- 1Gynaecology Department, Department Women-Mother-Child, Lausanne University Hospital (CHUV), Lausanne, Switzerland
- 2Institute of Pathology, Lausanne University Hospital (CHUV), Lausanne, Switzerland
- 3Institut de Recherche Expérimentale et Clinique (IREC), Université Catholique de Louvain, Brussels, Belgium
- 4Department of Obstetrics and Gynaecology, HFR Fribourg Hôpital Cantonal, Fribourg, Switzerland
- 5Department of Obstetrics and Gynaecology, University Hospital of Berne and University of Berne, Berne, Switzerland
- 6Department of Diagnostic and Interventional Radiology, Lausanne University Hospital (CHUV), Lausanne, Switzerland
- 7Faculty of Biology and Medicine (FBM), University of Lausanne, Lausanne, Switzerland
Objectives: The purpose of this study is to report nine patients of young women who underwent a surgical treatment of an accessory and cavitated uterine mass (ACUM) in our hospital between 2014 and 2022 and review all cases described in the literature.
Material and methods: The principal outcomes measured are the imaging techniques used to determine the diagnosis, the type of surgery used and the post-operative evolution of symptoms. We also report and analyse the 79 patients found in the literature since 1996 in addition to our 9 patients.
Results: Surgical excision is the only long-lasting treatment. Small invasive surgery with laparoscopic access is the gold standard and most widely used (83.0%). Some new therapeutic procedures have been recently described of which ethanol sclerotherapy seems very promising. Post-operatively, 54.5% of patients have a complete relief of symptoms. MRI is the best imaging technique to identify ACUM. Finally, we refine the description of this pathology and give a more precise definition of it.
Conclusion: Through our literature review and the analysis of our cases, we want to underline an important diagnostic criterion of this pathology: the fallopian tube on the homolateral side of the ACUM never communicates with the latter. It is a capital element for differential diagnosis.
1. Introduction
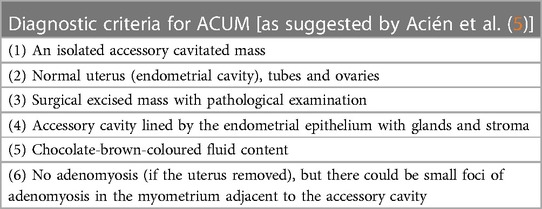
Accessory and cavitated uterine mass (ACUM) is a rare Müllerian duct anomaly of unknown incidence, which affects young women. Since its first description by Cullen in 1908 (1), different terminologies have been used to describe the same entity: juvenile or isolated cystic adenomyoma (2), uterus-like mass or accessory uterine cavity (3) and adenomyotic cyst or cystic adenomyosis (4). In 2010 Acién et al. (5) suggested the term accessory and cavitated uterine mass as a new terminology and defined it by the presence of a non-communicating accessory uterine mass located in the myometrium or within the broad ligament, close to the round ligament insertion, with an otherwise normal genital and urinary tract (3, 5). A list of the diagnostic criteria for ACUM as suggested by Acién et al. is presented in Table 1.
While most clinical manifestations for ACUM are non-specific, dysmenorrhea, which ranges from mild to severe, is reported as being the most common symptom. It typically starts soon after menarche and rapidly increases in severity thereafter. Chronic pelvic pain (CPP) and dysfunctional uterine bleeding are also frequent.
ACUM symptoms, such as dysmenorrhea and CPP, are often primarily or secondarily resistant to common analgesics and to classical hormonal treatment as progestogen-only pill (POP), combined oral contraceptive pill (COC) or gonadotropin-releasing hormone agonist (GnRH agonist), as it is the case with endometriosis.
According to Acién and his group, this anomaly required a separate classification and definition from the ESHRE 2013 consensus on congenital malformations of the female genital tract (6) as it does not include this anomaly. At the time of writing, it is considered as part of the unclassified uterine malformations (U6 class).
In their opinion, the origin of this uterine anomaly could be caused by a gubernaculum dysfunction during the embryogenesis expressed through a duplication and persistence of the ductal Müllerian tissue at the attachment level of the round ligament (7).
Our study objectives are (i) to describe nine new patients that we operated, (ii) to do a literature review starting from 1996 and (iii) to analyse and describe this rare pathology as precisely as possible in order to help with the differential diagnosis.
2. Materials and methods
We report on nine patients with ACUM treated in Lausanne in Switzerland. All of the patients gave their written consent for the care provided. The study was retrospective, based on medical file analysis, and the standard treatment for this pathology was performed. The written informed consent was obtained from the individuals’ and minors’ legal guardian for the publication of any potentially identifiable images or data included in this article.
For histological analysis, specimens were fixed in 10% neutral-buffered formalin (6–72 h). Formalin-fixed paraffin-embedded samples from specimens were stained with haematoxylin and eosin (HE) (Ventana HE 600 system). Immunohistochemistry (IHC) was performed with an anti-CD10 (56C6, mouse monoclonal, Ventana) antibody using the Ventana BenchMark automated stainer and revealed by the ultraView DAB detection kit (ref. 760-500).
Our literature review aimed to identify all reported cases of this pathology. The following terms were used to search the Medline database using PubMed: juvenile cystic adenomyoma (JCA), uterus-like mass, accessory uterine cavity, adenomyotic cyst, cystic adenomyosis and ACUM. Only the cases corresponding to Acién et al.'s diagnostic criteria of ACUM (5) were included. We found a total of 79 patients between 1996 and April 2020 to which we add our 9 patients. All authors declare no conflict of interest.
3. Results
3.1. Nine case descriptions
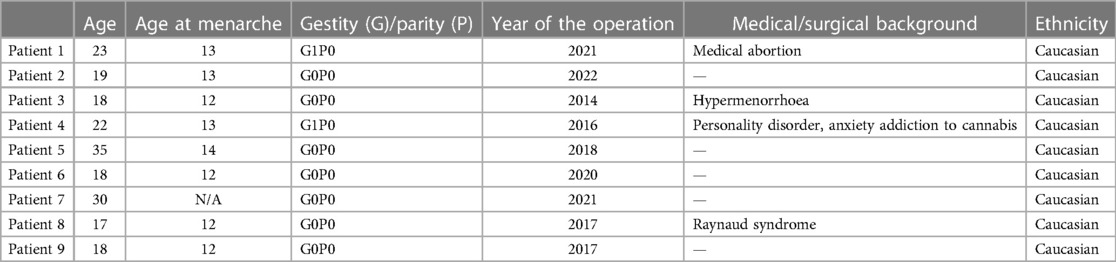
Nine patients who presented with ACUM were operated in our clinic between 2014 and 2022. Their characteristics are described in Table 2. The average age at the time of surgery was 22 years (range 17–35 years).
