- 1Division of Physical Activity and Weight Management, Department of Internal Medicine, University of Kansas Medical Center, Kansas City, MO, United States
- 2Division of Endocrinology, Diabetes and Clinical Pharmacology, Department of Internal Medicine, University of Kansas Medical Center, Kansas City, MO, United States
- 3Department of Biostatistics, University of Kansas Medical Center, Kansas City, MO, United States
- 4Department of Obstetrics and Gynecology, Center for Advanced Reproductive Medicine, University of Kansas Medical Center, Kansas City, MO, United States
Background: Obesity and visceral adiposity are associated with anovulation. The most common cause of anovulatory infertility in women of reproductive age is polycystic ovary syndrome (PCOS). We conducted this formative study to examine the effects of a remotely delivered, group-based lifestyle program for women with overweight/obesity and PCOS on ovulation, PCOS related quality of life (PCOSQ) and body composition.
Methods: Women with anovulatory infertility caused by PCOS (N = 12) were enrolled in a 6-month high-intensity weight management intervention. Participants were asked to attend 45 min., group behavioral lifestyle sessions, delivered remotely by a registered dietitian weekly across the 6-mo. study and comply with a reduced energy diet, increased physical activity (225 min/wk.), and self-monitoring of weight, physical activity and diet. Diets consisted of five portion-controlled meals (three shakes + two entrees), at least five servings of fruits/vegetables, and ad libitum non-caloric beverages daily. Wilcoxon signed-rank tests were used to assess changes in outcomes across the intervention.
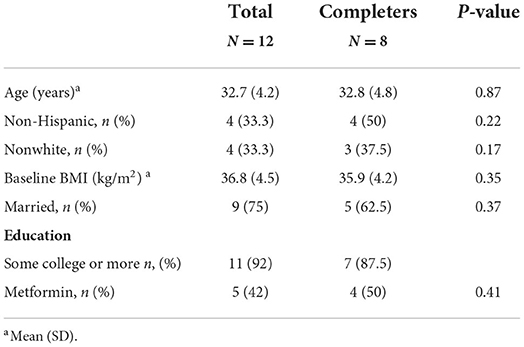
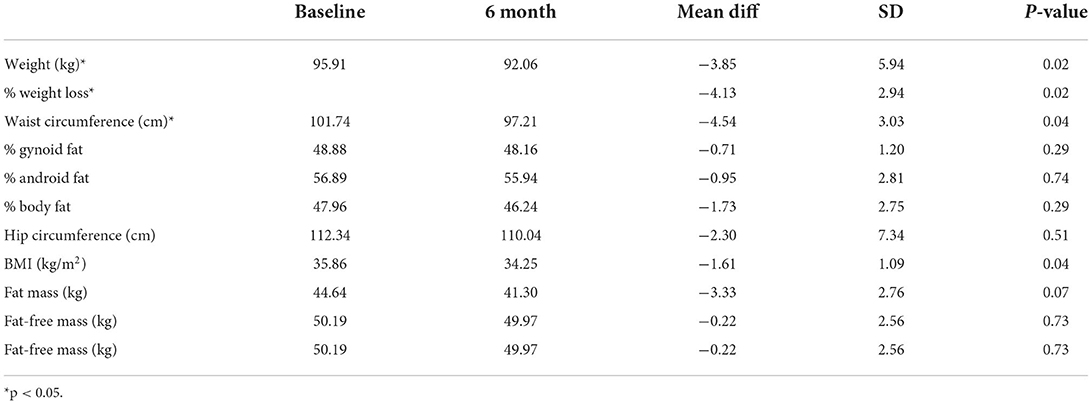
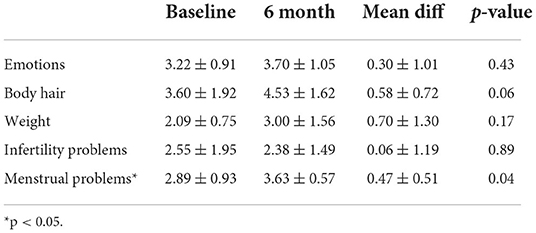
Results: Twelve women received the weight loss intervention (mean age = 32.7 ± 4.2 yrs., BMI = 36.8 ± 4.5 kg/m2, 92% college educated), and 8 completed the intervention. Eight (67%) women reported ovulating during the intervention with an average time to ovulation of 57 ± 45 days. Women lost an average of 3.85 ± 5.94 kg (p = 0.02), decreased their BMI (−1.61 ± 1.09 kg/m2; p = 0.04), and waist circumference (−4.54 ± 3.03 cm; p = 0.04) over the 6-mo. intervention. Additionally, self-reported menstrual problems measured by PCOSQ significantly improved over the study (p = 0.03).
Conclusion: A multicomponent group-based, remotely delivered, lifestyle intervention delivered remotely is a feasible and potentially scalable option to achieve clinically relevant (>3%) weight loss in women with PCOS.
Clinical trial registration: www.clinicaltrials.gov, identifier: NCT03677362.
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women of reproductive age characterized by ovarian dysfunction, hyperandrogenism and polycystic ovarian morphology (1, 2). Women with PCOS often have severe metabolic consequences including increased abdominal fat, insulin resistance and metabolic syndrome (3, 4). Due to a potential bidirectional relationship with obesity and PCOS (5), the prevalence of overweight and obesity is significantly higher in women with PCOS compared with women without PCOS, with estimates ranging from 40 to 80% (6). Obesity further heightens the reproductive, metabolic, and psychological dysfunction caused by PCOS (7), and women with both PCOS and obesity are more likely to suffer from anovulation compared to women with PCOS and a normal body weight (8). Lifestyle intervention targeting at least 5–15% weight loss is the recommended first line of treatment for women with PCOS and overweight or obesity (5, 9–11), though there is no established weight or weight loss threshold to induce ovulation or resumption of menstruation in women with PCOS and overweight/obesity.
