- Unit of Andrology and Reproductive Medicine, Department of Medicine, University of Padova, Padova, Italy
Obstructive sleep apnoea syndrome (OSAS) is an under-recognized medical disease. The main risk factors for OSAS are male sex, older age, obesity, and metabolic syndrome, that are also associated with male hypogonadism (MH). Therefore, obesity has been classically identified as the most evident link between OSAS and MH. However, OSAS is per se linked to the development of MH by a combined effect of hypoxia, increased night-time awakenings, reduced sleep efficiency and fragmented sleep. Similarly, MH might represent a risk factor for OSAS, mainly related to sleep disturbances that are frequently associated with low testosterone. Data on testosterone replacement therapy (TRT) in patients with OSAS are limited. Nevertheless, TRT is generally contraindicated by guidelines in the presence of untreated or severe OSAS. TRT might in fact worse OSAS symptoms in different ways. Furthermore, OSAS has been proposed to be a risk factor for secondary polycythaemia and TRT might exacerbate polycythaemia. Therefore, TRT in hypogonadal men affected by untreated OSAS or severe OSAS should be considered with caution and in a personalised way. Nevertheless, the type and dosage of TRT should be considered, as short-term high-dose TRT might worsen OSAS, whereas long-term lower doses could eventually determine a clinical improvement of symptoms of OSAS. Here we reviewed the data on the association between OSAS, MH and TRT, including the opportunity of assessment of patients who develop signs and symptoms of OSAS during TRT by polysomnography.
Introduction
Obstructive sleep apnoea syndrome
Obstructive sleep apnoea syndrome (OSAS) represents a common and often under-recognized and under-diagnosed medical disease which is characterised by sleep-dependent pauses and reductions in airflow (1, 2). In particular, the sleep-dependent pauses may be complete (apnoeas) or partial (hypopnoeas), further resulting, among other consequences of OSAS, in hypoxemia and sleep fragmentation (3). OSAS has a prevalence of about 15% in men and 5% in women in the adult age (4). Other data show how about 34% and 17% of middle-aged men and women, respectively, are affected by OSAS (3), whose prevalence has been increasing during the past decades (5). The prevalence of OSAS is higher in patients with systemic diseases, such as hypertension, heart failure, coronary artery disease, metabolic alterations and stroke (2–4, 6). On the other hand, OSAS is associated with an increased risk of hypertension, atrial fibrillation, myocardial infarction, insulin resistance, and stroke (2).
The main risk factors for OSAS are male sex, older age and obesity (3). In particular, regarding the association between obesity and OSAS, the risk of OSAS correlates with the body mass index (BMI), and obesity is probably the most relevant risk factor for OSAS (3). Epidemiological data show that about 50% of obese patients are affected by OSAS (3). The clinical symptoms of OSAS include, among others, snoring, nocturnal polyuria, daytime sleepiness, morning headache, neurocognitive deficits, reduced libido, irritability, and depressive symptoms (2, 4–6). In addition, excessive daytime sleepiness may cause motor vehicle and work-related accidents (7). Clinical categorization of OSAS is based upon apnoea-hypopnoea index (AHI), obtained by the polysomnography, that represent the ratio between the number of apnoeas and hypopnoea per hour which identifies mild OSAS (5–15), moderate OSAS (15–30) and severe OSAS (>30). Moreover, also an AHI > 15 per hour in the absence of symptoms may be diagnostic of OSAS (3).
The treatment of choice for OSAS is the application of continuous positive air pressure (cPAP) (3, 8, 9). Furthermore, it is mandatory to treat the underlying pathophysiological factors, such as obesity, in order to improve the symptoms and the severity of OSAS (8). Other approaches include, among others, mandibular advancement devices, maxillofacial surgery, bariatric surgery in case of morbid obesity, hypoglossal nerve stimulation (3, 8). In addition, an interesting future pharmacological approach might be based on histamine 3-receptor antagonist/inverse agonists (10).
OSAS and male hypogonadism
Male hypogonadism (MH) is defined as the failure of the testis to produce normal concentrations of testosterone and/or to produce a normal number of spermatozoa (11, 12). MH can be a primary disorder or a secondary one, resulting in testicular (or hypergonadotropic) or central (hypogonadotropic) hypogonadism, respectively, albeit combined forms may also occur (13). Moreover, another classification of MH distinguishes between organic—due to a permanent dysfunction—and functional hypogonadism—due to a reversible condition, and this is the typical form of the so-called late-onset hypogonadism (11). MH becomes increasingly prevalent in men over 40 or 50, but it might be underdiagnosed in clinical practice (14).
As seen above, the prevalence of OSAS is higher in patients with systemic diseases and even in patients affected by metabolic syndrome (MetS). MH is related to such diseases, in particular to diabetes, obesity and MetS (15, 16), creating an association between MH and obesity (17). Therefore, this association represents the first and major link between OSAS and MH, mediated by obesity itself. A recent meta-analysis, conducted to evaluate the association between OSAS and testosterone concentrations and considering 24 case-control studies with a total of 1,268 male patients and 745 male control individuals, found that serum testosterone concentrations in OSAS patients were significantly lower with respect to the control group, therefore suggesting a correlation between OSAS and serum testosterone concentrations (18). Another recent meta-analysis, conducted by Su et al. (19) considered 18 studies with 1,823 patients (1,119 with OSAS and 704 controls) and found an inverse correlation between OSAS and serum testosterone concentrations, independently from BMI and age, with the severity of OSAS also correlating with serum testosterone concentrations, which were notably reduced in patients with severe OSAS.
