- 1Department of Medicine, Center for AIDS Prevention Studies, University of California, San Francisco, San Francisco, CA, United States
- 2Department of Medicine, Center of Excellence for Transgender Health, University of California, San Francisco, San Francisco, CA, United States
- 3Friends Research Institute, Los Angeles, CA, United States
- 4Center for HIV Identification, Prevention and Treatment Services, University of California, Los Angeles, Los Angeles, CA, United States
- 5Department of Obstetrics, Gynecology, and Reproductive Sciences, Bixby Center for Global Reproductive Health, University of California, San Francisco, San Francisco, CA, United States
Introduction: Transgender women (assigned “male” at birth but who do not identify as male) are disproportionately impacted by HIV and experience unique barriers and facilitators to HIV care engagement. In formative work, we identified culturally specific and modifiable barriers to HIV treatment engagement among transgender women living with HIV (TWH), including prioritizing transition-related healthcare over HIV treatment, avoiding HIV care settings due to gender-related and HIV stigma, concerns about potential drug interactions with hormones, and inadequate social support. Grounded in the investigators' Models of Gender Affirmation and Health Care Empowerment, we developed the Healthy Divas intervention to optimize engagement in HIV care among TWH at risk for treatment failure and consequential morbidity, mortality, and onward transmission of HIV.
Methods and Analysis: We conducted a 2-arm randomized controlled trial (RCT) of the intervention's efficacy in Los Angeles and San Francisco to improve engagement in care among TWH (N = 278). The primary outcome was virologic control indicated by undetectable HIV-1 level (undetectability = < 20 copies/mL), at baseline and follow-up assessment for 12 months at 3-month intervals.
Ethics and Dissemination: This study was approved by University of California, San Francisco Institutional Review Board (15-17910) and Western Institutional Review Board (20181370). Participants provided informed consent before enrolment in the study. We are committed to collaboration with National Institutes of Health officials, other researchers, and health and social services communities for rapid dissemination of data and sharing of materials. The results will be published in peer-reviewed academic journals and scientific presentations. We will make our results available to researchers interested in transgender health to avoid unintentional duplication of research, as well as to others in health and social services communities, including HIV clinics, LGBT community-based organizations, and AIDS service organizations.
Clinical Trial Registration: Clinicaltrials.gov, identifier NCT03081559.
Introduction
Background and Rationale
Evidence of the disproportionate rates of HIV infection, AIDS-related mortality, and uncontrolled viral load among transgender women has been rapidly increasing. Transgender women have 49 times higher odds of HIV infection compared to other groups, a disparity that exists across cultures and socioeconomic boundaries (1). Disparate prevalence of HIV is particularly pronounced for African American transgender women (2). In San Francisco, high HIV incidence and prevalence persist among African American transgender women, at 1.4 per 100 person-years and more than 30%, respectively (3). Compared to other populations in the city, viral suppression is lowest among transgender women living with HIV (TWH) (68%) (4). Further, lack of engagement in care and not being on antiretroviral therapy (ART) is high among TWH in San Francisco at 14.3 and 13%, respectively (5). Among African American TWH who had ever enrolled in HIV care in nearby Alameda County, more than one third had not received ART (6). Similarly, in Los Angeles, HIV prevalence among transgender women is high at 16.7% (7). Although transgender individuals make up only 0.1% of the city's population, 1.3% of all people living with HIV and 1.4% of recently diagnosed individuals are transgender (7). Among African American people living with HIV in Los Angeles, 26.5% are transgender (7) and among street- and venue-recruited transgender women, overall HIV prevalence ranges from 21.9–26.1% (8, 9). Transgender women are estimated to have the lowest proportion of viral suppression of any behavioral risk group (69%) (10).
Active engagement in clinical care and high levels of ART adherence are critical to allow people with HIV to live longer, healthier lives. Among persons diagnosed with HIV infection, 63% have successful virologic control as evidenced by suppressed viral load (11). Inconsistent engagement and retention in care (i.e., defined as at least two HIV care visits within 12 months with each visit 3 months apart) has been associated with death among HIV+ adults (12). TWH are less likely to be retained in HIV care and receive ART than other groups, (13, 14) and TWH on ART demonstrate worse ART adherence (15) and viral suppression (16) than non-transgender people on ART and report less confidence in their abilities to integrate treatment regimens into their daily lives (17). Further, like other populations, TWH not on ART or are not adherent to ART are at increased risk for elevated viral load, which increases the risk of emergence of drug-resistant virus and transmission of HIV to others. Among TWH, experiencing higher levels of social adversity is significantly associated with lower odds of viral suppression (18), with African American/Black TWH less likely to be retained in care and to reach viral suppression than Black non-transgender counterparts (19).
Addressing the culturally unique barriers to HIV care and adherence is vital to improving health outcomes among TWH (20). TWH face a complex array of psychosocial challenges that complicate their access and adherence to HIV care, such as limited access to and refraining from healthcare due to stigma and past negative experiences with providers, prioritization of gender-related healthcare, and concerns about adverse interactions between ART medications and hormone therapy. (21, 22) Social and economic marginalization due to transphobia (negative societal attitudes toward transgender persons) often result in poverty and unstable housing, familial alienation, limited formal education, limited social support, mental illness, trauma and victimization, substance use, and introduction to sex work often at an early age (23–29). These factors can result in late or no presentation to HIV medical care and poor health outcomes (30). Culturally responsive intervention efforts, in addition to gender affirming services and caring relationships with interventionists (31), may be most effective for improving care engagement and health outcomes among TWH at the individual level (32). Recent evidence from Los Angeles and San Francisco indicate that among transgender women of color living with HIV, the implementation of peer-delivered interventions positively impacts HIV care visit attendance, receipt of ART prescriptions, and retention in HIV care (33–36).
The non-HIV related medical concerns of transgender women (regardless of HIV status) differ from non-transgender persons. The desire for gender-affirming healthcare, such as hormone therapy, is a critical factor that is sometimes at odds with effective engagement in HIV medical care (37). Anecdotal reports from healthcare providers indicate that hormone treatment can be an incentive for TWH to seek and adhere to ART (38). In our formative work, TWH repeatedly reported misinformed fears that ART can limit the effect of hormones, a serious concern for this population. Finally, in our prior work, TWH have reported that HIV medical providers often do not have expertise in transgender health and that providers specializing in transgender health do not also specialize in HIV care. When transgender women were compared to other respondents, regardless of the current medication regimen, they were significantly less likely to report positive interactions with healthcare providers (17). Taken together, TWH may feel lost between multiple medical settings that are not integrated. Faced with this dilemma, many TWH have reported that they may priorities gender-affirming healthcare over HIV treatment (39).
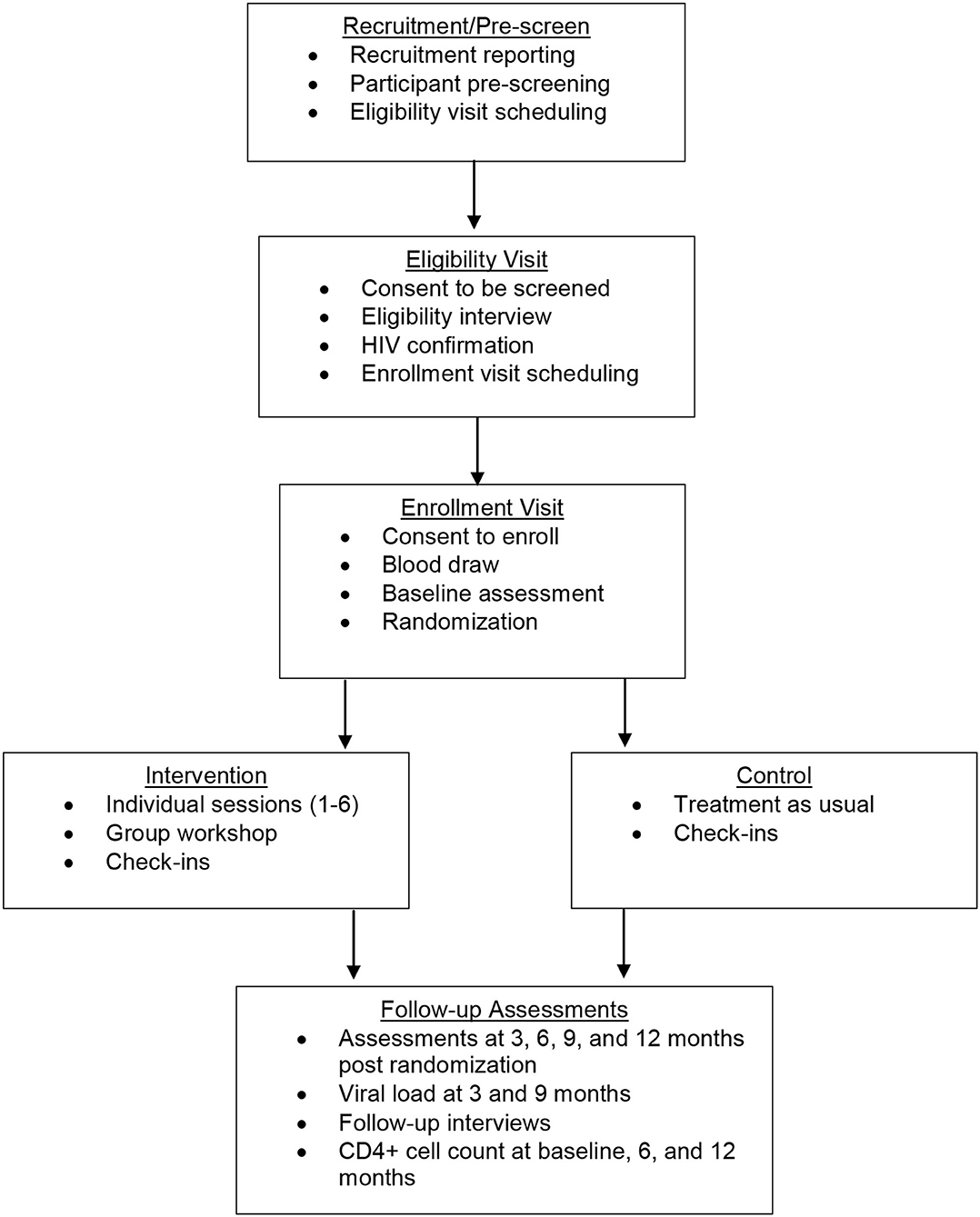
This study is a randomized controlled trial (RCT) to intervene systematically on complex barriers to optimal engagement in HIV care specifically for TWH (see Figure 1 for Schedule of enrolment, intervention, and assessments / CONSORT Diagram). It is grounded in the investigators' Models of Health Care Empowerment (HCE) (40–42) and Gender Affirmation (GA) (43) (see Figure 2 for the Healthy Divas conceptual model integrating the Models of Gender Affirmation and Health Care Empowerment). We developed the Healthy Divas intervention to optimize engagement in HIV care for TWH at elevated risk for virologic failure and consequential morbidity, mortality, and transmission of HIV. We conducted a feasibility pilot test of the intervention with TWH using the same eligibility criteria as this RCT. We did not design the pilot to detect an effect size with which to power this RCT, as such efforts are problematic given the high likelihood of false negatives/positives inherent with small samples (44). Results from this RCT will have the potential to transform gender-affirming HIV healthcare for a population at dramatically elevated risk for disparately negative personal and public health outcomes.
