- 1Department of Pathology, Institute of Infectious Disease and Molecular Medicine, University of Cape Town, Cape Town, South Africa
- 2Centre for the AIDS Programme of Research in South Africa (CAPRISA), Durban, South Africa
- 3Department of Medical Microbiology, University of KwaZulu-Natal, Durban, South Africa
- 4National Health Laboratory Service, Cape Town, South Africa
- 5Ragon Institute of MGH, MIT and Harvard, Cambridge, MA, United States
- 6Harvard Medical School, Boston, MA, United States
- 7Department of Obstetrics and Gynecology, Massachusetts General Hospital, Boston, MA, United States
Several soluble cytokines have been associated with microbicide-induced cervicovaginal inflammation, non-optimal vaginal microbiota, and risk of HIV acquisition. Many of these biomarkers are used in preclinical assays to estimate the safety of vaginally applied products. However, there are currently no validated biomarkers to evaluate the safety of novel vaginal products in clinical trials. This hinders the rapid and rational selection of novel products being tested in first-in-human trials. We reviewed available literature to assess how best to select and measure soluble immune markers to determine product safety in first in human clinical trials of novel vaginal products.
Introduction
Vaginally delivered products are used or are in development for contraception, sexual lubrication, prevention of sexually transmitted infections such as human immunodeficiency virus (HIV) and both treatment and prevention of vaginal infections. Currently, preclinical testing of novel products includes safety assessment in the laboratory using cell lines, cervicovaginal tissue or explant models, and small animal models to identify products with significant cytotoxic effects. While these may suffice for basic toxicity assays, humans are the only species in which a vaginal microbiota low in diversity and dominated by Lactobacillus species is commonly identified (1, 2). Thus, these models offer only a poor approximation of in vivo conditions.
The most well-described consideration for vaginal products is avoidance of hypertonic formulations which are cytotoxic, increase epithelial cell shedding and decrease epithelial integrity (3–5). Inflammation in the human female genital tract, regardless of the cause or presence of symptoms, creates an environment associated with a range of adverse health outcomes, including increased risk of HIV acquisition (6). Any new product should ensure no off-target, pro-inflammatory effects, however optimal safety biomarkers to ensure this are not well established. Currently, many studies of vaginal products measure clinical signs and symptoms. However, vaginal fluid biomarkers are likely more appropriate for this assessment, as there are often no overt clinical signs of inflammation in people at higher risk. The vaginal microbicide field made progress in characterizing markers suggestive of inflammation and mucosal damage, but a threshold for a “danger” signal has not been established (7–10).
Vaginally applied live biotherapeutic products to promote a Lactobacillus-dominant microbiota are in varying stages of development. The lack of an animal model for the human vagina means that few products have robust preclinical data describing their impact on inflammation in the human female genital tract in the context of existing microbial communities. In this review, we will address the following questions: Which soluble immune markers should the field focus on when assessing genital inflammation during first in human trials of novel vaginally applied products? How should these be measured, and what signals reflect an increased risk for adverse outcomes-or conversely, indicate the most promising intervention to reduce the risk for adverse outcomes?
The Cautionary Tale of Nonoxynol-9
The initial realization that a product presumed to be safe and protective could have an unforeseen impact on risk for viral infections occurred with the nonionic surfactant nonoxynol-9 (nonylphenoxypolyethoxyethanol; N-9), which was used as the active component of spermicides for almost 50 years. Early in vitro studies of N-9 demonstrated broad-spectrum activity against a number of STIs, including Chlamydia trachomatis, Neisseria gonorrhoeae, Herpes simplex virus (HSV)-2 and HIV (11–18). The in vitro data and the widespread use of N-9 as a contraceptive lead to development of this compound as a topical microbicide. Contrary to expectations, the final phase 2/3 clinical trial of N-9 vaginal gel showed an almost 2-fold greater increase in HIV acquisition with high-frequency use of N-9 (19), which was linked to an increase in genital mucosal inflammation.
The strength of the N-9 mediated induction of soluble pro-inflammatory markers was shown to be dependent on dosing and length of exposure (20, 21). A single application of N-9 caused a significant increase in Macrophage Inflammatory Protein (MIP)-1β and RANTES (Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted) by 12h in a mouse model (20). However, in humans, a single application of N-9 did not result in detectable increases in any of the pro-inflammatory markers measured, including interleukin (IL)-1α, IL-1β, IL-8, soluble tumor necrosis factor receptor (sTNF-R)I and sTNF-RII, or IL-1 receptor antagonist (RA). Repeated dosing (3 daily exposures) was needed to observe a significant increase of several markers, including IL-1α, IL-1β, IL-6, IL-8 and MIP-1β at varying times after the last application (12–60 h) (21). Prolonged use of N-9 was associated with increased IL-1β in cervicovaginal secretions and a subsequent increase in IL-1-mediated NFkB activation, resulting in chemokine-induced recruitment of immune cells (21).
Compound-induced damage to the mucosa generally begins with the production of IL-1 and other inflammatory cytokines by damaged epithelial cells. This is followed by NF-kB and AP-1-mediated release of pro-inflammatory cytokines [including IL-1, IL-6, and TNF-a] and chemokines [including IL-8, IL-10, and macrophage inflammatory protein (MIP)-3]. The inflammatory cascade results in the induction of endothelial vascular adhesion molecules, increase in lymphocyte trafficking markers, and the overall immune activation and infiltration of immune cells in the female genital tract (22). This suggests that these cytokines (IL-1, IL-6, and TNF-α), released early during the inflammatory cascade, might be good soluble immune markers to assess a ‘danger signal' during novel product testing in humans.