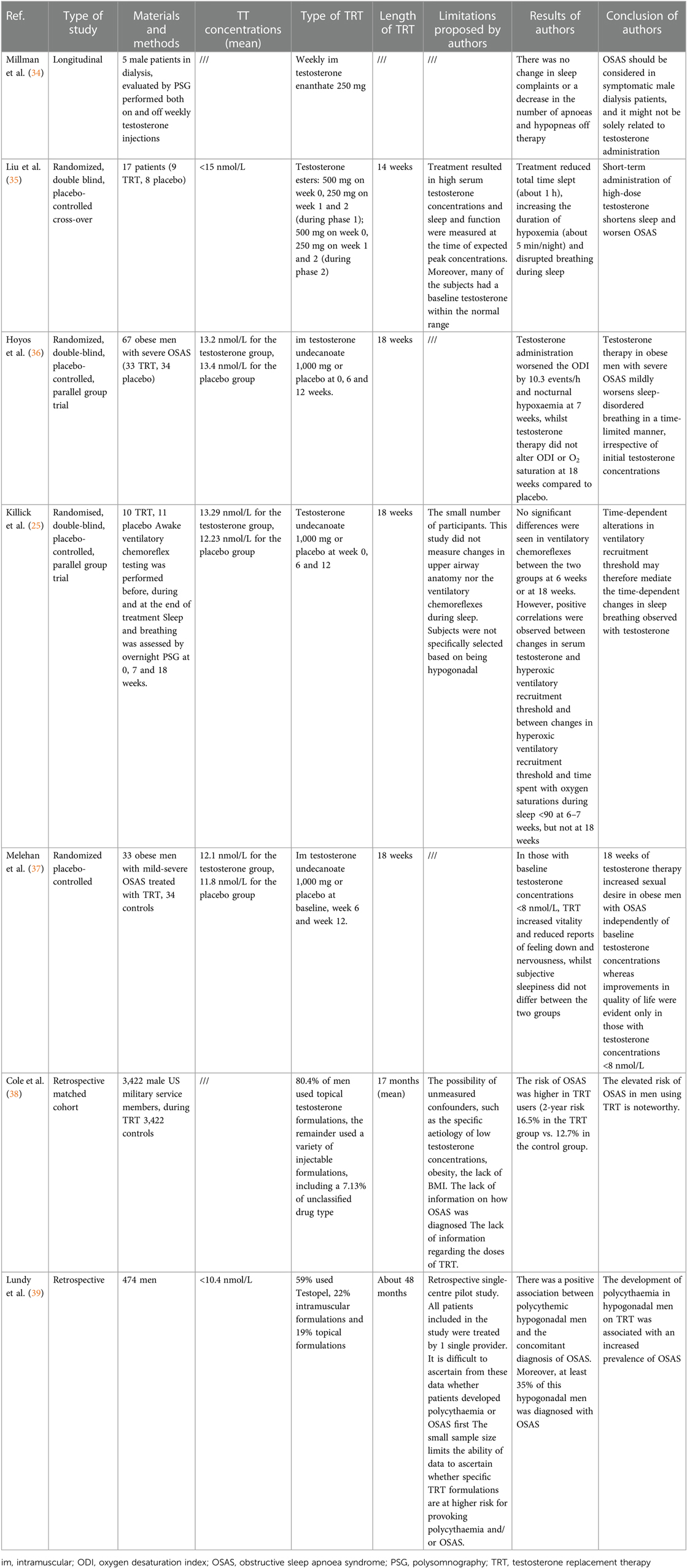
The epidemiological connection between OSAS and obesity has already been discussed above. Obesity is a risk factor for OSAS because (i) it induces enlargement of structures surrounding the airway, contributing to upper airway narrowing; (ii) an excess of fat deposition is also observed under the mandible and in the tongue, soft palate and uvula; (iii) lung volumes are reduced in obese patients, further decreasing longitudinal tracheal traction forces and pharyngeal wall tension thus leading to the narrowing of the airway; (iv) obesity-related increase in leptin and leptin resistance might contribute itself to the genesis of OSAS (20, 21). The relation of obesity and MH is well known and bidirectional. Obese men have lower serum testosterone concentrations than non-obese men (22), due to modifications in sex hormone binding globulin (SHBG), increase in the aromatase enzyme activity of adipocytes (23, 24), low-grade systemic inflammation, increase in oestradiol concentrations, hyperinsulinemia/insulin resistance, and hyperleptinemia/lleptin resistance (22–24) (Figure 1).
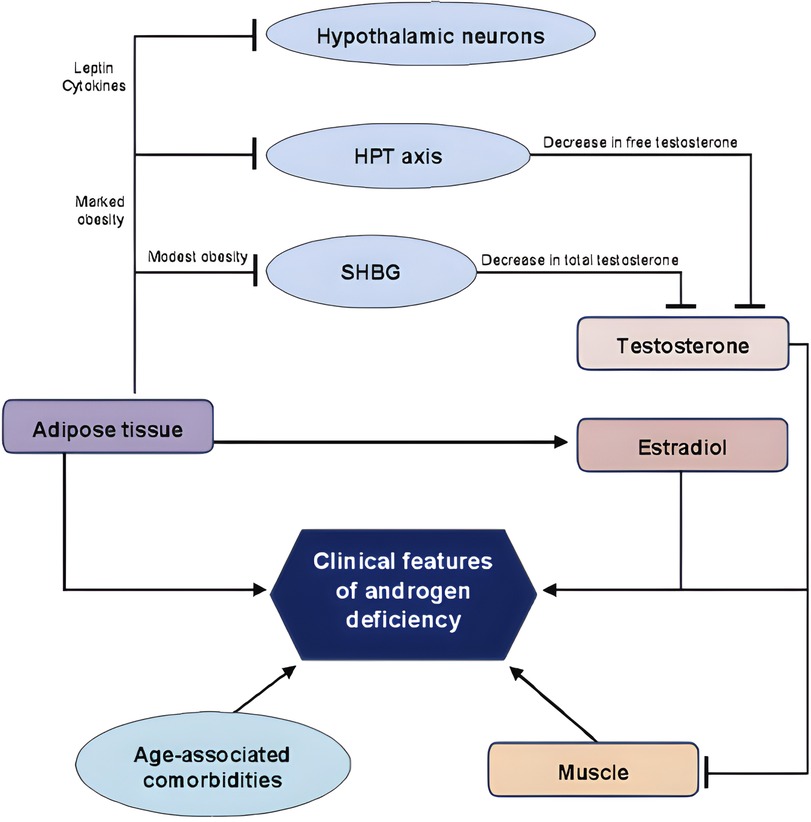
Figure 1. A schematic representation of the bidirectional association between adipose tissue and testosterone. HPT, hypothalamic pituitary axis; SHBG, sex hormone binding globulin. Lines: arrow line: stimulatory effect; non-arrow line: inhibitory effect. Adapted from Ref. (25).
