
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Reprod. Health , 23 July 2024
Sec. Adolescent Reproductive Health and Well-being
Volume 6 - 2024 | https://doi.org/10.3389/frph.2024.1394978
This article is part of the Research Topic Menstrual Pain View all articles
Introduction: Dysmenorrhoea affects many adolescents with significant impacts on education and well-being. In the UK, most of the adolescents who seek care (and many never do), will do so through general practice (primary care). Knowing how best to care for adolescents reporting menstrual pain is an area where UK general practitioners would like better guidance and resources.
Methods: This mixed-methods narrative synthesis collates community and specialist evidence from 320 papers about adolescent dysmenorrhoea, with a UK general practice community health perspective.
Results: We report a narrative summary of symptoms, cause, consequences and treatments for adolescent dysmenorrhoea. We highlight areas of tension or conflicted evidence relevant to primary care alongside areas of uncertainty and research gaps identified through this synthesis with input from lived experience advisers
Discussion: There is little evidence about primary care management of adolescent dysmenorrhoea or specific resources to support shared-decision making in general practice, although there are evidence-based treatments to offer. Primary care encounters also represent potential opportunities to consider whether the possibility of underlying or associated health conditions contributing to symptoms of dysmenorrhoea, but there is little epidemiological evidence about prevalence from within community health settings to inform this. The areas where there is little or uncertain evidence along the care journey for adolescent dysmenorrhoea, including at the interface between experience and expression of symptoms and potential underlying contributory causes warrant further exploration.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPEROFILES/256458_STRATEGY_20210608.pdf, identifier (CRD42021256458).
Dysmenorrhoea (menstrual pain) affects many adolescents who menstruate around the world, with potential adverse impacts on health, well-being, school, leisure, and work engagement (1). While dysmenorrhoea can occur in the absence of demonstrable underlying conditions, it can also be part of the symptom experience associated with conditions such as endometriosis, ovarian cysts, congenital genital tract developmental anomalies or pelvic inflammatory disease (2).
Our qualitative study with general practitioners working in England exploring how they navigate supporting patients with possible endometriosis elucidated their concern and uncertainty about how best to care for adolescents with dysmenorrhoea. This highlighted concerns about the lack of evidence about long-term outcomes and whether interventions in adolescence influence outcomes in adulthood. They identified a need for primary-care focussed evidence (3), a need this synthesis seeks to address.
This synthesis aims to document relevant evidence about adolescent dysmenorrhoea settings to create an evidence-based summary with a general practice focus. While this review does not focus on adolescent endometriosis, it considers the interface between associated health conditions and adolescent dysmenorrhoea in the context of presentation to primary care. We also sought to identify potential, or suggested, research gaps.
The majority of health contacts in the UK occur in general practice (4), typically the first port of call when people are presenting with symptoms and seeking care or treatment (5). In addition, general practice holds a gatekeeper function, coordinating referrals for more specialist investigations and care (6). This means that the population incidence of many conditions will be different in community and specialist care settings, risking a denominator error when data are extrapolated between them. This synthesis seeks to recognise this uncertainty and collate evidence from both community and specialist settings with a community health perspective.
PROSPERO 2021 CRD42021256458.
We undertook an integrative mixed methods synthesis (7, 8), reported as a narrative summary (9). The search strategy, approach taken to data selection, data extraction, and analysis is described in full in the study protocol. https://www.crd.york.ac.uk/PROSPEROFILES/256458_STRATEGY_20210608.pdf.
We conducted an exhaustive bibliographic search for: dysmenorrhoea, period pain, teenager, and adolescent. We conducted a parallel targeted purposive search using the terms adolescent, teenagers, dysmenorrhoea, and endometriosis. We searched the following databases: MEDLINE, Embase, PsycINFO, CINAHL and ERIC. We included all papers written in the English language. Our search strategy is available at: https://www.crd.york.ac.uk/PROSPEROFILES/256458_STRATEGY_20210608.pdf.
All study designs and typologies, including editorials and opinion pieces were considered for inclusion if they offered information that could inform the study research aim of documenting and assimilating existing evidence underpinning diagnosis and care of adolescents experiencing dysmenorrhoea in community settings. Because of the lack of direct primary care evidence, we adopted a broad inclusion strategy. We did not apply any date range or regional setting limits on data inclusion. We only included papers where the mean age of participants was between ten and nineteen, in line with the World Health Organisation definition of adolescence (10).
We included papers written in English and relevant to the context of UK general practice. Because complementary and alternative medications (treatments not offered in mainstream health settings in the UK, but which may be accessed alongside or instead of these services, such as homeopathy and chiropractic treatments) are not routinely accessible to or provided by UK NHS general practice (11), we did not include primary evidence documenting these. Whilst acupuncture is not routinely available in NHS or primary care clinics, it may be available or recommended within specialist settings, and so we included systematic reviews detailing evidence about this in adolescence. We included systematic reviews about treatments sanctioned in UK guidance if they included adolescent participants. We did not include studies of medications unavailable in the UK (listed in the British National Formulary).
The review team (SD, CD, NT) developed, piloted, and agreed a data extraction tool. All abstracts identified by searches were uploaded to Rayyan systematic review software. SD reviewed all abstracts, with twenty percent co-checked by CD/NT. We had a protocol plan for escalation for any disagreements or differing opinions or perspectives, but did not need to utilise this strategy. We had a shared understanding of the aim which was to document evidence and actively consider research gaps, and so we included any studies reporting menstrual pain during adolescence, and this broad inclusion facilitated our wide inclusion and minimised disagreements.
All full text documents were appraised by SD, with twenty percent checked by CD/NT. The wide approach we took to inclusion resulted in minimal discrepancies. All full text documents appraised were coded within NVivo 12. To encompass and represent the breadth of evidence included, SD undertook a thematic analysis of the narrative of the written studies and papers. Our final coding framework is represented as the thematic headings described in Table 1. Having identified the disparate nature of the studies, including variance of study type, geographical setting, chronological period, and nature of enquiry, along with the preponderance of surveys where the survey tool was not available, we elected to not formally undertake quality appraisal, but to instead adopt a broad inclusion approach for all potentially relevant studies and assimilate them into the descriptive analysis. We have documented and mapped the focus, typology and setting of each included paper (Table 2).
We report this narrative synthesis in thematic categories using illustrative references. These thematic categories are summarised in Table 2. These may not represent all of the references that contributed to the theme. The full reference list of studies that contributed evidence to each theme within this synthesis is included in Supplementary Appendix A.
Our included population is adolescents who menstruate. The majority of the studies we identified used gendered terms such as girls or women in describing their work, including in relation to study populations and when reporting findings. We identified one paper reporting specifically on endometriosis and dysmenorrhoea amongst trans-male adolescents. We recognise that gendered terms do not represent all experiences of menstruation.
This project is under-pinned by advice from a PPI group including adolescents with dysmenorrhoea and adults with lived experience of endometriosis and of adolescent dysmenorrhoea. They have assisted with interpreting findings, and in identifying potential gaps and research needs.
Our search yielded 2,565 unique abstracts. Following abstract screening, 382 papers were included for full text review, and 312 were included in the final synthesis. In addition, in line with our protocol, we added 6 papers from citation tracking (N = 2), purposive sampling (N = 3) and expert recommendation (N = 1). Noting an evidence gap, we undertook a detailed search to look for evidence about progestogen-only contraception and adolescent dysmenorrhoea, and included 2 studies from this search. These are annotated within the data extraction table, using the legend described in the PRISMA diagramme. Figure 1 summarises the search strategy and reasons for exclusion. Table 1 summarises and characterises the included papers. Supplementary Appendix A includes the data extraction sheet detailing all included studies. The study dates ranged from 1957 to 2023. Table 1 demonstrates the balance of evidence generated between community and specialist health settings and the relative lack of evidence from within community health settings. This demonstrates the relative lack of randomised controlled trial evidence and qualitative research within adolescents, although they are a population with both high prevalence and symptom burden of dysmenorrhoea. Table 2 details the thematic categories in which we present this narrative synthesis findings.
Dysmenorrhoea is a descriptive symptomatic term for the pain associated with menstruation. The term derives from the Greek words of dys (difficulty, pain or trouble), menos (month) and rrhoea (flow) (12, 13). The pain may be experienced as cramping, sharp, stabbing, aching, shooting or constant pains through the lower abdomen, pelvic, and inguinal region. Pain may also be experienced in the back and thighs. Pain typically begins between 2 and 3 days before and after the onset of bleeding, most commonly on the first day of bleeding (14–17).
Adolescents often experience extra-pelvic symptoms as part of dysmenorrhoea. These include tiredness, gastrointestinal symptoms [nausea, vomiting, changes in bowel habit (diarrhoea or constipation), altered appetite], bloating (oedema), dizziness, headaches, breast pain, insomnia and emotional changes and lability (mood changes, low mood, reduced concentration, irritability). For most affected adolescents, symptoms start with menstruation and last 1–3 days from onset (14–37). Some papers using participant experience to delineate symptoms associated with dysmenorrhoea also report pelvic pain at non-menstrual times of the cycle, suggesting that dysmenorrhoea can be associated with acyclic pain (mid-cycle and acyclic pain) (1, 21, 34, 38–40).
While previously regarded as a maladaptive response to menstruation (41–44), and as a psychosomatic or psychosocial phenomenon (45–50), evolving understanding contributed to a re-framing of dysmenorrhoea as a physiological (medical) entity (42, 51–59). Developments in understanding the pathogenesis of menstrual pain elucidated the role of prostaglandins and leukotrienes in menstrual pain (56, 57, 60, 61), although it is sometimes described as a “learned behaviour” (62). Studies do not show a consistent association between uterine morphology and dysmenorrhoea (63), although utero-cervical angle may predict severity (64).
As understanding of dysmenorrhoea has evolved, the ways in which it is categorised have also changed. Dysmenorrhoea is now typically categorised into primary dysmenorrhoea (no known or discernible underlying cause), and secondary dysmenorrhoea (menstrual pain in association with an identified underlying condition such as pelvic anatomical pathology). Causes of secondary dysmenorrhoea in adolescence include endometriosis, congenital developmental anomalies of the genitourinary tract, ovarian cysts, and pelvic inflammatory disease (2, 65, 66). Historically, primary dysmenorrhoea was further categorised as “spasmodic” (sometimes called true) dysmenorrhoea (cramping pains within first 48 h of onset of menstruation) (67) or “congestive” (more widespread bodily aches) (68). While this has largely now been abandoned (13), the terms do still sometimes appear (and sometimes interchangeably) (69) in the literature and so are mentioned here.
Data on the prevalence of dysmenorrhoea around the world is hard to compare directly, because it has been collected in a variety of settings, using different approaches, and with young people of different ages, and from different cultural contexts. There are studies that demonstrate different rates of dysmenorrhoea between rural and urban contiguous settings (70–74) or between different ethnic groups in one geographical setting (74–76). Menstrual stigma (secrecy) may also influence reporting of dysmenorrhoea (77–80), although this may be evolving with increasing recognition of menstrual health needs, making the year when the study was undertaken a relevant contextual factor. In the studies we identified that reported dysmenorrhoea prevalence, we observe a trend towards higher prevalence over time. Recognising the range of settings, temporal context, measuring scales, approaches, and aims of the included studies, we have not quantitatively assimilated the findings about dysmenorrhoea prevalence in community settings, but have visually mapped the figures for prevalence in the evidence we identified in Figure 2, Table 3 (references in Supplementary Appendix B). While there is a range of prevalence within and between countries, we note evidence of widespread documentation of symptomatic dysmenorrhoea. This aligns with a systematic review including 21,573 participants aged under 25 years which found an aggregate prevalence of 71.1% with no significant difference between Low and Middle Income Countries (LMIC) and High Income Countries (HIC). This review also reported no significant differences in prevalence related to the age of participants, or whether they were at school or University (77). In England, a 2021 study surveyed 442 secondary school students in Birmingham (a 53% response rate). Strikingly, 93.6% reported experiencing menstrual pain (46.2% reporting pain every month) and 63% believed their periods were normal with 27% unsure if they were normal and 30% unsure if they were regular (81). Papers included in this synthesis assert that 10% of adolescent dysmenorrhoea is secondary (90% primary) (54, 58), however we are unable to identify citations or evidence to substantiate this figure.

Figure 2 Recognising the range of settings, temporal context, measuring scales and approaches, and aims of the included studies, we have not quantitatively assimilated the findings about dysmenorrhoea prevalence in community settings, but have visually mapped the figures for prevalence in the evidence we identified.
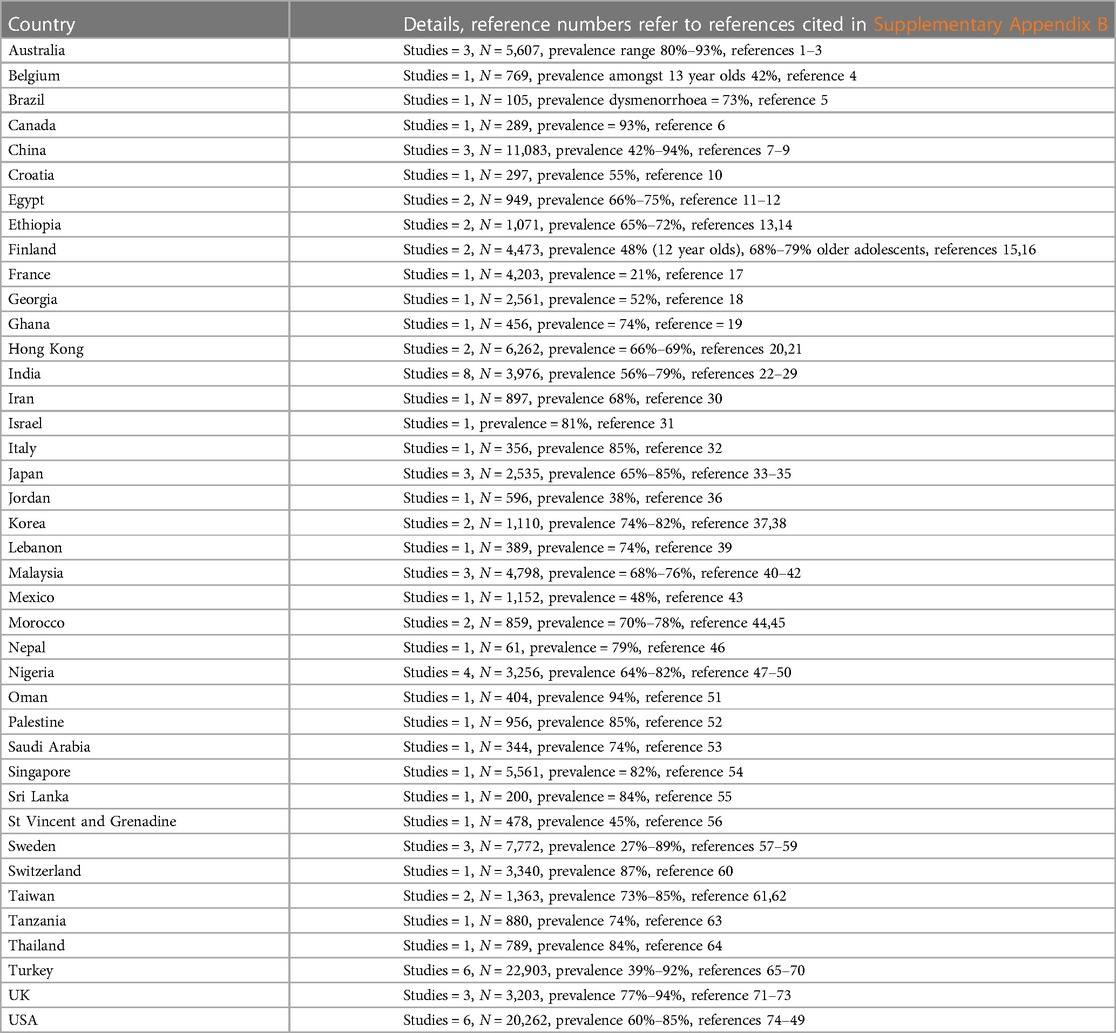
Table 3 References contributing evidence to this map, detailed in Supplementary Appendix B.
The relationship between socio-economic status (SES) and dysmenorrhoea is inconsistent, with some finding increased prevalence with increased SES (82, 83), and others no relationship (37, 84, 85). Differing variables to characterise socio-economic status are employed, such as household structure (45, 86) or income (29, 86) and paternal profession (87), which further complicates drawing conclusions. Potential confounders which could complicate understanding these potential associations include access to care and research inclusion. The literature documents other associations, including marital or relationship status (12, 88, 89), religious beliefs (2), family dynamics (45, 86, 87, 90, 91), educational level (90), ethnicity (including being in a minority ethnic group) (29), and perceptions of femininity and self-esteem (45, 46, 87). These are likely contextual and thus potentially confounded.
Dysmenorrhoea increases in incidence during adolescence with both chronological and gynaecological age (16, 31, 35, 57, 61, 85, 92–96). While the natural history of adolescent dysmenorrhoea is imperfectly understood (97), evidence suggests that rates of dysmenorrhoea peak in mid to late adolescence (2, 94, 98–103), becoming lower in adulthood than adolescence (66, 73, 104).
Early age at menarche is suggested as a risk factor for adolescent dysmenorrhoea (57, 105–107), however the evidence is inconsistent (19, 29, 70, 98). Some of this relationship may be attributable to the positive association between increasing gynaecological age (number of years since menarche) and dysmenorrhoea, with a large 2018 study reporting no association between age at menarche and dysmenorrhoea once gynaecological age was accounted for (94).
We found a range of assertions about the “typical” interval between menarche and the onset of menstrual pain, including what this time interval implies about the likelihood that dysmenorrhoea is primary or secondary. These include that (primary dysmenorrhoea) menstrual pain is variously expected to onset within 6–12 months of menarche (15 studies) (2, 103, 105, 107–118), 6–12 months after menarche (10 studies) (12, 25, 50, 119–125), within 6–24 months (2 studies) (65, 126), within 12–36 months (11 studies) (72, 82, 83, 98, 127–133), or after 12–36 months (several years) (11 studies) (13, 29, 55–58, 73, 102, 134–136). These frequencies are summarised in Table 4. Some authors advise that onset of pain within 6 months is abnormal and should be investigated (65). However, in several primary studies (28, 36, 87, 98, 101), including qualitative accounts (137, 138) many adolescents report that their pain started from their first period.
