
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Reprod. Health , 05 September 2023
Sec. HIV and STIs
Volume 5 - 2023 | https://doi.org/10.3389/frph.2023.1193183
 Esther Jebor Chongwo1*
Esther Jebor Chongwo1* Catherine J. Wedderburn2,3
Catherine J. Wedderburn2,3 Moses Kachama Nyongesa1
Moses Kachama Nyongesa1 Antipa Sigilai4
Antipa Sigilai4 Paul Mwangi1
Paul Mwangi1 Janet Thoya4
Janet Thoya4 Rachel Odhiambo1
Rachel Odhiambo1 Katana Ngombo4
Katana Ngombo4 Beatrice Kabunda4
Beatrice Kabunda4 Charles R. Newton4,5
Charles R. Newton4,5 Amina Abubakar1,4,5*
Amina Abubakar1,4,5*
Introduction: Globally, 1.7 million children are living with HIV, with the majority of them residing in sub-Saharan Africa. Due to reduced rates of vertical transmission of HIV, there is an increasing population of children born to HIV-infected mothers who remain uninfected. There is a growing concern around the development of these children in the antiretroviral therapy era. This study examined the neurocognitive outcomes of children who are HIV-exposed infected (CHEI), HIV-exposed uninfected (CHEU) and HIV-unexposed uninfected (CHUU) and explored the relationship between child neurocognitive outcomes and child's biomedical and caregivers’ psychosocial factors.
Methods: CHEI, CHUU and CHEU aged 3–5 years and their caregivers were recruited into the study. Neurocognitive outcomes were assessed using a validated battery of assessments. One-way analysis of variance and covariance (ANOVA and ANCOVA) were used to evaluate differences among the three groups by neurocognitive outcomes. Linear regression models were used to investigate the association between child neurocognitive outcomes and biomedical factors (nutritional status, HIV disease staging) and caregivers’ psychosocial factors [symptoms of common mental disorders (CMDs) and parenting behaviour].
Results: The study included 153 children and their caregivers: 43 (28.1%) CHEI, 52 (34.0%) CHEU and 58 (39.9%) CHUU. ANOVA and ANCOVA revealed a significant difference in cognitive ability mean scores across the child groups. Post hoc analysis indicated that CHEU children had higher cognitive ability mean scores than the CHUU group. Better nutritional status was significantly associated with higher cognitive ability scores (β = 0.68, 95% CI [0.18–1.18], p = 0.008). Higher scores of CMDs were negatively associated with inhibitory control (β = −0.28, 95% CI [−0.53 to 0.02], p = 0.036). While comparing HIV stages 2 and 3, large effect sizes were seen in working memory (0.96, CI [0.08–1.80]) and cognitive ability scores (0.83 CI [0.01–1.63]), indicating those in stage 3 had poor performance.
Conclusions: Neurocognitive outcomes were similar across CHEI, CHEU and CHUU, although subtle differences were seen in cognitive ability scores where CHEU had significantly higher cognitive mean scores than the CHUU. Well-designed longitudinal studies are needed to ascertain these findings. Nonetheless, study findings underscore the need for strategies to promote better child nutrition, mental health, and early antiretroviral therapy initiation.
HIV/AIDS remains a major public health issue and an important cause of disability globally (1, 2). Worldwide, approximately 38.4 million people live with HIV, the majority residing in sub-Saharan Africa (SSA) (3–5). Approximately 1.7 million children live with HIV globally (6). Kenya is one of the SSA countries with a high burden of HIV (7) with an average prevalence of 4.8% and an estimated 1.4 million people living with the disease (8). Among those living with HIV, approximately 83,000 are children aged 0–14 years (8).
The roll-out of antiretroviral therapy (ART) has tremendously prolonged the survival of individuals living with HIV, including children (9). With the decline in mortality rates attributable to ART, the impact of HIV on disability is becoming increasingly important. As children who are HIV-exposed and infected (CHEI) on ART survive longer, their neuropsychological functioning has become an area of great interest. HIV has been shown to cross the blood-brain barrier and enter the central nervous system (CNS) causing cerebral encephalopathy and impairment of the cerebral integrity which leads to neurodevelopmental delays (10, 11). This may be further exacerbated by infectious co-morbidities due to immune suppression. Parental HIV can also affect child-rearing practices with major implications for child development.
Neurodevelopmental delays reported in CHEI include impairment in cognition, language and motor functioning, among others (12). Before the availability of ART, more than 50% of CHEI had poor neurocognitive outcomes, in particular, delays in language and motor activity (13). Neurocognitive outcomes amongst CHEI in the modern ART era are less well described. A study by Whitehead and colleagues showed that CHEI infants on ART had greater neurodevelopmental impairment compared to children who were HIV-unexposed and uninfected (CHUU), implying that neurodevelopmental delays persist in the ART era (14). Generally, there has been inconsistent evidence on the neurocognitive outcomes of CHEU and CHUU. Some studies have suggested impairments in motor, language, cognitive and behavioural outcomes in CHEU compared to CHUU (15–17). In contrast, others have not reported any differences between the two groups (18–20). However, with early intervention the adverse effects of HIV can be mitigated. For instance, a study conducted by Strelau and colleagues in South Africa demonstrated that implementing a neurodevelopmental stimulation intervention for CHEI treated early CHEI resulted in a significant reduction in neurodevelopmental delays (7). These findings suggest that early initiation of ART alongside other early childhood stimulation programs has the potential to significantly enhance child developmental outcomes.
In SSA, data on the neurocognitive functioning of CHEI receiving ART in comparison with children who are HIV-exposed uninfected (CHEU) and CHUU pre-schoolers is scarce, yet the region bears a high burden of the disease (21). Data from studies conducted in other settings, particularly those in high-income countries, cannot be directly extrapolated to the SSA context as there exist significant differences in the social support structures, health care systems and the constellation of risks factors for child neurodevelopment including negative environmental factors such as pervasive poverty, malnutrition and malaria infection (22). Although previous studies on child neurodevelopment in the context of HIV have been conducted in Kenya, these have only assessed CHEI without recruiting appropriate comparison groups. One of these studies followed up CHEI for 2 years and observed that a compromised immune system was associated with poor developmental milestones for these children (23). Another study by Gomez and colleagues among newly diagnosed CHEI aged below 5.5 years found significantly improved neurodevelopment on the commencement of ART (24).
Despite HIV infection, improved nutrition has been linked with better cognitive development in CHEI infants (23–25), but there is a lack of comparative data on the nutritional status between children living with HIV and other child groups such as CHEU and CHUU from Kenya. Research on the unique neurodevelopmental profile of children living with HIV compared to their uninfected peers in low- and middle-income countries is needed since early child development (before the age of 5 years) is known to be critical for future scholastic achievement, social skills, behavioural outcomes, and quality of life (26–28). In addition, this stage is a timely period to intervene and take opportunity of the window of plasticity (29).
Furthermore, with strategies such as the prevention of mother-to-child transmission of HIV (PMTCT), there is a growing population of children born to HIV-infected mothers who remain uninfected due to the protective effects of ART in pregnancy. There are currently an estimated 15.9 million CHEU worldwide (30). Growing evidence suggests that CHEU have higher mortality and morbidity compared to CHUU (31). While the long-term neurocognitive outcomes of this population are unclear, there are reports of adverse outcomes in the early years, particularly in language and motor domains (32–35). These observations could plausibly be explained by: foetal exposure to HIV virus or maternal immune activation in utero; ART exposure in utero and prophylaxis during the neonatal period; or living in an HIV-affected household compounded with adverse psychosocial, socioeconomic and environmental factors (29, 32).
This study aimed to examine the neurocognitive outcomes of children aged 3–5 years exposed to and living with HIV compared to a representative sample of HIV-unexposed uninfected children on the Kenyan coast. The study also examined the relationship between child neurocognitive outcomes and the child's biomedical factors (nutritional status, HIV disease staging) and caregivers’ psychosocial factors [symptoms of common mental disorders (CMDs) and parenting behaviour].