Therefore, obesity induces a suppression of the hypothalamus–pituitary–gonadal axis (22, 24), representing one of the leading causes of secondary hypogonadism in men (23) with a biochemical picture characterised by normal or low concentrations of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) and reduced serum testosterone concentrations (22–24). Given evidence of causal association between MH and obesity, MH may represent an additional risk factor for the development of OSAS. Furthermore, OSAS is per se linked to development of MH (26–28). In fact, OSAS has a direct inhibitory effect on pituitary function (26) lowering LH pulse amplitude and decreasing mean serum LH concentrations (24). In addition, OSAS’ sleep alterations lead to low total serum testosterone concentrations (24, 29) and to higher circulating leptin concentrations (24). Therefore, OSAS patients have reduced amounts of LH and testosterone, and therefore secondary hypogonadism, due to a pituitary-gonadal dysfunction induced by OSAS itself (27). This is caused by multiple and combined effects of hypoxia, increased night-time awakenings, reduced sleep efficiency and fragmented sleep (26, 28). In addition to this direct mechanism, OSAS reduces testosterone concentrations indirectly when associated with obesity, insulin-resistance or MetS (28) (Figure 2).
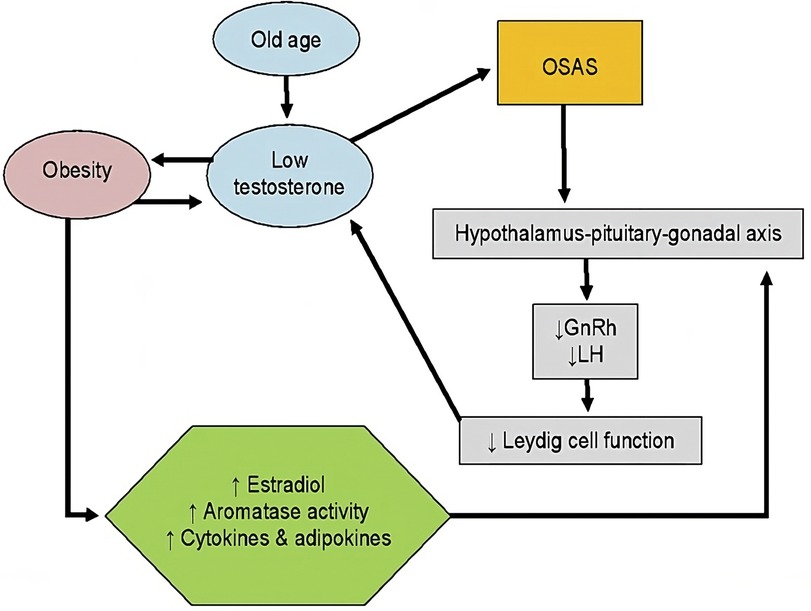
Figure 2. Association between testosterone, obesity and OSAS. GnRH, gonadotropin releasing hormone; LH, luteinizing hormone; OSAS, obstructive sleep apnoea syndrome. Lines: arrow line: stimulatory effect. Modified from Ref. (21).
Therefore, as seen above, obesity is a risk factor for OSAS, and it is also related to hypogonadism in male patients (30). OSAS severity is related to total testosterone serum concentrations: in particular, a higher AHI score correlates with a lower serum testosterone concentration (26, 28, 31, 32). Furthermore, the severity of hypoxia during sleep is correlated with a reduction in testosterone concentrations (26, 32). While the quantity and quality of sleep have been linked to testosterone concentrations, other evidence may suggest the reversal association. For instance, it has been reported that patients with low serum testosterone concentrations have a decreased sleep efficiency and increased frequency of night-time awakenings (33). A future development might be the proposal of considering sleep disturbances as one of the symptoms for hypogonadism.
Aim of the review
The relation between testosterone replacement therapy (TRT) and OSAS is more controversial. The aim of this review is to get more insight on this topic.
Material and methods
We conducted a literature review on PubMed, up to December 2022, using the words “OSAS and hypogonadism”, “OSAS and testosterone”, “obstructive sleep apnea syndrome and testosterone”, “sleep apnea and testosterone”.
Results
We collected the following results: “OSAS and hypogonadism” (6 results), “OSAS and testosterone” (14 results), “obstructive sleep apnea syndrome and testosterone” (151 results), “sleep apnea and testosterone” (235 results). After excluding non-English results, case reports, other studies/reviews, studies without available manuscripts and studies regarding TRT and other conditions, we included the clinical studies that reported the severity of OSAS pre/post TRT or changes in sleep functions or quality. The process of results’ collection is shown in Figure 3. We obtained 7 results, that are reported in Table 1.