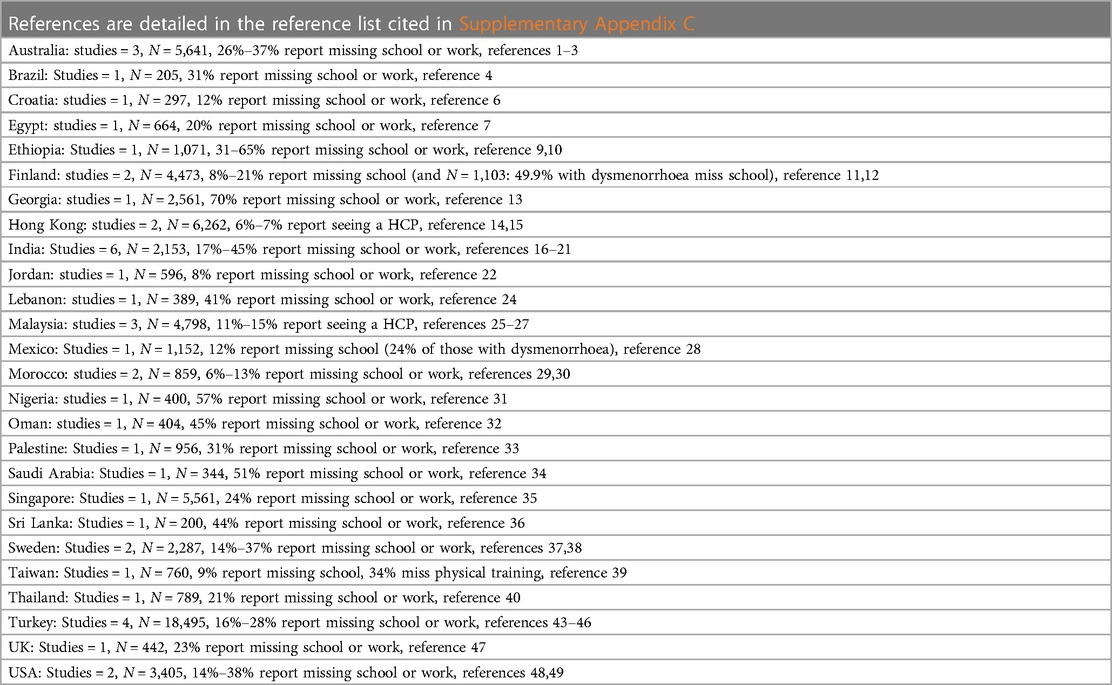
Table 4 Documented rates of missing school or work from references identified in this synthesis, full reference details in Supplementary Appendix C.
The relationship between menarche and onset (and causation) of primary dysmenorrhoea is frequently related to the onset of (regular) ovulatory cycles (13, 57, 58, 82, 117). However, recent evidence sheds doubt on this, with studies showing that anovulatory cycles are commonly painful (47, 131), irregular cycles are painful (139), that painful cycles are significantly more frequent than ovulatory ones (140), and that regular cycles do not reliably predict ovulation.
Studies differ as to whether heavy (37, 141) or longer duration (98) menstrual flow increases the likelihood of dysmenorrhoea (98), or alters the timing of pain relative to bleeding (139), although most suggest that pain is more likely with heavier flow and longer cycles (2, 28, 31, 85, 101, 104, 107, 142, 143). The relationship between pain and irregular cycles is inconsistent, with some studies finding an association between irregular cycles and pain (14, 16, 29, 32, 114, 144), and others no relationship (19, 33, 37, 106, 145). Irregular cycles can cause heavier unpredictable bleeds, which complicates this relationship (106).
There is a consistently documented relationship between pre-menstrual syndrome (PMS) or pre-menstrual dysphoric disorder (PMDD) and dysmenorrhoea (59, 92, 108, 146–148). This may be reciprocal, with PMS exacerbating pain and pain increasing the likelihood of PMS (106, 149).
A family history of menstrual pain is associated with an increased likelihood of dysmenorrhoea (30, 64, 83, 101, 150–153). This was historically attributed to maternal factors (anxiety, role modelling or dominance) (41, 45, 47, 62, 67, 83, 154) however a study comparing adolescents, their peers and their mothers found that adolescents experience significantly more symptoms than their mothers, and were less likely to view menstruation as a “positive event” (155). A family history of endometriosis also increases the likelihood of endometriosis (66, 156).
The relationship between dysmenorrhoea and BMI is inconsistent, with some studies reporting increased dysmenorrhoea in those with low (<16.5) (29, 35, 72, 157, 158) or raised BMIs (143, 158), while others not identifying a relationship (2, 13, 19, 98, 104, 113, 159, 160). This relationship may be complicated because being taller and thinner is also an identified epidemiological risk factor for endometriosis (156). Regardless of BMI, some authors report increased rates of dysmenorrhoea in young people who have dieted to lose weight (73, 158, 161, 162). Separate work finds that dysmenorrhoea is associated with an increased likelihood of “body image dissatisfaction” (163) or “negative self-perception” (164). Some authors relate the likelihood of dysmenorrhoea to eating junk food (110, 159). There are papers reporting an association between irregular meals or skipping breakfast with dysmenorrhoea (29, 165–167). While the authors conclude that the act of skipping meals may drive pain (165–167), we note that nausea (14, 18) and altered appetite (21, 24) are well documented symptoms associated with dysmenorrhoea which might cause young people to miss meals.
Two studies report that those with dysmenorrhoea drink more caffeine (62, 110), postulating the vasoconstrictor action of caffeine as a potential mechanism (110). In parallel, large observational studies demonstrate that dysmenorrhoea significantly adversely impacts quality and quantity of sleep, and is associated with significant daytime sleepiness (148, 168, 169). Reducing caffeine is used by some adolescents as a self-care strategy for dysmenorrhoea (170), while caffeine is a component of some medications used for dysmenorrhoea (171). Qualitative accounts demonstrate that dysmenorrhoea's impacts on sleep disruption are followed by fatigue (172), which could potentially contribute to the observed relationship between caffeine consumption and pain.
Evidence consistently links cigarette smoking with an increased incidence of dysmenorrhoea in teenagers (85, 104, 173–175). The associations between dysmenorrhoea and alcohol drinking in adolescence is less clear, with inconsistent findings about whether dysmenorrhoea is reduced or aggravated by alcohol (85, 143) and some find reducing alcohol effective in reducing pain (170). For both smoking and alcohol, the risk of social acceptability bias in self-reported evidence could under or overestimate the strength of these associations.
The relationship between exercise and the likelihood of dysmenorrhoea has been inconsistent (13, 104, 113, 141, 143, 145, 176–178). However, now two publications by Cochrane suggest that both high and low intensity exercise can alleviate menstrual pain, notably in young women, although whether this persists after exercise stops or impacts on quality of life is unknown (179, 180).
Historically, specific exercise programmes have been advocated as therapeutic interventions to treat or prevent menstrual pain (181–185). A trial studying yoga suggested therapeutic benefits for dysmenorrhoea (186). Both avoiding and doing exercise are commonly reported effective self-care strategies by adolescents with dysmenorrhoea. As well as impacts on fatigue and pain, potential contributory factors that could contribute to this include beliefs that people should avoid exercise whilst menstruating, or concerns about flow and leakage. In a survey study, Campbell showed that 90% of teenagers use resting to manage pain (with 60% finding this effective), while in the same study, 57% actively used exercise to relieve pain, (and 52% felt it worked) (170). Qualitative accounts similarly describe avoiding (138, 187, 188) and using exercise to manage menstrual pain (116). Dusek reports lower rates of dysmenorrhoea in elite athletes, compared with controls, however a third of elite athletes had amenorrhoea, reducing the number of painful cycles, which was not accounted for (189).
The impact of menstrual pain on emotional and psychological wellbeing is demonstrated in research, with dysmenorrhoea increasing the number and severity of depressive symptoms (90, 163, 168, 190, 191) and depression symptoms increasing menstrual symptoms (93) There is no consistent association between dysmenorrhoea and personality or psychosocial variables (46, 52, 66, 76). Pain is associated with (and can contribute to) negative expectations of menstruation (37, 192) and negative expectations of menstruation impact on menstrual experience (49, 193) Not accepting menstruation alongside holding stronger beliefs in external health locus of control was shown to predict seeking medical help (194). Dysmenorrhoea is related to lower perceptions of self-efficacy, with the level of pain predicting menstrual distress (91) and the extent to which this manifests as reduced engagement with activity. High levels of pain catastrophizing accompany higher levels of menstrual pain (195, 196), and flexible coping strategies may help mitigate against menstrual pain (197, 198). It has been suggested that the realisation that the onset of menstruation implies a recurring experience of dysmenorrhoea potentially exacerbates adolescent distress (49, 192), however others demonstrate that experience and habituation can diminish menstrual distress (73).
Menstrual pain increases stress (101, 150) and stress can aggravate menstrual pain (14, 86). For example, annual surveys in Japan reported increased dysmenorrhoea after the 2011 earthquake and tsunami (199). There is a reported relationship between childhood stressors and dysmenorrhoea, including sexual abuse (122) and conflicted domestic relationships (90, 112).
Research with young people with autism or cerebral palsy documents increases in difficult or distressed behaviours preceding and during the menstrual period (200–202), which can be helped by offering treatment for menstrual pain (200) (including hormonal treatments such as the intra-uterine system) (203).
We identified one paper associating ADHD symptoms (not diagnosis) from a screening tool (T-DSM-IV-S) in young people recruited from a child and adolescent service, which identified correlation between symptoms of dysmenorrhoea and inattention and impulsivity, impaired sleep and mood impacts (164).
An emergency room (uncontrolled) case series suggests a possible association between dysmenorrhoea, described as not responsive to non-steroidal anti-inflammatory medications (NSAIDs), and Familial Mediterranean Fever (FMF), noting that acute FMF pain episodes can triggered by the menstrual cycle (204).
Dysmenorrhoea is associated with other pain conditions [including chronic pelvic pain, IBS, fibromyalgia, temporomandibular joint (TMJ) pain, headaches and musculoskeletal pain] (205).
Dysmenorrhoea is associated with non-cyclical pain and chronic pelvic pain (47, 206). This relationship is complex, because pelvic pain may be a marker of adolescent endometriosis (207), as can dysmenorrhoea (208). However, in a large (1,785 adolescents) web survey in 2017, 44% of respondents reported acyclic pelvic pain (34). A correlation between irritable bowel syndrome (IBS) and both primary dysmenorrhoea and PMS has been reported (209).
Another condition linked with dysmenorrhoea is headache, especially menstrual and peri-menstrual headache (210, 211). Headache frequency increases with gynaecological age. Headaches are more common in combined hormonal contraception (CHC) users (210), (although headache as a side effect of CHC use may contribute to this observation).
In a systematic review, having dysmenorrhoea was associated with 2.5 times the odds of living with another chronic pain condition. While not exclusively focussed on adolescents, the majority of studies included adolescents, and three included only adolescents (reporting on IBS pain, TMJ pain, and migraine/headache). The authors reflected on the potential significance of adolescence as a time of neurodevelopmental plasticity as it relates to pain (205). Dysmenorrhoea is known to cause central pain sensitisation (205).
A systematic review of educational impacts of dysmenorrhoea including data from 11,226 young women aged <25 years in 19 studies found that 20.1% had missed school or university because of dysmenorrhoea and for 40.9%, dysmenorrhoea adversely affected academic work and classroom performance (77). This aligns with the studies included within this review, demonstrating a similar range of school or work absenteeism from 6%–70% in papers that reported this. The papers that differentiated school absence rates between mild, moderate, and severe dysmenorrhoea showed that absence rates tend to be higher for those who report more severe pain. There were adverse impacts reported on concentration, test performance and being able to study or do homework. This is summarised in Figure 3 and Table 4 (Reference list is cited in Supplementary Appendix C).
Figure 3 Visual mapping of documented figures for missed school or work because of adolescent dysmenorrhoea.
In a 2021 survey of 442 English school girls, 22.7% sometimes missed school because of their periods. Most absences were attributed to pain, but for 25% managing heavy bleeding contributed (81). The interface between bleeding and school attendance is illustrated in Li's qualitative study which highlights challenges experienced by school students in accessing pain relief and products to manage menstrual flow in school alongside negotiating necessary trips to the toilet (79). The potential contribution of menstrual poverty adds to the challenges of managing menstruation in school and work.
Menstrual pain reduces young people's engagement with leisure activities, including sports and hobbies (188), and socialising with peers and families (137, 172). It can impact on social interactions (172, 187) including sexual activity (212).
The experience of adolescent dysmenorrhoea was associated with a reduction in health-related quality of life in two studies, one of which found a reduction in the physical score domain (213), and one of which found reductions in both physical and social domains (115).
The lack of evidence describing the natural history of adolescent dysmenorrhoea is a documented research need (97). This includes whether adolescent dysmenorrhoea predicts health events or conditions in later life (including endometriosis, sub-fertility, pelvic pain or other pain conditions) and also whether interventions in adolescence influence any of these potential outcomes (214, 215). However, a small 1983 cohort study reported that irregular or painful periods in adolescence predict poorer gynaecological health in adulthood (42).
Longitudinal and cohort studies suggest a trend towards a reduction in severity and incidence of menstrual pain after adolescence. We identified one cohort study following up 148 adolescents seen in an Australian specialist tertiary care clinic for (presumed severe) dysmenorrhoea ten years later. Of the 70 who could be contacted, 30.4% had ongoing severe pain, but 27.1% had no, or slight, pain with menstruation, representing significant improvement. They did not identify characteristics in adolescence that predicted enduring severe menstrual pain (97).
Most young people learn about periods from their mothers, or other family members (20, 23, 38, 80, 100, 121, 216), followed by friends and teachers (26, 114). Only a minority receive menstrual information in healthcare settings (71, 80, 193). Education and knowledge improve self-care agency (217, 218), support effective use of treatments (123, 219), and increase awareness of endometriosis and attendance for healthcare (220). Information and education help young people feel prepared for menarche; encountering menarche without knowledge can be frightening and difficult (74, 138, 221).
Advertising is another potential source of menstrual information. A 1988 thematic analysis of menstrual product advertisements in a magazine targeted for adolescents concluded that menstrual adverts depict menstruation as a “hygiene crisis” requiring technology and science to ensure that leaks and odour are contained, while maintaining secrecy (and menstrual stigma). Menstruating people were characterised as full of energy, often wearing white and not allowing menstruation to hinder their activity or lifestyle, in stark contrast to the accounts of the impacts of menstrual pain on many young people (78).
In what is likely to be an evolving practice, adolescents reported using the internet to find information about menstruation (75). In a big data exploration mapping 1.9 billion enquiries made to a chat and answer platform in the USA in 2018, 84% of queries about menstruation were from adolescents, suggesting a significant unmet information need. Questions clustered around themes such as managing menses in school, treatment and self-care options and about possible underlying causes or consequences of menstrual pain, although queries about specific causes of dysmenorrhoea including endometriosis were rare (0.05% of female queries) (222). This aligns with the lack of knowledge and awareness of endometriosis, alongside a desire to know more about it, depicted by Randhawa et al. among English secondary school students (81). A UK study from 2011 evaluated the content of internet information about dysmenorrhoea for adolescents. Of the 23 websites they identified which advised on dysmenorrhoea, only one was targeted at adolescents. Although 65% of dysmenorrhoea sites advised seeking health professional advice, the paper concluded that, in 2011, the internet sites were of low quality and uncertain value in supporting adolescents experiencing pain (223).
The majority of young people with dysmenorrhoea do not seek medical help, with a systematic review reporting an aggregate figure of 11% seeking healthcare which did not significantly differ between high or low and middle income countries (224). Increasing pain predicts increasing attendance at healthcare (31, 194). In the UK, Randhawa's study found that 29.5% of young women had seen a GP (81), although little is known about what happens when they do. In the studies in this synthesis, we found that rates of having seen a doctor or nurse, varied between 0% and 34%. There are country specific factors which influence accessing healthcare, for example studies in China report young people choosing between traditional and Western practitioners (188), and in some health systems the cost of accessing healthcare is a deterrent. Figure 4 and Table 5 map documented attendance at healthcare settings (reference list is cited in Supplementary Appendix C).
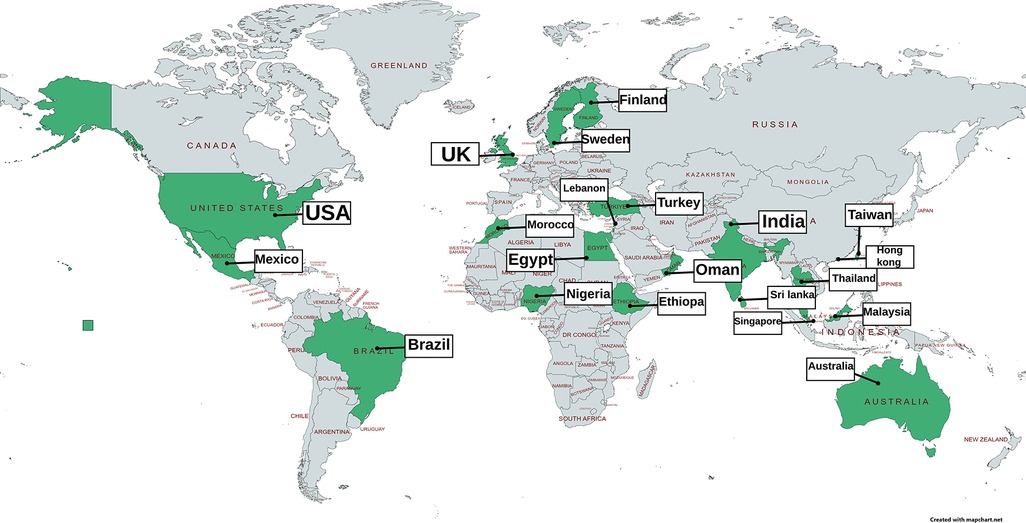
Figure 4 Visual mapping of documented figures of the proportion accessing healthcare services because of adolescent dysmenorrhoea.
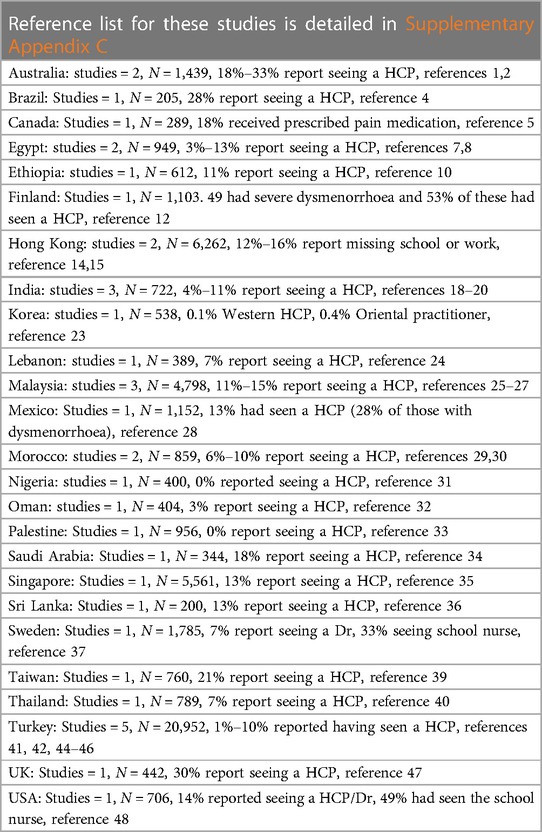
Table 5 Documented rates of attendance at healthcare settings for adolescent dysmenorrhoea from references identified in this synthesis, full reference details in Supplementary Appendix C.
