- 1Department of Experimental Pharmacology & Toxicology, Faculty of Pharmacy, University of Port Harcourt, Port Harcourt, Nigeria
- 2Department for Cardiovascular, Dysmetabolic and Aging-Associated Diseases, Istituto Superiore di Sanità, Rome, Italy
- 3African Centre of Excellence for Public Health and Toxicological Research (ACE-PUTOR), University of Port Harcourt, Port Harcourt, Nigeria
Exposures to heavy metals and metalloids have been associated with decreased fecundity and fertility in couples conceiving via assisted reproduction. Heavy metals and metalloids can alter the homeostasis of critical hormones controlling sexual maturation by binding to critical hormones and receptors. This may disrupt the time course of sexual maturation directly or indirectly affecting reproductive competence in males and females. The present review aims to provide a summarized overview of associations between heavy metal exposure, reproductive concerns, and IVF outcomes. A systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) in Google Scholar, Scopus, EMBASE and PubMed databases. Initial search produced 1,351 articles from which 30 articles were eligible to be included in the systematic review. From our results, 16 articles reported associations between selected heavy metals and IVF outcomes, while 14 articles summarized the role of heavy metals in reproductive concerns. For the studies on IVF outcomes, different human samples were examined for heavy metals. Heavy metals and metalloids (Pb, Hg, Cd, Cr, Mn, As) correlated negatively with oocyte fertilization/pregnancy rates in hair, follicular fluid, serum, urine and seminal plasma samples, while Cd and Hg in whole blood samples showed no associations. For the studies on reproductive concerns, high levels of heavy metals/metalloids were implicated in the following conditions: infertility (Cd, Pb, Ba, U), spontaneous abortion/miscarriage (Pb, Cd, Sb), congenital heart disease (Al, Mg, Cd), PCOS (As, Cd, Hg, Pb), endometriosis (Pb) and uterine leiomyomata (Hg). Taken together, the results of our study suggest that the impact of heavy metals and metalloids exposure on reproductive health may contribute to the failure rates of in vitro fertilization.
Introduction
Environmental heavy metals and metalloids pollution has been considered as one of the major challenges affecting assisted reproductive medicine in humans. Couples residing in residential areas of low environmental toxicants levels show more positive IVF outcomes (1). Couples residing in industrial areas with high contaminant levels, have less IVF success rates implying that environmental factors may have some adverse effects on IVF outcome (2).
Heavy metals and metalloids are known to possess high atomic numbers and densities of five times above the density of water (3). These are often grouped into essential (manganese, copper, chromium, zinc, selenium, etc) and non-essential metals (lead, cadmium mercury etc). Essential metals and metalloids play significant beneficial role in human health management and other living things at the bio-permissible levels set by standard regulatory bodies (4, 5). These include manganese, copper, chromium, zinc, selenium and others. Some of them are critical components of many metabolic regulating enzymes and signalling pathways. However, when concentration exceeds regulatory limits, biotoxicity ensues impacting on different organs and systems in biological systems (4, 5). The metals and metalloids classified as non-essential heavy metals and metalloids are toxic to biological systems even at trace levels (6). These include lead, arsenic, cadmium, chromium, mercury etc.
The mechanism underlying heavy metal toxicity in humans is mainly due to their interaction with the sulfhydryl (SH) enzyme groups in the non-enzymic antioxidant systems and subsequent inhibition of these enzymes required in energy generation in many metabolic and signal transduction pathways (7). Heavy metals preferentially replace H atom from the sulphydryl groups on the reduced glutathione moieties resulting to the formation of organo–metallic complexes with potentials of deactivating any further biochemical reactions (8).
Human gametes are said to be sensitive and error prone. Exposure to heavy metals and metalloids in the environment can further exacerbate this process directly or indirectly affecting gametogenesis, alter fertilization, depriving the embryo with essential nutrients and lower overall implantation rate in the uterine mucosa (9).
In vitro fertilization (IVF) is a type of Assisted Reproductive Technology (ART). It is the most effective and commonly used procedure to address issues of infertility especially in humans (10–12). The process involves multi-step medical procedures in which a matured oocyte (egg) from the ovaries of a female is collected and externally fertilized by sperm cells from a male and then implanted into the uterus of the female after undergoing embryo culture for two-seven days (13). The intention of this procedure is generally to establish pregnancy through an orchestrated sequence of events such as stimulation, egg retrieval, insemination, embryo culture and transfer (11, 12). Since its inception in 1978, IVF has steadily increased and has undergone significant transformation in many ways in aiding women to achieve pregnancy. An estimated 8–10 million children have been born through IVF and other types of ART (14). The success rate of this process has been determined by some factors which include age of the mother, cause of sterility, integrity of the fetus, and lifestyle. Overtime, the technology has expanded to also improve on the success rate of women who consider IVF as an alternative to conceive (12, 15).
Irrespective of the tremendous feat achieved in IVF technology, environmental heavy metals and metalloids pollution continue to pose significant threat on the integrity of the embryo cultured for implantation. Quality of the environment plays a significant role on the success rate of this technology in reproductive medicine. Environmental quality has been implicated in poor ovarian stimulation and response especially in women preparing for IVF (16). Metals and metalloids may restrain morphology of gametes resulting in the formation of poor-quality zygote with diminished viability in cell division (17). Environmental heavy metals and metalloids may cause retardation in cleavage rate of the zygote into a blastocyte with an attendant failure in implantation or recurrent miscarriages. Recurring pregnancy failures following repeated IVFs are often associated with psychological and emotional burden (18). Many reproductive endocrinologists are unaware of the influence of heavy metals and metalloids on the success of IVF treatments that may have surprisingly contributed to prevalence of women who drop out of IVF cited consistently in studies conducted across the globe (10, 19).
Prevalence and persistence nature of heavy metals and metalloids in the environment have been implicated in reproductive toxicity. Gametogenesis, preimplantation embryogenesis and culture are all orchestrated events that determine the success of IVF in human reproductive medicine. This study has delineated the critical concerns of environmental heavy metals and metalloids exposure, impact and outcomes on in vitro fertilization in human reproductive medicine. The present review aims to provide a summarized overview of associations between heavy metals and metalloids exposure, IVF outcomes, and reproductive concerns.
Method
Information source and database searching
This systematic review was done according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (20). Scientific databases such as Google Scholar, Scopus, EMBASE and PubMed were searched for relevant literature using the following combination of search terms: ‘exposure to heavy metals and metalloids OR ‘toxic trace elements’ and toxicological effects on human reproduction’, ‘heavy metals and reproductive concerns’, ‘heavy metals and reprotoxicity’, ‘in vitro fertilization (IVF)’, ‘heavy metals, and metalloids and preimplantation embryogenesis’, ‘heavy metals, and metalloids and gametogenesis in humans’, ‘heavy metals and IVF rate’, ‘heavy metals, and metalloids and pregnancy rate’, ‘heavy metals’ OR ‘toxic trace elements’ and ‘IVF outcome’, ‘effects of toxic/heavy metals and metalloids on reproductive outcome’. Hand-searching of appropriate publications from bibliographies of related international medical societies was also done and retrieved results screened.
