
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Reprod. Health , 06 April 2021
Sec. Gynecology
Volume 3 - 2021 | https://doi.org/10.3389/frph.2021.661360
This article is part of the Research Topic Highlights in Gynecology 2021/22 View all 5 articles
 Mira Mousa1
Mira Mousa1 Moamar Al-Jefout2,3
Moamar Al-Jefout2,3 Habiba Alsafar4,5
Habiba Alsafar4,5 Shona Kirtley6
Shona Kirtley6 Cecilia M. Lindgren7,8
Cecilia M. Lindgren7,8 Stacey A. Missmer9,10
Stacey A. Missmer9,10 Christian M. Becker1†
Christian M. Becker1† Krina T. Zondervan1,11†
Krina T. Zondervan1,11† Nilufer Rahmioglu1,11*†
Nilufer Rahmioglu1,11*†Introduction: High prevalence of gynecological conditions in women of Middle Eastern origin is reported, likely due to regional risk factors and mediators. The objective of this systematic review and meta-analysis is to investigate the prevalence of polycystic ovary syndrome (PCOS), endometriosis, uterine fibroids, and adenomyosis in women of Middle Eastern origin.
Methods: MEDLINE, EMBASE, PsycINFO, Global Health, and Google Scholar databases were searched from database inception until 14 February 2021 to identify relevant studies. Peer-reviewed research articles that reported the prevalence of PCOS, endometriosis, uterine fibroids, and adenomyosis in the Middle Eastern population were written in English or Arabic. The primary outcome was the estimated pooled prevalence of PCOS, endometriosis, uterine fibroids, and adenomyosis in the Middle Eastern populations. The secondary outcome was to assess the evidence in the data for the presence of heterogeneity, by conducting subtype-pooled analysis of prevalence estimates of the conditions. Total weighted prevalence was calculated via Freeman–Tukey arcsine transformation and heterogeneity through the I2 statistic. Quality control was performed using GRADE criteria.
Results: A total of 47 studies, 26 on PCOS, 12 on endometriosis, eight on uterine fibroids, and seven on adenomyosis, were included. The pooled prevalence of PCOS diagnosed according to the NIH criteria was 8.9% (95% CI: 6.5–11.7; prevalence range: 4.0–27.6%), with a higher prevalence from the Gulf Arab states (18.8%, 95% CI: 9.5–30.3; range: 12.1–27.6%). According to the Rotterdam criteria, the pooled prevalence of PCOS was 11.9% (95% CI: 7.1–17.7; range: 3.4–19.9%) with studies limited to the Persian and Levant regions. Endometriosis was diagnosed in 12.9% (95% CI: 4.2–25.4; range: 4.2–21.0%) of women undergoing laparoscopy, for any indication. Uterine fibroid and adenomyosis prevalence of women was 30.6% (95% CI: 24.9–36.7; range: 18.5–42.6%) and 30.8% (95% CI: 27.1–34.6, range: 25.6–37.7%), respectively. Heterogeneity was present between studies due to statistical and methodological inconsistencies between studies, and quality of evidence was low due to sample size and unrepresentative participant selection.
Conclusion: This is the first review that has reported the prevalence of gynecological diseases in the Middle Eastern population, suggesting that gynecological morbidity is a public health concern. Due to the health disparities in women, further research is required to understand the relative roles of environmental and genetic factors in the region to serve as a benchmark for evaluation and comparative purposes with other populations.
Polycystic ovary syndrome (PCOS), endometriosis, uterine fibroids, and adenomyosis are common benign gynecological conditions that affect women of reproductive age. They are often associated with dysfunctional uterine bleeding, pelvic pain, subfertility, psychological morbidity, and comorbid diseases (1–4). Genetic and environmental factors contribute to the risk of gynecological conditions, but none are currently specific enough to be clinically relevant. In addition, little is known regarding the reasons for the heterogeneity in symptomatology and contributing factors attributable to gynecological conditions in different populations of women, especially women of Middle Eastern origin. Table 1 summarizes the clinical epidemiology of PCOS, uterine fibroids, endometriosis, and adenomyosis, based on global statistics of mainly European ancestry populations.
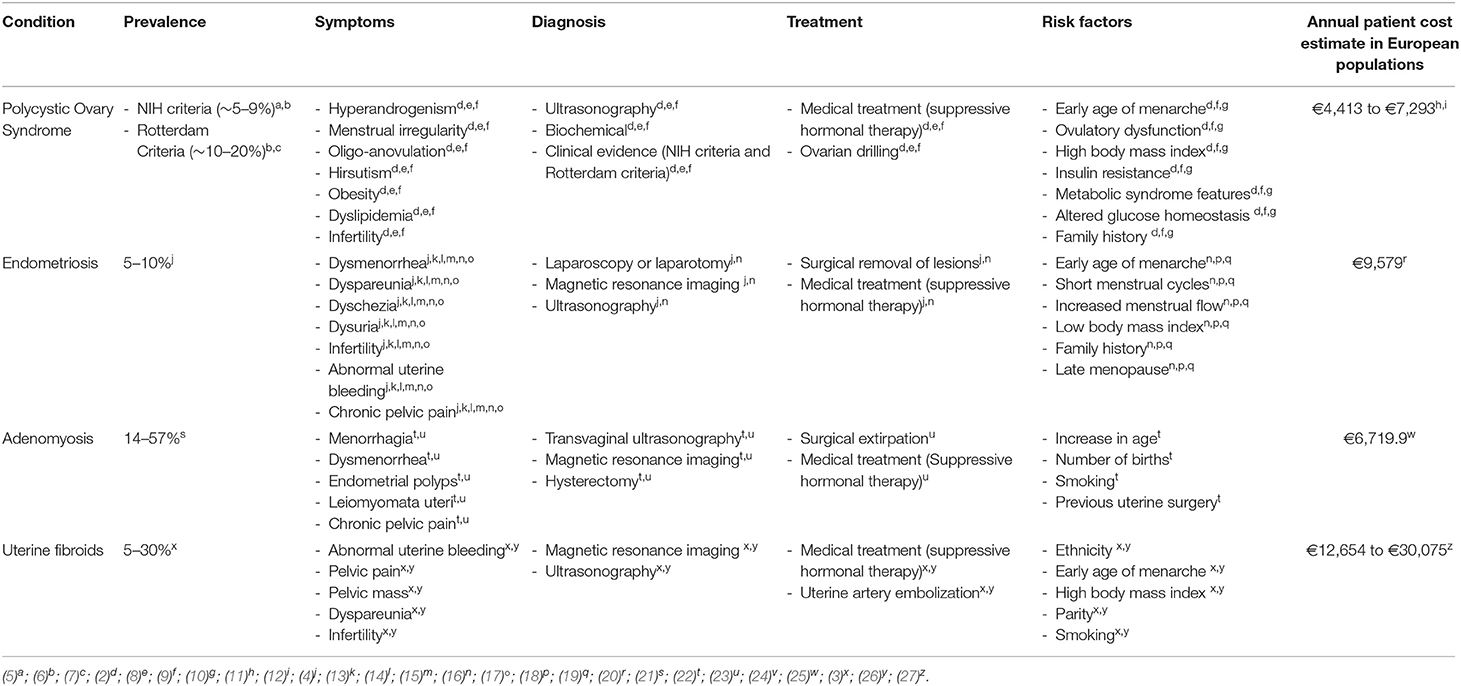
Table 1. Prevalence estimates, symptoms, diagnostic criteria, treatment method, risk factors, and average annual cost estimate of PCOS, endometriosis, adenomyosis, and uterine fibroids.
The Middle East represents 6.8% of the world's population (23 countries), yet it contributes <1% toward scientific research (28–31). Cross-population comparisons and regional differences, influenced by geographic, cultural, socioeconomic, genetic, and environmental factors, may alter health outcome measures associated with prevalence, symptomatology, diagnosis, and management of gynecological phenotypes. A high prevalence of gynecological conditions has been suggested in women of Middle Eastern origin, possibly due to consanguinity, obesity rates, environmental toxins from war exposure, and lack of awareness of reproductive health among adolescence (32–37).
