaction between environmental and nest size category but this was non-significant (p = 0.8) and removed from the presented model.
We evaluated if seeing many pollinators affected the feeling of having established a successful flower intervention (meadow or planting) using GLMs, with a binomial error distribution. We modeled the proportion of participants that stated they observed “many insects” (as opposed to “none, or few”), as a function of perceived intervention success.
We evaluated the accuracy of the simple pollinator assessments using a GLM with a negative binomial distribution, modeling meadows (N = 165) and plantings (N = 218) separately. We summed counts of the three major pollinator groups counted during surveys in 2021 (bees and wasps, butterflies, and hoverflies) to assess how pollinator abundance related to participants simple scores of insect abundance (“many insects,” as opposed to “none, or few”).
All analyses were carried out in R v 4.1.1 (R Core Team, 2021). Model assumptions were checked with package DHARMa (Hartig, 2020). Variance inflation factors (VIF) for models of meadows and plantings were checked with package car function vif (Fox and Weisberg, 2019), while VIF for bee hotels were checked with package performance (Lüdecke et al., 2021). Contrasts between groups (for example different sizes of plantings) were analyzed using Tukey's test for post-hoc analysis in the Emmeans package (Lenth, 2022). Test results were obtained from Analysis of Deviance Table using Wald Chi-square tests (package car function Anova, Fox and Weisberg, 2019) and the ggplot2 package was used to visualize data and create graphs (Wickham, 2016).
Respondents' gender, year of birth, and engagement in issues related to biodiversity (self-rated, Likert scale 1–5) were compiled to describe whom the campaigned had reached and involved. All respondents from the meadow- and flower-planting surveys were included, even if their answers had previously been removed from analyses of ecological questions due to incomplete data. We were interested in how changes to vegetation quality affects peoples' perception of their garden, and therefore did not include data from bee hotel-respondents. Moreover, in contrast to added flower resources, the benefits of bee hotels are contested.
In total, 3,758 survey responses were received for registered interventions: 898 for meadows, 1,281 for flower plantations, and 1,580 for bee hotels. After data curation (see above) 809 remained for meadows, 1,232 for plantations, and 1,210 for bee hotels. Approximately 19% of meadows, 23% of plantings, and 20% of bee hotels were situated in the 10 most populated cities/municipalities of Sweden (Stockholm, Göteborg, Malmö, Uppsala, Upplands Väsby and Sollentuna, Västerås, Örebro, Linköping, Helsingborg, and Jönköping), all situated in the southern third of the country. The vast majority of intervention were carried out in single-family residential gardens, in either urban or rural locations: 83% of meadows, 75% of plantings, and 82% of bee hotels.
The number of participants who saw many pollinators was related to the number of flowering species in the meadow (χ2 = 40.247, df = 4, p < 0.001). Post-hoc tests revealed that fewer participants had reported many pollinators in meadows that contained 1–5, as compared to >5, flowering plant species (Figure 1). The meadow size and its surrounding environment had no significant effect on the likelihood of reporting many pollinators (size: χ2 = 6.899, df = 4, p = 0.141; environment: χ2 = 2.057, df = 3, p = 0.561), while meadow age showed a non-significant positive trend (age: χ2 = 3.122, df = 1, p = 0.077). Meadow age and plant species richness were positively correlated (rho = 0.24, p < 0.001), but not strong enough to preclude inclusion in the same models (checked with VIFs, as above).
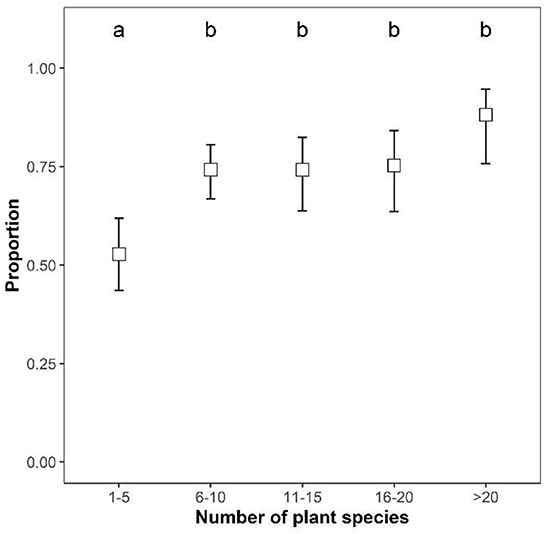
Figure 1. The proportion (estimated marginal means ± 95% confidence levels) of participants that reported having seen “many insects” in relation to flowering plant species richness in meadows. Pairwise comparisons of estimated marginal means are indicated by compact letter display, where plant species richness categories sharing a letter are not significantly different.
The proportion of participants that saw many pollinators was positively related to the age and size of the planting (age: χ2 = 9.35, df = 1, p = 0.002; size: χ2 = 31.24, df = 2, p < 0.001; Figures 2A, B). A lower proportion of participants saw many pollinators when plantings were situated in dense urban, compared to the other environments (χ2 = 19.08, df = 3, p < 0.001, Figure 2C). The number of flowering plant species had no significant effect on the abundance of pollinators seen (χ2 = 2.17, df = 4, p = 0.71). As for meadows, age and plant species richness were positively correlated (rho = 0.36, p < 0.001), but not strong enough to preclude inclusion in the same models (checked with VIFs, as above).