Selection criteria
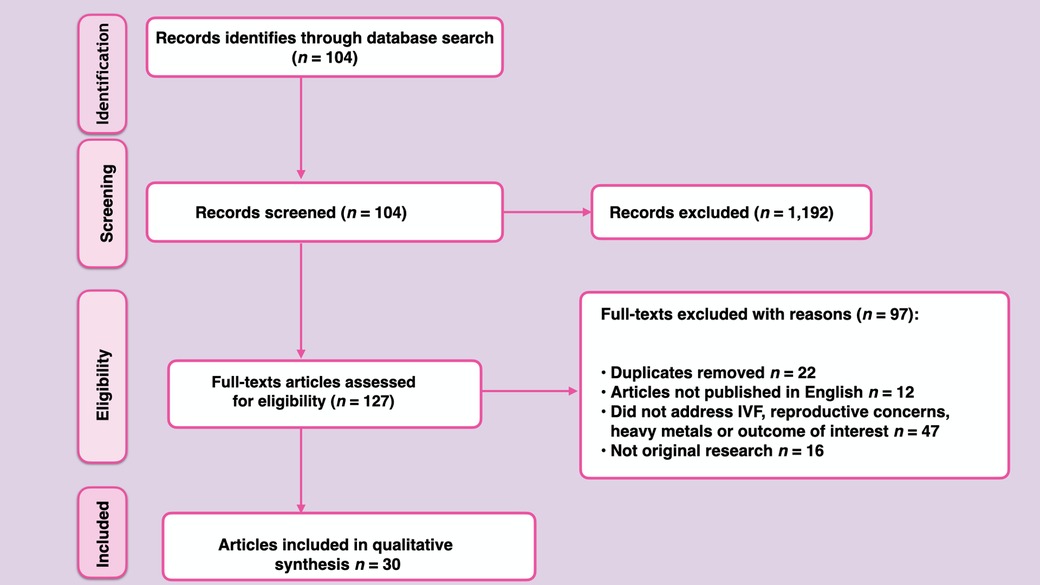
One thousand three hundred and nineteen (1,319) articles were pinpointed from Google Scholar, Scopus, EMBASE and PubMed. After going through abstracts and titles; 1,192 articles were excluded because the studies were irrelevant to the present review. Another 97 articles were excluded with reasons such as: duplicates (22), articles not published in English language (12) or did not address IVF outcome, reproductive concerns, heavy metals/metalloids or outcome of interest (47) or articles were not original research (16). Only 30 articles were eligible and were included in the present study. Taken together, the following guided the inclusion criteria; (1) Human studies that reported sufficient data, study design, sample size, study population, IVF outcomes and reproductive concerns (2) The names of the heavy metals/metalloids implicated were specified. No limits were applied to the year of study of articles included in the review. The PRISMA flow diagram summarizing the selection process is illustrated in Figure 1.
Quality assessment
The articles that met the inclusion criteria for both IVF outcomes and reproductive concerns were evaluated for quality using a 6-point score designed by the authors, specifically for the present review. Study characteristics listed below, were used for the quality assessment and a score of 0 or 1 was assigned if a particular study met a particular characteristic or not. These include: study design description (if study design was properly described or not, papers were scored 1 or 0), sampling strategy (studies with/without random selection were scored 1/0 respectively, as randomisation guards against selection bias), study population representativeness (if subjects/participants were good representatives of the population or not; papers were scored 1 or 0 respectively), ascertainment of exposure to heavy metals (assessment of heavy metal/metalloid exposure was scored 1 or 0), selection of non-exposed controls (papers were scored 1 or 0, if heavy metal/metalloid exposure was ascertained in participants or not), and appropriate control for variables was also scored 1 or 0 (whether adjustments were made for variables such as age, and socioeconomic status of participants).
Results
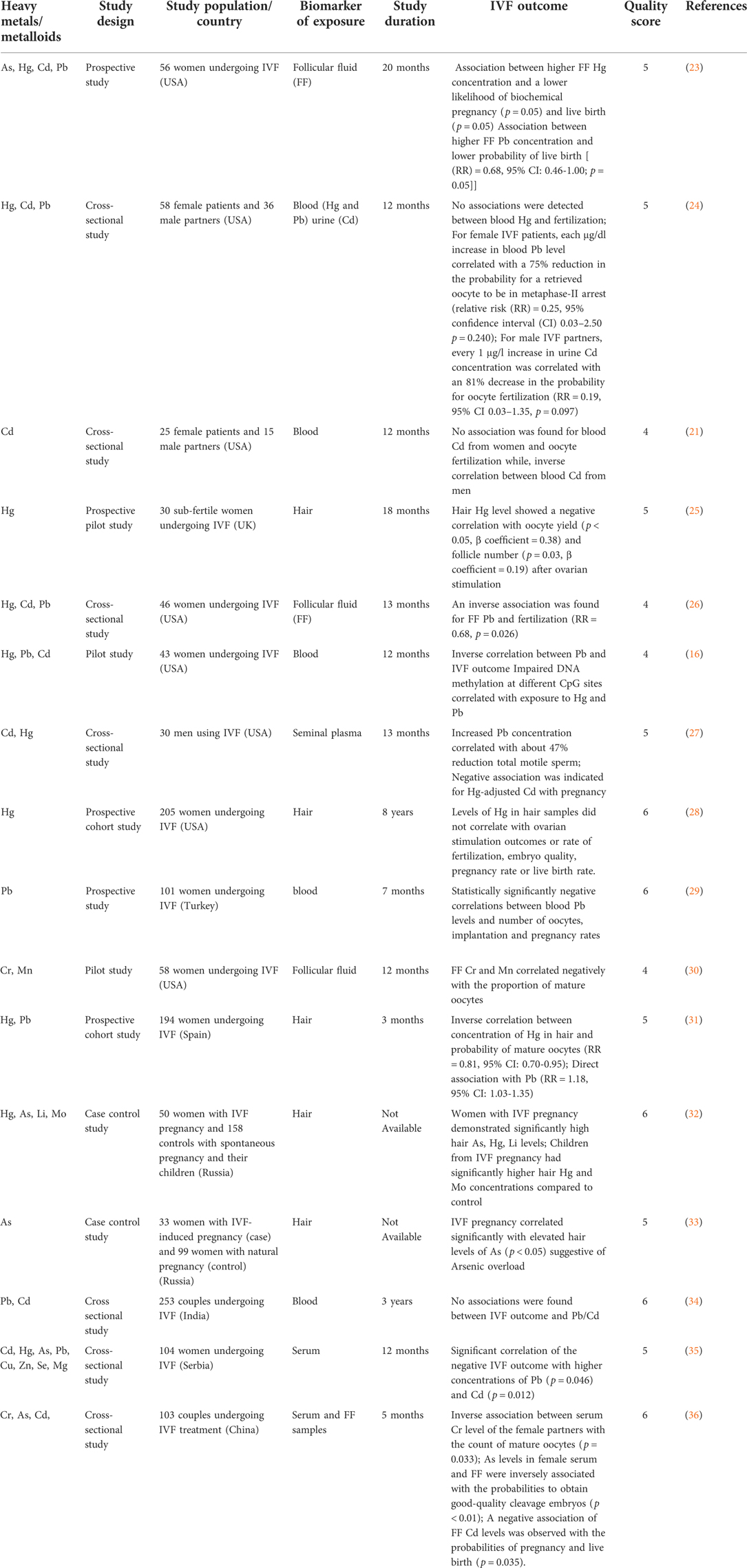
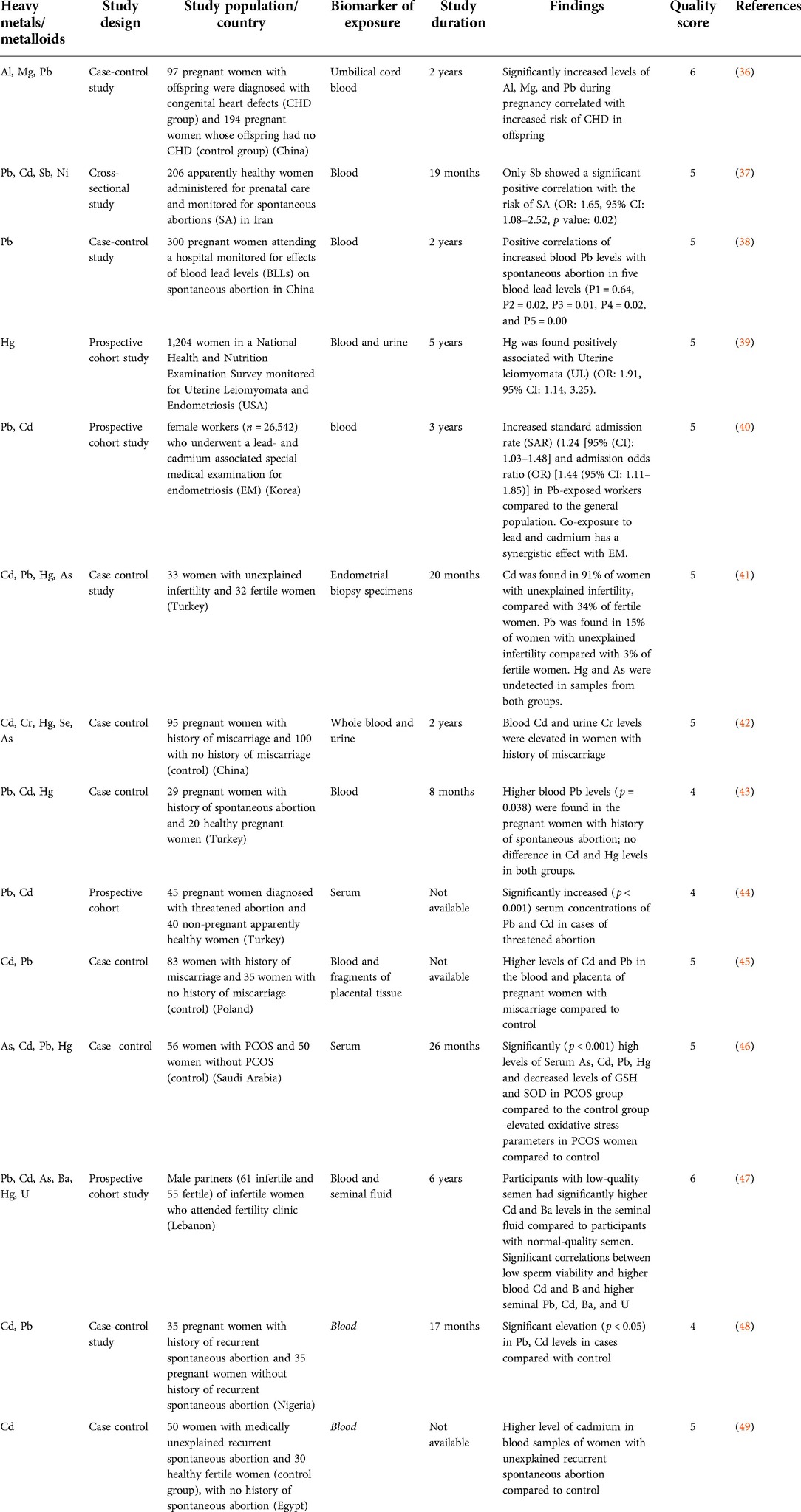
Out of 30 eligible articles included in the present systematic review, 16 articles focused on clinical studies investigating IVF outcomes (Table 1) (16, 21–35) while 14 articles focused on clinical studies that investigated reproductive concerns (Table 2) (36–49).
Discussion
Association of heavy metals/metalloids and in vitro fertilization outcome