The Effects of Endogenous Microbiota
Another source of data to define safety biomarkers are studies of mucosal responses to the vaginal microbiota. Diverse, non-Lactobacillus dominant vaginal microbial communities have been associated with epithelial barrier damage, in part through increases in matrix metalloproteinases (MMPs) (23) and apoptosis of vaginal epithelial cells through caspase-3 activation (24–26). Whether by culture, Gram stain or 16S rRNA gene sequencing, women with low abundance of diverse microbes and high abundance of vaginal lactobacilli–such as L. crispatus–have lower risk for these adverse outcomes (27–29).
In cross-sectional studies, women with bacterial vaginosis (BV) and/or more diverse, non-Lactobacillus dominant communities consistently had higher concentrations of IL-1β and IL-1α than women without BV, with consistently decreased IP-10 concentrations. This is true across studies from several geographic locations (Table 1). Other markers show more variability in studies across continents, and from different decades of analysis, including IL-6, IL-8, IL-10, TNF-α (Table 1).
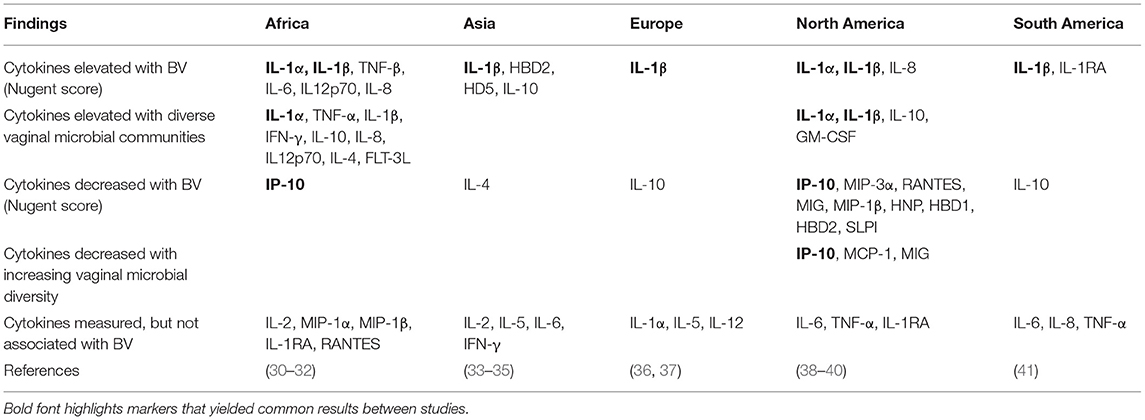
Table 1. Associations between vaginal immune markers and microbiota in cross-sectional studies of non-pregnant, HIV-uninfected women.
Longitudinal studies among HIV-uninfected, non-pregnant women showed that transition from a Lactobacillus-dominant to a non-Lactobacillus dominant microbial community is associated with significant increases in IL-1α, IL-1β, TNF-α, and IL12p70 (30, 42). The converse, a transition to a Lactobacillus-dominant community is associated with a decrease in IL-1α, IL-1β, IL-2, IL-4, IL-10, IL-18, TRAIL, TNF-α, and an increase in IP-10, MIG, MCP-1, MIP-1α, MIP-3α, and GROα (43–45). In a US study, treatment of BV was associated with a decrease in GM-CSF and IFN-γ, while in a Kenyan study the two markers increased with decreasing Nugent score (43, 44).
These observations from cross-sectional and longitudinal studies suggest that increases in IL-1α, IL-1β and possibly TNF-α and decreasing IP-10 might be suitable candidates to identify a danger signal in clinical trials.
Markers of vulnerability
Although several soluble immune markers are commonly increased in response to both N-9 and diverse vaginal microbiota, what matters most for the selection of safety biomarkers is whether those markers are also associated with the risk for acquisition of viral infections such as HIV, HSV and human papillomavirus (HPV). There are few longitudinal studies that assess markers prior to viral acquisition, thus data on the association between specific inflammatory markers and the risk for acquisition are limited.
HIV Acquisition
In the CAPRISA 004 study, which specifically evaluated HIV acquisition as an endpoint, women with the highest levels of at least five of nine markers of mucosal inflammation were at the highest risk for HIV infection. The panel of nine markers included IL-1α, IL-1β, IL-6, TNF-α, IL-8, IP-10, MCP-1, MIP-1α, MIP-1β, and cytokines were measured a median 4.5 months prior to HIV infection (6, 46). In another South African cohort, CAPRISA 002, increased concentrations of several cytokines and chemokines (IL-1β, IL-6, IL-8, and sCD40L) in cervicovaginal lavage (CVL) were also associated with a greater risk of HIV acquisition despite a long period (median ~300 days) between cytokine measurements in CVL specimens and subsequent HIV acquisition (47). In the FRESH cohort that also enrolled women from South Africa, individuals with a Lactobacillus-deficient cervicovaginal microbiota produced higher levels of inflammatory cytokines, particularly IL-1α, IL-1β, TNF-α, IFN-γ, IL-10, and IL-8 (30), and had a greater risk of subsequent HIV acquisition compared to women with L. crispatus-dominant genital microbiota (29). In Zimbabwean and Ugandan women, higher RANTES and lower secretory leukocyte protease inhibitor (SLPI) levels were associated with subsequent HIV seroconversion, while no associations between IL-1β, IL-6, IL-8, MIP-3α, ICAM-1, VEGF, and IL-1RA levels and later HIV seroconversion were observed (48–50). While there is some heterogeneity between studies and populations included, higher vaginal fluid IL-1α, IL-1β and IL-8 appear to be associated with increased risk of HIV acquisition. Current studies have not yet determined the absolute concentration threshold associated with increased HIV acquisition risk, or how long that concentration needs to persist to do so.