Severe dysmenorrhea (n = 5) and CPP (n = 4) were the most common presenting symptoms. As part of the clinical workup, the patients first underwent a pelvic ultrasound (Figure 1). A single lateralized intra-myometrial accessory cavity located under the insertion of the round ligament was found in all patients. The capsule of the lesion had the same echogenicity as the normal myometrium, and the content appeared as hypoechogenic.
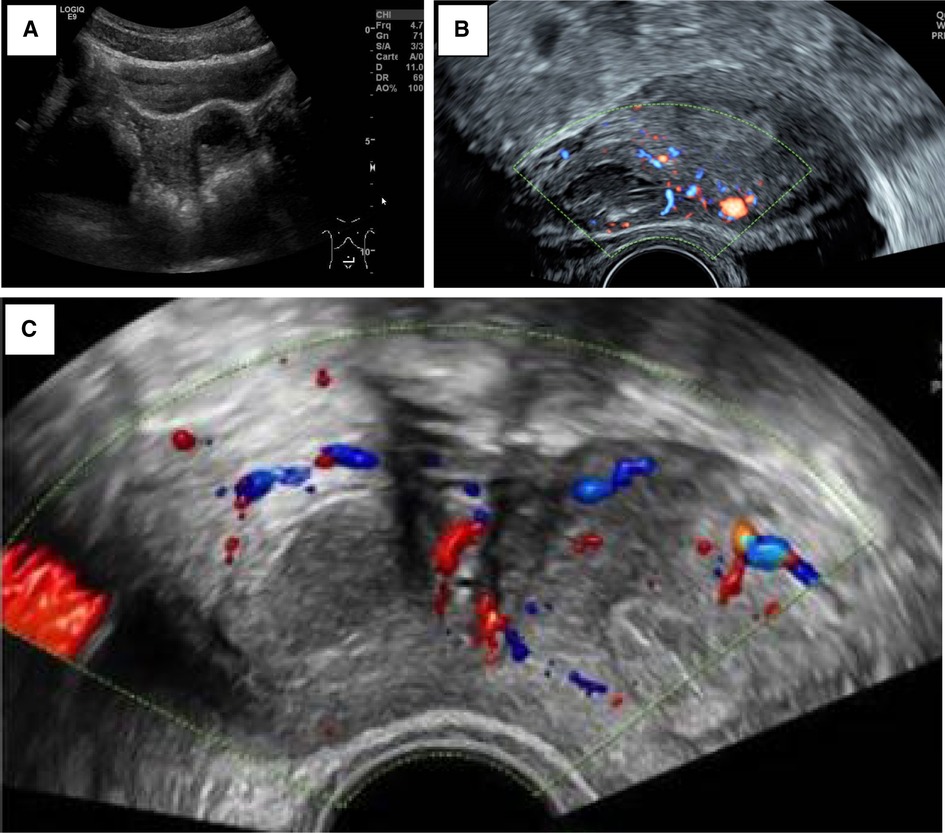
Figure 1. (A) Patient 3_TAUS shows a left antero-fundic sub-serous mass of 3.8 cm × 3.6 cm. (B) Patient 4_axial plan of TVUS showing the right-lateralized mass separated from the normal uterine cavity by a thick myometrial wall. (C) Patient 5_axial echography showing a round right-lateralized mass with hypoechogenic content surrounded by a ring-shaped vascularized capsule.
In addition to an ultrasound, all patients in our series underwent an MRI in order to have a precise description of the lesion (Figure 2). The lesion always had the same characteristics: the mass was isolated and composed of an external thick ring which had the same signal intensity as the junctional zone and regular boundaries. Its contents had a spontaneously hyper-intense signal on T1, T1 fat sat and T2-weighted images speaking for a haemorrhagic material. The rest of the genital and urinary tract was normal across all nine patients.
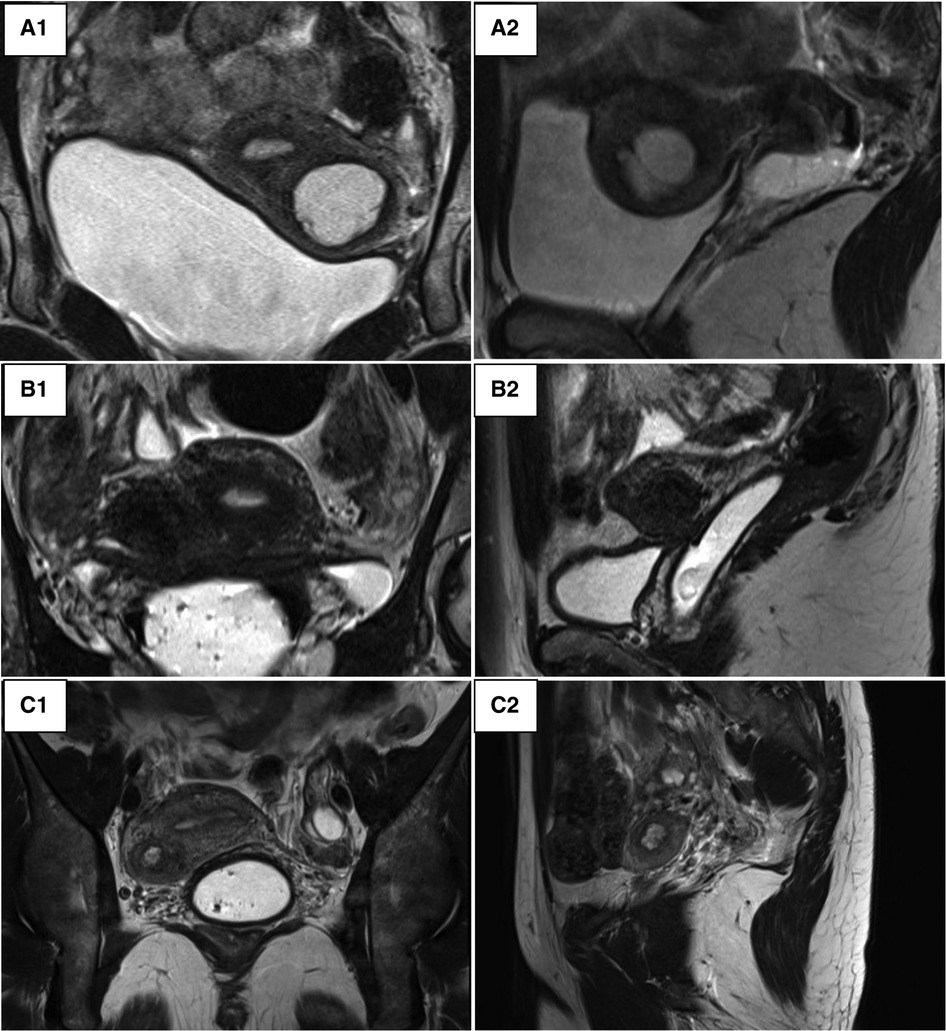
Figure 2. Pelvic MRI. (A) Patient 3_round mass in the left anterior myometrial wall suggestive of an accessory endometrial cavity within. (A1) T2-weighted coronal image. (A2) T2-weighted left lateral sagittal cut. (B) Patient 4_a round mass in the right anterior myometrial wall. (B1) T2-weighted coronal image. (B2) T2-weighted right lateral sagittal cut. (C) Patient 5_round mass in the right anterior myometrial wall. (C1) T2-weighted coronal image. (C2) T2-weighted right lateral sagittal cut.