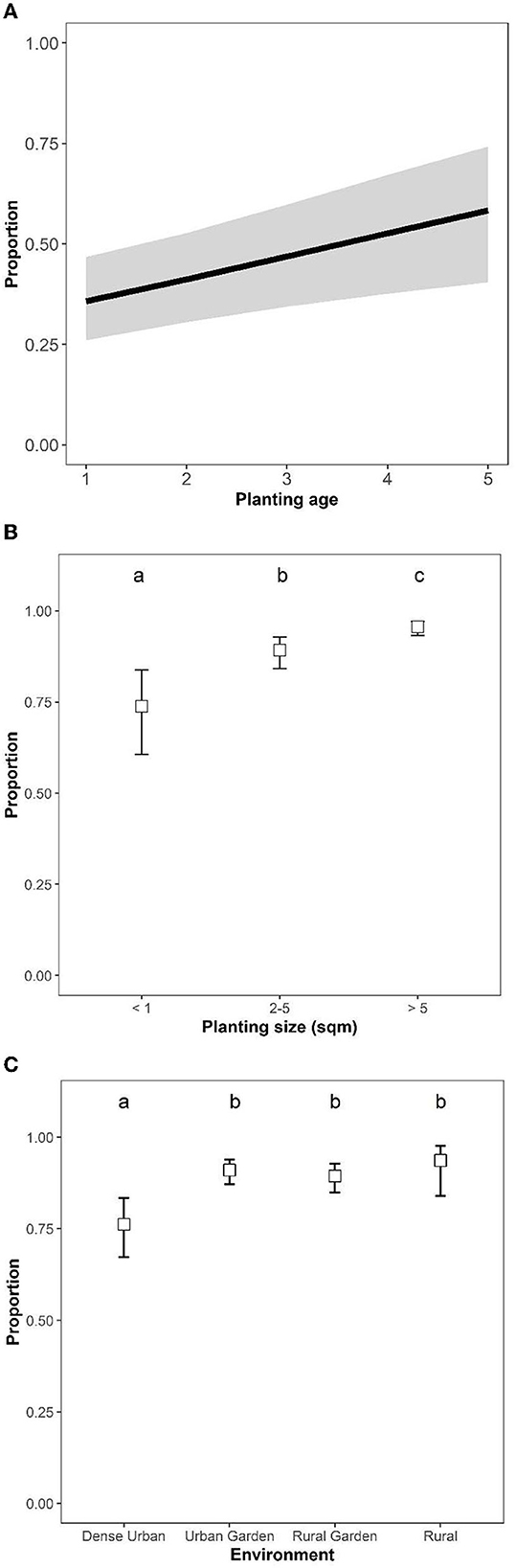
Figure 2. The proportion of participants (estimated marginal means ± 95% confidence levels) who reported having seen “many insects” in their plantings in relation to (A) planting age, (B) planting size, and (C) the surrounding environment. Pairwise comparisons of estimated marginal means are indicated by compact letter display such that environments sharing a letter are not significantly different.
A higher proportion of participants who reported having seen many pollinators also viewed their meadow as successfully established (χ2 = 108.46, df = 2, p < 0.001, Figure 3A). More participants reported they had observed many pollinators in meadows with the highest success rating, compared to meadows of either medium or low success, while participants with a medium success rate were in between the low and highly successful. Similarly, a higher proportion of participants who viewed their plantation as succesfull reported to have seen many insects, compared to those with a low or medium success rating (χ2 = 87.85, df = 2, p < 0.001, Figure 3B).
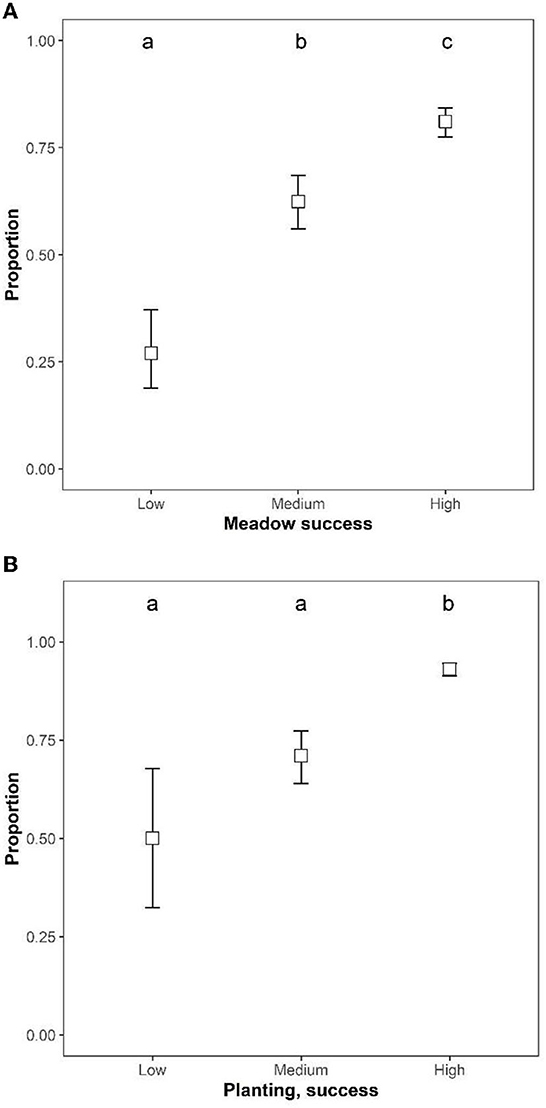
Figure 3. The proportion of participants (estimated marginal means ± 95% confidence levels), with meadows (A) or plantings (B) that reported having seen “many insects” in relation to their perception of intervention success. Pairwise comparisons of estimated marginal means are indicated by compact letter display. Success levels sharing a letter are not significantly different.
Bee hotel occupancy rates were positively related to both local flower availability (χ2 = 20.5, p < 0.001, Figure 4A) and bee hotel age (χ2 = 69.85, p < 0.001, Figure 4B). Furthermore, occupancy rates differed between nest size categories (χ2 = 101.99, p < 0.001, Figure 4C). Occupancy was significantly higher in small and medium sized holes (2–5, 6–10 mm diameter), than in large holes (11–15 mm). Occupancy rates differed significantly between environment types (χ2 = 74.69, p < 0.001, Figure 4D). In particular, occupancy was significantly higher in both rural environments than either of the urban environments.
