- 1Effective Care Research Unit, Department of Obstetrics and Gynaecology, Nnamdi Azikiwe University, Nnewi, Nigeria
- 2Institute of Maternal and Child Health, College of Medicine, University of Nigeria Ituku-Ozalla Campus, Enugu, Nigeria
- 3Department of Obstetrics and Gynaecology, Nnamdi Azikiwe University Teaching Hospital Nnewi, Nnewi, Nigeria
- 4Department of Obstetrics and Gynaecology, College of Medicine, University of Nigeria Ituku-Ozalla, Enugu, Nigeria
- 5Rural Community Clinical School, School of Medicine, Deakin University, Melbourne, VIC, Australia
Background: In low-and middle-income countries, no conclusive research explains the prevalence and associated factors of women with a history of recurrent pregnancy loss (RPL). Some authorities have recommended further scientific research on the effect of various definitions of RPL.
Objective: To assess prevalence and associated factors of RPL among pregnant women in Nigeria according to different national and international criteria: the American Society for Reproductive Medicine/ European Society for Human Reproduction and Embryology (ASRM/ESHRE; two losses) and the World Health Organization/ Royal College of Obstetricians and Gynecologists (WHO/RCOG; three consecutive losses) criteria.
Methods: This is a cross-sectional analytical study wherein, pregnant women with prior RPL were investigated. The outcome measures were prevalence and risk factors. The associations between independent variables and outcome variable were explored using bivariate and multivariable logistic regression models. The results of these analyses were reported as adjusted odds ratios (AORs) with 95% confidence intervals (95%CI). Factors associated with RPL were identified using multivariate regression models.
Result: Of the 378 pregnant women interviewed, the overall prevalence of RPL in this study was found to be 15.34% (95% confidence interval = 11.65%–19.84%). The prevalence of RPL was 15.34% (58/378; 95%CI = 11.65%–19.84%) and 5.29% (20/378; 95%CI = 3.23%–8.17) according to the ASRM and the WHO criterion respectively. Regardless of diagnostic criteria, unexplained (AOR = 23.04; 95%CI: 11.46–36.32), endocrine disturbances (AOR = 9.76; 95%CI: 1.61–63.19), uterine abnormalities (AOR = 13.57; 95%CI: 3.54–50.60), and antiphospholipid syndrome (AOR = 24.59; 95%CI: 8.45–71.04) were positively and independently associated with RPL. No significant risk factors were seen when the ASRM/ ESHRE criterion vs. WHO/RCOG criterion were compared. Advanced maternal age was significantly higher in secondary than in primary type of RPL.
Conclusion: The prevalence of RPL was 15.34% and 5.29% according to ASRM/ESHRE and WHO/RCOG criterion respectively, with secondary type predominating. No significant differences with regard to risk factors were seen according to diagnostic criteria studied, though advanced maternal age was significantly higher in secondary RPL. Further research is needed to confirm our findings and to better characterize the magnitude of differences.
Introduction
The definition of recurrent pregnancy loss (RPL) varies and has been debated among international societies (1, 2). For the World Health Organisation (WHO), and the Royal College of Obstetricians and Gynecologists (RCOG), RPL refers to three consecutive pregnancy losses, including nonvisualized ones (3). However, according to the American Society for Reproductive Medicine (ASRM) and the European Society for Human Reproduction and Embryology (ESHRE), it is defined as two or more clinical pregnancy losses (documented by ultrasound or histopathologic examination), but not necessarily consecutive (2–4).
Epidemiological studies of RPL are important to gain an understanding of the disorder and its occurrence in the population. In previous studies, RPL has been reported to affect up to 2% to 5% of couples (5, 6). In a very recent Swedish study by Rasmark et al., the authors suggested that it would be interesting to compare the frequencies of three consecutive miscarriages with two (7). The prevalence of RPL can also vary widely between reports because of the differences in whether the RPL is primary or secondary. Primary RPL refers to multiple losses in a pregnant woman without viable previous babies, while secondary RPL refers to multiple losses in a woman who has already had a pregnancy beyond age of viability (8). The determination of the prevalence of RPL is helpful for planning clinical investigations and treatment protocols or for cost-benefit designs for allocating resources for reproductive care.
The established risk factors of RPL include endocrine, anatomical, infection-related, genetic, hemostasis-related and immunological factors (9). In a previous study by Youssef et al., aimed at determining whether the distribution of RPL-associated factors was different in women with two vs. three or more pregnancy losses, no associated factor was found in 71.5% of couples with RPL and these did not differ statistically between women with two vs. three or more pregnancy losses (10). The distribution of investigated causes did not differ between the two groups too (10). In one systematic review and meta-analysis by van Dijk et al., it was revealed that a difference in prevalence in uterine abnormalities and antiphospholipid syndrome, chromosomal abnormalities, inherited thrombophilia and thyroid disorders was not seen in women with two vs. three pregnancy losses (11).
In low- and middle-income countries like Nigeria, no conclusive research explains the prevalence and associated factors of women with a history of RPL in the region. Furthermore, according to the most recent RPL guideline from the ASRM and the ESHRE, it recommended that RPL could be considered after the loss of two or more pregnancies and stresses the importance of further scientific research, including epidemiological studies on the effect of various definitions of RPL (12). Therefore, the general objective of this research is to test the hypothesis that there is no significant difference in the prevalence of RPL and its associated risk factors when diagnosed according to the ASRM/ESHRE criteria vs. the WHO/RCOG criteria.
Methods
Study period and area
The study was carried out from December 1, 2021, to May 31, 2022, at the Nnamdi Azikiwe University Teaching Hospital (NAUTH), Nnewi, Nigeria. The hospital was selected for the survey, as it is a multidisciplinary well equipped tertiary hospital with adequate pregnant women from all over Anambra state and its environs, and also a working center for the lead author. The research hospital also had a total of 44 consultants Obstetrician-gynecologist and so were equipped for management of RPL.
Study design and population
An institutional-based cross-sectional analytical study design was applied. The study was conducted following the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) statement for reports of cross-sectional studies recommendations. All booked pregnant women attending antenatal care at NAUTH, Nnewi, Nigeria during the study period were the study population.
Inclusion and exclusion criteria
All consenting pregnant women at a gestational age of 20 weeks and below were included. Post natal women, pregnant women with history of one or more induced or spontaneous abortions and pregnant women who are unsure of their date and without early (≤20 weeks) dating ultrasound were excluded from the study.