The Middle East is a transcontinental region that consists of the Gulf Cooperative Council (GCC) Region (Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, United Arab Emirates, Yemen), the Levant region (Cyprus, Iraq, Israel, Jordan, Lebanon, Palestine, Syria, Turkey), the North African region (Algeria, Egypt, Libya, Morocco, Somalia, Sudan, Tunisia), and the Persian region (Iran). The Middle Eastern populations have different degrees of genetic admixture, and higher prevalence of globally rare genetic variations with a highly conserved gene pool, due to consanguineous marriages and intercontinental migration (38–41). Consanguineous marriages account for 20–50% of marriages in the Arab ancestry Middle Eastern populations, resulting in higher frequency of recessive Mendelian diseases, large prevalence of deleterious genetic missense variants, and community-specific founder mutations (33, 42, 43). Nevertheless, the Middle East has a very diverse ancestral background, due to intercontinental migration between Africa, Asia, and Europe. Nine Middle Eastern countries have ranked highest in the global obesity statistics, with 70–75% of their populations being obese or overweight, with a 2-fold risk among women vs. men (36, 44–48). Physical inactivity and obesity rates in the region have been associated with gynecological diseases and menstrual disorders (49, 50). Additionally, exposure to war and environmental toxins may alter circulating hormone levels and the immune system of women (51).
This systematic review critically assesses the available evidence and quality of epidemiological studies that focus on the prevalence of common benign gynecological diseases in Middle Eastern populations, to inform public health policy and encourage studies into risk factors and prevention. The primary aim was to conduct a systematic review and meta-analysis of the prevalence of polycystic ovary syndrome, endometriosis, uterine fibroids, and adenomyosis in women of Middle Eastern origin. The secondary aim was to assess the evidence in the data for the presence of heterogeneity by conducting subtype-pooled analysis of prevalence estimates of the conditions.
This systematic review was reported in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (52, 53) and registered for inclusion in PROSPERO: CRD42019119804.
The electronic bibliographic databases, MEDLINE, EMBASE, PsycINFO, and GLOBAL HEALTH (through the OVID platform), and Google Scholar database, were searched from database inception until 14 February 2021. Search terms included a combination of free-text or controlled vocabulary terms (MeSH and EMTREE) for gynecological conditions including “Polycystic Ovary Syndrome,” “Endometriosis,” “Uterine Fibroids,” and “Adenomyosis.” We combined these with terms related to “Epidemiology” and “Prevalence” -related terms and terms for the “Middle East” -related countries. No other limits were applied to the search to obtain a comprehensive literature including the maximum possible number of studies. Details of the full search strategy used for the MEDLINE database are provided in Supplementary Text 1.
Peer-reviewed research articles that reported the prevalence of PCOS, endometriosis, uterine fibroids, and adenomyosis in women of Middle Eastern origin were included, in English and Arabic languages. The primary aim was to estimate the pooled prevalence estimate of PCOS, endometriosis, uterine fibroids, and adenomyosis in the Middle East, based on the following diagnostic criteria: NIH (54) and Rotterdam criteria (55) for PCOS; laparoscopic diagnosis for endometriosis; ultrasound sonography, MRI, and/or hysterectomy for adenomyosis; and ultrasound sonography, MRI, and/or hysterectomy for uterine fibroids. The secondary aim was to assess the evidence in the prevalence data for the presence of heterogeneity, in association to the following characteristics: ascertainment criteria (hospital-based/population-based), diagnostic criteria, age group of participants (adolescent/adult), country of origin (GCC region/Levant region/North Africa region/Persian region), type of study design (cross-sectional/cohort study), and availability of health insurance coverage.
The study outcomes to assess the pooled prevalence of PCOS were population-based study with the NIH criteria; population-based study with the Rotterdam criteria; hospital-based study with the Rotterdam criteria for any gynecological indication; and hospital-based study with the Rotterdam criteria for infertility investigation. For the pooled prevalence of endometriosis, distinction was made between population-based studies with a laparoscopic confirmation; hospital-based studies with a laparoscopic confirmation for any gynecological indication; and hospital-based studies with a laparoscopic confirmation for infertility investigation. The pooled prevalence of uterine fibroids and adenomyosis was divided into hospital-based studies with a hysterectomy or imaging diagnosis for any gynecological indication and hospital-based studies with a hysterectomy or imaging diagnosis for abnormal uterine bleeding investigation.
To reduce the risk of selection bias, methodological bias, and heterogeneity, studies were included in the meta-analysis when (i) the sample size involved >100 participants; (ii) diagnostic criteria were described; and (iii) prevalence estimates were reported. Reviews, editorials, and organizational guidelines were excluded to avoid bias toward more frequently cited publications. Case reports/series were excluded given that they use no control group to compare outcomes and have little statistical validity. Conference abstracts were excluded as there was no access to the full report and the studies were not peer-reviewed. To avoid over-representation of cases, when several studies on the same series of participants were published, only the report with the largest sample size was included in the meta-analysis.
Retrieved articles were uploaded to EndNote and duplicates deleted. One reviewer (M.M.) reviewed the full library for relevance. A second reviewer (N.R.) reviewed the retrieved articles; inter-reviewer concordance was checked, and the third reviewer (K.Z.) adjudicated discordant results. A second round of review was conducted, and full texts were assessed for inclusion. Data was extracted into a standard form including study design, participant characteristics, ascertainment method, nationality, diagnostic criteria, confounding factors, prevalence estimates, and outcome measures. Missing data were collected where possible by emailing the corresponding author, or through statistical calculations reported in the Cochrane handbook for systematic reviews (56). All procedures conformed to the guidelines for systematic review and meta-analysis of observational studies in epidemiology: the Meta-analysis Of Observational Studies in Epidemiology Checklist (57).
The quality control, assurance, and bias of the studies were assessed by two independent reviewers (M.M. and N.R.) using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria (58, 59). The quality of evidence was monitored for each study based on risk of bias, imprecision, inconsistency, indirectness, and publication bias and scored on four levels of evidence: very low, low, moderate, and high. The studies and outcomes were rated down for clinical heterogeneity, methodological heterogeneity, unrepresentative control groups, insufficient diagnostic criteria for case definition, lack of adjustment to confounding variables, unclear selection criteria, sample size, confidence interval around effect estimate, and selection bias.
Statistical analyses were conducted using the metaprop function in R (R package: meta, Schwarzer 2008, Version 2.7.1). Meta-analysis was used to synthesize the pooling proportion estimates of the gynecological condition. The Freeman–Tukey double arcsine transformation was applied for normalizing and variance stabilizing of the proportion sampling distribution. This transformation also provided confidence limits of proportions between zero and one. Heterogeneity among studies was assessed via I2 statistic. I2 of 50% or above was designated significant heterogeneity and 30–49% moderate heterogeneity (60). The random-effect model of DerSimonian and Laird was used to estimate the pooled prevalence and its 95% CI, and I2 heterogeneity was calculated for sensitivity analysis. Significant heterogeneity was explored by performing post-hoc subgroup meta-analyses to allow reliable conclusions to be drawn from analyses that involved subgroups. Forest plots demonstrate the pooled prevalence estimates of the meta-analysis results. P < 0.05 was used as the threshold for statistical significance.
The search strategy across all databases retrieved 9,307 studies, 6,754 studies after duplicates were removed (Figure 1); 6,269 failed to meet the inclusion criteria based on abstract review. Following a full-text review of 485 studies, 438 were excluded because they did not meet the inclusion criteria. Of the 47 articles, 26 covered prevalence of PCOS, 12 endometriosis, 8 uterine fibroids, and 7 adenomyosis. There were 25 retrospective studies using electronic medical records and 22 cross-sectional studies; 29 were hospital-based and 18 population-based. There were 14 studies published from the GCC region, 14 studies from the Persian region, 14 studies from the Levant region, and 5 studies from the North African region. Detailed characteristics of the studies included are given in Table 2.
