- 1Department of Psychiatry, University of Pittsburgh, Pittsburgh, PA, United States
- 2Department of Psychology, University of Pittsburgh, Pittsburgh, PA, United States
- 3Department of Biostatistics, University of Pittsburgh, Pittsburgh, PA, United States
- 4Department of Psychology, California State University Dominguez Hills, Carson, CA, United States
- 5Department of Psychiatry and Behavioral Neuroscience, University of Chicago, Chicago, IL, United States
The negative effects of prenatal stress on offspring health are well established, but there remains little understanding of the influence of stress prior to conception despite known effects on biological systems that are important for a healthy pregnancy. Furthermore, operational definitions of stress vary considerably, and exposure is often characterized via summed, ordinal scales of events. We hypothesized that type, severity, and consistency of preconception stress would be associated with birthweight and gestational age (GA) at birth. Data were drawn from a subsample of participants in the 21-year longitudinal Pittsburgh Girls Study (PGS, N = 2,450) that has followed women annually since childhood. Prior work in the PGS derived three domains of stress exposure between ages 7-17 years related to subsistence (e.g., resource strain, overcrowding), safety (e.g., community violence, inter-adult aggression), and caregiving (e.g., separation, maternal depression). We tested the effects of dimensions of preconception stress on birthweight and GA among offspring of 490 PGS participants who delivered at age 18 or older (n = 490; 76% Black, 20% White, 4% Multiracial). Our hypotheses were partially supported with results varying by stress type and severity and by infant sex. Severity of preconception exposure to subsistence stress was prospectively associated with lower offspring birthweight (B = −146.94, SE = 69.07, 95% CI = −282.66, −11.22). The association between severity of caregiving stress in childhood and adolescence and GA at birth was moderated by infant sex (B = 0.85, SE = .41, 95% CI = 0.04, 1.66), suggesting greater vulnerability to this type of stress for male compared to female infants. Exposure to safety stressors did not predict birth outcomes. Infants of Black compared with White mothers had lower birthweight in all models regardless of preconception stress type, severity or consistency. However, we observed no moderating effects of race on preconception stress-birth outcome associations. Demonstrating specificity of associations between preconception stress exposure and prenatal health has the potential to inform preventive interventions targeting profiles of exposure to optimize birth outcomes.
Introduction
Fetal programming provides a model for understanding the development of health and disease that is focused on prenatal conditions that impact the vulnerability of individuals to multiple pathologies (1, 2). Maternal exposure to environmental stressors during the prenatal period is one such condition that has been linked to various suboptimal birth outcomes such as preterm birth and low infant birthweight (3), as well as later neurodevelopmental impairments in childhood (4). The strength of the causal claim that maternal stress has a direct impact on fetal development is based on rigorous controlled experiments in animals, that distinguish prenatal from postpartum effects using methods such as cross-fostering or nursery rearing (5–8).
From a developmental and life-course perspective, however, stress exposure is unlikely to arise de novo following conception. In a similar vein, prenatal health is not independent of the health of the system prior to conception. For these reasons, the preconception period is emerging as an important focus for research on adverse birth outcomes and offspring development (9), as well as a model for understanding, and ultimately preventing, health disparities in pregnant women and their children (10, 11).
A small, but growing, evidence base in human studies provides preliminary support for the impact of preconception stress exposure on birth outcomes, although the majority of studies are based on maternal retrospective reports [see review (10)]. For example, at 9-months postpartum, adult participants in the United States’ Early Childhood Longitudinal Study, Birth Cohort (ECLS-B) reported on stressful life events that occurred prior to conception. Results indicated that any exposure to preconception stress was associated with heightened risk for very low infant birthweight, and that the cumulative number of life events was inversely related to infant birthweight (12).
Results of the few prospective longitudinal studies lend some additional support for a link between preconception stress exposure and suboptimal birth outcomes. The National Child Development Study in Great Britain included measures of financial, parenting, family, and community stressors at birth, ages 7, 11 and/or 16 years (13). At ages 33 or 41 years, female participants (n ≈ 5,000, 96% White) recalled the outcomes of any pregnancies to date. Results indicated that exposure to stressors across childhood and adolescence was associated with higher rates of preterm birth and lower birthweight, even after accounting for prenatal stress exposure (14). In a follow-up to the National Longitudinal Study of Adolescent Health (Add Health), chronic stressors (e.g., parent receipt of public assistance, high neighborhood unemployment) during adolescence and young adulthood, but not stressful life events were inversely associated with offspring birthweight among the female participants (n > 5,000, 57% White, 24% Black, 18% Latina), and also partially explained racial/ethnic disparities in birthweight (15). In the Australian Longitudinal Study of Women's Health, participants' perceptions of stress measured in the three years prior to delivery were linked to state-based data on birthweight for 3,622 women (mostly partnered, highly educated) (16). Results showed no differences in offspring birthweight among women reporting none or minimal stress (survey scores 0 or 1) vs. stress deemed moderate/high. Finally, in a racially diverse sample (44% Black, 30% Latina or Hispanic, 27% White) of low-income women (n = 360), earlier GA at delivery was associated with high levels of stress appraisal (i.e., perceived stress and parenting stress) and both high and low levels of exposure to stressors (e.g., life events, financial strain, interpersonal violence) during the interpregnancy interval (17). In general, results of these prospective studies suggest that stress experienced prior to conception can negatively influence birth outcomes, but differences in sample characteristics, conceptualizations of stress and the timing and duration of the exposure window highlight the need for further study.
There are long-standing racial disparities in birth outcomes in the United States with Black women at elevated risk for delivering low birthweight and preterm infants compared to White women (18–20). Increased focus on social determinants of health points to systemic racism and other structural processes as major contributors to these persistent disparities (21–24) relative to individual-level factors (e.g., health behaviors, prenatal characteristics) (25, 26). Despite some evidence that preconception stress explains more variability in birth outcome inequities than stress experienced during pregnancy (27), little is known about the differential impact of type and timing of preconception stress exposure on birth outcomes among Black and White women.
Fetal growth and risk for adverse birth outcomes are known to differ by sex. Whereas birthweights for female infants are generally lower than for males, preterm birth and stillbirths occur more often in male gestations (28–30). Male fetuses may also be particularly susceptible to the negative effects of prenatal stress exposure (31–33), consistent with reports of a decrease in the male-to-female birth ratio in contexts of maternal stress (34–36). However, evidence also suggests that female fetuses show a reduction in growth rate in response to early gestational stress, a response that is considered adaptive (37). Given that most research has focused on stress experienced during pregnancy, the extent to which fetal sex moderates the impact of exposure to preconception stress on birth outcomes remains unclear.
The impact of stress exposure on health varies significantly as a function of type, timing, and chronicity, as shown in rodent and non-human primate models (38–42). Results from animal studies also demonstrate that when chronic stress is predictable, rather than unexpected or inconsistent, responses can become habituated and attenuated (43, 44). The study of type, severity and consistency of stress exposure in humans is clearly more complex, given the lack of experimental control and a less developed approach to a functional taxonomy compared to animal studies (45). Extant studies that have measured type and timing of stress in the immediate preconception period suggest that such dimensions explain variance in birth outcomes (14, 15, 17, 46). However, there is a need for longitudinal studies that begin in childhood, assess multiple domains of stress exposure across development, and follow participants through pregnancy and birth to rigorously test the impact of type, severity and consistency of preconception stress exposure on birth outcomes. The Pittsburgh Girls Study (PGS), an ongoing community-based longitudinal study, now in its 21st year of annual data collection, is one such study. Participants (n = 2,450) were enrolled at ages 5–8 years and have been interviewed each year about multiple aspects of health and development including exposure to stress. In prior analyses with this sample, we characterized the dynamic nature of three domains stress exposure (subsistence, safety, and caregiving stress) among all PGS participants between ages 7 to 17 years (47). These domains extended from a substantial literature based on animal models (39, 40) and work in humans (48). Analyses indicated variability in initial severity levels, in consistency over time, and timing of shifts in exposure level within- and across-domains. Moreover, group membership differed in terms of racial composition with Black participants over-represented in groups exposed to high and inconsistent levels, especially in the domains of subsistence and safety stress.
In the current study, we examine the influence of these three domains of preconception stress assessed between ages 7 to 17 years on later birth outcomes in PGS participants. We focus on gestational age at birth and infant birthweight given the extensive research on prenatal stress exposure and birth outcomes, the relevance for later health, and clear operational definitions. We hypothesize that high levels of stress exposure (severity) across childhood and adolescence would be inversely associated with gestational age and birthweight. In addition, we expect that changing levels of moderate-high stress (inconsistent exposures over time) would be associated with more adverse birth outcomes given lack of opportunity for adaptation/habituation. We hypothesize that these effects would be evident after controlling for several confounds including maternal age, parity and pre-pregnancy BMI. Finally, we hypothesize that preconception stress effects on birth outcomes would be greater for Black than White women. Because there was insufficient evidence for sex-specific effects of preconception stress exposures, we did not propose hypotheses, but conducted exploratory analyses of potential interactions with infant sex.