There is a growing concern regarding the association of environmental heavy metals and metalloids contamination and IVF treatment outcomes. Exposure to heavy metals and metalloids in the environment has no doubt heavily impacted on fertility rate in humans (50, 51). Researchers have found out that overall decline in quality and quantity of the spermatozoa contribute greatly to infertility in men (52). Even with normal semen analysis, some couples undergoing IVF still fail to establish conception (53–55). The numerous external causes of impaired infertility have been linked to exposure to a number of heavy metals and metalloids via the alteration of fundamental principle of synchronization between the male and female gametes (56). Heavy metals and metalloids may evoke changes in physiological patterns and processes that elicit mutation by inhibiting DNA, RNA and protein synthesis during spermatogenesis (57). Furthermore, heavy metals and metalloids can induce oxidative stress which generate reactive oxygen species (ROS) (58). ROS generate peroxidation products which can cause depletion of antioxidant systems designed to protect the sperm from oxidative reactions and deterioration of sperm cells (59, 60). High levels of redox activity and aneuploidy rates due to heavy metals and metalloids exposure have been detected in defective spermatozoa and failure on oocyte interaction (61, 62). In females, exposure to heavy metals and metalloids can interfere with ovarian hyper-stimulation and cause poor response, as well as delay of ovulation subsequently contributing to decline in fertilization and conception rate (63).
Exposure to heavy metals and metalloids can trigger epigenetic changes (64) resulting in alteration in gene expression profiles (65). Heavy metals and metalloids such as arsenic and cadmium (66, 67) change DNA methylation in children of prenatally exposed mothers, suggesting that methylation may be a central mechanism by which genomes respond to the environment (66). A study by Hanna and co-workers, demonstrated that a decreased methylation of COL1A2 promoter region correlated with high Pb exposure in women undergoing IVF (16). The COL1A2 gene product is a key component of the uterine cervix and chorio-amniotic membranes (68). Earlier studies have reported that COL1A2 genes are up-regulated in the placental tissue of pregnant tobacco smokers (69). COL1A2 polymorphisms in women have also been associated with preterm prelabour rupture of membranes (which is a leading cause of preterm birth) (68). Therefore, environmentally-induced modifications of COL1A2 gene methylation, might compromise human reproduction (16). Differential DNA methylation with variation enriched at loci within CpG islands were observed in funiculus umbilicalis cord blood samples of infants exposed to low-levels of arsenic in utero (70). Recently, reports have also associated variation in DNA methylation in the placenta (as well as new-born health outcomes such as growth and neurobehavioral functioning) with different environmental exposures (71–73).
In the present review, 16 articles reported associations between selected heavy metals/metalloids and IVF outcomes in different human samples such as hair, follicular fluid, whole blood, serum, urine and seminal fluid. Heavy metals and metalloids investigated in these studies include Pb, Hg, Cd, Cr, Mn, and As.