Believing pain is inevitable or normal can deter people from seeking medical advice for menstrual pain (2, 71, 73, 123, 142, 224, 225). Young people may become habituated to pain with time, describing this as better (73). Where there are differentials in attendance reported by health setting, higher numbers seek care from school nurses than doctors (20, 34).
Documented barriers to accessing healthcare include previous adverse experiences of healthcare for dysmenorrhoea (188), fears of not being taken seriously (109) or of the pain or menstrual problems being normal (74, 226), and worry about “embarrassing” questions or being examined (22, 95).
Menstrual stigma and etiquette remain barriers to accessing advice and help (80, 226). This is reflected in the number of apologies for asking “embarrassing” questions on the anonymous chat assessment in an analysis of 1.9 billion menstruation queries on a USA question and answer internet platform (ChaCha) (222), and is also seen in the language young people use to describe their periods (e.g., one survey with free text box responses reported use of the words “disgusting”, “ridiculous” and “embarrassing”) (37).
Menstrual education has been shown to increase attendance at healthcare (220).
While developmental anomalies can present in a variety of ways and at any age, adolescence is a not uncommon time for identification and diagnosis of these, if they are associated with menstrual symptoms which develop following menarche (227–229). While some anomalies result in the absence of menarche, in this paper we consider developmental anomalies that may present with period pain in adolescence. For some anomalies, severe dysmenorrhoea was a prominent presenting symptom. For example, in their review of the literature about cystic adenomyosis, Brosens et al. identified 21 case reports, 18 of which presented with dysmenorrhoea (227). A number of case reports document presentation with pain which localises predominantly to one side (unilateral dysmenorrhoea) (230–232).
Pain onset is often marked and worsening from menarche (233–244), although some case reports document onset after menarche (245–250). This may depend on the type of anomaly, including for example whether there is obstruction to menstrual flow (251, 252). There is a wide range of anomalies that can present with dysmenorrhoea including uterine didelphys (252–255), those termed accessory cavitating uterine mass (230, 233, 234, 250), Roberts Uterus (228), Herlyn–Werner–Wunderlich syndrome (obstructing hemivaginal septum, uterus didelphys, and ipsilateral renal agenesis) (238, 244, 251), rudimentary horns (229, 231, 232, 237, 239, 243, 245, 256), and unicornuate uterus (Mullerian anomalies) (236, 253). These can co-exist and evaluating congenital anomalies requires specialist input and imaging (for example MRI or HSG), and treatment (227).
Pelvic anomalies can be associated with other developmental anomalies, especially of the genitourinary and renal tract (245, 251–253). Where ultrasound findings were documented in the case report, they reported anomalies that would clearly indicate a need for referral from primary to secondary care for specialist assessment (232, 235, 236, 239–242, 249, 250, 256–259). Congenital structural anomalies also increase the likelihood of endometriosis (118, 232, 251, 253, 260).
Endometriosis is a chronic inflammatory condition defined as the presence of endometrium like tissue outside of the uterus (66, 261). Endometriosis used to be considered rare in adolescents (262–264), but does occur: the majority of adult women with endometriosis report symptom onset in adolescents (265, 266). Like adult women, adolescents experience significant delays in diagnosis (262, 267).
There is uncertainty about the community prevalence of adolescent endometriosis (156, 214), in part because historically diagnosis required a laparoscopy (266), which could only be undertaken in specialist settings (214). Rates of endometriosis confirmed in the population of adolescents who are investigated with laparoscopy or imaging for chronic pelvic pain or dysmenorrhoea are strikingly high, with systematic reviews citing retrospective rates of 62% (207, 268) in adolescents with chronic pelvic pain, in 75% in those with chronic pelvic pain resistant to medical treatment (and 49% in others with chronic pelvic pain) and in 70% with (severe) dysmenorrhoea (268). However, the community prevalence of endometriosis is unknown (214), raising a significant denominator question (214, 265, 269). Adult women are often diagnosed with endometriosis through either a pain or fertility route, whilst adolescents tend to be diagnosed only when significantly symptomatic, which influences prevalence (214). In addition, the symptoms of those diagnosed with endometriosis in adolescence may differ from those diagnosed in adulthood, with a cross-sectional analysis of a longitudinal cohort identifying adolescents report more pain from menarche, more acyclic pelvic pain and more nausea (265). Across the cohort, 90% had dysmenorrhoea (265). Trans-masculine people experience endometriosis and the intersection between this and hormonal interventions in an area where greater understanding is needed (269).
Guidance and expert opinion pieces suggest referring any adolescent whose pain has not improved within 3–6 months of empirical therapy with NSAIDs and/or hormonal treatment for consideration of possible endometriosis (13, 65, 66, 109). We were unable to identify evidence about further discriminatory assessments for adolescents whose pain was resolved (or improved).
The interface between symptoms and response to empiric treatment is pivotal in guidance about referral for further investigation for endometriosis (109). The relationship between combined hormonal contraception and endometriosis is complex (156). Hormonal contraceptive medication is an evidence-based treatment for both dysmenorrhoea and endometriosis-associated pain (117). Whether these treatments influence the underlying processes of endometriosis, either to reduce progression or scarring and potential future sequelae, or contribute to disease progression directly whilst suppressing (or “masking”) symptoms is uncertain (117, 118). A systematic review (including predominantly adult women) reported a reduced risk of endometriosis diagnosis in current CHC users (OR 0.63) and an increased risk in past users (OR 1.21). The authors note the possibility that symptom suppression makes referral for investigation less likely (117) (and we note is embedded in clinical guidance) (3, 270). It is possible that adolescent CHC use is a marker for severe dysmenorrhoea and thus a confounder not cause of epidemiological associations between CHC use and endometriosis. A retrospective case series of 410 women found that previous CHC use for severe “primary” dysmenorrhoea was associated with an increased risk of a diagnosis of deep infiltrating endometriosis (OR 5.6) and other endometriosis (OR 2.6) in adulthood. They also note that this may be because CHC use for dysmenorrhoea functions as a marker for endometriosis and not necessarily a contributory cause (271).
The evidence associating non-response to hormonal contraception therapy as a marker of likely endometriosis is inconsistent. In retrospective case series, where the cases have a surgically confirmed diagnosis of endometriosis, many had dysmenorrhoea that was refractory to medical treatment (268). But, a prospective case series following young people with marked dysmenorrhoea seen in a specialist clinic, 92.2% achieved positive symptomatic responses to hormonal therapy, NSAIDs or tranexamic acid. Of the 8.8% (N = 2/16) of adolescents who had a laparoscopy, 2 had endometriosis (139). Knox followed up participants ten years after they were seen in tertiary care with adolescent dysmenorrhoea and found that using AND having a positive therapeutic response to CHC in adolescence (rather than a lack of response) predicted adult endometriosis (97). A prospective case series in Egypt found that of the 654 adolescents they interviewed, 48.9% had dysmenorrhoea and of these 68.8% reported severe dysmenorrhoea. Of the 320 adolescents they studied with dysmenorrhoea, 100 responded to medical therapy and were then not assessed further. Of the 220 whose pain did not resolve, 56 had USS findings suggestive of endometriosis and 34 of these agreed to laparoscopy (22 declined), and 27/34 (12%) had endometriosis. Those with a negative ultrasound scan were not investigated further in this study (272). In a 2013 systematic review, the authors report the “unexpected” finding that the prevalence of moderate or severe endometriosis was lower in girls with chronic pelvic pain or dysmenorrhoea resistant to CHC or NSAID treatment, when compared with a group with CPP not resistant to treatment, speculating that treatment may not prevent development of endometriosis, but may limit progression to severe disease (268).
In these studies, threshold for laparoscopy is a critical determinant of what we understand about endometriosis prevalence, and the interface between symptoms and response to treatment is an important component of determining this threshold. However, in a real-world case series of adolescents (aged 12–20) presenting to an out-patient ultrasound clinic between 2014 and 2019, ultrasound signs of endometriosis were found in 21% of the 147 adolescents referred with dysmenorrhoea, rising to 33% who also reported dyspareunia. It is worth noting that any young people taking hormonal medication were excluded from this study (273).
Once a diagnosis is made, there is evidence for surgery and hormonal therapy to reduce recurrence (208). There is a risk of recurrence after surgery, which may be higher in adolescence than in adulthood (274), which is a significant concern associated with risks of repeated surgeries and pain (215). Specialist treatment can also include menstrual suppression with Gn-RH analogues, possibly with add back hormone replacement therapy (208). This should be initiated within specialist care settings, who can advise on follow up, monitoring and treatment duration. However, general practice teams may be involved in supporting this process, for example administering treatment or prescriptions.
In general practice, awareness of the diagnosis of endometriosis can facilitate recognition and response to potential symptomatic recurrence and supports shared decision making (66). Recognising that endometriosis is a chronic and complex condition, alongside physical treatments, there is a need for ongoing educational, emotional and psychological support (275).
Adenomyosis is infiltration of the myometrium by endometrial tissue. Although currently considered rare in adolescence, there are case reports linking adolescent dysmenorrhoea to diagnoses of adenomyosis (276, 277).
The papers included in this review did not offer detail about other causes of secondary dysmenorrhoea beyond a descriptive listing, which includes sexually transmitted infections, pelvic inflammatory disease [which can cause dysmenorrhoea by increasing inflammatory markers and causing adhesions (278), and can present acutely with worsened dysmenorrhoea (279)], pelvic adhesions, and ovarian cysts, and uterine fibroids (109).
Self-care strategies are commonly employed by adolescents with menstrual pain. Self-care encompasses a wide range of potential actions, including physical interventions (exercise or rest) (116, 170, 172, 224), reflective interventions (prayer, distraction, meditation) (170, 172), dietary (food selection or avoidance) (170, 187, 188), pharmacological (herbal, complementary and alternative, OTC analgesia) (116, 171, 172, 187, 224, 225) and non-pharmacological (bathing, avoiding bathing, heat, cold) (17, 152, 170, 188, 225). Self-care strategies, were more likely to be adopted by young people with more severe period pain, by individuals with greater knowledge about dysmenorrhoea or who believed that self-care would be effective (120). Interventions that increase knowledge, such as education in schools, increase self-care behaviour (217, 280).
Many young people use over-the-counter remedies, such as paracetamol or ibuprofen (224, 225), albeit not always taken optimally (171). While offering the potential for self-efficacy, the potential risks of self-medication with inadequate support or knowledge can include harms from choice of painkiller (for example choosing aspirin which is contra-indicated in children), incorrect dosages, incorrect dose intervals, failing to recognise adverse reactions or not seeking healthcare advice when symptoms do not resolve (171, 225). Incomplete or inaccurate understandings of the causes of dysmenorrhoea may align with inappropriate selection of over the counter treatments for dysmenorrhoea (281). Qualitative accounts describe apprehension about side effects, safety and long-term health risks of taking medication including dependence (188), alongside concerns that medication use is unnatural (116, 172, 187). A systematic review showed that the medication most commonly used worldwide for dysmenorrhoea was paracetamol ahead of non-steroidal anti-inflammatory medications for which there is more evidence of benefit in dysmenorrhoea (224).
Non-pharmacological treatments used by adolescents include physical strategies (locally applied heat, massage, rest, and exercise) (187, 224, 282) and cognitive strategies (distraction, visualisation, seeking support and talking about pain, and keeping busy) (116, 188, 280, 282). In a study comparing methods used alongside perceived effectiveness, physical strategies were experienced as more effective, while psychologically focussed strategies enabled comfort and control (170). It is possible that the degree of pain influences the approaches selected, with more physical and non-pharmacological approaches used by adolescents experiencing more severe menstrual pain (170). Specific interventions with reported benefit include TENS machines (283, 284), external heat application (178, 284, 285), and yoga (186, 284). Systematic reviews evaluating the effectiveness of acupuncture for primary dysmenorrhoea do not consistently identify benefit (133), some noting that the conclusions are limited by methodological flaws in the contributing studies (133, 286), including finding no clear evidence of benefit including when compared to sham acupuncture (286), although reported possible benefits when compared with pharmacological treatment or no treatment (286, 287).
Small studies have explored the benefits of structured psychological therapies on menstrual pain in teenagers and dysmenorrhoea support programmes incorporating CBT methods (218) and relaxation therapy (288) have shown promise, in small un-blinded studies. A Cochrane review included five RCTs of behavioural interventions for dysmenorrhoea, including two trials where the participants mean age was in adolescence, and concluded that these showed promise in reducing pain, albeit with limited evidence because of small numbers of participants and methodological flaws (289).
Medications with an evidence base for treating adolescent dysmenorrhoea can be hormonal or non-hormonal. The non-hormonal medications trialled and shown to be effective in teenagers include non-steroidal anti-inflammatory medications (50, 68, 127, 290). There is physiological plausibility for the effectiveness of these medications, which act on prostaglandin pathways implicated in menstrual pain (55). Evidence suggests that NSAIDs are more effective than paracetamol (acetometnophen). Medications trialled and found to be ineffective include beta agonists (reviewed in a Cochrane review) (124) and montelukast (in an RCT including 22 adolescents) (291).
Combined hormonal contraception (CHC) medications reduce pain (292) [including reducing missed school (292) and associated symptoms of dysmenorrhoea such as back pain or nausea] (293). The combined hormonal contraceptive pill reduces prostaglandin levels associated with an improvement in clinical symptoms (56). Analgesic effectiveness has been shown across a range of formulations of CHC (134, 292), including low dose 20 mcg tablets (294). Combined contraception is available in non-oral formulations, including the vaginal ring, which has been shown to be acceptable to young people and effective in reducing dysmenorrhoea (295). Mitigating against dysmenorrhoea is identified by adolescents as a reason to use hormonal contraception even when they do not need contraception (296). When these pills are used primarily for contraception, they may be better accepted when their use is accompanied by non-contraceptive benefits including more tolerable period pain (297–299). There may be a therapeutic benefit from administering the CHC continually, rather than giving it cyclically, and this may be associated with less irregular bleeding (292). We observe that reducing bleeding frequency will by definition reduce dysmenorrhoea, which is pain associated with bleeding, however pragmatically, whilst there is uncertainty about whether pain would persist if bleeding did, this represents a reduction in pain and painful episodes.
Single agent progestogen hormonal contraceptives such as oral desogestrel or the subdermal implant (etonogestrel) are also suggested as potential treatment options for adolescent dysmenorrhoea (65). We identified limited evidence about the effectiveness of oral or subdermal progestogen-only methods in adolescence. A small open label trial of drospirenone in adolescents exploring safety and tolerability documented a reduction in dysmenorrhoea from baseline (300). Observational evidence suggests benefit (including reduced dysmenorrhoea and menstrual suppression) from oral desogestrel (278) and the progestogen-only injection (Depo-provera, DMPA) (55).
The IUS has been shown to be effective (in one case series of 48 teenagers not helped by other interventions 93% reported improved menstrual symptoms) and well tolerated (4% asked for removal within four months of insertion) (301). The IUS was also found to be effective and tolerable in a case series of 14 young people with medical disorders, learning or physical disabilities whose menstrual pain had not resolved with other interventions (203). Young people may experience (301) post-insertional pain after the IUS is fitted, so counselling about this is important.
A number of dietary supplements have been suggested and trialled for adolescent dysmenorrhoea, including zinc (302), Thiamine (vitamin B1) (69, 128), fish oil capsules (omega-3) (128, 303) and vita min E (129, 304). While some small trials have showed potentially beneficial findings, a Cochrane review in 2016 judged all of the available evidence to be of low or very low quality. They reported no evidence for vitamin E. However there was limited evidence for possible benefits for fish oil, Vitamin B1, and zinc, which warrant further research (125). A non-blinded non placebo controlled observational study in Iran treated adolescents (95% had vitamin D deficiency at baseline) with high dose vitamin D and reported improvements in PMS and menstrual pain symptoms (305).
A critical uncertainty that arises from this synthesis is how to characterise the likelihood that pain represents primary dysmenorrhoea rather than secondary. We identify inconsistencies in how primary dysmenorrhoea is described or characterised in research (Table 6).
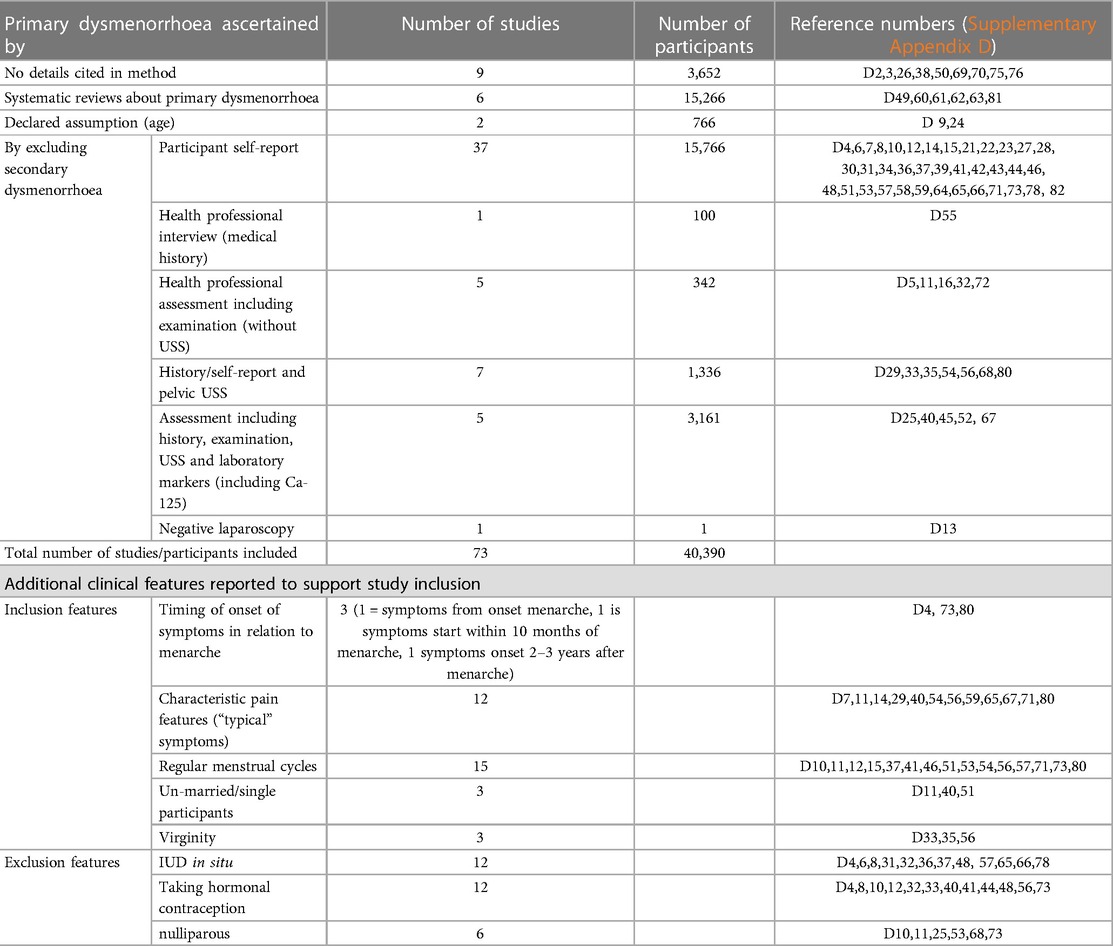
Table 6 Summarises the approaches taken by studies reporting evidence about primary dysmenorrhoea (references refer to reference list cited in Supplementary Appendix D).