The study was conducted in Kilifi County, Kenya, at the Comprehensive Care and Research Clinic (CCRC), Kilifi County Hospital, through the Centre for Geographic Medicine Research-Coast (CGMR-C). In Kilifi County, a largely rural setting, the overall prevalence of HIV is estimated as 4.5% (higher in women than men, 6.4% vs. 2.7%) and approximately 3,500 children live with the disease (36). Children and caregivers attending the CCRC were targeted for recruitment. The CCRC provides comprehensive care for adults and children living with or exposed to HIV. Some of the services offered include initiation and refill of ART medication, ART adherence monitoring, treatment and management of opportunistic infections, family planning, cervical cancer screening, nutritional counselling as well as HIV testing and counselling. Within the CGMR-C, is a neuro-assessment unit started in 1992 with well-trained and experienced staff for administering measures of neurological, mental, and cognitive functioning.
Power calculations for this study were conducted based on data from a previous study by Fishkin et al. to identify the effects of HIV on executive function (EF) (37). Using an effect size of 0.50 and a significance level of 0.05, the present study of 153 children (and caregivers) including 43 CHEI, 52 CHEU and 58 HIV-unexposed controls had a power of 85% to detect group differences.
The inclusion criteria for the children included in this study were: (i) age range 36–60 months; (ii) confirmation of positive HIV status for the CHEI and confirmation of negative HIV status for CHEU from the health records (iii) their caregivers spoke either Kiswahili, English or Mijikenda (local vernacular); and (iv) gave informed consent for child participation. Random sampling was used to recruit CHEI (n = 43) and CHEU (n = 52) during their scheduled HIV clinic visits at the Kilifi County Referral Hospital (CCRC). All CHEI had positive HIV tests confirmed at 18 months using the standard HIV antibody tests and virology assays. The CHEU group were those born to mothers living with HIV, but children tested HIV negative at 18 months. These tests are part of the routine clinical care under the PMTCT package. This information was confirmed using the documentation in the antenatal clinic (ANC) cards. Caregivers who consented for their children to take part in the study were recruited and invited to the neuro-assessment unit for assessments.
The CHUU group (n = 58) consisted of children not infected with or exposed to HIV. They were selected randomly from an existing database, the Kilifi Health and Demographic Surveillance System (KHDSS) (38). To ensure comparability, these children were matched to the CHEI based on age and geographical location. Subsequently, a field worker approached their caregivers for consenting at home. Due to ethical justifications and logistical difficulties, the HIV status of community controls was not directly tested during the time of assessment, but caution was exercised to minimise the chances of recruiting HIV-positive children. First, only children whose mothers had negative HIV tests during ANC visits (confirmed through clinic cards) were recruited. Second, a detailed history was taken to exclude children with severe childhood illnesses. Also, taking into account the 9% prevalence rate of HIV infection in mothers (39), high child mortality rate of CHEI (up to 50% by their second birthday) and expected mother-to-child transmission of 25%–40% (25), it was estimated that a maximum of two children would likely be HIV-positive in the control group. This number was deemed unlikely to invalidate the study results.
An overview of the study was introduced to all patients seeking services at the CRCC during the routine morning health talks (given before service initiation). Caregivers of CHEI and CHEU aged 3–5 years who showed interest in the study were approached by a member of the study team and taken through the study information sheet in detail in either the national (Swahili) or local (Giriama) language. Upon acceptance of study participation and signing of informed consent, the participants were booked for assessment dates at the neuro assessment clinic where the assessments were done. On the day of assessment, reaffirmation of consent was done before the start of any study-related activity. Study questionnaires and neurocognitive assessments were administered by a team of trained and experienced research assistants.
Permission to conduct this study in Kenya was sought and granted by the Institutional Review Board of the Kenya Medical Research Institute (KEMRI) called the Scientific and Ethics Review Unit (SERU), reference number: SSC No.2210. Information about the study was fully explained to all participants (in this case caregivers) and provided written informed consent for participation in their local languages (Swahili and Giriama).
Data on the child's age, sex, and schooling were collected. Information on maternal schooling and household socio-economic status was also documented.
All children's weight and height were taken using SECA digital scale. A medical examination of the children was done before the interview. This included physical examination of the participants including ear exams. Additionally, a review of previous medical history was done for all the children using a standard clinical questionnaire which had questions including history and the number of previous hospital admissions.
The World Health Organization's (WHO) clinical staging of HIV was used to assess the disease progression in CHEI. HIV is categorized from stage 1 (later/early stage) to stage 4 (advanced stage of AIDS) based on clinical signs and symptoms (40). This information was extracted from the medical records of the participants at the CCRC, with the prior permission of their caregivers.
The height for age Z-scores were computed using Anthro software following WHO standards [24]. HAZ was selected in this study because it measures stunting which has been found to be a good indicator of chronic malnutrition. In this study, a cut-off HAZ ≤2 SD was used. A systematic review by Perkins and colleagues demonstrated evidence of an association between stunting and child neurodevelopment (41).
The following battery of neurocognitive assessment tests was administered.
This is a simple measure of cognitive flexibility where the child identifies the hidden place for an object. The object is hidden in the child's view in one of two sites and the choice is then re-presented after a short delay to the child. The object's hiding place is changed between trials. The task is intended to elicit the A-not-B error, whereby the child continues to select a previously successful response despite seeing a new hiding place. The tool has 10 items, each scoring “one” for a correct response and “zero” for an incorrect response. The A-not-B task has been well-validated as a measure of cognitive flexibility, linked to frontal lobe functioning (42–44). In this study, the recommended procedures described by Espy and colleagues (45) were followed.
This task assesses set-shifting (46). The children were instructed to sort cards by numbers or by flowers and were scored based on whether they got them correct. The tool has 32 items each scoring “one” for a correct response and “zero” for an incorrect response.
This is a measure of working memory in which participants are presented with numbers and after a period of delay, they are asked to remember the numbers as many as possible (47). If they successfully repeat, the order list becomes longer. The tool has 24 items, each scoring “one” for a correct response and “zero” for an incorrect response. The tool has been validated in Kenyan children and showed a test-retest reliability of 0.70 (48).
This task uses a set of pictures showing big or small circles in black and the child is required to say the size of the picture as instructed by the assessor. This test was used because it is fairly easily understood by children and also, the picture sizes are distinctively opposite (49). The tool has 24 items each scoring “one” for a correct response.
Adapted from the third edition of the British Ability Scales (50), this tool measures cognitive ability. A child is required to construct wooden design blocks as shown by the assessor. The task has 16 items, and a correctly constructed design is awarded a point, giving a maximum score of 16. The tool has been validated in Ugandan children and demonstrated high reliability of 0.82 (51).
This tool has 24 items each with four pictures drawn in black and white: a target picture, a phonological distracter, a visual or semantic distracter, and an unrelated distracter (52). A child points to one of the items on every page as instructed by the assessor. This tool was adapted and validated in Kilifi, Kenya as a measure of receptive language and demonstrated good internal consistency reliability of 0.86 (48).
For these neurocognitive tests, all the assessments were conducted in Kiswahili or Giriama, based on what was most convenient to the participant. The number recall test was strictly carried out in Kiswahili to avoid method bias.
This is a 14-item screening tool for symptoms of common mental disorders (CMDs) that was developed in Zimbabwe (53). The measure has been used in various African settings (54–56) including Kenya (57). It is based on a “yes/no” response option, asking about symptoms such as suicidal ideations, failure to concentrate, unhappiness and thinking too much, among others, over the past 1 week. Higher scores indicate poor mental health. The Cronbach's alpha for SSQ in this study was 0.83.
Parenting behaviour was measured using 7 items (based on a “yes/no” response option) adapted from the UNICEF childcare module of the multiple indicator survey (58). We used items aimed at evaluating the cognitive stimulation that the child receives from the caregiver, for example, involvement with the child in activities such as reading, storytelling and singing. A score of one is given for a “No” response and two for a “Yes” response. Higher scores indicate better parenting behaviour.
