
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
GENERAL COMMENTARY article
Front. Reprod. Health, 02 June 2023
Sec. HIV and STIs
Volume 5 - 2023 | https://doi.org/10.3389/frph.2023.1158528
This article is part of the Research TopicMultipurpose Prevention Technologies for HIV, STIs & PregnanciesView all 13 articles
This article is a commentary on:
Multipurpose Prevention Technologies: Opportunities and Challenges to Ensure Advancement of the Most Promising MPTs
Over the past decade, developers have made advances in addressing sexual and reproductive health (SRH) needs through multipurpose prevention technologies (MPTs)—products designed to simultaneously prevent HIV, Sexually transmitted infections (STIs), and/or unintended pregnancy (1). Multiple studies have demonstrated users’ preference for methods that prevent pregnancy alongside HIV and/or STIs rather than a single indication (2–7). While there are limitations to interpreting results from hypothetical use studies, it is intuitive that individuals would prefer a product that offers multiple benefits. By leveraging contraceptive priorities, MPTs provide a potential solution to known challenges such as ongoing low uptake of HIV pre-exposure prophylaxis (PReP) (8) while reducing the stigma of prevention (5, 9–11).
The majority of MPTs under development incorporate HIV prevention (Figure 1) (12) consistent with stakeholder prioritization and funding allocation. U.S. government research funding for HIV/AIDS totaled $1.4 billion dollars in 2018, dwarfing funding for all other STIs and contraception combined (Table 1) (13). This heavy focus on HIV has been appropriate given the large global costs and burden. Global HIV infection causes 47.63 million DALYs in 2019, compared to an estimated 8.58 billion global DALYs accounted by non-HIV STIs (14). With recent promising advances in HIV treatment and prevention, MPT initiatives and appropriate funding must now shift to advance more products preventing non-HIV STIs to curb the growing STI epidemic.
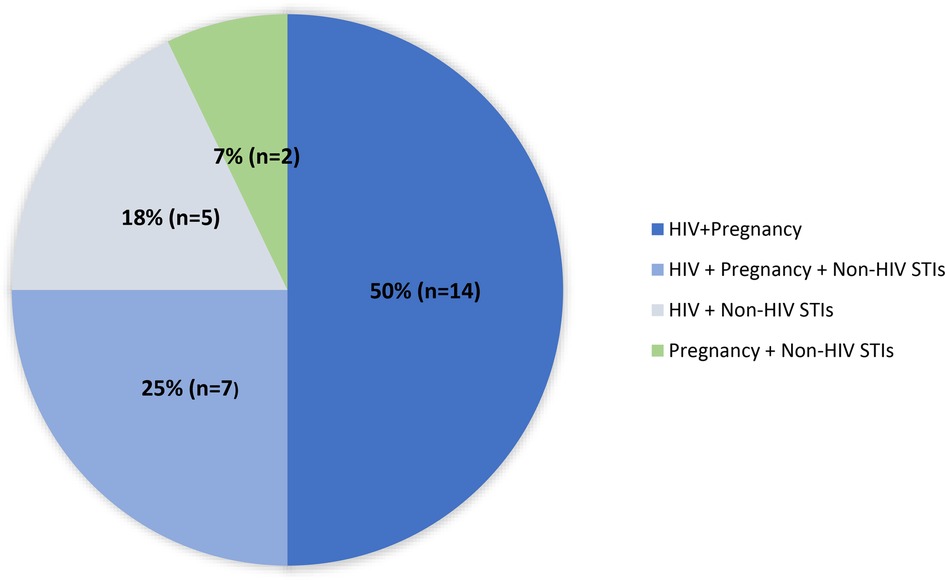
Figure 1. Multipurpose prevention technologies by indication (n = 28). Adapted from “MPT Pipeline by Indication Combination” by MPT 101, 2023 (https://mpts101.org/wp/wp-content/uploads/2023/01/MPT-Pipeline-Table_The-IMPT-Dec-2022_Indication-Combination.pdf). Copyright by IMPT for Reproductive Health. [Accessed January 29, 2023].
Non-HIV STIs account for 98% of all prevalent STIs worldwide (15, 16). In 2016, the World Health Organization (WHO) estimated there were 376 million new infections of four curable STIs: chlamydia (127 million), gonorrhea (87 million), syphilis (6 million), and trichomoniasis (156 million) (15). Viral STIs such as genital herpes simplex virus (HSV) and human papilloma virus (HPV) also have notably been increasing in prevalence (15). In the U.S. alone, 2.4 million new cases of chlamydia, gonorrhea, and syphilis were reported in 2020 despite underreporting and reduced access to screening during the COVID pandemic (17). Despite global efforts to combat STIs, infections are at an all-time high and cost $2 billion in treatment annually (18, 19). Untreated, STIs have broad-reaching health impacts ranging from increased risk of HIV acquisition, cancer, chronic pelvic pain, infertility, preterm delivery, and neonatal morbidity and mortality (15). Many infections may be asymptomatic and go undiagnosed. Even when identified, treatment may be complicated by challenges such as medication shortages, emerging antibiotic resistance, and reinfection after inadequate partner treatment (20–23). Adverse health impacts and barriers to diagnosis and treatment make renewed dedication to STI prevention strategies critical.
