- Wits RHI, University of the Witwatersrand, Johannesburg, South Africa
Introduction: South African women bear an intersecting burden of HIV, sexually transmitted infections (STIs) and unintended pregnancy. Multipurpose prevention technologies (MPTs) are a class of products that address multiple needs and have the potential to improve uptake and use of prevention products.
Methods: Analysing survey data from 703 HIV-negative women 18–40 years in three provinces in South Africa, collected between July and November 2022, this study explores their preferences for prevention methods and factors influencing choice of hypothetical prevention methods, including MPTs. Descriptive statistics and multinomial regression analyses were conducted to determine prevention method preferences and factors associated with choosing a pill, injectable or MPT-implant type prevention method.
Results: Most women wanted to prevent HIV, STIs and pregnancy. The most important factors when choosing a prevention product were whether it provided dual and long-term protection and if side effects were manageable. If choosing only one method, half of women would choose any MPT-implant and a quarter each would choose a pill or an injectable method, with method choices differing by population group.
Discussion: Prevention method choices were influenced by sexual-behavioural factors and current and prior contraceptive method use. Providing a choice of prevention methods and a population specific approach to new method development and introduction with access to accurate information could enhance their ability to fill a gap in prevention needs.
Introduction
Women in South Africa experience high rates of HIV, sexually transmitted infections (STIs) and unintended pregnancy (1–3). Addressing these coinciding epidemics through the integration of sexual and reproductive health (SRH) services has been a national focus (4, 5). Contraceptive methods available free of charge in public clinics in South Africa include condoms, oral and injectable contraceptives, intra-uterine devices and subdermal implants (6). Oral pre-exposure prophylaxis (PrEP) for the prevention of HIV was introduced to young women in South Africa in 2017 (7), with the dapivirine vaginal ring and long-acting injectable cabotegravir recently approved for use in country, but not yet available outside of implementation studies. Despite their availability, health system, interpersonal and product related barriers to uptake and effective use of contraceptives and oral PrEP remain, particularly among young people (8–13).
Multipurpose prevention technologies (MPTs) are a class of products that simultaneously prevent HIV, other STIs and/or unintended pregnancy (14). MPTs have the potential to improve uptake and use of prevention products by simplifying delivery and use, reducing stigma, providing discretion, and reducing user and health system burden (15–18). They may also offer additional protection among users who may otherwise have chosen a single-purpose product, in addition to being more cost-effective than single-purpose products (15–18). Currently, the condom is the only available MPT, although there are several MPTs in pre-clinical studies and clinical trials (14).
The development of new methods is complex and costly, and it is critical that in addition to expanding choice, investments are made in products most likely to be preferred and effectively used (19). Evidence from existing product introduction highlights that availability and efficacy do not always translate into uptake and effective use (19), and that they can be undermined by negative user experiences and misperceptions (20). In addition, health system considerations such as provider training requirements and community buy-in may impede new product introduction and use (21). Understanding end-user preferences is critical to inform considerations around which products should move forward in development; to ensure users are willing and able to use new products, and that they are appropriate for the contexts in which they will be implemented (17, 19, 21). This is even more important when effective single-purpose products are already available.
This study describes the prevention product choices and the factors associated with preferences for HIV prevention method types among women at risk of HIV in South Africa, with a particular focus on a hypothetical dual HIV prevention and contraceptive implant.
Methods
This work was part of a study funded by the Bill and Melinda Gates Foundation. The main study was cross-sectional, mixed-methods, formative research among women, men and health care providers which aimed to investigate the potential uptake of a hypothetical MPT-implant among PrEP eligible clients in South Africa. The study was conducted between July and November 2022. This paper focuses on the findings from surveys conducted among female participants. Additional findings from the other components of this formative research have been published elsewhere (22–24).
Study setting
The study was conducted in eight department of health primary care clinics and one key population clinic and their linked communities in three provinces in South Africa: Two clinics and a linked mobile clinic in peri-urban residential townships in Tshwane, Gauteng; two clinics and a linked mobile clinic in Mthatha in the rural Eastern Cape; and four clinics within two distinct rural communities in KwaZulu Natal. Recruitment of female sex workers (FSWs) was undertaken at a Wits RHI supported key populations clinic and linked mobile clinic in Tshwane. All study sites are in communities with a high burden of HIV, with antenatal prevalence rates ranging from 23% in Tshwane to 35% in Mthatha (3). All sites have been supported by Wits RHI to introduce oral PrEP through implementation science projects.
Study population and recruitment
Participants were HIV negative females aged 18–40 years. Female clients accessing services at recruiting sites were eligible if they were 18–40 years old, eligible for PrEP (self-reported HIV-negative) and willing and able to consent to study participation. Recruitment was conducted by trained fieldworkers and supported by peer educators at the key population site. For all target populations, two approaches to recruitment were used: consecutive sampling of clients accessing SRH services at study sites, and snowball sampling through already recruited participants, to allow for participation of women accessing health services as well as those from the community. Participants recruited at study sites were invited to present to a designated community venue on a set time and day to participate in study activities. Recruitment flyers were given to willing participants to take home to enable the recruitment of eligible family members or friends through snowballing. Flyers contained a study contact number, allowing participants to reach the study team for further information. If interested in participating, they were invited to present to the designated community venue on a set day and time, at which point they were screened and enrolled.
Study procedures
All participants were provided with a standard, interactive group information session of approximately 45 min, facilitated by a trained study team member. Information on new, existing and hypothetical HIV prevention and MPT methods, provided through a slide presentation, covered details of each potential method i.e., daily, monthly, and event-driven pill, two-monthly and six-monthly injectable, 1-year biodegradable, non-biodegradable and refillable MPT implant, mono-PrEP implant and 2-year biodegradable and non-biodegradable MPT implant. For each, information on product administration, conditions prevented, potential side effects, duration of prevention, anticipated availability and effectiveness (where known) was provided. This was followed by a question and answer session with each group to answer any questions related to the information provided.