Data analysis was carried out using STATA (version 15). Percentages and frequencies were used to summarize socio-demographic data. One-way analysis of variance (ANOVA) was utilized to identify group differences for continuous variables. Pearson's chi-squared test was used to assess group differences for categorical variables. ANOVA and analysis of covariance (ANCOVA), adjusting for the child's age and schooling, were done to identify group differences in the children's neurocognitive outcomes. Further, post hoc analysis using Bonferroni pairwise comparison was done for the scores that showed significant group differences in the ANOVA analysis. Linear regression models were used to investigate the association between child neurocognitive (outcome) and the following exposures: (i) the child's biomedical factors (nutritional status and HIV disease staging); and (ii) caregivers’ psychosocial factors (symptoms of CMDs and parenting behaviour). All the predictors were included in the adjusted model based on previous literature findings. We further computed Cohen's d to compare the effect sizes for child neurocognitive scores by HIV disease staging in the CHEI sub-group.
Table 1 provides details of the socio-demographic characteristics of the participants. A total of one hundred and fifty-three (153) children and their primary caregivers participated in this study including 43 CHEI children and caregivers, 52 CHEU and 58 CHUU. Table 1 summarizes participant characteristics by HIV infection status. The mean age (SD) of the children was 50.97 (9.70) months. Approximately half of the children were girls (52.3%) and the majority attended school (63.6%). Of the caregivers, 45.1% had a secondary or higher level of education. There was a statistically significant difference in the mean CMDs scores across the three caregiver groups (CHEI, CHEU and CHUU), showing higher CMD scores in caregivers of CHEI and CHEU groups. The mean scores of positive parenting behaviour also significantly differed across the three caregiver groups (CHEI, CHEU and CHUU).
Table 2 presents the mean (SD) scores for the neurocognitive battery tests. ANOVA results showed no statistically significant differences in the scores of receptive language, inhibitory control, set-shifting and cognitive flexibility across the three participant groups. However, there was a significant group difference in cognitive ability scores, p = 0.002. Post hoc analysis showed significantly higher cognitive ability scores among CHEU compared to (i) CHUU (p < 0.03801), and (ii) CHEI (p = 0.0028). After controlling for the child's characteristics (age, sex and schooling) and caregiver's characteristics (education level, CMD scores and parenting behaviour), ANCOVA results revealed that cognitive ability scores differed significantly between HIV groups, p = 0.006 (Table 2).
Table 3 presents the results of the unadjusted linear regression. The results revealed a significant association between the child's neurocognitive outcomes and HIV group, child age, child schooling, sex, nutrition status, HIV disease staging, caregiver level of education and positive parenting behaviour. The cognitive ability score for the CHEU group was 2.76 units higher than the CHUU group (β = 2.76, 95% CI [1.14–4.20], p = 0.001). Every 1-month increase in child's age was significantly associated with increased receptive language (β = 0.17, 95% CI [0.10–0.24], p < 0.001), working memory (β = 0.14, 95% CI [0.10–0.19], p < 0.001), inhibitory control (β = 0.17, 95% CI [0.07–0.26], p = 0.001), cognitive ability (β = 0.07, 95% CI [0.00–0.14], p = 0.049), set-shifting (β = 0.24, 95% CI [0.14–0.35], p < 0.001), and cognitive flexibility (β = 0.04, 95% CI [0.02–0.06], p < 0.001) scores, respectively. There was a significant increase in the receptive language (β = 0.75, 95% CI [0.23–1.27], p = 0.005), working memory (β = 0.41, 95% CI [0.02–0.79], p = 0.040), inhibitory control (β = 1.07, 95% CI [0.40–1.75], p = 0.002), cognitive ability (β = 0.91, 95% CI [0.42–1.41], p < 0.001), and set-shifting (β = 1.02, 95% CI [0.18–1.86], p = 0.018) scores for every unit increase in nutritional status. Children who were attending school had significantly increased scores in receptive language (β = 4.08, 95% CI [2.77–5.39], p < 0.001), working memory (β = 2.60, 95% CI [1.59–3.60], p < 0.001), inhibitory control (β = 4.89, 95% CI [3.18–6.60], p < 0.001), cognitive ability (β = 3.37, 95% CI [2.06–4.68], p < 0.001), set-shifting (β = 5.92, 95% CI [3.80–8.05], p < 0.001), and cognitive flexibility (β = 0.68, 95% CI [0.23–1.04], p = 0.002) compared to those who did not attend school. There was a significant increase in child receptive language (β = 2.51, 95% CI [0.21–4.81], p = 0.033) and inhibitory control scores (β = 3.54, 95% CI [0.67–6.40], p = 0.016) for caregivers with post-secondary education compared to those with no education. A unit increase in positive parenting behaviour significantly increased child's receptive language (β = 0.67, 95% CI [0.30–1.03], p < 0.001), inhibitory control (β = 0.68, 95% CI [0.22–1.15], p = 0.004) and working memory (β = 0.46, 95% CI [0.19–0.73], p = 0.001) scores.
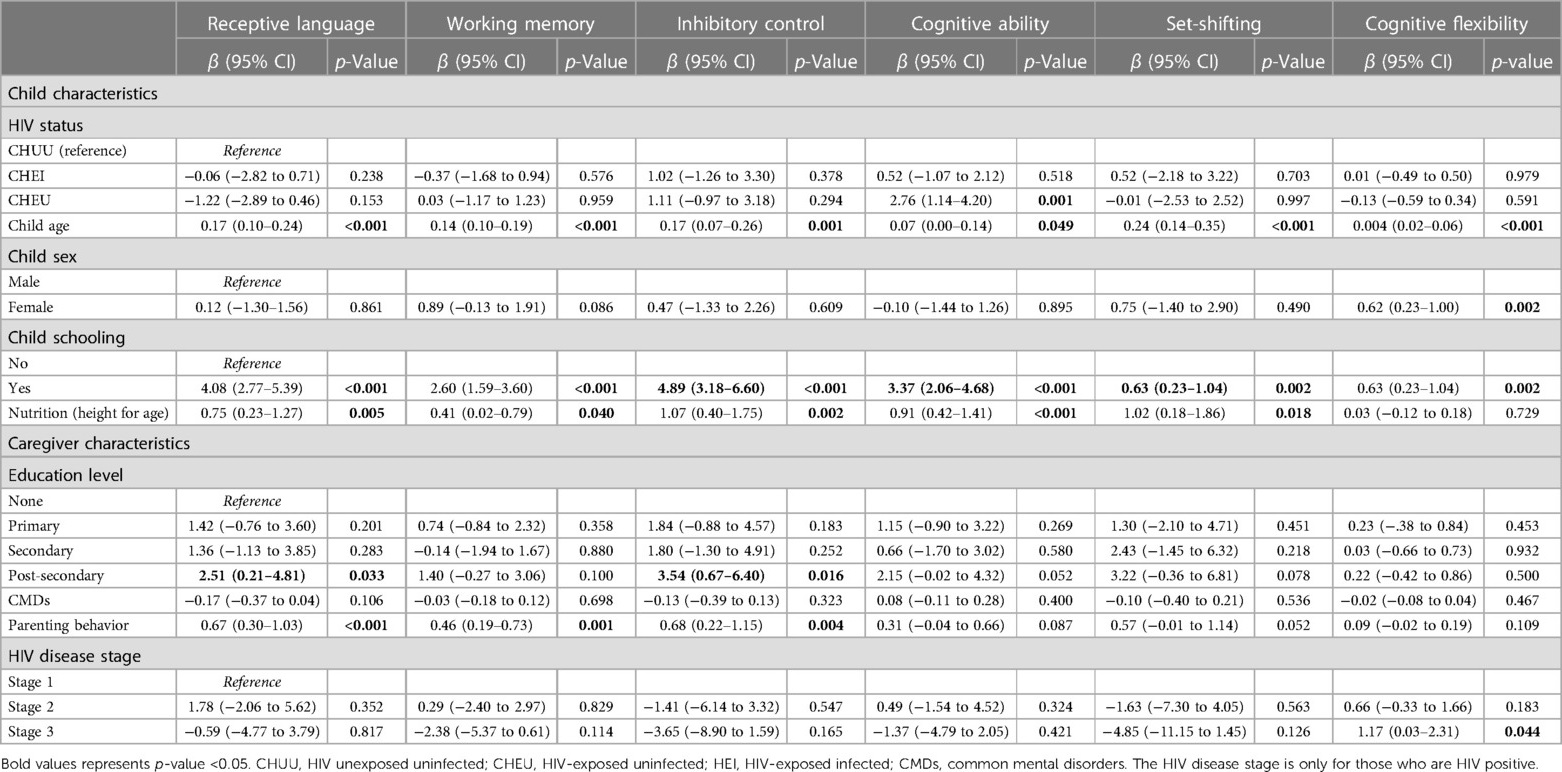
Table 3. Unadjusted linear regression for the association between neurocognitive outcomes and child biomedical factors and caregiver's psychosocial factors.