Non-HIV STIs are inextricably linked to HIV and contraception. Infection with some STIs such as HSV, chlamydia and gonorrhea may increase the risk of HIV acquisition (24, 25). Use of certain contraceptives may be associated with increased acquisition of chlamydia and HSV (26, 27). While some of these findings may be linked to more frequent access of diagnostic services, biological plausible mechanisms for altered risk exist (28, 29). As the linkages expand beyond epidemiologic and behavioral sexual risk factors, developers have an enhanced imperative to develop overlapping prevention tools.
While not universally available, the global market for HIV prevention includes medications taken pre- or post-exposure with different formulations including daily pill, long-acting injectables and vaginal rings (30–32). Prevention for other STIs still largely relies on promoting healthy sexual behaviors and barrier method use—traditional approaches that have been inadequate in curbing rise in infections (33). The current STI prevention pipeline leans heavily on vaccine development. While there are only two vaccines currently on the market, both of which act against viral STIs (Hepatitis B and HPV) (21), several vaccines in development offer hope for broader protection. Several MPTs are integrating non-vaccine prevention methods, however these are mostly in the preclinical stages. Promising options currently being explored for STI prevention include the recently FDA approved on-demand vaginal pH modulator (VPM) Phexxi® (34) and doxycycline as a possible pre- or post-exposure bacterial STI prophylaxis (35, 36).
Triple protection MPTs with broad pregnancy, non-HIV STIs, and HIV prevention make up about a quarter of all MPTs in development (Figure 1) (12). Advancing triple-indication products would align with the overlapping risks faced by many people around the world (37) and simultaneously confront concerns of risk compensation. Risk compensation theorizes that individuals who use STI prevention methods might engage in high-risk sexual behavior such as condomless sex and increased number of sexual partners. Although it is unclear to what extent risk compensation occurs, this potential raises concern that use of a prevention method for one STI might contribute to increasing rates of other STIs (38). Monitoring MPTs’ impact on other STIs is critical as newer methods enter the market. As it is unlikely there will be one method that protects against all STIs, a diverse range of MPTs must be developed to enable individuals to prioritize STIs that are highly prevalent in their community.
Efforts to counter the STI epidemic through MPTs rely on several key actions listed in Box 1. Exciting novel MPTs indicated for non-HIV STIs are already making their way through the development pipeline. Critical to success in the fight against STIs is momentum in R&D activities. This requires engaging researchers who are already focused on non-HIV STI projects as well as funders and impact investors who may be interested given their large potential market in developed countries, but who have not yet partnered with MPT developers. A key challenge to advancing these products is also ensuring that the regulatory environment facilitates approval of multipurpose prevention. The development of regulatory processes tailored specifically for the approval of MPTs will accelerate expansion of the STI prevention toolbox. With the traditional regulatory framework, multiple costly Phase 3 trials can be a barrier to advancing some indications, leading to the potential risk of secondary benefits not obtaining regulatory approval. As products advance, acknowledging that users value product characteristics other than effectiveness may open avenues for a broader array of options that address users’ needs. Ultimately, advancing MPTs indicated for non-HIV STIs will allow more individuals to achieve their sexual and reproductive health goals.
AL and LH: jointly drafted the manuscript and equally contributed to the writing and review. All authors contributed to the article and approved the submitted version.