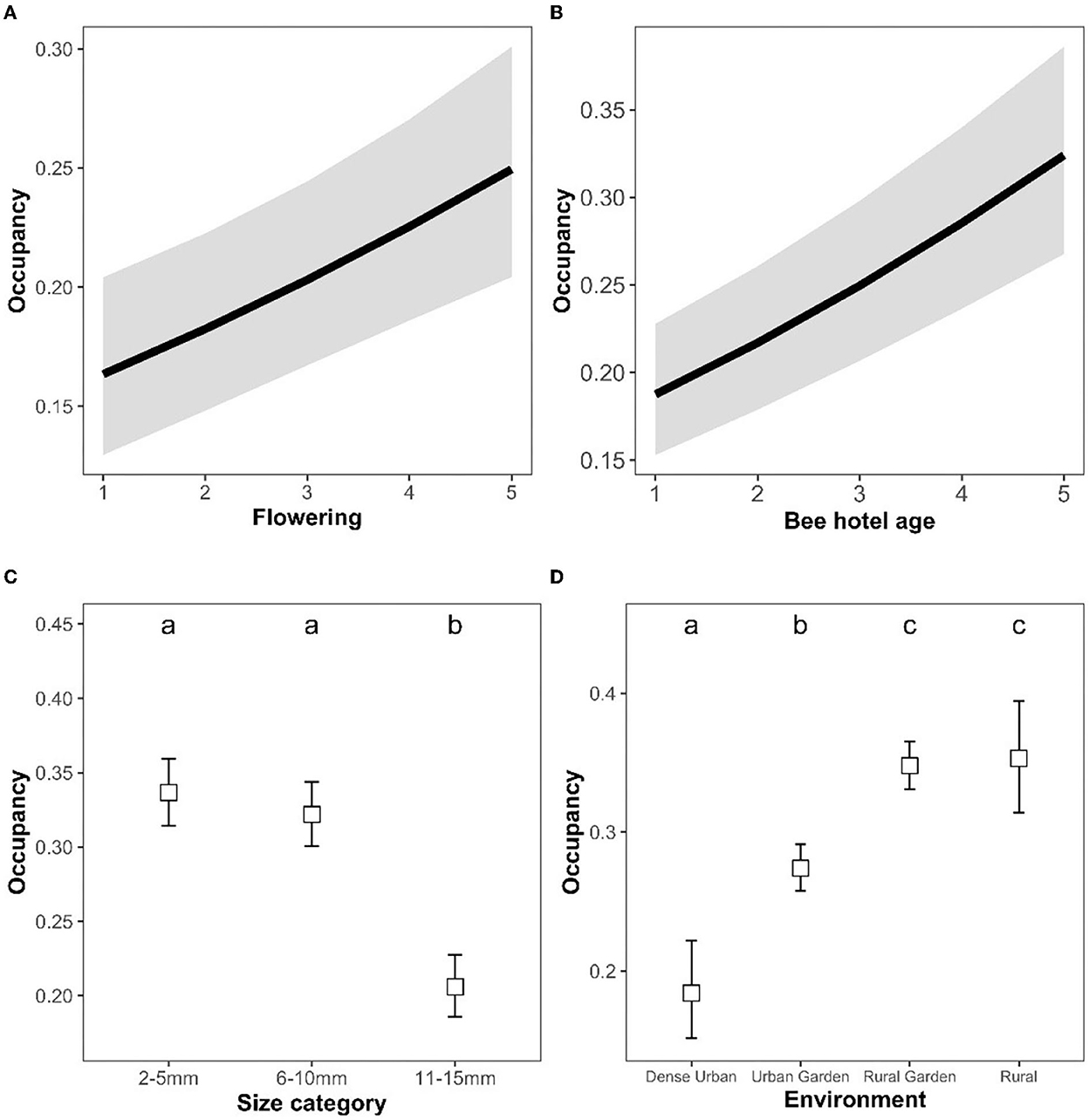
Figure 4. Bee hotel nest occupancy (estimated marginal means ± 95% confidence levels) in relation to (A) local flower availability, (B) bee hotel age, (C) nest hole size (diameter), and (D) surrounding environment. Pairwise comparisons of estimated marginal means (C, D) are indicated by compact letter display. Means (of size category and environment, respectively) sharing a letter is not significantly different.
For both meadows and plantings, the pollinator abundance assess by 10-min surveys was highly positively related to if participants reported having seen “none or few” or “many” insects (meadows: z = 3.668, p < 0.001; plantings: z = 5.167, p < 0.001).
The vast majority (80%) of survey respondents were women and most (47%) were aged 41–60 years, while the categories aged 20–40 and 61–80 years made up 27% and 25%, respectively. The majority (69%) considered themselves to have a strong engagement (4–5 on a 1–5 Likert scale) in issues related to biodiversity conservation, while 26% classified themselves as equally committed as the societal average (3).
Using data collected by citizens, we show that the ecological benefits of simple measures to enhance foraging resources for pollinators in private gardens and green spaces were moderated by flower species richness (for meadows), age and size (for plantings): older, more species rich, and larger flower interventions attracted more pollinators than newly established, species poor, or small ones. Similarly, the added nesting resources for bees (bee hotels) were more occupied when they were older and situated in more flower-rich gardens, compared to younger hotels in flower-poor gardens. In addition, smaller nest holes (2–10 mm wide), were more occupied than large ones (11–15 mm wide). There was a negative effect of urban environments, as both bee hotels and plantings situated in urban gardens and/or dense urban areas were less visited by pollinators. We also found that flowering interventions were perceived as more successful when they attracted many pollinators.
Positive effects of local flower species richness and abundance on the diversity and abundance of pollinator communities has previously been reported using traditional research methods, both in urban gardens (Quistberg et al., 2016; Del Toro and Ribbons, 2020), in rural experimental (Hegland and Boeke, 2006; Ebeling et al., 2008), and in agricultural settings (Potts et al., 2009; Jönsson et al., 2015). In addition, a citizen science project using standardized sampling methods showed that sown garden meadows enhanced local pollinator abundance and diversity over a 2-year period (Griffiths-Lee et al., 2022). Our results corroborate these findings, and in addition show that a very simple measurement, such as perceived pollinator abundance estimated by citizen scientists, may be used as a proxy for abundance to assess and compare the value of pollinator enhancement interventions.
The availability of local flower resources has been highlighted as a key factor for urban pollinator abundance and diversity (e.g., reviewed by Wenzel et al., 2019; but see Gathof et al., 2022) and may even buffer bee populations against the negative effects of landscape scale urbanization (Burdine and McCluney, 2019). Although we cannot evaluate the effects on pollinator populations in the wider landscape, even small-scale flower enhancements may result in population level effects if implemented on a large enough scale. For example, based on research in agricultural landscapes (e.g., Cong et al., 2014; Jönsson et al., 2015), one may expect that neighborhoods where uptake of interventions is high can support more pollinators at the landscape scale.
There was no effect of plant species richness in plantings on pollinator abundance. This may be because flowerbeds in general are highly dominated by ornamental and non-native plant species (Loram et al., 2007; Lowenstein and Minor, 2016), and therefore mainly cater for generalist pollinator species (Corbet et al., 2001; Wenzel et al., 2019). Adding more plant species to a flowerbed may then still only benefit the same part of the pollinator community and thus to a lesser degree attract more pollinators of other species (but see Simao et al., 2018; Staab et al., 2020). Simao et al. (2018) also show that, for small generalist bees, additions of urban flower resources had the strongest (positive) effect at low surrounding resource levels, whereas at higher levels the effect was unpredictable. Most (75%) of respondents reported plantings from single-family housing areas and we expect a generally high level of flowering of ornamental plants in such locations. In contrast, adding more plant species to a garden meadow dominated by native plants may increase the attractiveness of the garden to a wider array of pollinator species, including some specialists. Participants were only asked about the number of plant species present in their meadows or plantings, not about the abundance. It is therefore possible that we had seen a positive effect of flower abundance on pollinator activity in both meadows and plantings (as we did for occupancy of bee hotels), had we measured this variable. Indeed, the size of plantings, which is likely positively related to flower abundance, had a positive effect on pollinator abundance.