Table 4 shows the results for the adjusted linear regression model. In the multiple linear models, results showed that the child's HIV status, age and nutritional status were significantly associated with child neurocognitive outcomes. The CHEU group had a higher score in inhibitory control (β = 2.16, 95% CI [0.09–4.23], p = 0.041) and cognitive ability (β = 2.55, 95% CI [0.92–4.18], p = 0.002) compared to the CHUU group. For every 1-month increase in the child's age, there was a significant increase in the receptive language (β = 0.11, 95% CI [0.04–0.18], p = 0.004), working memory (β = 0.12, 95% CI [0.07–0.17], p < 0.001), inhibitory control (β = 0.12, 95% CI [0.02–0.21], p = 0.017), set shifting (β = 0.19, 95% CI [−0.07 to 0.30], p = 0.001), and cognitive flexibility (β = 0.03, 95% CI [0.01–0.05], p = 0.031) scores. A child being female was significantly associated with increased cognitive flexibility scores (β = 0.48, 95% CI [0.09–0.87], p = 0.031). Child school attendance was significantly associated with increased receptive language (β = 2.82, 95% CI [1.29–4.35], p < 0.001), working memory (β = 1.14, 95% CI [0.05–2.24], p = 0.040), inhibitory control (β = 3.27, 95% CI [1.26–5.27], p = 0.002), and set-shifting (β = 4.20, 95% CI [1.67–6.72], p = 0.001) scores. A unit increase in child nutritional status was significantly associated with increased cognitive ability scores (β = 0.68, 95% CI [0.18–1.18], p = 0.008). Increasing CMDs scores were significantly associated with reduced inhibitory control scores (β = −0.28, 95% CI [−0.53 to 0.02], p = 0.036).
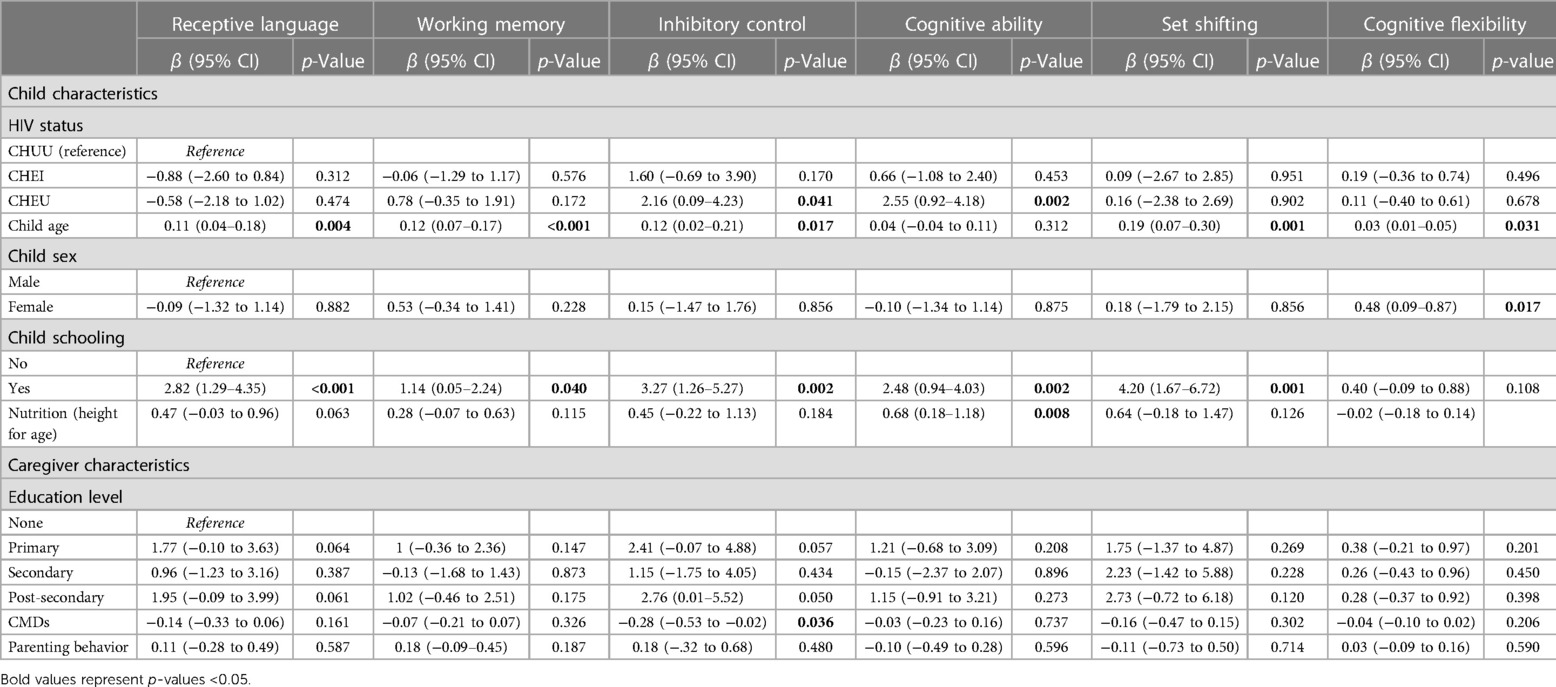
Table 4. Adjusted multivariable regression for the association between neurocognitive outcomes and child biomedical and caregiver's psychosocial factors.
Table 5 shows the effect sizes from a comparison of child neurocognitive scores by HIV disease staging. While comparing stages 2 and 3, large effect sizes were seen in working memory (0.96, CI [0.08–1.80]) and cognitive ability (0.83 CI [0.01–1.63] indicating worsening scores with the advanced disease stage. The effect sizes difference between stage 1 and stage 2 and stage 2 and stage 3 were negligible across all neurocognitive outcomes.
This study examined the neurocognitive outcomes of children exposed to and living with HIV compared to a representative HIV-unexposed control group. The project also explored the relationship between child neurocognitive outcomes and child biomedical factors (nutritional status) and caregivers’ caregiver's psychosocial factors (common mental disorders and parenting behaviour) on the Kenyan coast.
Overall, the results showed similar neurocognitive outcomes among the three child groups, however, there were some subtle differences between groups including cognitive ability scores whereby the CHEU group had significantly higher scores than the CHUU and CHEI children. Similar results have been reported in previous studies conducted in Africa showing comparable neurodevelopment in children regardless of HIV status (59, 60). However, these findings conflict with our prior hypothesis and with previous reports in the literature which indicate that CHEI group have poorer neurocognitive outcomes compared to HIV-uninfected children (15, 61–65). There are various potential explanations for these results. First, the majority of the CHEU were school-going compared to the CHEI and CHUU group. The schooling status of CHEU could have explained why they performed better in these neurocognitive outcomes as previously documented by other studies (66). Second, the CHEI in this study were on ART which has been reported to reduce the incidence of HIV-related encephalopathy (which is linked to neurodevelopmental delays) by up to 50% (13). Third, this could be attributed to the benefits of current comprehensive HIV health-care management practices in Kenya (including PMTCT care services) whereby substantial attention has been given to CHEI and CHEU leaving out the CHUU, which could have affected the neurocognitive scores. Random selection of study participants and matching the cases to controls based on age and geographical location hopefully mitigated selection bias, but as CHEI and CHEU were recruited from the CCRC, this may have overestimated outcomes as children or families in follow up may perform better than those not on follow up. However, these results could also be a true reflection of neurocognitive outcomes of the three child groups based on the follow up CHEI and CHEU receive. Empirical evidence has shown that even when individuals have had early brain insults, under supportive conditions, they may learn and acquire compensatory skills that ensure they perform well in neuropsychological tests (24, 67).