Sample size
The sample size was 346, which was determined using the Cochran formula: n = (z2*p*q)/e2; where p is the prevalence of secondary RPL taken from the previous study by Ticconi et al. in Italy, i.e., 34.1% (13), z is 1.96 at 95% confidence level, q is (1 − p), and e is the error margin, i.e., 5% and 381 when we considered 10% attrition rate.
Sampling procedure
The study participants were selected by simple random sampling using lists (sampling frame) of pregnant women from each selected unit.
Data collection tools, procedure and quality assurance
At the time of data collection, all participants were informed about the study and its objectives and informed written consent was obtained from those participants willing to volunteer for the study. The researchers had surveyed the participants physically using the pre-structured interview questionnaire in an English format by translating it into vernacular without disturbing the actual meaning of the sentence. The translation of the questionnaire was completed by the principal investigator.
Data collection questionnaire were modified from previous similar studies. Data was collected by using pre-tested and self-administered questionnaire design in English. The tool includes three sections; socio-demographic characteristics, life style, and personal habits, and reproductive and menstrual history were included. Data collectors explained the purpose of this study to study participants and have obtained consent from participants prior to data collection. The questionnaire was pre-tested at the NAUTH, Nnewi, Nigeria, using 10 pregnant women. A one-day training was given for data collectors and supervisors on objective of the study, methods of data collection, handling of data and ways of approaching the respondents. Trained nurses measured height and weight, using the stadiometer (Model RGZ-160, China) with participants wearing no shoes. The principal investigator checked the activities of each data collector and daily checked the completeness and clarity of the questionnaires during data collection period.
Operational definition
For the World Health Organisation (WHO) and the Royal College of Obstetricians and Gynecologists (RCOG), RPL refers to three consecutive pregnancy losses, including nonvisualized ones. However, according to the American Society for Reproductive Medicine (ASRM) and the European Society for Human Reproduction and Embryology (ESHRE), it is defined as two or more clinical pregnancy losses (documented by ultrasound or histopathologic examination), but not necessarily consecutive (2). Primary RPL refers to multiple losses in a pregnant woman with no previous viable infants, whereas secondary RPL refers to multiple losses in a woman who has already had a pregnancy beyond 28 weeks of gestation (7). Advanced maternal age was defined as an age greater than 35 years. Uterine anomalies were defined as cervical weakness, fuse intrauterine connections or uterine synechiae or Asherman's syndrome, uterine myomas, and/or endometrial polyps. Endocrine factors consist of diabetes mellitus, thyroid dysfunction, prolactin abnormalities, and/or polycystic ovary syndrome. Previous psychological pressure included maternal stress during prior pregnancies. Ectopic and molar pregnancies were excluded from the definition of recurrent pregnancy loss, whereas pregnancy loss after spontaneous conception and assisted reproductive treatment were included in the definition of RPL.
Study variables
The dependent variable in this study was prior recurrent pregnancy loss. Independent variables included the following; sociodemographic characteristics (age of the participant, marital status, level of education, occupation of the partner, socioeconomic class, and smoking status of the participant), obstetric factors (parity, primiparity [those who have delivered once], multiparity [between 2 and 4 deliveries] and grand multiparity [greater than or equal to 5 deliveries]), as well as the body mass index (BMI). Social class stratification was determined according to Olusanya et al. (14): classes 1, 2, and 3 were considered upper class, middle class and lower social class, respectively. Tertiary education was defined as polytechnic or university education. The body mass index (BMI) was calculated by dividing the weight with the square of the height and the quotient expressed in kg/m2 (WHO, 2000). The BMI was then interpreted and classified as underweight (less than 18.5 kg/m2); normal (18.5–24.9 kg/m2); overweight (25–29.9 kg/m2) and obese (30 kg/m2 and above) (15). Dependent variables included history of previous pregnancy losses, medical factors (body mass index, history of any of the following: uterine anomalies, endocrine disorders, psychological pressure, antiphospholipid antibody syndrome, and unexplained factors.
Methods of data processing and analysis
After data collection, the data was cleaned and coded before data entry. Excel Spreadsheet version 2013 was used for data entry and exported to Statistical Package for Social Sciences version 25 (IBM, Armonk, NY, USA) for analysis. Descriptive summary was used to describe the characteristics of the participants in terms of frequencies, proportion, mean and standard deviation, and the information was presented by text and tables. Socio-demographic data and severity of symptoms in women with prior RPL were compared with those of women without prior RPL. Prevalence was reported as percentage with a 95% confidence interval (95% CI). The logistic regression model of bivariate and multivariate analysis was used to identify factors associated with the outcome variable and were expressed in odds ratios (ORs). In bivariate analysis, all variables with p-value less than 0.05 were considered as a candidate for multivariable analysis. For the construction of logistic regression models to determine the associated factors for RPL, the dependent variable was the presence or absence of RPL. This was put against all the variables it depended upon. These significant factors were put in a model, and factors were removed one by one to produce best-fit multiple logistic models. An adjusted odds ratio (OR) with 95% confidence intervals and a p-value less than 0.05 was considered as statistically significant association.
Ethical approval
This study was approved by the NAUTH Ethics Committee, Nnewi, Nigeria on March 17, 2021, with a reference number NAUTH/CS/66/VOL.14/VER.3/06/2020/081.
Results
Socio-demographic characteristics of the respondents
A total of 3,961 women attended the antenatal clinic during the study period. Of these, 542 were assessed for eligibility to participate in the study. One hundred twenty-four participants whose gestational age was more than 20 weeks, 11 that had at least one previous induced or spontaneous pregnancy loss and 29 participants who came for their post natal visit were excluded from the study. Therefore, 378 women were enrolled in the study and were screened for previous recurrent pregnancy loss (RPL), including 58 that had prior RPL and 320 participants without prior RPL. Of the 378 pregnant women interviewed, the overall prevalence of RPL in this study was found to be 15.34% (95% confidence interval = 11.65%–19.84%).
The age ranges from 18 to 42 years with a mean of 31.72 (SD ± 5.10) years. The mean age for those with prior RPL was 32.24 (SD ± 4.65) years while those without prior RPL were 31.63 (SD ± 5.18) years (p = 0.501). Table 1 shows the bi-variable logistic regression of the sociodemographic distribution of participants across research groups. Most 241 (63.76%) of the participants were classified into the age group of 26–35 years. Most of the participants 370 (97.88%) were married and the majority of the participants 335 (88.62%) had at least secondary level of education. Approximately 20.0% of the study participants were of upper social class.