Our results highlight the enhanced benefit of older flowering elements and bee hotels, and thus the need for gardeners to make more lasting commitments to changes in garden design and management. The significant positive effect of planting age (and the non-significant positive trend for meadows) could be because perennial plants, which are often preferred by bumblebees (Fussell and Corbet, 1992), often require several years to establish and flower from seed. Gardeners may make several attempts at sowing or planting new species into an intervention, thus intentionally increasing plant richness over time. In addition, spontaneous establishment of plant species, especially in garden meadows, may lead to increased plant diversity over time (Norton et al., 2019). Age and plant diversity were indeed positively correlated. Another explanation could be that beneficial micro-habitats build up over time in gardens and flower beds, including bare patches of soil for ground nesting bees, dead wood and stems with hollows for cavity nesters, and dead organic matter for some hoverfly taxa, allowing a delayed response of pollinator populations to an intervention. For bee hotels, the philopatric behavior of many solitary bee species may explain why occupancy builds up over time. The increased occupancy of older nests has previously been described for the common species Osmia bicornis (Steffan-Dewenter and Schiele, 2004; Fortel et al., 2016).
The largest nest cavities (11–15 mm) were far less inhabited than smaller ones (2–5 and 6–10 mm). Most likely, this is due to there being few bee or wasp species in Sweden that use nests larger than 10 mm; Recommendations for bee hotels in temperate regions rarely stretch past 12 mm (e.g., Naturhistoriska Riksmuseet, 2013; Bauer et al., 2015; Winter, 2018). Clear information about preferred size and design of bee hotels may thus increase the occupancy of hotels in future campaigns.
Bee species differ in their requirements for nesting conditions. Of Sweden's approximately 250 solitary bee species, around 70% are ground nesters, and only a small fraction of species are known to nest in bee hotels (Linowski et al., 2004; Naturhistoriska Riksmuseet, 2013). Despite this, bee hotels may be useful bio-indicators for insect pollinators in general (Tscharntke et al., 1998). A garden where a bee hotel is highly occupied can thus be expected to host many other pollinating insects, either nesting in and/or visiting the garden to forage.
We found that the surrounding environment moderated pollinator abundances in flower interventions, such that plantings in single-family urban or rural gardens and rural natural environments were more visited by pollinators, compared to yards and green spaces in dense urban areas. Similarly, bee hotels in both rural gardens and natural environments were more occupied compared to those in both types of urban sites. Our results thus corroborate previous research showing that urbanization is generally negative for insect abundance and diversity, including pollinators (Fortel et al., 2014; Geslin et al., 2016; Fenoglio et al., 2020; Piano et al., 2020). However, pollinator taxa and trait groups differ in sensitivity to urbanization. Butterflies (Fenoglio et al., 2020; Piano et al., 2020) and hoverflies (e.g., Verboven et al., 2014; Persson et al., 2020) are generally negatively affected by urbanization and, while a recent meta-analysis show that bee diversity is negatively affected by urbanization (Fenoglio et al., 2020), other studies have shown that cavity nesting and long tongued bee species may actually benefit from intermediate to high levels of urbanization (Fortel et al., 2014; Wenzel et al., 2019). Our results show that urban bee hotels were less occupied than those in rural sites, indicating that cavity nesting species were actually less abundant in urban areas of Sweden. This could partly be explained by the large geographical uptake of the campaign, whereby we likely included rural sites spanning from those embedded in production landscapes to those rich in semi-natural or natural habitats, where the latter may harbor high bee abundance and diversity. Alternatively, and a bit speculative, bee hotels in urban areas may be of lower quality than those in rural or natural sites; e.g., they may more often be store-bought rather than home-made or place-built, and/or placed in too exposed or too shaded sites. This may make them less attractive to nesting bees compared to those in rural/natural sites (von Königslöw et al., 2019).
Regarding bee body size, results are so far inconclusive. While some studies show that small bodied species may benefit from highly urbanized areas (Banaszak-Cibicka and Zmihorski, 2012; Gathof et al., 2022), other studies find the opposite (reviewed by Wenzel et al., 2019). In addition, body size and nesting substrate may be correlated, such that small bees more often are ground nesters (Banaszak-Cibicka and Zmihorski, 2012). Our results do not indicate that any size class of cavity nesting bees benefit from urban areas (non-significant interaction for environment and size class). However, we have not assessed ground nesting species and may therefore have missed genera that are particularly well-adapted to urban environments.
Flowering interventions were seen as more successful when they attracted many pollinators, indicating that respondents evaluated their interventions based on the desired ecological outcome (to provide flowers and benefit pollinators). Previous research has shown that pro-pollinator actions may be conditional on the degree to which people perceive that their actions will indeed benefit pollinators (Knapp et al., 2021). Although not tested here, this may lead to a reinforcing loop, where perceived successful interventions remain, while less successful ones are terminated.
People who choose to design and manage their gardens to benefit biodiversity do so for a multitude of reasons, ranging from aesthetics and personal well-being to a sense of moral responsibility for nature (Freeman et al., 2012; Goddard et al., 2013; Knapp et al., 2021). People who are personally engaged and interested in biodiversity may also be more likely to perform acts beneficial to biodiversity (see e.g., Maiteny, 2002). This could explain why the majority of the respondents in this study stated that they are highly engaged in issues concerning biodiversity. Indeed, a growing number of studies highlight citizen science as a tool in the transition to a more sustainable society by strengthening, encouraging, and validating public participation in environmental and sustainability issues (Dickinson et al., 2012; Shulla et al., 2020).