The study found differences in cognitive scores between the CHEU compared to the CHEI and the CHUU. These results are comparable with previous findings from a prospective observational study on CHEU and uninfected community controls aged 24 months in Botswana (68). Arguably, the CHEU have frequent follow-ups as part of the PMTCT package which may provide avenues for implementation of additional services such as educational programs, which the community controls may not routinely access. Overall, these findings support the safety of in-utero exposure to maternal HIV and ART with respect to early cognitive development in CHEU. Of note, we did not measure gross motor and expressive language function which are the domains found to be affected in a recent meta-analysis of young CHEU (35). However, in this study the CHEU group scored lowest on receptive language, indicating the language domain may still be affected.
While comparing HIV stages 2 and 3, large effect sizes were seen in working memory and cognitive ability scores indicating worsening scores with the advanced disease stage. These results agree with previously reported findings whereby working memory and cognitive scores were related to the HIV disease stage (69, 70). For instance, Abubakar et al. (25) reported poorer psychomotor performance with advanced HIV disease staging in infants aged 6–35 months in Kilifi. This supports the continued need for early HIV treatment to halt the disease progression and slow down the negative impact of HIV on neurocognitive outcomes in children. Nonetheless, our findings demonstrate smaller effects in other neurocognitive outcomes compared with other research which showed evidence of an association between neurocognitive outcomes and HIV disease staging (71, 72). Notably, the results from the current study could be explained by the small sample size which could have lowered the power of the study to detect significant associations. Secondly, there could have been measurement bias in that the indicator used for disease staging may have been inadequate. In this study, disease staging was only measured using the WHO clinical staging which has been identified to have several limitations, including low sensitivity (73–75). The use of clinical disease staging with concurrent CD4 count and viral loads could have been better indicators of the HIV-disease stage and immunosuppression (76), but were not available in the current study.
There was a significant association between better nutritional status and increased cognitive ability scores. These results are consistent with previous findings from Kenyan infants which documented nutritional status as an independent predictor of various neurocognitive outcomes including language and psychomotor functioning (23–25). Other studies elsewhere have also reported the role of better nutrition on child neurodevelopmental outcomes (11, 70, 77–79). Our results, therefore, reiterate the substantial role of good nutrition in improving neurocognitive outcomes in children, suggesting the need to improve all children's nutritional status for optimal development.
There were no significant associations between parenting behaviour and neurocognitive outcomes. However, we noted a general trend of increased neurocognitive scores with improved parenting practices. Contrary to these findings, previous studies have documented the association between parenting behaviour and child neurodevelopment in children aged 6–12 years (80). Similarly, in a longitudinal study, parenting behaviour was significantly associated with executive function, implying that a supportive parenting environment can be a strong protective factor for a child's neurodevelopment amidst biological vulnerabilities (80). One possible explanation for the observed results is that young children are dependent on their caregivers for primary interactions which form the foundation for a healthy attachment and acquisition of developmental skills (81).
We observed a significant inverse association between CMDs and inhibitory control. We also observed a general reduction in scores across all neurocognitive scores with increased caregivers’ CMD scores, although not statistically significant. The lack of significant associations contrasts with previous findings from African studies which showed evidence of a relationship between caregiver's depression and child neurodevelopment (82–86). However, other studies have documented associations between maternal common mental disorders and poorer child neurocognition (87–90). The inconsistent evidence regarding the influence of caregivers’ mental health on child neurocognitive outcomes underscores the urgent need for further investigations in this topic. Currently, there is scarcity of research papers from LMICs in this area (87). Notably, in this study, caregivers of CHEI reported significantly higher CMDs scores and lower parenting behaviour scores compared to caregivers of CHUU and CHEU. The reportedly high CMDs scores in caregivers of CHEI and CHEU compared to CHUU have been described in the literature and could be partly attributed to the deterioration of their physical health, family breakdown, emotional distress and HIV-related stigma (91).
This study is among the few quantitative studies examining the neurocognitive outcomes of HIV-infected, HIV-exposed uninfected and HIV-unexposed uninfected children within SSA. It is the first study of such kind in Kenya. The inclusion of two comparison groups in the analysis (CHEU and CHUU) was a particular strength of the study, enabling the comparison of neurocognitive outcomes across three child groups in the same setting.
Given the cross-sectional nature of this study, it was not possible to infer causality for the associations observed. Furthermore, the community control group was not actively screened for HIV. This implies that it is possible there were HIV-infected or exposed children among the controls, however, this is unlikely given the measures put in place to prevent this. We also note the possibility of residual bias despite adjusting for potential confounders, and that the CHUU group may have been disadvantaged given the lower schooling attendance which could have influenced group comparisons. Although child schooling status was adjusted for in the multivariable regression model, the number of years at school would have been a better measure of education, however these data were not available. Again, the CHEI and CHEU children were recruited from CCRC which may have overestimated outcomes as children not in follow up may perform worse than those on follow up. Additionally, data regarding the participants’ residential areas, specifically whether they were from rural or peri-urban settings, was not collected. Another major limitation of this project was a small sample size. This might have lowered the power to detect significant neurocognitive outcomes among the groups and associations. Additionally, the use of self-reported interviewer-administered measures of depression, SES and parenting behaviour to caregivers may have led to social desirability bias.
This study demonstrated similar neurocognitive outcomes among the children living with HIV, those exposed to HIV but uninfected and the community controls, although some subtle differences were noted for cognitive abilities where the CHEU performed better than the other child groups. These results suggest that early use of ART and the comprehensive care services received by CHEI and CHEU may have mitigated potential neurodevelopmental deficits. The study further confirms the importance of child nutrition and caregiver mental health for optimising child neurodevelopment, underscoring the need for continued strategies to address these issues. Well-designed longitudinal studies are needed to further explore these findings.
Data used in this article should be made through the coordinator of the data governance committee of the KEMRI Wellcome Trust Research Programme. The committee will review the application and advise as appropriate and ensure that uses are compatible with the consent obtained from participants for data collection. Requests to access the datasets should be directed toZGdjQGtlbXJpLXdlbGxjb21lLm9yZw==.
The studies involving humans were approved by the Institutional Review Board of the Kenya Medical Research Institute (KEMRI) called the Scientific and Ethics Review Unit (SERU), reference number: SSC No.2210. Information about the study was fully explained to all participants (in this case caregivers) and provided written informed consent for participation in their local languages (Swahili and Giriama). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
AA and CN conceptualized and designed the study. AA, MN, and AS supervised data collection. JT and BK participated in the data collection. RO and PM managed the study data. EC and PM analyzed the data. AA, CW, EC, PM, and MN contributed to the interpretation of the data. The first draft was written by EC. All authors contributed to the article and approved the submitted version.