Of the 47 included studies, 27 received a GRADE score of “very low” and 20 studies received a GRADE score of “low” (Supplementary Table 1). The summary of the quality assessment for the studies included in the meta-analysis for each study outcome (N = 11; grouped based on multiple diagnostic criteria, sampling method, and definition of patient group prevalence estimation provided by each study, see Methods) also showed GRADE scores of “very low” and “low” (Table 3).
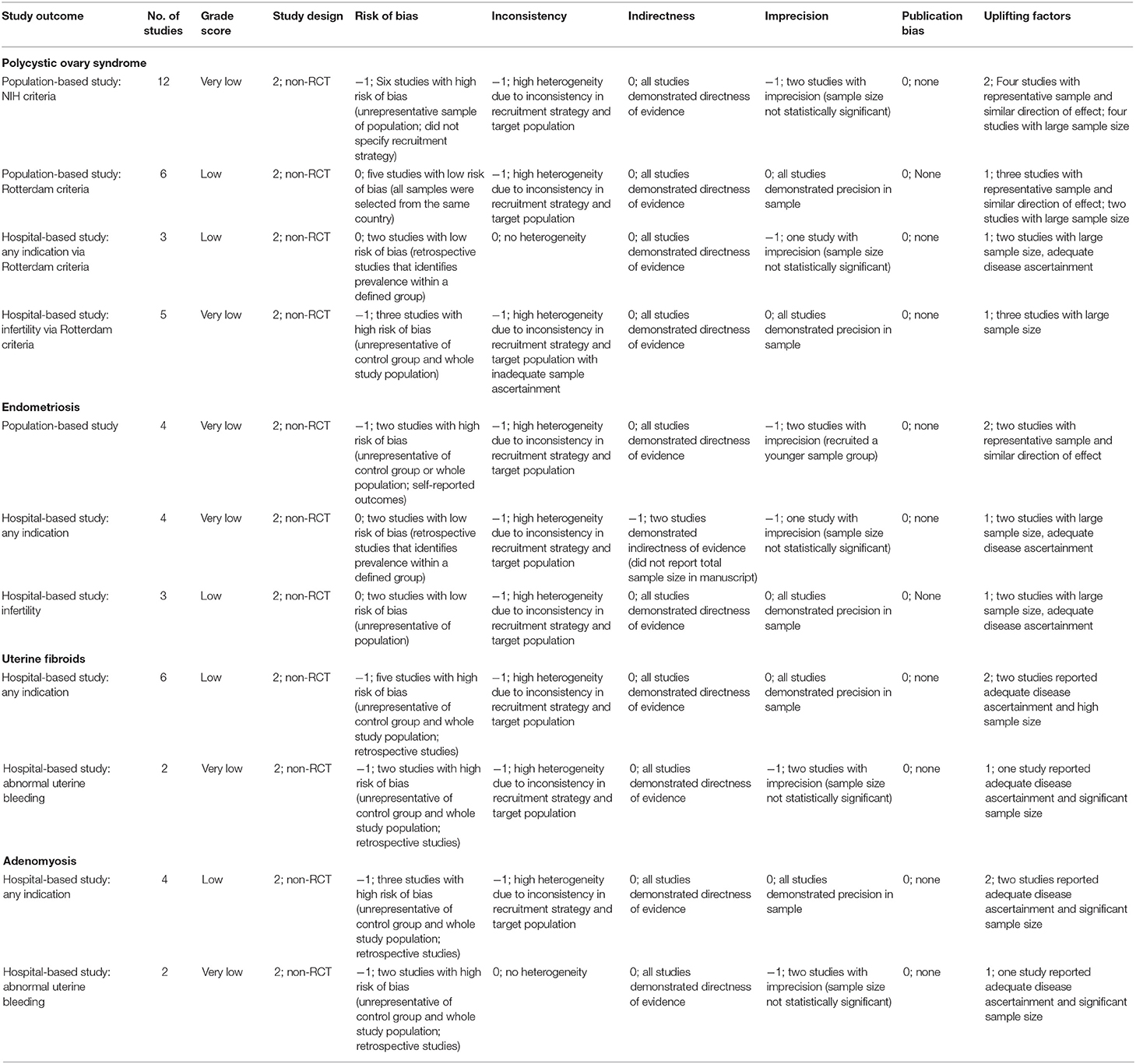
Table 3. Summary of the quality assessment of the studies reporting the outcomes of interest included in the meta-analyses.
Twenty-six studies reported prevalence estimates of PCOS, with eight reporting NIH criteria-based prevalence, 15 Rotterdam criteria-based prevalence, and three studies utilizing both the NIH and Rotterdam criteria (Figure 2). The population-based pooled prevalence of PCOS according to NIH criteria was 8.9% (95% CI: 6.5–11.7; range: 3.0–27.6%) (Figure 2A). Due to high heterogeneity (I2 = 94.0%) observed among the studies, results are presented from a random-effects model. Pooled prevalence of PCOS in the Levant region and Persian region was estimated at the lower range of the scale with 6.3% (95% CI: 4.4–8.6; range: 6.1–7.3%) (6, 91) and 6.7% (95% CI: 4.6–9.1; range: 3.0–11.3%) (69, 71, 74, 75, 77–79), respectively, whereas prevalence estimates in the Gulf Arab states were 18.8% (95% CI: 9.5–30.3; range: 12.0–27.6%) (92, 93, 105). The population-based pooled prevalence according to the Rotterdam criteria was 11.9% (95% CI: 7.1–17.6; range: 3.4–19.9%) but only included studies from Iran and Turkey, from the Persian and Levant regions (Figure 2B). Among post-menarche adolescent women (<18 years of age), the pooled prevalence was 5.6% (95% CI: 1.8–11.3; range: 3.0–8.3%) (76, 107), whereas among pre-menopausal adult women (>18 years of age), it was much higher, at 15.6% (95% CI: 13.6–17.7; range: 14.1–19.9%) (6, 74, 75, 78).