LBH is receiving support from the National institute of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development (P50HD106793, R01 HD095741) the National Institute of Allergy and Infectious diseases (R01AI150360), and Magee through MATRIX.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
2. Quaife M, Terris-Prestholt F, Eakle R, Cabrera Escobar MA, Kilbourne-Brook M, Mvundura M, et al. The cost-effectiveness of multi-purpose HIV and pregnancy prevention technologies in South Africa. J Int AIDS Soc. (2018) 21(3):1–13. doi: 10.1002/jia2.25064
3. van der Straten A, Agot K, Ahmed K, Weinrib R, Browne EN, Manenzhe K, et al. The tablets, ring, injections as options (TRIO) study: what young African women chose and used for future HIV and pregnancy prevention. J Int AIDS Soc. (2018) 21(3):e25094. doi: 10.1002/jia2.25094
4. Woodsong C, Musara P, Chandipwisa A, Montgomery E, Alleman P, Chirenje M, et al. Interest in multipurpose prevention of HIV and pregnancy: perspectives of women, men, health professionals and community stakeholders in two vaginal gel studies in southern Africa. BJOG. (2014) 121(Suppl 5):45–52. doi: 10.1111/1471-0528.12875
5. Hynes JS, Sales JM, Sheth AN, Lathrop E, Haddad LB. Interest in multipurpose prevention technologies to prevent HIV/STIs and unintended pregnancy among young women in the United States. Contraception. (2018) 97(3):277–84. doi: 10.1016/j.contraception.2017.10.006
6. Hynes JS, Sheth AN, Lathrop E, Sales JM, Haddad LB. Preferred product attributes of potential multipurpose prevention technologies for unintended pregnancy and sexually transmitted infections or HIV among U.S. Women. J Womens Health. (2019) 28(5):665–72. doi: 10.1089/jwh.2018.7001
7. Friedland BA. Women want choices: opinions from the share. Learn.shape global internet survey about multipurpose prevention technology (MPT) products in development. AIDS Behav. (2023) 27:1–15. doi: 10.1007/s10461-022-03951-8
8. Golub SA. PrEP stigma: implicit and explicit drivers of disparity. Curr HIV/AIDS Rep. (2018) 15(2):190–7. doi: 10.1007/s11904-018-0385-0
9. Clifton S, Mercer CH, Sonnenberg P, Tanton C, Field N, Gravningen K, et al. STI Risk perception in the British population and how it relates to sexual behaviour and STI healthcare use: findings from a cross-sectional survey (natsal-3). EClinicalMedicine. (2018) 2:29–36. doi: 10.1016/j.eclinm.2018.08.001
10. Guleria S, Faber MT, Hansen BT, Arnheim-Dahlstrom L, Liaw KL, Munk C, et al. Self-perceived risk of STIs in a population-based study of scandinavian women. Sex Transm Infect. (2018) 94(7):522–7. doi: 10.1136/sextrans-2017-053397
11. Gonzalez RP, Kadengye DT, Mayega RW. The knowledge-risk-behaviour continuum among young Ugandans: what it tells US about SRH/HIV integration. BMC Public Health. (2019) 19(Suppl 1):604. doi: 10.1186/s12889-019-6809-y
13. Chapman NDA, Goldstein M, Barnsley P, Oversteegen L, Chowdhary V, Rugarabamu G, Borri J, Hynen A, Ong M, Tjoeng I. Sexual and Reproductive Health Research and Development: Understanding the Spectrum. (2020).
14. Wu L, Zhang SQ, Zhao L, Ren ZH, Hu CY. Global, regional, and national burden of periodontitis from 1990 to 2019: results from the global burden of disease study 2019. J Periodontol. (2022) 93(10):1445–54. doi: 10.1002/JPER.21-0469
15. Organization WH. Global progress report on HIV, viral hepatitis and sexually transmitted infections. (2021).
16. Looker KJ, Johnston C, Welton NJ, James C, Vickerman P, Turner KME, et al. The global and regional burden of genital ulcer disease due to herpes simplex virus: a natural history modelling study. BMJ Glob Health. (2020) 5(3):e001875. doi: 10.1136/bmjgh-2019-001875
18. Collaborators GDaIIaP. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet. (2016) 388(10053):1545–602. doi: 10.1016/S0140-6736(16)31678-6
19. Sully EA BA, Darroch JE, Riley T, Ashford LS, Lince-Deroche N, Firestein L, Murro R. Adding it up: Investing in sexual and reproductive health 2019. New York, NY: Guttmacher Institute (2020).
20. Bradshaw CS, Vodstrcil LA, Hocking JS, Law M, Pirotta M, Garland SM, et al. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clin Infect Dis. (2013) 56(6):777–86. doi: 10.1093/cid/cis1030