Materials and methods
Sample
Participants in the PGS were identified in 1999–2000 via random household sampling, with over-sampling of households in low resourced neighborhoods. The PGS team enumerated 103,238 Pittsburgh households to locate girls between the ages of 5 and 8 years (49, 50). Neighborhoods in the City of Pittsburgh in which at least 25% of the families were living at or below the poverty level were fully enumerated (i.e., all homes were contacted to determine if the household contained an eligible girl), along with a random selection of 50% of households in all other city neighborhoods. The enumeration identified 3,118 separate households in which an eligible girl resided. From these households, families who moved out of state and families in which the girl would be age-ineligible by the start of the study were excluded. When two age-eligible girls were enumerated in a single household, one girl was randomly selected for participation. Of the 2,992 remaining families, 2,875 (96%) were successfully re-contacted to determine their willingness to participate in the longitudinal study. Of those families, 85% agreed to participate, resulting in a total sample size of 2,450.
As part of the PGS interview starting at age 11, participants were asked annually whether they had become pregnant or given birth in the past year. The present study included participants whose conception occurred at or after age 18 years to establish temporal precedence between stress exposures during childhood and adolescence (up through age 17) and later conception. Participants without birth data from electronic health records were excluded.
A total of 779 PGS participants were identified as having given birth between 2010 and 2021, of whom 59 gave birth before age 18 and had no subsequent births. For participants with multiple births, we focused on the earliest birth with available birth outcome data since the participant turned age 18. Birth outcome data were abstracted from electronic health records for participants who provided consent and delivered at a University of Pittsburgh Medical Center (UPMC) facility (N = 429), or for participants who were included in a PGS peripartum substudy and delivered out-of-network (N = 61). Among the 720 eligible participants, 490 participants had data for at least one birth outcome (of whom 98.8% had data for both gestational age and birthweight) and infant sex (see flowchart in Supplemental Figure). Thus, subsequent analyses focused on this analytic sample of 490 participants.
Missing birth outcome data were largely due to PGS participants delivering outside the UPMC network. Examination of patterns of missingness revealed that, compared with included participants (n = 490), participants without birth data (n = 230) were more likely to be living out of state [Χ2(1) = 51.94, p < .001], be primiparous [Χ2(1) = 20.76, p < .001], identify as White race [Χ2(2) = 30.18, p < .001] and to be younger age [F(1,718) = 52.89, p < .001]. Primary caregivers of excluded participants reported lower levels of perceived stress [F(1,718) = 52.89, p < .001]. There were no group differences in terms of the proportion of families receiving public assistance.
Measures
Birth outcomes. Gestational age at birth (GA) measured in days and partial weeks and infant birthweight measured in grams were obtained from maternal or child electronic health records.
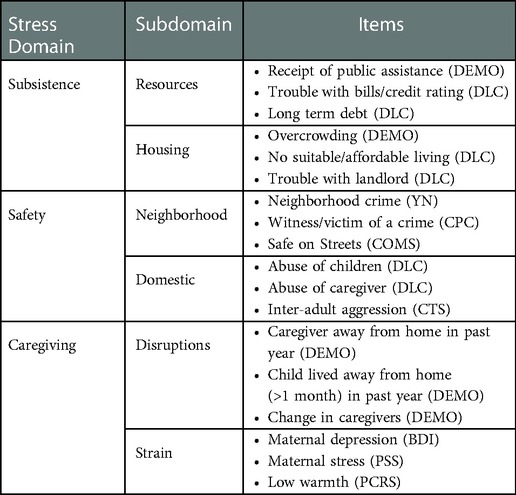
Stress domains were derived in analyses previously described in (47). Preconception stress exposures across three conceptual domains (subsistence, safety and caregiving stress) were obtained from annual PGS interviews with the caregivers when girls were aged between 7 and 17 years. Items within each stress domain are summarized in Table 1.
Subsistence Stress. Resource-related stress was based on caregivers' reports (yes/no) of receipt of public assistance (e.g., Nutrition Program for Women, Infants, and Children, food stamps, welfare, Medicaid) on the Demographic Questionnaire (DEMO; developed for the PGS), trouble with credit rating, and long-term debts (other than a mortgage) as reported by caregivers (yes/no) on the Difficult Life Circumstances measure (DLC) (51). Housing stress included overcrowding, defined as more than 2 people per bedroom as assessed on the DEMO, and suboptimal housing based maternal report of no suitable or affordable place to live (yes/no) and having trouble with the landlord (yes/no) on the DLC. Binary items measuring resource and housing stress were summed to yield a total score for subsistence stress.
Safety Stress. Neighborhood safety was assessed by caregiver report on the extent of illegal activities and neighborhood crime (e.g., vandalism, organized crime, drug-dealing, prostitution) using the Your Neighborhood questionnaire (YN) (52). Participants reported the extent to which 17 items were a problem on 3-point Likert scales. Scores falling in the upper quartile indicated neighborhood safety stress. Caregivers also indicated whether they had witnessed and/or were victimized by violent crime (e.g., homicide, assault, rape) (yes/no) on the Police Contacts measure (PC) (52). Finally, caregivers reported on lack of safety on neighborhood streets on the Community Survey (COMS) (53), defined as endorsing “disagree” or “strongly disagree” with the statement “I feel safe on the streets in my neighborhood.” Domestic safety was assessed using items from the DLC including whether any child was being emotionally, sexually or physically abused by anyone, and whether the caregiver had been physically abused by his/her partner. In addition, caregivers reported on inter-adult aggression on the revised Conflict Tactics Scale (CTS2) (54) with domestic violence coded from items: threatening to hit, throwing objects at the other, or slapping/hitting the other. Binary items measuring neighborhood and domestic safety were summed to yield a total score for safety stress.
Caregiving Stress. Disruptions in caregiving were based on reports of child separation/out-of-home care (e.g., foster home, special facility) for more than 1 month within a 12-month period (yes/no) and change in primary caregivers (yes/no) assessed via the DEMO measure. Caregiving strain was measured by low maternal warmth using six items from the Parent/Child Relationship Scale (PCRS) (55). Items were summed and the upper quartile defined low maternal warmth. Caregiver report of depression was measured using the Beck Depression Inventory-II (BDI-II) (56); a score ≥ 11 was used to indicate a significant level of depression symptoms. Caregiver stress appraisal was measured with the Perceived Stress Scale (PSS) (57). Fourteen items were rated on 3-point scales (1 = almost never, 2 = sometimes, 3 = never), summed, and cut at the upper quartile to index high perceived stress. The six binary items measuring caregiving stress were summed to yield a total score.
In prior analyses (47), we used a group-based trajectory modeling approach to identify the number of groups and shapes of trajectories (e.g., linear, quadratic) within each domain: model fit was compared using the Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC), with lower AIC and BIC values indicating better fit. The magnitude of decrease in AIC and BIC fit indices with each increase in number of groups was also considered to ensure parsimony. The stress trajectory groups were defined in terms of initial severity level: low (defined as z-scores below −0.5), average (z-scores between −0.5 and 0.5), moderate (z-scores above 0.5 but below 1.0), and high (z-scores at or above 1.0). Change in level of exposure over time was defined using a change of greater than 0.5 SD. Thus, consistent exposure was defined by scores that remained within 0.5 SD, and inconsistent (e.g., increasing, decreasing) exposure by changes of at least 0.5 SD.
Covariates. Maternal age at conception was estimated by subtracting 40 weeks from the participant's age on the date of delivery. Participants reported on racial identity as part of the PGS. Pre-pregnancy body mass index (i.e., BMI from the PGS wave prior to conception) was calculated from participants' height and weight measured using a stadiometer and digital scale (BMI; kg/m2). Infant sex assigned at birth was obtained from medical record and/or maternal report as for the birth outcomes. Given that nulliparity and adolescent childbirth are associated with higher risk of preterm birth and low birthweight (58, 59), we also covaried parity and history of childbirth prior to age 18 using data from annual PGS interviews with measures starting at age 11.
Procedure
Approval for all study procedures was obtained from the University of Pittsburgh Human Research Protection Office. Written informed consent from the caregiver and verbal assent from the girl were obtained through age 17, whereas all participants aged 18 and older provided written informed consent.