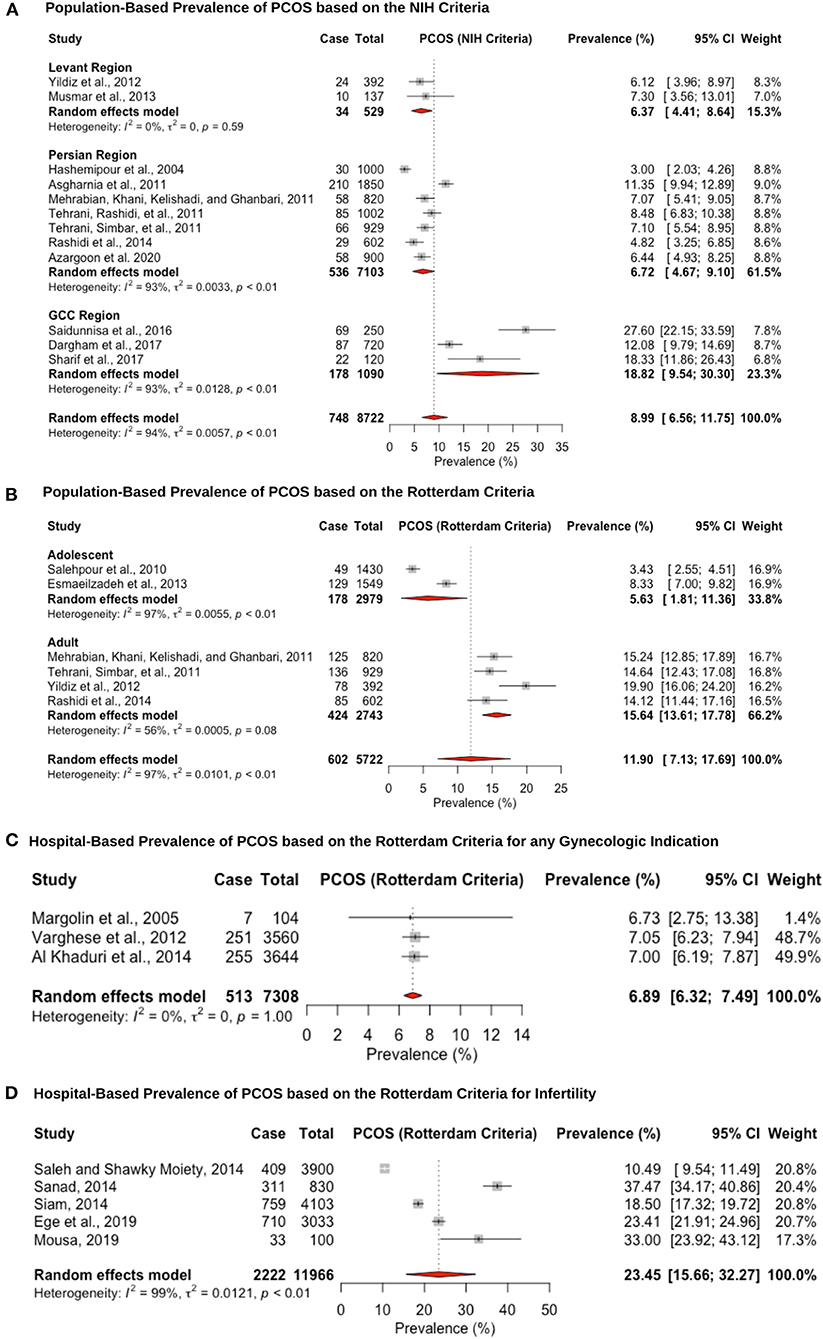
Figure 2. Forest plot of the pooled prevalence estimates of PCOS: (A) population-based study based on the NIH criteria, (B) population-based study based on the Rotterdam criteria, (C) hospital-based study (obstetrics and gynecology department) based on the Rotterdam criteria, and (D) hospital-based study (presenting with infertility) based on the Rotterdam criteria.
The hospital-based prevalence studies of PCOS were categorized into (i) women presenting to obstetrics and gynecology clinics with any indication: 6.8% (95% CI: 6.3–7.4; range: 6.7–7.0%) (Figure 2C) and (ii) infertile women: 23.4% (95% CI: 15.6–32.2; range: 10.4–37.4) (Figure 2D). The three hospital-based studies estimated lower prevalence rates, but one of the studies only recruited post-menopausal women (6.7%, 95% CI: 3.3–13.2) (83), and the other two analyzed medical records retrospectively (7.0%, 95% CI: 6.2–7.9 and 7.0%, 95% CI: 6.1–7.8) (89, 90). The high heterogeneity (I2 = 99%) that was observed for the infertile pooled prevalence estimate is likely due to the fact that two of the studies were recruiting participants from a specialized infertility clinic (10.4%, 95% CI: 9.5–11.44 and 23.4%, 95% CI: 21.9–24.9) (63, 99), whereas the other three studies were recruiting infertile women that were visiting the obstetrics and gynecology department in a University hospital (18.5%, 95% CI: 17.3–19.7; 37.4%, 95% CI: 34.1–40.8 and 33.0%, 95% CI: 23.9–43.1) (64, 65, 80). Hence, the former group may be women with severe PCOS phenotype that require specialized fertility services and assistive reproductive technology, whereas the latter group may be women who require fertility medical treatment. Since hyperandrogenism is part of the Rotterdam criteria, and hirsutism is caused by increased androgenicity in the pilosebaceous gland, the pooled prevalence within the hirsute patients diagnosed with PCOS was very large, at 70.2% (95% CI: 57.7–81.7; range: 62.5–82.1%; I2 = 96%) (67, 87, 96, 103, 108).
Twelve studies reported prevalence estimates of endometriosis diagnosed via laparoscopic surgical visualization, and four out of these 12 reported additional histological confirmation (Figure 3). The pooled prevalence for surgically confirmed endometriosis in population studies, which were validated through surgical records, was estimated at 1.2% (95% CI: 0.9–1.6, range: 0.8–2.4%) (Figure 3A). Two of these studies retrospectively extracted data from large-scale population-based databases in Israel (82) and Middle Eastern women living in Sweden (88). Two further cross-sectional studies in Jordan (84) and the U.A.E. (102) assessed women attending universities who could be affected but given the younger age may not have undergone laparoscopic intervention or sought medical help.
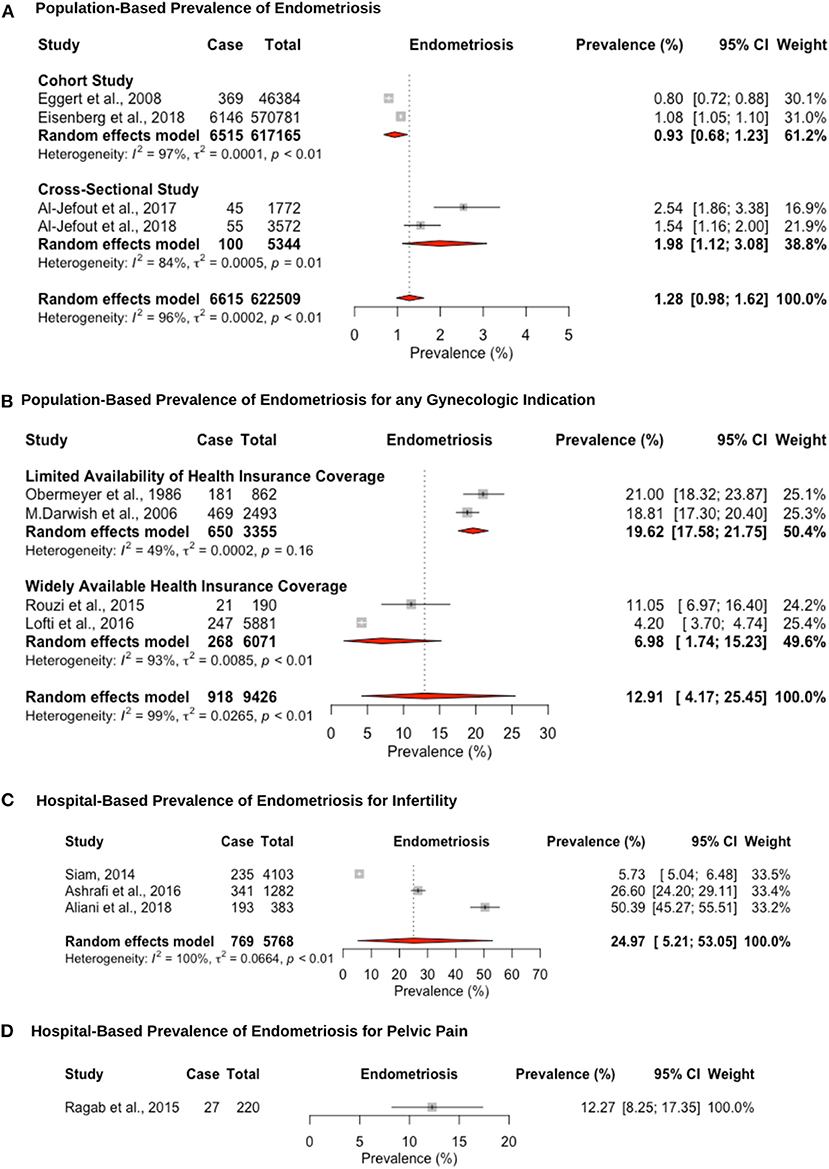
Figure 3. Forest plot of the pooled prevalence estimate of surgically confirmed endometriosis: (A) population-based study, (B) hospital-based study (undergoing laparoscopy for any reason), (C) hospital-based study (presenting with infertility), and (D) hospital-based study (presenting with pelvic pain).
