- 1Department of Neuroscience, Reproductive Science and Odontostomatology, School of Medicine and Surgery, University of Naples Federico II, Naples, Italy
- 2Department of Obstetrics and Gynecology, Center for Fetal Care and High-Risk Pregnancy, University of Chieti, Chieti, Italy
- 3Department of Medical and Surgical Sciences for Children and Adults, University of Modena and Reggio Emilia, Modena, Italy
We report a rare case of ovarian hyperstimulation syndrome (OHSS) in a 28-year-old woman with breast cancer and with a history of polycystic ovary syndrome (PCOS) despite treatment with letrozole and gonadotropin-releasing hormone agonist (GnRH-a) triggering in a GnRH antagonist (GnRH-ant) protocol without the administration of any human chorionic gonadotropin (hCG) for luteal-phase support. The patient, who underwent controlled ovarian syndrome (COS)-oocyte cryopreservation before chemotherapy, required hospitalization. Complete recovery was achieved after treatment with volume expanders, human albumin, and cabergoline. Based on our case and literature review, it is possible to establish that estradiol (E2) modulation with letrozole and GnRH-a triggering does not eliminate the risk of OHSS. Furthermore, it is advisable to postpone GnRH-a depot to minimize the risk of OHSS after the suspension of letrozole, following menstruation or at least 7–8 days after triggering. It would be desirable to identify high-risk patients, also on a genetic basis, in order to avoid delays in oncologic treatments that could strongly impact life expectancy.
Introduction
Ovarian hyperstimulation syndrome (OHSS) is a severe complication associated with controlled ovarian stimulation (COS) during assisted reproductive technology (ART) and occurs in approximately 1–5% of treatment cycles (1). OHSS is characterized by ovarian enlargement, ascites, hemoconcentration, hypercoagulability, and electrolyte imbalances. It appears with abdominal distension and discomfort, nausea, vomiting, dyspnea, and diarrhea. Most severe clinical presentations may lead to acute renal insufficiency, pleural effusion, and venous thromboembolism (1). Although human chorionic gonadotropin (hCG), estradiol (E2), and vascular endothelial growth factor (VEGF) represent the main actors of OHSS, the former seems to be the trigger of the process (2, 3). There are several associated risk factors such as young age, lower body mass index (BMI), polycystic ovary syndrome (PCOS), and elevated anti-müllerian hormone (AMH) values (4).
Ovarian hyperstimulation syndrome is considered an hCG-dependent phenomenon. According to the classical concept, neither early nor late OHSS can occur if exogenous hCG is not administered. Although it is widely accepted that triggering with gonadotropin-releasing hormone agonist (GnRH-a) represents the most efficient prevention in the high-risk patient (5, 6), several cases of early and severe OHSS following this approach are described in the literature study (7–14). The role of estrogens in the pathogenesis of OHSS is still a matter of debate. Despite not being the direct cause of the syndrome, a predicting role of E2 levels during COS has been documented (15, 16). More specifically, E2 values in patients with OHSS were >3,500 pg/ml (1).
Premature ovarian insufficiency (POI) is common in young women with cancer due to the therapies. Chemotherapy is the leading cause of follicular damage, which depends on age, ovarian reserve, chemotherapeutic drugs, and doses. The growing interest in treatments aimed at fertility preservation in patients with cancer has led to new protocols. In order to limit high hormone concentrations, letrozole has been approved to reduce estrogen levels in women affected by hormone-sensitive cancers (17). Furthermore, the use of GnRH-a to trigger oocyte maturation prior to egg retrieval during GnRH antagonist (GnRH-ant) protocol is recommended (1). These stimulation regimens produce results comparable to standard protocols associated with significantly lower E2 levels (18). We report a case of OHSS that occurred in a woman with breast cancer following COS performed in co-treatment with letrozole and triggered with GnRH-a. Furthermore, a review of similar cases reported in the literature studies was performed. All procedures were conducted in conformity with the Declaration of Helsinki, and the patient signed an informed consent form.