A sub-set of 299 (42.5%) participants (99 (33.1%) AGYW, 160 (53.5%) women >24 years and 40 (13.4%) FSWs) participated in a workshop immediately following the information session. The aim of the workshops was to explore demand creation messaging and channels as well as preferences for different attributes of a hypothetical MPT implant. There were four participants in the main study who completed a workshop, but for whom survey data was not included in this analysis as they did not complete the survey (n = 1) and were >40 years (n = 3). All FSWs participated in a workshop and recruitment for workshops among AGYW and women >24 years was conducted until data saturation was reached, after which participants completed only the information session. Workshops used participatory action research (PAR) methods and aimed to explore user preferences for prevention methods, perceptions of MPTs and to co-develop demand creation strategies for future MPTs. Workshops were approximately four hours, facilitated by two members of the study team and observed by three to four study team members. There were fourteen workshops in total, grouped according to population of interest: two among FSWs, six among AGYW and six among women >24 years. Each completed a set of activities which differed by population group. There was a median of 21 participants per workshop (ranging from 6 to 53). Mataboge et al. provide a full description of the workshop activities as well as the results of the PAR activities (22). In summary, this included an activity where participants voted for their favourite demand creation message for MPTs and an activity in which participants selected their most preferred, of three possible options, for each of eight identified attributes of a hypothetical MPT implant i.e., body placement, prevention characteristic (duration), side effects, service access point, removal options, replacement options, visibility and pain. FSWs participated in an activity which involved the identification and ranking of preferred characteristics of a hypothetical MPT. These activities were used to stimulate discussion and further enquiry, to gain a better understanding of participant choices and preferences.
Following workshops, participants completed a self-administered questionnaire on a handheld tablet, computer, or cell phone. For participants who did not participate in a workshop, the questionnaire was completed immediately following the information session. Questionnaire data were managed using REDCap electronic data capture tools hosted at the University of the Witwatersrand (25, 26). The questionnaire was in English and self-administered. Where assistance was requested, a study team member provided guidance in completing the survey.
Measures
Survey data included demographics and self-reported sexual behaviour. Self-perceived risk of HIV, STIs, and pregnancy was categorised as either “no perceived risk”, or “some perceived risk” based on participant's own risk perception. Early sexual debut was defined as having first sexual intercourse at age ≤14 years. Transactional sex was defined as having a sexual relationship in the last three months in exchange for any of the following items: food, clothing, cosmetics, cell phone, items for children or family, transport or tickets, school or residence fees, cash or somewhere to stay.
Data were collected on the factors influencing hypothetical prevention method choice. Participants viewed a list of 15 factors identified to influence pregnancy, HIV and STI prevention method decision making, based on a previous MPT acceptability study (27) and were asked to rank these in order of importance to their prevention method choice from 1 to 15. These were not specific to MPTs, but about prevention methods more broadly. The most important factor was defined as the factor ranked number one by each participant.
Data on whether participants would consider the use of various HIV and pregnancy prevention options were also collected. Participants viewed a list of different prevention methods, aligned to those presented in the participant information session, and were asked for each if they would consider using it to protect themselves from getting HIV. Prevention method options were limited to injectable, oral and implant products to reflect the most widely used existing methods with which women in South Africa have experience. These were a once daily pill; monthly pill; two-monthly, gluteal, intra-muscular injectable (in keeping with provision of cabotegravir); six-monthly sub-cutaneous injectable (2 injections per dose) (in keeping with provision of lenacapavir); They were then asked if they would consider the following MPT-implant methods to prevent HIV and pregnancy: 1-year non-biodegradable; 1-year biodegradable; 1-year refillable; 2-year non-biodegradable; 2-year biodegradable. Participants were then asked to select from the methods previously listed, the method they would choose if they could only choose one. From these responses, participants were categorized as either preferring MPT PrEP if they chose any of the MPT-implants; pill PrEP if they chose the pill products; or injectable PrEP if they chose any of the injectable products. Those that would use none of the products were categorized as not choosing any form of PrEP. Prevention methods included in the questionnaire were restricted to those provided through oral, injectable and implant forms, as these are currently the most frequently used biomedical contraception and HIV prevention method forms, and the method forms with which the population had some existing knowledge and experience.
Statistical analysis
A total of 703 females completed a study survey. Descriptive analyses of demographic and sexual behaviour characteristics, by population group of interest, were conducted. We determined the factors ranked most important when choosing a prevention product and which entity (HIV, STIs, or pregnancy) participants wanted to prevent. We determined the proportion of participants willing to consider each of the prevention methods presented, and their choice if choosing only one. We used multinomial logistic regression to assess the factors associated with choosing a pill, injectable or MPT-implant as a preferred prevention method among those who would consider any prevention product. The final regression model adjusted for age, study site and workshop participation a priori. Baseline factors with a p-value of 10% or less were included in the multivariable analysis and statistical significance was set at 5%. All statistical analyses were performed in STATA statistical software, version 15 (28).
Results
Of the 703 women enrolled, 193 (27%) were recruited through snowballing and 508 (72%) through direct recruitment at study sites. Data on recruitment type was missing for two participants. Compared to AGYW (89/289, 31%) and women >24 years (99/374, 26%), FSWs were recruited less frequently through snowballing (5/40, 12.5%).