The hospital-based prevalence for studies of endometriosis among women undergoing laparoscopy for unspecified indication was 12.9% (95% CI: 4.1–25.4, range: 4.2–21.0%). However, differences in prevalence estimates were observed based on the availability of healthcare insurance coverage (Figure 3B). Despite the importance of healthcare insurance coverage to provide cost-effective, quality healthcare services, most of the region did not provide or implement insurance coverage for the citizens and residents until the World Health Assembly passed a resolution in 2005 (30, 109, 110). Articles from countries that were published before 2006 had limited healthcare insurance coverage with a pooled prevalence estimate of 19.6% (95% CI: 17.5–21.7; range: 18.8–21.0%) (61, 86), as opposed to a prevalence estimate of 6.9% (95% CI: 1.7–15.2; range: 4.2–11.0%) (84, 102) for articles in countries published after 2007 with highly available private healthcare insurance coverage. Wider healthcare insurance coverage may reflect the lower prevalence estimate for surgically confirmed endometriosis, due to more accessibility to surgical procedures (111). The pooled hospital-based prevalence of endometriosis among infertile women undergoing laparoscopy was higher than for any indication, at 24.9% (95% CI: 5.2–53.0), although study estimates varied widely (range: 5.7–50.3%) (65, 66, 68) (Figure 3C). One large prospective study in Egypt (N = 2,493) showed that infertility is the primary reason for laparoscopic surgery in 80.9% of surgeries, followed by chronic pelvic pain in 12.2% and other indications in 14.5%. Endometriosis was diagnosed in 38.8, 46.6, and 14.5% of these three groups, respectively (61). This suggests that patients are more likely to seek medical treatment due to infertility compared to pelvic pain or other indications, which may be due to limited access of unmarried women to gynecology clinics. The hospital-based prevalence of endometriosis among women undergoing laparoscopy for pelvic pain was only estimated in a single study, at 12.2% (95% CI: 8.2–17.3) (62) (Figure 3D). The prevalence of endometriosis is likely overestimated among women undergoing surgery for pain symptoms and/or infertility; hence, there is an inherent selection bias in these groups compared to the general population.
Endometriosis was staged laparoscopically according to the four stages of the American Society of Reproductive Medicine (ASRM) staging system in 4 out of 12 studies (61, 68, 104, 112). The laparoscopic presentation of stage I/II vs. stage III/IV endometriosis was noted in one study (66). The endometriosis stage was not mentioned in eight of the 12 studies. Pooled prevalence of endometriosis for patients undergoing laparoscopy for any indication, according to the ASRM staging system, was 21.7% (95% CI: 13.8–30.8, I2 = 92.8%) for stage I, 34.1% (95% CI: 25.3–43.4, I2 = 73.7%) for stage II, 26.1% (95% CI: 14.9–39.1, I2 = 96.1%) for stage III, and 15.3% (95% CI: 8.03–24.3) for stage IV. Only one study reported the prevalence of endometriosis, according to ASRM criteria, for patients undergoing laparoscopy for infertility: 15.3% (52/341) for stage I, 24.9% (85/341) for stage II, 32.5% (111/341) for stage III, and 24.6% (84/341) for stage IV (68), and one study for patients undergoing laparoscopy for pelvic pain: 44.4% (12/27) for stage I, 25.9% (7/27) for stage II, and 29.6% (8/27) for stage III (62). Stage was not correlated with pelvic pain or infertility, as reported in previous studies (113).
Eight hospital-based studies estimated the prevalence of uterine fibroids by retrospectively screening electronic medical records of women (Figure 4). Seven studies utilized hysteroscopy as the diagnostic tool, and only one study utilized imaging and clinical assessment to diagnose uterine fibroids. The pooled hospital-based prevalence of uterine fibroids in women undergoing hysterectomy or ultrasound was 30.6% (95% CI: 24.9–36.6; range: 18.5–42.6%) for any indication (Figure 4A). The pooled hospital-based prevalence of uterine fibroids in women undergoing hysterectomy for abnormal uterine bleeding was 57.1% (95% CI: 45.5–68.4; range: 51.0–62.7%) (Figure 4B), while the prevalence of uterine fibroids diagnosed by ultrasound was 21.2% (95% CI: 18.8–23.7).
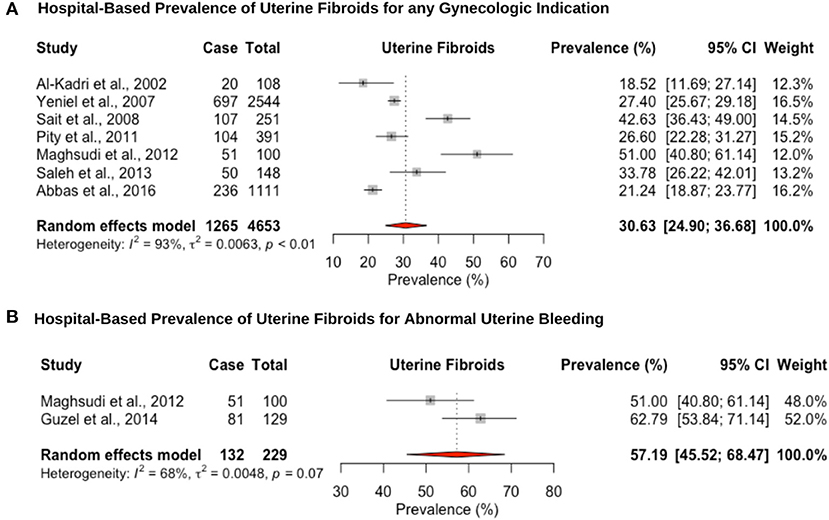
Figure 4. Forest plot of the pooled prevalence estimate of hysterectomy-confirmed uterine fibroids: (A) hospital-based study (undergoing hysteroscopy in a period of time) and (B) hospital-based study (presenting with abnormal uterine bleeding).
Five hospital-based studies reported the prevalence of adenomyosis by retrospectively screening electronic medical records of women attending a hospital for a hysterectomy (Figure 5). The hospital-based prevalence of adenomyosis in women undergoing hysterectomy for any indication was 30.8% (95% CI: 27.1–34.6, range: 25.5–37.7%) (Figure 5A) and 47.2% (95% CI: 40.7–53.7, range: 45.7–49.0%) for heavy menstrual bleeding (Figure 5B). Women diagnosed with adenomyosis often have other associated gynecological diseases, such as endometriosis or uterine fibroids, which complicates differential diagnosis and attribution of symptoms. In addition, no study provided an imaging-based diagnosis that could provide a more representative population-based estimate. While the gold standard for diagnosis has been histopathologic examination of the uterus after hysterectomy, the prevalence may be underestimated due to missed adenomyosis from cases that are not managed by hysterectomy (114).
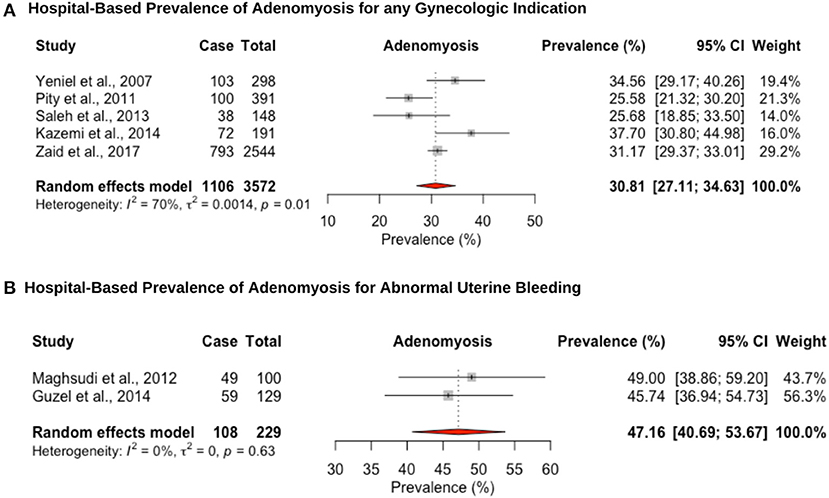
Figure 5. Forest plot of the pooled prevalence estimate of hysterectomy-confirmed adenomyosis: (A) hospital-based study (undergoing hysteroscopy in a period of time) and (B) hospital-based study (presenting with abnormal uterine bleeding).