Additional markers of interest in the context of HIV risk may include biomarkers of epithelial barrier integrity such as matrix metalloproteinases, tissue inhibitors of matrix metalloproteinases (51), cell-cell adhesion markers, and/or select pro-inflammatory cytokines or chemokines related to the influx of T cell targets for infection (6, 29, 51). However, there are fewer data to indicate which specific markers would be the best choice to serve as predictive biomarkers.
Genital HSV Acquisition
Fewer data are available to define markers for HSV risk. One study in which women were evaluated quarterly found that the highest levels of IL-6, SLPI, ICAM-1 and a higher IL-1RA/IL-1β ratio were associated with a significantly lower risk of genital HSV-2 acquisition by the subsequent quarterly visit (52). IFN-γ, and IFN-stimulated genes appear important for protection against infection with HSV (53, 54). As seen with HIV, BV is a risk factor for HSV acquisition (55). Although IFNs are rarely measured in vaginal fluid samples, IFN-γ induced protein (IP-10) is commonly found to be decreased when vaginal microbiota are diverse and lactobacilli are absent (Table 1).
HPV Acquisition and Persistence
Even fewer data are available to identify markers for risk of infection with HPV. In one longitudinal study, pre-acquisition levels of vaginal immune markers were not significantly different from those of women who never had HPV. However, post-clearance levels of IL-4, -5, -10, -12, and -13, IFN-γ, IFN-α2, MIP-1α, and TNF-α were significantly elevated compared to pre-acquisition or during infection visits (56). In the CAPRISA 004 study, women who cleared HPV infection had a significant increase in 40/48 measured cytokines and chemokines (57) compared to women who remained HPV negative. In this study, 10/48 cytokines measured were significantly elevated in women who acquired HPV compared to those remaining HPV negative: IL-6, IL12p70, MIF, MIG, MIP-1β, SDF-1α, IL-3, VEGF, IFN-γ, IL-13.
Variability in Measurement of Biomarkers
A major challenge to defining specific levels of concern for representative biomarkers in this field is the wide variety of mucosal sample types, collection methods, assays and sample processing methods that are used to evaluate immune events in the female genital tract (58, 59). The range of sampling methods for collecting cells and secretions from the FGT include CVL, disposable menstrual cups, swabs or sponge collection from the cervix or lateral vaginal wall, and endocervical cytobrushes (60–62). Within each of these collection methods, variability is further compounded by laboratory processing differences including the dilution factor and diluent used in down-stream processing (58, 59, 63–66).
Most CVL and swab collections cannot accurately adjust for dilution factor (60, 63), which can vary quite significantly from participant to participant (67), making the assessment of actual in vivo concentrations and comparison across studies difficult (61, 68–70). Additional participant-specific factors, such as vaginal pH, mucus, the presence of blood or semen (71, 72), or sampling device-specific factors, such as flocked swabs vs. Dacron swabs, or choice of phosphate-buffered saline [pH 7.2] vs. saline [pH 5.5] as a sample diluent have also been shown to impact measurement of cytokine concentrations (58, 63, 73). Inter-laboratory and inter-assay reproducibility of cytokine measurements from cervicovaginal samples is a further concern (58, 74). Nonetheless, although absolute concentrations differ, the relative concentrations of the majority of cytokines correlate between methods (61, 62, 68).
Also, when considering how immune biomarkers should be measured in a safety study, it is important to consider that significant biological variation occurs between and within women. Jespers et al. noted that cervicovaginal cytokine concentrations were more variable within women over time than between different women (42). This implies that the natural variation within a participant may be important context for understanding the impact of a novel product.
Identifying Biomarkers for Studies of Novel Vaginal Products
Clear cutoff values that would allow the use of biomarkers as a safety signal have not been established. The field needs a panel of markers that reflect the risk of adverse outcomes-a challenge given the lack of data available and the variability between cohorts. The strategy utilized by several groups of identifying a panel inflammatory markers, and categorizing high risk individuals as those with the highest levels of a minimum number of these markers (6, 75) works well within a cohort, identifying individuals with the highest risk relative to others. However, this does not work to relate the risk of participants between cohorts, nor over time.
Absolute values may be helpful at times and for some analyses. At least two studies show differences in vaginal fluid immune markers between healthy populations from different continents (76, 77), even after controlling for hormonal contraception and the presence of BV. These types of comparisons can suggest underlying differences between cohorts that may reflect unmeasured variables and may point to important biological considerations. However, as noted above, the variability in methods between studies means that such comparisons cannot always be made.