Apart from laparoscopy, none of these approaches will reliably exclude all causes of secondary dysmenorrhoea and therefore studies that define and delineate expectations of primary dysmenorrhoea likely include participants with both primary and secondary dysmenorrhoea. These research observations contribute to defining the expected characteristics of primary dysmenorrhoea, which are in turn embedded in clinical guidance (54, 89, 109). Examples include the inconsistent reporting of the association between ovulation and pain, age at menarche and pain, and age and pain, and how these relate to the likelihood of pain being attributable to primary dysmenorrhoea.
In some care settings, pelvic examination is advocated in sexually active adolescents with dysmenorrhoea as a routine part of care (47, 54), suggesting rectal examination to look for pelvic pathology if this is not acceptable and/or the young person is not sexually active (122, 261). Rectal examination is proposed as a stratagem to consider the possibility of recto-sigmoid endometriotic nodules or scarring (118). We identified no evidence about the sensitivity of pathology detection or on perceptions of acceptability of trans-rectal examination.
We have summarised possible unanswered questions for exploration and underpinning uncertainties following our review, positioned around a hypothetical journey to and through care in Figure 5. This has been developed with input and guidance from our PPI advisers.

Figure 5 This summarises the research gaps and uncertainties identified in this synthesis and with input from PPI advisers. These are represented along a journey to and through healthcare, situated within the overarching uncertainties identified.
We found a lack of evidence supporting progestogen-only contraception treatments, a gap noted by others (215).
We have also drafted a potential framework for primary care, which supports holding uncertainty and maintaining possibilities about whether pain is primary or secondary during clinical encounters and trials of treatment, and which is embedded in evidence from this systematic review. This is included as Supplementary Appendix E.
A strength of this synthesis is the breadth of evidence included, and in mapping the type and settings of published evidence. Considering the interface between symptoms and potential diagnoses with pragmatic treatment evidence summaries alongside uncertainties clinicians may encounter situates this review within a general practice setting, where there is little primary empiric evidence.
The focus on adolescence meant we excluded evidence from women in early adulthood. We observed within our searching that there is a significant body of observational evidence undertaken with university students. While there will be many areas of overlap between adolescents (often still in school) and young women at university, it is also possible that there are social and developmental differences between these groups, making the specific focus on this age group appropriate. This evidence synthesis excluded retrospective evidence from adults. The natural history of adolescent dysmenorrhoea is an established research uncertainty. We searched systematically and widely, but may not have identified all potentially relevant papers. Excluding papers written in languages other than English risked missing primary care relevant research or relevant contextual insights and experiences.
We did not identify research about adolescent dysmenorrhoea conducted within UK general practice, and very few studies within primary healthcare settings, although this is where a majority of UK health contacts occur. There is a known lack of research about adolescent gynaecology, with findings often extrapolated from adult women (306). This is not always appropriate in dysmenorrhoea, given the peak in incidence in adolescence, and within the life course context of the psychosocial development that occurs in adolescence. These concerns were identified by GPs, reflecting on uncertainty about managing adolescent dysmenorrhoea (3).
In a 2022 synthesis taking a bio social approach to representing the life course impacts of dysmenorrhoea, the authors recognise the potential value of a recognised diagnosis in affording validity to pain, to the detriment of those experiencing pain without a “identified pathology” (307). They note that most research considers secondary dysmenorrhoea, and that much research does not effectively delineate between primary and secondary (307). We expand this point by identifying points of tension and inconsistency in the literature which purports to be about primary dysmenorrhoea.
Subsequent systematic reviews have strengthened the evidence for an association between the number and severity of adverse childhood experiences experienced and the likelihood of dysmenorrhoea (308), and between IBS and endometriosis (309) Furthermore, adding to the systematic review reviewing evidence about the association between dysmenorrhoea and chronic pain conditions, there is also evidence about the association between menstrual pain and autonomic dysfunction and bladder pain, potentially starting from menarche (310).
Pain in young people is imperfectly understood and responded to, and there is likely to be a complex and bi-directional relationship between mood and pain (311). However the causal pathways align, if GPs ask about, recognise, and support young people with the pain itself and the emotional impacts of menstrual pain, this is likely to be an important component of care.
The use of Chinese herbal medicine is described as an effective self-care approach (188), and although the conclusions were tempered because of methodological limitations, a Cochrane review, including adolescents, found promising evidence to support the use of Chinese herbal medicine (312).
Evidence in adults, including young adults, supports the potential efficacy of progestogen-only contraception in reducing dysmenorrhoea, including oral desogestrel (313), including in those with dysmenorrhoea associated with endometriosis (314) and the subdermal implant (315).
We did not find qualitative evidence appraising teenagers' views and perspectives on priorities in dysmenorrhoea care. A UK thesis script included interviews with adolescents and mothers and highlighted that both experience uncertainty about when period pain is “normal” and when to ask for advice (316). The interface between representing menstrual pain as normal and seeking care was also highlighted by young adults, including some reporting disquiet about the acceptability of using hormonal contraception to reduce or alter menstrual bleeding (317). Mothers are a frequent source of information and guidance for adolescents with dysmenorrhoea, and so their perspectives and knowledge are important considerations. A 2012 survey study in China of 300 mothers of adolescent daughters identified low levels of knowledge and perceived acceptability of the use of hormonal contraception as a treatment for dysmenorrhoea (318). A survey of parents in Australia also highlighted parental concerns about medication and side effects, including hormonal contraception (319). Given the central place these treatments hold in guidelines for first line empirical therapy, this is important to explore further.
GPs express concern about holding uncertainty when caring for adolescents whose symptoms do improve with a trial of treatment. If symptoms recur if or when they stop hormonal treatment at some later point, and they are subsequently investigated and found to have endometriosis, these care journeys may be characterised as “delayed” diagnoses in retrospective analysis. However, the assumption that they inevitably represent missed opportunities or sub-standard care is overly simplistic (3), including in the context of guidance suggesting referral only if trials of treatment are unsuccessful (109, 270). The complex inter-relationship between exogenous hormones and whether they protect against or exacerbate endometriosis complications and progression remains a critical area of uncertainty (117, 156), which is also a concern for GPs (3).
A family history of dysmenorrhoea predicts dysmenorrhoea (30, 151, 153), and a family history of endometriosis predicts endometriosis (156, 320). NICE Clinical Knowledge Summaries position asking about a family history of dysmenorrhoea as a pointer towards primary dysmenorrhoea (321), and a family history of endometriosis is a risk factor and pointer towards endometriosis. However, noting the well-documented delays and likely incomplete ascertainment of endometriosis diagnoses (322), we consider that asking about a family history of either endometriosis or dysmenorrhoea is potentially clinically useful.
With increasing recognition of endometriosis and of the role and value of non-invasive modalities for diagnosis, such as ultrasound or MRI (322), it is hopeful that diagnostic journeys will improve, and require less invasive testing. This would be welcome, not least because diagnostic laparoscopy is associated with both immediate risks but also longer term risks. However, this requires the development of education and resources (including shared-decision making and patient information resources) that recognise the variability of endometriosis, are tailored for adolescents and for community settings, where the prevalence/population is different.
While there is striking uniformity worldwide about the prevalence and impacts of dysmenorrhoea, routes to care and support likely differ, and qualitative perspectives would help shine a light on this, and support the improvements to care and well-being that are demonstrably desperately needed.
This could usefully explore the reasons for school absenteeism, which would enable schools and services to consider if there are adaptations that they could make to facilitate participation in school, including health literacy and self-efficacy, period poverty, menstrual stigma, and access to care.
Dysmenorrhoea is common and impactful, affecting participation in education and leisure and on well-being. While important to consider whether there is an underlying or associated cause, recognising, validating, and treating pain is essential in it's own right. Earlier and non-invasive diagnostic tools will help us understand more about the community prevalence of endometriosis, and could potentially bring the capacity to make the diagnosis from specialist care into community settings. This is welcome but needs to be accompanied by evidence relevant to the population seen in primary care, with caution about extrapolating risks from tertiary care and specialist clinics (fertility, pelvic pain).
There are evidence-based treatments which can be instigated and supported in general practice, including NSAIDs and treatment with hormonal contraception. However, it is important to ensure care planning and follow up that enables review and re-appraisal of any ongoing concerns or symptoms. Marked symptoms or those not responding to empirical treatment (or if this is not acceptable, tolerated, or is contra-indicated) indicate the need for further assessment and specialist referral. An ultrasound scan can be arranged within general practice, and could identify a structural anomaly or endometrioma, but alone will not exclude endometriosis. There is both a need and an opportunity to develop menstrual education that enables young people to understand how and when to ask for help and advice, and to ensure that there are resources and services for them when they do.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
SD: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Visualization, Writing – original draft, Writing – review & editing. JH: Methodology, Supervision, Validation, Writing – review & editing. NT: Data curation, Formal Analysis, Methodology, Writing – review & editing. CD: Data curation, Formal Analysis, Methodology, Writing – review & editing. KV: Conceptualization, Formal Analysis, Methodology, Supervision, Writing – review & editing. SZ: Conceptualization, Investigation, Methodology, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study was funded as part of Sharon Dixon's NIHR Doctoral research fellowship NIHR301787.
Nia Roberts, Senior Outreach Librarian, Bodleian Health Care Libraries, University of Oxford, and to Frith Dixon for his help with figure production.
KV has received research funding from Bayer Healthcare and honoraria for talks and consultancy fees paid to her institution from Bayer Healthcare, AbbVie, Reckitts, Gedeon Richter and Eli Lilly.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frph.2024.1394978/full#supplementary-material
1. Armour M, Ferfolja T, Curry C, Hyman MS, Parry K, Chalmers KJ, et al. The prevalence and educational impact of pelvic and menstrual pain in Australia: a national online survey of 4202 young women aged 13–25 years. J Pediatr Adolesc Gynecol. (2020) 33(5):511–8. doi: 10.1016/j.jpag.2020.06.007
2. De Sanctis V, Soliman A, Bernasconi S, Bianchin L, Bona G, Bozzola M, et al. Primary dysmenorrhea in adolescents: prevalence, impact and recent knowledge. Pediatr Endocrinol Rev. (2015) 13(2):512–20. PMID: 26841639.26841639
3. Dixon S, McNiven A, Talbot A, Hinton L. Navigating possible endometriosis in primary care: a qualitative study of GP perspectives. Br J Gen Pract. (2021) 71(710):e668–e76. doi: 10.3399/BJGP.2021.0030
4. Activity in the NHS. Activity in the NHS | The King's Fund. Available online at: https://www.kingsfund.org.uk/projects/nhs-in-a-nutshell/NHS-activity (Accessed June 18, 2024).
5. Marshall M. A precious jewel–the role of general practice in the English NHS. N Engl J Med. (2015) 372(10):893–7. doi: 10.1056/NEJMp1411429
6. Sripa P, Hayhoe B, Garg P, Majeed A, Greenfield G. Impact of GP gatekeeping on quality of care, and health outcomes, use, and expenditure: a systematic review. Br J Gen Pract. (2019) 69(682):e294–303. doi: 10.3399/bjgp19X702209
7. Heyvaert M, Maes B, Onghena P. Mixed methods research synthesis: definition, framework, and potential. Qual Quant. (2013) 47:659–76. doi: 10.1007/s11135-011-9538-6
8. Pearson A, White H, Bath-Hextall F, Salmond S, Apostolo J, Kirkpatrick P. A mixed-methods approach to systematic reviews. Int J Evid Based Healthc. (2015) 13(3):121–31. doi: 10.1097/XEB.0000000000000052
9. Greenhalgh T, Thorne S, Malterud K. Time to challenge the spurious hierarchy of systematic over narrative reviews? Eur J Clin Investig. (2018) 48(6):e12931. doi: 10.1111/eci.12931
10. WHO. Adolescent health. Available online at: https://www.who.int/health-topics/adolescent-health#tab=tab_1 (accessed June 18, 2024).
11. Available online at: https://www.nhs.uk/conditions/complementary-and-alternative-medicine/?ref=vzk.ru (accessed April 27, 2024).
12. Gagua T, Tkeshelashvili B, Gagua D. Primary dysmenorreah-leading problem of adolescent gynecology (review). Georgian Med News. (2012) 207:7–14. PMID: 22859441.
13. Hillard PJ. Consultation with the specialist: dysmenorrhea. Pediatr Rev. (2006) 27(2):64–71. doi: 10.1542/pir.27.2.64
14. Helwa HAA, Mitaeb AA, Al-Hamshri S, Sweileh WM. Prevalence of dysmenorrhea and predictors of its pain intensity among Palestinian female university students. BMC Women’s Health. (2018) 18(1):18. doi: 10.1186/s12905-018-0516-1
15. Agarwal AK, Agarwal A. A study of dysmenorrhea during menikstruation in adolescent girls. Indian J Community Med. (2010) 35(1):159–64. doi: 10.4103/0970-0218.62586
16. El-Gilany AH, Badawi K, El-Fedawy S. Epidemiology of dysmenorrhoea among adolescent students in mansoura, Egypt. East Mediterr Health J. (2005) 11(1–2):155–63.16532684
17. Eryilmaz G, Ozdemir F. Evaluation of menstrual pain management approaches by Northeastern Anatolian adolescents. Pain Manag Nurs. (2009) 10(1):40–7. doi: 10.1016/j.pmn.2008.09.001
18. Parker MA, Sneddon AE, Arbon P. The menstrual disorder of teenagers (MDOT) study: determining typical menstrual patterns and menstrual disturbance in a large population-based study of Australian teenagers. BJOG. (2010) 117(2):185–92. doi: 10.1111/j.1471-0528.2009.02407.x
19. Al-Kindi R, Al-Bulushi A. Prevalence and impact of dysmenorrhoea among omani high school students. Sultan Qaboos Univ Med J. (2011) 11(4):485–91. PMID: 22087397; PMCID: PMC3206751.22087397
20. Banikarim C, Chacko MR, Kelder SH. Prevalence and impact of dysmenorrhea on Hispanic female adolescents. Arch Pediatr Adolesc Med. (2000) 154(12):1226–9. doi: 10.1001/archpedi.154.12.1226
21. Bata MS. Age at menarche, menstrual patterns, and menstrual characteristics in Jordanian adolescent girls. Int J Gynaecol Obstet. (2012) 119(3):281–3. doi: 10.1016/j.ijgo.2012.07.009
22. Chan SS, Yiu KW, Yuen PM, Sahota DS, Chung TK. Menstrual problems and health-seeking behaviour in Hong Kong Chinese girls. Hong Kong Med J. (2009) 15(1):18–23. PMID: 19197092.19197092
23. Demir SC, Kadayyfcy TO, Vardar MA, Atay Y. Dysfunctional uterine bleeding and other menstrual problems of secondary school students in Adana, Turkey. J Pediatr Adolesc Gynecol. (2000) 13(4):171–5. doi: 10.1016/S1083-3188(00)00061-9
24. Esen I, Oguz B, Serin HM. Menstrual characteristics of pubertal girls: a questionnaire-based study in Turkey. J Clin Res Pediatr Endocrinol. (2016) 8(2):192–6. doi: 10.4274/jcrpe.2026
25. Gagua T, Tkeshelashvili B, Gagua D. Primary dysmenorrhea: prevalence in adolescent population of Tbilisi, Georgia and risk factors. J Turk Gynecol Assoc. (2012) 13(3):162–8. doi: 10.5152/jtgga.2012.21
26. Gumanga SK, Kwame-Aryee RA. Menstrual characteristics in some adolescent girls in Accra, Ghana. Ghana Med J. (2012) 46(1):3–7. PMID: 22605882; PMCID: PMC3353505.22605882
27. Hillen TI, Grbavac SL, Johnston PJ, Straton JA, Keogh JM. Primary dysmenorrhea in young Western Australian women: prevalence, impact, and knowledge of treatment. J Adolesc Health. (1999) 25(1):40–5. doi: 10.1016/S1054-139X(98)00147-5
28. Hoppenbrouwers K, Roelants M, Meuleman C, Rijkers A, Van Leeuwen K, Desoete A, et al. Characteristics of the menstrual cycle in 13-year-old Flemish girls and the impact of menstrual symptoms on social life. Eur J Pediatr. (2016) 175(5):623–30. doi: 10.1007/s00431-015-2681-7
29. Hu Z, Tang L, Chen L, Kaminga AC, Xu H. Prevalence and risk factors associated with primary dysmenorrhea among Chinese female university students: a cross-sectional study. J Pediatr Adolesc Gynecol. (2020) 33(1):15–22. doi: 10.1016/j.jpag.2019.09.004
30. Lee JC, Yu BK, Byeon JH, Lee KH, Min JH, Park SH. A study on the menstruation of Korean adolescent girls in Seoul. Korean J Pediatr. (2011) 54(5):201–6. doi: 10.3345/kjp.2011.54.5.201
31. Lghoul S, Loukid M, Hilali MK. Prevalence and predictors of dysmenorrhea among a population of adolescent’s schoolgirls (Morocco). Saudi J Biol Sci. (2020) 27(7):1737–42. doi: 10.1016/j.sjbs.2020.05.022
32. Ortiz MI, Rangel-Flores E, Carrillo-Alarcon LC, Veras-Godoy HA. Prevalence and impact of primary dysmenorrhea among Mexican high school students. Int J Gynaecol Obstet. (2009) 107(3):240–3. doi: 10.1016/j.ijgo.2009.07.031
33. Pitangui AC, Gomes MR, Lima AS, Schwingel PA, Albuquerque AP, de Araujo RC. Menstruation disturbances: prevalence, characteristics, and effects on the activities of daily living among adolescent girls from Brazil. J Pediatr Adolesc Gynecol. (2013) 26(3):148–52. doi: 10.1016/j.jpag.2012.12.001
34. Soderman L, Edlund M, Marions L. Prevalence and impact of dysmenorrhea in Swedish adolescents. Acta Obstet Gynecol Scand. (2019) 98(2):215–21. doi: 10.1111/aogs.13480
35. Tangchai K, Titapant V, Boriboonhirunsarn D. Dysmenorrhea in Thai adolescents: prevalence, impact and knowledge of treatment. J Med Assoc Thail. (2004) 87:(Suppl 3):S69–73. PMID: 21218593.