21. Johnson JJ. Chlamydia and syphilis pipeline report 2019. New York, NY: Treatment Action Group (2020).
22. Orbe-Orihuela YC, Sanchez-Aleman MA, Hernandez-Pliego A, Medina-Garcia CV, Vergara-Ortega DN. Syphilis as re-emerging disease, antibiotic resistance, and vulnerable population: global systematic review and meta-analysis. Pathogens. (2022) 11(12):1–19. doi: 10.3390/pathogens11121546
23. Ratten LK, Plummer EL, Murray GL, Danielewski J, Fairley CK, Garland SM, et al. Sex is associated with the persistence of non-optimal vaginal microbiota following treatment for bacterial vaginosis: a prospective cohort study. BJOG. (2021) 128(4):756–67. doi: 10.1111/1471-0528.16430
24. Mayer KH, Venkatesh KK. Interactions of HIV, other sexually transmitted diseases, and genital tract inflammation facilitating local pathogen transmission and acquisition. Am J Reprod Immunol. (2011) 65(3):308–16. doi: 10.1111/j.1600-0897.2010.00942.x
25. Passmore JA, Jaspan HB, Masson L. Genital inflammation, immune activation and risk of sexual HIV acquisition. Curr Opin HIV AIDS. (2016) 11(2):156–62. doi: 10.1097/COH.0000000000000232
26. Akter T, Festin M, Dawson A. Hormonal contraceptive use and the risk of sexually transmitted infections: a systematic review and meta-analysis. Sci Rep. (2022) 12(1):20325. doi: 10.1038/s41598-022-24601-y
27. McCarthy KJ, Gollub EL, Ralph L, van de Wijgert J, Jones HE. Hormonal contraceptives and the acquisition of sexually transmitted infections: an updated systematic review. Sex Transm Dis. (2019) 46(5):290–6. doi: 10.1097/OLQ.0000000000000975
28. Mwatelah R, McKinnon LR, Baxter C, Abdool Karim Q, Abdool Karim SS. Mechanisms of sexually transmitted infection-induced inflammation in women: implications for HIV risk. J Int AIDS Soc. (2019) Suppl 6(Suppl Suppl 6):e25346. doi: 10.1002/jia2.25346
29. Calla NE Q, Miguel RD V, Boyaka PN, Hall-Stoodley L, Kaur B, Trout W, et al. Medroxyprogesterone acetate and levonorgestrel increase genital mucosal permeability and enhance susceptibility to genital herpes simplex virus type 2 infection. Mucosal Immunol. (2016) 9(6):1571–83. doi: 10.1038/mi.2016.22
30. Beymer MR, Holloway IW, Pulsipher C, Landovitz RJ. Current and future PrEP medications and modalities: on-demand, injectables, and topicals. Curr HIV/AIDS Rep. (2019) 16(4):349–58. doi: 10.1007/s11904-019-00450-9
31. WHO continues to support its conditional recommendation for the dapivirine vaginal ring as an additional prevention option for women at substantial risk of HIV. [press release]. 9 December 2021 (2021).
32. WHO recommends long-acting cabotegravir for HIV prevention [press release]. 28 July 2022 (2022).
33. Malcolm RK, Boyd P, McCoy CF, Murphy DJ. Beyond HIV microbicides: multipurpose prevention technology products. BJOG. (2014) 121(Suppl 5):62–9. doi: 10.1111/1471-0528.12852
34. Chappell BT, Mena LA, Maximos B, Mollan S, Culwell K, Howard B. EVO100 Prevents chlamydia and gonorrhea in women at high risk of infection. Am J Obstet Gynecol. (2021) 225(2):162 e1–e14. doi: 10.1016/j.ajog.2021.03.005
35. Bolan RK, Beymer MR, Weiss RE, Flynn RP, Leibowitz AA, Klausner JD. Doxycycline prophylaxis to reduce incident syphilis among HIV-infected men who have sex with men who continue to engage in high-risk sex: a randomized, controlled pilot study. Sex Transm Dis. (2015) 42(2):98–103. doi: 10.1097/OLQ.0000000000000216
36. Molina JM, Charreau I, Chidiac C, Pialoux G, Cua E, Delaugerre C, et al. Post-exposure prophylaxis with doxycycline to prevent sexually transmitted infections in men who have sex with men: an open-label randomised substudy of the ANRS IPERGAY trial. Lancet Infect Dis. (2018) 18(3):308–17. doi: 10.1016/S1473-3099(17)30725-9
37. Schelar E, Polis CB, Essam T, Looker KJ, Bruni L, Chrisman CJ, et al. Multipurpose prevention technologies for sexual and reproductive health: mapping global needs for introduction of new preventive products. Contraception. (2016) 93(1):32–43. doi: 10.1016/j.contraception.2015.09.002
Keywords: sexually transmitted infections, multipurpose prevention technology, gonorrhea, chlamydia, syphilis
Citation: Lu AMR and Haddad LB (2023) Commentary: Multipurpose prevention technologies—What about sexually transmitted infections?. Front. Reprod. Health 5:1158528. doi: 10.3389/frph.2023.1158528
Received: 4 February 2023; Accepted: 16 May 2023;
Published: 2 June 2023.
Edited by:
Andrea Ries Thurman, Eastern Virginia Medical School, United StatesReviewed by:
Bethany Young Holt, Public Health Institute, United States© 2023 Lu and Haddad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lisa B. Haddad bGhhZGRhZEBwb3Bjb3VuY2lsLm9yZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.