Heavy metals and metalloids in hair samples
Hair analysis provides a novel approach of probing the impact of long-term exposure to heavy metals and metalloids on reproductive outcomes (74, 75). Five studies (23, 26, 29–31) investigated heavy metals and metalloids in hair samples of women undergoing IVF. In a study to examine the heavy metals and metalloids status in hair samples of women with IVF pregnancy and their 9-month-old children, Skalny et al. (29) found out that women with IVF pregnancy demonstrated significantly high hair As, Hg, Li levels and their children also had significantly elevated hair Hg and Mo concentrations compared to control (30). Similarly, another case-control study by Skalny et al. (30) demonstrated that IVF pregnancy correlated significantly with elevated hair levels of As (p < 0.05) suggestive of arsenic overload in IVF patients (31). Garcia-Fortea et al. (28) in a prospective cohort study examined 194 women undergoing IVF and found an inverse correlation between concentration of Hg in hair and probability of mature oocytes (RR = 0.81, 95% CI: 0.70–0.95) while a direct association was demonstrated with Pb (RR = 1.18, 95% CI: 1.03–1.35) (29). Similarly, an earlier study by Dickerson et al. (22) revealed a negative correlation between hair Hg level with oocyte yield (p < 0.05, β coefficient = 0.38) and follicle number (p = 0.03, β coefficient = 0.19) following ovarian stimulation (23). Wright et al. (25) found no association between hair Hg concentrations with ovarian stimulation outcomes (total and mature oocyte yields, peak estradiol levels), fertilization rate, clinical pregnancy rate, embryo quality, or live birth rate (26).
Heavy metals and metalloids in whole blood samples
Blood, serum and urine sampling provide indications of short-term changes in heavy metal and metalloids levels (30). Heavy metals and metalloids concentrations in blood and urine are the most widely used biomarkers to document adverse health effects (29).
Five studies (16, 21, 22, 27, 34) investigated the associations between heavy metals and metalloids in whole blood samples and in vitro fertilization (IVF) outcomes. A study by Kim et al. (2010) in 25 female patients and 15 male partners showed no association between blood Cd in women and oocyte fertilization while, inverse correlation was found between blood Cd in men (22). Similarly, a later study by Kumar et al. (33), also showed no association between maternal and paternal blood Cd, Pb levels and IVF outcome (34). Bloom et al. (20) found no associations between blood Hg and fertilization; 1 μg/dl increase in blood Pb level correlated with a 75% reduction in the probability for a retrieved oocyte to be in metaphase-II arrest (relative risk (RR) = 0.25, 95% confidence interval (CI) 0.03–2.50, p = 0.240) (21). A study by Tolunay et al. (26) showed negative correlations between blood Pb concentrations and number of metaphase II (MII) oocytes, implantation, pregnancy rates (27). This data is consistent with results obtained by Hanna et al. (15) in which an inverse correlation between Pb and IVF outcome was obtained. In addition, impaired DNA methylation was also observed at different CpG sites which correlated with Hg and Pb exposure (16).
Heavy metals and metalloids in urine samples
A study by Bloom et al. (20) investigated impact of heavy metals and metalloids in male partners of IVF patients. Their results showed that every 1 μg/L increase in urine Cd concentration correlated with an 81% decrease in the probability for oocyte fertilization (RR = 0.19, 95% CI 0.03–1.35, p = 0.097) (21).
Heavy metals and metalloids in seminal plasma
Kim et al. (24) measured heavy metals in seminal plasma of men using IVF to probe associations between semen quality and IVF outcomes. While increased Pb concentration correlated with about 47% reduction in total motile sperm, a negative association was indicated for Hg-adjusted Cd with pregnancy (25).
Heavy metals and metalloids in serum samples
Two studies (32, 33) investigated the associations between heavy metals and metalloids in serum and in vitro fertilization (IVF) outcomes. A study by Wu et al. (32) revealed an inverse association between serum Cr level of IVF patients with the count of mature oocytes (p = 0.033), in addition, arsenic (As) concentration in female serum were inversely associated with the probabilities to obtain good-quality cleavage embryos (p < 0.01) (33). On the other hand, Tulic et al. (31) found a significant negative correlation between IVF outcome with higher serum concentrations of Pb (p = 0.046) and Cd (p = 0.012) (32).
Heavy metals and metalloids in follicular fluid (FF)
Follicular fluid (FF) samples provide additional information on the risk of infertility in women (29). However, it has a limitation of not being easily accessible and highly invasive, therefore, the use of other biomarkers are usually considered, especially when sampling does not pose any risk to patients, in addition to being performed at a minimal cost (23). Four studies (24, 28, 33, 35) investigated the associations between heavy metals and metalloids in follicular fluid and in vitro fertilization (IVF) outcomes. A cross-sectional study by Bloom et al. (23) conducted in 46 women undergoing IVF revealed an inverse association between FF Pb concentration and fertilization (RR = 0.68, p = 0.026) (24). Ingle et al. (27) found a negative correlation between FF Cr and Mn levels with the proportion of mature oocytes (28). Wu et al. (32) noted that As levels in FF samples were inversely associated with the probabilities to obtain good-quality cleavage embryos (p < 0.01) and a negative association of FF Cd levels found with the probabilities of pregnancy and live birth (p = 0.035) (33). A more recent study by Butts et al. (34) investigated the association between follicular fluid (FF) concentrations of As, Hg, Pb and Cd with IVF outcomes among women undergoing IVF. Their results showed lower probabilities of biochemical pregnancy (p = 0.05) and live birth (p = 0.05) at follicular fluid Hg concentrations greater than 0.51 µg/L Hg. In addition, higher follicular fluid Pb concentrations also correlated with a lower probability of live birth (RR = 0.68, 95% CI: 0.46–1.00; p = 0.05) (35).
Heavy metals/metalloids and reproductive concerns
Environmental contamination with heavy metals and metalloids has become a major area of public health concern especially for women of childbearing age, as it can cause infertility and reproductive dysfunction (76–79). The interference of heavy metals and metalloids on human reproduction ranges from uterine leiomyomata, spontaneous abortions, polycystic ovary syndrome (PCOS), birth defects, endometriosis, abnormal semen quality and functionality, impaired embryogenesis, as well as stillbirths (46, 75, 79–81). Recent reports have highlighted the endocrine-disrupting effect of heavy metals and metalloids on the pituitary ovarian axis indicative of their potential associations with female reproductive health (82, 83). Heavy metals and metalloids may trigger hormonal changes that alter the menstrual cycle, ovulation, and female fertility (84). Several studies have investigated the role of heavy metals and metalloids in altering hormonal levels (85, 86). For instance, a study by Chang (83) reported that women with blood Pb levels higher than 25 μg/L had a threefold increased risk of infertility compared to women with blood Pb levels less than 25 μg/(87). Similarly, another study reported that for every 1 μg/L elevation in Cd levels, a 21% increase in the levels of early follicular phase oestradiol (E2); serum follicle stimulating hormone (FSH) and luteinizing hormone (LH) concentrations were noted (88). Taken together, high levels of reproductive anomalies in women of reproductive age have driven the course of assisted reproduction such as in vitro fertilization.
In the present review, 14 studies investigated the impact of exposure of toxic metals on reproductive concerns. Two studies investigated the role of heavy metals and metalloids in infertility (41, 47). The study by Tanrikut et al. (40) revealed that Cd was found in 91% of women diagnosed with unexplained infertility against 34% of fertile women. Pb was found in 15% of women diagnosed with unexplained infertility and 3% of fertile women. Hg and As were undetected in endometrial samples from either groups of women. Results obtained suggested Cd to be a major contributing factor in the aetiology of unexplained infertility (41). Sukhn et al. (46) examined the impact of heavy metals and metalloids in male partners of infertile women attending a fertility clinic. Their data showed significant (p < 0.05) associations between low sperm viability and higher blood Cd and Ba, as well as higher seminal Pb, Cd, Ba, and U (47).