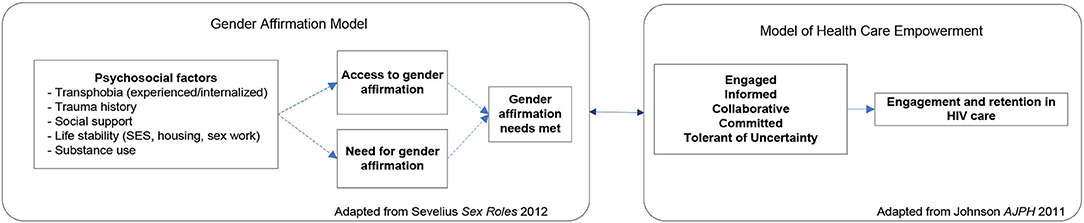
Figure 2. Healthy Divas conceptual model integrating the Models of Gender Affirmation and Health Care Empowerment.
Choice of Comparators
For our control condition, we considered several alternatives and weighed several issues, including Safer and Hugo's guidance for identifying active intervention elements while controlling for potential confounding effects (45). When considering a time and attention matched control condition, we found that identifying an inert intervention that would not directly or indirectly tap into the constructs of gender affirmation and healthcare empowerment through discussions of topics of interest was a challenge. Such a control intervention may have had non-specific effects that threatened our power to detect effects of the Healthy Divas intervention. Therefore, we decided on a treatment as usual (TAU) control condition in which participants randomized to the control condition were offered the intervention content after their final assessment point. As part of this design, we used measures to assess and control for exposure to adherence counselling and support outside of the trial for TWH in both conditions (46). We also accounted for variability in the treatment as usual control condition by assessing standard care quality (SCQ), as described by de Bruin et al., and used the SCQ measure as a covariate in our analyses of intervention effects. (46, 47) Recognizing that an effect on outcomes using this design will provide useful but incomplete evidence for this intervention, we identified potential future steps to evaluate the effectiveness of this intervention in comparison to other approaches. Given the relative strengths and weaknesses of any choice of control condition, our design represents a critical next step in the development of the evidence base for a high-risk population with disparately poor HIV outcomes.
Objectives
Building from our formative quantitative and qualitative interviews with patients and providers, focus groups, and piloting and refinement of the intervention, this RCT had the following aims:
Primary Aim 1: To evaluate odds of virologic control for TWH in response to the Healthy Divas intervention.
Hypothesis 1: TWH randomized to Healthy Divas will achieve or maintain higher odds of undetectable viral load than TWH assigned to a treatment as usual control condition.
Secondary Aim 2: To evaluate the efficacy of the intervention on HIV treatment engagement among TWH.
Secondary Hypothesis 2: TWH assigned to Healthy Divas will have higher engagement in HIV care (appointment attendance, ART uptake, adherence, and persistence) than the control group.
Secondary Aim 3: To explore the effect of the intervention on hypothesized mechanisms of action.
Secondary Hypothesis 3: TWH assigned to Healthy Divas will report more favorable scores on theory-based constructs of healthcare empowerment, gender affirmation, adherence self-efficacy, satisfaction with medical care, social support, HIV treatment information, and treatment beliefs and expectancies.
Trial Design
This study was designed as an interventional 2-arm randomized controlled superiority clinical trial with two parallel groups and a 1:1 allocation to compare Healthy Divas to a TAU control condition. The Healthy Divas intervention is a combination of six individual sessions with a peer counsellor and a peer-led small group workshop with other TWH, and a provider with expertise in HIV primary care and transgender healthcare, all completed within 3 months. TWH (N = 278) were enrolled at two sites (117 in San Francisco; 161 in Los Angeles) who were confirmed to be living with HIV and showed evidence of suboptimal engagement in care, defined as meeting one or more of the following three indicators: (a) not on ART, (b) on ART but reporting non-adherence, (c) reporting no HIV care appointments in the prior 6 months. The primary outcome was virologic control, defined as undetectable viral load. Secondary exploratory outcomes were (a) within-subject improvement in scores on our engagement in HIV clinical care composite index, which includes ART uptake, ART adherence, and self-reported attendance at HIV primary care appointments; and (b) change in hypothesized mechanisms of action (e.g., healthcare empowerment, gender affirmation). The intervention began immediately following randomization and was completed prior to the 3-month follow-up assessment. Follow-up assessments, including viral load assays and surveys, occurred for 12 months post-randomization at 3-month intervals (see Figure 1 for the Schedule of enrolment, intervention, and assessments). Monthly check-in visits promoted contact between visits and allowed regular monitoring of HIV medical care.
Methods
Participants, Interventions, and Outcomes
Study Setting
This study was conducted in San Francisco and Los Angeles, California. An estimated more than 900 transgender women live in the city of San Francisco (46, 47), not including hundreds of transgender women in the East Bay region. Epidemiological data from Los Angeles County estimates that there are over 7,000 transgender women living in Los Angeles County (DHSP, 2012). All study activities occurred in community-based research locations separate from clinical care sites, minimizing confounding with clinic attendance. In San Francisco, our field site was located in the Tenderloin area and in Los Angeles our field site was located on the border between Hollywood and West Hollywood, areas with high HIV prevalence and community viral load burdens in San Francisco and Los Angeles, respectively. Both sites have working relationships with dozens of community-based agencies and clinics in the area and are centrally located near public transportation access.
Eligibility Criteria
Potential participants were considered eligible if they were ≥18 years; assigned male sex at birth but did not currently identify as male; English- or Spanish-speaking; HIV-positive confirmed via antibody testing; and reported suboptimal engagement in HIV care, as indicated by one or more of the following: (a) Not on ART; (b) If on ART, reporting less than perfect adherence on a validated adherence rating scale; or (c) Reporting no HIV primary care appointments in the prior 6 months.
Potential participants were considered ineligible if they exhibited evidence of severe cognitive impairment or active psychosis, as determined by the Project Director in consultation with the Principal Investigator (PI), a licensed clinical psychologist.
Individuals were not excluded if they were actively engaged in substance use. Substance use is prevalent in TWH, and exclusion based on active substance use would have resulted in a sample that does not represent the target population. Informed by our prior work (21), the intervention includes a focus on the role of substance use and alcohol in optimizing engagement in care. However, if an individual was intoxicated at the time of a visit, we rescheduled the session to preserve the integrity of the data collection and intervention delivery.
We considered only enrolling TWH with detectable viremia but opted for inclusion based on indicators of poor engagement instead. This approach allowed the intervention to prevent potential treatment failure in TWH whose patterns of engagement in care put them at risk of poor outcomes. We contended that this was likely to be a more cost-effective long-term approach, as preventing virologic failure may have greater individual and public health benefit than waiting to intervene until treatment failure. It was also more exportable to community settings, thus enhancing external validity; if the intervention demonstrates efficacy, agencies will be able to adopt it with easier screening procedures than if eligibility had been based on clinical parameters such as viral load. From a methodological perspective, this decision allowed more statistical power, as the primary outcome could fluctuate in both directions for the two randomized groups, thereby increasing internal validity. We anticipated that 50% of the sample at baseline would have undetectable viral load.
Interventions
The Healthy Divas intervention was developed to address the unique HIV care needs of TWH by providing relevant information, support, and skills building for the identification and accomplishment of individualized healthcare goals related to both gender transition and HIV care and treatment. Healthy Divas consists of 6 peer-led individual sessions and one peer-led group workshop of 3–7 participants attended by a healthcare provider with expertise in HIV care and transgender health. As a group, participants have the opportunity to ask questions and receive accurate information and guidance to address barriers to engagement in healthcare. The intervention supports TWH in optimizing their health outcomes by increasing healthcare empowerment with a gender-specific and affirming approach. For example, in Healthy Divas, TWH build skills to cope with transphobia and HIV stigma, become active and collaborative in their treatment planning, and proactively address challenges to adherence and in their relationships with providers. Healthy Divas also incorporates transgender-specific concerns about substance use, provides opportunities for a participant to consider how substance use might be affecting her ability to reach her personal health goals, and supports her ability to access culturally competent treatment if she identifies this as a need.