When measuring the impact of a vaginal product, our goal is to understand how the product itself changes the risk profile of a given participant. In one small study that measured change in vaginal immune markers after treatment with N-9, cellulose sulfate (CS) or hydroxyethylcellulose (HEC) placebo, the absolute change in markers was not different between CS and placebo. In both placebo and CS arms, markers decreased in absolute value during product use, while N9 was associated with a 102–103 pg/ml increase in IL-1α, IL-1β, IL-1RA, MPO and IL-8 (78). Another strategy would be to measure a ratio of post:pre-treatment concentrations. In one study the comparison of the effect of condomless sex with vs. without lubricant did not identify any differences between the groups (75).
Conclusion
There are several barriers to providing easy answers to the questions of which biomarkers to use in evaluating novel vaginal products, and what threshold values to use when defining “safe.” These barriers include the lack of clear, definitive data on the exact pathways linking mucosal markers and risk for adverse outcomes; differences in vulnerability profiles for different outcomes; and variability of measurements across studies, which makes direct comparisons or aggregation of data challenging. Based on our review of existing data, we have developed a set of suggested guidelines for studies to use to identify safety signals using soluble vaginal fluid markers (Box 1) and would encourage regulators to support the collection of these markers to better identify what ranges are linked to adverse effects of vaginal products.
Box 1. Suggested guidelines for measuring soluble immune markers to define safety profiles for novel vaginal products.
1) Several baseline samples should be obtained prior to product administration to estimate longitudinal variation within a given participant.
2) Longitudinal samples should be collected to facilitate calculation of change in soluble immune markers due to product use. Calculating change in concentrations or ratios between pre/post intervention may allow comparisons between cohorts and minimize the impact of between-cohort variability due to sample processing and measurement methods (79).
3) The least dilute sample type, and one which allows quantification of the volume of vaginal fluid collected should be used to facilitate accurate comparison between measurements
4) The specific markers of interest will depend on what safety outcomes are of interest. To assess the impact of a novel product on risk for acquisition of viral STI, we suggest including measurement of IL-1α, IL-1β and IP-10.
Author Contributions
JP and CM conceived of the review. All authors contributed to literature review and writing.
Funding
JP and CM are supported by grants from the Bill & Melinda Gates Foundation.
Conflict of Interest
JP holds a patent for a Method for diagnosing an inflammatory condition in the female genital tract (EP3063542B1). CM receives grant funding from Merck, and has served as a consultant for Scynexis, Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yildirim S, Yeoman CJ, Janga SC, Thomas SM, Ho M, Leigh SR, et al. Primate vaginal microbiomes exhibit species specificity without universal Lactobacillus dominance. ISME J. (2014) 8:2431–44. doi: 10.1038/ismej.2014.90
2. Stumpf RM, Wilson BA, Rivera A, Yildirim S, Yeoman CJ, Polk JD, et al. The primate vaginal microbiome: comparative context and implications for human health and disease. Am J Phys Anthropol. (2013) 152(Suppl 57):119–34. doi: 10.1002/ajpa.22395
3. O'Hanlon DE, Brown SE, He X, Stennett CA, Robbins SJ, Johnston ED, et al. Observational cohort study of the effect of a single lubricant exposure during transvaginal ultrasound on cell-shedding from the vaginal epithelium. PLoS ONE. (2021) 16:e0250153. doi: 10.1371/journal.pone.0250153
4. Wilkinson EM, Łaniewski P, Herbst-Kralovetz MM, Brotman RM. Personal and clinical vaginal lubricants: impact on local vaginal microenvironment and implications for epithelial cell host response and barrier function. J Infect Dis. (2019) 220:2009–18. doi: 10.1093/infdis/jiz412
5. Dezzutti CS, Brown ER, Moncla B, Russo J, Cost M, Wang L, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV-1 activity. PLoS ONE. (2012) 7:e48328. doi: 10.1371/journal.pone.0048328
6. Masson L, Passmore JS, Liebenberg LJ, Werner L, Baxter C, Arnold KB, et al. Genital Inflammation and the Risk of HIV Acquisition in Women. Clin Infect Dis. (2015) 61:1–10. doi: 10.1093/cid/civ298