The same laparoscopic resection technique was used by four surgeons on all patients (Figure 3). Eight were performed by standard laparoscopy, whereas one of them was performed by a robotic-assisted approach. For the standard laparoscopies, we did a four-trocar approach. The upper abdomen, ovaries and fallopian tubes were macroscopically unremarkable in every patient. The uteruses were deformed by a mass bulging into their anterior part under the insertion of the round ligament. An incision was performed over the swelling zone on the uterus in order to remove the lesion. The progressive dissection around the mass was difficult due to the absence of a correct dissection plan. The average operative time was 128 min (range 80–240 min). No uterine cavity was opened during the procedures. No intraoperative or post-operative complication occurred except for one patient where a fundal uterine perforation by the manipulator occurred. After surgery, the patients were discharged between day 1 and day 3. In all patients, microscopic examination showed a cystic cavity lined by thin endometrium lining and stroma (Figure 4). The myometrial capsule contained small adenomyotic foci. Complementary IHC analysis was performed in patient number 5 to help for diagnosis. Anatomopathology confirmed the initial diagnoses of ACUM in all nine patients.
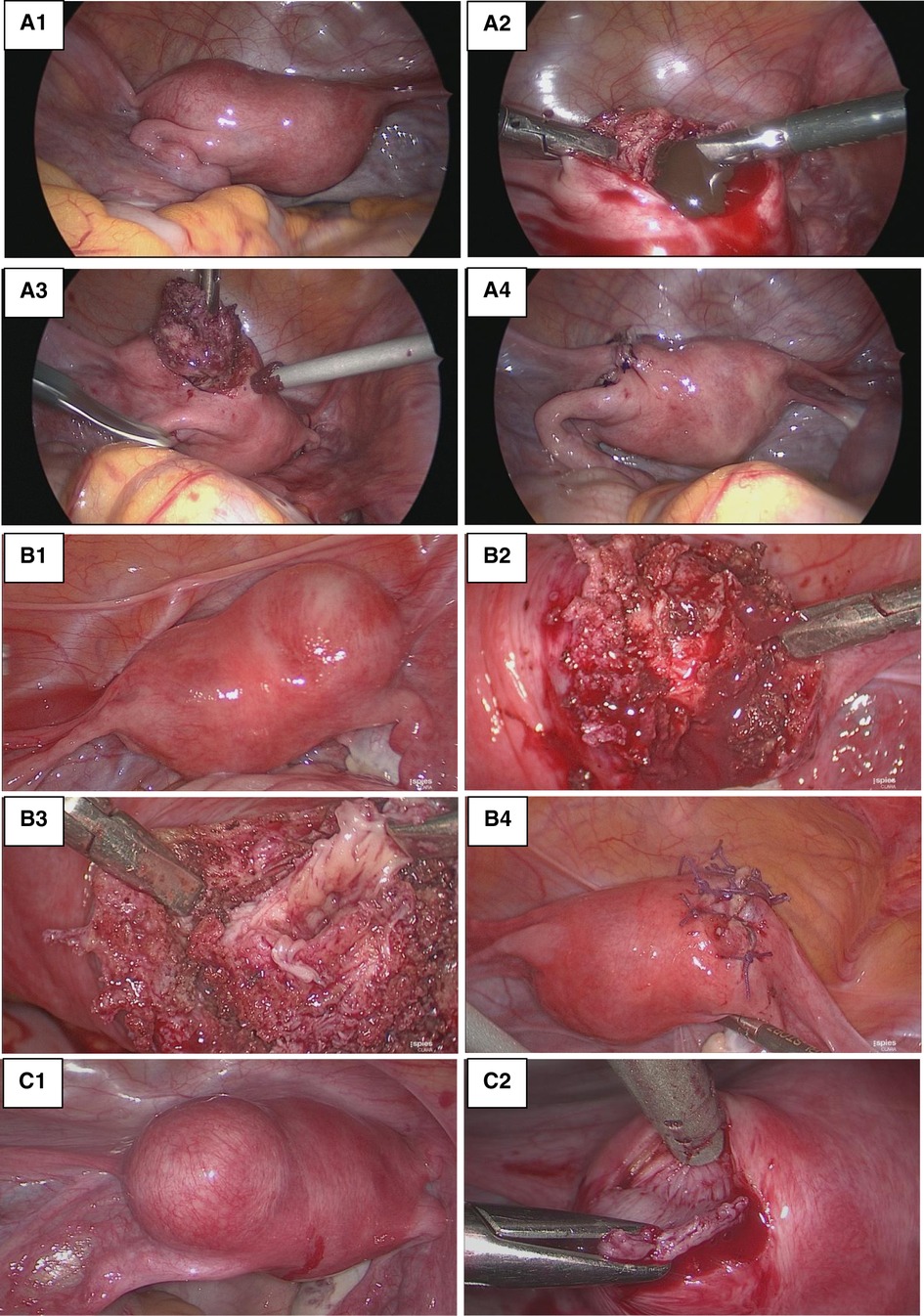
Figure 3. Laparoscopic resection. (A) Patient 3_(A1) Uterine left-sided mass bulging into the anterior part of the broad ligament under the insertion of the round ligament. (A2) Incision of the mass draining chocolate-brown fluid. (A3) Excision of the lesion wall. (A4) Myometrial defect sutured. (B) Patient 5: (B1) Right ACUM. (B2) Incision of the mass. (B3) Excision of the cyst wall with a view of the cystic cavity. (B4) Myometrial defect sutured. (C) Patient 6: (C1) Left ACUM. (C2) Excision of the lesions wall.