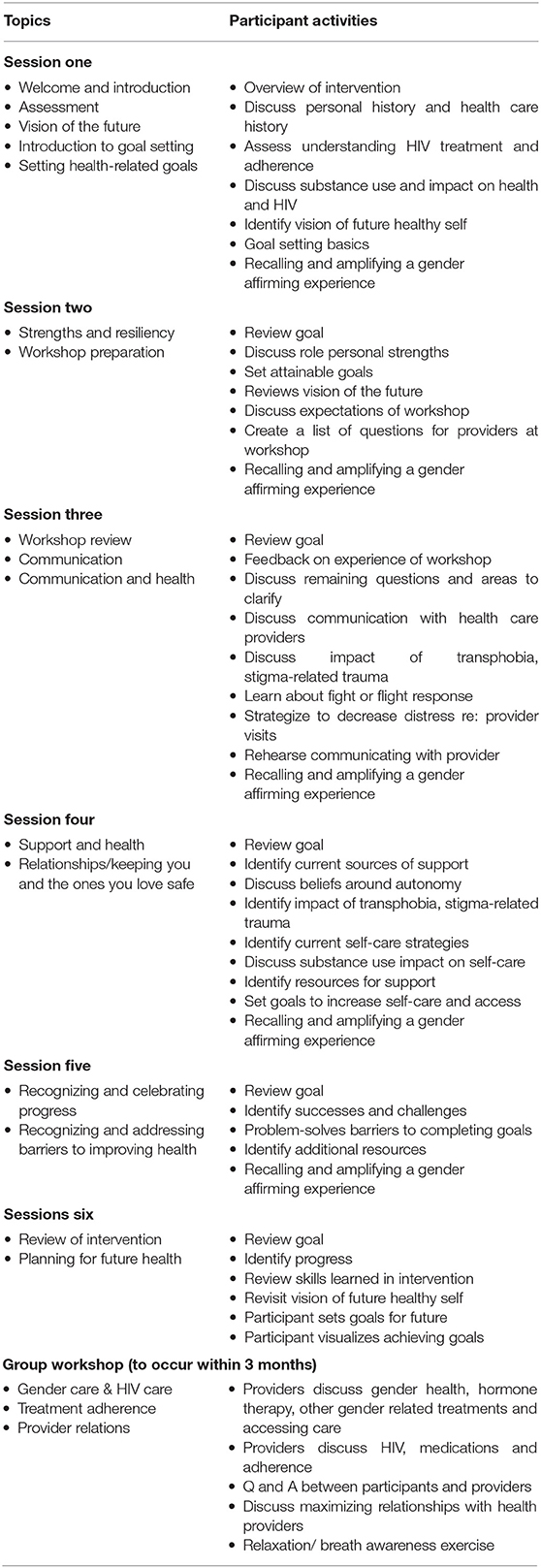
The combination of individual sessions and a single group workshop permitted tailoring and flexibility to address the specific concerns of each participant while also capitalizing on a supportive group process with the presence and contribution of knowledgeable providers. Each intervention session was standardized through the use of a detailed facilitator manual specifying session content, procedures, exercises, and activities (see Table 1 for Healthy Divas intervention activities outline).
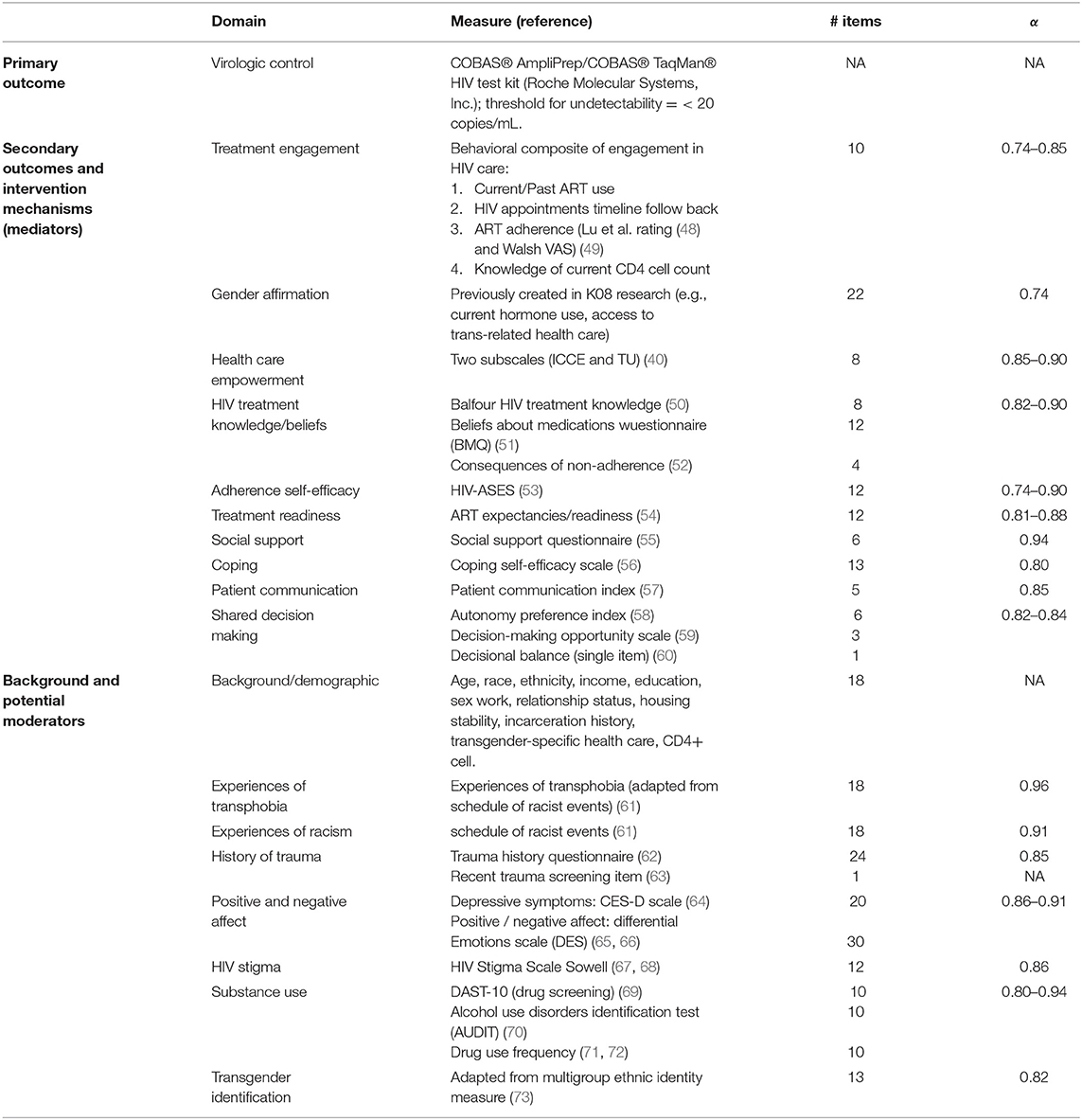
Individual sessions with the peer counsellor used consistent opening content, discussion points, and methods for generating individualized content from the participant's unique circumstances. Individual sessions also employed skills training which, depending on the session's focus, include role-playing, behavior rehearsal or practice exercises, and communication problem-solving. The group workshop provided an opportunity for participants to share experiences, brainstorm solutions with each other and with a provider to barriers to accessing health care and to optimal adherence and ask questions and receive accurate information. Workshop topics were generated by the participants and tailored to address their concerns, and included information about hormones and HIV treatment, transphobia, experiences with healthcare, concerns about HIV treatment, and how to communicate effectively with providers. In summary, the Healthy Divas intervention includes specific issues relevant to TWH and is organized along the constructs of the models of GA and HCE (see Table 2 for Outcomes and measures).
Intervention delivery training included detailed review of the intervention approach, procedures, and research ethics. Population-specific training included how to address trans-specific issues and cultural issues specific to African American and Latina TWH. Specific techniques for adhering to a manualized intervention while allowing for flexibility to address individual participant needs were be taught, role-played and supplemented by instructional readings including one written by Co-I Johnson (74). Ongoing training provided updates on HIV treatment and weekly group supervision; individual supervision addressed cases and procedural issues in intervention implementation. In this RCT, we offered the study in both English and Spanish.
Outcomes
This RCT's primary outcome was virologic control as indicated by an undetectable HIV-1 level on the COBAS® AmpliPrep/COBAS® TaqMan® HIV test kit (Roche Molecular Systems, Inc.), which has a threshold for undetectability = < 20 copies/mL and was conducted at each 3-month assessment visit. The secondary outcome was a behavioral composite of engagement in HIV care scale (75), which integrates current/past ART use, HIV appointments timeline follow back, ART adherence (adherence rating (48) and visual analog scale (49)), and knowledge of current CD4 cell count.
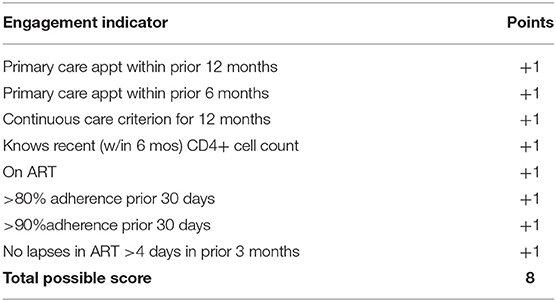
We developed a behavioral composite of engagement in HIV care scale (75) that operationalizes key behavioral dimensions of HIV care engagement: attendance in HIV primary care and ART-related engagement, which includes uptake, adherence, and persistence with ART. Importantly, the composite was designed to measure changes in engagement in response to intervention. For this reason, there is not a separate scale for those on ART vs. not (see Table 3. Behavioral composite of engagement in HIV care scoring). Indicators are additive and, by design, include some overlap. For example, if Participant A tested HIV-positive 2 years ago and has had a primary care visit in the prior 6 months, she would get one point for that indicator. This would also, by definition, give her one point for a primary care visit attended in the prior 12 months (two points total so far). If there were two visits in the prior 12 months that were at least 3 months apart (the current HRSA definition of retention in care), she would get one additional point (three points total so far). If she reports knowing her CD4+ count from a recent test (one point), is taking ART (one point), and her adherence is <80% (no point) but she reports no lapses in ART coverage of > 4 days in the prior 3 months (one point), her total engagement score is six.