7. Cohen CR, Wierzbicki MR, French AL, Morris S, Newmann S, Reno H, et al. Randomized trial of lactin-v to prevent recurrence of bacterial vaginosis. N Engl J Med. (2020) 382:1906–15. doi: 10.1056/NEJMoa1915254
8. Heczko PB, Tomusiak A, Adamski P, Jakimiuk AJ, Stefański G, Mikołajczyk-Cichońska A, et al. Supplementation of standard antibiotic therapy with oral probiotics for bacterial vaginosis and aerobic vaginitis: a randomised, double-blind, placebo-controlled trial. BMC Womens Health. (2015) 15:115. doi: 10.1186/s12905-015-0246-6
9. Happel A-U, Singh R, Mitchev N, Mlisana K, Jaspan HB, Barnabas SL, et al. Testing the regulatory framework in South Africa – a single-blind randomized pilot trial of commercial probiotic supplementation to standard therapy in women with bacterial vaginosis. BMC Infect Dis. (2020) 20:491. doi: 10.1186/s12879-020-05210-4
10. Hemalatha R, Mastromarino P, Ramalaxmi BA, Balakrishna NV, Sesikeran B. Effectiveness of vaginal tablets containing lactobacilli versus pH tablets on vaginal health and inflammatory cytokines: a randomized, double-blind study. Eur J Clin Microbiol Infect Dis. (2012) 31:3097–105. doi: 10.1007/s10096-012-1671-1
11. Benes S, McCormack WM. Inhibition of growth of Chlamydia trachomatis by nonoxynol-9 in vitro. Antimicrob Agents Chemother. (1985) 27:724–6. doi: 10.1128/AAC.27.5.724
12. Kelly JP, Reynolds RB, Stagno S, Louv WC, Alexander WJ. In vitro activity of the spermicide nonoxynol-9 against Chlamydia trachomatis. Antimicrob Agents Chemother. (1985) 27:760–2. doi: 10.1128/AAC.27.5.760
13. Asculai SS, Weis MT, Rancourt MW, Kupferberg AB. Inactivation of herpes simplex viruses by nonionic surfactants. Antimicrob Agents Chemother. (1978) 13:686–90. doi: 10.1128/AAC.13.4.686
14. Jennings R, Clegg A. The inhibitory effect of spermicidal agents on replication of HSV-2 and HIV-1 in-vitro. J Antimicrob Chemother. (1993) 32:71–82. doi: 10.1093/jac/32.1.71
15. Malkovsky M, Newell A, Dalgleish AG. Inactivation of HIV by nonoxynol-9. Vol. 1, Lancet. (1988) 331:645. doi: 10.1016/S0140-6736(88)91440-7
16. Krebs FC, Miller SR, Malamud D, Howett MK, Wigdahl B. Inactivation of human immunodeficiency virus type 1 by nonoxynol-9, C31G, or an alkyl sulfate, sodium dodecyl sulfate. Antiviral Res. (1999) 43:157–73. doi: 10.1016/S0166-3542(99)00044-3
17. Polsky B, Baron PA, Gold JW, Smith JL, Jensen RH, Armstrong D. In vitro of HIV-1 by contraceptive sponge containing nonoxynol-9. Vol. 1, Lancet. (1988) 1:1456. doi: 10.1016/S0140-6736(88)92261-1
18. Cook RL, Rosenberg MJ. Do spermicides containing nonoxynol-9 prevent sexually transmitted infections? A meta-analysis. Sex Transm Dis. (1998) 25:144–50. doi: 10.1097/00007435-199803000-00007
19. Van Damme L, Ramjee G, Alary M, Vuylsteke B, Chandeying V, Rees H, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. (2002) 360:971–7. doi: 10.1016/S0140-6736(02)11079-8
20. Cone RA, Hoen T, Wong X, Abusuwwa R, Anderson DJ, Moench TR. Vaginal microbicides: detecting toxicities in vivo that paradoxically increase pathogen transmission. BMC Infect Dis. (2006) 6:90. doi: 10.1186/1471-2334-6-90
21. Fichorova RN, Tucker LD, Anderson DJ. The molecular basis of nonoxynol-9-induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J Infect Dis. (2001) 184:418–28. doi: 10.1086/322047
22. Cummins JEJ, Doncel GF. Biomarkers of cervicovaginal inflammation for the assessment of microbicide safety. Sex Transm Dis. (2009) 36:S84–91. doi: 10.1097/OLQ.0b013e3181994191
23. Cherne MD, Cole AL, Newberry L, Schmidt-Owens M, Deichen M, Cole AM. Matrix metalloproteinases expressed in response to bacterial vaginosis disrupt the endocervical epithelium, increasing transmigration of HIV. Infect Immun. (2020) 88:e00041-20. doi: 10.1128/IAI.00041-20
24. Roselletti E, Sabbatini S, Perito S, Mencacci A, Vecchiarelli A, Monari C. Apoptosis of vaginal epithelial cells in clinical samples from women with diagnosed bacterial vaginosis. Sci Rep. (2020) 10:1978. doi: 10.1038/s41598-020-58862-2
25. Chen Z, Zhang Z, Zhang H, Xie B. Analysis of the oxidative stress status in nonspecific vaginitis and its role in vaginal epithelial cells apoptosis. Biomed Res Int. (2015) 2015:795656. doi: 10.1155/2015/795656
26. Ma X, Deng J, Cui X, Chen Q, Wang W. Berberine exhibits antioxidative effects and reduces apoptosis of the vaginal epithelium in bacterial vaginosis. Exp Ther Med. (2019) 18:2122–30. doi: 10.3892/etm.2019.7772
27. Atashili J, Poole C, Ndumbe PM, Adimora A, Jennifer S. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS. (2008) 22:1493–501. doi: 10.1097/QAD.0b013e3283021a37