Demographics
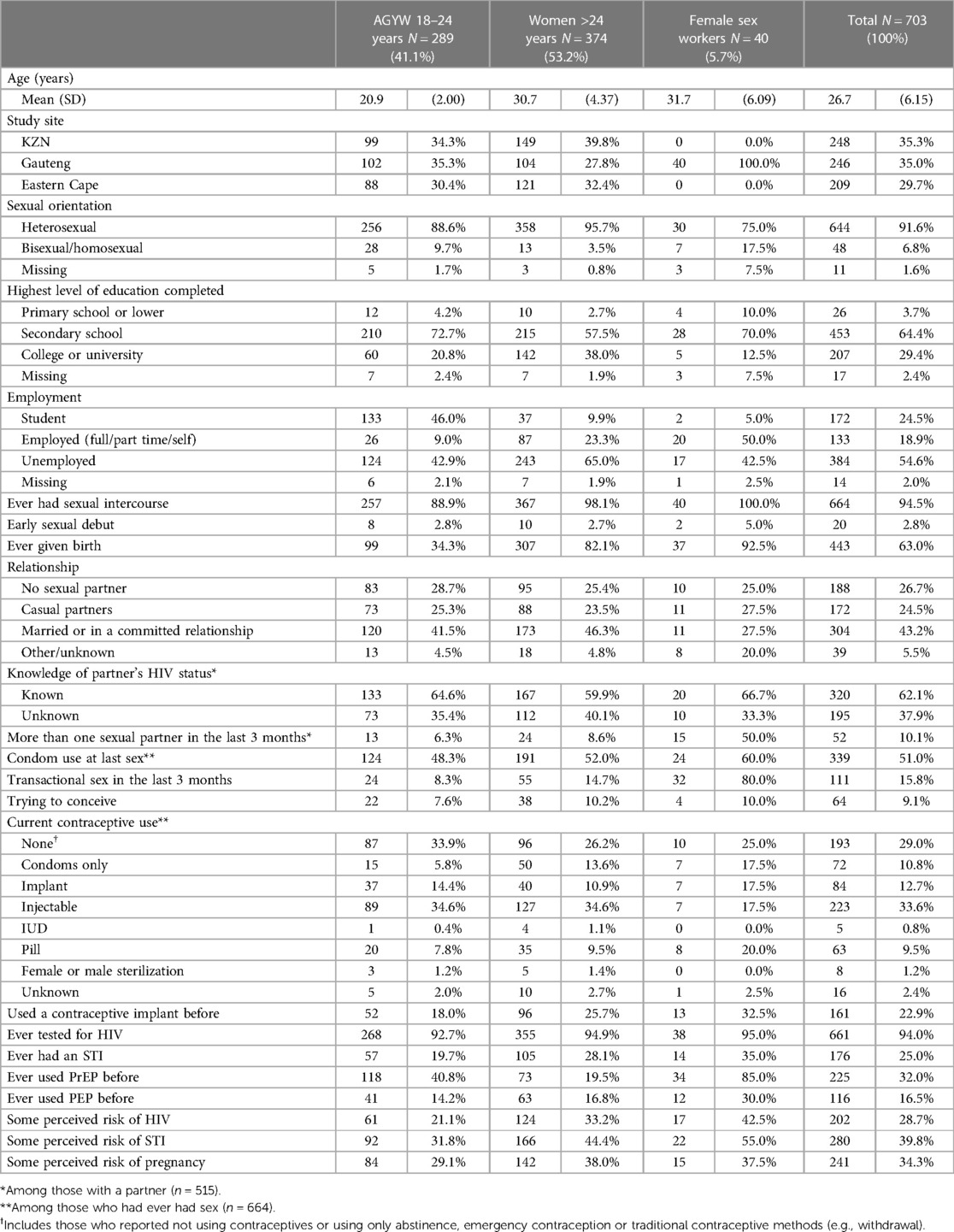
The demographic and sexual behavioural characteristics are presented in Table 1, stratified by population group. Most participants had completed secondary school, with completion of tertiary level education ranging from 38.0% (142/374) among women >24 years to 12.5% (5/40) among FSWs. Almost half of AGYW were students (46.0%, 133/289), whereas most women >24 years (65.0%, 243/374) were unemployed.
Sexual behaviour and perceived risk of HIV, STIs and pregnancy
Almost all participants reported having had sexual intercourse, with early sexual debut ranging from 2.7% (10/374) among women >24 years to 5.0% (2/40) among FSWs. Almost all (92.5%, 37/40) FSWs, 82.1% (307/374) of women >24 years and a third of AGYW (34.3%, 99/289) had given birth. Overall, 43.2% (304/703) were married or in committed relationships, 26.7% (188/703) had no sexual partner and 24.5% (172/703) had casual partners. The majority of participants with partners knew their partner's HIV status (62.1%, 320/515), and 10.1% (52/515) had more than one sexual partner in the last three months. Half of sexually active participants (51.0%, 339/664) used a condom at last sex. Transactional sex ranged from 8.3% (24/289) among AGYW to 80% (32/40) among FSWs. The injectable was the most commonly used contraceptive among AGYW (34.6%, 89/257) and among women >24 years (34.6%, 127/367); the oral pill was most commonly used among FSWs (20.0%, 8/40). The implant was used by 17.5% (7/40) of FSWs, 14.4% (37/257) of AGYW and 10.9% (40/367) of women >24 years. Approximately a quarter (22.9%, 161/703) had used a contraceptive implant before.
Almost all participants (94.0%, 661/703) had tested for HIV, and 25.0% (176/703) reported ever having an STI. A third (32.0%, 225/703) had ever used PrEP, ranging from 19.5% (73/374) among women >24 years, to 85% (34/40) among FSWs, with fewer (16.5%, 116/703) having used post exposure prophylaxis. Just under a third (28.7%, 202/703) perceived themselves to be at risk of HIV, ranging from 21.1% (61/289) among AGYW to 42.5% (17/40) among FSWs; over a third perceived themselves to be at risk of STIs (39.8%, 280/703) and pregnancy (34.3%, 241/703).
Compared to those who participated only in the information session, AGYW and women >24 years who participated in the information session and workshops were significantly older; they also differed significantly by study site, employment status, having had sex, having given birth, knowledge of their partner's HIV status, condom use, and perceived risk of STIs (Supplementary Table S1). All FSWs participated in an information session and workshop.