This research was funded by the Thrasher Research Fund Early Career Award to AA. The funders had no role in the study's design, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
The authors would like to acknowledge Fons Van de Vijver who supported in the acquisition of the funds, design and implementation of the study. We would also like to thank the participants for agreeing to take part in this study and the neuro-assessment team who contributed to the data collection of the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ortblad KF, Lozano R, Murray CJ. The burden of HIV: insights from the global burden of disease study 2010. AIDS. (2013) 27(13):2003–17. doi: 10.1097/QAD.0b013e328362ba67
2. Wang H, Wolock TM, Carter A, Nguyen G, Kyu HH, Gakidou E, et al. Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2015: the global burden of disease study 2015. Lancet HIV. (2016) 3(8):e361–e87. doi: 10.1016/S2352-3018(16)30087-X
3. Kharsany AB, Karim QA. HIV infection and AIDS in sub-saharan Africa: current status, challenges and opportunities. Open AIDS J. (2016) 10:34–48. doi: 10.2174/1874613601610010034
7. Strehlau R, Burke M, van Aswegen T, Kuhn L, Potterton J. Neurodevelopment in early treated HIV-infected infants participating in a developmental stimulation programme compared with controls. Child Care Health Dev. (2021) 47(2):154–62. doi: 10.1111/cch.12828
9. Quinn TC. HIV epidemiology and the effects of antiviral therapy on long-term consequences. AIDS. (2008) 22(Suppl 3):S7. doi: 10.1097/01.aids.0000327510.68503.e8
10. Epstein LG, Gelbard HA. HIV-1-induced neuronal injury in the developing brain. J Leukocyte Biol. (1999) 65(4):453–7. doi: 10.1002/jlb.65.4.453
11. Debeaudrap P, Bodeau-Livinec F, Pasquier E, Germanaud D, Ndiang ST, Nlend AN, et al. Neurodevelopmental outcomes in HIV-infected and uninfected African children. AIDS. (2018) 32(18):2749–57. doi: 10.1097/QAD.0000000000002023
12. Van Rie A, Harrington PR, Dow A, Robertson K. Neurologic and neurodevelopmental manifestations of pediatric HIV/AIDS: a global perspective. Eur J Paediatr Neurol. (2007) 11(1):1–9. doi: 10.1016/j.ejpn.2006.10.006
13. Chiriboga CA, Fleishman S, Champion S, Gaye-Robinson L, Abrams EJ. Incidence and prevalence of HIV encephalopathy in children with HIV infection receiving highly active anti-retroviral therapy (HAART). J Pediatr. (2005) 146(3):402–7. doi: 10.1016/j.jpeds.2004.10.021
14. Whitehead N, Potterton J, Coovadia A. The neurodevelopment of HIV-infected infants on HAART compared to HIV-exposed but uninfected infants. AIDS Care. (2014) 26(4):497–504. doi: 10.1080/09540121.2013.841828
15. Kerr SJ, Puthanakit T, Vibol U, Aurpibul L, Vonthanak S, Kosalaraksa P, et al. Neurodevelopmental outcomes in HIV-exposed-uninfected children versus those not exposed to HIV. AIDS Care. (2014) 26(11):1327–35. doi: 10.1080/09540121.2014.920949
16. Stanzi L, Donald K, Brittain K, Phillips TK, Zerbe A, Nguyen KK, et al. Neurodevelopment of breastfed HIV-exposed uninfected and HIV-unexposed children in South Africa: a prospective cohort. AIDS. (2018) 32(13):1781. doi: 10.1097/QAD.0000000000001872
17. Van Rie A, Mupuala A, Dow A. Impact of the HIV/AIDS epidemic on the neurodevelopment of preschool-aged children in Kinshasa, democratic republic of the Congo. Pediatrics. (2008) 122(1):e123–e8. doi: 10.1542/peds.2007-2558
18. Landes M, van Lettow M, Chan AK, Mayuni I, Schouten EJ, Bedell RA. Mortality and health outcomes of HIV-exposed and unexposed children in a PMTCT cohort in Malawi. PLoS One. (2012) 7(10):e47337. doi: 10.1371/journal.pone.0047337
19. Ngoma MS, Hunter JA, Harper JA, Church PT, Mumba S, Chandwe M, et al. Cognitive and language outcomes in HIV-uninfected infants exposed to combined antiretroviral therapy in utero and through extended breast-feeding. AIDS. (2014) 28:S323–S30. doi: 10.1097/QAD.0000000000000357
20. Springer PE, Slogrove AL, Laughton B, Bettinger JA, Saunders HH, Molteno CD, et al. Neurodevelopmental outcome of HIV-exposed but uninfected infants in the mother and infants health study, Cape Town, South Africa. Trop Med Int Health. (2018) 23(1):69–78. doi: 10.1111/tmi.13006
21. Wedderburn CJ, Evans C, Yeung S, Gibb DM, Donald KA, Prendergast AJ. Growth and neurodevelopment of HIV-exposed uninfected children: a conceptual framework. Curr HIV/AIDS Rep. (2019) 16(6):501–13. doi: 10.1007/s11904-019-00459-0
22. Kariuki SM, Abubakar A, Newton CRJC, Kihara M. Impairment of executive function in Kenyan children exposed to severe falciparum malaria with neurological involvement. Malar J. (2014) 13(1):365. doi: 10.1186/1475-2875-13-365
23. Benki-Nugent S, Eshelman C, Dalton Wamalwa MP, Langat A, Tapia K, Okinyi HM, et al. Correlates of age at attainment of developmental milestones in HIV-infected infants receiving early antiretroviral therapy. Pediatr Infect Dis J. (2015) 34(1):55. doi: 10.1097/INF.0000000000000526
24. Gómez LA, Crowell CS, Njuguna I, Cranmer LM, Wamalwa D, Chebet D, et al. Improved neurodevelopment following antiretroviral therapy in HIV-infected children. Pediatr Infect Dis J. (2018) 37(9):916. doi: 10.1097/INF.0000000000001942
25. Abubakar A, Holding P, Newton CR, Van Baar A, Van de Vijver FJ. The role of weight for age and disease stage in poor psychomotor outcome of HIV-infected children in Kilifi, Kenya. Dev Med Child Neurol. (2009) 51(12):968–73. doi: 10.1111/j.1469-8749.2009.03333.x
26. Yoshikawa H, Weiland C, Brooks-Gunn J, Burchinal MR, Espinosa LM, Gormley WT, et al. Investing in our future: the evidence base on preschool education. Soc Res Child Develop. (2013).
27. Nores M, Barnett WS. Benefits of early childhood interventions across the world:(under) investing in the very young. Econ Educ Rev. (2010) 29(2):271–82. doi: 10.1016/j.econedurev.2009.09.001
28. Engle PL, Fernald LC, Alderman H, Behrman J, O'Gara C, Yousafzai A, et al. Strategies for reducing inequalities and improving developmental outcomes for young children in low-income and middle-income countries. Lancet. (2011) 378(9799):1339–53. doi: 10.1016/S0140-6736(11)60889-1
29. Dehorter N, Del Pino I. Shifting developmental trajectories during critical periods of brain formation. Front Cell Neurosci. (2020) 14:283. doi: 10.3389/fncel.2020.00283
30. AIDSinfo U. Epidemiological status (2016). Available at: http://aidsinfo.unaids.org/
31. Evans C, Jones CE, Prendergast AJ. HIV-exposed, uninfected infants: new global challenges in the era of paediatric HIV elimination. Lancet Infect Dis. (2016) 16(6):e92–e107. doi: 10.1016/S1473-3099(16)00055-4
32. Le Doare K, Bland R, Newell M-L. Neurodevelopment in children born to HIV-infected mothers by infection and treatment status. Pediatrics. (2012) 130(5):e1326–e44. doi: 10.1542/peds.2012-0405
33. McHenry MS, McAteer CI, Oyungu E, McDonald BC, Bosma CB, Mpofu PB, et al. Neurodevelopment in young children born to HIV-infected mothers: a meta-analysis. Pediatrics. (2018) 141(2):e20172888. doi: 10.1542/peds.2017-2888
34. Wedderburn CJ, Yeung S, Rehman AM, Stadler JA, Nhapi RT, Barnett W, et al. Neurodevelopment of HIV-exposed uninfected children in South Africa: outcomes from an observational birth cohort study. Lancet Child Adolesc Health. (2019) 3(11):803–13. doi: 10.1016/S2352-4642(19)30250-0
35. Wedderburn CJ, Weldon E, Bertran-Cobo C, Rehman AM, Stein DJ, Gibb DM, et al. Early neurodevelopment of HIV-exposed uninfected children in the era of antiretroviral therapy: a systematic review and meta-analysis. Lancet Child Adolesc Health. (2022) 6(6):393–408. doi: 10.1016/S2352-4642(22)00071-2
37. Fishkin PE, Armstrong FD, Routh DK, Harris L, Thompson W, Miloslavich K, et al. Brief report: relationship between HIV infection and WPPSI-R performance in preschool-age children. J Pediatr Psychol. (2000) 25(5):347–51. doi: 10.1093/jpepsy/25.5.347
38. Scott JAG, Bauni E, Moisi JC, Ojal J, Gatakaa H, Nyundo C, et al. Profile: the Kilifi health and demographic surveillance system (KHDSS). Int J Epidemiol. (2012) 41(3):650–7. doi: 10.1093/ije/dys062
39. English M, Ngama M, Musumba C, Wamola B, Bwika J, Mohammed S, et al. Causes and outcome of young infant admissions to a Kenyan district hospital. Arch Dis Child. (2003) 88(5):438–43. doi: 10.1136/adc.88.5.438