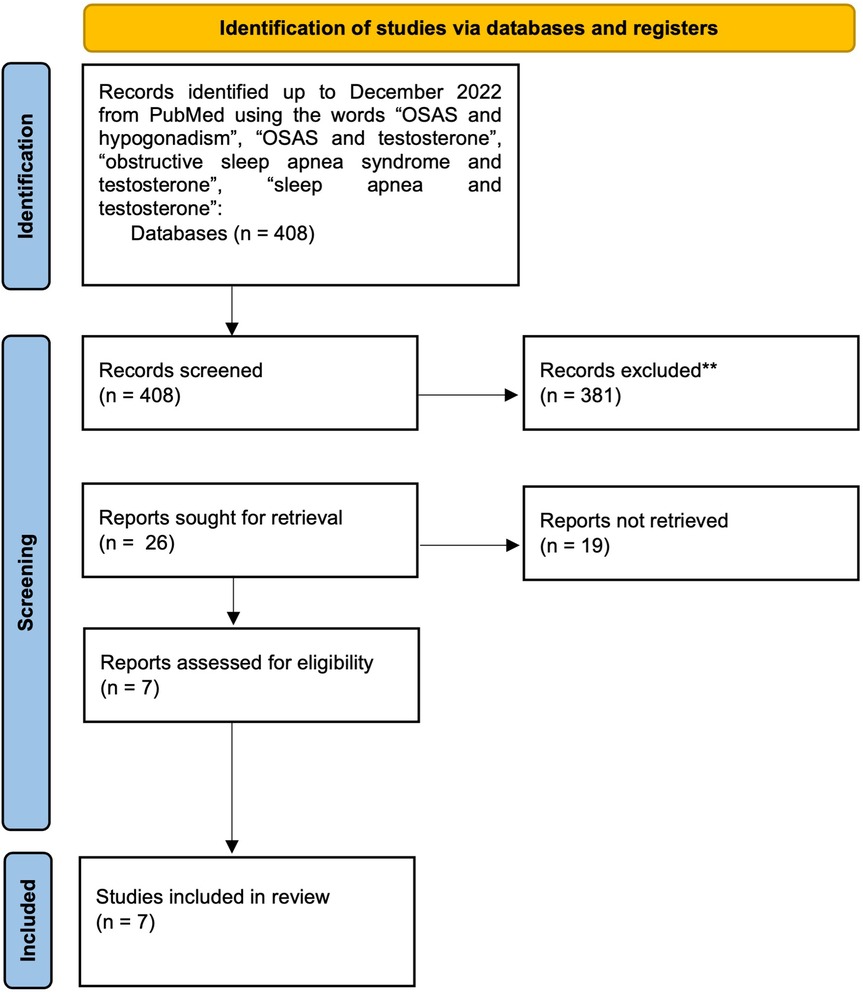
Figure 3. A schematic representation of the process of results’ collection regarding our literature search on PubMed. OSAS, obstructive sleep apnoea syndrome. *Reports excluded: non-English results, case reports, other studies/reviews, and studies regarding TRT and other conditions.
The first study was published in 1985 and evaluated 5 male patients in dialysis using polysomnography and found no change in sleep complaints or a decrease in the number of apnoeas and hypopneas during and off therapy (34). The second study, conducted by Liu et al. (35), evaluating 17 patients, found that testosterone treatment reduced total time slept (about 1 h), increased the duration of hypoxemia (about 5 min/night) and disrupted breathing during sleep, thus leading to the conclusion that short-term administration of high-dose testosterone might shorten sleep and worsen OSAS. On the other hand, Hoyos Cm et al. reported that testosterone therapy in 67 obese men with severe OSAS might mildly worsen sleep-disordered breathing in a time-limited manner, irrespective of initial testosterone concentrations. In fact, testosterone administration worsened ODI by 10.3 events/h and nocturnal hypoxaemia at 7 weeks, whilst testosterone therapy did not alter ODI or O2 saturation at 18 weeks compared to placebo administration (36). The fourth study was conducted by Killick et al. (25) and reported no significant differences in ventilatory chemoreflexes between the testosterone and placebo group evaluated at 6 weeks and at 18 weeks. Melehan et al. (37) evaluated 33 obese men with mild-severe OSAS under TRT and 34 controls and found that in patients with baseline testosterone concentrations below 8 nmol/L TRT increased vitality and reduced reports of feeling down and nervousness, whilst subjective sleepiness did not differ between TRT and placebo group. In the sixth study (38) a higher risk of OSAS in TRT uses was noted (2-year risk 16.5% in the TRT group vs. 12.7% in the control group). Finally, Lundy SD et al. evaluated 474 hypogonadal men and reported that the development of polycythaemia in men on TRT was associated with an increased prevalence of OSAS (39).
Regarding the type of studies, just one is longitudinal (34), two are retrospective (38, 39), and few are randomised placebo-controlled clinical studies (25, 35–37). Mean testosterone concentrations at baseline are not always specified (34, 38) and, in some cases, it is even in the normal reference range (25, 36). Therefore, in some studies, testosterone therapy is not a real replacement therapy for hypogonadism, but rather a supplementation therapy. Type (38, 39) and length (34) of TRT are not uniform and not always clear. The number of patients is generally low, except for two studies (38, 39) that, on the other hand, have several limitations, such as the retrospective nature and the lack of information regarding the type of TRT. Furthermore, data on free testosterone are lacking. Finally, the primary aims of the studies are only sometimes directly related to the relation between OSAS and TRT, therefore creating further difficulties in the comprehension of this complex relation. In addition, there is a lack of long-term studies, which might prevent for example to possibly notice a positive effect of chronic low-dose TRT on OSAS symptoms, as suggested by some studies.
Discussion
This review highlights that clinical data regarding TRT and OSAS are scarce and often lacking important clinical and/or biochemical data.