28. Cohen CR, Lingappa JR, Baeten JM, Ngayo MO, Spiegel CA, Hong T, et al. Bacterial vaginosis associated with increased risk of female-to-male HIV-1 transmission: a prospective cohort analysis among African Couples. PLoS Med. (2012) 9:e1001251. doi: 10.1371/journal.pmed.1001251
29. Gosmann C, Anahtar MN, Handley SA, Farcasanu M, Abu-Ali G, Bowman BA, et al. Lactobacillus-deficient cervicovaginal bacterial communities are associated with increased HIV acquisition in Young South African Women. Immunity. (2017) 46:29–37. doi: 10.1016/j.immuni.2016.12.013
30. Anahtar MN, Byrne EH, Fichorova RN, Kwon DS, Anahtar MN, Byrne EH, et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital article cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity. (2015) 42:965–76. doi: 10.1016/j.immuni.2015.04.019
31. Masson L, Mlisana K, Little F, Werner L, Mkhize NN, Ronacher K, et al. Defining genital tract cytokine signatures of sexually transmitted infections and bacterial vaginosis in women at high risk of HIV infection: a cross-sectional study. Sex Transm Infect. (2014) 90:1–8. doi: 10.1136/sextrans-2014-051601
32. Kyongo JK, Crucitti T, Menten J, Hardy L, Cools P, Michiels J, et al. Cross-sectional analysis of selected genital tract immunological markers and molecular vaginal microbiota in sub-Saharan African women, with relevance to HIV risk and prevention. Clin Vaccine Immunol. (2015) 22:526–38. doi: 10.1128/CVI.00762-14
33. Hemalatha R, Ramalaxmi BA, KrishnaSwetha G, Kumar PU, Rao D, Balakrishna N, et al. Cervicovaginal inflammatory cytokines and sphingomyelinase in women with and without bacterial vaginosis. Am J Med Sci. (2012) 344:35–9. doi: 10.1097/MAJ.0b013e318235597b
34. Fan SR, Liu XP, Liao QP. Human defensins and cytokines in vaginal lavage fluid of women with bacterial vaginosis. Int J Gynaecol Obstet. (2008) 103:50–4. doi: 10.1016/j.ijgo.2008.05.020
35. Yasodhara P, Raghunath M, Sreeramulu D, Venu L, Hemalatha R, Krishna TP. Local immunity in Indian women with bacterial vaginosis. J Reprod Immunol. (2006) 70:133–41. doi: 10.1016/j.jri.2005.11.001
36. Anton G, Rid J, Mylonas I, Friese K, Weissenbacher E-R. Evidence of a TH1-shift of local vaginal inflammatory response during bacterial vaginosis. Infection. (2008) 36:147–52. doi: 10.1007/s15010-007-7152-2
37. Weissenbacher T, Walter C, Mylonas I, Scholz C, Gingelmaier A, Friese K. Interleukin-6, interleukin-10 and interleukin-12 in vaginal fluid from women with bacterial vaginosis. Arch Gynecol Obstet. (2010) 281:77–80. doi: 10.1007/s00404-009-1072-6
38. Shannon B, Gajer P, Yi TJ, Ma B, Humphrys MS, Thomas-Pavanel J, et al. Distinct Effects of the cervicovaginal microbiota and herpes simplex type 2 infection on female genital tract immunology. J Infect Dis. (2017) 215:1366–75. doi: 10.1093/infdis/jix088
39. Valore EV, Wiley DJ, Ganz T. Reversible deficiency of antimicrobial polypeptides in bacterial vaginosis. Infect Immun. (2006) 74:5693–702. doi: 10.1128/IAI.00524-06
40. Hedges SR, Barrientes F, Desmond RA, Schwebke JR. Local and systemic cytokine levels in relation to changes in vaginal flora. J Infect Dis. (2006) 193:556–62. doi: 10.1086/499824
41. Santos-Greatti MMV, da Silva MG, Ferreira CST, Marconi C. Cervicovaginal cytokines, sialidase activity and bacterial load in reproductive-aged women with intermediate vaginal flora. J Reprod Immunol. (2016) 118:36–41. doi: 10.1016/j.jri.2016.08.005
42. Jespers V, Kyongo J, Joseph S, Hardy L, Cools P, Crucitti T, et al. A longitudinal analysis of the vaginal microbiota and vaginal immune mediators in women from sub-Saharan Africa. Sci Rep. (2017) 7:11974. doi: 10.1038/s41598-017-12198-6
43. Cherpes TL, Marrazzo JM, Cosentino LA, Meyn LA, Murray PJ, Hillier SL. Hormonal contraceptive use modulates the local inflammatory response to bacterial vaginosis. Sex Transm Infect. (2008) 84:57–61. doi: 10.1136/sti.2007.026625
44. Joag V, Obila O, Gajer P, Scott MC, Dizzell S, Humphrys M, et al. Impact of Standard Bacterial Vaginosis Treatment on the Genital Microbiota, Immune Milieu, and Ex Vivo Human Immunodeficiency Virus Susceptibility. Clin Infect Dis. (2018) 68:1675–83. doi: 10.1093/cid/ciy762
45. Garrett N, Mtshali A, Osman F, Masson L, McKinnon LR, Singh R, et al. Impact of point-of-care testing and treatment of sexually transmitted infections and bacterial vaginosis on genital tract inflammatory cytokines in a cohort of young South African women. Sex Transm Infect. (2021) 97:555–65. doi: 10.1136/sextrans-2020-054740