Data analysis plan
Linear regression models were used to test associations between types of preconception exposure (i.e., subsistence, safety, caregiving stress) and offspring birth outcomes (i.e., GA, birthweight). First, for each of the three stress types, we regressed GA on the stress trajectory groups (Models 1a, 2a, 3a in Tables 4–6 respectively), using the lowest severity group as the reference group. Parallel models were conducted with birthweight as the outcome. Covariates (i.e., maternal age, race, pre-pregnancy BMI, infant sex, birth number, and history of childbirth prior to age 18) were included in the models given their documented associations with birth outcomes. The analytic sample size after adjusting for covariates was N = 467. Because there were no differences in GA or birthweight for participants with complete covariates compared to those missing covariates, missing data were handled via listwise deletion.
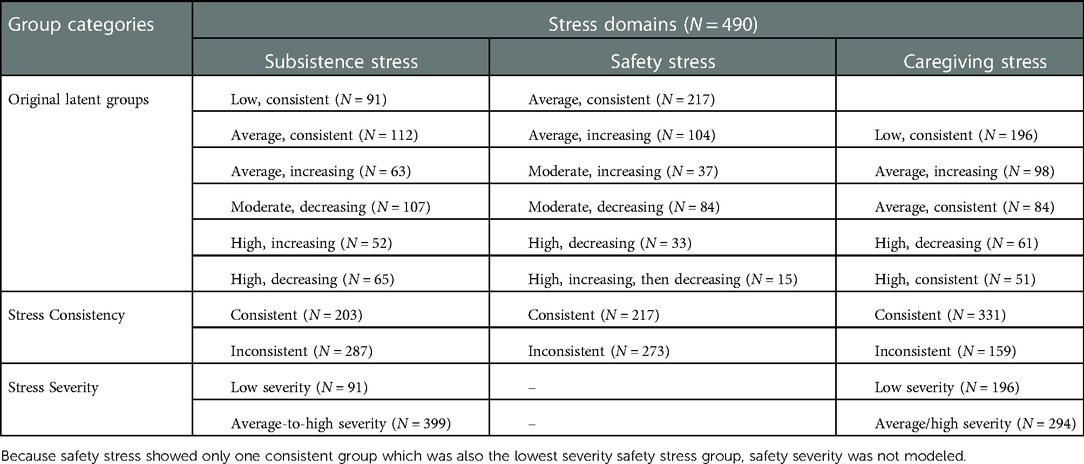
As illustrated in Figure 1, the original stress trajectory groups differed from each other with respect to the consistency of exposure, as well as severity of stress exposure, aspects of stress exposure that have been theorized to uniquely influence birth outcomes (10). In order to increase power, we regrouped the trajectories based on consistency and severity to probe these dimensions of stress exposure. Classification of the original latent stress groups and the binary stress groups used for the present study are shown in Table 2 along with sample sizes for each group. Specifically, to examine differences by consistency of exposure, we recoded the trajectory groups into a binary group variable (“stress consistency”) that compared all stress groups following a stable/consistent pattern to all inconsistent stress exposure groups (i.e., increasing and decreasing groups). We then regressed the birth outcomes (GA and birthweight) on the stress consistency group variable for each of the three stress types (Models 1b, 2b, 3b), adjusting for covariates. Next, to examine differences by severity of exposure, we recoded the trajectory groups into a binary group variable (“stress severity”) that compared the lowest severity trajectory with all other trajectories. We then regressed the birth outcomes (GA and birthweight) on the stress severity group variable for preconception subsistence and caregiving stress types (Models 1c and 3c) adjusting for covariates. Because safety stress showed only one consistent group, which was also the lowest severity safety stress group, a binary indicator of safety stress severity was not modeled.
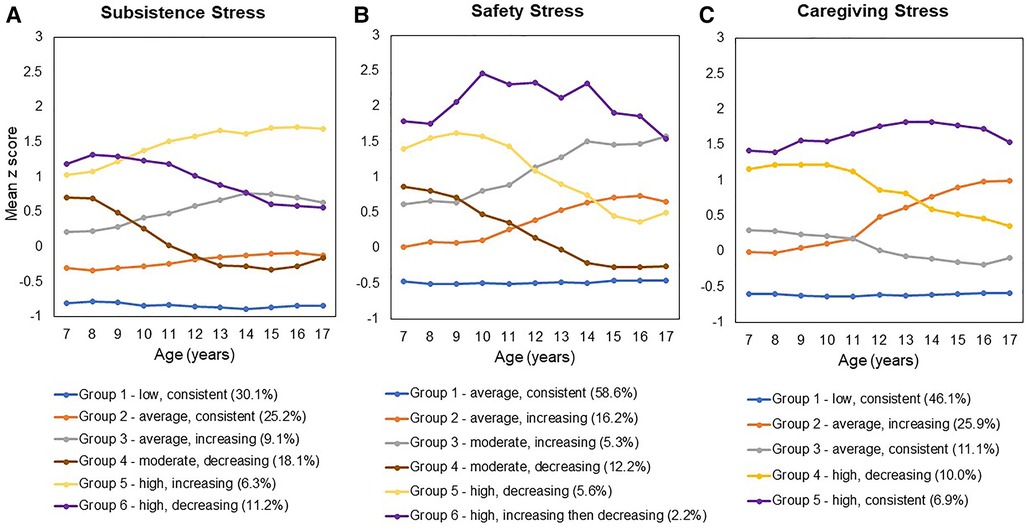
Figure 1. (A) Subsistence Stress, (B) Safety Stress, and (C) Caregiving Stress. [Figure reproduced from the authors' previously published work: Keenan et al. (47)].
Finally, to examine potential moderation effects by infant sex and maternal race, we conducted follow-up regression models for the stress severity and stress consistency models that included Stress x Infant Sex (male/female) and Stress x Maternal Race (Black/White) interaction terms. Following standard guidelines for interpreting interactions (60), significant Stress x Infant Sex interactions were probed by examining the associations between stress and birth outcomes when the binary sex variable was centered at 0 = male or 1 = female.
Results
Preliminary analyses
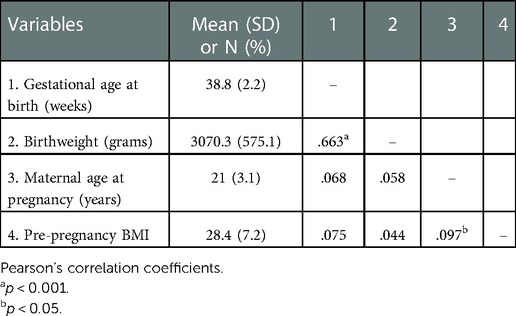
Descriptive statistics and bivariate correlations among continuous variables are included in Table 3. The sample included 371 Black mothers (76%), 98 White mothers (20%) and 20 Multiracial mothers (4%). One additional participant who identified as Asian American was excluded from the final analytic sample due to the small cell size preventing examination of group differences. Mean age at the time of conception was 22.8 years (SD = 2.8, range = 18–29.6 years). Approximately half (49%) of infants were female. Most of the sample (64.7%) was primiparous, 27.6% had one prior child and 7.7% had more than one prior child. A minority of mothers had had a prior birth before age 18 (15.3%). Gestational age at birth and birthweight were correlated in expected directions. Approximately 14.7% of infants in the current sample had low birthweights (<2,500 grams) and 15.9% of infants were born preterm (gestational age ≤ 37 weeks). Female infants had lower birthweights than male infants [M = 3014.91, SD = 594.16 vs. M = 3123.49, SD = 550.39, F(1,487) = 4.40, p = .036] but there were no sex differences in terms of GA at birth. Infants of Black mothers compared with infants of White mothers had significantly lower birthweights [M = 3017.72, SD = 582.00 vs. M = 3252.45, SD = 524.27, F(1,467) = 13.12, p < .001] and shorter gestations [M = 38.64, SD = 2.27 vs. M = 39.18, SD = 1.86, F(1,461) = 4.66, p = .031]. In categorical terms, low birthweight was more than twice as common for infants of Black mothers (17.0%) compared to infants of White mothers (8.2%), and preterm birth was more common for infants of Black mothers (17.2%) compared to infants of White mothers (11.3%).