One study investigated association of heavy metals with congenital heart defects (CHD) in offspring (36). In that study, high levels of Al, Mg, and Pb in umbilical cord blood significantly correlated with increased risk of CHD in offspring Al aOR (adjusted odds ratio) = 4.22, 95% CI: 1.35–13.16, p = 0.013), Mg (aOR = 8.00, 95% CI: 1.52–42.08, p = 0.014), and Pb (aOR = 3.82, 95% CI: 0.96–15.23, p = 0.049) (36).
Zhang et al. (38) probed the role of Hg in uterine leiomyomata (UL) (39). Data from that study revealed that Hg was found to be positively associated with UL (OR: 1.91, 95% CI: 1.14, 3.25).
Abudawood et al. (45) investigated the impact of heavy metals and metalloids in pregnant women diagnosed with PCOS (46). The role of heavy metals and metalloids in generating oxidative stress (an etiological factor in PCOS) was examined in that study. Results obtained from that study demonstrated high levels of serum As, Cd, Pb, and Hg which correlated with significantly diminished levels of serum glutathione (GSH) and superoxide dismutase (SOD) in the PCOS group compared to the control group at p < 0.001 (46).
Eight studies investigated association of heavy metals and metalloids with miscarriage/spontaneous abortions (37, 38, 42–45, 48, 49). Ajayi et al. (47) found significant increase (p < 0.05) in the serum Pb, and Cd in pregnant women with history of recurrent spontaneous abortion compared with controls (48). The study by Vigeh et al. (37) revealed that out of all the metals and metalloids evaluated, only Sb showed a significant positive correlation with the risk of spontaneous abortions (OR: 1.65, 95% CI: 1.08–2.52, p value: 0.02). Ou et al. (37) evaluated effects of blood lead levels (BLLs) on spontaneous abortion. The mean BLLs in both case and control groups were 27.17 μg/L and 17.28 μg/L respectively (p = 0.000). The odds ratios for spontaneous abortion in five blood lead levels (5–9, 10–14, 15–24, 25–39, and ≥40 μg/L) were 1.58 (0.23–10.90), 3.13 (2.11–9.08), 4.63 (1.45–14.83), 6.33 (1.95–20.56), and 22.56 (4.91–103.66), respectively, indicative of a significant trend (P1 = 0.64, P2 = 0.02, P3 = 0.01, P4 = 0.02, and P5 = 0.00) (38). Yildrim and Derici (42) found higher blood Pb levels (p = 0.038) in pregnant women with history of spontaneous abortion compared to control (43), while the study by Turan et al. (43) showed significantly increased (p < 0.001) serum concentrations of Pb and Cd in women with threatened abortion compared to control (44). Jie et al. (41) demonstrated that blood cadmium >0.4 µg/L or urine chromium >2 µg/L in pregnant women was indicative of a higher risk of spontaneous abortion (42). Omeljaniuk et al. (44) examined the role of Pb and Cd in women who had miscarriage. The mean concentrations of Pb (35.54 ± 11.0 µg/L) and Cd (2.730 ± 2.07 µg/L) in the blood of women who had miscarriage was higher compared to control (Cd 1.035 ± 0.59 µg/L; Pb 27.11 ± 4.6 µg/L). on the other hand, the mean concentrations of Pb (199.6 ± 348 µg/L) and Cd (214.4 ± 514 µg/L) in the placenta of women who had miscarriage was higher compared to control (Cd 127.4 ± 85 ng/L; Pb 26.35 ± 7.9 ng/L) (45). Similarly, Saad et al. (48) found higher levels of Cd in women who miscarried accompanied with increased levels of plasma malondialdehyde and decreased levels of antioxidants glutathione and glutathione peroxidase suggestive of oxidative stress as a major etiological factor (49). In these 8 studies, Pb and Cd stand out as major reoccurring metals in women with history of spontaneous abortion.
Two studies investigated the relationship between heavy metals/metalloids and endometriosis (39, 40). Zhang et al. (38) found no association between Hg and endometriosis (39) while Kim et al. (39) observed an increase in the standard admission rate (SAR) (1.24 (95% CI: 1.03–1.48) and admission odds ratio (OR) [1.44 (95% CI: 1.11–1.85)] for endometriosis in Pb exposed workers compared with that of the general population (40).
Conclusion
Chronic environmental exposure to heavy metals and metalloids can alter the function of sexual accessory tissues as well as reproductive hormones in humans. These delay the timecourse of sexual maturation directly or indirectly thereby affecting reproductive competence in males and females. Data collated in this review demonstrate that heavy metals and metalloids in body samples of women undergoing IVF correlated negatively with oocyte fertilization/pregnancy rates. Similarly, high concentrations of these metals were also associated with infertility, spontaneous abortion/miscarriage, congenital heart disease, PCOS, endometriosis as well as uterine leiomyomata. Therefore, couples undergoing IVF and those of reproductive age may be screened for heavy metals and metalloids load. In addition, therapeutic strategies to reduce body metals and metalloids burden should be undertaken to enhance pregnancy outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
CO Literature search, manuscript draft. CF manuscript draft. OO Literature search, manuscript draft and conceptualization. All authors contributed to the article and approved the submitted version
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Foster WG, Neal MS, Han M-S, Dominguez MM. Environmental contaminants and human infertility: hypothesis or cause for concern? J Toxicol Environ Health B Crit Rev. (2008) 11(3–4):162–76. doi: 10.1080/10937400701873274
2. Zitzmann M, Rolf C, Nordhoff V, Schräder G, Rickert-Föhring M, Gassner P, et al. Male smokers have a decreased success rate for in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril. (2003) 79:1550–4. doi: 10.1016/S0015-0282(03)00339-X
3. Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. In: Luch A, editor. Molecular, clinical and environmental toxicology. Basel, Switzerland: Springer (2012). p. 133–64.
4. Briffa J, Sinagra E, Blundell R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon. (2020) 6(9):e04691. doi: 10.1016/j.heliyon.2020.e04691
5. Singh BR, Gupta SK, Azaizeh H, Shilev S, Sudre D, Song WY, et al. Safety of food crops on land contaminated with trace elements. J Sci Food Agric. (2011) 91(8):1349–66. doi: 10.1002/jsfa.4355
6. Adepoju-Bello A, Issa O, Oguntibeju OO, Ayoola G, Adejumo O. Analysis of some selected toxic metals in registered herbal products manufactured in Nigeria. Afr J Biotechnol. (2012) 11(26):6918–22. doi: 10.5897/AJB11.3961
7. Kim HS, Kim YJ, Seo YR. An overview of carcinogenic heavy metal: molecular toxicity mechanism and prevention. J Cancer Prev. (2015) 20(4):232. doi: 10.15430/JCP.2015.20.4.232
8. Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. (2011) 283(2-3):65–87. doi: 10.1016/j.tox.2011.03.001
9. Nass R, Evans WS. Physiologic and pathophysiologic alterations of the neuroendocrine components of the reproductive axis. In: Strauss JF III, Barbieri RL, editors; Gargiulo RA, video editor. Yen and jaffe's reproductive endocrinology. Netherlands: Elsevier (2019). p. 473–519.e12.