46. McKinnon LR, Liebenberg LJ, Yende-Zuma N, Archary D, Ngcapu S, Sivro A, et al. Genital inflammation undermines the effectiveness of tenofovir gel in preventing HIV acquisition in women. Nat Med. (2018) 24:491–6. doi: 10.1038/nm.4506
47. Mlisana K, Naicker N, Werner L, Roberts L, Van Loggerenberg F, Baxter C, et al. Symptomatic vaginal discharge is a poor predictor of sexually transmitted infections and genital tract inflammation in high-risk women in South Africa. J Infect Dis. (2012) 206:6–14. doi: 10.1093/infdis/jis298
48. Mauck C, Chen P-L, Morrison CS, Fichorova RN, Kwok C, Chipato T, et al. Biomarkers of cervical inflammation and immunity associated with cervical shedding of HIV-1. AIDS Res Hum Retroviruses. (2016) 32:443–51. doi: 10.1089/aid.2015.0088
49. Morrison C, Fichorova RN, Mauck C, Chen P, Kwok C, Chipato T, et al. Cervical inflammation and immunity associated with hormonal contraception, pregnancy, and HIV-1 seroconversion. J Acquir Immune Defic Syndr. (2014) 66:109–17. doi: 10.1097/QAI.0000000000000103
50. Morrison CS, Fichorova R, Chen P-L, Kwok C, Deese J, Yamamoto H, et al. A longitudinal assessment of cervical inflammation and immunity associated with HIV-1 infection, hormonal contraception, and pregnancy. AIDS Res Hum Retroviruses. (2018) 34:889–99. doi: 10.1089/aid.2018.0022
51. Arnold KB, Burgener A, Birse K, Romas L, Dunphy LJ, Shahabi K, et al. Increased levels of inflammatory cytokines in the female reproductive tract are associated with altered expression of proteases, mucosal barrier proteins, and an influx of HIV-susceptible target cells. Mucosal Immunol. (2015) 9:194–205. doi: 10.1038/mi.2015.51
52. Fichorova RN, Morrison CS, Chen P-L, Yamamoto HS, Govender Y, Junaid D, et al. Aberrant cervical innate immunity predicts onset of dysbiosis and sexually transmitted infections in women of reproductive age. PLoS ONE. (2020) 15:e0224359. doi: 10.1371/journal.pone.0224359
53. Parr MB, Parr EL. The role of gamma interferon in immune resistance to vaginal infection by herpes simplex virus type 2 in mice. Virology. (1999) 258:282–94. doi: 10.1006/viro.1999.9739
54. Gopinath S, Kim MV, Rakib T, Wong PW, van Zandt M, Barry NA, et al. Topical application of aminoglycoside antibiotics enhances host resistance to viral infections in a microbiota-independent manner. Nat Microbiol. (2018) 3:611–21. doi: 10.1038/s41564-018-0138-2
55. Cherpes TL, Meyn LA, Krohn MA, Lurie JG, Hillier SL. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis. (2003) 37:319–25. doi: 10.1086/375819
56. Moscicki A-B, Shi B, Huang H, Barnard E, Li H. Cervical-vaginal microbiome and associated cytokine profiles in a prospective study of HPV 16 acquisition, persistence, and clearance. Front Cell Infect Microbiol. (2020) 10:569022. doi: 10.3389/fcimb.2020.569022
57. Liebenberg LJP, McKinnon LR, Yende-Zuma N, Garrett N, Baxter C, Kharsany ABM, et al. HPV infection and the genital cytokine milieu in women at high risk of HIV acquisition. Nat Commun. (2019) 10:5227. doi: 10.1038/s41467-019-13089-2
58. Fichorova RN, Richardson-Harman N, Alfano M, Belec L, Carbonneil C, Chen S, et al. Biological and technical variables affecting immunoassay recovery of cytokines from human serum and simulated vaginal fluid: a multicenter study. Anal Chem. (2008) 80:4741–51. doi: 10.1021/ac702628q
59. McKinnon LR, Hughes SM, De Rosa SC, Martinson JA, Plants J, Brady KE, et al. Optimizing viable leukocyte sampling from the female genital tract for clinical trials: an international multi-site study. PLoS ONE. (2014) 9:e85675. doi: 10.1371/journal.pone.0085675
60. Boskey ER, Moench TR, Hees PS, Cone RA. A self-sampling method to obtain large volumes of undiluted cervicovaginal secretions. Sex Transm Dis. (2003) 30:107–9. doi: 10.1097/00007435-200302000-00002
61. Jaumdally SZ, Masson L, Jones HE, Dabee S, Hoover DR, Gamieldien H, et al. Lower genital tract cytokine profiles in South African women living with HIV: influence of mucosal sampling. Sci Rep. (2018) 8:12203. doi: 10.1038/s41598-018-30663-8
62. Masson L, Barnabas S, De9ese J, Lennard K, Dabee S, Gamieldien H, et al. Inflammatory cytokine biomarkers of asymptomatic sexually transmitted infections and vaginal dysbiosis: a multicentre validation study. Sex Trans Infect. (2019) 95:5–12. doi: 10.1136/sextrans-2017-053506
63. Dezzutti CS, Hendrix CW, Marrazzo JM, Pan Z, Wang L, Louissaint N, et al. Performance of swabs, lavage, and diluents to quantify biomarkers of female genital tract soluble mucosal mediators. PLoS ONE. (2011) 6:e23136. doi: 10.1371/journal.pone.0023136