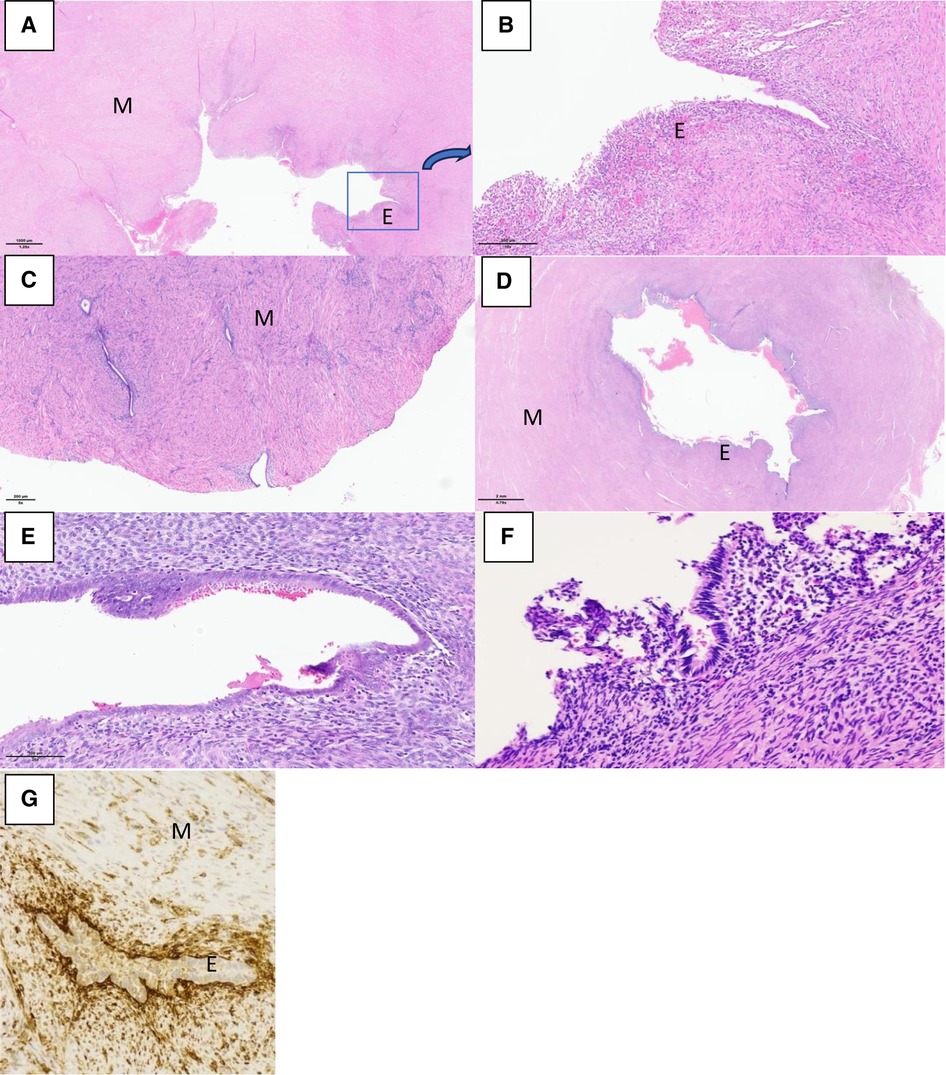
Figure 4. Histological sections with haematoxylin and eosin (HE) stain and immunohistological section showing the ACUM with endometrial epithelium “E”, surrounded by myometrium “M”. (A) Patient 1_HE × 1.25. (B) Patient 1_HE × 10, zooming in on the highlighted as found in exhibit A. (C) Patient 2_HE × 5. (D) Patient 3_HE × 0.79. (E) Patient 3_HE × 20. (F) Patient 5_HE × 5. (G) Patient 5_CD10 × 200 immunohistochemistry positivity confirming the presence of endometrial stroma.
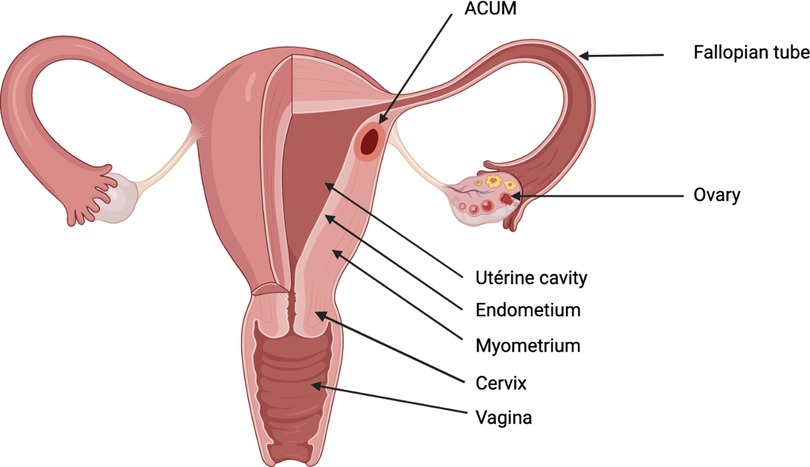
The schematic representation of the location of an ACUM in the reproductive tract is shown in Figure 5 (created with BioRender.com).