There is a complex interplay of physical and psychological symptoms in women with PCOS and obesity leading to high rates of depression, stress, anxiety, low self-esteem, low quality of life, and poor body image (12, 13). However, diet and exercise programs can result in weight loss, improved endocrine function, improved quality of life and wellbeing, improved nutrition, and increased pregnancy rates (5, 7, 14–18). Lifestyle interventions in infertile women without PCOS may restore ovulatory cycles, though the impact on live birth rates is inconclusive (19, 20). Still, resumption of menstruation and ovulation is an important starting point to treat infertility and 42–90% of women may resume ovulation during or following a diet and lifestyle intervention (15, 16, 19, 21, 22). Costs associated with a live birth in anovulatory women with obesity are 100% greater than their normal weight counterparts (23). First line medical therapy for anovulation is an ovulatory stimulant such as clomiphene citrate which has a comparable out-of-pocket cost to weight loss treatment over 6 months and results in similar rates of live births (24). However, reports indicate this medication causes depressed mood and/or mood swings in 41 and 45% of women, respectively (25). In contrast, it is well established that lifestyle change programs result in improvements in mood and quality of life (26).
The 2018 International Evidence-Based Guideline for Assessment and Management of Polycystic Ovary Syndrome recommends that a healthy lifestyle and prevention and management of excess weight should be prioritized in all people with PCOS (10). However, the management of PCOS is inconsistent and the needs of these women are not being adequately met (10). Barriers to implementation of lifestyle and/or weight management in those with PCOS are similar to those of the general population as the primary healthcare professionals tasked with treatment of PCOS are not well equipped to deliver lifestyle interventions (27). Insufficient utilization of allied health professionals, such as dietitians, exacerbates dissatisfaction in medical care for these women (27). Improving the availability and cost of programs or professionals to support lifestyle management is key to improving referrals from general practitioners and therefore improve health care for women with PCOS (27). In addition, women with PCOS suffer greater feelings of isolation compared to women without PCOS and may benefit from social support (28) and specifically treatment in a group setting with psychological support (29, 30). Further, increased attention has been given to the need for accessible lifestyle modification programs that decrease common barriers such as time and work commitments, while still producing beneficial psychological and physiological outcomes that are inexpensive for women with PCOS (10). Alternative delivery strategies may be especially important in women with PCOS as younger populations tend to have lower adherence, decreased retention and lower weight loss in lifestyle interventions (31). Thus, replicable, remotely delivered lifestyle change programs can fill a notable treatment gap for this population. Therefore, we conducted a single-arm formative study to examine the effects of a 6-month remotely delivered, multicomponent lifestyle group-based program tailored specifically for women with anovulatory infertility caused by PCOS on ovulation, quality of life and body composition.
Materials and methods
Overview
We delivered health education to women with PCOS to improve diet, physical activity, and behavior in a 6-month intervention. We instructed participants to use portion-controlled meals (PCMs), increase fruits and vegetables, increase moderate to vigorous physical activity (MVPA), and to self-report these behaviors weekly. Ovulation was tracked using ovulation prediction kits (OPKs; Pregmate, Fort Lauderdale, FL) and an OvuSense OvuCore™ fertility sensor (Fertility Focus Inc., Old Saybrook, CT). A registered dietitian led group behavioral counseling sessions remotely via video conferencing (Skype™, Palo Alto, CA). This study was conducted in the Kansas City Metropolitan Area and was approved by the Institutional Review Board at the University of Kansas Medical Center. The study was registered at clinicaltrials.gov (NCT03677362).
Participant eligibility
Participants satisfying the following criteria were eligible for this study. Inclusion: Age 21–42 years, BMI 25–44.9 kg/m2, diagnoses of anovulatory infertility caused by PCOS as assessed by Rotterdam criteria (2), verified by their physician, willing to delay fertility treatment for the 6-month intervention and weight stable (±4.6 kg) for the previous 3 months. Women taking metformin were included in the study. Exclusion: A history of another infertility diagnosis other than ovulatory dysfunction resulting from PCOS, unable to participate in moderate vigorous physical activity (such as brisk walking), currently participating in greater than three, 30-min bouts of planned physical activity per week, participating in a weight loss or physical activity program in the previous 6 months, taking a weight loss medication in the previous 2 months, evidence of binge eating disorder, or currently on birth control medication which would prevent ovulation from occurring. Women who became pregnant during the intervention were removed from the study.
Recruitment
Participants were recruited from September 2018 to September 2019 through reproductive endocrinology clinics, social media, University of Kansas Medical Center's Healthcare Enterprise Repository for Ontological Narration (HERON), an i2b2-based clinical integrated data repository (32), obstetrics and gynecology clinics, and PCOS support groups. All participants provided written informed consent before engaging in the study.
Study intervention
Participants completed a 6-month, group-based, weekly, and remotely delivered lifestyle intervention that recommended a reduced-energy diet and increased exercise and delivered behavioral change techniques to facilitate adherence to recommendations.
Diet
Guidance to follow a ~1,200–1,500 kcal/day diet as recommended by 2018 International Evidence-Based Guideline for Assessment and Management of Polycystic Ovary Syndrome (10) was provided. A generally healthy, nutrient-balanced, low-fat diet was chosen based on our experience implementing this dietary pattern and current evidence indicating that no specific energy-equivalent diet is better than another in this population (10). Participants were able to choose a combination of two commercially available PCMs, three low-calorie shakes, and at least five one-cup equivalent servings of fruits and vegetables per day. We provided directions and a list of examples to purchase PCMs that contained ~200–300 kcals and had ≤3 g saturated fat. We provided meal replacement shakes (Profile by Sanford Health, Sioux Falls, SD). Participants were able to order from a variety of shake flavors, and the shakes contained ~100 kcal and 15 g protein each.