36. Yucel G, Kendirci M, Gul U. Menstrual characteristics and related problems in 9- to 18-year-old turkish school girls. J Pediatr Adolesc Gynecol. (2018) 31(4):350–5. doi: 10.1016/j.jpag.2018.03.002
37. Santina T, Wehbe N, Ziade F. Exploring dysmenorrhoea and menstrual experiences among Lebanese female adolescents. East Mediterr Health J. (2012) 18(8):857–63. doi: 10.26719/2012.18.8.857
38. Farquhar CM, Roberts H, Okonkwo QL, Stewart AW. A pilot survey of the impact of menstrual cycles on adolescent health. Aust N Z J Obstet Gynaecol. (2009) 49(5):531–6. doi: 10.1111/j.1479-828X.2009.01062.x
39. Suvitie PA, Hallamaa MK, Matomaki JM, Makinen JI, Perheentupa AH. Prevalence of pain symptoms suggestive of endometriosis among Finnish adolescent girls (TEENMAPS study). J Pediatr Adolesc Gynecol. (2016) 29(2):97–103. doi: 10.1016/j.jpag.2015.07.001
40. Zannoni L, Giorgi M, Spagnolo E, Montanari G, Villa G, Seracchioli R. Dysmenorrhea, absenteeism from school, and symptoms suspicious for endometriosis in adolescents. J Pediatr Adolesc Gynecol. (2014) 27(5):258–65. doi: 10.1016/j.jpag.2013.11.008
41. Heald FP Jr., Masland RP Jr., Sturgis SH, Gallagher JR. Dysmenorrhea in adolescence. Pediatrics. (1957) 20(1):121–7. doi: 10.1542/peds.20.1.121
42. Gardner J. Adolescent menstrual characteristics as predictors of gynaecological health. Ann Hum Biol. (1983) 10(1):31–40. doi: 10.1080/03014468300006161
43. Fontana D, Rees V. Primary dysmenorrhea, educational performance, and cognitive and affective variables in adolescent schoolgirls. Br J Educ Psychol. (1982) 52:199–204. doi: 10.1111/j.2044-8279.1982.tb00826.x
44. Batt RE, Sturgis SH. Adolescents’ gynecologic problems. Medical aspects. Clin Pediatr (Phila). (1968) 7(1):17–23. doi: 10.1177/000992286800700108
45. Frisk M, Widholm O, Hortling H. Dysmenorrhea–psyche and soma in teenagers. Acta Obstet Gynecol Scand. (1965) 44(2):339–47. doi: 10.3109/00016346509155870
46. Holmlund U. The experience of dysmenorrhea and its relationship to personality variables. Acta Psychiatr Scand. (1990) 82(2):182–7. doi: 10.1111/j.1600-0447.1990.tb01379.x
47. Sloan D. Pelvic pain and dysmenorrhea. Pediatr Clin N Am. (1972) 19(3):669–80. doi: 10.1016/S0031-3955(16)32745-6
48. Lundstrom V, af Geijerstam G. Treatment of primary dysmenorrhea. Acta Obstet Gynecol Scand Suppl. (1983) 113:83–5. doi: 10.3109/00016348309155205
49. Sablosky L, Glanzberg P. Attitude and response to treatment of adolescents and adults with dysmenorrhea. J Am Med Womens Assoc. (1959) 14(5):415–8. PMID: 13654057.13654057
50. Kintis GA, Coutifaris B. Treatment of primary dysmenorrhea with mefenamic acid. Int J Gynaecol Obstet. (1980) 18(3):172–5. doi: 10.1002/j.1879-3479.1980.tb00274.x
51. Comerci GD. Symptoms associated with menstruation. Pediatr Clin N Am. (1982) 29(1):177–200. doi: 10.1016/S0031-3955(16)34116-5
52. Lawlor CL, Davis AM. Primary dysmenorrhea. Relationship to personality and attitudes in adolescent females. J Adolesc Health Care. (1981) 1(3):208–12. doi: 10.1016/S0197-0070(81)80058-7
53. Jay MS, DuRant RH. The patient with dysmenorrhea. Postgrad Med. (1983) 73(4):103–6; 9–11. doi: 10.1080/00325481.1983.11698351
54. Hewitt G. Dysmenorrhea and endometriosis: diagnosis and management in adolescents. Clin Obstet Gynecol. (2020) 63(3):536–43. doi: 10.1097/GRF.0000000000000540
55. Harel Z. Dysmenorrhea in adolescents and young adults: an update on pharmacological treatments and management strategies. Expert Opin Pharmacother. (2012) 13(15):2157–70. doi: 10.1517/14656566.2012.725045
56. Harel Z. Dysmenorrhea in adolescents and young adults: from pathophysiology to pharmacological treatments and management strategies. Expert Opin Pharmacother. (2008) 9(15):2661–72. doi: 10.1517/14656566.9.15.2661
57. Harel Z. Dysmenorrhea in adolescents and young adults: etiology and management. J Pediatr Adolesc Gynecol. (2006) 19(6):363–71. doi: 10.1016/j.jpag.2006.09.001
58. Harel Z. A contemporary approach to dysmenorrhea in adolescents. Paediatr Drugs. (2002) 4(12):797–805. doi: 10.2165/00128072-200204120-00004
59. Wilson CA, Keye WR Jr. A survey of adolescent dysmenorrhea and premenstrual symptom frequency. A model program for prevention, detection, and treatment. J Adolesc Health Care. (1989) 10(4):317–22. doi: 10.1016/0197-0070(89)90065-X
60. Stromberg P, Akerlund M, Forsling ML, Granstrom E, Kindahl H. Vasopressin and prostaglandins in premenstrual pain and primary dysmenorrhea. Acta Obstet Gynecol Scand. (1984) 63(6):533–8. doi: 10.3109/00016348409156715
61. Alvin PE, Litt IF. Current status of the etiology and management of dysmenorrhea in adolescence. Pediatrics. (1982) 70(4):516–25. doi: 10.1542/peds.70.4.516
62. Kartal YA, Akyuz EY. The effect of diet on primary dysmenorrhea in university students: a randomized controlled clinical trial. Pak J Med Sci. (2018) 34(6):1478–82. doi: 10.12669/pjms.346.16477
63. Senturk S. Relation between uterine morphology and severity of primary dysmenorrhea. Turk J Obstet Gynecol. (2020) 17(2):84–9. doi: 10.4274/tjod.galenos.2020.26032
64. Sahin ME, Sahin E, Madendag Y, Madendag IC, Tayyar AT, Ozdemir F, et al. The effect of anterior uterocervical angle on primary dysmenorrhea and disease severity. Pain Res Manag. (2018) 2018:9819402. doi: 10.1155/2018/9819402
65. Kho KA, Shields JK. Diagnosis and management of primary dysmenorrhea. JAMA. (2020) 323(3):268–9. doi: 10.1001/jama.2019.16921
66. de Sanctis V, Matalliotakis M, Soliman AT, Elsefdy H, Di Maio S, Fiscina B. A focus on the distinctions and current evidence of endometriosis in adolescents. Best Pract Res Clin Obstet Gynaecol. (2018) 51:138–50. doi: 10.1016/j.bpobgyn.2018.01.023
68. Jay MS, Durant RH, Shoffitt T, Linder CW. Differential response by adolescents to naproxen sodium therapy for spasmodic and congestive dysmenorrhea. J Adolesc Health Care. (1986) 7(6):395–400. doi: 10.1016/S0197-0070(86)80241-8
69. Gokhale LB. Curative treatment of primary (spasmodic) dysmenorrhoea. Indian J Med Res. (1996) 103:227–31. PMID: 8935744.8935744
70. Dambhare DG, Wagh SV, Dudhe JY. Age at menarche and menstrual cycle pattern among school adolescent girls in Central India. Glob J Health Sci. (2012) 4(1):105–11. doi: 10.5539/gjhs.v4n1p105
71. Wong LP. Attitudes towards dysmenorrhoea, impact and treatment seeking among adolescent girls: a rural school-based survey. Aust J Rural Health. (2011) 19(4):218–23. doi: 10.1111/j.1440-1584.2011.01213.x
72. Chauhan M, Kala J. Relation between dysmenorrhea and body mass index in adolescents with rural versus urban variation. J Obstet Gynaecol India. (2012) 62(4):442–5. doi: 10.1007/s13224-012-0171-7
73. Rani A, Sharma MK, Singh A. Practices and perceptions of adolescent girls regarding the impact of dysmenorrhea on their routine life: a comparative study in the urban, rural, and slum areas of Chandigarh. Int J Adolesc Med Health. (2016) 28(1):3–9. doi: 10.1515/ijamh-2014-0063
74. Wong LP. Premenstrual syndrome and dysmenorrhea: urban-rural and multiethnic differences in perception, impacts, and treatment seeking. J Pediatr Adolesc Gyneco. (2011) 24(5):272–7. doi: 10.1016/j.jpag.2011.03.009
75. Wong LP, Khoo EM. Dysmenorrhea in a multiethnic population of adolescent Asian girls. Int J Gynaecol Obstet. (2010) 108(2):139–42. doi: 10.1016/j.ijgo.2009.09.018
76. Goldstein-Ferber S, Granot M. The association between somatization and perceived ability: roles in dysmenorrhea among Israeli Arab adolescents. Psychosom Med. (2006) 68(1):136–42. doi: 10.1097/01.psy.0000197644.95292.00
77. Armour M, Parry K, Manohar N, Holmes K, Ferfolja T, Curry C, et al. The prevalence and academic impact of dysmenorrhea in 21,573 young women: a systematic review and meta-analysis. J Women’s Health. (2019) 28(8):1161–71. doi: 10.1089/jwh.2018.7615
78. Havens B, Swenson I. Imagery associated with menstruation in advertising targeted to adolescent women. Adolescence. (1988) 23(89):89–97. PMID: 3381691.3381691
79. Li AD, Bellis EK, Girling JE, Jayasinghe YL, Grover SR, Marino JL, et al. Unmet needs and experiences of adolescent girls with heavy menstrual bleeding and dysmenorrhea: a qualitative study. J Pediatr Adolesc Gynecol. (2020) 33(3):278–84. doi: 10.1016/j.jpag.2019.11.007
80. Abraham S, Fraser I, Gebski V, Knight C, Llewellyn-Jones D, Mira M, et al. Menstruation, menstrual protection and menstrual cycle problems. The knowledge, attitudes and practices of young Australian women. Med J Aust. (1985) 142(4):247–51. doi: 10.5694/j.1326-5377.1985.tb113322.x
81. Randhawa AE, Tufte-Hewett AD, Weckesser AM, Jones GL, Hewett FG. Secondary school Girls’ experiences of menstruation and awareness of endometriosis: a cross-sectional study. J Pediatr Adolesc Gynecol. (2021) 34(5):643–8. doi: 10.1016/j.jpag.2021.01.021
82. Klein JR, Litt IF. Epidemiology of adolescent dysmenorrhea. Pediatrics. (1981) 68(5):661–4. doi: 10.1542/peds.68.5.661
83. Widholm O, Kantero RL. A statistical analysis of the menstrual patterns of 8,000 Finnish girls and their mothers. Acta Obstet Gynecol Scand Suppl. (1971) 14(14):1–36. PMID: 5290914.
84. Doster ME, Mc NA, Lampe JM, Corliss LM. A survey of menstrual function among 1,668 secondary school girls and 720 women employees of the Denver public schools. Am J Public Health Nation’s Health. (1961) 51:1841–6. doi: 10.2105/AJPH.51.12.1841
85. Teperi J, Rimpela M. Menstrual pain, health and behaviour in girls. Soc Sci Med. (1989) 29(2):163–9. doi: 10.1016/0277-9536(89)90164-0
86. Borjigen A, Huang C, Liu M, Lu J, Peng H, Sapkota C, et al. Status and factors of menstrual knowledge, attitudes, behaviors and their correlation with psychological stress in adolescent girls. J Pediatr Adolesc Gynecol. (2019) 32(6):584–9. doi: 10.1016/j.jpag.2019.08.007
87. Sultan C, Jeandel C, Paris F, Trimeche S. Adolescent dysmenorrhea. Endocr Dev. (2004) 7:140–7. doi: 10.1159/000077082
88. Mou L, Lei W, Chen J, Zhang R, Liu K, Liang X. Mediating effect of interpersonal relations on negative emotions and dysmenorrhea in female adolescents. Gen Psychiatr. (2019) 32(1):e100008. doi: 10.1136/gpsych-2018-100008
89. Harada T. Dysmenorrhea and endometriosis in young women. Yonago Acta Med. (2013) 56(4):81–4. PMID: 24574576; PMCID: PMC3935015.24574576
90. Gagua T, Tkeshelashvili B, Gagua D, McHedlishvili N. Assessment of anxiety and depression in adolescents with primary dysmenorrhea: a case-control study. JPediatr Adolesc Gynecol. (2013) 26(6):350–4. doi: 10.1016/j.jpag.2013.06.018
91. Chen HM, Chen CH. Related factors and consequences of menstrual distress in adolescent girls with dysmenorrhea. Kaohsiung J Med Sci. (2005) 21(3):121–7. doi: 10.1016/S1607-551X(09)70288-8
92. Widholm O. Dysmenorrhea during adolescence. Acta Obstet Gynecol Scand Suppl. (1979) 87:61–6. doi: 10.3109/00016347909157792
93. Beal SJ, Dorn LD, Sucharew HJ, Sontag-Padilla L, Pabst S, Hillman J. Characterizing the longitudinal relations between depressive and menstrual symptoms in adolescent girls. Psychosom Med. (2014) 76(7):547–54. doi: 10.1097/PSY.0000000000000099
94. De Sanctis V, Rigon F, Bernasconi S, Bianchin L, Bona G, Bozzola M, et al. Age at menarche and menstrual abnormalities in adolescence: does it matter? The evidence from a large survey among Italian secondary schoolgirls. Indian J Pediatr. (2019) 86:34–41. doi: 10.1007/s12098-018-2822-x
95. Vicdan K, Kukner S, Dabakoglu T, Ergin T, Keles G, Gokmen O. Demographic and epidemiologic features of female adolescents in Turkey. J Adolesc Health. (1996) 18(1):54–8. doi: 10.1016/1054-139X(95)00127-E
96. Kazama M, Maruyama K, Nakamura K. Prevalence of dysmenorrhea and its correlating lifestyle factors in Japanese female junior high school students. Tohoku J Exp Med. (2015) 236(2):107–13. doi: 10.1620/tjem.236.107
97. Knox B, Ong YC, Bakar MA, Grover SR. A longitudinal study of adolescent dysmenorrhoea into adulthood. Eur J Pediatr. (2019) 178(9):1325–32. doi: 10.1007/s00431-019-03419-3
98. Strinic T, Bukovic D, Pavelic L, Fajdic J, Herman I, Stipic I, et al. Anthropological and clinical characteristics in adolescent women with dysmenorrhea. Coll Antropol. (2003) 27(2):707–11. PMID: 14746162.14746162
99. Flug D, Largo RH, Prader A. Symptoms related to menstruation in adolescent Swiss girls: a longitudinal study. Ann Hum Biol. (1985) 12(2):161–8. doi: 10.1080/03014468500007651
100. Lee LK, Chen PC, Lee KK, Kaur J. Menstruation among adolescent girls in Malaysia: a cross-sectional school survey. Singapore Med J. (2006) 47(10):869–74. PMID: 16990962.16990962
101. Jeon GE, Cha NH, Sok SR. Factors influencing the dysmenorrhea among Korean adolescents in middle school. J Phys Ther Sci. (2014) 26(9):1337–43. doi: 10.1589/jpts.26.1337
102. Negriff S, Dorn LD, Hillman JB, Huang B. The measurement of menstrual symptoms: factor structure of the menstrual symptom questionnaire in adolescent girls. J Health Psychol. (2009) 14(7):899–908. doi: 10.1177/1359105309340995
103. Svanberg L, Ulmsten U. The incidence of primary dysmenorrhea in teenagers. Arch Gynecol. (1981) 230(3):173–7. doi: 10.1007/BF02111800
104. Sundell G, Milsom I, Andersch B. Factors influencing the prevalence and severity of dysmenorrhoea in young women. Br J Obstet Gynaecol. (1990) 97(7):588–94. doi: 10.1111/j.1471-0528.1990.tb02545.x
105. Art MJ, Doerfler D. Incidences of menstrual cycle abnormalities in adolescence, and matches between the age at menarche and the development of menstrual cycle abnormalities. Wien Med Wochenschr. (2010) 160(15):406–13. PMID: 20812052.20812052
106. Yamamoto K, Okazaki A, Sakamoto Y, Funatsu M. The relationship between premenstrual symptoms, menstrual pain, irregular menstrual cycles, and psychosocial stress among Japanese college students. J Physiol Anthropol. (2009) 28(3):129–36. doi: 10.2114/jpa2.28.129
107. Balbi C, Musone R, Menditto A, Di Prisco L, Cassese E, D'Ajello M, et al. Influence of menstrual factors and dietary habits on menstrual pain in adolescence age. Eur J Obstet Gynecol Reprod Biol. (2000) 91(2):143–8. doi: 10.1016/S0301-2115(99)00277-8
108. Fujiwara T, Nakata R. Young Japanese college students with dysmenorrhea have high frequency of irregular menstruation and premenstrual symptoms. Open Med Inform J. (2007) 1:8–11. doi: 10.2174/1874431100701010008
109. Anonymous. ACOG Committee opinion No. 760: dysmenorrhea and endometriosis in the adolescent. Obstet Gynecol. (2018) 132(6):e249–e58. doi: 10.1097/AOG.0000000000002978
110. Monday I, Anthony P, Olunu E, Otohinoyi D, Abiodun S, Owolabi A, et al. Prevalence and correlation between diet and dysmenorrhea among high school and college students in saint vincent and grenadines. Open Access Maced J Med Sci. (2019) 7(6):920–4. doi: 10.3889/oamjms.2019.205
111. Fawole AO, Babarinsa IA, Fawole OI, Obisesan KA, Ojengbede OA. Menstrual characteristics of secondary school girls in Ibadan, Nigeria. J Pediatr Adolesc Gynecol. (2009) 28(2):92–6. doi: 10.1016/j.jpag.2010.04.001
112. Xu K, Chen L, Fu L, Xu S, Fan H, Gao Q, et al. Stressful parental-bonding exaggerates the functional and emotional disturbances of primary dysmenorrhea. Int J Behav Med. (2016) 23(4):458–63. doi: 10.1007/s12529-015-9504-0
113. Maruf FA, Ezenwafor NV, Moroof SO, Adeniyi AF, Okoye EC. Physical activity level and adiposity: are they associated with primary dysmenorrhea in school adolescents? Afr J Reprod Health. (2013) 17(4):167–74. PMID: 24558792.24558792
114. Chiou MH, Wang HH. Predictors of dysmenorrhea and self-care behavior among vocational nursing school female students. Jo Nurs Res. (2008) 16(1):17–25. doi: 10.1097/01.JNR.0000387286.30688.5b
115. Wong CL. Health-related quality of life among Chinese adolescent girls with Dysmenorrhoea. Reprod Health. (2018) 15(1):80. doi: 10.1186/s12978-018-0540-5
116. Aziato L, Dedey F, Clegg-Lamptey JN. Dysmenorrhea management and coping among students in Ghana: a qualitative exploration. J Pediatr Adolesc Gynecol. (2015) 28(3):163–9. doi: 10.1016/j.jpag.2014.07.002
117. Clemenza S, Vannuccini S, Capezzuoli T, Meleca CI, Pampaloni F, Petraglia F. Is primary dysmenorrhea a precursor of future endometriosis development? Gynecol Endocrinol. (2021) 37(4):287–93. doi: 10.1080/09513590.2021.1878134
118. Saridogan E. Endometriosis in teenagers. Women’s Health. (2015) 11(5):705–9. doi: 10.2217/whe.15.58
119. Eryilmaz G, Ozdemir F, Pasinlioglu T. Dysmenorrhea prevalence among adolescents in eastern Turkey: its effects on school performance and relationships with family and friends. J Pediatr Adolesc Gynecol. (2010) 23(5):267–72. doi: 10.1016/j.jpag.2010.02.009
120. Chang SF, Chuang MH. Factors that affect self-care behaviour of female high school students with dysmenorrhoea: a cluster sampling study. Int J Nurs Pract. (2012) 18(2):117–24. doi: 10.1111/j.1440-172X.2012.02007.x
121. De Sanctis V, Soliman AT, Elsedfy H, Soliman NA, Soliman R, El Kholy M. Dysmenorrhea in adolescents and young adults: a review in different country. Acta Biomed. (2016) 87(3):233–46. PMID: 28112688; PMCID: PMC10521891.28112688
122. Schroeder B, Sanfilippo JS. Dysmenorrhea and pelvic pain in adolescents. Pediatr Clin N Am. (1999) 46(3):555–71. doi: 10.1016/S0031-3955(05)70137-1
123. Johnson J. Level of knowledge among adolescent girls regarding effective treatment for dysmenorrhea. J Adolesc Health Care. (1988) 9(5):398–402. doi: 10.1016/0197-0070(88)90036-8
124. Fedorowicz Z, Nasser M, Jagannath VA, Beaman JH, Ejaz K, van Zuuren EJ. Beta2-adrenoceptor agonists for dysmenorrhoea. Cochrane Database Syst Rev. (2012) (5):CD008585. doi: 10.1002/14651858.CD008585.pub2
125. Pattanittum P, Kunyanone N, Brown J, Sangkomkamhang US, Barnes J, Seyfoddin V, et al. Dietary supplements for dysmenorrhoea. Cochrane Database Syst Rev. (2016) 3:CD002124. doi: 10.1002/14651858.CD002124.pub2
126. Khoiny FE. Adolescent dysmenorrhea. J Pediatr Health Care. (1988) 2(1):29–37. doi: 10.1016/0891-5245(88)90057-0
127. DuRant RH, Jay MS, Shoffitt T, Linder CW, Taylor W. Factors influencing adolescents’ responses to regimens of naproxen for dysmenorrhea. Am J Dis Child. (1985) 139(5):489–93. doi: 10.1001/archpedi.1985.02140070063035
128. Hosseinlou A, Alinejad V, Alinejad M, Aghakhani N. The effects of fish oil capsules and vitamin B1 tablets on duration and severity of dysmenorrhea in students of high school in Urmia-Iran. Glob J Health Sci. (2014) 6(7):124–9. doi: 10.5539/gjhs.v6n7p124
129. Ziaei S, Faghihzadeh S, Sohrabvand F, Lamyian M, Emamgholy T. A randomised placebo-controlled trial to determine the effect of vitamin E in treatment of primary dysmenorrhoea. BJOG. (2001) 108(11):1181–3. doi: 10.1111/j.1471-0528.2003.00279.x