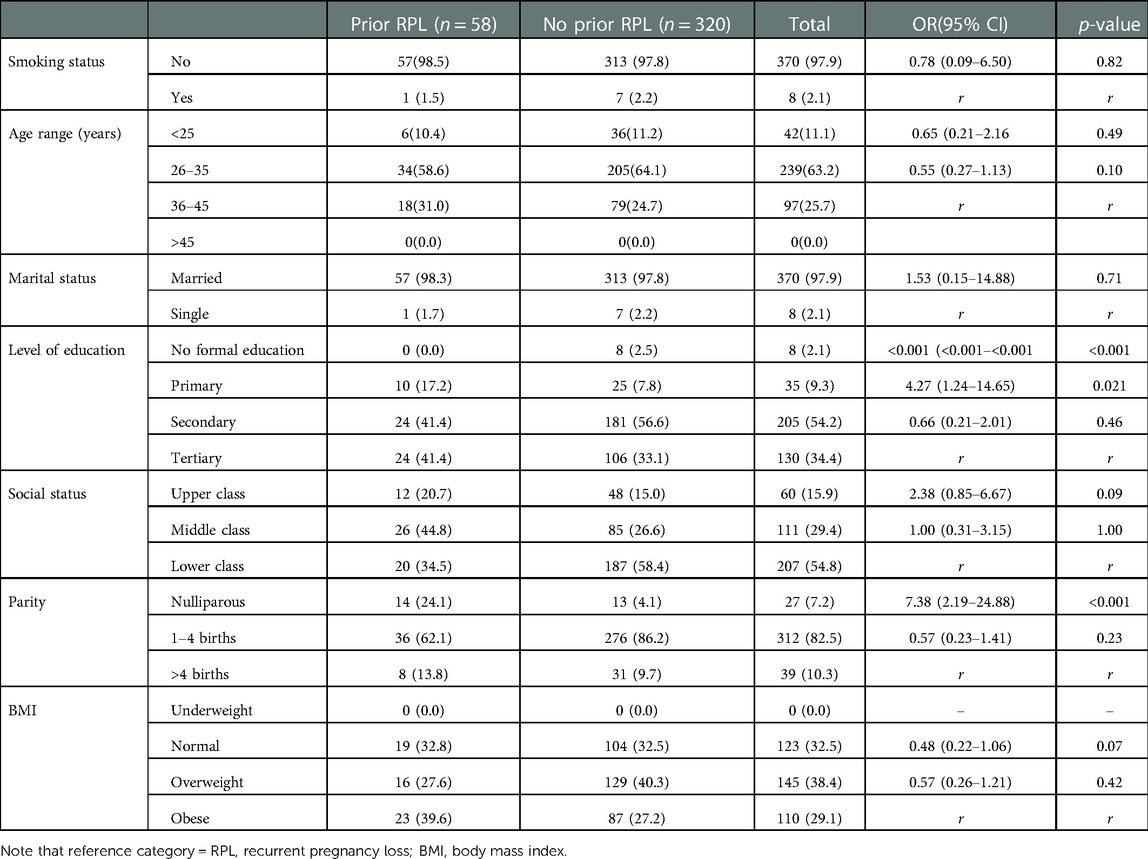
Table 1. Bi-variable logistic regression of sociodemographic distribution of participants across research groups.
Prevalence of recurrent pregnancy loss according to different international criteria and types
The overall prevalence of RPL in this study was found to be 15.34% (95% confidence interval = 11.65%–19.84%). This study identified that according to the ASRM criterion, 15.34% (95% confidence interval = 11.65%–19.84%) of participants had at least two previous RPL, while according to the WHO criterion, 5.29% (95% confidence interval = 3.23%–8.17) of the participants had at least three previous RPL. The comparison between such results revealed a significant difference of 10.05% (95% confidence interval = 7.11%–13.80%).
However, among those who had RPL, 15.52% (95% confidence interval = 7.10%–29.46%) had primary RPL while 84.48% (95% confidence interval = 62.50%–100.00%) had secondary RPL. Comparison between such results revealed a significant difference of 68.97% (95% confidence interval = 49.27%–93.91%).
Factors associated with recurrent pregnancy loss
A history of unexplained RPL, endocrine disorder, uterine anomalies, psychological pressure, and antiphospholipid syndrome were significantly associated with RPL in bivariate logistic regression analysis (Table 2). All these variables with a p-value of <0.05 in the bivariate analysis were entered to multivariable logistic regression analysis.
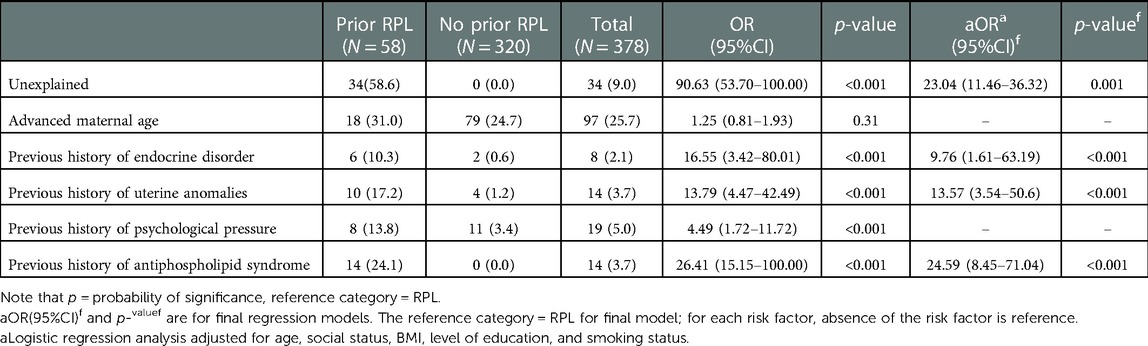
Table 2. Bi-variable and multivariable logistic regression of risk factors for RPL in the whole participants.
In multivariate analysis; history of unexplained RPL, endocrine disorder, uterine anomalies, and antiphospholipid syndrome were the factors independently associated with RPL.
Pregnant women who had RPL had 23.04 increased odds of having unexplained RPL as compared to pregnant women who had no RPL [AOR = 23.04; 95% CI (11.46, 36.32)]. Pregnant women who have a history of RPL had 9.76 increased odds of it being caused by endocrine disorder compared with those without prior RPL (AOR = 4.67; 95% CI: 2.33–9.37). Pregnant women who have prior history of RPL had 13.57 increased odds of it being caused by uterine anomalies as compared with those without prior RPL [AOR = 13.57; 95% CI (3.54, 50.60)]. Pregnant women who have a history of RPL had 24.59 increased probability of it being caused by antiphospholipid syndrome compared with those without prior RPL [AOR = 24.59;95% CI (8.41, 71.04)] (Table 2).