10. Domar AD, Smith K, Conboy L, Iannone M, Alper M. A prospective investigation into the reasons why insured United States patients drop out of in vitro fertilization treatment. Fertil Steril. (2010) 94(4):1457–9. doi: 10.1016/j.fertnstert.2009.06.020
11. Elder K, Dale B. In: Buster JE, editor. In-vitro fertilization. Cambridge: Cambridge University Press (2020).
12. Pandey S. Human chorionic gonadotrophin (hcg) trigger-mediated ovulation induction in infertility management in south Indian women undergoing ivf/icsi regimens: a pilot sexual medicine study with public health perspective. J Psychosexual Health. (2021) 3(1):57–64. doi: 10.1177/2631831821990501
13. Su Y, Li J-J, Wang C, Haddad G, Wang W-H. Aneuploidy analysis in day 7 human blastocysts produced by in vitro fertilization. Reprod Biol Endocrinol. (2016) 14(1):20. doi: 10.1186/s12958-016-0157-x
14. Kushnir VA, Barad DH, Albertini DF, Darmon SK, Gleicher N. Systematic review of worldwide trends in assisted reproductive technology 2004–2013. Reprod Biol Endocrinol. (2017) 15(1):6. doi: 10.1186/s12958-016-0225-2
15. Wade JJ, MacLachlan V, Kovacs G. The success rate of ivf has significantly improved over the last decade. Aust N Z J Obstet Gynaecol. (2015) 55(5):473–6. doi: 10.1111/ajo.12356
16. Hanna CW, Bloom MS, Robinson WP, Kim D, Parsons PJ, vom Saal FS, et al. DNA methylation changes in whole blood is associated with exposure to the environmental contaminants, mercury, lead, cadmium and bisphenol a, in women undergoing ovarian stimulation for ivf. Hum Reprod. (2012) 27(5):1401–10. doi: 10.1093/humrep/des038
17. Chojnacka K, Zarzycka M, Mruk DD. Biology of the sertoli cell in the fetal, pubertal, and adult mammalian testis. In: Piprek RP, editor. Molecular mechanisms of cell differentiation in gonad development. Cham: Springer (2016). p. 225–51.
18. Pasch LA, Gregorich SE, Katz PK, Millstein SG, Nachtigall RD, Bleil ME, et al. Psychological distress and in vitro fertilization outcome. Fertil Steril. (2012) 98(2):459–64. doi: 10.1016/j.fertnstert.2012.05.023
19. Ying L, Wu LH, Loke AY. Gender differences in emotional reactions to in vitro fertilization treatment: a systematic review. J Assist Reprod Genet. (2016) 33(2):167–79. doi: 10.1007/s10815-015-0638-4
20. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021):372. doi: 10.1136/bmj.n71
21. Bloom MS, Parsons PJ, Steuerwald AJ, Schisterman EF, Browne RW, Kim K, et al. Toxic trace metals and human oocytes during in vitro fertilization (IVF). Reprod Toxicol. (2010) 29(3):298–305. doi: 10.1016/j.reprotox.2010.01.003
22. Kim K, Fujimoto VY, Parsons PJ, Steuerwald AJ, Browne RW, Bloom MS. Recent cadmium exposure among male partners may affect oocyte fertilization during in vitro fertilization (IVF). J Assist Reprod Genet. (2010) 27(8):463–8. doi: 10.1007/s10815-010-9437-0
23. Dickerson E, Sathyapalan T, Knight R, Maguiness S, Killick S, Robinson J, et al. Endocrine disruptor & nutritional effects of heavy metals in ovarian hyperstimulation. J Assist Reprod Genet. (2011) 28(12):1223–8. doi: 10.1007/s10815-011-9652-3
24. Bloom MS, Kim K, Kruger PC, Parsons PJ, Arnason JG, Steuerwald AJ, et al. Associations between toxic metals in follicular fluid and in vitro fertilization (IVF) outcomes. J Assist Reprod Genet. (2012) 29(12):1369–79. doi: 10.1007/s10815-012-9882-z
25. Kim K, Bloom MS, Kruger PC, Parsons PJ, Arnason JG, Byun Y, et al. Toxic metals in seminal plasma and in vitro fertilization (IVF) outcomes. Environ Res. (2014) 133:334–7. doi: 10.1016/j.envres.2014.06.014
26. Wright DL, Afeiche MC, Ehrlich S, Smith K, Williams PL, Chavarro JE, et al. Hair mercury concentrations and in vitro fertilization (IVF) outcomes among women from a fertility clinic. Reprod Toxicol. (2015) 51:125–32. doi: 10.1016/j.reprotox.2015.01.003
27. Tolunay HE, Şükür YE, Ozkavukcu S, Seval MM, Ateş C, Türksoy VA, et al. Heavy metal and trace element concentrations in blood and follicular fluid affect art outcome. Eur J Obstet Gynecol Reprod Biol. (2016) 198:73–7. doi: 10.1016/j.ejogrb.2016.01.001
28. Ingle ME, Bloom MS, Parsons PJ, Steuerwald AJ, Kruger P, Fujimoto VY. Associations between IVF outcomes and essential trace elements measured in follicular fluid and urine: a pilot study. J Assist Reprod Genet. (2017) 34(2):253–61. doi: 10.1007/s10815-016-0853-7
29. García-Fortea P, Cohen-Corcia I, Córdoba-Doña JA, Reche-Rosado A, González-Mesa E. Toxic elements in hair and in vitro fertilization outcomes: a prospective cohort study. Reprod Toxicol. (2018) 77:43–52. doi: 10.1016/j.reprotox.2018.02.001
30. Skalny AV, Tinkov AA, Bohan TG, Shabalovskaya MB, Terekhina O, Leshchinskaia SB, et al. Toxicological and nutritional status of trace elements in hair of women with in vitro fertilization (IVF) pregnancy and their 9-month-old children. Reprod Toxicol. (2018) 82:50–6. doi: 10.1016/j.reprotox.2018.10.004
31. Skalny AV, Tinkov AA, Voronina I, Terekhina O, Skalnaya MG, Kovas Y. Hair trace element and electrolyte content in women with natural and in vitro fertilization-induced pregnancy. Biol Trace Elem Res. (2018) 181(1):1–9. doi: 10.1007/s12011-017-1032-0
32. Tulić L, Vidaković S, Tulić I, Ćurčić M, Bulat Z. Toxic metal and trace element concentrations in blood and outcome of in vitro fertilization in women. Biol Trace Elem Res. (2019) 188(2):284–94. doi: 10.1007/s12011-018-1421-z
33. Wu S, Wang M, Deng Y, Qiu J, Zhang X, Tan J. Associations of toxic and essential trace elements in serum, follicular fluid, and seminal plasma with in vitro fertilization outcomes. Ecotoxicol Environ Saf. (2020) 204:110965. doi: 10.1016/j.ecoenv.2020.110965
34. Kumar S, Mishra V, Thaker R, Gor M, Perumal S, Joshi P, et al. Role of environmental factors & oxidative stress with respect to in vitro fertilization outcome. Indian J Med Res. (2018) 148(Suppl 1):S125. doi: 10.4103/ijmr.IJMR_1864_17