3.2. Literature review
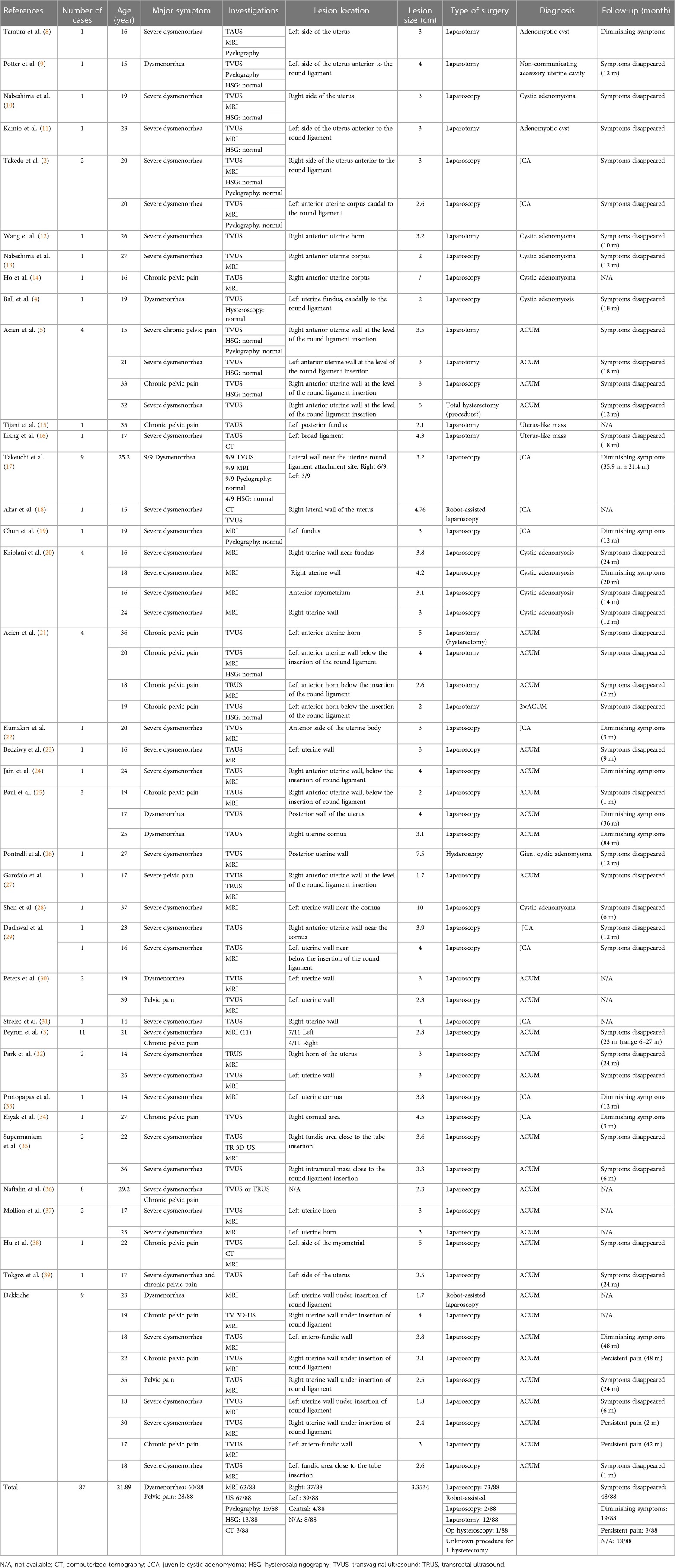
The characteristics of the 79 patients retrieved from the literature and our nine patients are presented in Table 3. The mean age at diagnosis is 21.9 years (range 14–39 years).
The clinical manifestations are always some form of pelvic pain; dysmenorrhea is the most prevalent symptom (68.2%), associated or not with CPP (31.8%).
The two most useful radiological procedures are 2D ultrasound and MRI. The latter was performed for 70.5% of the patients.
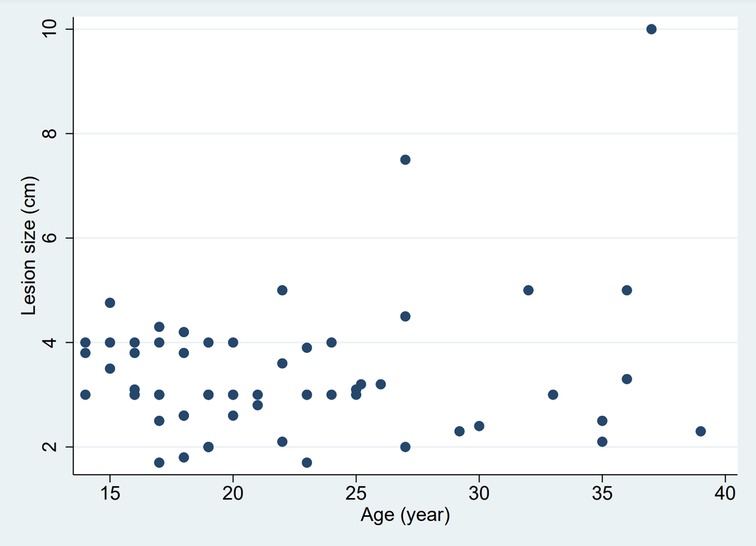
Usually, the mass is unique, but in rare cases, it can also be biloculated [3/88, 3.4% (26, 40)]. The lesion was lateralized 86% of the time, 42.0% right, 44.3% left, and astonishingly 4.5% were central. The mean size of the lesion was 3.4 cm. No relation between the variables “age” and “size of the lesion” was noted as shown in Figure 6. Linear regression analysis also found no relation between these two variables (R-squared = 0.03, p-value = 0.14).
Surgical resection was in 83.0% of the patients performed by laparoscopy which should be the privileged approach, in 13.6% of patients by laparotomy, in 2.3% of patients by robot-assisted laparoscopy and in 1.1% of patients by operative hysteroscopy. Clinical improvement occurred in almost all patients after surgical resection, except for a few patients (n = 3). To this day, no other aetiology was found for these three patients presenting persistent pain (endometriosis was excluded during laparoscopy). They are treated with conservative medical treatment.
4. Discussion
We consider Acién et al.'s physiopathologic hypothesis and their definition of this anomaly as the most appropriate for now and therefore decided to adopt the terminology and concept of ACUM. As opposed to some authors who consider this pathology as a focal or cystic form of adenomyosis, we do not, mainly because of its absence of recurrence, the young age of the affected patients and its pathological characteristics (point 4 of Acién's definition) which are clearly different from adenomyosis.