Physical activity
A recommendation to achieve ≥ 225 min/week of moderate-vigorous physical activity (MVPA) was provided as recommended in the “2009 ACSM Position Stand on Physical Activity Interventions for Weight Loss and Prevention of Weight Regain in Adults” (33). This recommendation is line with the 2018 International Evidence-Based Guideline for Assessment and Management of Polycystic Ovary Syndrome to achieve ≥ 150 min/week of MVPA for health and prevention of weight gain, or ≥250 min/week of MVPA for weight loss. The recommendation progressed from 20 min/day-3 days/wk. the first week to 45 min/day-5 days/wk. by week 6 and remained at that level through 6 months. For physical activity to count toward this weekly number, the physical activity had to be aerobic in nature and last ≥10 min in duration. While not counting toward their weekly minutes, participants were also encouraged to include muscle strengthening exercises at least 2 days per week. Participants were asked to increase their daily steps by 10% each week from their current level until reaching a goal of 10,000 steps/day to reduce sedentary activity. Pedometers (Omron HJ-320, Lake Forest, IL) were provided to participants as both a motivational tool and to self-monitor daily activity and were worn over the non-dominant hip.
Behavioral intervention
Participants were given a comprehensive program notebook which included general guidelines regarding participation in the study, calendars and timelines for class meetings and data reporting, lessons for each session, and detailed instructions for the diet plan, including appropriate recipes, handouts, worksheets, and assignments specific to the topic for each session. Registered dietitians delivered behavioral counseling sessions via group video conferencing (Skype) weekly for 24 weeks. These ~45 min. group meetings utilized behavioral strategies based on Social Cognitive theory (SCT) to promote beneficial lifestyle changes in diet and exercise (34). Counseling strategies included goal setting, self-monitoring, promotion of self-efficacy, manipulation of the environment to promote behavioral change, and reflection on outcome expectations and outcome values. Sessions began with a check-in question designed to identify barriers to diet and exercise to allow the group to work together to identify solutions and to promote group cohesion and social support. Each session included education on a weight management topic, such as reading nutrition labels, planning for social situations, exercising while on vacation, etc. Additionally, PCOS-specific education material was provided, such as the role of nutrition and physical activity on insulin resistance. We provided weekly homework assignments to increase self-efficacy for diet, exercise, and self-monitoring and to encouraging practicing behavioral strategies. Lastly, participants reported their self-monitoring data (diet, PA and weight) and received one-on-one feedback via email from the dietitian to promote intervention adherence.
Outcome assessments
All outcome measures were collected at the University of Kansas Medical Center from December 2018 to February 2020. Demographic information was collected prior to the intervention (baseline) and outcome assessments were completed at baseline and 6 months by trained research assistants. Attendance at group sessions, ovulation, and self-monitoring data were collected weekly via self-report using REDCap electronic data capture tools hosted at the University of Kansas Medical Center (35).
Ovulation
Each participant was provided both ovulation prediction kits (OPKs; Pregmate, Fort Lauderdale, FL) and OvuSense OvuCore™ fertility sensor (Fertility Focus Inc., Old Saybrook, CT) to predict ovulation occurrence (36). OPKs have a high accuracy (97%) of detecting ovulation, are accessible, non-invasive, and affordable (37) and were used to assess ovulation as the primary outcome. OPKs predict ovulation by detecting the presence of luteinizing hormone (LH) in urine, or the LH surge, which occurs 24–36 h prior to ovulation (38). Participants were instructed to begin urine LH testing on menstrual cycle day 10 and to test every morning until a positive LH surge was seen or until cycle day 18. Participants collected a urine sample each morning upon rising and used the test strip to detect LH.
The OvuSense OvuCore™ fertility sensor provided a second method to detect ovulation through the monitoring of core body temperature. This was used as a secondary device to assess utility and acceptability. This device may help predict ovulation based on the principle that the rise in progesterone in the second half of the cycle results in a rise in basal body temperature (39). The OvuCore™ fertility sensor device measuring 118 mm tip to tail, with a maximum outside diameter of 23 mm was self-inserted into the vagina each night, and recorded core body temperature at 5-min intervals during the night. Once removed upon waking, the sensor was cleaned with soap and water, dried, and connected by the near-field communication (NFC) reader to upload data to the patient's smart phone via the OvuSense app. Participants were instructed to start using the sensor the day after menstruation ended and continue using each night until the start of the next menstruation period each month throughout the duration of the study. Ovulation occurrence results from both the OPK's and OvuSense device were self-reported by participants via their weekly REDCap survey.
Feasibility, attendance, safety, and satisfaction
Retention was measured as the number of women who completed outcome assessment at both baseline and 6 months. Attendance at group sessions was recorded by the dietitian. Group session attendance was defined as being logged in to the video conference and remaining on the screen for the entire 45-min session. Safety was measured as the number of adverse and serious adverse events occurring during the study. Data on intervention acceptance and satisfaction were assessed through an end-of-study survey.
Program adherence
Participants tracked their total weekly servings of shakes, entrees, fruits, vegetables, minutes of MVPA, days off plan, weight and steps. Days off plan was defined as eating anything that was not a portion-controlled shake, entrée, fruit, vegetable, non-caloric beverage or allotted 60 kcals of condiments. In addition, weight was recorded by a home scale. These data were self-reported weekly via a REDCap survey (35, 40). Weekly totals were reviewed by the dietitian and feedback was provided both individually and in the group meetings to identify barriers and promote social support.