Exposure to preconception subsistence stress and offspring birth outcomes
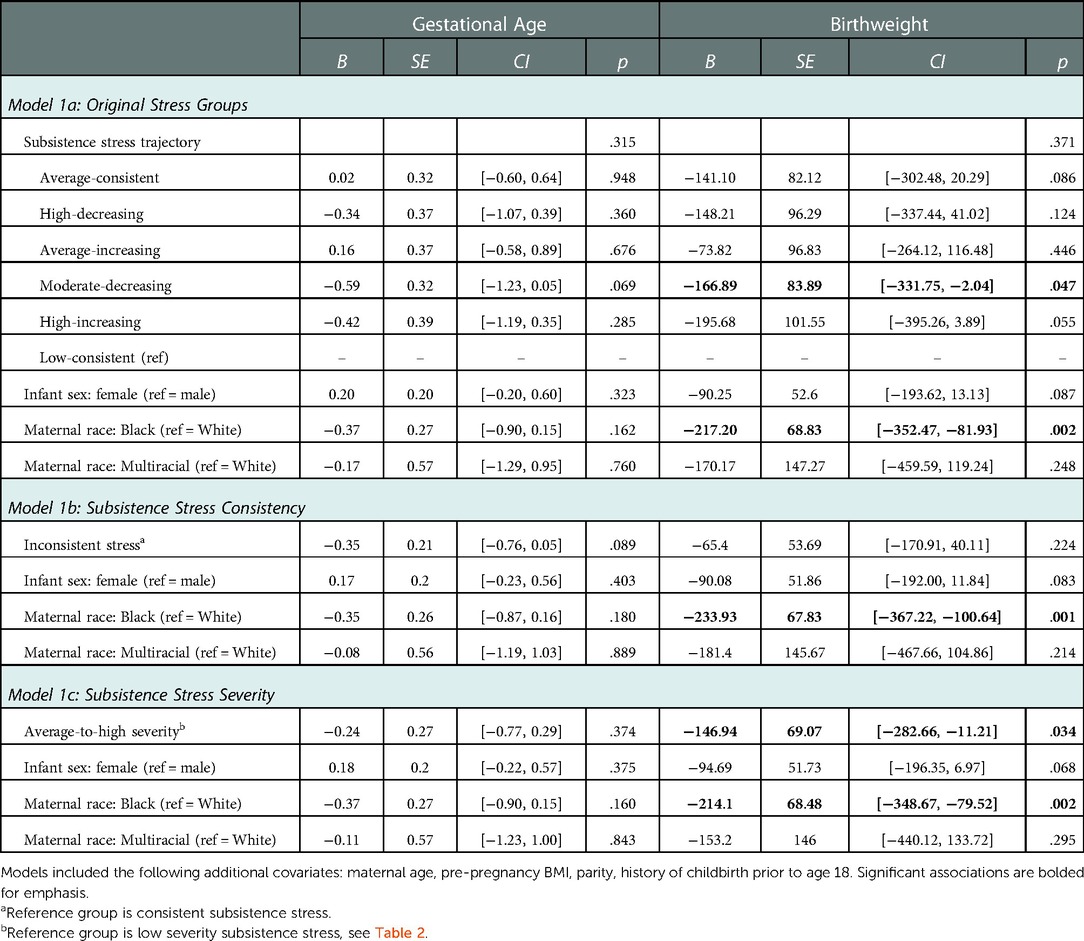
Results from the models testing preconception exposure to subsistence stress as a predictor of offspring birth outcomes are shown in Table 4. When comparing the original six subsistence stress trajectories, none of the trajectory groups differed from the low-consistent reference group with respect to predicting offspring GA. In addition, most of the groups did not differ from the low-consistent reference group with respect to birthweight, although offspring of the moderate-decreasing group showed significantly lower birthweight than the low-consistent reference group (Table 4 Model 1a).
To examine differences by consistency of exposure, we compared the low and average consistent exposure groups (N = 203) with women exposed to inconsistent subsistence stress (N = 287). There was a trend for women with inconsistent subsistence stress exposure in childhood to have offspring with earlier gestational age at birth (B = −0.35, SE = 0.21, p = 0.089). The groups did not differ for birthweight (Table 4 Model 1b). Next, we compared differences by low vs. average-to-high severity of exposure to subsistence stress. Compared to the low severity group, mothers with a history of exposure to average-to-high levels of subsistence stress had offspring with significantly lower birthweights (Table 4 Model 1c).
Female infants had marginally lower birthweights than male infants in all models, but there were no sex differences in GA. Offspring birthweight (but not GA) was significantly lower for Black women compared to White women in all models, beyond the main effect of subsistence stress severity (Table 4). Finally, when adding in the stress x infant sex and stress x race interaction terms, neither infant sex nor maternal race moderated the associations between subsistence stress and offspring birth outcomes (all p's > .10).
Exposure to preconception safety stress and offspring birth outcomes
The results of models testing prediction from preconception exposure to safety stress to birth outcomes are shown in Table 5. None of the original safety stress trajectories differed from the low-consistent trajectory reference group with respect to predicting either GA or birthweight (Table 5 Model 2a). Dichotomizing the safety stress groups into consistent vs. inconsistent exposure also did not significantly explain variability in GA or birthweight (Table 5 Model 2b). Furthermore, inconsistent exposure to safety stress across childhood and adolescence did not interact with infant sex or maternal race to predict either birth outcome (all interaction p's > .10).
Exposure to preconception caregiving stress and offspring birth outcomes
Results from the models testing maternal exposure to preconception caregiving stress in childhood and adolescence predicting offspring birth outcomes are provided in Table 6. None of the original caregiving stress trajectories differed from the low consistent reference group with respect to predicting GA and birthweight (Table 6 Model 3a). Dichotomizing the groups into consistent vs. inconsistent exposure to caregiving stress did not yield significant differences in GA or birthweight (Table 6 Model 3b). Similarly, there was no main effect of average vs. high severity of caregiving stress on birth outcomes (Table 6 Model 3c). However, follow-up analyses showed that the association between severity of preconception exposure to caregiving stress and offspring GA was moderated by infant sex (B = 0.85, SE = .41, p = .039, Cohen's f = 0.097). As shown in Figure 2, the pattern of association between severity of caregiving during childhood and adolescence on GA at delivery differed between male infants (B = −0.62, SE = 0.48, p = .198) and female infants (B = 0.23, SE = 0.51, p = .651), although neither simple slope was statistically significant. Infant sex did not moderate the association between severity of caregiving stress and offspring birthweight (B = 103.35, SE = 105.90, p = .330). Finally, maternal race did not interact with severity or consistency of caregiving stress in predicting offspring birth outcomes.
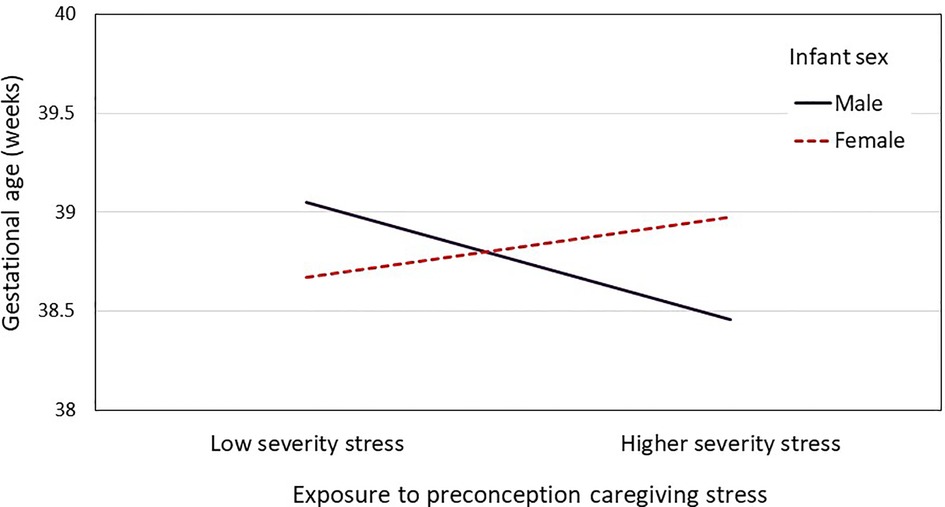
Figure 2. A two-way interaction plot (infant sex and preconception stress severity) for the mean gestational age.
Discussion
We used multiple waves of repeated data from a longitudinal study to examine the effects of type, timing and consistency of stress exposures during childhood and adolescence on later birth outcomes in a racially and socioeconomically diverse sample of urban-living women. We tested hypotheses that severity and inconsistent exposure to preconception stress would be associated with shorter GA at birth and lower birthweight. Results provided modest support for our hypotheses, showing negative effects of average-to-high levels of preconception subsistence stress (i.e., resource and housing stress) on offspring birthweight. This finding aligns with an established literature describing the pervasive effects of exposure to financial strain in childhood on health across the lifespan (61), as well as results from prior longitudinal and registry studies showing heightened risk for subsequent poor birth outcomes (14, 62, 63). The association is consistent with stress-sensitization and life-course models (64) contending that growing up in a household with income-related physical and social risks may potentiate physiological stress and alter stress regulatory systems (e.g., neuroendocrine, immunological, cardiometabolic) that, in turn, influence prenatal health, placental development and ultimately birth outcomes (65–68). Such a conceptualization integrates early programming (i.e., exposures most impactful during sensitive periods in childhood or adolescence), cumulative exposure (e.g., “wear and tear”) and specificity of type and timing models (10). Early life exposure to subsistence stress may also influence an individual's preparedness for pregnancy via long-term deficiencies in nutrition (e.g., dietary fatty acids, vitamin D), infection risk (69, 70), restricted access to quality health care, health screenings, and reproductive health preparations (e.g., folic acid supplementation) (71, 72) and via health behaviors such as smoking, disrupted sleep or depression that have also been linked to adverse birth outcomes (73–76). Important next steps will be research focused on subsistence stressors experienced before and during pregnancy that may influence fetal growth via additive, multiplicative or interactive effects, as well as factors that help explain heterogeneity in birth outcomes despite early adversity.