64. Snowhite I V, Jones WE, Dumestre J, Dunlap K, Braly PS, Hagensee ME. Comparative analysis of methods for collection and measurement of cytokines and immunoglobulins in cervical and vaginal secretions of HIV and HPV infected women. J Immunol Methods. (2002) 263:85–95. doi: 10.1016/S0022-1759(02)00038-8
65. Marks MA, Eby Y, Howard R, Gravitt PE. Comparison of normalization methods for measuring immune markers in cervical secretion specimens. J Immunol Methods. (2012) 382:211–5. doi: 10.1016/j.jim.2012.05.012
66. Birse KM, Burgener A, Westmacott GR, McCorrister S, Novak RM, Ball TB. Unbiased proteomics analysis demonstrates significant variability in mucosal immune factor expression depending on the site and method of collection. PLoS ONE. (2013) 8:e79505. doi: 10.1371/journal.pone.0079505
67. Mitchell C, Paul K, Agnew K, Gaussman R, Coombs RW, Hitti J. Estimating Volume of Cervicovaginal Secretions in Cervicovaginal Lavage Fluid Collected for Measurement of Genital HIV-1 RNA Levels in Women. J Clin Microbiol. (2011) 49:735–6. doi: 10.1128/JCM.00991-10
68. Faro CJ, Hollier LM, Bishop K. Comparison of vaginal cytokine collection methods. Am J Reprod Immunol. (2006) 55:315–20. doi: 10.1111/j.1600-0897.2006.00365.x
69. Archary D, Liebenberg LJ, Werner L, Tulsi S, Majola N, Naicker N, et al. Randomized cross-sectional study to compare HIV-1 specific antibody and cytokine concentrations in female genital secretions obtained by menstrual cup and cervicovaginal lavage. PLoS ONE. (2015) 10:e0131906. doi: 10.1371/journal.pone.0131906
70. Short CS, Quinlan R, Bennett P, Shattock RJ, Taylor GP. Optimising the collection of female genital tract fluid for cytokine analysis in pregnant women. J Immunol Methods. (2018) 458:15–20. doi: 10.1016/j.jim.2018.03.014
71. Jewanraj J, Ngcapu S, Osman F, Ramsuran V, Fish M, Mtshali A, et al. Transient association between semen exposure and biomarkers of genital inflammation in South African women at risk of HIV infection. J Int AIDS Soc. (2021) 24:e25766. doi: 10.1002/jia2.25766
72. Mngomezulu K, Mzobe GF, Mtshali A, Osman F, Liebenberg LJP, Garrett N, et al. Recent semen exposure impacts the cytokine response and bacterial vaginosis in women. Front Immunol. (2021) 12:695201. doi: 10.3389/fimmu.2021.695201
73. Ginsburg KA, Wolf NA, Fidel PL. Potential effects of midcycle cervical mucus on mediators of immune reactivity. Fertil Steril. (1997) 67:46–50. doi: 10.1016/S0015-0282(97)81854-7
74. Scott ME, Wilson SS, Cosentino LA, Richardson BA, Moscicki A-B, Hillier SL, et al. Interlaboratory reproducibility of female genital tract cytokine measurements by Luminex: implications for microbicide safety studies. Cytokine. (2011) 56:430–4. doi: 10.1016/j.cyto.2011.06.011
75. Tuddenham S, Stennett CA, Cone RA, Ravel J, Macintyre AN, Ghanem KG, et al. Vaginal cytokine profile and microbiota before and after lubricant use compared with condomless vaginal sex: a preliminary observational study. BMC Infect Dis. (2021) 21:973. doi: 10.1186/s12879-021-06512-x
76. Murphy K, Richardson BA, Dezzutti CS, Marrazzo J, Hillier SL, Hendrix CW, et al. Levels of genital tract defensins and cytokines differ between hiv-uninfected us and african women. Am J Reprod Immunol. (2015) 74:313–22. doi: 10.1111/aji.12411
77. Cohen CR, Moscicki A-B, Scott ME, Ma Y, Shiboski S, Bukusi E, et al. Increased levels of immune activation in the genital tract of healthy young women from sub-Saharan Africa. AIDS. (2010) 24:2069–74. doi: 10.1097/QAD.0b013e32833c323b
78. Mauck CK, Lai JJ, Weiner DH, Chandra N, Fichorova RN, Dezzutti CS, et al. Toward early safety alert endpoints: exploring biomarkers suggestive of microbicide failure. AIDS Res Hum Retroviruses. (2013) 29:1475–86. doi: 10.1089/aid.2012.0345
Keywords: female genital tract, live biotherapeutics, product development, safety, cytokines
Citation: Happel A-U, Sivro A, Liebenberg L, Passmore JA and Mitchell CM (2022) Considerations for Choosing Soluble Immune Markers to Determine Safety of Novel Vaginal Products. Front. Reprod. Health 4:899277. doi: 10.3389/frph.2022.899277
Received: 18 March 2022; Accepted: 11 April 2022;
Published: 17 May 2022.
Edited by:
Carolina Herrera, Imperial College London, United KingdomReviewed by:
Jack David Sobel, Wayne State University, United StatesCopyright © 2022 Happel, Sivro, Liebenberg, Passmore and Mitchell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caroline M. Mitchell, caroline.mitchell@mgh.harvard.edu
 Anna-Ursula Happel
Anna-Ursula Happel Aida Sivro
Aida Sivro Lenine Liebenberg
Lenine Liebenberg Jo Ann Passmore
Jo Ann Passmore Caroline M. Mitchell
Caroline M. Mitchell