130. Blythe MJ. Common menstrual problems of adolescence. Adolesc Med State Art Rev. (1997) 8(1):87–109. PMID: 10360010.
131. Akman AO, Bozdag G, Kizilkan MP, Akgul S, Derman O, Kanbur N. Menstrual cycle pain is independent of ovulation in adolescents with primary dysmenorrhea. J Pediatr Adolesc Gynecol. (2021) 25(25):635–43. doi: 10.1016/j.jpag.2021.04.001
132. Smith CA, Zhu X, He L, Song J. Acupuncture for primary dysmenorrhoea. Cochrane Database Syst Rev. (2011) (1):CD007854. doi: 10.1002/14651858.CD007854.pub2
133. Smith CA, Armour M, Zhu X, Li X, Lu ZY, Song J. Acupuncture for dysmenorrhoea. Cochrane Database Syst Rev. (2016) 4:CD007854. doi: 10.1002/14651858.CD007854.pub3
134. Uysal G, Akkaya H, Cagli F, Tutus S, Tayyar AT. A comparison of two different oral contraceptives in patients with severe primary dysmenorrhoea. J Obstet Gynaecol. (2018) 38(6):828–32. doi: 10.1080/01443615.2017.1410533
135. Jacks TH, Obed JY, Agida ET, Petrova GV. Dysmenorrhoea and menstrual abnormalities among postmenarcheal secondary school girls in Maiduguri Nigeria. Afr J Med Med Sci. (2005) 34(1):87–9. PMID: 15971560.15971560
136. Andres Mde P, Podgaec S, Carreiro KB, Baracat EC. Endometriosis is an important cause of pelvic pain in adolescence. Rev Assoc Med Bras. (2014) 60(6):560–4. doi: 10.1590/1806-9282.60.06.015
137. Aziato L, Dedey F, Clegg-Lamptey JN. The experience of dysmenorrhoea among Ghanaian senior high and university students: pain characteristics and effects. Reprod Health. (2014) 11:58. doi: 10.1186/1742-4755-11-58
138. Chang YT, Chen YC, Hayter M, Lin ML. Menstrual and menarche experience among pubescent female students in Taiwan: implications for health education and promotion practice. J Clin Nurs. (2009) 18(14):2040–8. doi: 10.1111/j.1365-2702.2008.02545.x
139. Sachedina A, Abu Bakar M, Dunford AM, Morris A, Nur Azurah AG, Grover SR. Dysmenorrhea in young people: experiences from a tertiary center with a focus on conservative management. J Obstet Gynaecol Res. (2021) 47(1):352–8. doi: 10.1111/jog.14532
140. Gunn HM, Tsai MC, McRae A, Steinbeck KS. Menstrual patterns in the first gynecological year: a systematic review. J Pediatr Adolesc Gynecol. (2018) 31(6):557–65.e6. doi: 10.1016/j.jpag.2018.07.009
141. Vannuccini S, Fondelli F, Clemenza S, Galanti G, Petraglia F. Dysmenorrhea and heavy menstrual bleeding in elite female athletes: quality of life and perceived stress. Reprod Sci. (2020) 27(3):888–94. doi: 10.1007/s43032-019-00092-7
142. Durain D. Primary dysmenorrhea: assessment and management update. J Midwifery Women’s Health. (2004) 49(6):520–8. doi: 10.1016/j.jmwh.2004.08.013
143. Harlow SD, Park M. A longitudinal study of risk factors for the occurrence, duration and severity of menstrual cramps in a cohort of college women. Br J Obstet Gynaecol. (1996) 103(11):1134–42. doi: 10.1111/j.1471-0528.1996.tb09597.x
144. Azagew AW, Kassie DG, Walle TA. Prevalence of primary dysmenorrhea, its intensity, impact and associated factors among female students’ at Gondar town preparatory school, Northwest Ethiopia. BMC Women’s Health. (2020) 20(1):5. doi: 10.1186/s12905-019-0873-4
145. Wilson C, Emans SJ, Mansfield J, Podolsky C, Grace E. The relationships of calculated percent body fat, sports participation, age, and place of residence on menstrual patterns in healthy adolescent girls at an independent New England high school. J Adolesc Health Care. (1984) 5(4):248–53. doi: 10.1016/S0197-0070(84)80126-6
146. Vichnin M, Freeman EW, Lin H, Hillman J, Bui S. Premenstrual syndrome (PMS) in adolescents: severity and impairment. J Pediatr Adolesc Gynecol. (2006) 19(6):397–402. doi: 10.1016/j.jpag.2006.06.015
147. Freeman EW, Rickels K, Sondheimer SJ. Premenstrual symptoms and dysmenorrhea in relation to emotional distress factors in adolescents. J Psychosom Obstet Gynecol. (1993) 14(1):41–50. doi: 10.3109/01674829309084429
148. Sharma P, Malhotra C, Taneja DK, Saha R. Problems related to menstruation amongst adolescent girls. Indian J Pediatr. (2008) 75(2):125–9. doi: 10.1007/s12098-008-0018-5
149. Kitamura M, Takeda T, Koga S, Nagase S, Yaegashi N. Relationship between premenstrual symptoms and dysmenorrhea in Japanese high school students. Arch Women’s Men Health. (2012) 15(2):131–3. doi: 10.1007/s00737-012-0266-2
150. Katwal PC, Karki NR, Sharma P, Tamrakar SR. Dysmenorrhea and stress among the Nepalese medical students. Kathmandu Univ Med J. (2016) 14(56):318–21. PMID: 29336418.153.
151. Andersch B, Milsom I. An epidemiologic study of young women with dysmenorrhea. Am J Obstet Gynecol. (1982) 144(6):655–60. doi: 10.1016/0002-9378(82)90433-1
152. Ching-Hsing H, Meei-Ling G, Hsin-Chun M, Chung-Yi L. The development and psychometric testing of a self-care scale for dysmenorrhic adolescents. J Nurs Res. (2004) 12(2):119–30. doi: 10.1097/01.JNR.0000387495.01557.aa
153. Chaudhuri A, Singh A. How do school girls deal with dysmenorrhoea? J Indian Med Assoc. (2012) 110(5):287–91. PMID: 23360019.23360019
154. Schauffler GC. Dysmenorrhea in and near puberty. Ann N Y Acad Sci. (1967) 142(3):794–800. doi: 10.1111/j.1749-6632.1967.tb14692.x
155. Stoltzman SM. Menstrual attitudes, beliefs, and symptom experiences of adolescent females, their peers, and their mothers. Health Care Women Int. (1986) 7(1):97–114. doi: 10.1080/07399338609515726
156. Cramer DW, Missmer SA. The epidemiology of endometriosis. Ann N Y Acad Sci. (2002) 955:11–22; discussion 34–6, 396–406. doi: 10.1111/j.1749-6632.2002.tb02761.x
157. Singh M, Rajoura OP, Honnakamble RA. Menstrual patterns and problems in association with body mass index among adolescent school girls. J Family Med Prim Care. (2019) 8(9):2855–8. doi: 10.4103/jfmpc.jfmpc_474_19
158. Vani KR, Veena KS, Subitha L, Hemanth Kumar VR, Bupathy A. Menstrual abnormalities in school going girls—are they related to dietary and exercise pattern? J Clin Diagn Res. (2013) 7(11):2537–40. doi: 10.7860/JCDR/2013/6464.3603
159. Negi P, Mishra A, Lakhera P. Menstrual abnormalities and their association with lifestyle pattern in adolescent girls of Garhwal, India. J Family Med Prim Care. (2018) 7(4):804–8. doi: 10.4103/jfmpc.jfmpc_159_17
160. Nooh AM, Abdul-Hady A, El-Attar N. Nature and prevalence of menstrual disorders among teenage female students at Zagazig University, Zagazig, Egypt. J Pediatr Adolesc Gynecol. (2016) 29(2):137–42. doi: 10.1016/j.jpag.2015.08.008
161. Montero P, Bernis C, Fernandez V, Castro S. Influence of body mass index and slimming habits on menstrual pain and cycle irregularity. J Biosoc Sci. (1996) 28(3):315–23. doi: 10.1017/S0021932000022380
162. Fujiwara T. Diet during adolescence is a trigger for subsequent development of dysmenorrhea in young women. Int J Food Sci Nutr. (2007) 58(6):437–44. doi: 10.1080/09637480701288348
163. Ambresin AE, Belanger RE, Chamay C, Berchtold A, Narring F. Body dissatisfaction on top of depressive mood among adolescents with severe dysmenorrhea. J Pediatr Adolesc Gynecol. (2012) 25(1):19–22. doi: 10.1016/j.jpag.2011.06.014
164. Kabukcu C, Kabukcu Basay B, Basay O. Primary dysmenorrhea in adolescents: association with attention deficit hyperactivity disorder and psychological symptoms. Taiwan J Obstet Gynecol. (2021) 60(2):311–7. doi: 10.1016/j.tjog.2021.01.033
165. Fujiwara T. Skipping breakfast is associated with dysmenorrhea in young women in Japan. Int J Food Sci Nutr. (2003) 54(6):505–9. doi: 10.1080/09637480310001622369
166. Fujiwara T, Nakata R. Skipping breakfast is associated with reproductive dysfunction in post-adolescent female college students. Appetite. (2010) 55(3):714–7. doi: 10.1016/j.appet.2010.08.005
167. Fujiwara T, Sato N, Awaji H, Sakamoto H, Nakata R. Skipping breakfast adversely affects menstrual disorders in young college students. Int J Food Sci Nutr. (2009) 60:23–31. doi: 10.1080/09637480802260998
168. Bahrami A, Sadeghnia H, Avan A, Mirmousavi SJ, Moslem A, Eslami S, et al. Neuropsychological function in relation to dysmenorrhea in adolescents. Eur J Obstet Gynecol Reprod Biol. (2017) 215:224–9. doi: 10.1016/j.ejogrb.2017.06.030
169. Liu X, Chen H, Liu ZZ, Fan F, Jia CX. Early menarche and menstrual problems are associated with sleep disturbance in a large sample of Chinese adolescent girls. Sleep. (2017) 40(9). doi: 10.1093/sleep/zsx107
170. Campbell MA, McGrath PJ. Non-pharmacologic strategies used by adolescents for the management of menstrual discomfort. Clin J Pain. (1999) 15(4):313–20. doi: 10.1097/00002508-199912000-00008
171. Campbell MA, McGrath PJ. Use of medication by adolescents for the management of menstrual discomfort. Arch Pediatr Adolesc Med. (1997) 151(9):905–13. doi: 10.1001/archpedi.1997.02170460043007
172. Allyn K, Evans S, Seidman LC, Payne LA. Tomorrow, I'll be Fine": impacts and coping mechanisms in adolescents and young adults with primary dysmenorrhoea. J Adv Nurs. (2020) 76(10):2637–47. doi: 10.1111/jan.14460
173. Charlton A, While D. Smoking and menstrual problems in 16-year-olds. J R Soc Med. (1996) 89(4):193–5. doi: 10.1177/014107689608900405
174. Dorn LD, Negriff S, Huang B, Pabst S, Hillman J, Braverman P, et al. Menstrual symptoms in adolescent girls: association with smoking, depressive symptoms, and anxiety. J Adolesc Health. (2009) 44(3):237–43. doi: 10.1016/j.jadohealth.2008.07.018
175. Qin LL, Hu Z, Kaminga AC, Luo BA, Xu HL, Feng XL, et al. Association between cigarette smoking and the risk of dysmenorrhea: a meta-analysis of observational studies. PLoS One. (2020) 15(4):e0231201. doi: 10.1371/journal.pone.0231201
176. Blakey H, Chisholm C, Dear F, Harris B, Hartwell R, Daley AJ, et al. Is exercise associated with primary dysmenorrhoea in young women? BJOG. (2010) 117(2):222–4. doi: 10.1111/j.1471-0528.2009.02220.x
177. Izzo A, Labriola D. Dysmenorrhoea and sports activities in adolescents. Clin Exp Obstet Gynecol. (1991) 18(2):109–16. PMID: 1914207.1914207
178. Chaudhuri A, Singh A, Dhaliwal L. A randomised controlled trial of exercise and hot water bottle in the management of dysmenorrhoea in school girls of Chandigarh, India. Indian J Physiol Pharmacol. (2013) 57(2):114–22. PMID: 24617160.24617160
179. Armour M, Ee CC, Naidoo D, Ayati Z, Chalmers KJ, Steel KA, et al. Exercise for dysmenorrhoea. Cochrane Database Syst Rev. (2019) (9):CD004142. doi: 10.1002/14651858.CD004142.pub4
180. Exercise for period pain: new evidence to support exercising to reduce painful cramping, Zauniddin, Carter. Available online at: https://www.evidentlycochrane.net/exercise-for-period-pain/ (accessed June 18, 2024).