Prevention product preferences
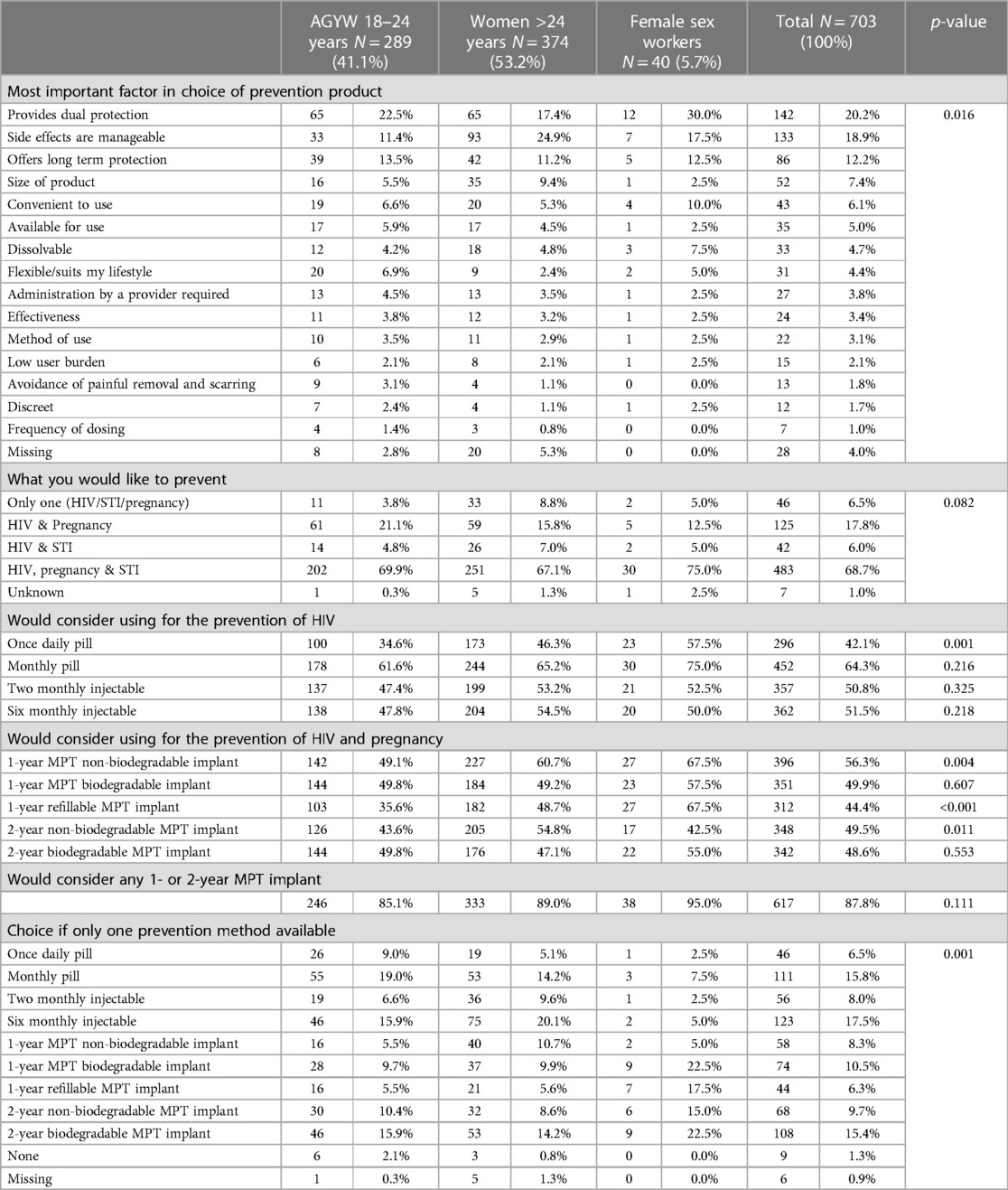
The factors reported as the most important in decision making around prevention product choice are presented in Table 2, together with prevention method preferences. Dual protection was reported by 20.2% (142/703) as the most important factor when choosing a prevention product, followed by whether side effects were manageable (18.9%, 133/703) and whether it offers long term protection (12.2%, 86/703). These factors differed significantly by population group (p = 0.016).
Most participants (68.7%, 483/703) wanted to prevent HIV, pregnancy and STIs. Almost half would consider using each of the prevention method options presented, ranging from 42.1% (296/703) who would consider the daily oral pill to 64.3% (452/703) who would consider the monthly pill. Across all participants, the monthly pill was most frequently considered, ranging from 61.6% (178/289) among AGYW to 75% (30/40) among FSWs. Methods noted to differ significantly across population groups were the daily pill (p = 0.001), the 1 and 2-year non-biodegradable (p = 0.004 and p = 0.011) and the refillable MPT-implant (p < 0.001). Methods noted to differ significantly between those who participated in the information session, and those who participated in the information session and workshop were the 6-monthly injectable (p = 0.010), the 1-year non-biodegradable (p < 0.001) and the 1-year refillable MPT-implant (p = 0.028) (Supplementary Table S2).
When choosing only one prevention method, the 6-monthly injectable was chosen most frequently (17.5%, 123/703), followed by the monthly oral pill (15.8%, 111/703) and the 2-year biodegradable MPT-implant (15.4%, 108/703). The methods chosen least frequently were the 1-year refillable MPT-implant (6.3%, 44/703), the daily oral pill (6.5%, 46/703), and the two monthly injectable (8.0%, 56/703). Overall, any MPT-implant was chosen by 50.1% (352/703) of participants. Choices differed significantly by population group (p < 0.001), with AGYW preferring the monthly oral pill (19.0%, 55/289), women >24 years the 6-monthly injectable (20.1%, 75/374) and FSWs the 1-year and 2-year biodegradable MPT-implants (each 22.5%, 9/40). Choices differed significantly between those who participated in an information session, and those who participated in an information session and workshop (p < 0.001) (Supplementary Table S2). Those who participated only in the information session preferred a monthly pill (19.3%, 78/404), whilst those who participated in the information session and workshop preferred the 2-year biodegradable MPT-implant (15.7%, 47/299).