Case Report
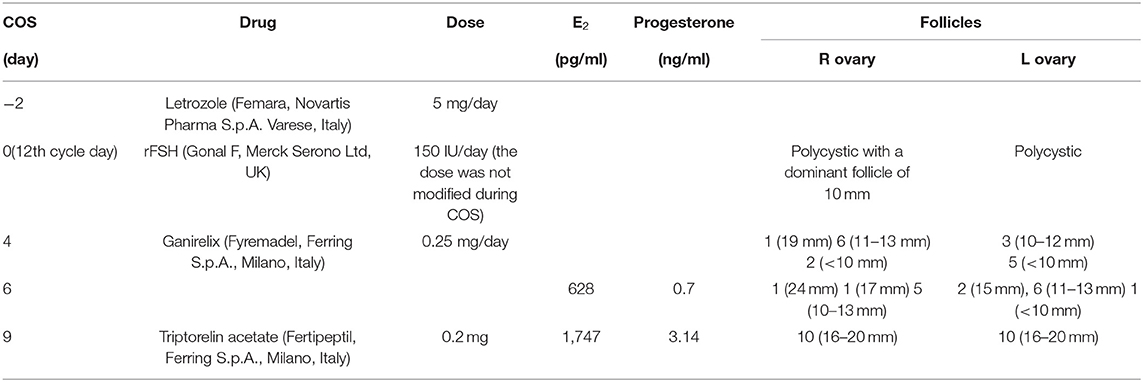
A 28-year-old woman, diagnosed with breast cancer, did a consultation for fertility preservation at our oncofertility clinic (University of Naples Federico II). The patient had a history of oligomenorrhea and PCOS. Clinical features of the patient are reported in Table 1. Transvaginal ultrasonography (USG-TV) revealed polycystic ovaries and central hyperthecosis (19). She had no family history of breast and/or female genital tract tumors. In order to preserve her oocytes, the patient underwent COS and oocyte cryopreservation. The procedure was performed between breast surgery and chemotherapy. A unilateral quadrantectomy was performed, and subsequently, the patient began treatment with letrozole (Femara, Novartis Pharma S.p.A., Varese, Italy; 5 mg daily). The ovarian stimulation protocol is shown in Table 2. On the 11th day after stimulation, about 20 follicles were aspirated. A total of 16 oocytes were retrieved, and 13 mature oocytes were cryopreserved. At the post-pick up (PU) ultrasound check, there was a flap in the Douglas (26 mm x 16 mm) and there was an anterior-free fluid collection (34 × 39 mm). Her hemoglobin (Hb), hematocrit (Ht), and white blood cell count (WBC) values were 12.8 g/dl, 38.1%, and 5,200/μl, respectively. Treatment with letrozole was suspended on the day of GnRH-a triggering and was restarted after collecting eggs until E2 levels were below 50 pg/ml. On the day after PU, triptorelin (Decapeptyl, Ipsen S.p.A., Milan, Italy; 3.75 mg/28 days) was administered for ovarian protection during the chemotherapy, which the patient would have started on the following day. E2 and progesterone levels on the 5th day after the administration of triptorelin depot (17th after starting COS) were 195 pg/ml and 562 ng/ml, respectively.
On the 18th day, the patient came to the hospital emergency room complaining of abdominal bloating, pelvic pain, and mild dyspnea. On physical examination, ascites were found. Hospitalization was required. Her Hb, Ht, and WBC levels were 15.7 g/dl, 47.3%, and 13,240/μl, respectively. USG-TV revealed markedly enlarged ovaries (70 mm × 55 mm, bilaterally) and significant anterior (70 × 50 mm) and posterior (70 mm x 60 mm) free fluid. Furthermore, the collection of a subphrenic (82 mm) and subhepatic (119 mm) fluid was found.