Anthropometrics and body composition
Anthropometric and body composition outcomes were collected after a 12-h fast at baseline and month 6. Weight was obtained with participant in a hospital gown by a digital scale (Befour, Inc Model #PS6600, Saukville, WI). Height was measured using a stadiometer. Waist and hip circumference were obtained using the protocol by Lohman et al. (41). Dual-energy x-ray absorptiometry (DXA) was used to measure body composition and specifically fat-free mass, fat mass, and percent body fat (Lunar DPX-IQ, GE Healthcare, Madison, WI). Pregnancy tests were conducted on all women and verified as negative prior to administering the DXA scans. DXA scans were analyzed using native software for Lunar Prodigy Advance (GE Healthcare, Madison, WI). Total fat mass and fat-free mass determined by the sum of lean soft tissue plus bone mineral content were used in the analysis.
Dietary intake
Energy and macronutrient intake was measured using self-administered, web-based VioScreen Graphical Food Frequency System (Viocare Technologies, Inc., Princeton, NJ) (42). Additionally, Healthy Eating Index 2010 (HEI-2010) scores were calculated with the data collected in VioScreen. Participants completed the survey at home on a tablet or personal computer within 1 or 2 days following the baseline and 6-month testing visits.
Questionnaires
The three-factor eating questionnaire (TFEQ) (43) was used to assess behaviors related to eating, including cognitive restraint of eating, uncontrolled eating or disinhibition, and emotional eating. There are sub-categories within each factor to better understand each of these eating behaviors. Item responses on the TFEQ are scored as 0 or 1 and summed. Higher scores indicate higher levels of restrained eating, disinhibited eating, and predisposition to hunger. PCOS health-related quality of life questionnaire (PCOSQ) (44) was used to assess quality of life and included questions specific to women with PCOS related to body hair, infertility, emotions, menstruation, and weight management.
Statistics
All statistical tests were 2-tailed and P < 0.05 was considered the level of statistical significance. Baseline and demographic characteristics were summarized for the entire sample and completers only (N = 8) in Table 1 with continuous variables presented as mean ± standard deviation and categorical variables are presented as percentages and proportions. Primary analyses were conducted on completers only. Time to ovulation was calculated as the difference between the date of self-reported ovulation minus the start date of the intervention. Changes in anthropometrics, body composition, PCOSQ and TFEQ, from baseline to 6 months were assessed via Wilcoxon signed-rank tests which were corrected for multiple testing using Hommel's adjustment. Exploratory Wilcoxon signed-rank tests were conducted to assess differences in weight loss between those who met attendance recommendations (attended at least 75% of sessions) and those taking Metformin. Statistical analyses were conducted using SAS 9.4 and R 3.6.3.3.
Results
Participants/retention/safety
Out of 192 volunteers screened, 14 women met initial eligibility criteria. Primary reasons for exclusion were (1) no longer interested in the study or no response after initial contact (33.7%), (2) outside of BMI range (21.9%), (3) no PCOS diagnosis (18.5%) and 4) prohibitive dietary restrictions (10.1%). Twelve women completed baseline testing and enrolled in the study, with an average age of 33 years, a BMI of 36.8 kg/m2, 8 (66%) were white and 11 (92%) were college educated. Eight women (67%) completed 6-month testing. Of the 4 women who did not complete the intervention, 1 became pregnant and 3 did not respond when contacted for 6-month outcome testing. No serious adverse events were reported. There were no baseline differences in those who completed the intervention and those who did not complete the intervention (Table 1).
Ovulation and menstruation rates
On average women reported 2.9 ± 2.0 menstrual cycles during the intervention. Ovulation occurred 1.2 ± 1.2 times as measured by the OPK, and 0.8 ± 0.8 times as measured by the OvuSense OvuCore™ device. Eight (67%) women reported ovulating measured by OPK during the intervention with an average time to ovulation of 57 ± 45 days. Days since starting weight loss intervention and cumulative ovulation rates measured via OPK are shown in Figure 1.
Anthropometrics and body composition
Women lost an average of −3.85 ± 5.94 kg (4.13%, p = 0.02), decreased their BMI by 1.61 ± 1.09 kg/m2 (p = 0.04), and decreased their waist circumference by 4.54 ± 3.03 cm (p = 0.04) during the intervention (Table 2). No differences in weight loss were observed between those taking Metformin (N = 4; −4.20 ± 2.32%) and those not taking Metformin (N = 4; −4.07 ± 3.83%) (p = 0.73). The intervention resulted in a loss of fat mass that did not reach statistical significance (−3.33 ± 2.76 kg; P = 0.07). There were no significant changes across the intervention for gynoid fat percentage, android fat percentage, body fat percentage, hip circumference or fat-free mass.
PCOSQ
Self-reported menstrual problems measured by PCOSQ significantly improved over the study (p = 0.04) as shown in Table 3. These problems included headaches related to their previous menstrual cycle, irregular menstrual periods, abdominal bloating and menstrual cramps. We did not see any other significant differences across the other domains measured including emotions, body hair, weight and infertility.