While citizen science projects with appropriate organization and design have been shown to provide data with similar quality as that collected by professionals (Danielsen et al., 2014; Henckel et al., 2020), problems concerning data reliability and quality may occur (Bonney et al., 2014; MacPhail and Colla, 2020). For example, Mason and Arathi (2019) show that a citizen science program that included volunteer training gave reliable data concerning pollinator presence only at the level of morpho-species, while species specific mapping was less accurate. The campaign “Operation: Save the bees” did not include training of participants in doing insect observations. Respondents will thus have very different levels of understanding and knowledge about flower visiting insects and the research methods, and answers may therefore vary between rough estimates and exact answers. The lack of definition of “few” vs. “many” insects is another weakness. On the other hand, the questions asked here were kept simple precisely in order not to require prior knowledge on pollinator or plant species identification, and only 1% of respondents specifically stated that they found certain questions difficult to answer (data not shown). Our results align with expectations based on previous scientific studies, indicating that data is of acceptable quality in relation to the questions asked and statistical models used. However, our study may suffer from so called “expectation bias” regarding insect observations in flower interventions, such that respondents that have a more species rich flower interventions also expect to see more insects, and thereby report too high abundances. Our evaluation of the simple pollinator estimate, using a more structured flower visiting insect survey, indicate that the simple measure was valid. Even so, using a standardized survey protocol and adding surveys by trained staff as a control, would make possible proper evaluation of the simple method used. Training, e.g., through (online) instruction videos or workshops and interactions with campaign staff, and using photo and expert identification, could further improve both data quality and reporting frequency and allow the study of more complex research questions (e.g., Deguines et al., 2016; MacPhail and Colla, 2020). Training participants using multimedia, and using social media to promote citizen science projects and help participants with insect identifications, has been shown to be successful both in terms of project outreach and an increased interest and awareness of the benefits provided by insects (Griffin et al., 2021, 2022).
The majority of the respondents were middle-aged women highly engaged in biodiversity. There can be both age and gender differences regarding engagement, knowledge, roles, and responsibility in relation to biodiversity and (wildlife) gardening (Soga and Gaston, 2018; Jones and Niemiec, 2020; Hanson et al., 2021). Although we do not know to what extent respondents singlehandedly established and surveyed interventions, the results indicates that engagement and uptake of interventions could be further increased by engaging a more diverse group of participants, including more men and younger people in the campaign. Indeed, a recent study in Great Britain suggest that men are overrepresented in environmental citizen science programs (Pateman et al., 2021). Highlighting the science part of the campaign, e.g., through multi-media resources and outreach such as developed by Griffin et al. (2021), could therefore attract and involve more men.
Our results show that data collected by citizens can be a useful tool for evaluation of small-scale conservation interventions in private green spaces. Larger and more species rich flower interventions that last for multiple years, attracted more pollinators, and should thus be promoted in future campaigns. Supporting information and assistance on how to establish and manage garden meadows (e.g., through online fora) could increase the success rate and promote more species rich and long lasting interventions. In addition, differentiating the recommendations regarding plant choice based on soil type and surrounding environment (e.g., urban, rural, latitude/climate zone) may improve outcomes.
The results indicate that the flower interventions registered through “Operation: Save the bees” may have a positive effect on local insect pollinator abundance. The fact that private gardens can be efficient tools in supporting biodiversity in general (Goddard et al., 2010) and pollinators in particular (e.g., Samnegård et al., 2011; Martins et al., 2017; Baldock et al., 2019) merits further work on how to engage the public in biodiversity friendly gardening practices.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
SSNC and Lund University share the ownership and responsibility for the data collected through the campaign. The data used does not contain any personal information. People who participated in the campaign and completed the survey did so voluntarily.
AP conceived the idea, designed the study, and collected data together with officers at SSNC. IL, VH, and LK analyzed the data with input from AP and LN. IL wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
AP was funded by Formas (Grant no. 2019-01524). VH was partly funded by the European Research Council (ERC) under the European Union's Horizon 2020 Research and Innovation Programme (Grant Agreement no. 819374).
The authors are grateful to all participants in the campaign, and for the collaboration and help from several officers at Svenska Naturskyddsföreningen (SSNC) throughout the project. The authors are affiliated with the Strategic Research Area BECC (Biodiversity and Ecosystem services in a Changing Climate).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frsc.2022.1099100/full#supplementary-material
Baldock, K. C. (2020). Opportunities and threats for pollinator conservation in global towns and cities. Curr. Opin. Insect Sci. 38, 63–71. doi: 10.1016/j.cois.2020.01.006
Baldock, K. C., Goddard, M. A., Hicks, D. M., Kunin, W. E., Mitschunas, N., Morse, H., et al. (2019). A systems approach reveals urban pollinator hotspots and conservation opportunities. Nat. Ecol. Evol. 3, 363–373. doi: 10.1038/s41559-018-0769-y
Banaszak-Cibicka, W., and Zmihorski, M. (2012). Wild bees along an urban gradient: Winners and losers. J. Insect Conserv. 16, 331–343. doi: 10.1007/s10841-011-9419-2
Barnes, M. R., Nelson, K. C., and Dahmus, M. E. (2020). What's in a yardscape? A case study of emergent ecosystem services and disservices within resident yardscape discourses in Minnesota. Urban Ecosyst. 23, 1167–1179. doi: 10.1007/s11252-020-01005-2
Bartsch, H. (2009). Tvåvingar: Blomflugor: Eristalinae and Mictodontinae. Diptera: Syrphidae: Eristalinae and Mictodontinae. Uppsala: ArtdataBanken, Sveriges Lantbruksuniversitet (SLU).
Bates, A. J., Lakeman Fraser, P., Robinson, L., Tweddle, J. C., Sadler, J. P., West, S. E., et al. (2015). The OPAL bugs count survey: Exploring the effects of urbanisation and habitat characteristics using citizen science. Urban Ecosyst. 18, 1477–1497. doi: 10.1007/s11252-015-0470-8
Bauer, E. C., Lynch, L. I., Golick, D. A., and Weissling, T. J. (2015). Creating a Solitary Bee Hotel. University of Nebraska-Lincoln Extension, Institute of Agriculture and Natural Resources. Available online at: https://extensionpublications.unl.edu/assets/pdf/g2256.pdf (accessed November 10, 2022).