Although the data on TRT in patients with OSAS are limited and not uniform, as evidenced in this review, and there is a lack of convincing evidence that TRT causes and/or aggravates OSAS (25, 40–42), TRT is generally considered contraindicated in the presence of untreated or severe OSAS. The recent clinical practice guideline of the Italian Society of Andrology and Sexual Medicine (SIAMS) and the Italian Society of Endocrinology (SIE), regarding adult and late-onset hypogonadism, suggests not considering a treated OSAS as an absolute contraindication for TRT, albeit considering the lack of data regarding the role of TRT in men with OSAS (13). Other guidelines, surprisingly, such as the Society for Endocrinology guidelines for TRT in male hypogonadism, do not even mention OSAS among contraindication of TRT (43) whilst the Endocrine Society Clinical Practice Guideline (40) recommends against starting TRT in patients with untreated severe OSAS and considers the presence of the induction or worsening of obstructive sleep apnoea as an uncommon adverse with a weak association with TRT. Furthermore, a recent systematic review, conducted by Twitchell et al. (44) and evaluating the controversies about TRT, reported a positive association between TRT and OSAS. In addition, the European Academy of Andrology (EAA) guidelines on investigation, treatment and monitoring of functional hypogonadism in males does not consider the presence of OSAS as a contraindication for TRT but suggests evaluating the patient for hypoxia and sleep apnoea development during TRT (45). There are a variety of possible pathophysiological mechanisms by which TRT might exacerbate OSAS. TRT has been proposed to alter the central chemoreceptors (42, 46), therefore contributing to the development or worsening of OSAS via central mechanisms (26). A promising theory focuses on the role of androgens in neural response pathways to hypoxemia, but, according to the most recent data, the administration of testosterone has variable effects on ventilatory chemo-responsiveness and single unifying conclusion has not been reached yet. Notwithstanding, the evidence suggests that TRT might alter chemoreceptor stimulation thresholds or the ventilatory response to chemoreceptor stimulation by decreasing breathing stability (40).
One of the most debated parameters that seems to worsen after TRT is the morphological change in the upper airway. In particular, testosterone seems to impact the contraction of the airway dilator muscles and the following airway collapsibility. However, a consensus has not been found yet (26). A further connection between TRT and OSAS resides in the risk of polycythaemia. In fact, OSAS is a risk factor for secondary polycythaemia and TRT exacerbates polycythaemia in some patients (39, 40, 44). Furthermore, TRT may also induce higher metabolic rates, resulting in greater oxygen consumption leading to hypoxia (26). Finally, another possible explanation regarding the worsening of OSAS during TRT deals with a possible role of testosterone on the alteration of ventilatory response to hypoxia and hypercapnia, the alteration of serotonergic pathway and the reduction in time slept (26, 35).
Although the possible risks of TRT in subjects with OSAS are far to be clearly elucidated, it is important to note the benefit of restoring normal testosterone concentrations in men with hypogonadism. TRT is suggested in hypogonadal men for its clinical benefits regarding, among others, cardiometabolic risk, sexual function, bone metabolism and body composition (43, 47). Moreover, a recent clinical study (48) reported an improvement in sleep disturbance [defined as three or more points in question 4 of the ageing male symptoms (AMS) questionnaire] after 1 year of TRT in male affected by MH without OSAS. Therefore, while caution in TRT prescription should be maintained in patients with OSAS (38), a balanced and personalised counselling between risks and benefits should be discussed with the patients, taking also in consideration the possible co-morbidities and concomitant therapies. To this regard, it seems reasonable to delay TRT in hypogonadal men affected by untreated OSAS or severe OSAS. This review highlights also that modulation of type and dosage of TRT in the different patients is advisable. For example, it seems that short-term high-dose TRT could worsen OSAS, but these adverse effects could disappear with time (13, 42) and eventually determinate a clinical improvement of OSAS’ symptoms after 1 year of TRT (48). Therefore, a low dose TRT in the form of transdermal gel, which is more malleable if the clinical condition worsens, seems preferable. Finally, we suggest considering the assessment by polysomnography in patients who develop signs and symptoms of OSAS during TRT, with possible reduction or discontinuation of TRT if necessary.
Further considerations are necessary in this context. The severity of OSAS seems related to bone mineral density (BMD), as a recent prospective case–control study, enrolling 93 individuals (59 with OSAS and 34 as controls), reported lower BMD and vitamin D in patients with OSAS than controls, with a negative correlation between AHI and BMD (49). The risk of fracture and osteoporosis appears to be increased in patients with OSAS, probably due to different mechanisms, such as hypoxia, OSAS-related respiratory acidosis, leptin, OSAS-related comorbidities and, of course, hypogonadism (49). The assessment of bone status may become, in future, a parameter to evaluate when considering TRT in patients affected by OSAS, since the well-known positive effects of TRT in improving the bone status in hypogonadal patients (13, 50). On the other hand, the therapy of OSAS may improve the gonadal status and sexual functions. In fact, OSAS-related sleep fragmentation might disrupt the testosterone rhythm, with noteworthy attenuation of the nocturnal increase in testosterone (26). Studies showed a linear association between weight loss and increased serum testosterone concentrations in obese men (26). Few studies showed that the treatment of OSAS—both by surgery (51) and cPAP (52)—improve the gonadal function and testosterone concentrations. Nevertheless, other studies reported opposite results (26, 32, 46). In particular, a recent systematic review and meta-analysis (53) found that the cPAP use was not associated with a significant change in total testosterone concentrations, suggesting against the hypothesis of a direct interaction between OSAS and testosterone, albeit the conclusion that cPAP has no effect on testosterone concentrations is highly premature due to the low-quality available studies (54).
In addition, OSAS has been associated with altered HPG function and sexual dysfunction, manifested primarily as erectile dysfunction and decreased libido (22). Sexual functions of patients with OSAS and MH are thought to improve upon TRT, albeit this result should be confirmed in larger studies (37, 55). On the other hand, the therapy of OSAS might improve the OSAS-related erectile and sexual dysfunctions (26, 46). Finally, there are new concerns regarding testosterone hormone therapy in transgender males (assigned females at birth). In particular, Robertson et al. (56) reported two cases of transgender males in whom OSAS developed following initiation of testosterone therapy, with documented absence of OSAS before the sex affirming therapy.