Participant satisfaction
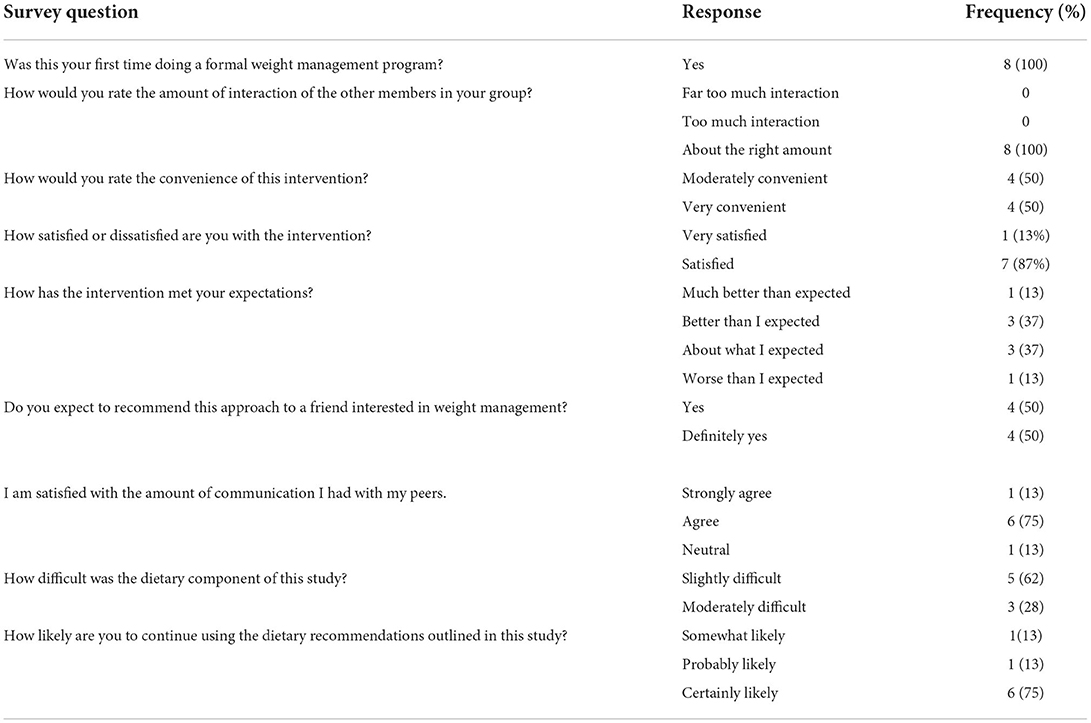
A custom end-of-intervention survey was conducted to measure intervention satisfaction (Table 4). Overall study completers found the intervention to be moderately convenient 4 (50%) or very convenient 4 (50%). All participants were very satisfied 1 (12%) or satisfied 7 (88%) with the intervention. Five (63%) of the participants rated the dietary component slightly difficult, while 3 (37%) rated it moderately difficult. When asked if participants would recommend this approach to weight management to their friends of family, 4 (50%) said yes and the other 4 (50%) reported definitely yes.
Attendance
On average, attendance at weekly behavioral lessons was 68% (~16 sessions attended out of 24 total). Six (50%) of participants attended the recommended 75% or more of behavioral sessions. Percent weight loss did not differ between those who attended 75% or more behavioral sessions (−4.63 ± 3.28%) compared to who did not (−2.65 ± 0.90%) (p = 0.55).
Three-factor eating
Changes in cognitive restraint of eating, uncontrolled eating or disinhibition, and emotional eating were assessed at baseline and 6-months. We found no differences across the intervention in cognitive restraint of eating (p = 0.23), uncontrolled eating (p = 0.16), or emotional eating (p = 0.09).
Diet
Valid dietary data was obtained from 4 people at baseline and 6 months. At baseline participants consumed 2.7 servings of fruit/day, 2.9 servings of vegetables/day, had a mean HEI-2010 score of 55.5, and reported energy intake of 1,971 kcals/day. After the 6-month intervention fruit intake increased by ~1 serving per day, vegetable intake increased by ~2.0 servings per day and HEI-2010 scores increased by ~14.5 points per day.
Adherence to program recommendations
Average weekly totals for entrees, shakes, fruits and vegetables, number of days eating off the meal plan, MVPA and steps are reported in Table 5. Overall, participants did not meet the recommendations for entrees, shakes, and fruits and vegetables; however, they exceeded the recommended MVPA target of 225 min per week with an average of 260 ± 133 min per week during the intervention. The listed amounts of shakes, fruit, vegetable, moderate-vigorous physical activity (MVPA) and steps were the minimum weekly recommendation. Participants were encouraged not to deviate from the allowed foods (off plan) and to attend each week.
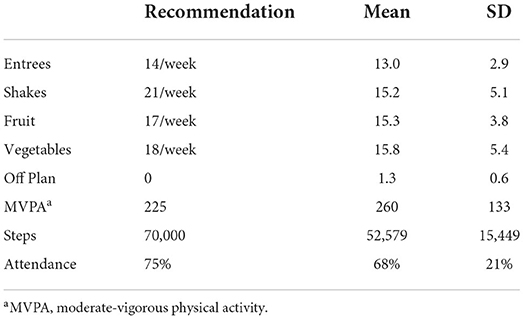
Table 5. Program recommendations, diet and physical activity adherence and weekly self-monitoring (n = 12).
Discussion
In this study, we investigated the feasibility of a remotely delivered 6-month lifestyle intervention in adult women with PCOS and overweight/obesity and the impact on ovulation rates and time-to-ovulation. First, we observed 66% of the previously anovulatory women in our study resumed ovulation as measured by at least one of our two ovulation detection methods. The average time to ovulation was 57 days with an average self-reported weight loss of −5.3% at time to ovulation. However, we cannot rule out that these women ovulated randomly, and it was independent of the lifestyle intervention. The ovulation rates we observed are lower than a single arm trial by Clark et al. where 81% of the women were anovulatory, 90% of the total sample resumed spontaneous ovulation (16, 45). Our results are more favorable than Mutsaetrs et al. (22) where a diet and exercise intervention prompted resumption of ovulation in 42% of the women perhaps due to a more structured diet and physical activity program.
In our study, women with overweight/obesity and PCOS lost significant weight (4.2%) and decreased their BMI over 6 months following a remotely delivered multicomponent lifestyle intervention. This amount of weight loss is clinically significant and resulted in positive metabolic and psychological outcomes. Our weight loss results at 6 months are similar to Jiskoot et al. in their recently published randomized controlled trial where they found 5.1% weight loss at 12-months in women randomized to a 3-component lifestyle intervention delivered in-person (46).