The patient was treated with volume expanders (tetramido 500 ml/day), prophylactic anticoagulation (enoxaparin sodium 4,000 UI/day), and antibiotic therapy (cefazoline 2 g/day). The following day, 40 g of human albumin was administered. Subsequently, once Ht was <37%, 20 mg of furosemide and 0.5 mg cabergoline were added to therapy. After 5 days, probably due to chemotherapy, the patient developed severe leukopenia (530/μl), for which filgrastrim (1/day for 7 days) was started. Fluid therapy and cabergoline were continued for 12 days. She was discharged on the 30th day after starting COS when Hb, Ht, and WBC levels were 8 g/dl, 23.9%, and 17,420/μl, respectively. On ultrasound, the size of the ovaries and the free fluid were reduced, with the disappearance of the collection of subhepatic and subphrenic fluid. Her chemotherapy regimen was scheduled for the same day.
Discussion
We report a case of OHSS arising in a patient who received COS in association with letrozole and GnRH-a triggering. More specifically, severe OHSS occurred in the high-risk patient (young age, low BMI, and PCOS), despite modulated E2 peak (1,747 pg/ml), and all the precautions were put in place to prevent the syndrome. In fact, letrozole is considered a valid option for modulating E2 levels, whereas GnRH-ant protocol associated with GnRH-a triggering represents a safer way of performing COS in both patients with PCOS and those with breast cancer (20–22). The day after ovum PU, the patient restarted letrozole administration and received triptorelin depot 3.75 mg. The OHSS occurred on the 6th day after the depot.
Several cases of different grade of OHSS have been reported following treatment with GnRH-ant associated with GnRH-a triggering in non-oncologic patients (7–14). These cases raise questions about the dogma that the presence of exogenous or endogenous hCG is required for the onset of OHSS. Possible explanations for these observations include variations of genes, such as allelic variants of hCGs and their receptors, estrogen receptors, and VEGF gene and its receptor.
Some differences characterized our case: The syndrome occurred in a woman with breast cancer who received co-treatment with letrozole for modulating estrogen levels. An analogous observation was reported by Kim et al. The authors described a patient (35 years old, BMI 19.3 Kg/m2, AMH 12 ng/ml), with a history of PCOS, who developed severe OHSS following stimulation conducted with recombinant follicle-stimulating hormone (rFSH) (Gonal F, Serono Inc., Rockland, MA; 225 UI daily) and letrozole (Femara, Novartis Pharma S.p.A., Italy; 5 mg) despite low E2 levels (636 pg/ml) (23). The main difference between the two cases was that the triggering was performed with GnRH-a in our case and hCG (Ovidrel, Serono Inc., Rockland, MA; 250 μg) in the other case. The appearance of OHSS in patients receiving letrozole seems to reinforce the idea that modulating E2 levels, despite necessary for preventing cancer progression, have an unpredictable impact on the prevention of OHSS. Papanikolaou et al. had already shown that E2 levels are less reliable in predicting OHSS (24). Sen et al. have shown that androgens not only increase the expression of FSH receptor (FSHR) on the follicles and, consequently, increase the growth and development of the follicle mediated by FSH but also prevent follicular atresia by inducing the expression of an antiapoptotic microRNA (miR-125b). Furthermore, these authors observed a positive effect of androgens on ovulation rates in women with reduced ovarian reserve. This finding could explain the unregulated follicle growth observed in a disease with excess androgen, such as PCOS (25). The dosage of androgen levels in the days preceding the administration of GnRH-a depot could be useful in the decision-making process.