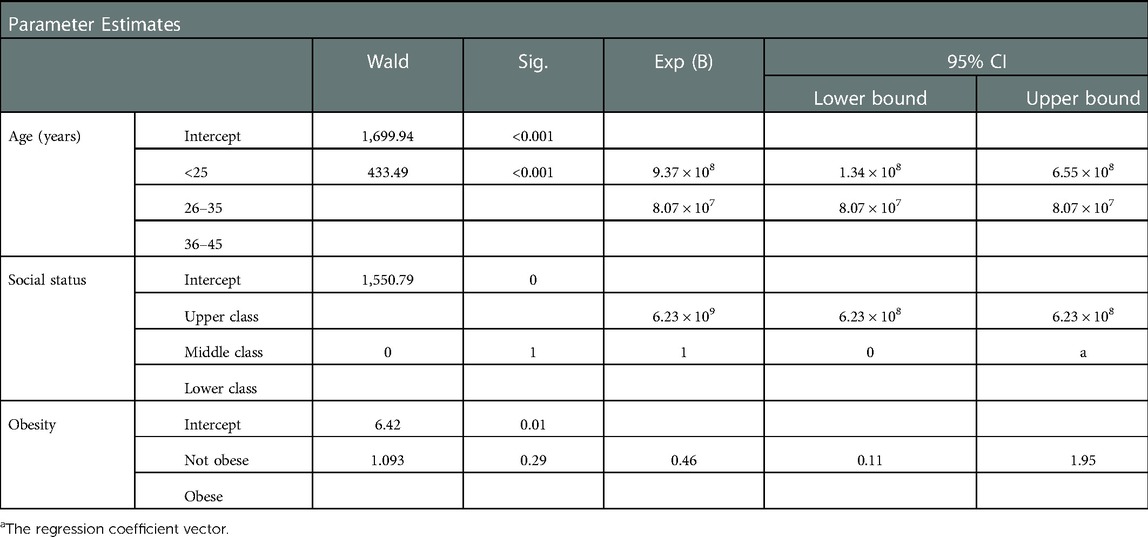
Factors associated with recurrent pregnancy loss according to definition of RPL (the WHO/RCOG and the ASRM/ESHRE criteria)
Table 3 shows the socio-demographic characteristics of participants with prior RPL according to ASRM/ESHRE (2 RPL) and WHO (≥3 RPL) criterion. In this analysis stratified by definition of RPL (ASRM/ESHRE criterion vs. WHO/RCOG criterion) the association between RPL according to the ASRM/ESHRE vs. WHO/RCOG criterion was similar in both socio-demographic parameters without significant differences. The risk factors of RPL among participants with prior RPL according to ASRM/ESHRE (2 RPL) and WHO/RCOG (≥3 RPL) criterion is shown in Table 4. Table 5 shows the multinomial regression analysis influence of factors on RPL according to ASRM/ESHRE (2 RPL) and WHO/RCOG (≥3 RPL) criterion. In these analyses, the association between RPL according to the ASRM/ESHRE vs. WHO/RCOG criterion was similar in risk factor parameters without significant differences.
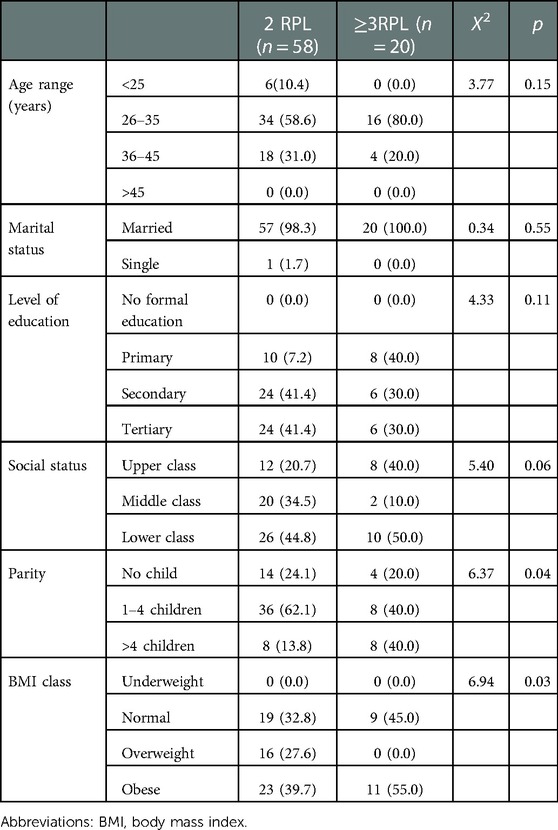
Table 3. Socio-demographic characteristics of participants with prior RPL according to ASRM (2 RPL) and wHO (≥3 RPL) criterion.
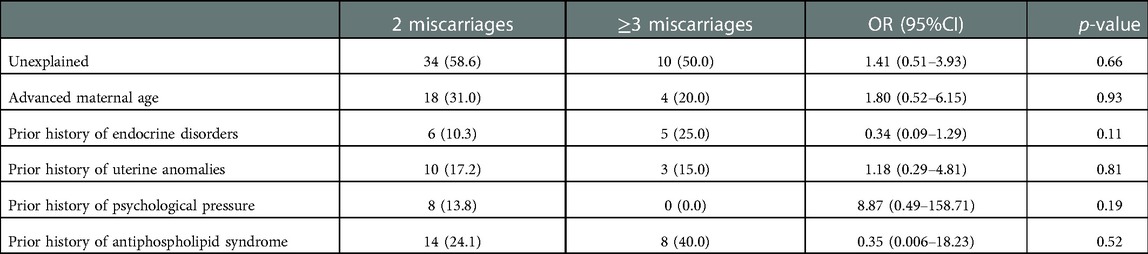
Table 4. Risk factors of RPL among participants with prior RPL according to ASRM/ESHRE (2 RPL) and wHO/RCOG (≥3 RPL) criterion.