Factors associated with prevention product choices
The MPT-implant group was used as the reference category in the multinomial regression analysis (Table 3). All models adjusted for age, study site and workshop participation. Sex workers were less likely to choose an injectable compared to MPT-implant (RRR: 0.21, 95% CI: 0.05–0.78) and women who had sex before were less likely to choose an oral pill compared to an MPT-implant (RRR: 0.32, 95% CI: 0.12–0.89). Compared to those that were single, women who had casual partners were also less likely to choose an oral pill over an MPT-implant (RRR: 0.54, 95% CI: 0.30–0.97). Women who reported using condoms at last sex and who were trying to conceive were more likely to choose an oral pill over an MPT-implant (RRR: 1.70, 95% CI: 1.10–2.64: and RRR: 2.14, 95% CI: 1.03–4.48, respectively). Compared to those not using a contraceptive method, women who were currently using an implant (RRR: 0.38, 95% CI: 0.17–0.88) or an injectable contraceptive (RRR: 0.55, 95% CI: 0.31–0.96) were less likely to choose an oral pill over an MPT-implant. Women who had ever used a contraceptive implant were less likely to choose an injectable compared to MPT-implant (RRR: 0.54, 95% CI: 0.31–0.97). Relative to those who participated in the information session only, those who participated in both the information session and workshop were less likely to choose either a pill or an injectable compared to an MPT-implant (RRR: 0.48, 95% CI: 0.31–0.76 and RRR: 0.46, 95% CI: 0.31–0.70).
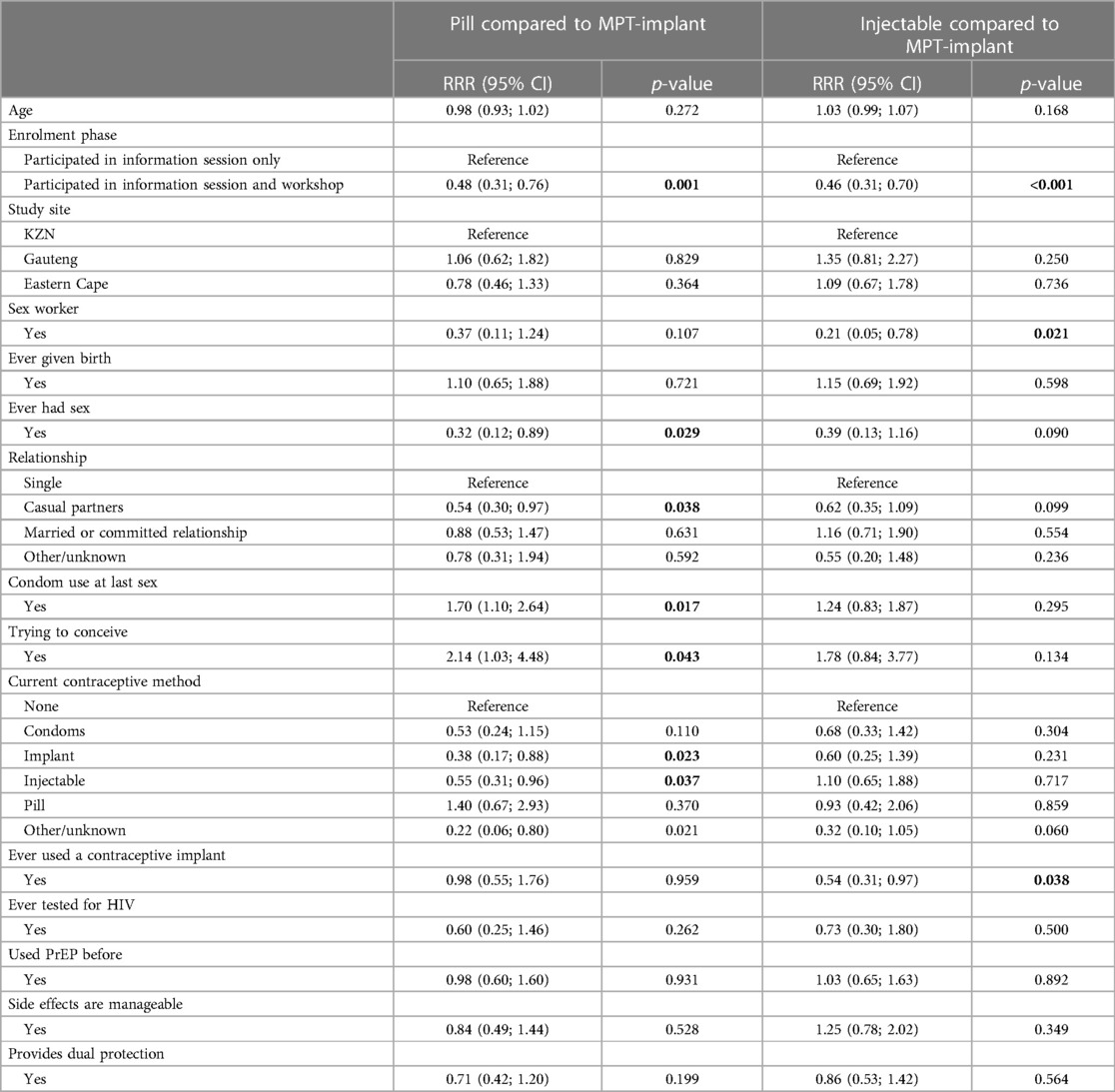
Table 3 Fully adjusted analyses for factors associated with hypothetical prevention method choice among females who would consider PrEP (N = 688).
Discussion
MPTs present an opportunity to address multiple prevention needs among women. The results from this study are consistent with others, which provide a strong indication that women want to prevent pregnancy, HIV and STIs, and would prefer an MPT over single-purpose products (17, 18, 27, 29–33). The prevention methods most preferred by women in this study were a 6-monthly sub-cutaneous injection, followed by a monthly pill and a 2-year biodegradable implant, with half of women choosing any MPT-implant method and a quarter each choosing a pill or an injectable method. Women who had casual partners, who were not using condoms, who were not trying to conceive and who were engaged in sex work were significantly more likely to consider an MPT-implant as a prevention method.
Our findings confirm that, when looking at products that require consistent use, there is a preference among women for prevention methods that are long-acting, that provide dual protection, and have minimal side effects (16–18, 29, 30, 32, 34–36). Whilst having minimal side effects was noted to be of high importance to women in this study, and has been raised as a concern among young women in prior research (37), women may be willing to accept some product side effects if they are adequately counselled and supported to manage them (38). In contrast to studies conducted among men and women in the region (30, 31, 36, 39, 40), method efficacy was not reported to be one of the most important factors influencing choice in this study. This highlights the important role that other product attributes may play among women and reiterates the notion that product efficacy alone will not necessarily translate to its acceptability and use (40). Further evaluation of end-users understanding of efficacy may also be needed. There may be an assumption among end-users that longer-acting products have higher efficacy (34), or that products would only be made available if they met certain efficacy requirements.