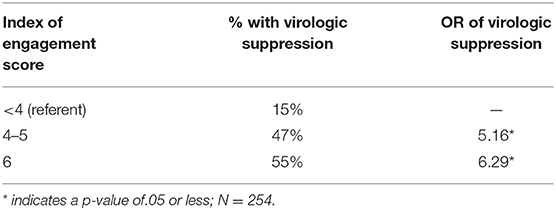
Another example is Participant B who tested HIV-positive 8 months prior, has never seen a provider for HIV care and thus does not know her CD4+ cell count and is not taking ART. She would score a zero on the index. The goal is to help Participant A increase her ART adherence and for Participant B to link to an HIV primary care provider. If successful, both of these participants would increase their scores on the composite index of engagement by at least one point. Thus, the goal of the intervention is to increase the individual's score on the index of engagement along the HIV care continuum. Favorable score changes on the index of engagement in care is a secondary outcome of this RCT and was hypothesized to be the main driver of improvements in viral suppression (our primary outcome). Using an abbreviated form of the index in one of our available datasets of 253 HIV-infected adults, we found that scores of 4–5 were associated with five-fold increased odds of virologic control and a score of six with a six-fold increase in odds of virologic control, compared to those scoring <4 on the index (see Table 4. Behavioral composite scores and virologic control). The eight-point index allows greater precision to distinguish between levels of engagement in care. We hypothesized that if TWH improve their index scores in response to the Healthy Divas intervention, they will increase their likelihood of virologic control.
Participant Timeline
From the date that we enrolled our first participant to the conclusion of the final participant's 12-month follow-up assessment, this RCT took 47 months to implement. Follow-up assessments, including viral load assays and surveys, occurred for 12 months post-randomization at 3-month intervals (For measures, see Table 2. Outcomes and measures). A 29-day window, defined as 14 days before and 15 days after the due date, was available for participants to complete follow-up assessments (Figure 1 provides a schematic diagram of the schedule of enrolment, intervention, control, and assessments). At the time of this manuscript submission, RCT analyses were starting, and we anticipate all data analyses and dissemination of primary trial results to conclude in 12 months.
Sample Size
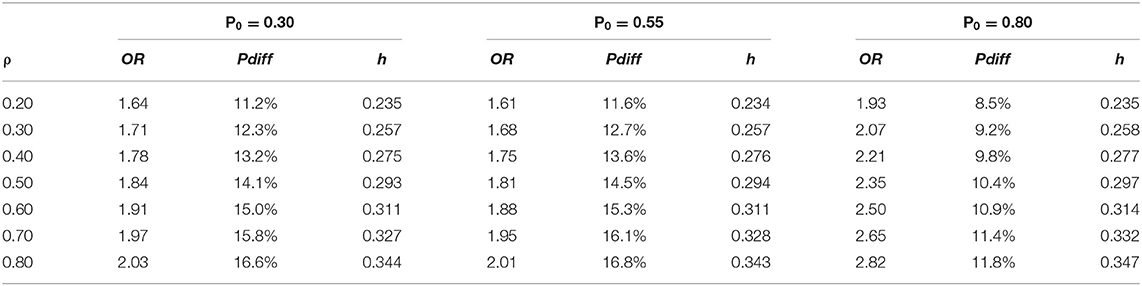
Our sample size was selected to accommodate a range of 40–60% undetectable viral load at baseline, which we monitored in consultation with our Data Safety Monitoring Board (DSMB) to ensure enrolment of a sample with sufficient variability in viral load. Power analyses were generated using the two-group repeated proportions module in NCSS PASS 12 (76) to compute minimum detectable effect sizes at the proposed N and α = 0.05 and power = 0.80 for the primary analysis to evaluate Hypothesis 1 (TWH randomized to Healthy Divas will achieve or maintain higher odds of undetectable viral load than TWH assigned to a treatment as usual control condition). The study enrolled 278 participants evenly assigned to the intervention and control groups. Prior to starting implementation, we assumed 20% attrition, so that data from 228 participants would be available for analysis at all time points (i.e., 114 participants per group). We computed the minimum detectable odds ratio (OR), proportion difference (pdiff), and standardized proportion difference (h) for a time-averaged comparison, assuming four post-baseline measurements and assuming a range of virologic control base rates P0 and the within-subject correlation ρ was varied between.20 and.80 (see Table 5 for minimum detectable effect size estimates). Minimum detectable effect size estimates for our primary analyses fall between cutoffs of 0.20 and 0.50 for small and medium standardized effect sizes (77), respectively, suggesting that primary analyses have sufficient power to detect small to small-medium effects.
Sample size calculations described above to evaluate Hypothesis 1 were also used for Hypothesis 2 (TWH assigned to Healthy Divas will have higher engagement in HIV care) and Hypothesis 3 (TWH assigned to Healthy Divas will report more favorable scores on theory-based constructs of healthcare empowerment, gender affirmation, adherence self-efficacy, satisfaction with medical care, social support, HIV treatment information, and treatment beliefs and expectancies). For secondary mediation analyses to address the mediation component of Aim 2 (TWH assigned to Healthy Divas will have higher engagement in HIV care than the control group, which will lead to higher odds of virologic control), the R function getn.logistic.bcs (78) was used to compute the minimum detectable odds ratio for the indirect effect of the intervention on virologic control via a mediator for the worst case scenario depicted in Table 5, P0 = 0.80 and OR for the direct effect = 2.82. Assuming α = 0.05, power = 0.80, a minimum N = 228 balanced on a binary intervention arm predictor and a high correlation of 0.80 between the mediator and intervention predictor, the minimum detectable OR = 2.49, pdiff = 10.9%, and h = 0.313. Alternative scenarios with lower base rates, smaller direct effects, or lower predictor-mediator correlations would be able to detect smaller mediation effects at the same power.
Recruitment
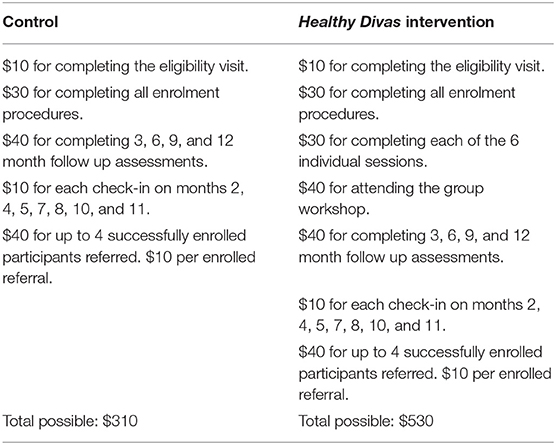
Consistent with our prior work, we took a multi-pronged approach to recruitment: posting flyers in targeted areas; performing outreach to agencies, clinics, community-based organizations, shelters, single room occupancy hotels; and accepting direct peer and provider referrals. Recruitment was conducted by experienced teams of transgender field staff. All interviews and intervention procedures were conducted at trans-friendly field sites where many transgender women live or congregate. In San Francisco, our field site was located in the Tenderloin area, and in Los Angeles our field site was located on the border between Hollywood and West Hollywood, areas with high HIV prevalence and community viral load burdens in San Francisco and Los Angeles. Both sites had working relationships with dozens of community-based agencies and clinics in the area. These connections, along with input from our community advisory boards and our strong working relationships with local agencies that serve transgender women, were used in recruitment efforts to meet project timelines. The enrolment period was conducted in 34 months. Recruitment efforts were monitored by study investigators. Individuals were paid $10 for completing eligibility activities and $30 for completing enrolment procedures. For complete incentive information, see Table 6. Healthy Divas incentive schedule.
Patient and Public Involvement
The development of the Healthy Divas intervention and the implementation of the RCT were informed by a Community Advisory Board comprised of transgender women living with HIV.
Assignment of Interventions
Allocation
Sequence Generation
Participants were randomly assigned via stratified randomization with randomly permuted block sizes of 2, 4, and 6. There were eight strata based on site, age <40 years old vs. not, and binary race (Black vs. not for San Francisco and Latina vs. not for Los Angeles) with a 1:1 allocation to either Healthy Divas or control condition using a computerized secure and fraud-resistant procedure employed in our team's prior studies. Our experience with over 700 participants in three RCTs has indicated that this method is successful for achieving balance across demographic, mediating, and outcome variables.
Concealment Mechanism
Allocation concealment was ensured, as the randomization procedure did not release the randomization code (to participants or study staff) until the participant had been recruited into the RCT after all baseline measurements had been completed.
Implementation
The study statistician generated the randomization scheme using SAS v.9.3. The resulting allocation list was stored in the data collection software package, REDCap, and maintained by the statistician. The research team did not have access to the randomization scheme at any time. The next available randomization status from the appropriate strata for each individual participant was delivered to the research assistant at the time of randomization only by REDCap. The research assistants enrolled participants into their assigned study arms.
Masking
Masking was not used in this trial; therefore emergency unmasking was also not used in this trial. Outcome assessors were not masked in this RCT because they informed participants of their study condition assignment. Peer counsellors did not serve as outcome assessors.
Data Collection, Management, Analysis
Data Collection Methods
Potential participants gave consent to be screened for eligibility. An eligibility survey was administered by study staff; confirmation that participants were living with HIV was achieved via evidence of one of the following: HIV ART medicine prescribed to the participant; other medical documentation; rapid HIV testing provided by study staff; having previously participated in another research study for people living with HIV at either study site; or contact with the participant's care provider through signing a release of medical information form. If found eligible and following consent to enroll in the study, participants had their blood drawn, completed the baseline survey, provided extensive locator information, and were randomized to either intervention or control group.
Primary Outcome
To determine whether TWH randomized to Healthy Divas will achieve or maintain higher rates of undetectable viral load than TWH assigned to a treatment as usual control condition, the primary outcome was assessed. This RCT's primary outcome was virologic control as indicated by an undetectable HIV-1 level (undetectability = < 20 copies/mL). Virologic control was conducted at baseline and at each follow-up assessment for 12 months post-randomization at 3-month intervals. A study phlebotomist performed peripheral venous blood sample collections. This process included: completing laboratory requisitions forms with collection date, time, and other relevant information; preparing two tubes for HIV-RNA; performing venipuncture and blood draw; spinning and freezing each specimen; placing each labelled specimen and requisition form in a shipment bag for pick up. Results were securely accessed through the Care360TM platform in San Francisco and Foundation Laboratory in Los Angeles and entered and saved in a study password-protected Access form.