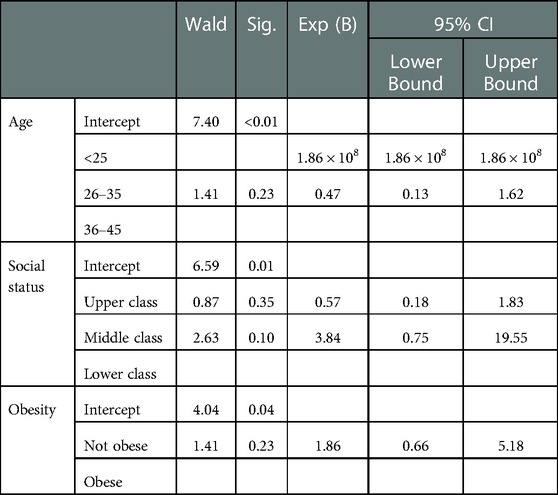
Table 5. Multinomial regression analysis influence of factors on RPL according to ASRM/ESHRE (2 RPL) and wHO/RCOG (≥3 RPL) criterion.
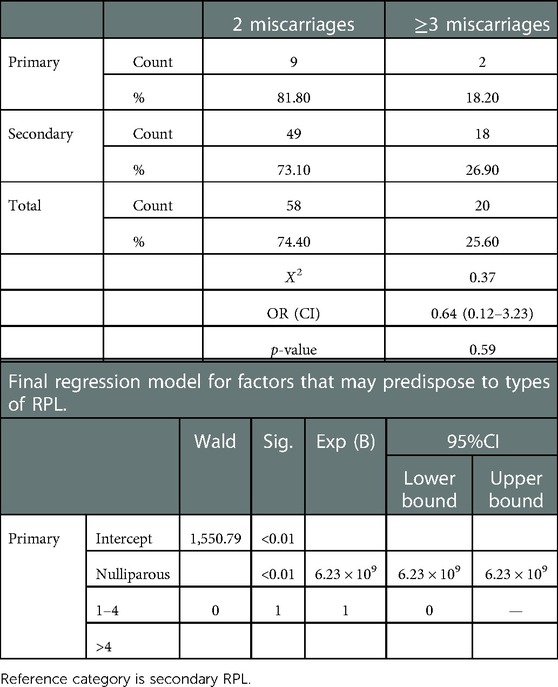
Factors associated with recurrent pregnancy loss according to type of RPL (primary vs. secondary)
Table 6 shows the socio-demographic variables by type of miscarriage (primary vs. secondary) while the risk factors for RPL among participants with prior RPL according to type of miscarriage (primary vs. secondary) is shown in Table 7. Table 8 shows the multinominal analysis of socio-demographic variables on type of RPL. In these analyses stratified by type of RPL (primary vs. secondary), the association between primary RPL and secondary RPL was observed similarly in both socio-demographic and risk factor parameters with only advanced maternal age significantly higher in women with secondary RPL.
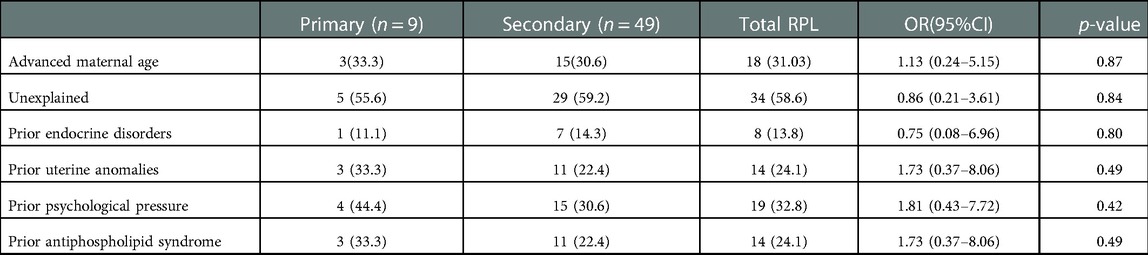
Table 7. Risk factors for RPL among participants with prior RPL according to type of miscarriage (primary vs secondary).
Association between type of RPL and RPL classification criterion
Table 9 shows the association between type of RPL and RPL classification criterion. The analysis shows there was no significant differences.
Discussion
Recurrent pregnancy loss is one of the most common infertility problems facing couples of reproductive age. Previously, there had been no study conducted in Nigeria on the prevalence and associated factors among pregnant women with a history of recurrent pregnancy loss. We have found that in pregnant women with prior history of RPL, the prevalence was 15.34% and 5.29% according to the ASRM/ESHRE and the WHO/RCOG criterion, respectively. Overall, the established significant risk factors included unexplained RPL, endocrine disorder, uterine anomalies, and antiphospholipid syndrome. A risk factor can be found in approximately 41.38% of pregnant women, while it remains unclear in the other 58.62%. This study on prevalence and risk factors of RPL is justified because the management of recurrent miscarriage should be individualized and there is currently no adequate intervention to prevent all types of recurrent miscarriage in the study environment.
The overall prevalence of RPL in this study was found to be 15.34%, which means out of hundred pregnant women around 15% of pregnant women had been affected by at least two previous pregnancy losses. This prevalence was not in keeping with the previous study conducted in Sweden with the prevalence of RPL ranges from 0.478% to 0.875%, while the mean prevalence was 0.65%. The exceedingly variability in this result could be attributed to varying age of the pregnant women at 18 years or at 42 years, respectively (7). However, when we take into account three successive losses in accordance with the WHO criterion, the prevalence was 5.29%. In a previous study, recurrent pregnancy loss affects approximately 1 to 2% of women taking into consideration three consecutive pregnancy losses occurring before 20 weeks of gestation (16). A prevalence of 0.8 to 1.4% was reported when only clinical miscarriages, i.e., pregnancy losses confirmed by ultrasound were taken into account, including biochemical losses, increasing the prevalence from 2 to 3%. The prevalence of RPL is expectedly higher in pregnant women in low and middle-income countries, because of suspected RPL or undiagnosed condition, as the required part of the diagnosis or other investigation may be lacking. Furthermore, in these low- and middle-income countries such as Nigeria, the age of fetal viability is 28 weeks, instead of 20 or 22–24 weeks agreed in high-income countries (17).