Our findings support others which suggest that prior use of a method type may increase its acceptability and use (33). The TRIO study, a randomised cross over study of placebo MPTs found that prior use of a product was associated with a preference for that product at baseline (41) and that product ratings increased with use, indicating that the experience of using a product may increase its acceptability (16). A discrete choice experiment among women in South Africa and Kenya also found product experience to influence preferences for prevention method attributes (30). Formative studies exploring acceptability of MPT implants in clinical development have also noted the influence of prior implant use on user acceptability (42). It is notable that participation in this study's workshop was associated with choosing an MPT-implant. This may be due to the inherent differences in the characteristics of the population that participated in the workshops. However, may indicate that providing comprehensive information on new products as well as participatory activities reflecting on new products could influence choice, and may improve acceptability. As has been noted by others, community experiences and efforts to support new product users are likely to influence demand and adoption of new products (20, 30).
The findings from this study further highlight that daily pills are not a preferred prevention method (16, 30, 31), and reiterate the need for additional types of prevention methods to be made available. Although reported by women in similar contexts to be a less important attribute of HIV prevention implants (35), our findings are in keeping with existing literature which suggests that there may be some preference for a biodegradable compared to a non-biodegradable implant, an attribute that may be linked to women's perception of method privacy or discretion (21, 36–38, 42, 43).
The differences noted across population groups, and the influence of factors such as sexual behaviour, condom use, and trying to conceive, indicate that preferences are not homogenous, and that women need a choice of prevention methods to suit their different and likely changing preferences and needs.
Limitations
The following limitations should be considered. Firstly, preferences and product choices were self-reported and hypothetical. Real life decision making may be influenced by social and contextual factors and may differ from what is self-reported. Although the influence of workshop participation was adjusted for in the final models, this may have resulted in an over reporting of the proportion of women who would consider an MPT-implant if they were not provided with sufficient opportunity to discuss new method choices. We acknowledge that participation in the workshop activities allowed those participants to engage more in discussions on product attributes. The activities also differed slightly between workshops, and there may have been a social desirability bias among workshop attendees, or dominant workshop participants who may have influenced the groups' preferences. Participants were predominantly recruited from health facilities, and thus may reflect a population already accessing prevention services, who may differ from the general population. Participants were all English speaking, and the results may therefore not accurately reflect those of individuals who could not speak English. In the statistical analysis, we did not account for the different sampling methods, or the potential associations between participants recruited through the snowball sampling, which may limit the representativeness of the sample. The restriction of this study to pill, injectable and implant products limits the considerations and influence that the availability of a vaginal ring method may have on women's prevention product choices. In addition, the study did not include event-driven and on-demand methods, for which women have shown interest (44).
Conclusions
In this study exploring women's preferences for prevention methods, women indicated a preference for products which provide dual and long term protection, with few side effects. Their method choices were influenced be sexual-behavioural factors and current and prior contraceptive method use. Providing a prevention method choice and a population specific approach to new method development and introduction could enhance their ability to fill a gap in prevention needs. Ensuring access to accurate, client-centred information around products prior to their introduction may also enhance uptake and use.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was approved by the Human Research Ethics Committee at the University of the Witwatersrand (M220305). The study was conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CM: Formal Analysis, Writing – original draft, Writing – review & editing. AK: Conceptualization, Methodology, Project administration, Writing – original draft, Writing – review & editing. PM: Project administration, Writing – original draft, Writing – review & editing. GC: Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. NM: Writing – original draft, Writing – review & editing. RB: Writing – original draft, Writing – review & editing. SM: Conceptualization, Methodology, Writing – original draft, Writing – review & editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article.
This study was funded through the Bill and Melinda Gates Foundation [INV-022667]. Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission. The findings and conclusions contained within are those of the authors and do not necessarily reflect positions or policies of the Bill & Melinda Gates Foundation.
Acknowledgments
The authors would like to thank the field staff for their hard work and dedication, and the staff at the study sites for their support and collaboration. We thank the study participants for contributing their time, effort and opinions to this study. We thank Ms. Vusile Butler for support with grant management and implementation, Ms. Samantha Jack and Ms. Mbali Mazibuko for data cleaning and management and Prof. Mags Beksinska for her review and input on a manuscript draft.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frph.2024.1368889/full#supplementary-material
Abbreviations
AGYW, adolescent girls and young women; FSW, female sex worker; MPT, multipurpose prevention technology; PrEP, pre-exposure prophylaxis; SRH, sexual and reproductive health; STI, sexually transmitted infection.
References
1. Simbayi LC, Zuma K, Zungu N, Moyo S, Marinda E, Jooste S, et al. South African National HIV Prevalence, Incidence, Behaviour and Communication Survey. Cape Town: HSRC Press (2017).
2. Francis SC, Mthiyane TN, Baisley K, Mchunu SL, Ferguson JB, Smit T, et al. Prevalence of sexually transmitted infections among young people in South Africa: a nested survey in a health and demographic surveillance site. PLoS Med. (2018) 15:e1002512. doi: 10.1371/journal.pmed.1002512
3. Woldesenbet SA, Lombard C, Manda S, Kufa T, Ayalew K, Cheyip M, et al. The 2019 National Antenatal Sentinel HIV Survey. South Africa: National Department of Health (2021).
4. Department of Health South Africa. Welcome to she conquers (2022). Available online at: http://sheconquerssa.co.za/ (accessed December 6, 2022).
6. National Department of Health (NDoH), Statistics South Africa (Stats SA), South African Medical Research Council (SAMRC), and ICF. South Africa Demographic and Health Survey 2016. Pretoria, South Africa, and Rockville, MD: NDoH, Stats SA, SAMRC, and ICF (2019).
7. Department of Health Republic of South Africa. Guidelines for Expanding Combination Prevention and Treatment Options: Oral Pre-Exposure Prophylaxis (PrEP) and Test and Treat (T&T). Pretoria, South Africa: National Department of Health (2017).