181. Golub LJ, Lang WR, Menduke H. Dysmenorrhea in high school and college girls; relationship to sports participation. West J Surg Obstet Gynecol. (1958) 66(3):163–5. PMID: 13558092.13558092
182. Golub LJ, Lang WR, Menduke H, Brown JO. Therapeutic exercises for teen-age dysmenorrhea; a statistical comparison of the billing and mosher techniques in the Philadelphia school system. Am J Obstet Gynecol. (1958) 76(3):670–4. doi: 10.1016/0002-9378(58)90089-9
183. Golub LJ, Menduke H, Lang WR. Exercise and dysmenorrhea in young teenagers: a 3-year study. Obstet Gynecol. (1968) 32(4):508–11. PMID: 5754610.5754610
184. Golub LJ, Menduke H, Lang WR. One component of an exercise for dysmenorrhea: twisting with bending. Obstet Gynecol. (1963) 22:324–6. PMID: 14059735.14059735
185. Golub LJ, Menduke H, Lang WR. Further evaluation of a therapeutic exercise for teen-age dysmenorrhea. Obstet Gynecol. (1960) 16:469–71. PMID: 13706745.13706745
186. Chien LW, Chang HC, Liu CF. Effect of yoga on serum homocysteine and nitric oxide levels in adolescent women with and without dysmenorrhea. J Altern Complement Med. (2013) 19(1):20–3. doi: 10.1089/acm.2011.0113
187. Chen CH, Lin YH, Heitkemper MM, Wu KM. The self-care strategies of girls with primary dysmenorrhea: a focus group study in Taiwan. Health Care Women Int. (2006) 27(5):418–27. doi: 10.1080/07399330600629583
188. Wong CL, Ip WY, Lam LW. Self-care strategies among Chinese adolescent girls with dysmenorrhea: a qualitative study. Pain Manag Nurs. (2016) 17(4):262–71. doi: 10.1016/j.pmn.2016.04.001
189. Dusek T. Influence of high intensity training on menstrual cycle disorders in athletes. Croat Med J. (2001) 42(1):79–82. PMID: 11172662.11172662
190. Balik G, Ustuner I, Kagitci M, Sahin FK. Is there a relationship between mood disorders and dysmenorrhea? J Pediatr Adolesc Gynecol. (2014) 27(6):371–4. doi: 10.1016/j.jpag.2014.01.108
191. Sahin N, Kasap B, Kirli U, Yeniceri N, Topal Y. Assessment of anxiety-depression levels and perceptions of quality of life in adolescents with dysmenorrhea. Reprod Health. (2018) 15(1):13. doi: 10.1186/s12978-018-0453-3
192. Nathan LA. Dysmenorrhea and pre-menstrual tension in adolescents. IMJ Ill Med J. (1967) 131(2):184–6. PMID: 4382881.4382881
193. Houston AM, Abraham A, Huang Z, D'Angelo LJ. Knowledge, attitudes, and consequences of menstrual health in urban adolescent females. J Pediatr Adolesc Gynecol. (2006) 19(4):271–5. doi: 10.1016/j.jpag.2006.05.002
194. White PA, Wildman BG. Factors related to medical help-seeking in women with menstrual discomfort. Behav Res Ther. (1986) 24(4):471–4. doi: 10.1016/0005-7967(86)90012-4
195. Payne LA, Rapkin AJ, Lung KC, Seidman LC, Zeltzer LK, Tsao JC. Pain catastrophizing predicts menstrual pain ratings in adolescent girls with chronic pain. Pain Med. (2016) 17(1):16–24. doi: 10.1111/pme.12869
196. Walsh TM, LeBlanc L, McGrath PJ. Menstrual pain intensity, coping, and disability: the role of pain catastrophizing. Pain Med. (2003) 4(4):352–61. doi: 10.1111/j.1526-4637.2003.03039.x
197. Kennett DJ, O'Hagan FT, Meyerhoff TJ. Managing menstruation: moderating role of symptom severity on active coping and acceptance. West J Nurs Res. (2016) 38(5):553–71. doi: 10.1177/0193945915620055
198. Kato T. Effects of flexibility in coping with menstrual pain on depressive symptoms. Pain Pract. (2017) 17(1):70–7. doi: 10.1111/papr.12412
199. Takeda T, Tadakawa M, Koga S, Nagase S, Yaegashi N. Relationship between dysmenorrhea and posttraumatic stress disorder in Japanese high school students 9 months after the Great East Japan Earthquake. J Pediatr Adolesc Gynecol. (2013) 26(6):355–7. doi: 10.1016/j.jpag.2013.06.020
200. Burke LM, Kalpakjian CZ, Smith YR, Quint EH. Gynecologic issues of adolescents with Down syndrome, autism, and cerebral palsy. J Pediatr Adolesc Gynecol. (2010) 23(1):11–5. doi: 10.1016/j.jpag.2009.04.005
201. Hamilton A, Marshal MP, Murray PJ. Autism spectrum disorders and menstruation. J Adolesc Health. (2011) 49(4):443–5. doi: 10.1016/j.jadohealth.2011.01.015
202. Power R, Wiley K, Muhit M, Heanoy E, Karim T, Badawi N, et al. ‘Flower of the body': menstrual experiences and needs of young adolescent women with cerebral palsy in Bangladesh, and their mothers providing menstrual support. BMC Women’s Health. (2020) 20(1):160. doi: 10.1186/s12905-020-01032-3
203. Pillai M, O'Brien K, Hill E. The levonorgestrel intrauterine system (Mirena) for the treatment of menstrual problems in adolescents with medical disorders, or physical or learning disabilities. BJOG. (2010) 117(2):216–21. doi: 10.1111/j.1471-0528.2009.02372.x
204. Ocak Z, Ocak T, Duran A, Ozlu T, Kocaman EM. Frequency of MEFV mutation and genotype-phenotype correlation in cases with dysmenorrhea. J Obstet Gynaecol Res. (2013) 39(8):1314–8. doi: 10.1111/jog.12061
205. Li R, Li B, Kreher DA, Benjamin AR, Gubbels A, Smith SM. Association between dysmenorrhea and chronic pain: a systematic review and meta-analysis of population-based studies. Am J Obstet Gynecol. (2020) 223(3):350–71. doi: 10.1016/j.ajog.2020.03.002
206. Smorgick N, As-Sanie S. Pelvic pain in adolescents. Semin Reprod Med. (2018) 36(2):116–22. doi: 10.1055/s-0038-1676088
207. Hirsch M, Dhillon-Smith R, Cutner AS, Yap M, Creighton SM. The prevalence of endometriosis in adolescents with pelvic pain: a systematic review. J Pediatr Adolesc Gynecol. (2020) 33(6):623–30. doi: 10.1016/j.jpag.2020.07.011
208. Yeung P Jr, Gupta S, Gieg S. Endometriosis in adolescents: a systematic review. J Endo Pelvic Pain Disord. (2017) 9(1):17–29. doi: 10.5301/je.5000264
209. Bahrami A, Gonoodi K, Khayyatzadeh SS, Tayefi M, Darroudi S, Bahrami-Taghanaki H, et al. The association of trace elements with premenstrual syndrome, dysmenorrhea and irritable bowel syndrome in adolescents. Eur J Obstet Gynecol Reprod Biol. (2019) 233:114–9. doi: 10.1016/j.ejogrb.2018.12.017
210. Bianchin L, Bozzola M, Battistella Pier A, Bernasconi S, Bona G, Buzi F, et al. Menstrual cycle and headache in teenagers. Indian J Pediatr. (2019) 86:25–33. doi: 10.1007/s12098-018-2829-3
211. Carman KB, Arslantas D, Unsal A, Atay E, Ocal EE, Demirtas Z, et al. Menstruation-related headache in adolescents: point prevalence and associated factors. Pediatr Int. (2018) 60(6):576–80. doi: 10.1111/ped.13572
212. Woods JL, Hensel DJ, Fortenberry JD. Gynecological symptoms and sexual behaviors among adolescent women. J Pediatr Adolesc Gynecol. (2010) 23(2):93–5. doi: 10.1016/j.jpag.2009.06.004
213. Nur Azurah AG, Sanci L, Moore E, Grover S. The quality of life of adolescents with menstrual problems. J Pediatr Adolesc Gynecol. (2013) 26(2):102–8. doi: 10.1016/j.jpag.2012.11.004
214. Shah DK, Missmer SA. Scientific investigation of endometriosis among adolescents. J Pediatr Adolesc Gynecol. (2011) 24(5):S18–S9. doi: 10.1016/j.jpag.2011.07.008
215. Sarıdoğan E. Adolescent endometriosis. Eur J Obstet Gynecol Reprod Biol. (2017) 209:46–9. doi: 10.1016/j.ejogrb.2016.05.019
216. Wijesiri HS, Suresh TS. Knowledge and attitudes towards dysmenorrhea among adolescent girls in an urban school in Sri Lanka. Nurs Health Sci. (2013) 15(1):58–64. doi: 10.1111/j.1442-2018.2012.00736.x
217. Chiou MH, Wang HH, Yang YH. Effect of systematic menstrual health education on dysmenorrheic female adolescents’ knowledge, attitudes, and self-care behavior. Kaohsiung J Med Sci. (2007) 23(4):183–90. doi: 10.1016/S1607-551X(09)70395-X
218. Yilmaz B, Sahin N. The effects of a dysmenorrhea support program on university students who had primary dysmenorrhea: a randomized controlled study. J Pediatr Adolesc Gynecol. (2020) 33(3):285–90. doi: 10.1016/j.jpag.2019.12.008
219. Sinaga TR, Damanik E, Sitorus MEJ. Relationship between anxiety and knowledge levels about primary dysmenorrhea with prevention of illness in adolescents Bosar Maligas district, Simalungun district. Enferm Clin. (2020) 30:147–50. doi: 10.1016/j.enfcli.2019.11.042
220. Bush D, Brick E, East MC, Johnson N. Endometriosis education in schools: a New Zealand model examining the impact of an education program in schools on early recognition of symptoms suggesting endometriosis. Aus N Z J Obstet Gynaecol. (2017) 57(4):452–7. doi: 10.1111/ajo.12614
221. Bellis EK, Li AD, Jayasinghe YL, Girling JE, Grover SR, Peate M, et al. Exploring the unmet needs of parents of adolescent girls with heavy menstrual bleeding and dysmenorrhea: a qualitative study. J Pediatr Adolesc Gynecol. (2020) 33(3):271–7. doi: 10.1016/j.jpag.2019.12.007
222. Chen CX, Groves D, Miller WR, Carpenter JS. Big data and dysmenorrhea: what questions do women and men ask about menstrual pain? J Women’s Health. (2018) 27(10):1233–41. doi: 10.1089/jwh.2017.6732
223. Henderson EM, Rosser BA, Keogh E, Eccleston C. Internet sites offering adolescents help with headache, abdominal pain, and dysmenorrhoea: a description of content, quality, and peer interactions. J Pediatr Psychol. (2012) 37(3):262–71. doi: 10.1093/jpepsy/jsr100
224. Armour M, Parry K, Al-Dabbas MA, Curry C, Holmes K, MacMillan F, et al. Self-care strategies and sources of knowledge on menstruation in 12,526 young women with dysmenorrhea: a systematic review and meta-analysis. PLoS One. (2019) 14(7):e0220103. doi: 10.1371/journal.pone.0220103
225. De Sanctis V, Soliman AT, Daar S, Di Maio S, Elalaily R, Fiscina B, et al. Prevalence, attitude and practice of self-medication among adolescents and the paradigm of dysmenorrhea self-care management in different countries. Acta Biomed. (2020) 91(1):182–92. doi: 10.23750/abm.v91i1.9242
226. Gupta J, Cardoso LF, Harris CS, Dance AD, Seckin T, Baker N, et al. How do adolescent girls and boys perceive symptoms suggestive of endometriosis among their peers? Findings from focus group discussions in New York City. BMJ open. (2018) 8(6):e020657. doi: 10.1136/bmjopen-2017-020657
227. Brosens I, Gordts S, Habiba M, Benagiano G. Uterine cystic adenomyosis: a disease of younger women. J Pediatr Adolesc Gynecol. (2015) 28(6):420–6. doi: 10.1016/j.jpag.2014.05.008
228. John SK, Prabhu PS, Virmani S, Kumar V, Thotan SP. Misdiagnosed roberts uterus leading to surgical misadventures. J Pediatr Adolesc Gynecol. (2017) 30(4):508–10. doi: 10.1016/j.jpag.2017.01.005
229. Borah T, Das A, Panda S, Singh S. A case of unilateral dysmenorrhea. J Hum Reprod Sci. (2010) 3(3):158–9. doi: 10.4103/0974-1208.74162
230. Park JC, Kim DJ. Successful laparoscopic surgery of accessory cavitated uterine mass in young women with severe dysmenorrhea. Yeungnam Univ J Med. (2021) 38(3):235–9. doi: 10.12701/yujm.2020.00696
231. McRae MA, Kim MH. Dysmenorrhea in unicornis with rudimentary uterine cavity. Obstet Gynecol. (1979) 53(1):134–7. PMID: 760014.760014
232. Liatsikos SA, Tsikouras P, Souftas V, Ammari A, Prassopoulos P, Maroulis G, et al. Diagnosis and laparoscopic management of a rudimentary uterine horn in a teenage girl, presenting with haematometra and severe endometriosis: our experience and review of literature. Minim Invasive Ther Allied Technol. (2010) 19(4):241–7. doi: 10.3109/13645701003644491
233. Acien P, Bataller A, Fernandez F, Acien MI, Rodriguez JM, Mayol MJ. New cases of accessory and cavitated uterine masses (ACUM): a significant cause of severe dysmenorrhea and recurrent pelvic pain in young women. Hum Reprod. (2012) 27(3):683–94. doi: 10.1093/humrep/der471
234. Bedaiwy MA, Henry DN, Elguero S, Pickett S, Greenfield M. Accessory and cavitated uterine mass with functional endometrium in an adolescent: diagnosis and laparoscopic excision technique. J Pediatr Adolesc Gynecol. (2013) 26(4):e89–91. doi: 10.1016/j.jpag.2012.11.003
235. Coppola L. Unique case of imperforate hymen. J Pediatr Adolesc Gynecol. (2016) 29(1):e1–3. doi: 10.1016/j.jpag.2015.07.004
236. Dadhwal V, Mittal S, Kumar S, Barua A. Hematometra in postmenarchal adolescent girls: a report of two cases. Gynecol Obstet Invest. (2000) 50(1):67–9. doi: 10.1159/000010284
237. Nadkarni D, Spitzer RF, Kives S, Colgan T, Haider MA, Allen L. Uterine asymmetry and dysmenorrhea in a young woman. J Obstet Gynaecol Canada. (2008) 30(4):301. doi: 10.1016/S1701-2163(16)32792-X
238. Nishu DS, Uddin MM, Akter K, Akter S, Sarmin M, Begum S. Herlyn-Werner-Wunderlich syndrome presenting with dysmenorrhea: a case report. J Med Case Rep. (2019) 13(1):323. doi: 10.1186/s13256-019-2258-6
239. Obeidat RA, Aleshawi AJ, Tashtush NA, Alsarawi H. Unicornuate uterus with a rudimentary non-communicating cavitary horn in association with VACTERL association: case report. BMC Women’s Health. (2019) 19(1):71. doi: 10.1186/s12905-019-0768-4
240. Perino A, Chianchiano N, Simonaro C, Cittadini E. Endoscopic management of a case of ‘complete septate uterus with unilateral haematometra’. Hum Reprod. (1995) 10(8):2171–3. doi: 10.1093/oxfordjournals.humrep.a136256
241. Takeda A, Sakai K, Mitsui T, Nakamura H. Laparoscopic management of juvenile cystic adenomyoma of the uterus: report of two cases and review of the literature. J Minim Invasive Gynecol. (2007) 14(3):370–4. doi: 10.1016/j.jmig.2007.01.005
242. Wilcox A, Schmidt M, Luciano D. Identification of juvenile cystic adenomyoma using high-resolution imaging. Obstet Gynecol. (2020) 136(5):1021–4. doi: 10.1097/AOG.0000000000004121
243. Frontino G, Bianchi S, Ciappina N, Restelli E, Borruto F, Fedele L. The unicornuate uterus with an occult adenomyotic rudimentary horn. J Minim Invasive Gynecol. (2009) 16(5):622–5. doi: 10.1016/j.jmig.2009.04.015
244. Nabeshima H, Nishimoto M, Shiga N, Utsunomiya H, Yaegashi N. Laparoscopic Strassman metroplasty in a postmenarcheal adolescent girl with Herlyn-Werner-Wunderlich mullerian anomaly variant, obstructed noncommunicating didelphic uterus without gartner duct pseudocyst. J Minim Invasive Gynecol. (2013) 20(2):255–8. doi: 10.1016/j.jmig.2012.10.016
245. Balasch J, Martinez-Roman S, Vanrell JA. Hematometra in an unattached rudimentary uterine horn and ipsilateral renal agenesis. Eur J Obstet Gynecol Reprod Biol. (1994) 54(2):150–2. doi: 10.1016/0028-2243(94)90257-7
246. Branquinho MM, Marques AL, Leite HB, Silva IS. Juvenile cystic adenomyoma. BMJ Case Rep. (2012) 19:19. doi: 10.1136/bcr-2012-007006
247. Eisenberg E, Farber M, Mitchell GW Jr., Turksoy RN, Rule AH. Complete duplication of the uterus and cervix with a unilaterally imperforate vagina. Obstet Gynecol. (1982) 60(2):259–62. PMID: 7155489.7155489
248. Liang YJ, Hao Q, Wu YZ, Wu B. Uterus-like mass in the left broad ligament misdiagnosed as a malformation of the uterus: a case report of a rare condition and review of the literature. Fertil Steril. (2010) 93(4):1347.e13–6. doi: 10.1016/j.fertnstert.2009.10.040
249. Ryan GL, Stolpen A, Van Voorhis BJ. An unusual cause of adolescent dysmenorrhea. Obstet Gynecol. (2006) 108(4):1017–22. doi: 10.1097/01.AOG.0000237163.98010.b3
250. Shah N, Joshi A, Bansode S, Bansode V. Accessory cavitated uterine mass: an emerging differential diagnosis of dysmenorrhea in adolescents. J Minim Invasive Gynecol. (2021) 28(6):1131–2. doi: 10.1016/j.jmig.2020.12.021
251. Tong J, Zhu L, Lang J. Clinical characteristics of 70 patients with Herlyn-Werner-Wunderlich syndrome. Int J Gynaecol Obstet. (2013) 121(2):173–5. doi: 10.1016/j.ijgo.2012.11.023
252. Tzialidou-Palermo I, von Kaisenberg CS, Garcia-Rocha GJ, Schloesser HW, Baehr I, Schippert C. Diagnostic challenges of hemihematocolpos and dysmenorrhea in adolescents: obstructed hemivagina, didelphys or bicornuate uterus and renal aplasia is a rare female genital malformation. Arch Gynecol Obstet. (2012) 286(3):785–91. doi: 10.1007/s00404-012-2392-5
253. Kapczuk K, Friebe Z, Iwaniec K, Kedzia W. Obstructive mullerian anomalies in menstruating adolescent girls: a report of 22 cases. J Pediatr Adolesc Gynecol. (2018) 31(3):252–7. doi: 10.1016/j.jpag.2017.09.013
254. Deutsch M, Beck D, Paldi E, Brandes JM. Uterus duplex with a unilaterally imperforate vagina–diagnosis and treatment. Report of two cases and review of the literature. Ann Chir Gynaecol. (1985) 74(5):247–9. PMID: 3909911.3909911
255. Morgan MA, Thurnau GR, Smith ML. Uterus didelphys with unilateral hematocolpos, ipsilateral renal agenesis and menses. A case report and literature review. J Reprod Med. (1987) 32(1):47–58. PMID: 3560063.3560063
256. Wozniakowska E, Stepniak A, Czuczwar P, Milart P, Paszkowski T. Secondary dysmenorrhea due to a rudimentary, non-communicating functional uterine horn. Ginekol Pol. (2017) 88(7):404–5. doi: 10.5603/GP.a2017.0075
257. Agarwal M, Das A, Singh AS. Dysmenorrhea due to a rare mullerian anomaly. Niger J Clin Pract. (2011) 14(3):377–9. doi: 10.4103/1119-3077.86788
258. Potter DA, Schenken RS. Noncommunicating accessory uterine cavity. Fertil Steril. (1998) 70(6):1165–6. doi: 10.1016/S0015-0282(98)00380-X
259. Tamura M, Fukaya T, Takaya R, Ip CW, Yajima A. Juvenile adenomyotic cyst of the corpus uteri with dysmenorrhea. Tohoku J Exp Med. (1996) 178(3):339–44. doi: 10.1620/tjem.178.339
260. Song XC, Yu X, Luo M, Yu Q, Zhu L. Clinical characteristics and postoperative symptoms of 85 adolescents with endometriosis. J Pediatr Adolesc Gynecol. (2020) 33(5):519–23. doi: 10.1016/j.jpag.2020.06.021
261. Shim JY, Laufer MR. Adolescent endometriosis: an update. J Pediatr Adolesc Gynecol. (2020) 33(2):112–9. doi: 10.1016/j.jpag.2019.11.011
262. Benagiano G, Guo SW, Puttemans P, Gordts S, Brosens I. Progress in the diagnosis and management of adolescent endometriosis: an opinion. Reprod Biomed Online. (2018) 36(1):102–14. doi: 10.1016/j.rbmo.2017.09.015
263. Bullock JL, Massey FM, Gambrell RD. Symptomatic endometriosis in teen-agers. A reappraisal. Obstet Gynecol. (1974) 43(6):896–900. PMID: 4275237.4275237
264. Davis GD, Thillet E, Lindemann J. Clinical characteristics of adolescent endometriosis. J Adolesc Health. (1993) 14(5):362–8. doi: 10.1016/S1054-139X(08)80008-0
265. DiVasta AD, Vitonis AF, Laufer MR, Missmer SA. Spectrum of symptoms in women diagnosed with endometriosis during adolescence vs adulthood. Am J Obstet Gynecol. (2018) 218(3):324.e1–e11. doi: 10.1016/j.ajog.2017.12.007
266. Sachedina A, Todd N. Dysmenorrhea, endometriosis and chronic pelvic pain in adolescents. J Clin Res Pediatr Endocrinol. (2020) 12:7–17. doi: 10.4274/jcrpe.galenos.2019.2019.S0217
267. Dun EC, Kho KA, Morozov VV, Kearney S, Zurawin JL, Nezhat CH. Endometriosis in adolescents. JSLS. (2015) 19(2):e2015.00019. doi: 10.4293/JSLS.2015.00019
268. Janssen E, Rijkers A, Hoppenbrouwers K, Meuleman C, d'Hooghe T. Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: a systematic review. Hum Reprod Update. (2013) 19(5):570–82. doi: 10.1093/humupd/dmt016
269. Shim JY, Laufer MR, Grimstad FW. Dysmenorrhea and endometriosis in transgender adolescents. J Pediatr Adolesc Gynecol. (2020) 33(5):524–8. doi: 10.1016/j.jpag.2020.06.001
270. NICE Clinical guideline. Endometriosis: diagnosis and management NG73. (2017). Available online at: https://www.nice.org.uk/guidance/ng73/evidence/full-guideline-pdf-4550371315 (Accessed June 19, 2024).