In our study, only 15.52% of the women with RPL had primary RPL while 84.48% had secondary RPL. Although comparable to Shapira et al. study in Israel that reported 39% prevalence of primary RPL and 61% prevalence of secondary RPL, the prevalence of primary RPL in our findings was lower (18). Similarly, this finding did not corroborate a previous Italian study by Vaquero et al., which had 75.70% as primary RPL and 24.30% being secondary RPL (19). In another Italian study by Ticconi et al., 65.9% of women had primary RPL while 34.1% had secondary RPL (13). Our study is also not in line with a previous study conducted in Sweden that revealed that the proportion of primary RPL and secondary RPL is 51.4% and 48.6%, respectively (6). Similarly, in an Indian study, the prevalence of primary RPL was 74.7% while secondary was 25.3% (20). The possible justification for these variations could be differences of the study population and race, diagnostic criteria, patient selection criteria, lifestyle of the participants, data collection methods and self-report nature of the study (6).
In this study, the odd of advanced maternal age is not significantly higher for pregnant women with prior RPL compared to those without RPL. This is inconsistent with report in other study in Norway (21). This finding of no significant difference for maternal age risk factor is surprising. This is because recurrent miscarriages could be due to the decreased ovarian reserve seen in advanced maternal age. Another mechanism is that advanced maternal age can lead to embryonic aneuploidy or could be due to poor egg quality leading to chromosomal (genetic) abnormalities.
Furthermore, this study showed that endocrine disorder is associated with increased chances of RPL compared to their counterparts without prior RPL. This finding is consistent with the findings of other studies and reviews (2, 3, 4, 20). This might be due to polycystic ovary syndrome, thyroid diseases, diabetes mellitus, prolactin abnormalities and implantation failure seen commonly in women with endocrine disease. Mechanisms thought to be involved are insulin resistance, hyperinsulinemia, hyperandrogenemia, or increased plasminogen activator inhibitor-1 activity (22).
In this study, pregnant women with a history of RPL had 24.59 higher odds of it being caused by antiphospholipid syndrome compared with those without a history of RPL. This finding is consistent with a previous Nigerian study by Abdulahi et al. that reported a prevalence of 14.1% for APA among women with RPL (23). Another study by Zolghadri et al. in Iran also corroborates with our findings (24). Antiphospholipid Syndrome is an autoimmune condition comprising of acquired thrombophilia and accounting for 5%–20% of recurrent pregnancy loss. The probable mechanisms of antiphospholipid antibodies causing RPL involves inducing damage of the trophoblast leading to impaired trophoblast mediated functions like spiral artery formation, secretion of growth factors, human chorionic gonadotrophin, early apoptosis of trophoblasts and abnormal inflammatory response resulting in impaired pregnancy support (25).
According to this study, the prevalence of RPL was significantly affected by the psychological pressure, but in multivariable logistic regression, adjusted for confounders, the association between RPL and the psychological pressure did not remain significant. Thus, RPL can have a significant psychological effect on the personal and professional life of the affected pregnant woman, and various feelings have been reported, such as grief and depression, hopelessness, guilt, anxiety, and anger toward the partner, friends, or the treating physician (26). Recurrent pregnancy loss has a significant psychological and emotional impact on couples (27). Several reports have looked at a possible psychological etiology for RPL, but such associations are very difficult to prove with the presence of various variables and confounding factors (7, 28).
However, some studies have showed that the causes of miscarriage that can be recognized after two pregnancy losses are like the causes that can be recognized after three consecutive pregnancy losses. For instance, a study involving 351 participants with consecutive second trimester miscarriages found the causes were idiopathic (51%), antiphospholipid syndrome (33%), cervical weakness (8%), uterine anomaly 267 (4%), bacterial vaginosis (3%), and hypothyroidism (1%) (29). Our study has confirmed similar report because the association between RPL according to the ASRM vs. WHO criterion was similarly observed in both socio-demographic and risk factor parameters without any significant differences. Therefore, it has been recommended that couples with two or more consecutive spontaneous miscarriages warrant an evaluation to identify any factor that may be associated with their poor reproductive history.
The clinical implications for these findings are that when we consider the ASRM/ESHRE criterion for RPL diagnosis, the prevalence will be significantly higher than when the WHO/RCOG definition criteria are used. However, the risk factors in each international criteria remain the same for both. This means that we can expend more resources in the use of ASRM/ESHRE than WHO/RCOG criteria, but with expectedly high chance of preventing further pregnancy loss if adequate intervention is put in place during subsequent pregnancies. The findings highlight that obstetric care providers should adopt a holistic and couple-focused approach in their prevention of subsequent RPL and include attention to the cumulative effect of multiple pregnancy losses on the woman (30). In addition, couples with RPL usually express concern about the cause and risk of recurrence. RPL requires medical intervention encompassing access to specialized clinics, investigations, and enhanced support and monitoring during future pregnancies. Most women with a history of RPL are likely to receive care from tertiary or specialist centers as they will be able to provide them with the care they need to prevent future occurrence. Women with unexplained RPL recognize that no specific cause could be identified in previous diagnostic workups after previous losses (31).
This study appears to be the first study that examined hospital based prevalence of RPL and distribution of associated risk factors in Nigeria, as no conclusive research explains the prevalence and associated factors of women with a history of RPL. These findings are vital for reproductive health policy design and program planning in low and middle-income settings. As many Nigerian states are contemplating including either two or three previous pregnancy losses in the routine antenatal high-risk program work up for RPL, our findings are important to make evidence based decisions.
Our study provided information on the novelty of the prevalence of RPL in low-income settings according to two different international and national criteria for the diagnosis of RPL. There is also the collection of comprehensive clinical data on the types and criteria for RPL. Furthermore, this research has provided baseline information on pregnant women with a history of RPL, which will help to recognize and treat underlying issues and help when allocating resources for reproductive care and preventive healthcare strategies for women with RPL.
We acknowledge certain limitations of our study. Given the cross-sectional design and our study being a single-center hospital-based study; therefore, the findings may not be applicable around the country or globally. Also, this simple cross-sectional study could not establish the determining causal relationship in all cases. Additionally, our findings should be taken with caution because the prevalence of RPL varies a lot in different studies and depends on definition of the condition (two or three losses, consecutive or non-consecutive losses, biochemical losses included or excluded) as well as the study design (cross-sectional, observational, case-control, registry and /or hospital based reports). Furthermore, the lack of comparison groups limits its control over unobserved heterogeneity among respondents. Furthermore, although some women were referred with prior diagnosis of RPL, we did not have access to data on diagnostic measures used to confirm various risk factors or causes. Most of the miscarriages were self-reported and it is based on questionnaires completed by the participants, with the known methodological problems of potential recall and selection bias. Even though 30% of pregnant women initially approached for screening did not participate in the study, we were able to recruit a relatively large sample of participants. Lifestyle and obesity also play an important significant role in RPL, although this was not revealed in our present study (32). Another limitations of the study was that we did not obtain any information regarding the paternal factors as well as information regarding cases reported with problems in the male counterpart with regards to smoking, obesity and diabetes mellitus in the absence of male factor abnormalities. This is because recent studies have shown that advanced paternal age is also associated with an increased risk of spontaneous recurrent miscarriage (33–35).