Secondary Outcomes
Secondary outcomes included a behavioral composite of engagement in HIV care scale (75), which integrates current/past ART use, HIV appointments timeline follow back, ART adherence (adherence rating (48) and visual analog scale (49)), and knowledge of current CD4 cell count, in addition to the intervention's hypothesized mechanisms of action. Surveys were administered at baseline and at each follow-up assessment for 12 months post-randomization at 3-month intervals using the audio computer-assisted self-interviewing (ACASI) system and REDCap. Data were collected and managed using REDCap electronic data capture tools at UCSF (79). REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing: (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for importing data from external sources. Research assistants set participants up to use the ACASI and REDCap systems for baseline and follow-up survey assessments. see Table 2 for a full list of the measures used, including scale names/sources, number of items, and Cronbach's alpha. Data quality was promoted through initial, follow-up, and ongoing training of staff members conducting assessments, in addition to regularly held quality assurance meetings.
Retention
Our retention plan, developed by our team, has successfully retained >90% of research cohorts per year, and we conservatively estimated 80% retention for the 12-month period (80, 81). The retention plan included: presence at a central location close to public transit; maintenance of detailed contact information; skilled staff trackers; a reliable monthly check-in program (see below); graduated incentive payments (see Table 6. Healthy Divas incentive schedule); and efforts to foster positive attachments to the study.
Each participant was scheduled for brief monthly check-in visits in months in which there was otherwise no scheduled visit. At this visit, which was highly flexible and could occur in person or by phone, we updated contact information, clarified the next study visit date and time, documented 30-day self-reported adherence and any changes in medication regimens, and recorded any provider visits that had occurred in the prior month. Participants were paid a small incentive for this 10-min visit ($10). For this study, each monthly check-in visit had the added benefit of documenting HIV care appointment attendance while the events were still recent, which improved precision of our self-reported retention in care measurement. This timeline follow-back procedure was used in our pilot with positive results. Staff members had previously reported information available to them to facilitate this data collection. For example, an interviewer was able to say, “The last time we spoke was on [date] and at that time you said you had seen Dr. [provider's name here] at [clinic or hospital name] on [date previously provided]. Since that visit with Dr. X, have you attended any appointments?” We also inquired about missed appointments (i.e., scheduled but not attended) during each time period.
Data Management
All data were password protected and backed up daily to an encrypted secure server. Electronic copies of the data were stored on a secure server and any paper copies were destroyed at the end of the study. Participants' identity and data were handled with highest levels of care and confidentiality. The exceptions to confidentiality were those defined by law and include a suspicion of child abuse, elder abuse, and threat of imminent action on suicidal or homicidal ideation. The information provided by participants is coded with a number to help protect privacy; the link listing names with numbers is kept in locked files. Only study staff had access to study files. Other entities who may access research data include UCSF Committee on Human Research; Western Institutional Review Board; National Institutes of Health (NIH); University of California; and Friends Research Institute. Reports and publications about the study will never refer to participants by name. All staff members were trained in procedures for maintaining confidentiality of participant information.
Statistical Methods
Analysis Population and Missing Data
For preliminary analyses and missing data, one-way frequency tables for all variables and measures of central tendency and variability for continuous variables will characterize the sample. These analyses will be stratified by randomization group (i.e., intervention vs. control) to check for imbalances. If the intervention and control groups are found to differ significantly at baseline on one or more covariates (e.g., on ART vs. not), we will use methods based on the Rubin causal model (e.g., propensity scores, targeted maximum likelihood estimation, double-robust estimation) to obtain the desired marginal effect estimates under the counterfactual assumption of balanced groups (82–86). In general, we will address incomplete data multiple imputation (MI) (87) because MI makes the relatively mild assumption that incomplete data arise from a conditionally random (MAR) mechanism rather than the completely random process (MCAR) assumed by ad hoc methods such as listwise data deletion (88). Auxiliary variables will be included in analyses involving incomplete data to help meet the MAR assumption (89, 90) and sensitivity analyses will be conducted with pattern-mixture models to assess the robustness of the MAR assumption (91). SAS (92) will be used to perform the proposed analyses.
Outcomes
We estimated based on our prior work with the population that 20% of the sample will not be on ART at baseline and thus would have detectable viral load. The remaining estimated 80% of the enrolled sample would report limited engagement in care as evidenced by low ART adherence or poor appointment attendance. Therefore, we conservatively estimated that at least 40% (and as many as 60%) will have detectable viral load at baseline. Those who meet eligibility criteria were at increased risk of virologic failure resulting from poor engagement in care. If our intervention is successful, a higher proportion of intervention participants will display virologic control relative to control group participants.
Therefore, we hypothesized that, following the intervention, the odds of undetectable viral load will be higher for intervention participants than for control participants (Hypothesis 1). Our primary interest was to estimate the marginal or population-average effect of intervention participation on each outcome rather than the effect for a hypothetical average subject. (93) Moreover, within-subject correlations among outcomes are considered nuisance parameters rather than quantities of interest to be modeled explicitly. Accordingly, generalized estimating equations (GEE) will be used to perform the proposed primary analysis, which is a planned time-averaged comparison of post-baseline measurements across the intervention and control groups to test primary Hypothesis 1. Alpha will be set at 0.05 for this planned comparison. Any additional post-hoc comparisons (e.g., paired comparisons of the control and intervention groups at each time point) will maintain a nominal alpha level of 0.05 through the use of simulation-based stepdown multiple comparison methods (94). Reflecting the binary nature of the virologic control outcome, a binomial distribution and logit link will be used for this analysis. Though GEE estimates are consistent even if the correlation structure is misspecified, GEE's statistical efficiency improves as the working correlation structure more closely approximates the actual correlation structure (95). Thus, working correlation structures suitable for the study's design will be considered (e.g., unstructured, AR (1), exchangeable, M-dependent) (96). The QIC statistic will be used to select the final working correlation structure (97). Robust Huber-White “sandwich” standard errors will be used to obtain correct inferences even if the chosen correlation structure remains slightly misspecified. GEE case deletion diagnostics (e.g., DFBetas, Cook's D) will be used to investigate whether influential cases are present; if such cases are found, results will be reported with and without influential cases included (98). As noted above, standard care quality (SCQ) will be included as a covariate in this analysis. In addition, randomization strata indicators will be included as covariates to obtain unbiased results (99). Additional covariates may be included if they improve QIC.
For the secondary outcome of HIV care engagement, we hypothesized that women assigned to Healthy Divas will have higher engagement in HIV care at follow-up than those assigned to the control condition as measured by appointment attendance and ART uptake, adherence and persistence (Hypothesis 2). We will use the same GEE methods described above to explore whether TWH in the Healthy Divas intervention group have higher HIV treatment engagement relative to those in the control group.
Additional Analyses
To explore the effect of the intervention on hypothesized mechanisms of action, secondary analyses will use GEE to evaluate whether TWH assigned to the intervention report higher post-intervention mean scores on theory-based constructs such as health care empowerment, gender affirmation, adherence self-efficacy, social support, HIV treatment information, and treatment beliefs and expectancies (Hypothesis 3). These analyses will also investigate whether these constructs mediate the relationship between intervention group assignment and virologic control. Statistical mediation will be assessed using the causal inference-based approach of Valeri and VanderWeele, which yields optimal estimates of indirect effects in the presence of binary outcomes and moderator-mediator interactions (100). In these analyses mediators will always temporally precede outcomes to uphold temporal ordering assumptions. For instance, mediators at 3 months will be considered for analyses involving virologic control at 9 months and mediators at 9 months will be considered for analyses involving virologic control measured at 12 months.
Monitoring
Data Monitoring
Recruitment goals, missing data, and follow-up failures were monitored monthly throughout the trial.
Formal Committee
The study's data and safety monitoring board (DSMB), the “University of California, Los Angeles (UCLA) DSMB for Addiction Medicine,” is independent from the sponsor; members do not have any competing interests. Board members were chosen for their relevant expertise on study content, target populations, and methodologies. The roles of our DSMB include: reviewing analyses of outcome data and data safety to determine whether the trial should continue as originally designed, should be changed, or should be terminated based on these data; reviewing trial performance information, such as accrual information; determining whether and to whom results should be released prior to the reporting of the study results; reviewing reports of related studies to determine whether the monitored study needs to be changed or terminated; reviewing major proposed modifications to the study prior to their implementation; and providing study leadership with written summary information following board meetings.
Interim Analysis
No interim analyses took place during the trial.
Harms
All safety-related risks of harm were monitored routinely at the time of the assessment or intervention session. The security of confidential information was monitored regularly. Study staff were trained in asking questions about sensitive topics in a caring and non-threatening manner and stopping questioning at the first sign of discomfort or on request. Privacy, confidentiality, and disclosure comfort were emphasized in every session. All participants were reminded that they were not required to disclose their HIV status (or any other personal information) to anyone at any time. Group facilitators requested that participants agree to respect the privacy and confidentiality of other members of the group and to not disclose anyone's personal information to anyone outside of the group. Participants were informed that assessment responses are kept confidential and are not used against them in any manner, including for reasons of legal action or medical treatment. Study staff were trained to identify a participant who reports distress. For participants who report distress or suicidality, a protocol guided staff action, including steps to assess the level of distress, to obtain emergency contact information for clinical supervisors, and to obtain up-to-date phone numbers for crisis centers, hotlines, and referral agencies.
Study staff were trained to report breach of confidentiality risks incurred by participants to the Project Director, who in turn was trained to inform the PI. Any participant in need of treatment due to distress was referred for appropriate services after staff followed the participant distress protocol and informed the Project Director and PI. Finally, the PI was responsible for informing the DSMB chair, IRB through the IRB adverse event reporting procedure, and the sponsor Project Officer through immediate email of any life-threatening incidents and through annual reports of other incidents. The PI was prepared to take appropriate action to stop the study, release a participant from the study, or modify procedures to reduce and/or eliminate the abovementioned risks if they occurred at an unacceptable level.
Auditing
No trial audits were planned or conducted, but participating institutions had the authority to perform random audits of research protocols.