271. Chapron C, Borghese B, Streuli I, de Ziegler D. Markers of adult endometriosis detectable in adolescence. J Pediatr Adolesc Gynecol. (2011) 24(5):S7–12. doi: 10.1016/j.jpag.2011.07.006
272. Ragab A, Shams M, Badawy A, Alsammani MA. Prevalence of endometriosis among adolescent school girls with severe dysmenorrhea: a cross sectional prospective study. Int J Health Sci. (2015) 9(3):273–81. doi: 10.12816/0024694
273. Martire FG, Lazzeri L, Conway F, Siciliano T, Pietropolli A, Piccione E, et al. Adolescence and endometriosis: symptoms, ultrasound signs and early diagnosis. Fertil Steril. (2020) 114(5):1049–57. doi: 10.1016/j.fertnstert.2020.06.012
274. Tandoi I, Somigliana E, Riparini J, Ronzoni S, Vigano P, Candiani M. High rate of endometriosis recurrence in young women. J Pediatr Adolesc Gynecol. (2011) 24(6):376–9. doi: 10.1016/j.jpag.2011.06.012
275. Endometriosis Guideline of European Society of Human Reproduction and Embryology. ESHRE Endometriosis Guideline Development Group. (2022). Available online at: https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Endometriosis-guideline.aspx (accessed June 19, 2024).
276. Itam SP 2nd, Ayensu-Coker L, Sanchez J, Zurawin RK, Dietrich JE. Adenomyosis in the adolescent population: a case report and review of the literature. J Pediatr Adolesc Gynecol. (2009) 22(5):e146–7. doi: 10.1016/j.jpag.2009.01.067
277. Ho ML, Ratts V, Merritt D. Adenomyotic cyst in an adolescent girl. J Pediatr Adolesc Gynecol. (2009) 22(3):e33–8. doi: 10.1016/j.jpag.2008.05.011
278. Kulkarni A, Deb S. Dysmenorrhoea. Obstet Gynaecol Reprod Med. (2019) 29(10):286–91. doi: 10.1016/j.ogrm.2019.06.002
279. Brunham RC, Gottlieb SL, Paavonen J. Pelvic inflammatory disease. N Engl J Med. (2015) 372(21):2039–48. doi: 10.1056/NEJMra1411426
280. Wong CL, Ip WY, Choi KC, Lam LW. Examining self-care behaviors and their associated factors among adolescent girls with dysmenorrhea: an application of Orem’s self-care deficit nursing theory. J Nurs Scholarsh. (2015) 47(3):219–27. doi: 10.1111/jnu.12134
281. Ogunfowokan AA, Babatunde OA. Management of primary dysmenorrhea by school adolescents in ILE-IFE, Nigeria. J School Nurs. (2010) 26(2):131–6. doi: 10.1177/1059840509349723
282. O'Connell K, Davis AR, Westhoff C. Self-treatment patterns among adolescent girls with dysmenorrhea. J Pediatr Adolesc Gynecol. (2006) 19(4):285–9. doi: 10.1016/j.jpag.2006.05.004
283. Kaplan B, Rabinerson D, Lurie S, Peled Y, Royburt M, Neri A. Clinical evaluation of a new model of a transcutaneous electrical nerve stimulation device for the management of primary dysmenorrhea. Gynecol Obstet Invest. (1997) 44(4):255–9. doi: 10.1159/000291539
284. Kannan P, Claydon LS. Some physiotherapy treatments may relieve menstrual pain in women with primary dysmenorrhea: a systematic review. J Physiother. (2014) 60(1):13–21. doi: 10.1016/j.jphys.2013.12.003
285. Potur DC, Komurcu N. The effects of local low-dose heat application on dysmenorrhea. J Pediatr Adolesc Gynecol. (2014) 27(4):216–21. doi: 10.1016/j.jpag.2013.11.003
286. Cho SH, Hwang EW. Acupuncture for primary dysmenorrhoea: a systematic review. BJOG. (2010) 117(5):509–21. doi: 10.1111/j.1471-0528.2010.02489.x
287. Woo HL, Ji HR, Pak YK, Lee H, Heo SJ, Lee JM, et al. The efficacy and safety of acupuncture in women with primary dysmenorrhea: a systematic review and meta-analysis. Medicine. (2018) 97(23):e11007. doi: 10.1097/MD.0000000000011007
288. Ben-Menachem M. Treatment of dysmenorrhea: a relaxation therapy program. Int J Gynaecol Obstet. (1980) 17(4):340–2. doi: 10.1002/j.1879-3479.1980.tb00296.x
289. Proctor M, Murphy PA, Pattison HM, Suckling JA, Farquhar C. Behavioural interventions for dysmenorrhoea. Cochrane Database Syst Rev. (2007) 3:CD002248. doi: 10.1002/14651858.CD002248.pub3
290. Marjoribanks J, Ayeleke RO, Farquhar C, Proctor M, Gynaecology C, Group F. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. (2015) (7):CD001751. doi: 10.1002/14651858.CD001751.pub3
291. Harel Z, Riggs S, Vaz R, Flanagan P, Harel D. The use of the leukotriene receptor antagonist montelukast (singulair) in the management of dysmenorrhea in adolescents. J Pediatr Adolesc Gynecol. (2004) 17(3):183–6. doi: 10.1016/j.jpag.2004.03.037
292. Schroll JB, Black AY, Farquhar C, Chen I. Combined oral contraceptive pill for primary dysmenorrhoea. Cochrane Database Syst Rev. (2023) (7):CD002120. doi: 10.1002/14651858.CD002120.pub4
293. Anthuber S, Schramm GA, Heskamp ML. Six-month evaluation of the benefits of the low-dose combined oral contraceptive chlormadinone acetate 2 mg/ethinylestradiol 0.03 mg in young women: results of the prospective, observational, non-interventional, multicentre TeeNIS study. Clin Drug Investig. (2010) 30(4):211–20. doi: 10.2165/11532910-000000000-00000
294. Davis AR, Westhoff C, O'Connell K, Gallagher N. Oral contraceptives for dysmenorrhea in adolescent girls: a randomized trial. Obstet Gynecol. (2005) 106(1):97–104. doi: 10.1097/01.AOG.0000165826.03915.65
295. Merki-Feld GS, Hund M. Clinical experience with NuvaRing® in daily practice in Switzerland: cycle control and acceptability among women of all reproductive ages. Eur J Contracept Reprod Health Care. (2007) 12(3):240–7. doi: 10.1080/13625180701440180
296. van Hooff MH, Hirasing RA, Kaptein MB, Koppenaal C, Voorhorst FJ, Schoemaker J. The use of oral contraceptives by adolescents for contraception, menstrual cycle problems or acne. Acta Obstet Gynecol Scand. (1998) 77(9):898–904. doi: 10.1034/j.1600-0412.1998.770905.x
297. Davis AR, Westhoff CL. Primary dysmenorrhea in adolescent girls and treatment with oral contraceptives. J Pediatr Adolesc Gynecol. (2001) 14(1):3–8. doi: 10.1016/S1083-3188(00)00076-0
298. Robinson JC, Plichta S, Weisman CS, Nathanson CA, Ensminger M. Dysmenorrhea and use of oral contraceptives in adolescent women attending a family planning clinic. Am J Obstet Gynecol. (1992) 166(2):578–83. doi: 10.1016/0002-9378(92)91676-2
299. Lara-Torre E, Schroeder B. Adolescent compliance and side effects with quick start initiation of oral contraceptive pills. Contraception. (2002) 66(2):81–5. doi: 10.1016/S0010-7824(02)00326-8
300. Apter D, Colli E, Gemzell-Danielsson K, Peters K. Multicenter, open-label trial to assess the safety and tolerability of drospirenone 4.0 mg over 6 cycles in female adolescents, with a 7-cycle extension phase. Contraception. (2020) 101(6):412–9. doi: 10.1016/j.contraception.2020.02.004
301. Aslam N, Blunt S, Latthe P. Effectiveness and tolerability of levonorgestrel intrauterine system in adolescents. J Obstet Gynaecol. (2010) 30(5):489–91. doi: 10.3109/01443615.2010.484107
302. Zekavat OR, Karimi MY, Amanat A, Alipour F. A randomised controlled trial of oral zinc sulphate for primary dysmenorrhoea in adolescent females. Aus N Z J Obstet Gynaecol. (2015) 55(4):369–73. doi: 10.1111/ajo.12367
303. Harel Z, Biro FM, Kottenhahn RK, Rosenthal SL. Supplementation with omega-3 polyunsaturated fatty acids in the management of dysmenorrhea in adolescents. Am J Obstet Gynecol. (1996) 174(4):1335–8. doi: 10.1016/S0002-9378(96)70681-6
304. Ziaei S, Zakeri M, Kazemnejad A. A randomised controlled trial of vitamin E in the treatment of primary dysmenorrhoea. BJOG. (2005) 112(4):466–9. doi: 10.1111/j.1471-0528.2004.00495.x
305. Bahrami A, Avan A, Sadeghnia HR, Esmaeili H, Tayefi M, Ghasemi F, et al. High dose vitamin D supplementation can improve menstrual problems, dysmenorrhea, and premenstrual syndrome in adolescents. Gynecol Endocrinol. (2018) 34(8):659–63. doi: 10.1080/09513590.2017.1423466
306. Williams CE, Creighton SM. Menstrual disorders in adolescents: review of current practice. Horm Res Paediatr. (2012) 78(3):135–43. doi: 10.1159/000342822
307. Gagnon MM, Moussaoui D, Gordon JL, Alberts NM, Grover SR. Dysmenorrhea across the lifespan: a biopsychosocial perspective to understanding the dysmenorrhea trajectory and association with comorbid pain experiences. Pain. (2022) 163(11):2069–75. doi: 10.1097/j.pain.0000000000002649
308. Moussaoui D, Grover SR. The association between childhood adversity and risk of dysmenorrhea, pelvic pain, and dyspareunia in adolescents and young adults: a systematic review. J Pediatr Adolesc Gynecol. (2022) 35(5):567–74. doi: 10.1016/j.jpag.2022.04.010
309. DiVasta AD, Zimmerman LA, Vitonis AF, Fadayomi AB, Missmer SA. Overlap between irritable bowel syndrome diagnosis and endometriosis in adolescents. Clin Gastroenterol Hepatol. (2021) 19(3):528–37.e1. doi: 10.1016/j.cgh.2020.03.014
310. Oladosu FA, Hellman KM, Ham PJ, Kochlefl LE, Datta A, Garrison EF, et al. Persistent autonomic dysfunction and bladder sensitivity in primary dysmenorrhea. Sci Rep. (2019) 9(1):2194. doi: 10.1038/s41598-019-38545-3
311. Eccleston C, Fisher E, Howard RF, Slater R, Forgeron P, Palermo TM, et al. Delivering transformative action in paediatric pain: a lancet child & adolescent health commission. Lancet Child Adolesc Health. (2021) 5(1):47–87. doi: 10.1016/S2352-4642(20)30277-7
312. Zhu X, Proctor M, Bensoussan A, Wu E, Smith CA. Chinese Herbal medicine for primary dysmenorrhoea. Cochrane Database Syst Rev. (2007) (2):CD005288. doi: 10.1002/14651858.CD005288.pub3
313. Ahrendt H-J, Karck U, Pichl T, Mueller T, Ernst U. The effects of an oestrogen-free, desogestrel-containing oral contraceptive in women with cyclical symptoms: results from two studies on oestrogen-related symptoms and dysmenorrhoea. Eur J Contracept Reprod Health Care. (2007) 12(4):354–61. doi: 10.1080/13625180701536771
314. Vannuccini S, Biagiotti C, Esposto MC, La Torre F, Clemenza S, Orlandi G, et al. Long-term treatment of endometriosis-related pain among women seeking hormonal contraception. Gynecol Endocrinol. (2022) 38(5):398–402. doi: 10.1080/09513590.2022.2047172
315. Yildizbas B, Sahin HG, Kolusari A, Zeteroglu S, Kamacı M. Side effects and acceptability of implanon®: a pilot study conducted in eastern Turkey. Eur J Contracept Reprod Health Care. (2007) 12(3):248–52. doi: 10.1080/13625180701442228
316. Hardy-Johnson PL. Data to Support Thesis: dysmenorrhea and its impact on the health-related quality of life of adolescent girls (2022).
317. Chéileachair FN, McGuire BE, Durand H. Coping with dysmenorrhea: a qualitative analysis of period pain management among students who menstruate. BMC Women’s Health. (2022) 22(1):407. doi: 10.1186/s12905-022-01988-4
318. Yiu K, Chan SS, Chung TK. Mothers’ attitude to the use of a combined oral contraceptive pill by their daughters for menstrual disorders or contraception. Hong Kong Med J. (2017) 23(2):150–7. doi: 10.12809/hkmj164993
319. Girling JE, Hawthorne SC, Marino JL, Azurah AGN, Grover SR, Jayasinghe YL. Paternal understanding of menstrual concerns in young women. J Pediatr Adolesc Gynecol. (2018) 31(5):459–67. doi: 10.1016/j.jpag.2018.04.001
320. Shafrir AL, Farland L, Shah D, Harris H, Kvaskoff M, Zondervan K, et al. Risk for and consequences of endometriosis: a critical epidemiologic review. Best Pract Res Clin Obstet Gynaecol. (2018) 51:1–15. doi: 10.1016/j.bpobgyn.2018.06.001
321. Dysmenorrhoea, NICE Clinical knowledge Summaries. Dysmenorrhoea | Health topics A to Z | CKS | NICE. Available online at: https://cks.nice.org.uk/topics/dysmenorrhoea/diagnosis/assessment/ (Accessed June 18, 2024).
Keywords: adolescent, dysmenorrhoea, period pain, primary care, general practice, narrative synthesis, uncertainties
Citation: Dixon S, Hirst J, Taghinejadi N, Duddy C, Vincent K and Ziebland S (2024) What is known about adolescent dysmenorrhoea in (and for) community health settings?. Front. Reprod. Health 6: 1394978. doi: 10.3389/frph.2024.1394978
Received: 2 March 2024; Accepted: 31 May 2024;
Published: 23 July 2024.
Edited by:
Kevin M. Hellman, NorthShore University HealthSystem, United StatesReviewed by:
Akmal El-Mazny, Cairo University, Egypt© 2024 Dixon, Hirst, Taghinejadi, Duddy, Vincent and Ziebland. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sharon Dixon, c2hhcm9uLmRpeG9uQHBoYy5veC5hYy51aw==
†These authors share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.