Perspectives and clinical messages
This manuscript underlines the need of an active collaboration between different specialists, including endocrinologists, general physicians, obesity physicians, otolaryngologists, pneumologist and sleep specialists. Furthermore, in the light of the evidence that OSAS represents a complex clinical condition—and not just a sleep disorder—and that nowadays multidisciplinary groups are created in order to offer a better care for complex patients, a multidisciplinary evaluation for patients with OSAS might be evaluated and even become mandatory in patients with OSAS and other complex diseases. Regarding the gonadal evaluation in patients with OSAS, albeit sleep disorders are not considered to be main signs/symptoms of hypogonadism (13), we would like to share the interplay between signs/symptoms that might be associated with both OSAS and MH, such as sexual dysfunction, low BMD, low motivation and vitality, poor concentration and memory, and even fatigue. Therefore, albeit for further studies are needed, a complete endocrine and metabolic evaluation in patients with OSAS might be suggested, in order to (i) evaluated endocrine-metabolic alterations caused by OSAS itself and (ii) evaluated possible endocrine-metabolic underlying disorders that might be even the cause of OSAS.
Conclusion
In this review, we described the relation between OSAS and MH, which are two under-recognized and inter-connected medical disorders, with particular attention to TRT and OSAS. The analysis of the literature highlights the general lack of well-done studies and therefore the evidence is of low quality. We cannot confirm or reject the guidelines that suggest avoiding TRT in the presence of untreated or severe OSAS, as this appears a reasonable clinical practice. Indeed, some evidence suggests that short-time high-dose TRT might indeed worsen OSAS, whereas chronic low-dose TRT might improve OSAS. Notwithstanding, the therapy of OSAS might benefit the gonadal status and sexual functions. In the light of the few and sometimes inconclusive studies in this field, and therefore of the limited evidence and large grey areas, we wish for further studies and more active collaboration between endocrinologists and otolaryngologists.
Author contributions
AG contributed to research design, bibliographic analysis and acquisition of data, analysis and interpretation of the data, and wrote the paper. GG contributed to interpretation of the data, wrote and revised the manuscript critically. AF contributed to research design, interpretation of the data, revised the manuscript critically, and supervised the study. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lee JJ, Sundar KM. Evaluation and management of adults with obstructive sleep apnea syndrome. Lung. (2021) 199:87–101. doi: 10.1007/s00408-021-00426-w
2. Singh P, Bonitati A. Obstructive sleep apnea syndrome—a review for primary care physicians and pulmonologists. R I Med J. (2021) 104:10–3. PMID: 34437659.
3. Yeghiazarians Y, Jneid H, Tietjens JR, Redline S, Brown DL, El-Sherif N, et al. Obstructive sleep apnea and cardiovascular disease: a scientific statement from the American heart association. Circulation. (2021) 144. doi: 10.1161/CIR.0000000000000988
4. Koka V, De Vito A, Roisman G, Petitjean M, Filograna Pignatelli GR, Padovani D, et al. Orofacial myofunctional therapy in obstructive sleep apnea syndrome: a pathophysiological perspective. Medicina. (2021) 57:323. doi: 10.3390/medicina57040323
5. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. (2013) 177:1006–14. doi: 10.1093/aje/kws342
6. Liu X, Ma Y, Ouyang R, Zeng Z, Zhan Z, Lu H, et al. The relationship between inflammation and neurocognitive dysfunction in obstructive sleep apnea syndrome. J Neuroinflammation. (2020) 17:229. doi: 10.1186/s12974-020-01905-2
7. Pollis M, Lobbezoo F, Aarab G, Ferrari M, Marchese-Ragona R, Manfredini D. Correlation between apnea severity and sagittal cephalometric features in a population of patients with polysomnographically diagnosed obstructive sleep apnea. J Clin Med. (2022) 11:4572. doi: 10.3390/jcm11154572
8. Antonaglia C, Passuti G. Obstructive sleep apnea syndrome in non-obese patients. Sleep Breath. (2022) 26:513–8. doi: 10.1007/s11325-021-02412-1
9. Santilli M, Manciocchi E, D’Addazio G, Di Maria E, D’Attilio M, Femminella B, et al. Prevalence of obstructive sleep apnea syndrome: a single-center retrospective study. Int J Environ Res Public Health. (2021) 18:10277. doi: 10.3390/ijerph181910277
10. Dauvilliers Y, Verbraecken J, Partinen M, Hedner J, Saaresranta T, Georgiev O, et al. Pitolisant for daytime sleepiness in patients with obstructive sleep apnea who refuse continuous positive airway pressure treatment. A randomized trial. Am J Respir Crit Care Med. (2020) 201:1135–45. doi: 10.1164/rccm.201907-1284OC
11. Duca Y, Aversa A, Condorelli RA, Calogero AE, La Vignera S. Substance abuse and male hypogonadism. J Clin Med. (2019) 8:732. doi: 10.3390/jcm8050732
12. Grinspon RP, Bergadá I, Rey RA. Male hypogonadism and disorders of sex development. Front Endocrinol. (2020) 11. doi: 10.3389/fendo.2020.00211
13. Isidori AM, Aversa A, Calogero A, Ferlin A, Francavilla S, Lanfranco F, et al. Adult- and late-onset male hypogonadism: the clinical practice guidelines of the Italian Society of Andrology and Sexual Medicine (SIAMS) and the Italian Society of Endocrinology (SIE). J Endocrinol Invest. (2022) 45:2385–403. doi: 10.1007/s40618-022-01859-7