Discussion
The Healthy Divas intervention and corresponding RCT protocols were developed with extensive formative work, including input from TWH, providers, and other community stakeholders. The forthcoming results will represent a critical step in the development of an intervention that specifically addresses the unique and wide-ranging challenges experienced by TWH, a group whose disproportionate rates of HIV infection and poor outcomes warrant focused efforts. This work fills a significant public health gap through the evaluation of a theory-driven, piloted, culturally tailored intervention to improve engagement in HIV care among TWH. The project was established within two novel and complementary theoretical models developed by the investigative team; the Model of Gender Affirmation and the Health Care Empowerment Model offered a rich foundation on which to build the Healthy Divas intervention. We also implemented a behavioral composite of engagement in HIV care scale, which is supported by preliminary data and offers clear direction for promoting greater engagement in HIV. This RCT tested an innovative engagement in HIV care intervention designed specifically for TWH. It, therefore, potentially represents an important contribution in providing an evidence-based approach to improving HIV clinical outcomes among this population at heightened risk for treatment failure and onward transmission of HIV. In addition to the individual-level intervention described here, structural interventions are urgently necessary to improve HIV care outcomes among TWH (101).
Although the results of this RCT are not yet available, the Healthy Divas intervention is already being replicated in three US cities: Birmingham, Alabama; Newark, New Jersey; and Oakland, California. Funded by the Health Resources and Services Administration (HRSA) / HIV/AIDS Bureau (HAB) Ryan White HIV/AIDS Program (U69HA31067; U90HA31099), Healthy Divas is being implemented for 4 years (August 2017–July 2021). We attribute this early replication to the urgent need for effective gender affirming and transgender-specific peer led interventions focused on HIV treatment engagement and outcomes among TWH. We anticipate that the results from this RCT will have the potential to transform gender-affirming HIV health care for a population at dramatically elevated risk for disparately negative personal and public health outcomes.
Strengths and Limitations of This Study
• Our multidisciplinary team integrated support for improvement of engagement in HIV care with gender affirmation to develop the Healthy Divas intervention and achieve optimal outcomes, rather than requiring transgender women living with HIV to compartmentalize their needs for HIV and transition-related support and care.
• This RCT was implemented in two urban settings with large numbers of transgender women living with HIV, making replication and scale-up relatively straightforward in other urban areas of the US where HIV care and gender affirming services are available for transgender women.
• A limitation of the intervention approach is that many challenges that contribute to HIV-related disparities among transgender women include social, economic, and structural factors, which are beyond the scope of individual-level interventions; however, individual-level interventions are urgently needed to help transgender women living with HIV develop skills and coping resources for navigating existing systems.
Ethics and Dissemination
Research Ethics Approval
This study was reviewed and approved by the University of California, San Francisco Institutional Review Board (15-17910) and the Western Institutional Review Board (20181370).
Protocol Amendments
All protocol modifications were reviewed and approved by NIH prior to implementation of amendments. All modifications approved by NIH were submitted to the University of California, San Francisco and the Western Institutional Review Boards. The Principal Investigator made the appropriate changes (e.g., to extend the study period) to the protocol via clinicaltrials.gov. Major changes that would have warranted re-consenting participants did not take place.
Consent or Assent
Two consent processes took place; signed informed consent was conducted for the eligibility visit and the enrolment visit. After an individual was screened and expressed interest in participating in the study, she provided signed informed consent for the eligibility visit. Signed informed consent for enrolment took place at the baseline enrolment visit. Study staff members who conducted assessments (i.e., “assessors”) completed the consent procedures.
Ancillary Studies
Participant data and biospecimens were collected solely for the purposes of this study; no ancillary studies were planned or conducted.
Confidentiality
The following confidentiality protection steps were implemented: study staff participated in training, ongoing monitoring, and supervision to ensure understanding of the ethical issues involved in this research; only trained staff knew the name, identification number, and contact information of participants; consent forms were kept in locked files; personal identifiers linked to data were removed and replaced by code numbers in all records. Electronic copies of data were stored on a secure password-protected server. Paper copies of data will be destroyed at the end of the study.
Declaration of Interests
There are no competing interests among the study investigators.
Access to Data
All investigators have been given access to the cleaned data sets. Project data sets are housed in REDCap; all data sets are password protected. Study investigators have direct access to data originating from both study sites. To ensure confidentiality, any identifying participant information has been removed from data dispersed to project team members.
Ancillary and Post-trial Care
During study enrolment, participants received referrals to emergency health and psychological services if needed. Participants received a brochure with information about culturally appropriate and transgender-sensitive health, psychological, and social services at enrolment. No provisions for post-trial care were planned or provided.
Dissemination Policy
Trial Results
Following study completion and publication of primary reports, research data will be shared in accordance with NIH guidelines (http://grants.nih.gov/grants/policy/data_sharing/). As Principal Investigator, Dr. Sevelius shared information about this trial via timely registration and updates in ClinicalTrials.gov and will provide results in accordance with NIH policy. The results will be published and provided in peer-reviewed academic journals and scientific presentations at national conferences. We will make our results available to the community of researchers and general public interested in transgender health to avoid unintentional duplication of research, as well as to others in the health and social services community, including HIV clinics, LGBT community-based organizations, and AIDS service organizations.
Authorship
The Investigators of the study will follow International Committee of Medical Journal Editors guidelines for determining authorship eligibility and order. Final authorship decisions will be made by the Principal Investigator. No professional writers will be used.
Reproducible Research
We will share protocols and study forms in response to specific requests. Requests for study data will be evaluated on an individual basis, and de-identified study data will be made available as appropriate only after publication of all study outcome analyses.
Ethics Statement
The studies involving human participants were reviewed and approved by University of California, San Francisco Institutional Review Board (15-17910), Western Institutional Review Board (20181370). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JS and MJ conceived of the trial and designed the study. JS is PI and grant holder. TN was study statistician. CR was site PI in Los Angeles. DC contributed to the design of the intervention. SD was data manager. RK compiled the first draft of the study protocol manuscript. All authors contributed to the manuscript and consented to final publication.
Funding
This work was supported by National Institute of Mental Health (NIMH) R01MH106373 and University of California, San Francisco. The funding source had no role in the design of this study, its execution, analyses, interpretation of the data, or decision to submit results.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The study team would like to especially thank our participants and out Community Advisory Board for dedicating their time and energy to this study. San Francisco Study Team Members: Breonna McCree, Akira Jackson, Carla Clynes, Azze Ngo. La Study Team Members: Raymond P. Mata, Larissa Harrison, Prudence Mendiola, Angel Santana, Workshop Facilitators: Royce Lin, MD, Layla Welborn, FNP, and William Hernandez, FNP.
References
1. Baral SD, Poteat T, Stromdahl S, Wirtz AL, Guadamuz TE, Beyrer C. Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect Dis. (2013) 13:214–22. doi: 10.1016/S1473-3099(12)70315-8
2. California Department of Health Services. California HIV Counseling and Testing Annual Report: January - December 2003. Sacramento, CA: Office of AIDS (2006).
3. Wilson E, Turner C, Lin J, Veras MA, Yan H, McFarland W. Persistently High Hiv Incidence Among Trans Women, San Francisco. Mexico City, MX: International AIDS Society (2019).
4. San Francisco Department of Public Health. HIV/AIDS Epidemiology Annual Report. San Francisco, CA: San Francisco Department of Public Health (2008).
5. Baguso GN, Turner CM, Santos GM, Raymond HF, Dawson-Rose C, Lin J, et al. Successes and final challenges along the HIV care continuum with transwomen in San Francisco. J Int AIDS Soc. (2019) 22:e25270. doi: 10.1002/jia2.25270
6. Nemoto T, Iwamoto M, Suico S, Stanislaus V, Piroth K. Sociocultural contexts of access to hiv primary care and participant experience with an intervention project: african american transgender women living with hiv in alameda county, california. AIDS Behav. (2020) 25(Suppl 1):84–95. doi: 10.1007/s10461-019-02752-w
7. Husted CE. Los Angeles County Comprehensive HIV Plan (2017-2021). Los Angeles, California: Los Angeles County Commission on HIV and Los Angeles County Department of Public Health Division of HIV and STD Programs (2016).
8. Bowers JR, Branson CM, Fletcher J, Reback CJ. Differences in substance use and sexual partnering between men who have sex with men, men who have sex with men and women, and transgender women. Cult Health Sex. (2011) 13:629–42. doi: 10.1080/13691058.2011.564301
9. Perez MJ. State of the HIV/AIDS Epidemic: Future Directions for Los Angeles County. Los Angeles, CA: Los Angeles County, Department of Public Health, Division of HIV and STD Programs (2011).
10. Division of HIV and STD Programs The Los Angeles County Commission on HIV Committee tLACHPP. Los Angeles County Five-Year Comprehensive HIV Plan (2013-2017). Los Angeles County Department of Public Health (2013).