Biesmeijer, J. C., Roberts, S. P. M., Reemer, M., Ohlemuller, R., Edwards, M., Peeters, T., et al. (2006). Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313, 351–354. doi: 10.1126/science.1127863
Blackmore, L. M., and Goulson, D. (2014). Evaluating the effectiveness of wildflower seed mixes for boosting floral diversity and bumblebee and hoverfly abundance in urban areas. Insect Conserv. Divers. 7, 480–484. doi: 10.1111/icad.12071
Bonney, R., Cooper, C. B., Dickinson, J., Kelling, S., Phillips, T., Rosenberg, K. V., et al. (2009). Citizen science: A developing tool for expanding science knowledge and scientific literacy. Bioscience 59, 977–984. doi: 10.1525/bio.2009.59.11.9
Bonney, R., Shirk, J. L., Phillips, T. B., Wiggins, A., Ballard, H. L., Miller-Rushing, A. J., et al. (2014). Next steps for citizen science. Science 343, 1436–1437. doi: 10.1126/science.1251554
Burdine, J. D., and McCluney, K. E. (2019). Interactive effects of urbanization and local habitat characteristics influence bee communities and flower visitation rates. Oecologia 190, 715–723. doi: 10.1007/s00442-019-04416-x
Cong, R.-G., Smith, H. G., Olsson, O., and Brady, M. (2014). Managing ecosystem services for agriculture: Will landscape-scale management pay? Ecol. Econ. 99, 53–62. doi: 10.1016/j.ecolecon.2014.01.007
Corbet, S. A., Bee, J., Dasmahapatra, K., Gale, S., Gorringe, E., La Ferla, B., et al. (2001). Native or exotic? Double or single? Evaluating plants for pollinator-friendly gardens. Ann. Bot. 87, 219–232. doi: 10.1006/anbo.2000.1322
Danielsen, F., Jensen, P. M., Burgess, N. D., Altamirano, R., Alviola, P. A., Andrianandrasana, H., et al. (2014). A multicountry assessment of tropical resource monitoring by local communities. Bioscience 64, 236–251. doi: 10.1093/biosci/biu001
Deguines, N., Julliard, R., de Flores, M., and Fontaine, C. (2012). The whereabouts of flower visitors: Contrasting land-use preferences revealed by a country-wide survey based on citizen science. PLoS ONE 7:e45822. doi: 10.1371/journal.pone.0045822
Deguines, N., Julliard, R., de Flores, M., and Fontaine, C. (2016). Functional homogenization of flower visitor communities with urbanization. Ecol. Evol. 6, 1967–1976. doi: 10.1002/ece3.2009
Del Toro, I., and Ribbons, R. R. (2020). No Mow May lawns have higher pollinator richness and abundances: An engaged community provides floral resources for pollinators. PeerJ 8, e10021. doi: 10.7717/peerj.10021
Dickinson, J. L., Shirk, J., Bonter, D., Bonney, R., Crain, R. L., Martin, J., et al. (2012). The current state of citizen science as a tool for ecological research and public engagement. Front. Ecol. Environ. 10, 291–297. doi: 10.1890/110236
Dunn, R. R., Gavin, M. C., Sanchez, M. C., and Solomon, J. N. (2006). The pigeon paradox: Dependence of global conservation on urban nature. Conserv. Biol. 20, 1814–1816. doi: 10.1111/j.1523-1739.2006.00533.x
Ebeling, A., Klein, A.-M., Schumacher, J., Weisser, W. W., and Tscharntke, T. (2008). How does plant richness affect pollinator richness and temporal stability of flower visits? Oikos 117, 1808–1815. doi: 10.1111/j.1600-0706.2008.16819.x
Fauviau, A., Baude, M., Bazin, N., Fiordaliso, W., Fisogni, A., Fortel, L., et al. (2022). A large-scale dataset reveals taxonomic and functional specificities of wild bee communities in urban habitats of Western Europe. Sci. Rep. 12, 18866. doi: 10.1038/s41598-022-21512-w
Fenoglio, M. S., Rossetti, M. R., and Videla, M. (2020). Negative effects of urbanization on terrestrial arthropod communities: A meta-analysis. Glob. Ecol. Biogeogr. 29, 1412–1429. doi: 10.1111/geb.13107
Fischer, L. K., Eichfeld, J., Kowarik, I., and Buchholz, S. (2016). Disentangling urban habitat and matrix effects on wild bee species. PeerJ 4, e2729. doi: 10.7717/peerj.2729
Fortel, L., Henry, M., Guilbaud, L., Guirao, A. L., Kuhlmann, M., Mouret, H., et al. (2014). Decreasing abundance, increasing diversity and changing structure of the wild bee community (Hymenoptera: Anthophila) along an urbanization gradient. PLoS ONE 9:e104679. doi: 10.1371/journal.pone.0104679
Fortel, L., Henry, M., Guilbaud, L., Mouret, H., and Vaissière, B. E. (2016). Use of human-made nesting structures by wild bees in an urban environment. J. Insect Conserv. 20, 239–253. doi: 10.1007/s10841-016-9857-y
Fox, J., and Weisberg, S. (2019). An R Companion to Applied Regression, 3rd Edn. Thousand Oaks, CA: Sage.
Freeman, C., Dickinson, K. J. M., Porter, S., and van Heezik, Y. (2012). “My garden is an expression of me”: Exploring householders' relationships with their gardens. J. Environ. Psychol. 32, 135–143. doi: 10.1016/j.jenvp.2012.01.005
Fussell, M., and Corbet, S. A. (1992). Flower usage by bumble-bees: A basis for forage plant management. J. Appl. Ecol. 29, 451–465. doi: 10.2307/2404513
Garbuzov, M., Fensome, K. A., and Ratnieks, F. L. W. (2015). Public approval plus more wildlife: Twin benefits of reduced mowing of amenity grass in a suburban public park in Saltdean, UK. Insect Conserv. Divers. 8, 107–119. doi: 10.1111/icad.12085
Gathof, A. K., Grossmann, A. J., Herrmann, J., and Buchholz, S. (2022). Who can pass the urban filter? A multi-taxon approach to disentangle pollinator trait–environmental relationships. Oecologia 199, 165–179. doi: 10.1007/s00442-022-05174-z
Geslin, B., Le Féon, V., Folschweiller, M., Flacher, F., Carmignac, D., Motard, E., et al. (2016). The proportion of impervious surfaces at the landscape scale structures wild bee assemblages in a densely populated region. Ecol. Evol. 6, 6599–6615. doi: 10.1002/ece3.2374
Goddard, M. A., Dougill, A. J., and Benton, T. G. (2010). Scaling up from gardens: Biodiversity conservation in urban environments. Trends Ecol. Evol. 25, 90–98. doi: 10.1016/j.tree.2009.07.016
Goddard, M. A., Dougill, A. J., and Benton, T. G. (2013). Why garden for wildlife? Social and ecological drivers, motivations and barriers for biodiversity management in residential landscapes. Ecol. Econ. 86, 258–273. doi: 10.1016/j.ecolecon.2012.07.016
Goulson, D., Nicholls, E., Botias, C., and Rotheray, E. L. (2015). Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347, 1435–1444. doi: 10.1126/science.1255957
Griffin, B., LaTora, A. G., Bhattarai, U., and Braman, S. K. (2022). Knowledge gleaned from the first great Georgia pollinator census. J. Entomol. Sci. 57, 39–63. doi: 10.18474/JES21-05
Griffin, B, Braman, R., Griffin, M., and Sarieh, Y. (2021). The strategic use of multimedia in the great Georgia pollinator census citizen science project. Citizen Sci. Theory Pract. 6, 1–13. doi: 10.5334/cstp.334
Griffiths-Lee, J., Nicholls, E., and Goulson, D. (2022). Sown mini-meadows increase pollinator diversity in gardens. J. Insect Conserv. 26, 299–314. doi: 10.1007/s10841-022-00387-2
Hanley, M. E., Awbi, A. J., and Franco, M. (2014). Going native? Flower use by bumblebees in English urban gardens. Ann. Bot. 113, 799–806. doi: 10.1093/aob/mcu006
Hanson, H. I., Eckberg, E., Widenberg, M., and Olsson, J. A. (2021). Gardens' contribution to people and urban green space. Urban For. Urban Green. 63, 127198. doi: 10.1016/j.ufug.2021.127198
Hartig, F. (2020). DHARMa: Residual Diagnostics for Hierarchical (Multi-level/Mixed) Regression Models. Available online at: http://florianhartig.github.io/DHARMa/ (accessed November 11, 2022).