Conclusion
The prevalence of RPL was 15.34% and 5.29% according to the ASRM/ESHRE and the WHO/RCOG criterion respectively, with secondary type predominating. Unexplained loss, endocrine disorder, uterine anomalies and antiphospholipid syndrome were significantly associated with RPL. No significant differences with regard to risk factors were seen according to the two different international diagnostic criteria except advanced maternal age which was significantly higher in secondary type of RPL than in the primary type. Further research is needed to confirm our findings and to better characterize the magnitude of the differences in the prevalence and any possible differences in the risk factors according to different national and international criteria for RPL.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Nnamdi Azikiwe University Teaching Hospital Ethics committee with approval number of NAUTH/CS/66/VOL.14/VER.3/06/2020/081. The patients/participants provided their written informed consent to participate in this study.
Author contributions
GUE contributed to the conception of the study, design and manuscript writing. UIN supervised the study contributed to the design and manuscript writing. EOU, DEM, EPI, EUN, JOU and JII contributed to manuscript writing and revision. GUE and DEM contributed to data collection, analysis and manuscript writing. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors appreciate the help of the staff of Nnamdi Azikiwe University Teaching Hospital, Nnewi, Nigeria and participants involved in the study. The study was developed from the author's PhD Dissertation, “A cohort study of serum biomarkers, psychological health profile and pregnancy outcomes in women with recurrent pregnancy loss in NAUTH, Nnewi, Anambra state”, whose proposal was presented for the Doctorate in Human Reproduction & Women Health at the Institute of Maternal and Child Health, School of Medicine, University of Nigeria Ituku-Ozalla, Enugu, Nigeria.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Elsharkawy NB, Mohamed SM, Awad MH, Ouda MMA. Effect of happiness counseling on depression, anxiety, and stress in women with recurrent miscarriage. Int J Womens Health. (2021) 13:287–95. doi: 10.2147/IJWH.S283946
2. Youssef A, Vermeulen N, Lashley EELO, Goddijn M, van der Hoorn MLP. Comparison and appraisal of (inter)national recurrent pregnancy loss guidelines. Reprod Biomed Online. (2019) 39(3):497–503. doi: 10.1016/j.rbmo.2019.04.008
3. Green DM, O'Donoghue K. A review of reproductive outcomes of women with two consecutive miscarriages and no living child. J Obstet Gynaecol. (2019) 39(6):816–21. doi: 10.1080/01443615.2019.1576600
4. Toth B, Würfel W, Bohlmann M, Zschocke J, Rudnik-Schöneborn S, Nawroth F, et al. Recurrent miscarriage: diagnostic and therapeutic procedures. Guideline of the DGGG, OEGGG and SGGG (S2k-level, AWMF registry number 015/050). Geburtshilfe Frauenheilkd. (2018) 78(4):364–81. doi: 10.1055/a-0586-4568
5. Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. (2012) 98(5):1103–11. doi: 10.1016/j.fertnstert.2012.06.048
6. Royal College of Obstetricians and Gynaecologists, Scientific Advisory Committee, Guideline No. 17. The Investigation and treatment of couples with recurrent miscarriage (2011). Available at: http://www.rcog.org.uk/womens-health/clinical-guidance/investigation-and-treatmentcouples-recurrent-miscarriage-green-top- (Accessed June 28, 2022).
7. Rasmark Roepke E, Matthiesen L, Rylance R, Christiansen OB. Is the incidence of recurrent pregnancy loss increasing? A retrospective register-based study in Sweden. Acta Obstet Gynecol Scand. (2017) 96(11):1365–72. doi: 10.1111/aogs.13210
8. Hachem H E, Crepaux V, May-Panloup P, Descamps P, Legendre G, Bouet PE. Recurrent pregnancy loss: current perspectives. Int J Womens Health. (2017) 9:331–45. doi: 10.2147/IJWH.S100817
9. Vomstein K, Aulitzky A, Strobel L, Bohlmann M, Feil K, Rudnik-Schöneborn S, et al. Recurrent spontaneous miscarriage: a comparison of international guidelines. Geburtshilfe Frauenheilkd. (2021) 81(7):769–79. doi: 10.1055/a-1380-3657
10. Youssef A, Lashley L, Dieben S, Verburg H, van der Hoorn ML. Defining recurrent pregnancy loss: associated factors and prognosis in couples with two versus three or more pregnancy losses. Reprod Biomed Online. (2020) 41(4):679–85. doi: 10.1016/j.rbmo.2020.05.016
11. van Dijk MM, Kolte AM, Limpens J, Kirk E, Quenby S, van Wely M, et al. Recurrent pregnancy loss: diagnostic workup after two or three pregnancy losses? A systematic review of the literature and meta-analysis. Hum Reprod Update. (2020) 26(3):356–67. doi: 10.1093/humupd/dmz048
12. ESHRE Guideline Group on RPL, Bender Atik R, Christiansen OB, Elson J, Kolte AM, Lewis S, Middeldorp S, et al. ESHRE Guideline: recurrent pregnancy loss. Hum Reprod Open. (2018) 2018(2):hoy004. doi: 10.1093/hropen/hoy004
13. Ticconi C, Pietropolli A, Specchia M, Nicastri E, Chiaramonte C, Piccione E, et al. Pregnancy-Related complications in women with recurrent pregnancy loss: a prospective cohort study. J Clin Med. (2020) 9(9):2833. doi: 10.3390/jcm9092833
14. Olusanya O, Okpere EE, Ezimokhai M. The importance of social class in voluntary fertility control in a developing country. West Afr J Med. (1985) 4(4):205–12.