11. Harris NS, Johnson AS, Huang YA, Kern D, Fulton P, Smith DK, et al. Vital signs: status of human immunodeficiency virus testing, viral suppression, and HIV preexposure prophylaxis — United States, 2013-2018. MMWR Morb Mortal Wkly Rep. (2019) 68:1117–23. doi: 10.15585/mmwr.mm6848e1
12. Giordano TP, Gifford AL, White AC Jr, Suarez-Almazor ME, Rabeneck L, Hartman C, et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. (2007) 44:1493–9. doi: 10.1086/516778
13. Melendez R, Exner T, Ehrhardt A, Dodge B, Remien R, Rotheram-Borus M, et al. Health and health care among male-to-female transgender persons who are HIV positive. Am J Public Health. (2005) 95:5–7. doi: 10.2105/AJPH.2004.042010
14. Poteat T, Hanna DB, Rebeiro PF, Klein M, Silverberg MJ, Eron JJ, et al. Characterizing the human immunodeficiency virus care continuum among transgender women and cisgender women and men in clinical care: a retrospective time-series analysis. Clin Infect Dis. (2020) 70:1131–8. doi: 10.1093/cid/ciz322
15. Dowshen N, Matone M, Luan X, Lee S, Belzer M, Fernandez MI, et al. Behavioral and health outcomes for HIV+ young transgender women (YTW) linked to and engaged in medical care. LGBT Health. (2016) 3:162–7. doi: 10.1089/lgbt.2014.0062
16. Kalichman SC, Hernandez D, Finneran S, Price D, Driver R. Transgender women and HIV-related health disparities: falling off the HIV treatment cascade. Sex Health. (2017) 14:469–76. doi: 10.1071/SH17015
17. Sevelius J, Carrico A, Johnson M. Antiretroviral therapy adherence among transgender women. J Assoc Nurses AIDS Care. (2010) 21:256–64. doi: 10.1016/j.jana.2010.01.005
18. Hotton AL, Perloff J, Paul J, Parker C, Ducheny K, Holloway T, et al. Patterns of exposure to socio-structural stressors and HIV care engagement among transgender women of color. AIDS Behav. (2020). doi: 10.1007/s10461-020-02874-6
19. Klein PW, Psihopaidas D, Xavier J, Cohen SM. HIV-related outcome disparities between transgender women living with HIV and cisgender people living with HIV served by the health resources and services administration's ryan white HIV/AIDS program: a retrospective study. PLoS Med. (2020) 17:e1003125. doi: 10.1371/journal.pmed.1003125
20. Bockting W, MacCrate C, Israel H, Mantell JE, Remien RH. Engagement and retention in HIV care for transgender women: perspectives of medical and social service providers in new york city. AIDS Patient Care STDS. (2020) 34:16–26. doi: 10.1089/apc.2019.0067
21. Sevelius J, Patouhas E, Keatley J, Johnson M. Barriers and facilitators to engagement and retention in care among transgender women living with human immunodeficiency virus. Ann Behav Med. (2013) 47:5–16. doi: 10.1007/s12160-013-9565-8
22. Hines DD, Laury ER, Habermann B. They just don't get me: a qualitative analysis of transgender women's health care experiences and clinician interactions. J Assoc Nurses AIDS Care. (2019) 30:e82–95. doi: 10.1097/JNC.0000000000000023
23. Nuttbrock L, Bockting W, Rosenblum A, Hwahng S, Mason M, Macri M, et al. Gender abuse, depressive symptoms, and hiv and other sexually transmitted infections among male-to-female transgender persons: a three-year prospective study. Am J Public Health. (2012). doi: 10.2105/AJPH.2011.300568. [Epub ahead of print].
24. Brennan J, Kuhns LM, Johnson AK, Belzer M, Wilson EC, Garofalo R. Syndemic theory and HIV-related risk among young transgender women: the role of multiple, co-occurring health problems and social marginalization. Am J Public Health. (2012) 102:1751–7. doi: 10.2105/AJPH.2011.300433
25. Operario D, Nemoto T. HIV in transgender communities: syndemic dynamics and a need for multicomponent interventions. J Acquir Immune Defic Syndr. (2010) 55(Suppl 2):S91–93. doi: 10.1097/QAI.0b013e3181fbc9ec
26. Operario D, Soma T, Underhill K. Sex work and HIV status among transgender women: systematic review and meta-analysis. J Acquir Immune Defic Syndr. (2008) 48:97–103. doi: 10.1097/QAI.0b013e31816e3971
27. Bockting WO, Miner MH, Swinburne Romine RE, Hamilton A, Coleman E. Stigma, mental health, and resilience in an online sample of the US transgender population. Am J Public Health. (2013). doi: 10.2105/AJPH.2013.301241. [Epub ahead of print].
28. Nemoto T, Bödeker B, Iwamoto M. Social support, exposure to violence and transphobia, and correlates of depression among male-to-female transgender women with a history of sex work. Am J Public Health. (2011) 101:1980–8. doi: 10.2105/AJPH.2010.197285
29. Nuttbrock L, Hwahng S, Bockting W, Rosenblum A, Mason M, Macri M, et al. Psychiatric impact of gender-related abuse across the life course of male-to-female transgender persons. J Sex Res. (2010) 47:12–23. doi: 10.1080/00224490903062258
30. Stone VE. Optimizing the care of minority patients with HIV/AIDS. Clin Infect Dis. (2004) 38:400–4. doi: 10.1086/380969
31. Maiorana A, Sevelius J, Keatley J, Rebchook G. She is like a sister to me. Gender-affirming services and relationships are key to the implementation of HIV care engagement interventions with transgender women of color. AIDS Behav. (2020):1–12. doi: 10.1007/s10461-020-02777-6. [Epub ahead of print].
32. Tanner AE, Mann-Jackson L, Song EY, Alonzo J, Schafer KR, Ware S, et al. Supporting health among young men who have sex with men and transgender women with HIV: lessons learned from implementing the wecare intervention. Health Promot Pract. (2020) 21:755–63. doi: 10.1177/1524839920936241
33. Wilson EC, Turner C, Arayasirikul S, Woods T, Tryon J, Franza K, et al. Care engagement among trans women of color in san francisco bay area demonstration projects: findings from the brandy martell project and transaccess. AIDS Behav. (2019) 25(Suppl 1):31–9. doi: 10.1007/s10461-019-02697-0
34. Hirshfield S, Contreras J, Luebe RQ, Swartz JA, Scheinmann R, Reback CJ, et al. Engagement in HIV care among new york city transgender women of color: findings from the peer-led, TWEET intervention, a SPNS trans women of color initiative. AIDS Behav. (2019). doi: 10.1007/s10461-019-02667-6
35. Holloway IW, Jordan SP, Dunlap SL, Ritterbusch A, Reback CJ. Leveraging social networks and technology for HIV prevention and treatment with transgender women. AIDS Educ Prev. (2020) 32:83–101. doi: 10.1521/aeap.2020.32.2.83
36. Reback CJ, Kisler KA, Fletcher JB. A novel adaptation of peer health navigation and contingency management for advancement along the HIV care continuum among transgender women of color. AIDS Behav. (2019) 25(Suppl 1):40–51. doi: 10.1007/s10461-019-02554-0
37. Poteat TC, Radix A. HIV antiretroviral treatment and pre-exposure prophylaxis in transgender individuals. Drugs. (2020) 80:965–72. doi: 10.1007/s40265-020-01313-z
38. Tom Waddell Health Center. Protocols for Hormonal Reassignment of Gender. San Francisco: San Francisco Department of Public Health (2006).
39. Sevelius J, Johnson M. A Qualitative Investigation of Barriers to Treatment Initiation and Engagement Among Transgender Women Living with HIV. 8th International Conference on HIV Treatment and Prevention Adherence. Miami Beach, FL (2013).
40. Johnson MO, Dawson Rose C, Dilworth SE, Neilands TB. Advances in the conceptualization and measurement of health care empowerment: development and validation of the health care empowerment inventory. PLoS ONE. (2012) 7:e45692. doi: 10.1371/journal.pone.0045692
41. Johnson MO. The shifting landscape of health care: toward a model of health care empowerment. Am J Public Health. (2011) 101:265–70. doi: 10.2105/AJPH.2009.189829
42. Johnson MO, Sevelius JM, Dilworth SE, Saberi P, Neilands TB. Preliminary support for the construct of health care empowerment in the context of treatment for human immunodeficiency virus. Patient Prefer Adherence. (2012) 6:395–404. doi: 10.2147/PPA.S30040
43. Sevelius J. Gender affirmation: a framework for conceptualizing risk behavior among transgender women of color. Sex Roles. (2012):1–15. doi: 10.1007/s11199-012-0216-5. [Epub ahead of print].
44. Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiatr Res. (2011) 45:626–9. doi: 10.1016/j.jpsychires.2010.10.008
45. Safer DL, Hugo EM. Designing a control for a behavioral group therapy. Behav Ther. (2006) 37:120–30. doi: 10.1016/j.beth.2005.06.001
46. de Bruin M, Viechtbauer W, Schaalma H, Kok G, Abraham C, Hospers H. Standard care impact on effects of highly active antiretroviral therapy adherence interventions: a meta-analysis of randomized controlled trials. Arch Inter Medi. (2010) 170:240–50. doi: 10.1001/archinternmed.2009.536
47. Wesson P, Qabazard RF, Wilson EC, McFarland W, Raymond HF. Estimating the population size of transgender women in San Francisco using multiple methods, 2013. Int J Transgenderism. (2018) 19:107–12. doi: 10.1080/15532739.2017.1376729
48. Lu M, Safren SA, Skolnik PR, Rogers WH, Coady W, Hardy H, et al. Optimal recall period and response task for self-reported HIV medication adherence. AIDS Behav. (2008) 12:86–94. doi: 10.1007/s10461-007-9261-4
49. Walsh JC, Mandalia S, Gazzard BG. Responses to a 1 month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS. (2002) 16:269–77. doi: 10.1097/00002030-200201250-00017
50. Balfour L, Kowal J, Tasca GA, Cooper CL, Angel JB, Macpherson PA, et al. Development and psychometric validation of the HIV treatment knowledge scale. AIDS Care. (2007) 19:1141–8. doi: 10.1080/09540120701352241
51. Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. (1999) 14:1–24. doi: 10.1080/08870449908407311
52. Johnson MO, Catz SL, Remien RH, Rotheram-Borus MJ, Morin SF, Charlebois E, et al. Theory-guided, empirically supported avenues for intervention on HIV medication nonadherence: findings from the healthy living project. AIDS Patient Care STDS. (2003) 17:645–56. doi: 10.1089/108729103771928708
53. Johnson MO, Neilands TB, Dilworth S, Morin SF, Remien RH, Chesney MA. The role of self-efficacy in HIV treatment adherence: validation of the HIV treatment adherence self-efficacy scale (HIV-ASES). J Behav Med. (2007) 30:359–70. doi: 10.1007/s10865-007-9118-3
54. Johnson MO, Dilworth SE, Stephens E, Lum PJ, Neilands TB. Expectancy and readiness-based predictors of treatment uptake among the urban poor living with HIV. J Acquir Immune Defic Syndr. (2011) 58:469–71. doi: 10.1097/QAI.0b013e3182365671
55. Sarason I, Sarason B, Shearin E, Pierce G. A brief measure of social support: practical and theoretical implications. J Soc Pers Relat. (1987) 4:497–510. doi: 10.1177/0265407587044007
56. Chesney M, Neilands T, Chambers D, Taylor J, Folkman S. A validity and reliability study of the coping self-efficacy scale. Br J Health Psychol. (2006) 11(Pt 3):421–37. doi: 10.1348/135910705X53155
57. Galassi JP, Schanberg R, Ware WB. The patient reactions assessment: a brief measure of the quality of the patient∧provider medical relationship. Psychol Assess. (1992) 4:346–51. doi: 10.1037/1040-3590.4.3.346
58. Ende J, Kazis L, Ash A, Moskowitz MA. Measuring patients' desire for autonomy: decision making and information-seeking preferences among medical patients. J Gen Intern Med. (1989) 4:23–30. doi: 10.1007/BF02596485
59. Harvey RM, Kazis L, Lee AF. Decision-making preference and opportunity in VA ambulatory care patients: association with patient satisfaction. Res Nurs Health. (1999) 22:39–48. doi: 10.1002/(SICI)1098-240X(199902)22:1<39::AID-NUR5>3.0.CO;2-J
60. Beach MC, Duggan PS, Moore RD. Is patients' preferred involvement in health decisions related to outcomes for patients with HIV? J Gen Intern Med. (2007) 22:1119–24. doi: 10.1007/s11606-007-0241-1
61. Landrine H, Klonoff E. The schedule of racist events: a measure of racial discrimination and a study of its negative physical and mental health consequences. J Black Psychol. (1996) 22:144–68. doi: 10.1177/00957984960222002
62. Green BL. Psychometric review of trauma history questionnaire (self-report). In: Stamm BH, Varra EM, editors. Measurement of Stress, Trauma and Adaptation. Lutherville, MD: Sidran (1996).