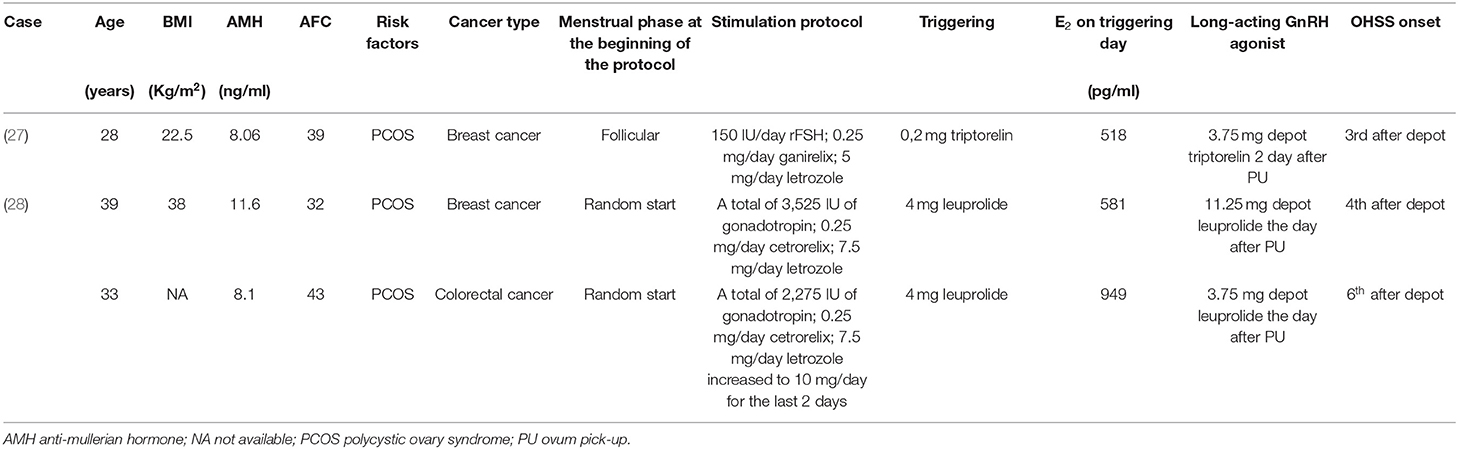
Another important observation is related to the administration of GnRH-a depot after ovum PU and during the “luteal phase” administration of letrozole. The finding that letrozole addition in the luteal phase decreases serum estrogen levels of patients after oocyte retrieval but cannot delete the risk of severe OHSS that had been previously reported (26). Recently, three cases have been reported in which, despite GnRH-a triggering and use of letrozole, the administration of GnRH-a depot shortly after PU led to OHSS (Table 3) (27, 28). As in our case, the onset of OHSS followed the use of GnRH-a depot, administered for chemoprotection, after PU.
The waiting duration before safely administering GnRH-a depot after PU is not well established, but the authors assumed that waiting 7–8 days before the administration may be sufficient to avoid an OHSS.
However, it is interesting to note that, in the case reported by Friedler et al., OHSS occurred after the administration of a GnRH-a, which is used as luteal phase support (LPS) (8).
Regarding the LPS, it is important to introduce the concept of “luteal costing,” which could be adapted to patients with cancer (29, 30). The idea of individualizing the LPS by monitoring serum progesterone levels could be used in these patients to identify the best timing for the administration of GnRH-a depot to prevent the onset of OHSS.
Although an etiology linked to hCG has been ruled out, the molecular mechanisms that triggered the syndrome remain unclarified. A pathogenic role for the VEGF system has been also hypothesized. Stouffs et al. analyzed the DNA samples of three women from the studies of Gurbuz et al. and Santos-Ribeiro et al. that have been reported with a rare variant in VEGFR3 (9, 13, 31). The study of genetic variants of VEGF receptors could open new perspectives on the mechanisms involved in the syndrome. Borgwardt et al. (32) evaluated the presence of genetic variants predisposing to the development of OHSS and found an association with the integrin-linked kinase (ILK) signaling pathway (2020). ILK signaling stimulated VEGF expression in tumor cells and VEGF-mediated endothelial activation (33). ILK was identified as a critical regulator of VEGFR3 signaling (34).
According to the literature review, PCOS is the most common risk factor. PCOS is characterized by ovarian abnormal vascularization and significant differences on levels of multiple pro-angiogenic factors (VEGF, placental growth factor, angiopoietins, transforming growth factor-beta, platelet-derived growth factor, and basic fibroblast growth factor) (35). In a normal ovary, vascular homeostasis is highly regulated by Notch signaling, which inhibits ovary angiogenesis and maintains the integrity of the ovarian vasculature (36). Notch activation induces increased expression of VEGFR1 and soluble VEGFR1 in cultured endothelial cells and provides negative feedback on the activity of the VEGF/VEGFR2 pathway (37). Furthermore, the Notch pathway seems to affect the expression of VEGFR3 through regulating VEGFR3 promoters. Therefore, it is feasible to hypothesize that the Notch pathway is dysregulated in PCOS. Future research studies are needed to establish a clinically useful connection between the receptor mutations and the occurrence and intensity of OHSS.