We conducted the first systematic review and meta-analysis of pooled prevalence of PCOS, endometriosis, uterine fibroids, and adenomyosis in women from the Middle East, a region representing 6.8% of the world's population but underserved in clinical science (28–31). Much of the scientific research featured poorly designed studies and methodological and statistical heterogeneity. The region lacks reliable, population-based data on the major causes of women's health diseases due to limited reporting regulations and lack of national registries (29, 31).
Our findings indicate that the population-based prevalence of PCOS according to NIH and Rotterdam diagnostic criteria in the Middle Eastern populations is estimated at 8.9% (95% CI: 6.5–11.7) and 11.9% (95% CI: 7.1–17.6), respectively, which are similar to the estimated global prevalence (5–9% and 10–20%, respectively) (2). However, among women from the Gulf Region, there was a substantially higher prevalence of PCOS (18.8%; 95% CI: 9.5–30.3) in comparison to women from the Levant (6.3%; 95% CI: 4.4–8.6) and Persian Region (6.7%; 95% CI: 4.6–9.1), based on the NIH criteria. Obesity rates in the Arab Gulf countries exceed those of the remaining Middle Eastern region, with a gender disparity among women, for all age groups (115), where the prevalence of overweight and obesity among adults is 35–48 and 24–40%, respectively (116). Obesity is a common clinical feature in women affected by PCOS, due to its association to the onset of oligomenorrhea and hyperandrogenism (117). Comorbid conditions of PCOS, such as hyperinsulinemia, type 2 diabetes mellitus, cardiovascular disease, and dyslipidemia, have also been reported to have the highest prevalence in the Arab Gulf countries (118–120). Hence, it is likely that the prevalence differences of PCOS between the regions are at least in part due to the pathogenetic role on obesity in the subsequent development of PCOS (117). Analysis of a genome-wide association study (GWAS) on PCOS demonstrated that the relative severity of the PCOS phenotypes, in particular severe hirsute-hyperandrogenism, may result from different combinations of SNPs more commonly present in women of Middle Eastern origin. This may be a basis for the higher prevalence of PCOS that is dominated by obesity, high blood pressure, diabetes mellitus, and hirsutism (121, 122). Genetic influences in populations of diverse origin are important to study alongside environmental influences, to understand the effect of their interaction in causing different PCOS phenotypes (123). Additionally, high heterogeneity was reported due to variability of age group of the participants among studies, where post-menarche adolescent women and post-menopausal women reported a lower prevalence of PCOS compared to pre-menopausal adult women. Adolescents with PCOS manifest similar clinical, metabolic, and endocrine features to adult women (124); hence, the significant difference could be attributed to delayed diagnosis of the condition among young, single women who are unable to access reproductive health care due the hegemony of traditions that may prevent them from discussing their symptoms and seeking care.
Prevalence for surgically confirmed endometriosis (12.9% among women undergoing laparoscopy for any indication; 24.% among infertile women; 12.2% among women hospitalized for pelvic pain; 1.28% in a population-based setting) was similar to European ancestry populations (5–10% among women undergoing laparoscopy; 5–50% among infertile women; 5–21% among women hospitalized for pelvic pain; 2–43% among asymptomatic women seeking tubal ligation) (4, 17, 125). However, the reliance on surgical confirmation of endometriosis, access to clinics that is mostly limited to married women, and consequent selection biases affect these estimates, as is demonstrated by the heterogeneity estimates in our meta-analyses. As the main presenting symptomatology of endometriosis and mode of diagnosis are both influenced by psychosocial factors, women of different cultural backgrounds may present with different clinical phenotypes. Further epidemiological studies must be conducted among women of different populations, to explore the diagnostic pathways for women with endometriosis and be able to obtain an accurate prevalence estimate for the population. In addition, given that endometriosis has an estimated heritability of ~50% and the region has an estimated consanguinity rate of 20–50%, increased prevalence of endometriosis may be mediated by numerous deleterious recessive alleles due to increased homozygosity levels (126–128). Most GWAS datasets have been generated from women of European ancestry, with only two in women of East Asian ancestry. It is important to define the extent of the effects of specific risk alleles in different ethnic groups and conduct trans-ethnic mapping to restrict signal to causal variants of endometriosis (129–131).
The hospital-based pooled prevalence of uterine fibroids for women was estimated at 28.2% (95% CI: 20.4–36.7), vs. 57.1% (95% CI: 45.5–68.4) among women with heavy menstrual bleeding, which are on the high end of the estimated global prevalence 5–30% (3) and 37–59% (132), respectively. Detection bias may explain the overestimation of uterine fibroid prevalence as nearly all studies were from women presenting for hysterectomy. Considering the consistently observed greater risk of uterine fibroids among black women, it is important to conduct further more robust epidemiological and genetic studies in women of Middle Eastern origin, to investigate whether they also exhibit a higher prevalence and whether this is associated with certain risk factors (3, 132, 133). The hospital-based prevalence of adenomyosis for women undergoing hysterectomy was high (any indication: 31.7% [95% CI: 20.6–43.9; for heavy menstrual bleeding: 47.2% (95% CI: 40.7–53.7)], but within the range previously reported for studies assessing prevalence at hysterectomy (14–57%) (21). The restriction of hospital-based studies to women undergoing hysterectomy for adenomyosis likely underestimates the prevalence as tissue may not have been routinely investigated for the condition. In addition, prevalence studies of uterine fibroids and adenomyosis all recruited multiparous women in their fourth and fifth decades of life, making comparison of estimates with global figures difficult. The lack of well-conducted, population-based studies limits the understanding of the public health impact of these conditions and prioritize diagnostic and management pathways.
This is the first systematic review of the prevalence of common gynecological conditions in Middle Eastern women. Study and reporting quality were generally low; however, the moderate/high heterogeneity that were present in the pooled data was reduced after completing subgroup analysis using relevant variables to limit bias. Potential explanations of residual heterogeneity among the studies are likely due to differences in study populations, sampling schemes, and study designs (case definition and ascertainment). Most of the studies selected women through convenience sampling, from hospitals, which may either overestimate the prevalence of the diseases or underestimate the disease risk due to missed asymptomatic cases. Prevalence estimates from retrospectively screened medical records are potentially affected by selection and information biases, as well as methodological issues, such as a lack of specific inclusion and exclusion criteria and data abstraction protocols. Beyond the limitations of the existing literature, there are fundamental issues with the diagnostic method used for the gynecological conditions, which hinders derivation of true population prevalence. The heterogeneous nature of PCOS in terms of its definition, diagnostic criteria, and ethnic variability likely leads to variable PCOS prevalence estimates in different populations. The lack of a non-invasive diagnostic modality for endometriosis creates diagnostic bias for those that are unable to access surgical evaluation. In addition, much of the information available on uterine fibroids and adenomyosis prevalence came from hospital-based studies that lacked imaging and symptomatology-based diagnosis and were not representative of the general population.