15. de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. (2007) 85(9):660–7. doi: 10.2471/blt.07.043497
16. Li J, Wang L, Ding J, Cheng Y, Diao L, Li L, et al. Multiomics studies investigating recurrent pregnancy loss: an effective tool for mechanism exploration. Front Immunol. (2022) 13:826198. doi: 10.3389/fimmu.2022.826198
17. Ikechebelu JI, Eleje GU, Ugochukwu EF, Edokwe ES. Should we Re-define age of fetal viability in Nigeria? A case report of newborn survival from Pre-viable Pre-labor rupture of membranes. J Womens Health Issues Care. (2014) 3:3. doi: 10.4172/2325-9795.1000142
18. Shapira E, Ratzon R, Shoham-Vardi I, Serjienko R, Mazor M, Bashiri A. Primary vs. Secondary recurrent pregnancy loss–epidemiological characteristics, etiology, and next pregnancy outcome. J Perinat Med. (2012) 40(4):389–96. doi: 10.1515/jpm-2011-0315
19. Vaquero ME, Valentini B, Lazzarin N, Nuccitelli A, Valensise HC. Are HLA-G polymorphisms associated to recurrent pregnancy loss? JGynaecol Obstet. (2021) 33(1):26–35. doi: 10.36129/jog.33.01.03
20. Ali S, Majid S, Niamat Ali M, Taing S, El-Serehy HA, Al-Misned FA. Evaluation of etiology and pregnancy outcome in recurrent miscarriage patients. Saudi J Biol Sci. (2020) 27(10):2809–17. doi: 10.1016/j.sjbs.2020.06.049
21. Magnus MC, Wilcox AJ, Morken NH, Weinberg CR, Håberg SE. Role of maternal age and pregnancy history in risk of miscarriage: prospective register based study. Br Med J. (2019) 364:l869. doi: 10.1136/bmj.l869
22. Sultana S, Nallari P, Ananthapur V. Recurrent pregnancy loss (RPL): an overview. J Women's Health Dev. (2020) 3:302–15. doi: 10.26502/fjwhd.2644-28840038
23. Abdullahi ZG, Abdul MA, Aminu SM, Musa BO, Amadu L, Jibril E-BM. Antiphospholipid antibodies among pregnant women with recurrent fetal wastage in a tertiary hospital in northern Nigeria. Ann Afr Med. (2016) 15(3):133–7. doi: 10.4103/1596-3519.188894
24. Zolghadri J, Gharesi-Fard B, Parsanezhad M, Alborzi S. The prevalence of antiphospholipi D syndrome in patients with recurrent pregnancy loss: a report from south of Iran. Med J Islam Repub Iran. (2004) 18(2):119–21.
25. Sultana S, Nallari P, Ananthapur V. Recurrent pregnancy loss (RPL): an overview. J Women's Health Dev. (2020) 3:302–15. doi: 10.26502/fjwhd.2644-28840038
26. Kolte AM, Olsen LR, Mikkelsen EM, Christiansen OB, Nielsen HS. Depression and emotional stress is highly prevalent among women with recurrent pregnancy loss. Hum Reprod. (2015) 30(4):777–82. doi: 10.1093/humrep/dev014
27. Kuhlmann E, Scharli P, Schick M, Ditzen B, Langer L, Strowitzki T, et al. The posttraumatic impact of recurrent pregnancy loss in both women and men. Geburtshilfe Frauenheilkd. (2023) 83(1):88–96. doi: 10.1055/a-1916-9180
28. Hada K, Kuse E, Nakatsuka M. Women with recurrent pregnancy loss: their psychology during late pregnancy and the supportive behavior of their partners. Acta Med Okayama. (2018) 72(4):387–94. doi: 10.18926/AMO/56176
29. McNamee KM, Dawood F, Farquharson RG. Mid-trimester pregnancy loss. Obstet Gynecol Clin North Am. (2014) 41(1):87–102. doi: 10.1016/j.ogc.2013.10.007
30. Koert E, Malling GMH, Sylvest R, Krog MC, Kolte AM, Schmidt L, et al. Recurrent pregnancy loss: couples’ perspectives on their need for treatment, support and follow up. Hum Reprod. (2019) 34(2):291–6. doi: 10.1093/humrep/dey362
31. Deng T, Liao X, Zhu S. Recent advances in treatment of recurrent spontaneous abortion. Obstet Gynecol Surv. (2022) 77(6):355–66. doi: 10.1097/OGX.0000000000001033
32. Eleje GU, Eke AC, Ikechebelu JI, Ezebialu IU, Okam PC, Ilika CP. Cervical stitch (cerclage) in combination with other treatments for preventing spontaneous preterm birth in singleton pregnancies. Cochrane Database Syst Rev. (2020) 9(9):CD012871. doi: 10.1002/14651858.CD012871
33. du Fossé NA, van der Hoorn MP, van Lith JMM, le Cessie S, Lashley EELO. Advanced paternal age is associated with an increased risk of spontaneous miscarriage: a systematic review and meta-analysis. Hum Reprod Update. (2020) 26(5):650–69. doi: 10.1093/humupd/dmaa010
34. Nguyen BT, Chang EJ, Bendikson KA. Advanced paternal age and the risk of spontaneous abortion: an analysis of the combined 2011–2013 and 2013–2015 national survey of family growth. Am J Obstet Gynecol. (2019) 221(5):476.e1–476.e7. doi: 10.1016/j.ajog.2019.05.028
Keywords: abortions, antiphospholipid syndrome, counselling, miscarriage, recurrent
Citation: Eleje GU, Ugwu EO, Igbodike EP, Malachy DE, Nwankwo EU, Ugboaja JO, Ikechebelu JI and Nwagha UI (2023) Prevalence and associated factors of recurrent pregnancy loss in Nigeria according to different national and international criteria (ASRM/ESHRE vs. WHO/RCOG). Front. Reprod. Health 5:1049711. doi: 10.3389/frph.2023.1049711
Received: 21 September 2022; Accepted: 9 January 2023;
Published: 21 February 2023.
Edited by:
Singh Rajender, Central Drug Research Institute (CSIR), IndiaReviewed by:
Mohamed Farghali, Ain Shams University, EgyptGayatri Mohanty, University of Massachusetts Amherst, United States
© 2023 Eleje, Ugwu, Igbodike, Malachy, Nwankwo, Ugboaja, Ikechebelu and Nwagha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: George Uchenna Eleje Z2VvcmdlbDIxQHlhaG9vLmNvbQ==; Z3UuZWxlamVAdW5pemlrLmVkdS5uZw==
Specialty Section: This article was submitted to Reproductive Epidemiology, a section of the journal Frontiers in Reproductive Health
 George Uchenna Eleje
George Uchenna Eleje Emmanuel Onyebuchi Ugwu2,4
Emmanuel Onyebuchi Ugwu2,4 Emeka Philip Igbodike
Emeka Philip Igbodike Joseph Ifeanyichukwu Ikechebelu
Joseph Ifeanyichukwu Ikechebelu