It is also not possible to exclude that, in patients with cancer, there is a higher risk profile linked to increased vascular permeability and angiogenic stimulus.
Youssef et al. reported that cabergoline, a dopaminergic agent that blocks the binding of VEGF to the VEGF receptor, prevented an increase in vascular permeability and reduced the incidence and severity of the syndrome, without completely preventing it when hCG was administered (38). The best time to start administration of dopaminergic agents is a few hours before triggering (11). Administration of cabergoline as a prophylactic measure against OHSS in high-risk patients appears to be effective without compromising pregnancy rates (39).
In conclusion, at the moment, we can hypothesize that the onset of OHSS in this case was related to the administration of GnRH-a depot shortly after ovum PU. However, on the basis of this case and the other three previously reported cases, it is not yet possible to identify the molecular mechanism underlying the syndrome. In addition, we were unable to identify parameters that suggest the onset of the syndrome. Is it possible that the GnRH-a depot acts on some peripheral receptors? (40).
Anyway, as a precaution, it is possible to suggest that [1) OHSS is still possible even after modulating estrogen production with letrozole and using GnRH-ant and that the use of GnRH-a triggering is to be preferred due to the necessity to prevent comorbidities and delays in adjuvant therapy and [2) it is advisable to postpone GnRH-a depot for minimizing the risk for OHSS. Should it be the case, an ideal timing has to be identified (i.e., after the suspension of letrozole, following menstruation, or at least 7–8 days after triggering). Finally, it would be desirable to identify high-risk patients, also on a genetic basis, in order to avoid delays in oncologic treatments that could strongly impact life expectancy.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
GI and CA contributed to the conception and design of the study. MR, RV, RD, FC, and FB collected the clinical data. GI and MR reviewed the literature. GI and MR wrote the first draft of the manuscript. LC and AC wrote sections of the manuscript. GI, AL, and CA did the final editing of the manuscript. All authors contributed to the review of the manuscript, read and approved the version sent.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer HF declared a past co-authorship with one of the authors AL to the handling Editor.
References
1. Pfeifer S, Butts S, Dumesic D, Fossum G, Gracia C, Barbera AL, et al. Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertil Steril. (2016) 106:1634–47. doi: 10.1016/j.fertnstert.2016.08.048
2. Geva E, Jaffe RB. Role of vascular endothelial growth factor in ovarian physiology and pathology. Fertil Steril. (2000) 74:429–38. doi: 10.1016/S0015-0282(00)00670-1
3. Neulen J, Yan Z, Raczek S, Weindel K, Keck C, Weich HA, et al. Human chorionic gonadotropin-dependent expression of vascular endothelial growth factor/vascular permeability factor in human granulosa cells: importance in ovarian hyperstimulation syndrome. J Clin Endocrinol Metab. (1995) 80:1967–71. doi: 10.1210/jcem.80.6.7775647
4. Luke B, Brown MB, Morbeck DE, Hudson SB, Coddington CC, Stern JE. Factors associated with ovarian hyperstimulation syndrome (OHSS) and its effect on assisted reproductive technology (ART) treatment and outcome. Fertil Steril. (2010) 94:1399–404. doi: 10.1016/j.fertnstert.2009.05.092
5. Lainas TG, Sfontouris IA, Zorzovilis IZ, Petsas GK, Lainas GT, Alexopoulou E, et al. Flexible GnRH antagonist protocol versus GnRH agonist long protocol in patients with polycystic ovary syndrome treated for IVF: a prospective randomised controlled trial (RCT). Hum Reprod. (2010) 25:683–9. doi: 10.1093/humrep/dep436