40. Weinberg JL, Kovarik CL. The WHO clinical staging system for HIV/AIDS. AMA J Ethics. (2010) 12(3):202–6. doi: 10.1001/virtualmentor.2010.12.3.cprl1-1003
41. Perkins JM, Kim R, Krishna A, McGovern M, Aguayo VM, Subramanian S. Understanding the association between stunting and child development in low-and middle-income countries: next steps for research and intervention. Soc Sci Med. (2017) 193:101–9. doi: 10.1016/j.socscimed.2017.09.039
42. Diamond A. The development and neural bases of memory functions as indexed by the AB and delayed response tasks in human infants and infant monkeys a. Ann N Y Acad Sci. (1990) 608(1):267–317. doi: 10.1111/j.1749-6632.1990.tb48900.x
43. Diamond A. Looking closely at infants’ performance and experimental procedures in the A-not-B task. Behav Brain Sci. (2001) 24(1):38–41. doi: 10.1017/S0140525X01253916
44. Diamond A. The early development of executive functions. Lifespan Cogn Mech Change. (2006) 210:70–95. doi: 10.1093/acprof:oso/9780195169539.003.0006
45. Espy KA, Kaufmann PM, McDiarmid MD, Glisky ML. Executive functioning in preschool children: performance on A-not-B and other delayed response format tasks. Brain Cogn. (1999) 41(2):178–99. doi: 10.1006/brcg.1999.1117
46. Rennie DA, Bull R, Diamond A. Executive functioning in preschoolers: reducing the inhibitory demands of the dimensional change card sort task. Dev Neuropsychol. (2004) 26(1):423–43. doi: 10.1207/s15326942dn2601_4
47. Conway AR, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, Engle RW. Working memory span tasks: a methodological review and user’s guide. Psychon Bull Rev. (2005) 12(5):769–86. doi: 10.3758/BF03196772
48. Alcock KJ, Holding P, Mung'ala-Odera V, Newton C. Constructing tests of cognitive abilities for schooled and unschooled children. J Cross Cult Psychol. (2008) 39(5):529–51. doi: 10.1177/0022022108321176
49. Gerstadt CL, Hong YJ, Diamond A. The relationship between cognition and action: performance of children 312–7 years old on a stroop-like day-night test. Cognition. 1994;53(2):129–53. doi: 10.1016/0010-0277(94)90068-X
51. Nampijja M, Apule B, Lule S, Akurut H, Muhangi L, Elliott A, et al. Adaptation of western measures of cognition for assessing 5-year-old semi-urban Ugandan children. Br J Educ Psychol. (2010) 80(1):15–30. doi: 10.1348/000709909X460600
52. Holding PA, Taylor HG, Kazungu SD, Mkala T, Gona J, Mwamuye B, et al. Assessing cognitive outcomes in a rural African population: development of a neuropsychological battery in Kilifi district, Kenya. J Int Neuropsychol Soc. (2004) 10:246–60. doi: 10.1017/S1355617704102166
53. Patel V, Simunyu E, Gwanzura F, Lewis G, Mann A. The Shona symptom questionnaire: the development of an indigenous measure of common mental disorders in Harare. Acta Psychiatr Scand. (1997) 95(6):469–75. doi: 10.1111/j.1600-0447.1997.tb10134.x
54. Chibanda D, Mesu P, Kajawu L, Cowan F, Araya R, Abas MA. Problem-solving therapy for depression and common mental disorders in Zimbabwe: piloting a task-shifting primary mental health care intervention in a population with a high prevalence of people living with HIV. BMC Public Health. (2011) 11(1):828. doi: 10.1186/1471-2458-11-828
55. Nhiwatiwa S, Patel V, Acuda W. Predicting postnatal mental disorder with a screening questionnaire: a prospective cohort study from Zimbabwe. J Epidemiol Community Health. (1998) 52(4):262–6. doi: 10.1136/jech.52.4.262
56. Uriyo JG, Abubakar A, Swai M, Msuya SE, Stray-Pedersen B. Prevalence and correlates of common mental disorders among mothers of young children in Kilimanjaro region of Tanzania. PLoS One. (2013) 8(7):e69088. doi: 10.1371/journal.pone.0069088
57. Nyongesa MK, Sigilai A, Hassan AS, Thoya J, Odhiambo R, Van de Vijver FJ, et al. A mixed methods approach to adapting and evaluating the functional assessment of HIV infection (FAHI), swahili version, for use with low literacy populations. PLoS One. (2017) 12(4):e0175021. doi: 10.1371/journal.pone.0175021
58. Kariger P, Frongillo EA, Engle P, Britto P, Sywulka SM, Menon P. Indicators of family care for development for use in multicountry surveys. J Health Popul Nutr. (2012) 30(4):472–86. doi: 10.3329/jhpn.v30i4.13417
59. Bagenda D, Nassali A, Kalyesubula I, Sherman B, Drotar D, Boivin MJ, et al. Health, neurologic, and cognitive status of HIV-infected, long-surviving, and antiretroviral-naive Ugandan children. Pediatrics. (2006) 117(3):729–40. doi: 10.1542/peds.2004-2699
60. Boivan M, Green D, Davies A, Giordani B, Mokili J, Cutting W. A preliminary evaluation of the cognitive and motor effects of pediatric HIV infection in zairian children. Health Psychol. (1995) 14:13–21. doi: 10.1037/0278-6133.14.1.13
61. Rice ML, Buchanan AL, Siberry GK, Malee KM, Zeldow B, Frederick T, et al. Language impairment in children perinatally infected with HIV compared to children who were HIV-exposed and uninfected. J Dev Behav Pediatr. (2012) 33(2):112. doi: 10.1097/DBP.0b013e318241ed23
62. Hoare J, Ransford GL, Phillips N, Amos T, Donald K, Stein DJ. Systematic review of neuroimaging studies in vertically transmitted HIV positive children and adolescents. Metab Brain Dis. (2014) 29(2):221–9. doi: 10.1007/s11011-013-9456-5
63. Smith R, Chernoff M, Williams PL, Malee KM, Sirois PA, Kammerer B, et al. Impact of human immunodeficiency virus severity on cognitive and adaptive functioning during childhood and adolescence. Pediatr Infect Dis J. (2012) 31(6):592–8. doi: 10.1097/INF.0b013e318253844b
64. Drotar D, Olness K, Wiznitzer M, Guay L, Marum L, Svilar G, et al. Neurodevelopmental outcomes of Ugandan infants with human immunodeficiency virus type 1 infection. Pediatrics. (1997) 100(1):e5–e. doi: 10.1542/peds.100.1.e5
65. Brahmbhatt H, Boivin M, Ssempijja V, Kigozi G, Kagaayi J, Serwadda D, et al. Neurodevelopmental benefits of anti-retroviral therapy in Ugandan children 0–6 years of age with HIV. J Acquir Immune Defic Syndr. (2014) 67(3):316. doi: 10.1097/QAI.0000000000000295
66. Luyten H. An empirical assessment of the absolute effect of schooling: regression-discontinuity applied to TIMSS-95. Oxf Rev Educ. (2006) 32(3):397–429. doi: 10.1080/03054980600776589
67. Chan RC, Shum D, Toulopoulou T, Chen EY. Assessment of executive functions: review of instruments and identification of critical issues. Arch Clin Neuropsychol. (2008) 23(2):201–16. doi: 10.1016/j.acn.2007.08.010
68. Chaudhury S, Williams PL, Mayondi GK, Leidner J, Holding P, Tepper V, et al. Neurodevelopment of HIV-exposed and HIV-unexposed uninfected children at 24 months. Pediatrics. (2017) 140(4):e20170988. doi: 10.1542/peds.2017-0988
69. Foster C, Biggs R, Melvin D, Walters M, Tudor-Williams G, Lyall E. Neurodevelopmental outcomes in children with HIV infection under 3 years of age. Dev Med Child Neurol. (2006) 48(8):677–82. doi: 10.1017/S0012162206001423
70. Struyf T, Dube Q, Cromwell EA, Sheahan AD, Heyderman RS, Van Rie A. The effect of HIV infection and exposure on cognitive development in the first two years of life in Malawi. Eur J Paediatr Neurol. (2020) 25:157–64. doi: 10.1016/j.ejpn.2019.11.004
71. Foster CJ, Biggs RL, Melvin D, Walters MD, Tudor-Williams G, Lyall EG. Neurodevelopmental outcomes in children with HIV infection under 3 years of age. Dev Med Child Neurol. (2006) 48(8):677–82. doi: 10.1017/S0012162206001423
72. Coscia JM, Christensen BK, Henry RR, Wallston K, Radcliffe J, Rutstein R. Effects of home environment, socioeconomic status, and health status on cognitive functioning in children with HIV-1 infection. J Pediatr Psychol. (2001) 26(6):321–9. doi: 10.1093/jpepsy/26.6.321
73. Kagaayi J, Makumbi F, Nakigozi G, Wawer MJ, Gray RH, Serwadda D, et al. WHO HIV clinical staging or CD4 cell counts for antiretroviral therapy eligibility assessment? An evaluation in rural Rakai district, Uganda. AIDS. (2007) 21(9):1208–10. doi: 10.1097/QAD.0b013e32810c8dce
74. Martinson N, Heyer A, Steyn J, editors. Does WHO clinical stage reliably predict who should receive ARV treatment?[WeFo0304]. 3rd IAS conference on HIV pathogenesis and treatment. (2005).