Social and cultural factors play a role in diagnostic bias. Given that women in the region will more likely consult a physician for fertility-related issues, as opposed to pain or menstrual irregularities (61), the studies conducted in a hospital-based setting are based on highly selected populations that cannot be generalized broadly. In addition, gynecological conditions and associated clinical phenotypes may present differently in various populations. Given the lack of epidemiological studies in the region focusing on symptomatology and phenotypes, the standard clinical definitions based on mainly Western population health data may not adequately capture the symptom presence and severity affecting diagnostic pathways of these heterogeneous conditions (134, 135). In fact, treatment regimens and responses vary widely between regions and among ethnic groups (136). There is an underrepresentation of studies from low- and middle-income regions in the Middle East that have been subject to war and political instability (Iraq, Syria, Libya, Sudan, Lebanon, Yemen, and Palestine), as opposed to higher-income regions, such as the Gulf countries, Turkey, and Egypt, where women have greater access to health care and may be more intensively screened.
This review highlights the importance of investigating gynecological health in the Middle East, and the need for well-designed studies that address both prevalence and the impact of gynecological condition on women's lives. An extensive search was conducted in two languages, to avoid missing any relevant information. We performed a broad search, including manuscripts from gray literature. Potential sources of bias were identified, and significant clinical and statistical heterogeneity was discussed to indicate considerable variability in the data due to residual confounding and study design. It is essential to prioritize improved and unbiased quantification of gynecological condition prevalence and incidence extrapolation, to improve generalizability and improve public health focus.
Further epidemiological studies must be conducted to investigate symptomatology and risk factors in women of this region, to deliver more culturally sensitive healthcare (137). Specific areas in need of attention include improving the clinical definition, diagnostic techniques, and treatment methods to provide the best practice for this diverse patient population. More generally, given the potential effects of these common chronic conditions on personal lives, economies, healthcare systems, and global community, it must be a public health priority to advance our understanding of the complex pathways leading to diagnosis, develop targeted prevention, and provide early detection guidelines in different populations (138–143). Key to this endeavor is advancing our understanding of how genetic and non-genetic factors interplay to disease predisposition and mechanisms in different populations (144).
To our knowledge, this is the first systematic review study to investigate the prevalence of common gynecological diseases in women in Middle Eastern populations, highlighting a paucity in reliable data. With 200 million women in the region, the importance of conducting more research in the area of women's health is clear and is needed to serve as a benchmark for evaluation of future research activities and comparative purposes with Middle Eastern immigrants and other populations. A gynecology clinic in the Middle East remains the realm mostly of married women. Young women may suffer from chronic pain and menstrual irregularities, but the hegemony of traditions may prevent them from discussing these and thus prolong suffering. To continue to decrease health disparities, researchers should acknowledge the limitations in scientific research that investigate the health disparities in women of Middle Eastern region. Robust data will urge policymakers in the Middle East to invest in health information systems to better identify risk factors for gynecology diseases, help set targets for policymaking, impact public awareness, and enable educational and evaluation strategies.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
MM and NR were involved in all parts of the study: conception, study design, acquisition of data, information extraction, quality assessment, analysis, meta-analysis plots, interpretation, drafting of manuscript, and final approval. KZ and CB were responsible for conception, study design, analysis methods, interpretation, drafting of manuscript, and final approval. SK identified the search terms, developed the search strategies, conducted the search on each database to find relevant studies, reviewed the literature search method section of the manuscript, and did the final approval. MA-J, HA, CL, and SM were involved in study design, interpretation, reviewing of the manuscript, and final approval. All authors contributed to the article and approved the submitted version.
SM has been a member of advisory/scientific boards for AbbVie. CB reports grant from Bayer AG, others from AbbVie Inc., grants from Volition Rx, grants from MDNA Life Sciences, grants from Roche Diagnostics Inc., nonfinancial support from Population Diagnostics Ltd, others from ObsEva, and others from Flo Health, outside the submitted work. KZ reports grants from Bayer Healthcare, MDNA Life Sciences, Roche Diagnostics Inc., Volition Rx, and Evotec (Lab282 - Partnership program with Oxford University), nonfinancial support from AbbVie Ltd, all outside the submitted work, and is a Board member (Secretary) of the World Endometriosis Society and World Endometriosis Research Foundation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frph.2021.661360/full#supplementary-material
2. Azziz R, Carmina E, Chen Z, Dunaif A, Laven JSE, Legro RS, et al. Polycystic ovary syndrome. Nat Rev Dis Prim. (2016) 2:16057. doi: 10.1038/nrdp.2016.57
3. Stewart EA, Laughlin-Tommaso SK, Catherino WH, Lalitkumar S, Gupta D, and Vollenhoven B. Uterine fibroids. Nat Rev Dis Prim. (2016) 2:16043. doi: 10.1038/nrdp.2016.43
4. Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, and Viganò P. Endometriosis. Nat Rev Dis Prim. (2018) 4:9. doi: 10.1038/s41572-018-0008-5
5. Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, and Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. (2016) 106:6–15. doi: 10.1016/j.fertnstert.2016.05.003
6. Yildiz BO, Bozdag G, Yapici Z, Esinler I, and Yarali H. Prevalence, phenotype and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Hum Reprod. (2012) 27:3067–73. doi: 10.1093/humrep/des232
7. Bozdag G, Mumusoglu S, Zengin D, Karabulut E, and Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. (2016) 31:1–15. doi: 10.1093/humrep/dew218
8. Dewailly D, Hieronimus S, Mirakian P, and Hugues J-N. Polycystic ovary syndrome (PCOS). Ann Endocrinol. (2010) 71:8–13. doi: 10.1016/j.ando.2009.12.003
9. Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, and Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. (2015) 36:487–525. doi: 10.1210/er.2015-1018
10. Yen SSC, and Jaffe RB. Yen and Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. Philadelphia, PA: Elsevier Health Sciences (2009).
11. Azziz R, Marin C, Hoq L, Badamgarav E, and Song P. Health care-related economic burden of the polycystic ovary syndrome during the reproductive life Span. J Clin Endocrinol Metab. (2005) 90:4650–8. doi: 10.1210/jc.2005-0628
12. Jason J. Polycystic ovary syndrome in the united states: clinical visit rates, characteristics, and associated health care costs. Arch Intern Med. (2011) 171:1209. doi: 10.1001/archinternmed.2011.288
13. Duignan NM, Jordan JA, Coughlan BM, and Logan-Edwards R. One thousand consecutive cases of diagnostic laparoscopy. BJOG An Int J Obstet Gynaecol. (1972) 79:1016–24. doi: 10.1111/j.1471-0528.1972.tb11880.x
15. Meuleman C, Vandenabeele B, Fieuws S, Spiessens C, Timmerman D, and D'Hooghe T. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners. Fertil Steril. (2009) 92:68–74. doi: 10.1016/j.fertnstert.2008.04.056
16. Missmer SA, and Cramer DW. The epidemiology of endometriosis. Obstet Gynecol Clin North Am. (2003) 30:1–19. doi: 10.1016/S0889-8545(02)00050-5
17. Shafrir AL, Farland LV, Shah DK, Harris HR, Kvaskoff M, Zondervan K, et al. Risk for and consequences of endometriosis: a critical epidemiologic review. Best Pract Res Clin Obstet Gynaecol. (2018) 51:1–15. doi: 10.1016/j.bpobgyn.2018.06.001
18. Prescott J, Farland LV, Tobias DK, Gaskins AJ, Spiegelman D, Chavarro JE, et al. A prospective cohort study of endometriosis and subsequent risk of infertility. Hum Reprod. (2016) 31:1475–82. doi: 10.1093/humrep/dew085
19. Shah DK, Correia KF, Vitonis AF, and Missmer SA. Body size and endometriosis: results from 20 years of follow-up within the Nurses' Health Study II prospective cohort. Hum Reprod. (2013) 28:1783–92. doi: 10.1093/humrep/det120
20. Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. (2012) 27:1292–9. doi: 10.1093/humrep/des073
21. Vercellini P, Viganò P, Somigliana E, Daguati R, Abbiati A, and Fedele L. Adenomyosis: epidemiological factors. Best Pract Res Clin Obstet Gynaecol. (2006) 20:465–77. doi: 10.1016/j.bpobgyn.2006.01.017
22. Ferenczy A. Pathophysiology of adenomyosis. Hum Reprod Update. (1998) 4:312–22. doi: 10.1093/humupd/4.4.312
23. Vercellini P, Chatenoud L, Panazza S, Parazzini F, Crosignani PG, and Oldani S. Risk factors for adenomyosis. Hum Reprod. (2002) 12:1275–9. doi: 10.1093/humrep/12.6.1275
25. Liu XF, Huang LH, Zhang C, Huang GH, Yan LM, and He J. A comparison of the cost–utility of ultrasound-guided high-intensity focused ultrasound and hysterectomy for adenomyosis: a retrospective study. BJOG An Int J Obstet Gynaecol. (2017) 124:40–5. doi: 10.1111/1471-0528.14746
26. Parazzini F, Negri E, La Vecchia C, Chatenoud L, Ricci E, and Guarnerio P. Reproductive factors and risk of uterine fibroids. Epidemiology. (1996) 7:440–2. doi: 10.1097/00001648-199607000-00018
27. Lee DW, Carls GS, Stewart E, Gibson TB, Ozminkowski RJ, and Wang S. What are the total costs of surgical treatment for uterine fibroids? J Women's Heal. (2008) 17:1119–32. doi: 10.1089/jwh.2008.0456
28. World Population Prospects. Available online at: http://www.un.org/en/development/desa/population/
29. Habibzadeh F. Geopolitical changes and trends in middle eastern countries' contributions to world science over the past three decades. Arch Iran Med. (2011) 14:310–1.