Regarding the age at diagnosis, which is considered for Takeuchi et al. in 2010 (17) as a diagnostic criterion when under 30, we would not be that restrictive. All the more since this diagnosis is often delayed after months or years of investigations or symptomatic treatments such as pain killers or hormonal treatments.
We want to insist on an important characteristic of an ACUM, which may not be clear enough in Acién et al.'s definition (5); the tube on the homolateral side of the lesion is always connected to the normal uterine cavity and is patent. This was already described by Takeuchi's definition of JCA (17). It also means that an ectopic pregnancy is not possible in the cavity of an ACUM. This is the principal criteria that distinguishes it from a uterine malformation type U4 (6), another rare type of Müllerian duct anomaly (also known as non-communicating rudimentary uterine horn or Robert's uterus) which is the principal differential diagnosis. It is also important to note that for now no ACUM has ever been associated with urinary tract malformation.
MRI is known as the imaging modality of choice to achieve complete exploration of female genital anomalies (41). It allows for a precise localization of the tumour and therefore helps for an appropriate curative and fertility-sparing laparoscopic resection (3). Indeed, MRI has a higher correlation with surgical findings compared with echography (42).
In case of an unclear diagnosis, complementary investigations with a hysterosalpingo-foam sonography, hysterosalpingography or per-operative chromopertubation must be performed. Fertility-preserving and non-invasive surgery is essential in these young patients.
In our experience, IHC is not mandatory for the diagnosis of ACUM, but it can help if the endometrium and the cytogenic chorion are difficult to locate on HE alone.
Salpingectomy is not indicated and definitely has to be avoided (except in the case of a coexisting tubal pathology of another ethology). Both the homolateral uterine artery and the round ligament must be preserved as much as possible. Nevertheless, if the size of the lesion is important, it can be difficult to stay minimally invasive while doing a complete resection.
Pontrelli et al. (26) described the only case of a successful hysteroscopic resection of an ACUM. This method was chosen because the MRI findings were suggestive of a bicornuate uterus with cornual hematometra in a non-communicating horn, so they planned to remove the wall of the lesion. The undeniable advantage of this technique is its short operative time and its minimal invasive character. One can question the quality of resection of the capsule which must be difficult to obtain. If this latter is incomplete, there might be a risk of recurrence. There is also the remaining issue of the future obstetrical outcome for these young patients because no sutures are made to reinforce the myometrium. This technique might also expose the patient to a higher risk of uterine rupture in case of future pregnancy than with an intra-abdominal access.
Transvaginal ultrasound-guided alcohol sclerotherapy is an interesting procedure gaining momentum in the treatment of ACUM. In 2020, the first patients was described by Merviel et al. (43) who used the same technique as for the treatment of ovarian endometriomas. In 2021, Naftalin et al. (36) reported on another four women treated with this procedure. One of them had a recurrence of symptoms 6 months after the sclerotherapy and therefore needed a laparoscopic resection. It is possible that the surgical intervention was planned due to lesion reappearance; however this is not specified by the authors. For these four patients, the diagnosis of ACUM was based on the haemorrhagic content of the mass found in cytology. As a definitive histologic diagnosis cannot be obtained with sclerotherapy, we did not include these patients in our review. This technique has several benefits over laparoscopy; it is shorter in duration, is performed under local anaesthesia, does not add an iatrogenic myometrial injury and therefore might not negatively affect the future obstetrical outcome, although information on the obstetrical risk after surgical resection of ACUM is still unknown.
While there are no reported cases of uterine rupture during pregnancy in the literature to date, one can imagine that the risk exists and is similar to that observed after an intramural myomectomy [0.93% according to Gambacorti-Passerini et al. (44)]. Patients need to be informed of this risk and be aware of it.
Finally, the incidence of ACUM is still unknown, but in the last two decades, there has been more and more literature available on this pathology, and the number of cases is increasing. This can be explained by the improvement of imaging techniques and improved knowledge of this pathology despite its rarity.
ACUM is now a well-defined uterine malformation with precise characteristics that should be known by gynaecologists and should be evoked in the differential diagnosis of severe dysmenorrhea and CPP. The decision of whether a conservative or a surgical therapy should be done has to be made with the patient according to their preferences. Long-term outcome for these patients is still unknown and has to be especially studied regarding the potential recurrence of the lesions and the obstetrical outcomes. Hysteroscopic resection and ethanol sclerotherapy are two new interesting therapeutic approaches that need to be explored in the future to treat ACUM.
ACUM is certainly underdiagnosed, because it is a poorly known pathology hardly ever researched in a context of acute and early dysmenorrhea. With our cases, we also want to stress that ACUM has to be thought of and looked for in the case of atypical, chronic pelvic pain, in pre-menopausal women.
Concerning the limitations of this study, we would highlight its retrospective character. Moreover, the heterogeneous qualitative description of the cases found in the literature makes the comparison between them difficult and limits the potential of meaningful statistical analysis. Finally, as ACUM is a rare pathology, the number of studied cases is relatively small, which makes its understanding still incomplete.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individuals’ and minors’ legal guardian for the publication of any potentially identifiable images or data included in this article.
Author contributions
SD and PM organized the database and contributed to the conception and design of the study. SD wrote the manuscript. MK contributed to the proofreading. ED, AF, MM, J-YM, and PM collected the data. All authors contributed to the article and approved the submitted version.