63. Machtinger EL, Haberer JE, Wilson TC, Weiss DS. Recent trauma is associated with antiretroviral failure and HIV transmission risk behavior among HIV-positive women and female-identified transgenders. AIDS Behav. (2012). 16:2160–70. doi: 10.1007/s10461-012-0158-5
64. Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. (1977) 1:385–401.
66. Izard CE, Dougherty F, Bloxom BM, Kotsch NE. The Differential Emotions Scale: A Method of Measuring the Subjective Experience of Discrete Emotions. (1974).
67. Sowell RL, Lowenstein A, Moneyham L, Demi A, Mizuno Y, Seals BF. Resources, stigma, and patterns of disclosure in rural women with HIV infection. Public Health Nurs. (1997) 14:302–12. doi: 10.1111/j.1525-1446.1997.tb00379.x
68. Emlet CA. Measuring stigma in older and younger adults with HIV/AIDS: an analysis of an HIV stigma scale and initial exploration of subscales. Res Soc Work Pract. (2005) 15:291–300. doi: 10.1177/1049731504273250
69. Yudko E, Lozhkina O, Fouts A. A comprehensive review of the psychometric properties of the drug abuse screening test. J Subst Abuse Treat. (2007) 32:189–98. doi: 10.1016/j.jsat.2006.08.002
70. Saunders JB, Aasland OG, Babor TF. de la Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): who collaborative project on early detection of persons with harmful alcohol consumption—II. Addiction. (1993) 88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x
71. Carrico AW, Johnson MO, Moskowitz JT, Neilands TB, Morin SF, Charlebois ED, et al. Affect regulation, stimulant use, and viral load among HIV-positive persons on anti-retroviral therapy. Psychosom Med. (2007) 69:785–92. doi: 10.1097/PSY.0b013e318157b142
72. Lightfoot M, Rogers T, Goldstein R, Rotheram-Borus MJ, May S, Kirshenbaum S, et al. Predictors of substance use frequency and reductions in seriousness of use among persons living with HIV. Drug Alcohol Depend. (2005) 77:129–38. doi: 10.1016/j.drugalcdep.2004.07.009
73. Phinney J. The multigroup ethnic identity measure: a new scale for use with diverse groups. J Adolesc Res. (1992) 7:156–76. doi: 10.1177/074355489272003
74. Johnson MO, Remien RH. Adherence to research protocols in a clinical context: challenges and recommendations from behavioral intervention trials. Am J Psychother. (2003) 57:348–60. doi: 10.1176/appi.psychotherapy.2003.57.3.348
75. Saberi P, Johnson MO. Moving toward a novel and comprehensive behavioral composite of engagement in HIV care. AIDS Care. (2015) 27:660–4. doi: 10.1080/09540121.2014.986052
76. NCSS Statistical Software. NCSS PASS 2008 [Computer Program]. Version 2008. Kaysville, Utah: NCSS Statistical Software (2008).
77. Crosby RA, Rothenberg R. In STI interventions, size matters. Sex Transm Infect. (2004) 80:82–5. doi: 10.1136/sti.2003.007625
78. Vittinghoff E, Sen S, McCulloch CE. Sample size calculations for evaluating mediation. Stat Med. (2009) 28:541–57. doi: 10.1002/sim.3491
79. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) 42:377–81. doi: 10.1016/j.jbi.2008.08.010
80. Johnson MO, Dilworth SE, Neilands TB, Chesney MA, Rotheram-Borus MJ, Remien RH, et al. Predictors of attrition among high risk HIV-infected participants enrolled in a multi-site prevention trial. AIDS Behav. (2008) 12:974–7. doi: 10.1007/s10461-007-9356-y
81. Olem D, Sharp K, Johnson MO. Challenges with engaging participants in behavioral intervention research trials. Open Access J Clin Trials. (2009) 1:17–21. doi: 10.2147/OAJCT.S6841
82. Shadish WR, Luellen JK, Clark MH. Propensity scores and quasi-experiments: a testimony to the practical side of lee sechrest. In: Bootzin RR, McKnight PE, editors. Strengthening Research Methodology: Psychological Measurement and Evaluation. Washington, DC, United States: American Psychological Association (2006): p. 143–57. doi: 10.1037/11384-008
83. Luellen JK, Shadish WR, Clark MH. Propensity scores: an introduction and experimental test. Eval Rev. (2005) 29:530–58. doi: 10.1177/0193841X05275596
84. Rubin DB. On principles for modeling propensity scores in medical research. Pharmacoepidemiol Drug Saf. (2004) 13:855–7. doi: 10.1002/pds.968
85. Weitzen S, Lapane KL, Toledano AY, Hume AL, Mor V. Principles for modeling propensity scores in medical research: a systematic literature review. Pharmacoepidemiol Drug Saf. (2004) 13:841–53. doi: 10.1002/pds.969
86. Seaman S, Copas A. Doubly robust generalized estimating equations for longitudinal data. Stat Med. (2009) 28:937–55. doi: 10.1002/sim.3520
87. Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. (2002) 7:147–77. doi: 10.1037/1082-989X.7.2.147
88. Little JWA, Rubin DA. Statistical Analysis With Missing Data. New York: John Wiley and Sons (1987).
89. Collins LM, Schafer JL, Kam C-M. A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychol Methods. (2001) 6:330–51. doi: 10.1037/1082-989X.6.4.330
90. Graham JW. Adding missing-data relevant variables to FIML-based structural equation models. Struct Equation Model. (2003) 10:80–100. doi: 10.1207/S15328007SEM1001_4
91. Hedeker D, Gibbons RD. Application of random-effects pattern-mixture models for missing data in longitudinal studies. Psychol Methods. (1997) 2:64–78. doi: 10.1037/1082-989X.2.1.64
93. Young ML, Preisser JS, Qaqish BF, Wolfson M. Comparison of subject-specific and population averaged models for count data from cluster-unit intervention trials. Stat Methods Med Res. (2007) 16:167–84. doi: 10.1177/0962280206071931
94. Westfall PH, Young SS. Resmpling Based Multiple Testing: Examples and Methods for P-Value Adjustment. New York, NY: John Wiley and Sons (1993).
95. Hardin J, Hilbe J. Generalized Estimating Equations. New York: Chapman & Hall/CRC (2003). doi: 10.1201/9781420035285
96. Shults J, Sun W, Tu X, Kim H, Amsterdam J, Hilbe JM, et al. Comparison of several approaches for choosing between working correlation structures in generalized estimating equation analysis of longitudinal binary data. Stat Med. (2009) 28:2338–55. doi: 10.1002/sim.3622
97. Pan W. Akaike's information criterion in generalized estimating equations. Biometrics. (2001) 57:120–5. doi: 10.1111/j.0006-341X.2001.00120.x
98. Hammill B, Preisser JS. A SAS/IML software program for GEE and regression diagnostics. Comput Stat Data Anal. (2006) 51:1197–212. doi: 10.1016/j.csda.2005.11.016
99. Kahan BC, Morris TP. Improper analysis of trials randomised using stratified blocks or minimisation. Stat Med. (2012) 31:328–40. doi: 10.1002/sim.4431
100. Valeri L, VanderWeele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. (2013) 18:137–50. doi: 10.1037/a0031034
Keywords: transgender women, gender affirmation, health care empowerment, behavioral composite of engagement in HIV care, virologic control, HIV care engagement, two-arm randomized controlled superiority clinical trial
Citation: Sevelius JM, Neilands TB, Reback CJ, Castro D, Dilworth SE, Kaplan RL and Johnson MO (2021) An Intervention by and for Transgender Women Living With HIV: Study Protocol for a Two-Arm Randomized Controlled Trial Testing the Efficacy of “Healthy Divas” to Improve HIV Care Outcomes. Front. Reprod. Health 3:665723. doi: 10.3389/frph.2021.665723
Received: 25 March 2021; Accepted: 16 September 2021;
Published: 27 October 2021.
Edited by:
Edith Nakku-Joloba, Makerere University, UgandaReviewed by:
Elizabeth Joseph-Shehu, National Open University of Nigeria, NigeriaMaria Pyra, Howard Brown Health Center, United States
Copyright © 2021 Sevelius, Neilands, Reback, Castro, Dilworth, Kaplan and Johnson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jae M. Sevelius, amFlLnNldmVsaXVzQHVjc2YuZWR1
 Jae M. Sevelius
Jae M. Sevelius Torsten B. Neilands1
Torsten B. Neilands1 Rachel L. Kaplan
Rachel L. Kaplan