Hegland, S. J., and Boeke, L. (2006). Relationships between the density and diversity of floral resources and flower visitor activity in a temperate grassland community. Ecol. Entomol. 31, 532–538. doi: 10.1111/j.1365-2311.2006.00812.x
Henckel, L., Bradter, U., Jönsson, M., Isaac, N. J. B., and Snäll, T. (2020). Assessing the usefulness of citizen science data for habitat suitability modelling: Opportunistic reporting versus sampling based on a systematic protocol. Divers. Distrib. 26, 1276–1290. doi: 10.1111/ddi.13128
IPBES (2016). Summary for Policymakers of the Assessment Report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services on Pollinators, Pollination and Food Production. Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services, Bonn, Germany.
Jones, M. S., and Niemiec, R. M. (2020). Social-psychological correlates of personal-sphere and diffusion behavior for wildscape gardening. J. Environ. Manage. 276, 111271. doi: 10.1016/j.jenvman.2020.111271
Jönsson, A. M., Ekroos, J., Dänhardt, J., Andersson, G. K. S., Olsson, O., and Smith, H. G. (2015). Sown flower strips in southern Sweden increase abundances of wild bees and hoverflies in the wider landscape. Biol. Conserv. 184, 51–58. doi: 10.1016/j.biocon.2014.12.027
Klein, A. M., Vaissiere, B. E., Cane, J. H., Steffan-Dewenter, I., Cunningham, S. A., Kremen, C., et al. (2007). Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 274, 303–313. doi: 10.1098/rspb.2006.3721
Knapp, J. L., Phillips, B. B., Clements, J., Shaw, R. F., and Osborne, J. L. (2021). Socio-psychological factors, beyond knowledge, predict people's engagement in pollinator conservation. People Nat. 3, 204–220. doi: 10.1002/pan3.10168
Lenth, R. V. (2022). emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.7.2. Available online at: https://CRAN.R-project.org/package=emmeans (accessed November 11, 2022).
Lin, B. B., and Egerer, M. H. (2017). “Urban agriculture - An opportunity for biodiversity and food provision in urban landscapes,” in Urban Biodiversity - From Research to Practice, eds A. Ossola and J. Niemelä (London, UK: Routledge), 71–86.
Linowski, W. I., Pettersson, M. W. C. B., and Nilsson, L. A. (2004). Nyskapande av Livsmiljöer och Aktiv Spridning av Vildbin. Avdelningen för Växtekologi, Uppsala University, Uppsala, Sweden.
Loram, A., Tratalos, J., Warren, P. H., and Gaston, K. J. (2007). Urban domestic gardens (X): The extent and structure of the resource in five major cities. Landsc. Ecol. 22, 601–615. doi: 10.1007/s10980-006-9051-9
Lowenstein, D. M., and Minor, E. S. (2016). Diversity in flowering plants and their characteristics: Integrating humans as a driver of urban floral resources. Urban Ecosyst. 19, 1735–1748. doi: 10.1007/s11252-016-0563-z
Lüdecke, D., Ben-Shachar, M. S., Patil, I., Waggoner, P., and Makowski, D. (2021). performance: An R package for assessment, comparison and testing of statistical models. J. Open Source Softw. 6, 3139. doi: 10.21105/joss.03139
MacIvor, J. S., and Packer, L. (2015). 'Bee hotels' as tools for native pollinator conservation: A premature verdict? PLoS ONE 10, e0122126. doi: 10.1371/journal.pone.0122126
MacPhail, V. J., and Colla, S. R. (2020). Power of the people: A review of citizen science programs for conservation. Biol. Conserv. 249, 108739. doi: 10.1016/j.biocon.2020.108739
Maiteny, P. T. (2002). Mind in the Gap: Summary of research exploring 'inner' influences on pro-sustainability learning and behaviour. Environ. Educ. Res. 8, 299–306. doi: 10.1080/13504620220145447
Martins, K. T., Gonzalez, A., and Lechowicz, M. J. (2017). Patterns of pollinator turnover and increasing diversity associated with urban habitats. Urban Ecosyst. 20, 1359–1371. doi: 10.1007/s11252-017-0688-8
Mason, L., and Arathi, H. S. (2019). Assessing the efficacy of citizen scientists monitoring native bees in urban areas. Glob. Ecol. Conserv. 17, e00561. doi: 10.1016/j.gecco.2019.e00561
Naturhistoriska Riksmuseet (2013). Bin och Biholkar. Stockholm: Naturhistoriska Riksmuseet. Available online at: https://www.nrm.se/faktaomnaturenochrymden/djur/insekterochspindeldjur/steklargetingar/binochbiholkar.420.html (accessed November 10, 2022).