Stress related to subsistence or financial strain can be relatively stable across the life course (77), and this was evident for approximately 40% of the current sample. Our results, based on annual assessments showed a trend for inconsistent exposure to subsistence stress predicting shorter GA at birth, raising the intriguing possibility that unpredictability is an important feature of subsistence stress that could have implications for prenatal health (10). With a larger sample, future research could probe associations between patterns of inconsistent stress exposure (e.g., early vs. late increasing, childhood increasing and adolescent decreasing trajectories) on birth outcomes, and the extent to which unpredictability in stress exposure continues through pregnancy. In addition, dimensional measures may provide a more sensitive test of the impact of stress related to basic needs across development on perinatal health including birth outcomes.
Our findings also revealed an association between severity of preconception exposure to caregiving stress and GA that was moderated by infant sex. Although the magnitude of the effect was small (Cohen's f ≤ 0.25), the direction was consistent with prenatal stress studies that have highlighted male vulnerability (31, 32, 78, 79). Although not examined in the current study, it is possible that prenatal stressors related to early caregiving experiences mediated the effect of preconception stress exposure on birth outcomes. For example, early separation from a parent or exposure to caregiver depression may increase risk for interpersonal difficulties or could activate stressful feelings about parenting that emerge during pregnancy. Mechanistic studies are needed to extend these results and investigate the ways in which stress experienced across the lifespan could alter biological systems that support the healthy development of both male and female fetuses. Improving our understanding of potential selection effects of preconception stress on pregnancy status, pregnancy health and fetal outcomes are also important areas that warrant further research.
Our results showed further indication that the type of preconception stress has relevance for birth outcomes. We observed no effects of preconception exposure to neighborhood and domestic safety-related stress on birth outcomes. This result differs from studies documenting associations between exposure to violence in the prenatal period and risk for low birthweight and preterm birth (80) as well as retrospectively reported associations between preconception exposure to trauma (i.e., adult abuse and child maltreatment) on later birth outcomes (81). However, the literature on neighborhood safety is somewhat mixed and small but significant associations are most often reported in studies linking geocoded birth and police-recorded crime data [e.g. (82–84),]. In our prospective cohort study, it is possible that caregiver experiences of safety stress differed from those of the developing child. For example, some caregivers who perceive safety threats engage in higher levels of protective parenting behaviors, which appear to attenuate the impact of the exposure on child health and development [see (85) for a review]. Our dataset includes multiple measures of potential resilience factors (e.g., family support) that will be important for identifying the family and community contexts that mitigate the risks from stress exposure on birth outcomes.
Taken together, the observed patterns of association between type of preconception stress and birth outcomes suggest that there may be differential effects on health systems (e.g., maternal nutrition, endocrine, cardiovascular, or immune functioning) and/or epigenetic modification that warrants further investigation. In prior work with a different sample, we have shown that type of prenatal stress exposure is differentially related to neuroendocrine and cardiac response to a controlled stressor (86), and this may also be the case for preconception stress types. Increasing clarity in operational definitions of stress and a greater focus on the preconception period are critical steps towards filling these gaps and yielding precise, developmentally specific targets for effective preventive interventions.
Birthweight was lower in infants of Black compared with White mothers as has been observed in prior research (21, 22); in the current study, this effect was evident while also accounting for the type, severity and consistency of preconception stress exposures during childhood and adolescence. We previously documented racial differences in stress exposure trajectories based on the full PGS sample (47), and it is also widely recognized that cumulative wear and tear (e.g., weathering, accelerated aging) associated with systemic racism and other structural processes contributes to inequities in Black women's reproductive outcomes (87, 88). In the present PGS subsample of young women giving birth, differences in severity and consistency of stress exposures measured between ages 7–17 years did not account for the risk for low birthweight. Several explanations are possible. First, it is unlikely that our measures of stress, examined as categorical variables in separate models, fully captured the pervasive and multifaceted racial inequalities and lifelong exposure to discrimination experienced by Black women. Second, there may have been important unmeasured influences that occurred before age 7 (e.g., fetal programming, early trauma) and/or after age 17 (e.g., work, education, relationship stress, pregnancy complications and pregnancy-related stress, lack of access to quality prenatal care) that contributed to birth outcomes. Third, it is possible that specific developmental features of childhood-adolescent stress exposures (e.g., moderate or high increasing trajectories) that were associated with observed race differences in the full PGS could not be detected in the smaller childbearing subsample due to reduced statistical power. Large-scale studies with culturally sensitive, multi-level measures of stress and systemic racism across the lifespan are clearly warranted to understand the preconception and prenatal mechanisms that underlie these persistent racial disparities.
Limitations
Despite some unique strengths of the current longitudinal study, several limitations should also be noted. First, we focused on stress exposures during the formative developmental periods of childhood and adolescence but did not model exposures through to the time of conception or through pregnancy. For example, it is possible that stress type, or changes in severity or consistency in the immediate preconception period contributed to the causal pathway. Results from European population-based registry studies suggest that exposure to stressful life events (i.e., death or serious illness in a close relative) in the 6- to 18-months prior to pregnancy may carry especially high risk as far as preterm birth and low infant birthweight are concerned (46, 89, 90). Understanding the salience and influence of stressors experienced during both the preconception and prenatal periods is critical for informing optimal timing of preventive interventions. Our findings contribute to this effort in demonstrating that, although effects were generally modest, stress exposures in childhood and adolescence may have implications for later birth outcomes and suggest potential benefits of preventive interventions even during the school years. Second, pre-pregnancy BMI was included as a proxy for overall health, but we recognize that BMI is a non-specific measure of perinatal health, especially for Black women (91). Moreover, chronic health conditions (e.g., hypertension or diabetes) that are known risk factors for low birthweight and GA (92) were not included. Third, several factors may have impacted the generalizability of our findings. For example, we focused on births occurring after age 18 to retain temporality between the independent and dependent variables. In doing so, however, we may have excluded births that occurred following especially high levels of preconception stress exposure (93), which probably also reduced our model estimates. Similarly, our focus on live births may have introduced sample bias, including potential bias related to infant sex given that male fetuses are often more vulnerable to loss. In addition, patterns of missing birth outcome data suggested that our results may be most relevant to Black or multi-racial, multiparous, women living in the Pittsburgh region. Fourth, the sizeable, but nonsignificant, birthweight coefficients suggest that the study may have been underpowered to detect causal effects. Finally, while the trajectory modeling approach used in the current study provided new information about severity and consistency of exposures across childhood and adolescence, applying the trajectories to the smaller PGS subsample of pregnant women necessitated reduction into relatively small binary groups that also prevented us from pinpointing developmental periods of heightened plasticity or vulnerability. For example, animal studies have shown that in the peripubertal period, plasticity in the hypothalamic-pituitary-adrenal (HPA) axis increases (94, 95), the effects of which may persist into adulthood (96). Human studies have also shown unique responses to stress around the time of puberty (97).
Conclusions
The current study supports a life-course perspective for understanding the impact of stress on women's reproductive health with relevance for the next generation. As in other areas of health such as cardiovascular health (98–100), testing the effects of the type, severity and consistency of stress across childhood and adolescence will help to incorporate life history in our understanding and prevention of health problems in pregnancy and the neonatal period. Such an approach is consistent with decade-long calls for the need for preconception physical health care [e.g. (101, 102)], in light of the often modest successes of pregnancy-specific behavioral health interventions (103, 104).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Approval for all study procedures was obtained from the University of Pittsburgh Human Research Protection Office. Written informed consent from the caregiver and verbal assent from the girl were obtained through age 17, whereas all participants aged 18 and older provided written informed consent.
Author contributions
AEH contributed to conceptualization, validation, investigation, writing, project administration and funding acquisition; HF contributed to conceptualization, validation, formal analysis, writing and visualization; IT contributed to conceptualization, validation, investigation and writing; AS contributed to validation and data curation; KK contributed to the conceptualization, validation, investigation, writing, project administration and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Institutes of Health (UH3 OD023244, MH056630, HL157787). During the preparation of this manuscript, I.T. received support from an early career development award from the National Institute of Mental Health (K01 MH123505). The content is solely the responsibility of the authors and does not necessarily represent the official views the funding organization.
Acknowledgments
We are grateful to all the families of the Pittsburgh Girls Study for their participation in this research and to our dedicated research team.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/frph.2022.1007788/full#supplementary-material.