6. Toftager M, Bogstad J, Bryndorf T, Løssl K, Roskær J, Holland T, et al. Risk of severe ovarian hyperstimulation syndrome in GnRH antagonist versus GnRH agonist protocol: RCT including 1050 first IVF/ICSI cycles. Hum Reprod. (2016) 31:1253–64. doi: 10.1093/humrep/dew051
7. Fatemi HM, Popovic-Todorovic B, Humaidan P, Kol S, Banker M, Devroey P, et al. Severe ovarian hyperstimulation syndrome after gonadotropin-releasing hormone (GnRH) agonist trigger and “freeze-all” approach in GnRH antagonist protocol. Fertil Steril. (2014) 101:1008–11. doi: 10.1016/j.fertnstert.2014.01.019
8. Friedler S, Grin L. Luteal phase support with GnRH agonist does not eliminate the risk for ovarian hyperstimulation syndrome. Gynecol Endocrinol. (2019) 35:368–9. doi: 10.1080/09513590.2018.1548591
9. Gurbuz AS, Gode F, Ozcimen N, Isik AZ. Gonadotrophin-releasing hormone agonist trigger and freeze-all strategy does not prevent severe ovarian hyperstimulation syndrome: a report of three cases. Reprod Biomed Online. (2014) 29:541–4. doi: 10.1016/j.rbmo.2014.07.022
10. Ling LP, Phoon JW, Lau MS, Chan JK, Viardot-Foucault V, Tan TY, et al. GnRH agonist trigger and ovarian hyperstimulation syndrome: relook at 'freeze-all strategy'. Reprod Biomed Online. (2014) 29:392–4. doi: 10.1016/j.rbmo.2014.05.012
11. Mahajan N, Gupta S, Sharma S, Rani K, Naidu P, Arora PR. Early onset ovarian hyperstimulation syndrome despite use of segmentation approach and ovarian hyperstimulation syndrome prophylaxis. J Hum Reprod Sci. (2015) 8:234–8. doi: 10.4103/0974-1208.170415
12. Orvieto R, Vanni VS. Ovarian hyperstimulation syndrome following GnRH agonist trigger—think ectopic. J Assist Reprod Genet. (2017) 34:1161–5. doi: 10.1007/s10815-017-0960-0
13. Santos-Ribeiro S, Polyzos NP, Stouffs K, De Vos M, Seneca S, Tournaye H, et al. Ovarian hyperstimulation syndrome after gonadotropin-releasing hormone agonist triggering and “freeze-all”: in-depth analysis of genetic predisposition. J Assist Reprod Genet. (2015) 32:1063–8. doi: 10.1007/s10815-015-0498-y
14. Singh N, Dogra Y, Saini M, Govindarajan M. Severe early-onset ovarian hyperstimulation syndrome with liver dysfunction in an IVF segmentation cycle. BMJ Case Rep. (2020) 13:e233379. doi: 10.1136/bcr-2019-233379
15. Hendriks DJ, Klinkert ER, Bancsi LFJMM, Looman CWN, Habbema JDF, te Velde ER, et al. Use of stimulated serum estradiol measurements for the prediction of hyper-response to ovarian stimulation in in vitro fertilization (IVF). J Assist Reprod Genet. (2004) 21:65–72. doi: 10.1023/B:JARG.0000027016.65749.ad
16. Lee TH, Liu C-H, Huang CC, Wu YL, Shih YT, Ho HN, et al. Serum anti-Müllerian hormone and estradiol levels as predictors of ovarian hyperstimulation syndrome in assisted reproduction technology cycles. Hum Reprod. (2008) 23:160–7. doi: 10.1093/humrep/dem254
17. Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol Off J Am Soc Clin Oncol. (2005) 23:4347–53. doi: 10.1200/JCO.2005.05.037
18. Kahnberg A, Enskog A, Brännström M, Lundin K, Bergh C. Prediction of ovarian hyperstimulation syndrome in women undergoing in vitro fertilization. Acta Obstet Gynecol Scand. (2009) 88:1373–81. doi: 10.3109/00016340903287482
19. Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. (2004) 19:41–7. doi: 10.1093/humrep/deh098
20. Alviggi C, Marci R, Vallone R, Conforti A, Di Rella F, Strina I, et al. High progesterone levels during the luteal phase related to the use of an aromatase inhibitor in breast cancer patients. Eur Rev Med Pharmacol Sci. (2017) 21:3134–8.