Norton, B. A., Bending, G. D., Clark, R., Corstanje, R., Dunnett, N., Evans, K. L., et al. (2019). Urban meadows as an alternative to short mown grassland: Effects of composition and height on biodiversity. Ecol. Appl. 29, e01946. doi: 10.1002/eap.1946
Ollerton, J., Winfree, R., and Tarrant, S. (2011). How many flowering plants are pollinated by animals? Oikos 120, 321–326. doi: 10.1111/j.1600-0706.2010.18644.x
Pateman, R., Dyke, A., and West, S. (2021). The diversity of participants in environmental citizen science. Citizen Sci. Theory Pract. 6, 9. doi: 10.5334/cstp.369
Persson, A. S., Ekroos, J., Olsson, P., and Smith, H. G. (2020). Wild bees and hoverflies respond differently to urbanisation, human population density and urban form. Landsc. Urban Plan. 204, 103901. doi: 10.1016/j.landurbplan.2020.103901
Piano, E., Souffreau, C., Merckx, T., Baardsen, L. F., Backeljau, T., Bonte, D., et al. (2020). Urbanization drives cross-taxon declines in abundance and diversity at multiple spatial scales. Glob. Chang. Biol. 26, 1196–1211. doi: 10.1111/gcb.14934
Potts, S. G., Woodcock, B. A., Roberts, S. P. M., Tscheulin, T., Pilgrim, E. S., Brown, V. K., et al. (2009). Enhancing pollinator biodiversity in intensive grasslands. J. Appl. Ecol. 46, 369–379. doi: 10.1111/j.1365-2664.2009.01609.x
Quistberg, R. D., Bichier, P., and Philpott, S. M. (2016). Landscape and local correlates of bee abundance and species richness in urban gardens. Environ. Entomol. 45, 592–601. doi: 10.1093/ee/nvw025
Rader, R., Bartomeus, I., Garibaldi, L. A., Garratt, M. P. D., Howlett, B. G., Winfree, R., et al. (2016). Non-bee insects are important contributors to global crop pollination. Proc. Natl. Acad. Sci. U.S.A. 113, 146–151. doi: 10.1073/pnas.1517092112
Samnegård, U., Persson, A. S., and Smith, H. G. (2011). Gardens benefit bees and enhance pollination in intensively managed farmland. Biol. Conserv. 144, 2602–2606. doi: 10.1016/j.biocon.2011.07.008
Shulla, K., Leal Filho, W., Sommer, J. H., Lange Salvia, A., and Borgemeister, C. (2020). Channels of collaboration for citizen science and the sustainable development goals. J. Clean. Prod. 264, 121735. doi: 10.1016/j.jclepro.2020.121735
Simao, M.-C. M., Matthijs, J., and Perfecto, I. (2018). Experimental small-scale flower patches increase species density but not abundance of small urban bees. J. Appl. Ecol. 55, 1759–1768. doi: 10.1111/1365-2664.13085
Soga, M., and Gaston, K. J. (2018). Shifting baseline syndrome: Causes, consequences, and implications. Front. Ecol. Environ. 16, 222–230. doi: 10.1002/fee.1794
SSNC. (2022). Bisysslor – Räddningsinsatser för Bin Och Pollinerare. Svenska Naturskyddsföreningen. Available online at: https://www.naturskyddsforeningen.se/artiklar/bisysslor-raddningsinsatser-for-bin-och-pollinerare (accessed November 10, 2022).
Staab, M., Pereira-Peixoto, M. H., and Klein, A.-M. (2020). Exotic garden plants partly substitute for native plants as resources for pollinators when native plants become seasonally scarce. Oecologia 194, 465–480. doi: 10.1007/s00442-020-04785-8
Steffan-Dewenter, I., and Schiele, S. (2004). Nest-site fidelity, body weight and population size of the red mason bee, Osmia rufa (Hymenoptera: Megachilidae), evaluated by Mark-Recapture experiments. Entomol. Gen. 27, 123–132. doi: 10.1127/entom.gen/27/2004/123
Steffan-Dewenter, I., and Schiele, S. (2008). Do resources or natural enemies drive bee population dynamics in fragmented habitats. Ecology 89, 1375–1387. doi: 10.1890/06-1323.1
Tscharntke, T., Gathmann, A., and Steffan-Dewenter, I. (1998). Bioindication using trap-nesting bees and wasps and their natural enemies: Community structure and interactions. J. Appl. Ecol. 35, 708–719. doi: 10.1046/j.1365-2664.1998.355343.x
Verboven, H. A. F., Uyttenbroeck, R., Brys, R., and Hermy, M. (2014). Different responses of bees and hoverflies to land use in an urban-rural gradient show the importance of the nature of the rural land use. Landsc. Urban Plan. 126, 31–41. doi: 10.1016/j.landurbplan.2014.02.017
von Königslöw, V., Klein, A.-M., Staab, M., and Pufal, G. (2019). Benchmarking nesting aids for cavity-nesting bees and wasps. Biodivers. Conserv. 28, 3831–3849. doi: 10.1007/s10531-019-01853-1
Wenzel, A., Grass, I., Belavadi, V. V., and Tscharntke, T. (2019). How urbanization is driving pollinator diversity and pollination – A systematic review. Biol. Conserv. 241, 108321. doi: 10.1016/j.biocon.2019.108321
Wickham, H. (2016). ggplot2: Elegant Graphics for Data Analysis. New York, NY: Springer-Verlag. doi: 10.1007/978-3-319-24277-4_9
Winter, C. (2018). Gynna solitärbin. Jordbruksverket. Available online at: https://www2.jordbruksverket.se/download/18.377b10d8163f4deaf8923e72/1528877358751/jo18_8.pdf (accessed November 10, 2022).
Keywords: pollinator conservation, urban green space (UGS), bee hotel, garden meadow, flower plantings
Citation: Persson AS, Hederström V, Ljungkvist I, Nilsson L and Kendall L (2023) Citizen science initiatives increase pollinator activity in private gardens and green spaces. Front. Sustain. Cities 4:1099100. doi: 10.3389/frsc.2022.1099100
Received: 15 November 2022; Accepted: 20 December 2022;
Published: 11 January 2023.
Edited by:
Léo Correia da Rocha-Filho, Federal University of Uberlandia, BrazilReviewed by:
Kris Braman, University of Georgia, United StatesCopyright © 2023 Persson, Hederström, Ljungkvist, Nilsson and Kendall. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna S. Persson,  YW5uYS5wZXJzc29uQGNlYy5sdS5zZQ==
YW5uYS5wZXJzc29uQGNlYy5sdS5zZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.