References
1. Barker DJ. Editorial: the developmental origins of adult disease. Eur J Epidemiol. (2003) 18(8):733–6. doi: 10.1023/A:1025388901248
2. Gluckman PD, Hanson MA, Buklijas T. A conceptual framework for the developmental origins of health and disease. J Dev Orig Health Dis. (2010) 1(1):6–18. doi: 10.1017/S2040174409990171
3. Graignic-Philippe R, Dayan J, Chokron S, Jacquet A, Tordjman S. Effects of prenatal stress on fetal and child development: a critical literature review. Neurosci Biobehav Rev. (2014) 43:137–62. doi: 10.1016/j.neubiorev.2014.03.022
4. Talge NM, Neal C, Glover V. Early stress translational research and prevention science network. Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? JChild Psychol Psychiatry. (2007) 48(3-4):245–61. doi: 10.1111/j.1469-7610.2006.01714.x
5. Chapillon P, Patin V, Roy V, Vincent A, Caston J. Effects of pre-and postnatal stimulation on developmental, emotional, and cognitive aspects in rodents: a review. Dev Psychobiol. (2002) 41(4):373–87. doi: 10.1002/dev.10066
6. Coe CL, Lulbach GR, Schneider ML. Prenatal disturbance alters the size of the corpus callosum in young monkeys. Dev Psychobiol. (2002) 41(2):178–85. doi: 10.1002/dev.10063
7. Keenan K, Hipwell AE. Modulation of prenatal stress via docosahexaenoic acid supplementation: implications for child mental health. Nutr Rev. (2015) 73(3):166–74. doi: 10.1093/nutrit/nuu020
8. Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun. (2005) 19(4):296–308. doi: 10.1016/j.bbi.2004.09.006
9. Grandjean P, Barouki R, Bellinger DC, Casteleyn L, Chadwick LH, Cordier S, et al. Life-long implications of developmental exposure to environmental stressors: new perspectives. Endocrinology. (2015) 156(10):3408–15. doi: 10.1210/en.2015-1350
10. Keenan K, Hipwell AE, Class QA, Mbayiwa K. Extending the developmental origins of disease model: impact of preconception stress exposure on offspring neurodevelopment. Dev Psychobiol. (2018) 60(7):753–64. doi: 10.1002/dev.21773
11. Vaivada T, Gaffey MF, Das JK, Bhutta ZA. Evidence-based interventions for improvement of maternal and child nutrition in low-income settings: what's New? Curr Opin Clin Nutr Metab Care. (2017) 20(3):204–10. doi: 10.1097/MCO.0000000000000365
12. Witt WP, Cheng ER, Wisk LE, Litzelman K, Chatterjee D, Mandell DS, et al. Maternal stressful life events prior to conception and the impact on infant birth weight in the United States. Am J Public Health. (2014) 104(S1):S81–S9. doi: 10.2105/AJPH.2013.301544
13. Atherton K, Fuller E, Shepherd P, Strachan D, Power C. Loss and representativeness in a biomedical survey at age 45 years: 1958 British birth cohort. J Epidemiol Community Health. (2008) 62(3):216–23. doi: 10.1136/jech.2006.058966
14. Harville EW, Boynton-Jarrett R, Power C, Hyppönen E. Childhood hardship, maternal smoking, and birth outcomes: a prospective cohort study. Arch Pediatr Adolesc Med. (2010) 164(6):533–9. doi: 10.1001/archpediatrics.2010.61
15. Strutz KL, Hogan VK, Siega-Riz AM, Suchindran CM, Halpern CT, Hussey JM. Preconception stress, birth weight, and birth weight disparities among US women. Am J Public Health. (2014) 104(8):e125–e32. doi: 10.2105/AJPH.2014.301904
16. Harris ML, Hure AJ, Holliday E, Chojenta C, Anderson AE, Loxton D. Association between preconception maternal stress and offspring birth weight: findings from an Australian longitudinal data linkage study. BMJ Open. (2021) 11(3):e041502. doi: 10.1136/bmjopen-2020-041502
17. Mahrer NE, Guardino C, Hobel C, Dunkel Schetter C. Maternal stress before conception is associated with shorter gestation. Ann Behav Med. (2021) 55(3):242–52. doi: 10.1093/abm/kaaa047
18. Osterman MJ, Hamilton BE, Martin JA, Driscoll AK, Valenzuela CP. Births: final data for 2020. Natl Vital Stat Rep. (2022) 70(17):1–49. doi: 10.15620/cdc:112078
19. Anderson JG, Rogers EE, Baer RJ, Oltman SP, Paynter R, Partridge JC, et al. Racial and ethnic disparities in preterm infant mortality and severe morbidity: a population-based study. Neonatology. (2018) 113(1):44–54. doi: 10.1159/000480536
20. Burris HH, Lorch SA, Kirpalani H, Pursley DM, Elovitz MA, Clougherty JE. Racial disparities in preterm birth in USA: a biosensor of physical and social environmental exposures. Arch Dis Child. (2019) 104(10):931–5. doi: 10.1136/archdischild-2018-316486
21. Alhusen JL, Bower KM, Epstein E, Sharps P. Racial discrimination and adverse birth outcomes: an integrative review. J Midwifery Women's Health. (2016) 61(6):707–20. doi: 10.1111/jmwh.12490
22. Braveman PA, Heck K, Egerter S, Marchi KS, Dominguez TP, Cubbin C, et al. The role of socioeconomic factors in black–white disparities in preterm birth. Am J Public Health. (2015) 105(4):694–702. doi: 10.2105/AJPH.2014.302008
23. Mehra R, Boyd LM, Ickovics JR. Racial residential segregation and adverse birth outcomes: a systematic review and meta-analysis. Soc Sci Med. (2017) 191:237–50. doi: 10.1016/j.socscimed.2017.09.018
24. Misra DP, Slaughter-Acey J, Giurgescu C, Sealy-Jefferson S, Nowak A. Why do black women experience higher rates of preterm birth? Curr Epidemiol Rep. (2017) 4(2):83–97. doi: 10.1007/s40471-017-0102-3
25. Goldenberg RL, Cliver SP, Mulvihill FX, Hickey CA, Hoffman HJ, Klerman LV, et al. Medical, psychosocial, and behavioral risk factors do not explain the increased risk for low birth weight among black women. Am J Obstet Gynecol. (1996) 175(5):1317–24. doi: 10.1016/S0002-9378(96)70048-0
26. Giscombe CL, Lobel M. Explaining disproportionately high rates of adverse birth outcomes among African Americans: the impact of stress, racism, and related factors in pregnancy. Psychol Bull. (2005) 131(5):662–83. doi: 10.1037/0033-2909.131.5.662
27. Lu MC, Halfon N. Racial and ethnic disparities in birth outcomes: a life-course perspective. Matern Child Health J. (2003) 7(1):13–30. doi: 10.1023/A:1022537516969
28. Engel PJ, Smith R, Brinsmead MW, Bowe SJ, Clifton VL. Male sex and pre-existing diabetes are independent risk factors for stillbirth. Aust New Zealand J Obstet Gynaecol. (2008) 48(4):375–83. doi: 10.1111/j.1479-828X.2008.00863.x
29. Aibar L, Puertas A, Valverde M, Carrillo MP, Montoya F. Fetal sex and perinatal outcomes. J Perinat Med. (2012) 40:271–6. doi: 10.1515/jpm-2011-0137
30. Chien EK, Jayakrishnan A, Dailey TL, Raker CA, Phipps MG. Racial and ethnic disparity in male preterm singleton birth. J Reprod Med. (2011) 56(1-2):58–64.21366129