8. Makhakhe NF, Sliep Y, Meyer-Weitz A. “Whatever is in the ARVs, is also in the PrEP” challenges associated with oral pre-exposure prophylaxis use among female sex workers in South Africa. Front Public Health. (2022) 10:691729. doi: 10.3389/fpubh.2022.691729. eCollection 2022.35784260
9. Pillay D, Stankevitz K, Lanham M, Ridgeway K, Murire M, Briedenhann E, et al. Factors influencing uptake, continuation, and discontinuation of oral PrEP among clients at sex worker and MSM facilities in South Africa. PLoS One. (2020) 15:e0228620. doi: 10.1371/journal.pone.0228620
10. Dollah A, Ongolly F, Ngure K, Odoyo J, Irungu E, Mugwanya K, et al. “I just decided to stop:” understanding PrEP discontinuation among individuals initiating PrEP in HIV care centers in Kenya and its implications for a public health approach to prevention. J Int AIDS Soc. (2021) 24:19–20. doi: 10.1097/QAI.0000000000002625
11. Musakwa NO, Bor J, Nattey C, Lönnermark E, Nyasulu P, Long L, et al. Perceived barriers to the uptake of health services among first-year university students in Johannesburg, South Africa. PLoS One. (2021) 16:e0245427. doi: 10.1371/journal.pone.0245427
12. Chersich MF, Wabiri N, Risher K, Shisana O, Celentano D, Rehle T, et al. Contraception coverage and methods used among women in South Africa: a national household survey. S Afr Med J. (2017) 107:307–14. doi: 10.7196/SAMJ.2017.v107i4.12141
13. Jonas K, Duby Z, Maruping K, Harries J, Mathews C. Rumours, myths, and misperceptions as barriers to contraceptive use among adolescent girls and young women in South Africa. Front Reprod Health. (2022) 4:960089. doi: 10.3389/frph.2022.960089
14. The IMPT. MPT product development database. Public Health Institute/CAMI Health/IMPT Secretariat (2023).
15. Quaife M, Terris-Prestholt F, Eakle R, Cabrera Escobar MA, Kilbourne-Brook M, Mvundura M, et al. The cost-effectiveness of multi-purpose HIV and pregnancy prevention technologies in South Africa. J Int AIDS Soc. (2018) 21(3):e25064. doi: 10.1002/jia2.25064
16. Minnis AM, Roberts ST, Agot K, Weinrib R, Ahmed K, Manenzhe K, et al. Young women’s ratings of three placebo multipurpose prevention technologies for HIV and pregnancy prevention in a randomized, cross-over study in Kenya and South Africa. AIDS Behav. (2018) 22:2662–73. doi: 10.1007/s10461-018-2078-5
17. Woodsong C, Musara P, Chandipwisa A, Montgomery E, Alleman P, Chirenje M, et al. Interest in multipurpose prevention of HIV and pregnancy: perspectives of women, men, health professionals and community stakeholders in two vaginal gel studies in Southern Africa. BJOG: Int J Obstet Gynaecol. (2014a) 121(Suppl 5):45–52. doi: 10.1111/1471-0528.12875
18. Minnis AM, Krogstad E, Shapley-Quinn MK, Agot K, Ahmed K, Danielle Wagner L, et al. Giving voice to the end-user: input on multipurpose prevention technologies from the perspectives of young women in Kenya and South Africa. Sex Reprod Health Matters. (2021) 29:1927477. doi: 10.1080/26410397.2021.1927477
19. The IMPT. Toward a roadmap for biomedical HIV prevention investment standards: strategic insights from key industry stakeholders (2019). Initiative for MPTs (IMPT) for the USAID Office of HIV/AIDS Research Division.
20. Pleaner M, Morroni C, Smit J, Lince-Deroche N, Chersich M, Mullick S, et al. Lessons learnt from the introduction of the contraceptive implant in South Africa. S Afr Med J. (2017) 107:933–8. doi: 10.7196/SAMJ.2017.v107i11.12805
21. Humphries H, Upfold M, Mahlase G, Mdladla M, Gengiah TN, Abdool Karim Q. Implants for HIV prevention in young women: provider perceptions and lessons learned from contraceptive implant provision. PLoS One. (2022) 17:e0262043. doi: 10.1371/journal.pone.0262043
22. Mataboge P, Mthimkhulu N, Kutywayo A, Martin CE, Mazibuko M, Kwatsha K, et al. Preferences, educational messaging, and demand creation channels for multipurpose-prevention technologies (MPTs) among women in South Africa. BMC Public Health. (2023) 23:2090. doi: 10.1186/s12889-023-16904-0
23. Kutywayo A, Mataboge P, Mthimkhulu N, Martin CE, Muhwava LS, Mazibuko M, et al. Key programmatic and policy considerations for introducing multipurpose prevention (MPT) methods: reflections from healthcare providers and key stakeholders in South Africa. Front Reprod Health. (2024) 6:1249750. doi: 10.3389/frph.2024.1249750
24. Mthimkhulu N, Chidumwa G, Kutywayo A, Mataboge P, Martin CE, Kwatsha K, et al. Factors influencing the uptake of a mono-PrEP implant for the prevention of HIV: males’ perspectives from three South African provinces. PLoS One. (2024) 19:e0296341. doi: 10.1371/journal.pone.0296341
25. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) 42:377–81. doi: 10.1016/j.jbi.2008.08.010
26. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. (2019) 95:103208. doi: 10.1016/j.jbi.2019.103208
27. El-Sahn M, Lucas J, Aikenheada M, Nemadeb R, Van Damme L. Understanding the potential for multipurpose prevention of pregnancy and HIV: results from surveys assessing four hypothetical concept profiles of multipurpose prevention technologies (MPTs) in Uganda, Nigeria and South Africa (2014).