35. Butts CD, Bloom MS, McGough A, Lenhart N, Wong R, Mok-Lin E, et al. Toxic elements in follicular fluid adversely influence the likelihood of pregnancy and live birth in women undergoing IVF. Hum Reprod Open. (2021) 2021(3):hoab023. doi: 10.1093/hropen/hoab023
36. Liu JT, Zhou YW, Wang WD, Mao BH, Hu YG. Association of the levels of heavy metals and trace elements during pregnancy with congenital heart defects in offspring: a prospective cohort study. Zhongguo Dang Dai Er Ke Za Zhi. (2022) 24(2):147–54. doi: 10.7499/j.issn.1008-8830.2109053
37. Vigeh M, Yunesian M, Matsukawa T, Shamsipour M, Jeddi MZ, Rastkari N, et al. Prenatal blood levels of some toxic metals and the risk of spontaneous abortion. J Environ Health Sci Eng. (2021) 19(1):357–63. doi: 10.1007/s40201-020-00608-3
38. Ou J, Peng P, Qiu L, Teng L, Li C, Han J, et al. Effect of lead exposure on spontaneous abortion: a case-control study. Clin Lab. (2020) 66(5):1–7. doi: 10.7754/Clin.Lab.2019.190940
39. Zhang Y, Lu Y, Ma H, Xu Q, Wu X. Combined exposure to multiple endocrine disruptors and uterine leiomyomata and endometriosis in us women. Front Endocrinol. (2021) 12:726876. doi: 10.3389/fendo.2021.726876
40. Kim MG, Min YS, Ahn YS. Does exposure of lead and cadmium affect the endometriosis? Int J Environ Res Public Health. (2021) 18(17):9077. doi: 10.3390/ijerph18179077
41. Tanrıkut E, Karaer A, Celik O, Celik E, Otlu B, Yilmaz E, et al. Role of endometrial concentrations of heavy metals (cadmium, lead, mercury and arsenic) in the aetiology of unexplained infertility. Eur J Obstet Gynecol Reprod Biol. (2014) 179:187–90. doi: 10.1016/j.ejogrb.2014.05.039
42. Jie O, Peng P, Qiu L, Teng L, Li C, Han J, et al. Biomarkers of metal toxicity in embryos in the general population. J Clin Lab Anal. (2019) 33(8):e22974. doi: 10.1002/jcla.22974
43. Yıldırım E, Derici MK. The effect of heavy metals on miscarriage. JCOG. (2019) 29(1):31–8. doi: 10.5336/jcog.2018-64175
44. Turan K, Arslan A, Uçkan K, Demir H, Demir C. Change of the levels of trace elements and heavy metals in threatened abortion. J Chin Med Assoc. (2019) 82(7):554–7. doi: 10.1097/JCMA.0000000000000077
45. Omeljaniuk WJ, Socha K, Soroczynska J, Charkiewicz AE, Laudanski T, Kulikowski M, et al. Cadmium and lead in women who miscarried. Clin Lab. (2018) 64(1):59–67. doi: 10.7754/Clin.Lab.2017.170611
46. Abudawood M, Tabassum H, Alanazi AH, Almusallam F, Aljaser F, Ali MN, et al. Antioxidant status in relation to heavy metals induced oxidative stress in patients with polycystic ovarian syndrome (PCOS). Sci Rep. (2021) 11(1):22935. doi: 10.1038/s41598-021-02120-6
47. Sukhn C, Awwad J, Ghantous A, Zaatari G. Associations of semen quality with non-essential heavy metals in blood and seminal fluid: data from the environment and male infertility (EMI) study in Lebanon. J Assist Reprod Genet. (2018) 35(9):1691–701. doi: 10.1007/s10815-018-1236-z
48. Ajayi OO, Charles-Davies MA, Arinola OG. Progesterone, selected heavy metals and micronutrients in pregnant Nigerian women with a history of recurrent spontaneous abortion. Afr Health Sci. (2012) 12(2):153–9. doi: 10.4314/ahs.v12i2.12
49. Saad AA, Hegazy N, Amer N, Gaber K, Youssef A, Sharaf N, et al. The role of cadmium exposure on spontaneous abortion. World J Med Sci. (2012) 7(4):6. doi: 10.5829/idosi.wjms.2012.7.4.1111
50. Sadeu J, Hughes CL, Agarwal S, Foster WG. Alcohol, drugs, caffeine, tobacco, and environmental contaminant exposure: reproductive health consequences and clinical implications. Crit Rev Toxicol. (2010) 40(7):633–52. doi: 10.3109/10408444.2010.493552
51. Pandey S, Murdia K, Murdia A, Chandra V, Murdia N. RE: partner smoking influences whether mothers quit smoking during pregnancy: a prospective cohort study. BJOG. (2018) 125(7):904. doi: 10.1111/1471-0528.15030
52. Bay B, Mortensen EL, Kesmodel US. Assisted reproduction and child neurodevelopmental outcomes: a systematic review. Fertil Steril. (2013) 100(3):844–53. doi: 10.1016/j.fertnstert.2013.05.034
53. Schulte RT, Ohl DA, Sigman M, Smith GD. Sperm DNA damage in male infertility: etiologies, assays, and outcomes. J Assist Reprod Genet. (2010) 27(1):3–12. doi: 10.1007/s10815-009-9359-x
54. Lazaros LA, Vartholomatos GA, Hatzi EG, Kaponis AI, Makrydimas GV, Kalantaridou SN, et al. Assessment of sperm chromatin condensation and ploidy status using flow cytometry correlates to fertilization, embryo quality and pregnancy following in vitro fertilization. J Assist Reprod Genet. (2011) 28(10):885–91. doi: 10.1007/s10815-011-9611-z
55. Franken DR, Oehninger S. Semen analysis and sperm function testing. Asian J Androl. (2012) 14(1):6. doi: 10.1038/aja.2011.58
56. Mehta JG. Laboratory aspects of in vitro fertilization and intracytoplasmic sperm injection. In: Panchal S, editor. Donald school textbook of human reproductive & gynecological endocrinology India: JP Medical Publishers (2018). p. 197.
57. Bhargava P, Gupta N, Vats S, Goel R. Health issues and heavy metals. Austin J Environ Toxicol. (2017) 3(1):3018.
58. Phaniendra A, Jestadi DB, Periyasamy L. Free radicals: properties, sources, targets, and their implication in Various diseases. Indian J Clin Biochem. (2015) 30(1):11–26. doi: 10.1007/s12291-014-0446-0
59. Hammadeh ME, Hamad M, Refaat K, Said TM, Fischer-Hammadeh C. Effect of oxidative stress on art outcome. In: Parinandi N, editor. Studies on men's health and fertility. New Jersey: Springer (2012). p. 449–83.
60. Durairajanayagam D. Physiological role of reactive oxygen Species in Male reproduction. In: Henkel R, Samanta L, Agarwal A, editors. Oxidants, antioxidants and impact of the oxidative status in male reproduction. Netherlands: Elsevier (2019). p. 65–78.