14. Sizar O, Schwartz J. Hypogonadism. In: Dulebohn S, editor. StatPearls. Treasure Island (FL): StatPearls (2022).
15. Lotti F, Marchiani S, Corona G, Maggi M. Metabolic syndrome and reproduction. Int J Mol Sci. (2021) 22:1988. doi: 10.3390/ijms22041988
16. Louters M, Pearlman M, Solsrud E, Pearlman A. Functional hypogonadism among patients with obesity, diabetes, and metabolic syndrome. Int J Impot Res. (2022) 34(7):714–20. doi: 10.1038/s41443-021-00496-7
17. Lamm S, Chidakel A, Bansal R. Obesity and hypogonadism. Urol Clin N Am. (2016) 43:239–45. doi: 10.1016/j.ucl.2016.01.005
18. Wang H, Lu J, Xu L, Yang Y, Meng Y, Li Y, et al. Obstructive sleep apnea and serum total testosterone: a system review and meta-analysis. Sleep Breath. (2023) 27(3):789–97. doi: 10.1007/s11325-022-02655-6
19. Su L, Meng Y, Zhang S, Cao Y, Zhu J, Qu H, et al. Association between obstructive sleep apnea and male serum testosterone: a systematic review and meta-analysis. Andrology. (2022) 10:223–31. doi: 10.1111/andr.13111
20. Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea. J Am Coll Cardiol. (2013) 62:569–76. doi: 10.1016/j.jacc.2013.05.045
21. Kuvat N, Tanriverdi H, Armutcu F. The relationship between obstructive sleep apnea syndrome and obesity: a new perspective on the pathogenesis in terms of organ crosstalk. Clin Respir J. (2020) 14:595–604. doi: 10.1111/crj.13175
22. Grossmann M. Hypogonadism and male obesity: focus on unresolved questions. Clin Endocrinol. (2018) 89:11–21. doi: 10.1111/cen.13723
23. Genchi VA, Rossi E, Lauriola C, D’Oria R, Palma G, Borrelli A, et al. Adipose tissue dysfunction and obesity-related male hypogonadism. Int J Mol Sci. (2022) 23:8194. doi: 10.3390/ijms23158194
24. Molina-Vega M, Muñoz-Garach A, Damas-Fuentes M, Fernández-García J, Tinahones F. Secondary male hypogonadism: a prevalent but overlooked comorbidity of obesity. Asian J Androl. (2018) 20:531. doi: 10.4103/aja.aja_44_18
25. Killick R, Wang D, Hoyos CM, Yee BJ, Grunstein RR, Liu PY. The effects of testosterone on ventilatory responses in men with obstructive sleep apnea: a randomised, placebo-controlled trial. J Sleep Res. (2013) 22:331–6. doi: 10.1111/jsr.12027
26. Kim S-D, Cho K-S. Obstructive sleep apnea and testosterone deficiency. World J Mens Health. (2019) 37:12. doi: 10.5534/wjmh.180017
27. Luboshitzky R, Aviv A, Hefetz A, Herer P, Shen-Orr Z, Lavie L, et al. Decreased pituitary-gonadal secretion in men with obstructive sleep apnea. J Clin Endocrinol Metab. (2002) 87:3394–8. doi: 10.1210/jcem.87.7.8663
28. Molina FD, Suman M, de Carvalho TBO, Piatto VB, Taboga SR, Maniglia JV, et al. Avaliação dos níveis séricos de testosterona em pacientes com síndrome da apneia obstrutiva do sono. Braz J Otorhinolaryngol. (2011) 77:88–95. doi: 10.1590/S1808-86942011000100015
29. Wittert G. The relationship between sleep disorders and testosterone. Curr Opin Endocrinol Diabetes Obes. (2014) 21:239–43. doi: 10.1097/MED.0000000000000069
30. Fernandez CJ, Chacko EC, Pappachan JM. Male obesity-related secondary hypogonadism—pathophysiology, clinical implications and management. Eur Endocrinol. (2019) 15:83. doi: 10.17925/EE.2019.15.2.83
31. Gambineri A, Pelusi C, Pasquali R. Testosterone levels in obese male patients with obstructive sleep apnea syndrome: relation to oxygen desaturation, body weight, fat distribution and the metabolic parameters. J Endocrinol Invest. (2003) 26:493–8. doi: 10.1007/BF03345209
32. Tančić-Gajić M, Vukčević M, Ivović M, Marina LV, Arizanović Z, Soldatović I, et al. Obstructive sleep apnea is associated with low testosterone levels in severely obese men. Front Endocrinol. (2021) 12. doi: 10.3389/fendo.2021.622496
33. Barrett-Connor E, Dam T-T, Stone K, Harrison SL, Redline S, Orwoll E. The association of testosterone levels with overall sleep quality, sleep architecture, and sleep-disordered breathing. J Clin Endocrinol Metab. (2008) 93:2602–9. doi: 10.1210/jc.2007-2622
34. Millman RP, Kimmel PL, Shore ET, Wasserstein AG. Sleep apnea in hemodialysis patients: the lack of testosterone effect on its pathogenesis. Nephron. (1985) 40:407–10. doi: 10.1159/000183509
35. Liu PY, Yee B, Wishart SM, Jimenez M, Jung DG, Grunstein RR, et al. The short-term effects of high-dose testosterone on sleep, breathing, and function in older men. J Clin Endocrinol Metab. (2003) 88:3605–13. doi: 10.1210/jc.2003-030236
36. Hoyos CM, Killick R, Yee BJ, Grunstein RR, Liu PY. Effects of testosterone therapy on sleep and breathing in obese men with severe obstructive sleep apnoea: a randomized placebo-controlled trial. Clin Endocrinol. (2012) 77:599–607. doi: 10.1111/j.1365-2265.2012.04413.x
37. Melehan KL, Hoyos CM, Yee BJ, Wong KK, Buchanan PR, Grunstein RR, et al. Increased sexual desire with exogenous testosterone administration in men with obstructive sleep apnea: a randomized placebo-controlled study. Andrology. (2016) 4:55–61. doi: 10.1111/andr.12132