29. van der Straten A, Agot K, Ahmed K, Weinrib R, Browne EN, Manenzhe K, et al. The tablets, ring, injections as options (TRIO) study: what young African women chose and used for future HIV and pregnancy prevention. J Int AIDS Soc. (2018) 21:e25094. doi: 10.1002/jia2.25094
30. Minnis AM, Browne EN, Boeri M, Agot K, van der Straten A, Ahmed K, et al. Young women’s stated preferences for biomedical HIV prevention: results of a discrete choice experiment in Kenya and South Africa. J Acquir Immune Defic Syndr. (2019) 80:394–403. doi: 10.1097/QAI.0000000000001945
31. Quaife M, Eakle R, Cabrera Escobar MA, Vickerman P, Kilbourne-Brook M, Mvundura M, et al. Divergent preferences for HIV prevention: a discrete choice experiment for multipurpose HIV prevention products in South Africa. Med Decis Making. (2018a) 38:120–33. doi: 10.1177/0272989X17729376
32. Little KM, Hanif H, Anderson SM, Clark MR, Gustafson K, Doncel GF. Preferences for long-acting PrEP products among women and girls: a quantitative survey and discrete choice experiment in Eswatini, Kenya, and South Africa. AIDS Behav. (2024) 28:936–50. doi: 10.1007/s10461-023-04202-0
33. Bhushan NL, Ridgeway K, Luecke EH, Palanee-Phillips T, Montgomery ET, Minnis AM. Synthesis of end-user research to inform future multipurpose prevention technologies in sub-Saharan Africa: a scoping review. Front Reprod Health. (2023) 5:1156864. doi: 10.3389/frph.2023.1156864
34. Minnis AM, Atujuna M, Browne EN, Ndwayana S, Hartmann M, Sindelo S, et al. Preferences for long-acting pre-exposure prophylaxis (PrEP) for HIV prevention among South African youth: results of a discrete choice experiment. J Int AIDS Soc. (2020) 23:e25528. doi: 10.1002/jia2.25528
35. Browne EN, Manenzhe K, Makoni W, Nkomo S, Mahaka I, Ahmed K, et al. Incorporating end-users’ voices into the development of an implant for HIV prevention: a discrete choice experiment in South Africa and Zimbabwe. BMC Womens Health. (2023) 23:58. doi: 10.1186/s12905-023-02181-x
36. Little KM, Flomen L, Hanif H, Anderson SM, Thurman AR, Clark MR, et al. HIV pre-exposure prophylaxis implant stated preferences and priorities: results of a discrete choice experiment among women and adolescent girls in Gauteng province, South Africa. AIDS Behav. (2022) 26:3099–109. doi: 10.1007/s10461-022-03658-w
37. Krogstad EA, Atujuna M, Montgomery ET, Minnis A, Ndwayana S, Malapane T, et al. Perspectives of South African youth in the development of an implant for HIV prevention. J Int AIDS Soc. (2018) 21:e25170. doi: 10.1002/jia2.25170
38. Brown MS, Hanif H, Little KM, Clark MR, Thurman AR, Flomen L, et al. End-user research in support of long-acting systemic antiretroviral delivery systems: insights from qualitative research with providers and target users in South Africa. BMC Infect Dis. (2022) 22:919. doi: 10.1186/s12879-022-07907-0
39. Montgomery ET, Atujuna M, Krogstad E, Hartmann M, Ndwayana S, O'Rourke S, et al. The invisible product: preferences for sustained-release, long-acting pre-exposure prophylaxis to HIV among South African youth. J Acquir Immune Defic Syndr. (2019) 80:542–50. doi: 10.1097/QAI.0000000000001960
40. Browne EN, Montgomery ET, Mansfield C, Boeri M, Mange B, Beksinska M, et al. Efficacy is not everything: eliciting women’s preferences for a vaginal HIV prevention product using a discrete-choice experiment. AIDS Behav. (2020) 24:1443–51. doi: 10.1007/s10461-019-02715-1
41. Weinrib R, Minnis A, Agot K, Ahmed K, Owino F, Manenzhe K, et al. End-users’ product preference across three multipurpose prevention technology delivery forms: baseline results from young women in Kenya and South Africa. AIDS Behav. (2018) 22:133–45. doi: 10.1007/s10461-017-1911-6
42. Nkomo S, Makoni W, Shapley-Quinn MK, Luecke E, Mbatsane E, Manenzhe K, et al. Prospective acceptability of a multipurpose technology (MPT) implant in preclinical development to prevent HIV and unplanned pregnancy: qualitative insights from women end users and health care providers in South Africa and Zimbabwe. PLoS One. (2023) 18:e0285711. doi: 10.1371/journal.pone.0285711. eCollection 2023.37195918
43. Krogstad EA, Montgomery ET, Atujuna M, Minnis AM, O’Rourke S, Ahmed K, et al. Design of an implant for long-acting HIV pre-exposure prophylaxis: input from South African health care providers. AIDS Patient Care STDS. (2019) 33:157–66. doi: 10.1089/apc.2018.0177
Keywords: multipurpose prevention technology, MPT, PrEP, HIV prevention, contraception
Citation: Martin CE, Kutywayo A, Mataboge P, Chidumwa G, Mthimkhulu N, Bothma R and Mullick S (2024) Prevention method preferences and factors influencing hypothetical choice among women in South Africa: a survey exploring opportunities for a multipurpose prevention technology implant. Front. Reprod. Health 6:1368889. doi: 10.3389/frph.2024.1368889
Received: 11 January 2024; Accepted: 31 May 2024;
Published: 25 June 2024.
Edited by:
Z. Mike Chirenje, University of California, San Francisco, United StatesReviewed by:
Margaret Kasaro, UNC Global Projects Zambia, ZambiaKathryn Therese Mngadi, Aurum Institute, South Africa
Ariane Van Der Straten, University of California, San Francisco, United States
© 2024 Martin, Kutywayo, Mataboge, Chidumwa, Mthimkhulu, Bothma and Mullick. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Catherine E. Martin, Y21hcnRpbkB3cmhpLmFjLnph
 Catherine E. Martin
Catherine E. Martin Alison Kutywayo
Alison Kutywayo Paballo Mataboge
Paballo Mataboge Glory Chidumwa
Glory Chidumwa Nqaba Mthimkhulu
Nqaba Mthimkhulu Rutendo Bothma
Rutendo Bothma Saiqa Mullick
Saiqa Mullick