21. Reddy J, Turan V, Bedoschi G, Moy F, Oktay K. Triggering final oocyte maturation with gonadotropin-releasing hormone agonist (GnRHa) versus human chorionic gonadotropin (hCG) in breast cancer patients undergoing fertility preservation: an extended experience. J Assist Reprod Genet. (2014) 31:927–32. doi: 10.1007/s10815-014-0248-6
22. Youssef MAFM, Van der Veen F, Al-Inany HG, Mochtar MH, Griesinger G, Mohesen MN, et al. Gonadotropin-releasing hormone agonist versus HCG for oocyte triggering in antagonist-assisted reproductive technology. Cochrane Database Syst. Rev. (2014) CD008046. doi: 10.1002/14651858.CD008046.pub4
23. Kim J, Steiner AZ, Fritz M, Mersereau JE. Severe ovarian hyperstimulation syndrome after letrozole-gonadotropin stimulation: a case report. J Assist Reprod Genet. (2012) 29:127–9. doi: 10.1007/s10815-011-9676-8
24. Papanikolaou EG, Pozzobon C, Kolibianakis EM, Camus M, Tournaye H, Fatemi HM, et al. Incidence and prediction of ovarian hyperstimulation syndrome in women undergoing gonadotropin-releasing hormone antagonist in vitro fertilization cycles. Fertil Steril. (2006) 85:112–20. doi: 10.1016/j.fertnstert.2005.07.1292
25. Sen A, Prizant H, Light A, Biswas A, Hayes E, Lee HJ, et al. Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microRNA-125b expression. Proc Natl Acad Sci USA. (2014) 111:3008–13. doi: 10.1073/pnas.1318978111
26. Wang YQ, Yang J, Xu WM, Xie QZ, Yan WJ, Yin TL, et al. Luteal letrozole administration decreases serum estrogen level but not the risk of ovarian hyperstimulation syndrome. Beijing Da Xue Xue Bao Yi Xue Ban. (2013) 45:869–72. Chinese.
27. Marin L, Vitagliano A, Capobianco G, Dessole F, Ambrosini G, Andrisani A. Which is the optimal timing for starting chemoprotection with gonadotropin-releasing hormone agonists after oocyte cryopreservation? Reflections on a critical case of ovarian hyperstimulation syndrome. J Gynecol Obstet Hum Reprod. (2020) 17:101815. doi: 10.1016/j.jogoh.2020.101815
28. Christ J, Herndon CN, Yu B. Severe ovarian hyperstimulation syndrome associated with long-acting GnRH agonist in oncofertility patients. J Assist Reprod Genet. (2021) 38:751–6. doi: 10.1007/s10815-020-02051-7
29. Lawrenz B, Ruiz F, Engelmann N, Fatemi HM. Individual luteolysis post GnRH-agonist-trigger in GnRH-antagonist protocols. Gynecol Endocrinol. (2017) 33:261–4. doi: 10.1080/09513590.2016.1266325
30. Lawrenz B, Humaidan P, Kol S, Fatemi HM. GnRHa trigger and luteal coasting: a new approach for the ovarian hyperstimulation syndrome high-risk patient? Reprod Biomed Online. (2018) 36:75–7. doi: 10.1016/j.rbmo.2017.09.014
31. Stouffs K, Daelemans S, Santos-Ribeiro S, Seneca S, Gheldof A, Gürbüz AS, et al. Rare genetic variants potentially involved in ovarian hyperstimulation syndrome. J Assist Reprod Genet. (2019) 36:491–7. doi: 10.1007/s10815-018-1372-5
32. Borgwardt L, Olsen KW, Rossing M, Helweg-Larsen RB, Toftager M, Pinborg A, et al. Rare genetic variants suggest dysregulation of signaling pathways in low- and high-risk patients developing severe ovarian hyperstimulation syndrome. J Assist Reprod Genet. (2020) 37:2883–92. doi: 10.1007/s10815-020-01941-0