Acknowledgements
We want to thank A. Cavicchioli for the proof reading, Mrs. K. Lepigeon for the statistical analysis, Dr C. Matthey-Page, Dr K. Athanasiou and Dr A. Bouzerda Kawthar who helped for the data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Takeda A, Sakai K, Mitsui T, Nakamura H. Laparoscopic management of juvenile cystic adenomyoma of the uterus: report of two cases and review of the literature. J Minim Invasive Gynecol. (2007) 14:370–4. doi: 10.1016/j.jmig.2007.01.005
3. Peyron N, Jacquemier E, Charlot M, Devouassoux M, Raudrant D, Golfier F, et al. Accessory cavitated uterine mass: MRI features and surgical correlations of a rare but under-recognised entity. Eur Radiol. (2019) 23:1144–52. doi: 10.1007/s00330-018-5686-6
4. Ball E, Ganji M, Janik G, Koh C. Laparoscopic resection of cystic adenomyosis in a teenager with arcurate uterus. Gynecol Surg. (2009) 6:367–70. doi: 10.1007/s10397-009-0505-3
5. Acién P, Acién M, Fernández F, José Mayol M, Aranda I. The cavitated accessory uterine mass: a Müllerian anomaly in women with an otherwise normal uterus. Obstet Gynecol. (2010) 116:1101–09. doi: 10.1097/AOG.0b013e3181f7e735
6. Grimbizis GF, Gordts S, Di Spiezio Sardo A, Brucker S, De Angelis C, Gergolet M, et al. The ESHRE/ESGE consensus on the classification of female genital tract congenital anomalies. Hum Reprod Oxf Engl. (2013) 28:2032–44. doi: 10.1093/humrep/det098
7. Acién P, Sánchez del Campo F, Mayol MJ, Acién M. The female gubernaculum: role in the embryology and development of the genital tract and in the possible genesis of malformations. Eur J Obstet Gynecol Reprod Biol. (2011) 159:426–32. doi: 10.1016/j.ejogrb.2011.07.040
8. Tamura M, Fukaya T, Takaya R, Ip CW, Yajima A. Juvenile adenomyotic cyst of the corpus uteri with dysmenorrhea. Tohoku J Exp Med. (1996) 176:339–44. doi: 10.1620/tjem.178.339
9. Potter DA, Schenken RS. Noncommunicating accessory uterine cavity. Fertil Steril. (1998) 70:1165–6. doi: 10.1016/S0015-0282(98)00380-X
10. Nabeshima H, Murakami T, Terada Y, Noda T, Yaegashi N, Okamura K. Total laparoscopic surgery of cystic adenomyoma under hydroultrasonographic monitoring. J Am Assoc Gynecol Laparosc. (2003) 10:195–9. doi: 10.1016/S1074-3804(05)60298-8
11. Kamio M, Taguchi S, Oki T, Tsuji T, Iwamoto I, Yoshinaga M, et al. Isolated adenomyotic cyst associated with severe dysmenorrhea. J Obstet Gynaecol Res. (2007) 33:388–91. doi: 10.1111/j.1447-0756.2007.00543.x
12. Wang JH, Wu RJ, Xu KH, Lin J. Single large cystic adenomyoma of the uterus after cornual pregnancy and curettage. Fertil Steril. (2007) 88:965–7. doi: 10.1016/j.fertnstert.2006.12.085
13. Nabeshima H, Murakami T, Nishimoto M, Sugawara N, Sato N. Successful total laparoscopic cystic adenomyomectomy after unsuccessful open surgery using transtrocar ultrasonographic guiding. J Minim Invasive Gynecol. (2008) 15:227–30. doi: 10.1016/j.jmig.2007.10.007
14. Ho ML, Raptis C, Hulett R, McAlister WH, Moran K, Bhalla S. Adenomyotic cyst of the uterus in an adolescent. Pediatr Radiol. (2008) 38:1239–42. doi: 10.1007/s00247-008-0948-0
15. Tijani EH, Meryem T, Lamya G, Abdelouahed J. Giant uterus-like mass of the uterus. Indian J Pathol Microbiol. (2010) 53:793. doi: 10.4103/0377-4929.72095
16. Liang YJ, Hao Q, Wu YZ, Wu B. Uterus-like mass in the left broad ligament misdiagnosed as a malformation of the uterus: a case report of a rare condition and review of the literature. Fertil Steril. (2010) 93:1347.e13–1347.e16. doi: 10.1016/j.fertnstert.2009.10.040
17. Takeuchi H, Kitade M, Kikuchi I, Kumakiri J, Kuroda K, Jinushi M. Diagnosis, laparoscopic management, and histopathologic findings of juvenile cystic adenomyoma: a review of nine cases. Fertil Steril. (2010) 94:862–8. doi: 10.1016/j.fertnstert.2009.05.010
18. Akar ME, Leezer KH, Yalcinkaya TM. Robot-assisted laparoscopic management of a case with juvenile cystic adenomyoma. Fertil Steril. (2010) 93:e55–e56. doi: 10.1016/j.fertnstert.2010.06.001
19. Chun SS, Hong DG, Seong WJ, Choi MH, Lee TH. Juvenile cystic adenomyoma in a 19-year-old woman: a case report with a proposal for new diagnostic criteria. J Laparoendosc Adv Surg Tech. (2011) 21:771–4. doi: 10.1089/lap.2011.0014
20. Kriplani A, Mahey R, Agarwal N, Bhatla N, Yadav R, Singh MK. Laparoscopic management of juvenile cystic adenomyoma: four cases. J Minim Invasive Gynecol. (2011) 18:343–8. doi: 10.1016/j.jmig.2011.02.001
21. Acién P, Bataller A, Fernandez F, Acien MI, Rodriguez JM, Mayol MJ. New cases of accessory and cavitated uterine masses (ACUM): a significant cause of severe dysmenorrhea and recurrent pelvic pain in young women. Hum Reprod. (2012) 27:683–94. doi: 10.1093/humrep/der471
22. Kumakiri J, Kikuchi I, Sogawa Y, Jinushi M, Aoki Y, Kitade M, et al. Single-incision laparoscopic surgery using an articulating monopolar for juvenile cystic adenomyoma. Minim Invasive Ther Allied Technol. (2013) 22:312–5. doi: 10.3109/13645706.2013.789060
23. Bedaiwy MA, Henry DN, Elguero S, Pickett S, Greenfield M. Accessory and cavitated uterine mass with functional endometrium in an adolescent: diagnosis and laparoscopic excision technique. J Pediatr Adolesc Gynecol. (2013) 26:e89–e91. doi: 10.1016/j.jpag.2012.11.003