Additionally, our results for improvement in menstrual cycle symptoms in the menstrual domain of the PCOSQ match results found by Dokras et al. (47) when analyzing the OWL-PCOS study which was a three-arm randomized controlled trial to examine pre-pregnancy interventions including face-to-face lifestyle modification to induce weight loss, hormonal contraception to suppress androgens and the combination of face-to-face lifestyle modification and hormonal contraception on live birth rates in overweight/obese women with PCOS. Additionally, we saw a borderline significant improvement in the body hair domain (p = 0.06) of the PCOSQ. Excess body hair (hirsutism) in women with PCOS has been associated with anxiety and depression therefore our lifestyle intervention has the potential to offer psychological benefits as well. Dokras et al. also noted significant improvement in menstrual, weight and fertility domains over a 16-week lifestyle intervention and improvement in all domains (emotion, body hair, menstrual, weight and infertility) in the combined lifestyle intervention and oral contraception pill group (47).
Women with PCOS face many of the same barriers to lifestyle modifications as women without PCOS such as work commitments, time, costs, childcare and access to opportunities for physical activity (48). However, women with PCOS have higher rates of body dissatisfaction, low referral rates to dietitians, more sedentary time per day and consume an additional ~60 kcals/day compared to women without PCOS. Disappointed by the lack of information provided on lifestyle management from the healthcare system, women with PCOS are a notable population that need more resources and innovative options such as remotely delivered lifestyle interventions to improve their health care and quality of life.
There are several strengths of this study, including following the AHA/ACC/TOS guidelines for lifestyle interventions (14). The lifestyle intervention was delivered remotely via Skype which allowed participants in a broad geographical region to participate. Body composition was measured using DXA providing insight to the reduction in fat mass across the intervention. We had a very diverse sample (33% minority). PCOS is very heterogeneous, and presentation varies among race and ethnicity therefore it is extremely important to include diverse women in clinical research. End of study surveys noted great acceptance and participant satisfaction. Participants enjoyed the health education component from a registered dietitian and dietary curriculum focused on PCOS.
Limitations of this pilot study include a small sample size and lack of control group due, in part, to stringent eligibility criteria. Many of the patients who initially expressed interest in the study did not qualify because they were on a hormonal form of contraception (oral, intrauterine devices or implant) or declined to participate. In addition, we elected not to include a control group due to the time course nature of fertility. Women must be trying to conceive unsuccessfully for 12 months before being seen and treatment started with a reproductive endocrinologist. For this study, women had to delay treatment further (6 months) for participation in the intervention to test the impact of weight loss on ovulation rates. Therefore, we thought it was unethical to enroll a control group undergoing no fertility treatment or lifestyle intervention for 6 months which is crucial time for this population. Due to the anovulatory nature of this cohort, we were unable to take into account where each participant was at in their current menstrual cycle which led to left censoring/truncation of the data and inability to assess impact of weight loss on time-to-ovulation.
Attrition in this remotely delivered lifestyle intervention was 33%. This level of attrition is similar to lifestyle interventions in the general population (~31%) (49, 50) but lower than lifestyle interventions in women with PCOS that can be anywhere from 50 to 67% (46, 50). Reasons for attrition included becoming pregnant and non-response for the 6-month measurements. Additionally, we noticed that those participants who did not come in for 6-month measurements were not successful with weight loss toward the end of the intervention which is consistent with other reports in the literature. There should be more focus on retention efforts during lifestyle interventions in women with PCOS as those who remain in interventions are more likely to be successful at weight loss.
Implementation of this remotely delivered lifestyle intervention for women with PCOS provides a feasible option for health care providers interested in weight loss for improvement of metabolic, reproductive and psychological features for overweight and obese women with PCOS. Larger and adequately powered trials are necessary to determine the threshold of weight loss necessary induce ovulation and regulate menstruation in overweight and obese women with PCOS.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board of University of Kansas Medical Center. The patients/participants provided their written informed consent to participate in this study.