75. McGrath N, Kranzer K, Saul J, Crampin AC, Malema S, Kachiwanda L, et al. Estimating the need for antiretroviral treatment and an assessment of a simplified HIV/AIDS case definition in rural Malawi. AIDS. (2007) 21(Suppl 6):S105. doi: 10.1097/01.aids.0000299417.69432.65
76. Baveewo S, Ssali F, Karamagi C, Kalyango JN, Hahn JA, Ekoru K, et al. Validation of World Health Organisation HIV/AIDS clinical staging in predicting initiation of antiretroviral therapy and clinical predictors of low CD4 cell count in Uganda. PLoS One. (2011) 6(5):e19089. doi: 10.1371/journal.pone.0019089
77. Missmer SA, Spiegelman D, Gorbach SL, Miller TL. Predictors of change in the functional status of children with human immunodeficiency virus infection. Pediatrics. (2000) 106(2):e24–e. doi: 10.1542/peds.106.2.e24
78. Govender R, Eley B, Walker K, Petersen R, Wilmshurst JM. Neurologic and neurobehavioral sequelae in children with human immunodeficiency virus (HIV-1) infection. J Child Neurol. (2011) 26(11):1355–64. doi: 10.1177/0883073811405203
79. Horacio R-E, Itziar F-L, Alla S, Nikita J, Noelline N, Robert O, et al. Nutritional and immunological correlates of memory and neurocognitive development among HIV infected children living in Kayunga, Uganda. J Acquir Immune Defic Syndr. (2016) 71(5):522. doi: 10.1097/QAI.0000000000000905
80. Chavez-Arana C, Catroppa C, Yáñez-Téllez G, Prieto-Corona B, de León MA, García A, et al. Parenting and the dysregulation profile predict executive functioning in children with acquired brain injury. Child Neuropsychol. (2019) 25(8):1125–43. doi: 10.1080/09297049.2019.1589442
81. Coyl DD, Roggman LA, Newland LA. Stress, maternal depression, and negative mother–infant interactions in relation to infant attachment. Infant Mental Health J. (2002) 23(1-2):145–63. doi: 10.1002/imhj.10009
82. Familiar I, Nakasujja N, Bass J, Sikorskii A, Murray SM, Ruisenor-Escudero H, et al. Caregivers’ depressive symptoms and parent-report of child executive function among young children in Uganda. Learn Individ Differ. (2016) 46:17–24. doi: 10.1016/j.lindif.2015.01.012
83. Mebrahtu H, Simms V, Chingono R, Mupambireyi Z, Weiss HA, Ndlovu P, et al. Postpartum maternal mental health is associated with cognitive development of HIV-exposed infants in Zimbabwe: a cross-sectional study. AIDS Care. (2018) 30(sup2):74–82. doi: 10.1080/09540121.2018.1468015
84. Richter LM, Griesel RD, Barbarin O. 10 Behavioral problems among preschool children in South Africa: a six-year longitudinal perspective from birth to age five. Intern Perspect Child Adolesct Mental Health. (2000) 1:159–82. doi: 10.1016/S1874-5911(00)80011-1
85. Servili C, Medhin G, Hanlon C, Tomlinson M, Worku B, Baheretibeb Y, et al. Maternal common mental disorders and infant development in Ethiopia: the P-MaMiE birth cohort. BMC Public Health. (2010) 10:1–12. doi: 10.1186/1471-2458-10-693
86. Hadley C, Tegegn A, Tessema F, Asefa M, Galea S. Parental symptoms of common mental disorders and children’s social, motor, and language development in sub-saharan Africa. Ann Hum Biol. (2008) 35(3):259–75. doi: 10.1080/03014460802043624
87. Burger M, Hoosain M, Einspieler C, Unger M, Niehaus D. Maternal perinatal mental health and infant and toddler neurodevelopment-evidence from low and middle-income countries. A systematic review. J Affect Disord. (2020) 268:158–72. doi: 10.1016/j.jad.2020.03.023
88. Quevedo L, Silva R, Godoy R, Jansen K, Matos M, Tavares Pinheiro K, et al. The impact of maternal post-partum depression on the language development of children at 12 months. Child Care Health Dev. (2012) 38(3):420–4. doi: 10.1111/j.1365-2214.2011.01251.x
89. Patel V, DeSouza N, Rodrigues M. Postnatal depression and infant growth and development in low income countries: a cohort study from goa, India. Arch Dis Child. (2003) 88(1):34–7. doi: 10.1136/adc.88.1.34
90. Bhopal S, Roy R, Verma D, Kumar D, Avan B, Khan B, et al. Impact of adversity on early childhood growth & development in rural India: findings from the early life stress sub-study of the SPRING cluster randomised controlled trial (SPRING-ELS). PLoS One. (2019) 14(1):e0209122. doi: 10.1371/journal.pone.0209122
Keywords: HIV, children, caregivers, mental health, nutrition, parenting, neurocognitive
Citation: Chongwo EJ, Wedderburn CJ, Nyongesa MK, Sigilai A, Mwangi P, Thoya J, Odhiambo R, Ngombo K, Kabunda B, Newton CR and Abubakar A (2023) Neurocognitive outcomes of children exposed to and living with HIV aged 3–5 years in Kilifi, Kenya. Front. Reprod. Health 5:1193183. doi: 10.3389/frph.2023.1193183
Received: 24 March 2023; Accepted: 14 August 2023;
Published: 5 September 2023.
Edited by:
Stephanie Shiau, The State University of New Jersey - Busch Campus, United StatesReviewed by:
Florence Bodeau-Livinec, École des Hautes Etudes en Santé Publique, France© 2023 Chongwo, Wedderburn, Nyongesa, Sigilai, Mwangi, Thoya, Odhiambo, Ngombo, Kabunda, Newton and Abubakar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Esther Jebor Chongwo ZXN0aGVyLmNob25nd29AYWt1LmVkdQ== Amina Abubakar YWFidWJha2FyQGtlbXJpLXdlbGxjb21lLm9yZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.