24. Jain N, Verma R. Imaging diagnosis of accessory and cavitated uterine mass, a rare Mullerian anomaly. Indian J Radiol Imaging. (2014) 2:178. doi: 10.4103/0971-3026.134411
25. Paul PG, Chopade G, Das T, Dhivya N, Patil S, Thomas M. Accessory cavitated uterine mass: a rare cause of severe dysmenorrhea in young women. J Minim Invasive Gynecol. (2015) 22:1300–03. doi: 10.1016/j.jmig.2015.06.007
26. Pontrelli G, Bounous VE, Scarperi S, Minelli L, Di Spiezio Sardo A, Florio P. Rare case of giant cystic adenomyoma mimicking a uterine malformation, diagnosed and treated by hysteroscopy. J Obstet Gynaecol Res. (2015) 41:1300–04. doi: 10.1111/jog.12698
27. Garofalo A, Alemanno MG, Sochirca O, Pilloni E, Garofalo G, Chiadò fiorio tin M, et al. Accessory and cavitated uterine mass in an adolescent with severe dysmenorrhoea: from the ultrasound diagnosis to surgical treatment. J Obstet Gynaecol. (2016) 37:259–61. doi: 10.1080/01443615.2016.1239074
28. Shen J, Masuda K, Onoue M, Yano Y, Hatta K, Takayama T, et al. A case of difficult to diagnose cystic adenomyoma treated with laparoscopic surgery. Clin Obstet Gynecol Reprod Med. (2017) 3:1–3. doi: 10.15761/COGRM.1000199
29. Dadhwal V, Sharma A, Khoiwal K. Juvenile cystic adenomyoma mimicking a uterine anomaly: a report of two cases. Eurasian J Med. (2017) 49:59–61. doi: 10.5152/eurasianjmed.2017.17028
30. Peters A, Rindos NB, Guido RS, Donnellan NM. Uterine-sparing laparoscopic resection of accessory cavitated uterine masses. J Minim Invasive Gynecol. (2018) 25:24–5. doi: 10.1016/j.jmig.2017.06.001
31. Strelec M, Banović M, Banović V, Sirovec A. Juvenile cystic adenomyoma mimicking a Mullerian uterine anomaly successfully treated by laparoscopic excision. Int J Gynecol Obstet. (2019) 146:265–6. doi: 10.1002/ijgo.12880
32. Park JC, Kim DJ. Successful laparoscopic surgery of accessory cavitated uterine mass in young women with severe dysmenorrhea. Yeungnam Univ J Med. (2020) 38:235–9. doi: 10.12701/yujm.2020.00696
33. Protopapas A, Kypriotis K, Chatzipapas I, Kathopoulis N, Sotiropoulou M, Michala L. Juvenile cystic adenomyoma vs blind uterine horn: challenges in the diagnosis and surgical management. J Pediatr Adolesc Gynecol. (2020) 33:735–8. doi: 10.1016/j.jpag.2020.08.010
34. Kiyak H, Seckin KD, Karakis L, Karacan T, Ozyurek ES, Resit Asoglu M. Decidualized juvenile cystic adenomyoma mimicking a cornual pregnancy. Fertil Steril. (2020) 113:463–5. doi: 10.1016/j.fertnstert.2019.10.026
35. Supermaniam S, Thye WL. Diagnosis and laparoscopic excision of accessory cavitated uterine mass in young women: two case reports. Case Rep Womens Health. (2020) 26:e00187. doi: 10.1016/j.crwh.2020.e00187
36. Naftalin J, Bean E, Saridogan E, Barton-Smith P, Arora R, Jurkovic D. Imaging in gynecological disease (21): clinical and ultrasound characteristics of accessory cavitated uterine malformations. Ultrasound Obstet Gynecol. (2021) 57:821–8. doi: 10.1002/uog.22173
37. Mollion M, Host A, Faller E, Garbin O, Ionescu R, Roy C. Report of two cases of accessory cavitated uterine mass (ACUM): diagnostic challenge for MRI. Radiol Case Rep. (2021) 16:3465–9. doi: 10.1016/j.radcr.2021.07.071
38. Hu YL, Wang A, Chen J. Diagnosis and laparoscopic excision of accessory cavitated uterine mass in a young woman: a case report. World J Clin Cases. (2021) 9:9122–8. doi: 10.12998/wjcc.v9.i30.9122
39. Tokgoz VY, Tekin AB. A rare case of the new entity of Müllerian anomalies mimicking the noncommunicating rudimentary cavity with hemi-uterus: accessory cavitated uterine mass. Fertil Steril. (2022) 117:646–8. doi: 10.1016/j.fertnstert.2021.11.028
40. Acién P, Acién M. The presentation and management of complex female genital malformations. Hum Reprod Update. (2016) 22:48–69. doi: 10.1093/humupd/dmv048
41. Grimbizis GF, Di Spiezio Sardo A, Saravelos SH, Gordts S, Exacoustos C, Van Schoubroeck D, et al. The Thessaloniki ESHRE/ESGE consensus on diagnosis of female genital anomalies. Hum Reprod Oxf Engl. (2016) 31:2–7. doi: 10.1093/humrep/dev264
42. Santos XM, Krishnamurthy R, Bercaw-Pratt JL, Dietrich JE. The utility of ultrasound and magnetic resonance imaging versus surgery for the characterization of Müllerian anomalies in the pediatric and adolescent population. J Pediatr Adolesc Gynecol. (2012) 25:181–4. doi: 10.1016/j.jpag.2011.12.069
43. Merviel P, Lelievre C, Cambier T, Thomas-Kergastel I, Dupré PF. The first ethanol sclerotherapy of an accessory cavitated uterine mass. Clin Case Rep. (2020) 9:19–22. doi: 10.1002/ccr3.3371
Keywords: ACUM, Müllerian anomalies, uterine malformations, dysmenorrhea, chronic pelvic pain
Citation: Dekkiche S, Dubruc E, Kanbar M, Feki A, Mueller M, Meuwly J-Y and Mathevet P (2023) Accessory and cavitated uterine masses: a case series and review of the literature. Front. Reprod. Health 5:1197931. doi: 10.3389/frph.2023.1197931
Received: 31 March 2023; Accepted: 4 July 2023;
Published: 17 August 2023.
Edited by:
Takeshi Kurita, The Ohio State University, United States© 2023 Dekkiche, Dubruc, Kanbar, Feki, Mueller, Meuwly and Mathevet. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Souad Dekkiche c291YWQuZGVra2ljaGVAZ21haWwuY29t
 S. Dekkiche
S. Dekkiche E. Dubruc2
E. Dubruc2 M. Kanbar
M. Kanbar A. Feki
A. Feki P. Mathevet
P. Mathevet