Author contributions
AG, LP, CM, and JD: conceptualization. AG and RNM: formal analysis. AG and FS: writing—original draft preparation. AG, LP, FS, CM, JD, RM, RNM, and PS: writing—review and editing. AG: supervision. FS, RM, and PS: project administration. AG and CM: funding acquisition. All authors have read and agreed to the published version of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by an NIH Clinical and Translational Science Award grant (UL1 TR002366) awarded to the University of Kansas Medical Center (KUMC), and an internal Lied Basic Science Grant Program of the KUMC Research Institute. Additionally, this study was supported by funding from the Department of Obstetrics and Gynecology and Division of Physical Activity and Weight Management at KUMC.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Azziz R, Marin C, Hoq L, Badamgarav E, Song P. Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J Clin Endocrinol Metab. (2005) 90:4650–8. doi: 10.1210/jc.2005-0628
2. Rotterdam EA-SPCWG. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. (2004) 81:19–25. doi: 10.1016/j.fertnstert.2003.10.004
3. Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. (2010) 16:347–63. doi: 10.1093/humupd/dmq001
4. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. (2012) 33:981–1030. doi: 10.1210/er.2011-1034
5. Teede HJ, Joham AE, Paul E, Moran LJ, Loxton D, Jolley D, et al. Longitudinal weight gain in women identified with polycystic ovary syndrome: results of an observational study in young women. Obesity. (2013) 21:1526–32. doi: 10.1002/oby.20213
6. Sam S. Obesity and polycystic ovary syndrome. Obes Manag. (2007) 3:69–73. doi: 10.1089/obe.2007.0019
7. Lim SS, Norman RJ, Davies MJ, Moran LJ. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. (2013) 14:95–109. doi: 10.1111/j.1467-789X.2012.01053.x
8. Al-Azemi M, Omu FE, Omu AE. The effect of obesity on the outcome of infertility management in women with polycystic ovary syndrome. Arch Gynecol Obstet. (2004) 270:205–10. doi: 10.1007/s00404-003-0537-2
9. Moran LJ, Pasquali R, Teede HJ, Hoeger KM, Norman RJ. Treatment of obesity in polycystic ovary syndrome: a position statement of the androgen excess and polycystic ovary syndrome society. Fertil Steril. (2009) 92:1966–82. doi: 10.1016/j.fertnstert.2008.09.018
10. Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. (2018) 33:1602–18. doi: 10.1093/humrep/dey256
11. Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. (2016) 22 (Suppl. 3):1–203. doi: 10.4158/EP161365.GL
12. Kocelak P, Chudek J, Naworska B, Bak-Sosnowska M, Kotlarz B, Mazurek M, et al. Psychological disturbances and quality of life in obese and infertile women and men. Int J Endocrinol. (2012) 2012:236217. doi: 10.1155/2012/236217
13. Barnard L, Ferriday D, Guenther N, Strauss B, Balen AH, Dye L. Quality of life and psychological well being in polycystic ovary syndrome. Hum Reprod. (2007) 22:2279–86. doi: 10.1093/humrep/dem108
14. Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the obesity society. J Am Coll Cardiol. (2014) 63:2985–3023. doi: 10.1016/j.jacc.2013.11.004
15. Huber-Buchholz MM, Carey DG, Norman RJ. Restoration of reproductive potential by lifestyle modification in obese polycystic ovary syndrome: role of insulin sensitivity and luteinizing hormone. J Clin Endocrinol Metab. (1999) 84:1470–4. doi: 10.1210/jc.84.4.1470
16. Clark AM, Thornley B, Tomlinson L, Galletley C, Norman RJ. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod. (1998) 13:1502–5. doi: 10.1093/humrep/13.6.1502
17. Galletly C, Clark A, Tomlinson L, Blaney F. Improved pregnancy rates for obese, infertile women following a group treatment program. An open pilot study. Gen Hosp Psychiatry. (1996) 18:192–5. doi: 10.1016/0163-8343(96)00006-0
18. Kim SY, Park ES, Kim HW. Effectiveness of non-pharmacological interventions for overweight or obese infertile women: a systematic review and meta-analysis. Int J Environ Res Public Health. (2020) 17:7438. doi: 10.3390/ijerph17207438
19. Smithson DS, Vause TDR, Cheung AP. No. 362-ovulation induction in polycystic ovary syndrome. J Obstet Gynaecol Can. (2018) 40:978–87. doi: 10.1016/j.jogc.2017.12.004
20. Kim EJ, Jang M, Choi JH, Park KS, Cho IH. An improved dehydroepiandrosterone-induced rat model of polycystic ovary syndrome (PCOS): post-pubertal improve PCOS's features. Front Endocrinol. (2018) 9:735. doi: 10.3389/fendo.2018.00735
21. Best D, Avenell A, Bhattacharya S. How effective are weight-loss interventions for improving fertility in women and men who are overweight or obese? A systematic review and meta-analysis of the evidence. Hum Reprod Update. (2017) 23:681–705. doi: 10.1093/humupd/dmx027
22. Mutsaerts MA, van Oers AM, Groen H, Burggraaff JM, Kuchenbecker WK, Perquin DA, et al. Randomized trial of a lifestyle program in obese infertile women. N Engl J Med. (2016) 374:1942–53. doi: 10.1056/NEJMoa1505297
23. Koning AM, Kuchenbecker WK, Groen H, Hoek A, Land JA, Khan KS, et al. Economic consequences of overweight and obesity in infertility: a framework for evaluating the costs and outcomes of fertility care. Hum Reprod Update. (2010) 16:246–54. doi: 10.1093/humupd/dmp053
24. Karimzadeh MA, Javedani M. An assessment of lifestyle modification versus medical treatment with clomiphene citrate, metformin, and clomiphene citrate-metformin in patients with polycystic ovary syndrome. Fertil Steril. (2010) 94:216–20. doi: 10.1016/j.fertnstert.2009.02.078
25. Choi SH, Shapiro H, Robinson GE, Irvine J, Neuman J, Rosen B, et al. Psychological side-effects of clomiphene citrate and human menopausal gonadotrophin. J Psychosom Obstet Gynaecol. (2005) 26:93–100. doi: 10.1080/01443610400022983
26. Martin CK, Bhapkar M, Pittas AG, Pieper CF, Das SK, Williamson DA, et al. Effect of calorie restriction on mood, quality of life, sleep, and sexual function in healthy nonobese adults: the CALERIE 2 randomized clinical trial. JAMA Intern Med. (2016) 176:743–52. doi: 10.1001/jamainternmed.2016.1189
27. Blackshaw LCD, Chhour I, Stepto NK, Lim SS. Barriers and facilitators to the implementation of evidence-based lifestyle management in polycystic ovary syndrome: a narrative review. Med Sci. (2019) 7:76. doi: 10.3390/medsci7070076
28. Elsenbruch S, Hahn S, Kowalsky D, Offner AH, Schedlowski M, Mann K, et al. Quality of life, psychosocial well-being, and sexual satisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. (2003) 88:5801–7. doi: 10.1210/jc.2003-030562
29. Domar AD, Clapp D, Slawsby EA, Dusek J, Kessel B, Freizinger M. Impact of group psychological interventions on pregnancy rates in infertile women. Fertil Steril. (2000) 73:805–11. doi: 10.1016/S0015-0282(99)00493-8
30. Hartmann-Boyce J, Johns DJ, Jebb SA, Aveyard P, Behavioural Weight Management Review Group. Effect of behavioural techniques and delivery mode on effectiveness of weight management: systematic review, meta-analysis and meta-regression. Obes Rev. (2014) 15:598–609. doi: 10.1111/obr.12165
31. Gokee-LaRose J, Gorin AA, Raynor HA, Laska MN, Jeffery RW, Levy RL, et al. Are standard behavioral weight loss programs effective for young adults? Int J Obes. (2009) 33:1374–80. doi: 10.1038/ijo.2009.185
32. Waitman LR, Warren JJ, Manos EL, Connolly DW. Expressing observations from electronic medical record flowsheets in an i2b2 based clinical data repository to support research and quality improvement. AMIA Annu Symp Proc. (2011) 2011:1454–63.
33. Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exercise. (2009) 41:459–71. doi: 10.1249/MSS.0b013e3181949333
34. Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ, US: Prentice-Hall, Inc. (1986). xiii, 617-xiii, p.
35. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) 42:377–81. doi: 10.1016/j.jbi.2008.08.010
36. Papaioannou S, Aslam M, Al Wattar B, Milnes R, Knowles T. Ovulation assessment by vaginal temperature analysis (the ovusense advanced fertility monitoring system) in comparison to oral temperature measurement. Fertil Steril. (2012) 98:S160. doi: 10.1016/j.fertnstert.2012.07.590
37. Bonnar J, Flynn A, Freundl G, Kirkman R, Royston R, Snowden R. Personal hormone monitoring for contraception. Br J Fam Plann. (1999) 24:128–34.
38. Su HW, Yi YC, Wei TY, Chang TC, Cheng CM. Detection of ovulation, a review of currently available methods. Bioeng Transl Med. (2017) 2:238–46. doi: 10.1002/btm2.10058
39. Papaioannou S, Aslam M, Al Wattar BH, Milnes RC, Knowles TG. User's acceptability of OvuSense: a novel vaginal temperature sensor for prediction of the fertile period. J Obstet Gynaecol. (2013) 33:705–9. doi: 10.3109/01443615.2013.817984
40. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. (2019) 95:103208. doi: 10.1016/j.jbi.2019.103208
41. Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics (1988).
42. Kristal AR, Kolar AS, Fisher JL, Plascak JJ, Stumbo PJ, Weiss R, et al. Evaluation of web-based, self-administered, graphical food frequency questionnaire. J Acad Nutr Diet. (2014) 114:613–21. doi: 10.1016/j.jand.2013.11.017
43. Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. (1985) 29:71–83. doi: 10.1016/0022-3999(85)90010-8
44. Cronin L, Guyatt G, Griffith L, Wong E, Azziz R, Futterweit W, et al. Development of a health-related quality-of-life questionnaire (PCOSQ) for women with polycystic ovary syndrome (PCOS). J Clin Endocrinol Metab. (1998) 83:1976–87. doi: 10.1210/jc.83.6.1976
45. Clark AM, Ledger W, Galletly C, Tomlinson L, Blaney F, Wang X, et al. Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Hum Reprod. (1995) 10:2705–12. doi: 10.1093/oxfordjournals.humrep.a135772
46. Jiskoot G, Timman R, Beerthuizen A, Dietz de Loos A, Busschbach J, Laven J. Weight reduction through a cognitive behavioral therapy lifestyle intervention in PCOS: the primary outcome of a randomized controlled trial. Obesity. (2020) 28:2134–41. doi: 10.1002/oby.22980
47. Dokras A, Sarwer DB, Allison KC, Milman L, Kris-Etherton PM, Kunselman AR, et al. Weight loss and lowering androgens predict improvements in health-related quality of life in women with PCOS. J Clin Endocrinol Metab. (2016) 101:2966–74. doi: 10.1210/jc.2016-1896
48. Pirotta S, Joham AJ, Moran LJ, Skouteris H, Lim SS. Implementation of evidence-based PCOS lifestyle management guidelines: perceived barriers and facilitators by consumers using the theoretical domains framework and COM-B model. Patient Educ Couns. (2021) 104:2080–8. doi: 10.1016/j.pec.2021.01.036
49. Elobeid MA, Padilla MA, McVie T, Thomas O, Brock DW, Musser B, et al. Missing data in randomized clinical trials for weight loss: scope of the problem, state of the field, and performance of statistical methods. PLoS ONE. (2009) 4:e6624. doi: 10.1371/journal.pone.0006624
Keywords: polycystic ovary syndrome, obesity, weight loss, ovulation, quality of life
Citation: Gorczyca AM, Steger FL, Ptomey LT, Montgomery RN, Mickelsen R, Smith P, Donnelly JE and Marsh CA (2022) The impact of a group based, remotely delivered weight loss intervention in women with polycystic ovary syndrome on ovulation, quality of life and body composition. Front. Reprod. Health 4:940945. doi: 10.3389/frph.2022.940945
Received: 10 May 2022; Accepted: 04 July 2022;
Published: 22 July 2022.
Edited by:
Melanie Cree-Green, University of Colorado, United StatesReviewed by:
Admire Takuranenhamo Chikandiwa, Wits Health Consortium (WHC), South AfricaEleni Memi, National and Kapodistrian University of Athens, Greece
Rhonda Garad, Monash University, Australia
Copyright © 2022 Gorczyca, Steger, Ptomey, Montgomery, Mickelsen, Smith, Donnelly and Marsh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna M. Gorczyca, YWdvcmN6eWNhQGt1bWMuZWR1
 Anna M. Gorczyca
Anna M. Gorczyca Felicia L. Steger2
Felicia L. Steger2 Robert N. Montgomery
Robert N. Montgomery Joseph E. Donnelly
Joseph E. Donnelly