31. Lee A, Chiu Y-HM, Rosa MJ, Jara C, Wright RO, Coull BA, et al. Prenatal and postnatal stress and asthma in children: temporal-and sex-specific associations. J Allergy Clin Immunol. (2016) 138(3):740–7. e3. doi: 10.1016/j.jaci.2016.01.014
32. Bronson SL, Bale TL. The placenta as a mediator of stress effects on neurodevelopmental reprogramming. Neuropsychopharmacology. (2016) 41(1):207–18. doi: 10.1038/npp.2015.231
33. Robinson M, Carter KW, Pennell CE, Jacoby P, Moore HC, Zubrick SR, et al. Maternal prenatal stress exposure and sex-specific risk of severe infection in offspring. PloS one. (2021) 16(1):e0245747. doi: 10.1371/journal.pone.0245747
34. James WH. Proximate causes of the variation of the human sex ratio at birth. Early Hum Dev. (2015) 91(12):795–9. doi: 10.1016/j.earlhumdev.2015.10.004
36. Wainstock T, Shoham-Vardi I, Glasser S, Anteby E, Lerner-Geva L. Fetal sex modifies effects of prenatal stress exposure and adverse birth outcomes. Stress. (2015) 18(1):49–56. doi: 10.3109/10253890.2014.974153
37. Torche F, Kleinhaus K. Prenatal stress, gestational age and secondary sex ratio: the sex-specific effects of exposure to a natural disaster in early pregnancy. Hum Reprod. (2012) 27(2):558–67. doi: 10.1093/humrep/der390
38. Badache S, Bouslama S, Brahmia O, Baïri AM, Tahraoui AK, Ladjama A. Prenatal noise and restraint stress interact to alter exploratory behavior and balance in juvenile rats, and mixed stress reverses these effects. Stress. (2017) 20(3):320–8. doi: 10.1080/10253890.2017.1307962
39. Boersma G, Bale T, Casanello P, Lara H, Lucion A, Suchecki D, et al. Long-term impact of early life events on physiology and behaviour. J Neuroendocrinol. (2014) 26(9):587–602. doi: 10.1111/jne.12153
40. Parker KJ, Maestripieri D. Identifying key features of early stressful experiences that produce stress vulnerability and resilience in primates. Neurosci Biobehav Rev. (2011) 35(7):1466–83. doi: 10.1016/j.neubiorev.2010.09.003
41. Pennington KA, van der Walt N, Pollock KE, Talton OO, Schulz LC. Effects of acute exposure to a high-fat, high-sucrose diet on gestational glucose tolerance and subsequent maternal health in mice. Biol Reprod. (2017) 96(2):435–45. doi: 10.1095/biolreprod.116.144543
42. Veru F, Laplante DP, Luheshi G, King S. Prenatal maternal stress exposure and immune function in the offspring. Stress. (2014) 17(2):133–48. doi: 10.3109/10253890.2013.876404
43. Girotti M, Pace T, Gaylord R, Rubin B, Herman J, Spencer R. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience. (2006) 138(4):1067–81. doi: 10.1016/j.neuroscience.2005.12.002
44. Harris RB, Gu H, Mitchell TD, Endale L, Russo M, Ryan DH. Increased glucocorticoid response to a novel stress in rats that have been restrained. Physiol Behav. (2004) 81(4):557–68. doi: 10.1016/j.physbeh.2004.01.017
45. Grant KE, Compas BE, Thurm AE, McMahon SD, Gipson PY. Stressors and child and adolescent psychopathology: measurement issues and prospective effects. J Clin Child Adolesc Psychol. (2004) 33(2):412–25. doi: 10.1207/s15374424jccp3302_23
46. Precht DH, Andersen PK, Olsen J. Severe life events and impaired fetal growth: a nation-wide study with complete follow-up. Acta Obstet Gynecol Scand. (2007) 86(3):266–75. doi: 10.1080/00016340601088406
47. Keenan K, Fu H, Tung I, Berona J, Krafty RT, Hipwell AE, et al. Capturing the dynamic nature of stress exposure in the Pittsburgh girls study. Soc Sci Med Popul Health. (2021) 16:100983. doi: 10.1016/j.ssmph.2021.100983
48. McLaughlin KA, Sheridan MA, Alves S, Mendes WB. Child maltreatment and autonomic nervous system reactivity: identifying dysregulated stress reactivity patterns using the biopsychosocial model of challenge and threat. Psychosom Med. (2014) 76(7):538. doi: 10.1097/PSY.0000000000000098
49. Keenan K, Hipwell A, Chung T, Stepp S, Stouthamer-Loeber M, Loeber R, et al. The Pittsburgh girls study: overview and initial findings. J Clin Child Adolesc Psychol. (2010) 39(4):506–21. doi: 10.1080/15374416.2010.486320
50. Hipwell AE, Loeber R, Stouthamer-Loeber M, Keenan K, White HR, Kroneman L. Characteristics of girls with early onset disruptive and antisocial behaviour. Crim Behav Mental Health. (2002) 12(1):99–118. doi: 10.1002/cbm.489
52. Loeber R, Farrington DP, Stouthamer-Loeber M, Van Kammen WB. Antisocial behavior and mental health problems: Explanatory factors in childhood and adolescence. Manwah, NJ: Lawrence Erlbaum Associates (1998).
53. Gorman-Smith D, Tolan PH, Henry DB. A developmental-ecological model of the relation of family functioning to patterns of delinquency. J Quant Criminol. (2000) 16(2):169–98. doi: 10.1023/A:1007564505850
54. Straus M, Hamby SL, Boney-McCoy S, Sugarman DB. The revised conflict tactics scales (CTS2). J Fam Issues. (1996) 17:283–316. doi: 10.1177/019251396017003001
55. Hipwell A, Keenan K, Kasza K, Loeber R, Stouthamer-Loeber M, Bean T. Reciprocal influences between girls’ conduct problems and depression, and parental punishment and warmth: a six year prospective analysis. J Abnorm Child Psychol. (2008) 36(5):663–77. doi: 10.1007/s10802-007-9206-4
56. Beck AT, Brown G, Steer RA. Beck depression inventory-II manual. San antonio, TX: Psychological Corporation (1996).
57. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. (1983) 24(4):385–96. doi: 10.2307/2136404
58. Hinkle SN, Albert PS, Mendola P, Sjaarda LA, Yeung E, Boghossian NS, et al. The association between parity and birthweight in a longitudinal consecutive pregnancy cohort. Paediatr Perinat Epidemiol. (2014) 28(2):106–15. doi: 10.1111/ppe.12099
59. Chen X-K, Wen SW, Fleming N, Demissie K, Rhoads GG, Walker M. Teenage pregnancy and adverse birth outcomes: a large population based retrospective cohort study. Int J Epidemiol. (2007) 36(2):368–73. doi: 10.1093/ije/dyl284
61. Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull. (2011) 137(6):959. doi: 10.1037/a0024768
62. Morton SM, De Stavola BL, Leon DA. Intergenerational determinants of offspring size at birth: a life course and graphical analysis using the Aberdeen children of the 1950s study (ACONF). Int J Epidemiol. (2014) 43(3):749–59. doi: 10.1093/ije/dyu028
63. Astone NM, Misra D, Lynch C. The effect of maternal socio-economic status throughout the lifespan on infant birthweight. Paediatr Perinat Epidemiol. (2007) 21(4):310–8. doi: 10.1111/j.1365-3016.2007.00821.x
64. Elder GH, George LK, Shanahan MJ. Psychosocial stress over the life course. In: Kaplan HB, editors. Psychosocial stress: perspectives on structre, theory, life-course, and methods. Newyork: Academic Press (1996). p. 247–92.
65. McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. (2003) 43(1):2–15. doi: 10.1016/S0018-506X(02)00024-7
66. Chrousos GP, Torpy DJ, Gold PW. Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: clinical implications. Ann Intern Med. (1998) 129(3):229–40. doi: 10.7326/0003-4819-129-3-199808010-00012
67. Wadhwa PD, Culhane JF, Rauh V, Barve SS, Hogan V, Sandman CA, et al. Stress, infection and preterm birth: a biobehavioural perspective. Paediatr Perinat Epidemiol. (2001) 15:17–29. doi: 10.1046/j.1365-3016.2001.00005.x
68. Dunkel-Schetter C. Psychological science on pregnancy: stress processes, biopsychosocial models, and emerging research issues. Annu Rev Psychol. (2011) 62(1):531–58. doi: 10.1146/annurev.psych.031809.130727
69. Culhane JF, Rauh VA, Goldenberg RL. Stress, bacterial vaginosis, and the role of immune processes. Curr Infect Dis Rep. (2006) 8(6):459–64. doi: 10.1007/s11908-006-0020-x
70. Lindsay KL, Buss C, Wadhwa PD, Entringer S. The interplay between maternal nutrition and stress during pregnancy: issues and considerations. Ann Nutr Metab. (2017) 70(3):191–200. doi: 10.1159/000457136
71. Catov JM, Bodnar LM, Ness RB, Markovic N, Roberts JM. Association of periconceptional multivitamin use and risk of preterm or small-for-gestational-age births. Am J Epidemiol. (2007) 166(3):296–303. doi: 10.1093/aje/kwm071
72. Berghella V, Buchanan E, Pereira L, Baxter JK. Preconception care. Obstet Gynecol Surv. (2010) 65:119–31. doi: 10.1097/OGX.0b013e3181d0c358
73. Li D, Liu L, Odouli R. Presence of depressive symptoms during early pregnancy and the risk of preterm delivery: a prospective cohort study. Hum Reprod. (2009) 24(1):146–53. doi: 10.1093/humrep/den342
74. Witt WP, Wisk LE, Cheng ER, Hampton JM, Hagen EW. Preconception mental health predicts pregnancy complications and adverse birth outcomes: a national population-based study. Matern Child Health J. (2012) 16(7):1525–41. doi: 10.1007/s10995-011-0916-4
75. August EM, Salihu HM, Biroscak BJ, Rahman S, Bruder K, Whiteman VE. Systematic review on sleep disorders and obstetric outcomes: scope of current knowledge. Am J Perinatol. (2013) 30(04):323–34. doi: 10.1055/s-0032-1324703
76. Brand JS, Gaillard R, West J, McEachan RR, Wright J, Voerman E, et al. Associations of maternal quitting, reducing, and continuing smoking during pregnancy with longitudinal fetal growth: findings from Mendelian randomization and parental negative control studies. PLoS Med. (2019) 16(11):e1002972. doi: 10.1371/journal.pmed.1002972
77. Surachman A, Wardecker B, Chow S-M, Almeida D. Life course socioeconomic status, daily stressors, and daily well-being: examining chain of risk models. J Gerontol. (2019) 74(1):126–35. doi: 10.1093/geronb/gby014
78. Bale TL. Placental sex differences and risk of neurodevelopmental disorders. Dialogues Clin Neurosci. (2016). 18(4) 459–464. doi: 10.31887/DCNS.2016.18.4/tbale
79. Weinstock M. Sex-dependent changes induced by prenatal stress in cortical and hippocampal morphology and behaviour in rats: an update. Stress. (2011) 14(6):604–13. doi: 10.3109/10253890.2011.588294
80. Hill A, Pallitto C, McCleary-Sills J, Garcia-Moreno C. A systematic review and meta-analysis of intimate partner violence during pregnancy and selected birth outcomes. Int J Gynecol Obstet. (2016) 133(3):269–76. doi: 10.1016/j.ijgo.2015.10.023
81. Nesari M, Olson JK, Vandermeer B, Slater L, Olson DM. Does a maternal history of abuse before pregnancy affect pregnancy outcomes? A systematic review with meta-analysis. BMC Pregnancy Childbirth. (2018) 18(1):1–11. doi: 10.1186/s12884-018-2030-8
82. Messer LC, Kaufman JS, Dole N, Herring A, Laraia BA. Violent crime exposure classification and adverse birth outcomes: a geographically-defined cohort study. Int J Health Geogr. (2006) 5(1):1–12. doi: 10.1186/1476-072X-5-22
83. Mayne SL, Pool LR, Grobman WA, Kershaw KN. Associations of neighbourhood crime with adverse pregnancy outcomes among women in Chicago: analysis of electronic health records from 2009 to 2013. J Epidemiol Community Health. (2018) 72(3):230–6. doi: 10.1136/jech-2017-209801
84. Goin DE, Gomez AM, Farkas K, Zimmerman S, Matthay EC, Ahern J. Exposure to community homicide during pregnancy and adverse birth outcomes: a within-community matched design. Epidemiology. (2019) 30(5):713. doi: 10.1097/EDE.0000000000001044
85. Ozer EJ, Lavi I, Douglas L, Wolf JP. Protective factors for youth exposed to violence in their communities: a review of family, school, and community moderators. J Clin Child Adolesc Psychol. (2017) 46(3):353–78. doi: 10.1080/15374416.2015.1046178
86. Ilyumzhinova R, Mbayiwa K, Fowle J, Jones C, Hipwell AE, Keenan K. Phentoyping stress exposures related to perinatal health disparities. Dev Psychobiol. (2021), 63(5):1006–12. doi: 10.1002/dev.22060
87. Geronimus AT. Black/white differences in the relationship of maternal age to birthweight: a population-based test of the weathering hypothesis. Soc Sci Med. (1996) 42(4):589–97. doi: 10.1016/0277-9536(95)00159-X
88. Geronimus AT. The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethn Dis. (1992) 2(3):207–21. http://www.jstor.org/stable/454030511467758
89. Khashan AS, McNamee R, Abel KM, Pedersen MG, Webb RT, Kenny LC, et al. Reduced infant birthweight consequent upon maternal exposure to severe life events. Psychosom Med. (2008) 70(6):688–94. doi: 10.1097/PSY.0b013e318177940d
90. Khashan AS, McNamee R, Abel K, Mortensen P, Kenny L, Pedersen M, et al. Rates of preterm birth following antenatal maternal exposure to severe life events: a population-based cohort study. Hum Reprod. (2009) 24(2):429–37. doi: 10.1093/humrep/den418
91. Mayo JA, Stevenson DK, Shaw GM. Population-based associations between maternal pre-pregnancy body mass index and spontaneous and medically indicated preterm birth using restricted cubic splines in California. Ann Epidemiol. (2022) 72:65–73. doi: 10.1016/j.annepidem.2022.05.009
92. Graham J, Zhang L, Schwalberg R. Association of maternal chronic disease and negative birth outcomes in a non-hispanic black-white Mississippi birth cohort. Public Health Nurs. (2007) 24(4):311–7. doi: 10.1111/j.1525-1446.2007.00639.x
93. Woodward L, Fergusson DM, Horwood LJ. Risk factors and life processes associated with teenage pregnancy: results of a prospective study from birth to 20 years. J Marriage Family. (2001) 63(4):1170–84. doi: 10.1111/j.1741-3737.2001.01170.x
94. Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, et al. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. (2006) 147(4):1664–74. doi: 10.1210/en.2005-1432
95. Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiol Behav. (2009) 97(3-4):484–94. doi: 10.1016/j.physbeh.2009.03.025
96. Hollis F, Isgor C, Kabbaj M. The consequences of adolescent chronic unpredictable stress exposure on brain and behavior. Neuroscience. (2013) 249:232–41. doi: 10.1016/j.neuroscience.2012.09.018
97. Gunnar MR, Frenn K, Wewerka SS, Van Ryzin MJ. Moderate versus severe early life stress: associations with stress reactivity and regulation in 10–12-year-old children. Psychoneuroendocrinology. (2009) 34(1):62–75. doi: 10.1016/j.psyneuen.2008.08.013
98. Doom JR, Rivera KM, Blanco E, Burrows R, Correa-Burrows P, East PL, et al. Sensitive periods for psychosocial risk in childhood and adolescence and cardiometabolic outcomes in young adulthood. Dev Psychopathol. (2020) 32(5):1864–75. doi: 10.1017/S0954579420001248
99. Jenkins ND, Rogers EM, Banks NF, Tomko PM, Sciarrillo CM, Emerson SR, et al. Childhood psychosocial stress is linked with impaired vascular endothelial function, lower SIRT1, and oxidative stress in young adulthood. Am J Physiol Heart Circ Physiol. (2021) 321(3):H532–H41. doi: 10.1152/ajpheart.00123.2021
100. Pierce JB, Kershaw KN, Kiefe CI, Jacobs DR Jr, Sidney S, Merkin SS, et al. Association of childhood psychosocial environment with 30-year cardiovascular disease incidence and mortality in middle age. J Am Heart Assoc. (2020) 9(9):e015326. doi: 10.1161/JAHA.119.015326
101. Atrash HK, Johnson K, Adams MM, Cordero JF, Howse J. Preconception care for improving perinatal outcomes: the time to act. Matern Child Health J. (2006) 10(1):3–11. doi: 10.1007/s10995-006-0100-4
102. Cheng TL, Kotelchuck M, Guyer B. Preconception women's Health and pediatrics: an opportunity to address infant mortality and family health. Acad Pediatr. (2012) 12(5):357–9. doi: 10.1016/j.acap.2012.04.006
103. Spring B, Doran N, Pagoto S, Schneider K, Pingitore R, Hedeker D. Randomized controlled trial for behavioral smoking and weight control treatment: effect of concurrent versus sequential intervention. J Consult Clin Psychol. (2004) 72(5):785. doi: 10.1037/0022-006X.72.5.785
Keywords: preconception, stress exposure, birth outcomes, trajectories, Pittsburgh Girls Study
Citation: Hipwell AE, Fu H, Tung I, Stiller A and Keenan K (2023) Preconception stress exposure from childhood to adolescence and birth outcomes: The impact of stress type, severity and consistency. Front. Reprod. Health 4:1007788. doi: 10.3389/frph.2022.1007788
Received: 30 July 2022; Accepted: 14 December 2022;
Published: 11 January 2023.
Edited by:
Linda G. Kahn, New York University, United StatesReviewed by:
Emily Harville, Tulane University, United StatesTeresa Janevic, Icahn School of Medicine at Mount Sinai, United States
© 2023 Hipwell, Fu, Tung, Stiller and Keenan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alison E. Hipwell aGlwd2FlQHVwbWMuZWR1
†ORCID Alison E. Hipwell orcid.org/0000-0001-7179-1151
Specialty Section: This article was submitted to Reproductive Epidemiology, a section of the journal Frontiers in Reproductive Health
 Alison E. Hipwell
Alison E. Hipwell Haoyi Fu
Haoyi Fu Irene Tung4
Irene Tung4 Kate Keenan
Kate Keenan