33. Hannigan G, Troussard AA, Dedhar S. Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nat Rev Cancer. (2005) 5:51–63. doi: 10.1038/nrc1524
34. Urner S, Planas-Paz L, Hilger LS, Henning C, Branopolski A, Kelly-Goss M, et al. Identification of ILK as a critical regulator of VEGFR3 signalling and lymphatic vascular growth. EMBO J. (2019) 38:e99322. doi: 10.15252/embj.201899322
35. Tal R, Seifer DB, Arici A. The emerging role of angiogenic factor dysregulation in the pathogenesis of polycystic ovarian syndrome. Semin Reprod Med. (2015) 33:195–207. doi: 10.1055/s-0035-1552582
36. Xie Q, Cheng Z, Chen X, Lobe CG, Liu J. The role of Notch signalling in ovarian angiogenesis. J Ovarian Res. (2017) 10:13. doi: 10.1186/s13048-017-0308-5
37. Harrington LS, Sainson RCA, Williams CK, Taylor JM, Shi W, Li J-L, et al. Regulation of multiple angiogenic pathways by Dll4 and Notch in human umbilical vein endothelial cells. Microvasc Res. (2008) 75:144–54. doi: 10.1016/j.mvr.2007.06.006
38. Youssef MAFM, van Wely M, Hassan MA, Al-Inany HG, Mochtar M, Khattab S, et al. Can dopamine agonists reduce the incidence and severity of OHSS in IVF/ICSI treatment cycles? A systematic review and meta-analysis. Hum Reprod Update. (2010) 16:459–66. doi: 10.1093/humupd/dmq006
39. Gaafar S, El-Gezary D, El Maghraby HA. Early onset of cabergoline therapy for prophylaxis from ovarian hyperstimulation syndrome (OHSS): a potentially safer and more effective protocol. Reprod Biol. (2019) 19:145–8. doi: 10.1016/j.repbio.2019.03.005
Keywords: ovulation induction, OHSS, breast cancer, fertility preservation, GnRH agonist, vascular endothelial growth factor
Citation: Iorio GG, Rovetto MY, Conforti A, Carbone L, Vallone R, Cariati F, Bagnulo F, Di Girolamo R, La Marca A and Alviggi C (2021) Severe Ovarian Hyperstimulation Syndrome in a Woman With Breast Cancer Under Letrozole Triggered With GnRH Agonist: A Case Report and Review of the Literature. Front. Reprod. Health 3:704153. doi: 10.3389/frph.2021.704153
Received: 01 May 2021; Accepted: 10 June 2021;
Published: 06 July 2021.
Edited by:
Zeev Blumenfeld, Technion Israel Institute of Technology, IsraelReviewed by:
Human Mousavi Fatemi, ART Fertility Clinics, United Arab EmiratesMitchell Rosen, University of California, San Francisco, United States
Copyright © 2021 Iorio, Rovetto, Conforti, Carbone, Vallone, Cariati, Bagnulo, Di Girolamo, La Marca and Alviggi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giuseppe Gabriele Iorio, Z2l1c2VwcGVnLmlvcmlvOTRAZ21haWwuY29t; orcid.org/0000-0002-1723-3420
 Giuseppe Gabriele Iorio
Giuseppe Gabriele Iorio Marika Ylenia Rovetto1
Marika Ylenia Rovetto1 Alessandro Conforti
Alessandro Conforti Luigi Carbone
Luigi Carbone Roberta Vallone
Roberta Vallone Raffaella Di Girolamo
Raffaella Di Girolamo Antonio La Marca
Antonio La Marca Carlo Alviggi
Carlo Alviggi