61. Aitken RJ, Curry BJ. Redox regulation of human sperm function: from the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxid Redox Signaling. (2011) 14(3):367–81. doi: 10.1089/ars.2010.3186
62. Aitken RJ, Smith TB, Jobling MS, Baker MA, De Iuliis GN. Oxidative stress and male reproductive health. Asian J Androl. (2014) 16(1):31. doi: 10.4103/1008-682X.122203
63. Gleicher N, Kushnir V, Barad D. Worldwide decline of IVF birth rates and its probable causes. Hum Reprod Open. (2019) 2019(3):hoz017. doi: 10.1093/hropen/hoz017
64. Szyf M. The dynamic epigenome and its implications in toxicology. Toxicol Sci. (2007) 100(1):7–23. doi: 10.1093/toxsci/kfm177
65. Genchi G, Sinicropi MS, Lauria G, Carocci A, Catalano A. The effects of cadmium toxicity. Int J Environ Res Public Health. (2020) 17(11):3782. doi: 10.3390/ijerph17113782
66. Litzky JF, Marsit CJ. Epigenetically regulated imprinted gene expression associated with IVF and infertility: possible influence of prenatal stress and depression. J Assist Reprod Genet. (2019):1–15. doi: 10.1007/s10815-019-01483-0
67. Fei DL, Koestler DC, Li Z, Giambelli C, Sanchez-Mejias A, Gosse JA, et al. Association between in utero arsenic exposure, placental gene expression, and infant birth weight: a us birth cohort study. Environ Health. (2013) 12(1):58. doi: 10.1186/1476-069X-12-58
68. Romero R, Friel LA, Edwards DRV, Kusanovic JP, Hassan SS, Mazaki-Tovi S, et al. A genetic association study of maternal and fetal candidate genes that predispose to preterm prelabor rupture of membranes (PROM). Am J Obstet Gynecol. (2010) 203(4):361.e1–361.e30. doi: 10.1016/j.ajog.2010.05.026
69. Bruchova H, Vasikova A, Merkerova M, Milcova A, Topinka J, Balascak I, et al. Effect of maternal tobacco smoke exposure on the placental transcriptome. Placenta. (2010) 31(3):186–91. doi: 10.1016/j.placenta.2009.12.016
70. Koestler DC, Avissar-Whiting M, Houseman EA, Karagas MR, Marsit CJ. Differential DNA methylation in umbilical cord blood of infants exposed to low levels of arsenic in utero. Environ Health Perspect. (2013) 121(8):971–7. doi: 10.1289/ehp.1205925
71. Simpkin AJ, Suderman M, Gaunt TR, Lyttleton O, McArdle WL, Ring SM, et al. Longitudinal analysis of DNA methylation associated with birth weight and gestational age. Hum Mol Genet. (2015) 24(13):3752–63. doi: 10.1093/hmg/ddv119
72. Appleton AA, Lester BM, Armstrong DA, Lesseur C, Marsit CJ. Examining the joint contribution of placental Nr3c1 and Hsd11b2 methylation for infant neurobehavior. Psychoneuroendocrinology. (2015) 52:32–42. doi: 10.1016/j.psyneuen.2014.11.004
73. Lesseur C, Paquette AG, Marsit CJ. Epigenetic regulation of infant neurobehavioral outcomes. Med Epigenet. (2014) 2(2):71–9. doi: 10.1159/000361026
74. Mohmand J, Eqani SAMAS, Fasola M, Alamdar A, Mustafa I, Ali N, et al. Human exposure to toxic metals via contaminated dust: bio-accumulation trends and their potential risk estimation. Chemosphere. (2015) 132:142–51. doi: 10.1016/j.chemosphere.2015.03.004
75. Amadi CN, Igweze ZN, Orisakwe OE. Heavy metals in miscarriages and stillbirths in developing nations. Middle East Fertil Soc J. (2017) 22(2):91–100. doi: 10.1016/j.mefs.2017.03.003
76. Mendola P, Messer LC, Rappazzo K. Science linking environmental contaminant exposures with fertility and reproductive health impacts in the adult female. Fertil Steril. (2008) 89(2):e81–94. doi: 10.1016/j.fertnstert.2007.12.036
77. Bloom MS, Louis GMB, Sundaram R, Kostyniak PJ, Jain J. Associations between blood metals and fecundity among women residing in New York state. Reprod Toxicol. (2011) 31(2):158–63. doi: 10.1016/j.reprotox.2010.09.013
78. Pandey S, Murdia K, Murdia A. Tobacco as a significant predictor of infertility: a public health perspective in an Indian scenario. Fertil Steril Res. (2017) 4(1):15. doi: 10.4103/fsr.fsr_15_17
79. Pandey S. Toll-like receptor-2 and tissue inhibitor of matrix metalloproteinase-2 genetic variants as predictors of tobacco-mediated female infertility amongst mycobacterium tuberculi-positive Asian Indian cohort. Fertil Steril. (2020) 114(3):e541. doi: 10.1016/j.fertnstert.2020.09.061
80. Bede-Ojimadu O, Amadi CN, Orisakwe OE. Blood lead levels in women of child-bearing age in sub-saharan Africa: a systematic review. Front Public Health. (2018) 6:367. doi: 10.3389/fpubh.2018.00367
81. Ettinger AS, Egan KB, Homa DM, Brown MJ. Blood lead levels in us women of childbearing age, 1976–2016. Environ Health Perspect. (2020) 128(1):017012. doi: 10.1289/EHP5925
82. Dutta S, Gorain B, Choudhury H, Roychoudhury S, Sengupta P. Environmental and occupational exposure of metals and female reproductive health. Environ Sci Pollut Res. (2021):1–26. doi: 10.1007/s11356-021-16581-9
83. Green MP, Harvey AJ, Finger BJ, Tarulli GA. Endocrine disrupting chemicals: impacts on human fertility and fecundity during the peri-conception period. Environ Res. (2021) 194:110694. doi: 10.1016/j.envres.2020.110694
84. Sengupta P, Banerjee R, Nath S, Das S, Banerjee S. Metals and female reproductive toxicity. Hum Exp Toxicol. (2015) 34(7):679–97. doi: 10.1177/0960327114559611
85. Gallagher CM, Moonga BS, Kovach JS. Cadmium, follicle-stimulating hormone, and effects on bone in women age 42–60 years, nhanes iii. Environ Res. (2010) 110(1):105–11. doi: 10.1016/j.envres.2009.09.012
86. Guo Z, Guo H, Xia Y. Effects on endocrine system of female rats exposed to chronic arsenic. J Hyg Res. (2011) 40(2):178–9.
87. Chang S-H, Cheng B-H, Lee S-L, Chuang H-Y, Yang C-Y, Sung F-C, et al. Low blood lead concentration in association with infertility in women. Environ Res. (2006) 101(3):380–6. doi: 10.1016/j.envres.2005.10.004
Keywords: heavy metals and metalloids, toxicological concerns, in vitro fertilization, human reproductive medicine, pregnancy rate, public health
Citation: Obasi CN, Frazzoli C and Orisakwe OE (2022) Heavy metals and metalloids exposure and in vitro fertilization: Critical concerns in human reproductive medicine. Front. Reprod. Health 4:1037379. doi: 10.3389/frph.2022.1037379
Received: 5 September 2022; Accepted: 17 October 2022;
Published: 21 November 2022.
Edited by:
Bianca Bianco, Faculdade de Medicina do ABC, BrazilReviewed by:
Donatella Caserta, Sapienza University of Rome, ItalySaumya Pandey, IndiraIVF Hospital, India
© 2022 Obasi, Frazzoli and Orisakwe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Orish Ebere Orisakwe orishebere@gmail.com, orish.orisakwe@uniport.edu.ng
Specialty Section: This article was submitted to Assisted Reproduction, a section of the journal Frontiers in Reproductive Health
 Cecilia Nwadiuto Obasi
Cecilia Nwadiuto Obasi Chiara Frazzoli
Chiara Frazzoli Orish Ebere Orisakwe
Orish Ebere Orisakwe