38. Cole AP, Hanske J, Jiang W, Kwon NK, Lipsitz SR, Kathrins M, et al. Impact of testosterone replacement therapy on thromboembolism, heart disease and obstructive sleep apnoea in men. BJU Int. (2018) 121:811–8. doi: 10.1111/bju.14149
39. Lundy SD, Parekh NV, Shoskes DA. Obstructive sleep apnea is associated with polycythemia in hypogonadal men on testosterone replacement therapy. J Sex Med. (2020) 17:1297–303. doi: 10.1016/j.jsxm.2020.03.006
40. Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, et al. Testosterone therapy in men with hypogonadism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2018) 103:1715–44. doi: 10.1210/jc.2018-00229
41. Hanafy HM. Testosterone therapy and obstructive sleep apnea: is there a real connection? J Sex Med. (2007) 4:1241–6. doi: 10.1111/j.1743-6109.2007.00553.x
42. Payne K, Lipshultz LI, Hotaling JM, Pastuszak AW. Obstructive sleep apnea and testosterone therapy. Sex Med Rev. (2021) 9:296–303. doi: 10.1016/j.sxmr.2020.04.004
43. Jayasena CN, Anderson RA, Llahana S, Barth JH, MacKenzie F, Wilkes S, et al. Society for endocrinology guidelines for testosterone replacement therapy in male hypogonadism. Clin Endocrinol. (2022) 96:200–19. doi: 10.1111/cen.14633
44. Twitchell DK, Pastuszak AW, Khera M. Controversies in testosterone therapy. Sex Med Rev. (2021) 9:149–59. doi: 10.1016/j.sxmr.2020.09.004
45. Corona G, Goulis DG, Huhtaniemi I, Zitzmann M, Toppari J, Forti G, et al. European Academy of Andrology (EAA) guidelines on investigation, treatment and monitoring of functional hypogonadism in males. Andrology. (2020) 8:970–87. doi: 10.1111/andr.12770
46. Burschtin O, Wang J. Testosterone deficiency and sleep apnea. Sleep Med Clin. (2016) 11:525–9. doi: 10.1016/j.jsmc.2016.08.003
47. Thirumalai A, Berkseth KE, Amory JK. Treatment of hypogonadism: current and future therapies. F1000Res. (2017) 6:68. doi: 10.12688/f1000research.10102.1
48. Shigehara K, Konaka H, Sugimoto K, Nohara T, Izumi K, Kadono Y, et al. Sleep disturbance as a clinical sign for severe hypogonadism: efficacy of testosterone replacement therapy on sleep disturbance among hypogonadal men without obstructive sleep apnea. Aging Male. (2018) 21:99–105. doi: 10.1080/13685538.2017.1378320
49. Sadaf S, Shameem M, Siddiqi SS, Anwar S, Mohd S. Effect of obstructive sleep apnea on bone mineral density. Turk Thorac J. (2021) 22:301–10. doi: 10.5152/TurkThoracJ.2021.20051
50. Corona G, Vena W, Pizzocaro A, Giagulli VA, Francomano D, Rastrelli G, et al. Testosterone supplementation and bone parameters: a systematic review and meta-analysis study. J Endocrinol Invest. (2022) 45:911–26. doi: 10.1007/s40618-021-01702-5
51. Santamaria JD, Prior JC, Fleetham JA. Reversible reproductive dysfunction in men with obstructive sleep apnoea. Clin Endocrinol. (1988) 28:461–70. doi: 10.1111/j.1365-2265.1988.tb03680.x
52. Grunstein RR, Handelsman DJ, Lawrence SJ, Blackwell C, Caterson ID, Sullivan CE. Neuroendocrine dysfunction in sleep apnea: reversal by continuous positive airways pressure therapy. J Clin Endocrinol Metab. (1989) 68:352–8. doi: 10.1210/jcem-68-2-352
53. Cignarelli A, Castellana M, Castellana G, Perrini S, Brescia F, Natalicchio A, et al. Effects of CPAP on testosterone levels in patients with obstructive sleep apnea: a meta-analysis study. Front Endocrinol. (2019) 10. doi: 10.3389/fendo.2019.00551
54. Liu PY, Reddy RT. Sleep, testosterone and cortisol balance, and ageing men. Rev Endocr Metab Disord. (2022) 23:1323–39. doi: 10.1007/s11154-022-09755-4
55. Zhuravlev VN, Frank MA, Gomzhin AI. Sexual functions of men with obstructive sleep apnoea syndrome and hypogonadism may improve upon testosterone administration: a pilot study. Andrologia. (2009) 41:193–5. doi: 10.1111/j.1439-0272.2008.00914.x
Keywords: OSAS, hypogonadism, testosterone, testosterone replacement therapy, obesity
Citation: Graziani A, Grande G and Ferlin A (2023) The complex relation between obstructive sleep apnoea syndrome, hypogonadism and testosterone replacement therapy. Front. Reprod. Health 5:1219239. doi: 10.3389/frph.2023.1219239
Received: 8 May 2023; Accepted: 22 August 2023;
Published: 10 October 2023.
Edited by:
Peter Natesan Pushparaj, King Abdulaziz University, Saudi ArabiaReviewed by:
Andrea Delbarba, Asst degli Spedali Civili di Brescia, ItalyPoonam Mehta, Central Drug Research Institute (CSIR), India
© 2023 Graziani, Grande and Ferlin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alberto Ferlin YWxiZXJ0by5mZXJsaW5AdW5pcGQuaXQ=
†ORCID Giuseppe Grande orcid.org/0000-0003-3264-0937 Alberto Ferlin orcid.org/0000-0001-5817-8141
 Andrea Graziani
Andrea Graziani Giuseppe Grande
Giuseppe Grande Alberto Ferlin
Alberto Ferlin