30. Batniji R, Khatib L, Cammett M, Sweet J, Basu S, Jamal A, et al. Health in the Arab world: a view from within 1: governance and health in the Arab world. Lancet. (2014) 383:343–55. doi: 10.1016/S0140-6736(13)62185-6
31. Meo SA, Hassan A, Aqil M, and Usmani AM. Medical education research in GCC countries. BMC Med Educ. (2015) 15:8. doi: 10.1186/s12909-015-0293-6
32. Roudi-Fahimi F. Women's reproductive health in the Middle East and North Africa. Popul. Ref. Bur. (2003) 1:4–7.
33. Tadmouri GO, Nair P, Obeid T, Al Ali MT, Al Khaja N, and Hamamy HA. Consanguinity and reproductive health among Arabs. Reprod Health. (2009) 6:17. doi: 10.1186/1742-4755-6-17
34. Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, and Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. (2012) 9:e1001356. doi: 10.1371/journal.pmed.1001356
35. Abdul Rahim HF, Sibai A, Khader Y, Hwalla N, Fadhil I, Alsiyabi H, et al. Non-communicable diseases in the Arab world. Lancet. (2014) 383:356–67. doi: 10.1016/S0140-6736(13)62383-1
36. Alzaman N, and Ali A. Obesity and diabetes mellitus in the Arab world. J Taibah Univ Med Sci. (2016) 11:301–9. doi: 10.1016/j.jtumed.2016.03.009
37. Report: obesity rates by country – 2017. (2017). Renew Bariatrics. Available online at: https://renewbariatrics.com/obesity-rank-by-countries/
38. Fakhro KA, Staudt MR, Ramstetter MD, Robay A, Malek JA, Badii R, et al. The Qatar genome: a population-specific tool for precision medicine in the Middle East. Hum Genome Var. (2016) 3:16016. doi: 10.1038/hgv.2016.16
39. Zayed H. The Arab genome: health and wealth. Gene. (2016) 591:239–43. doi: 10.1016/j.gene.2016.07.007
40. Saxena R, Plenge RM, Bjonnes AC, Dashti HS, Okada Y, Gad El Haq W, et al. A multinational Arab genome-wide association study identifies new genetic associations for rheumatoid arthritis. Arthritis Rheumatol. (2017) 69:976–85. doi: 10.1002/art.40051
41. Al-Ali M, Osman W, Tay GK, and Alsafar HS. A 1000 Arab genome project to study the Emirati population. J Hum Genet. (2018) 63:533–6. doi: 10.1038/s10038-017-0402-y
42. Shawky RM, Elsayed SM, Zaki ME, Nour El-Din SM, and Kamal FM. Consanguinity and its relevance to clinical genetics. Egypt J Med Hum Genet. (2013) 14:157–64. doi: 10.1016/j.ejmhg.2013.01.002
43. Anwar WA, Khyatti M, and Hemminki K. Consanguinity and genetic diseases in North Africa and immigrants to Europe. Eur J Public Health. (2014) 24:57–63. doi: 10.1093/eurpub/cku104
44. Martorell R, Kettel Khan L, Hughes ML, and Grummer-Strawn LM. Obesity in women from developing countries. Eur J Clin Nutr. (2000) 54:247–52. doi: 10.1038/sj.ejcn.1600931
45. Ng SW, Zaghloul S, Ali H, Harrison G, Yeatts K, El Sadig M, et al. Nutrition transition in the United Arab Emirates. Eur J Clin Nutr. (2011) 65:1328–37. doi: 10.1038/ejcn.2011.135
46. Boutayeb A, Boutayeb S, and Boutayeb W. Multi-morbidity of non communicable diseases and equity in WHO Eastern Mediterranean countries. Int J Equity Health. (2013) 12:1–13. doi: 10.1186/1475-9276-12-60
47. Majeed A, El-Sayed AA, Khoja T, Alshamsan R, Millett C, and Rawaf S. Diabetes in the Middle-East and North Africa: an update. Diabetes Res Clin Pract. (2014) 103:218–22. doi: 10.1016/j.diabres.2013.11.008
49. Vrbikova J, and Hainer V. Obesity and polycystic ovary syndrome. Obes Facts. (2009) 2:26–35. doi: 10.1159/000194971
50. Rojas J, Chávez M, Olivar L, Rojas M, Morillo J, Mejías J, et al. Polycystic ovary syndrome, insulin resistance, and obesity: navigating the pathophysiologic labyrinth. Int J Reprod Med. (2014) 2014:1–17. doi: 10.1155/2014/719050
51. Proctor SP, Heeren T, White RF, Wolfe J, Borgos MS, Davis JD, et al. Health status of Persian Gulf War veterans: self-reported symptoms, environmental exposures and the effect of stress. Int J Epidemiol. (1998) 27:1000–10. doi: 10.1093/ije/27.6.1000
52. Moher D, Liberati A, Tetzlaff J, Altman DG, and Group TP. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement (PRISMA 2009 checklist). Public Libr Sci. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
53. Vrabel M. Preferred reporting items for systematic reviews and meta-analyses. Oncol Nurs Forum. (2015) 42:552–4. doi: 10.1188/15.ONF.552-554
54. Zawadski JK, and Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Haseltine JR, editor. Polycystic Ovary Syndrome. Boston, MA: Blackwell Science (1992). pp. 377–84.
55. Rotterdam E-S. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome Rotterdam Consensus on Diagnostic Criteria for PCOS. Fertil Steril. (2004) 81:19–25. doi: 10.1016/j.fertnstert.2003.10.004
56. Higgins J, and Green S, (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (2011). Available online at: http://www.cochrane-handbook.org
57. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson G, Moher D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting - Meta-Analysis of Observational Studies in Epidemiology (MOOSE) Group B. JAMA Neurol. (2000) 283:2008–012. doi: 10.1001/jama.283.15.2008
58. Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, and Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. (2011) 64:380–2. doi: 10.1016/j.jclinepi.2010.09.011
59. Ryan R, and Hill S. How to GRADE the quality of the evidence. Cochrane Consum. Commun. Gr. Vers. (2016) 3:1–24.
60. Higgins JPT, Thompson SG, Deeks JJ, and Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:55. doi: 10.1136/bmj.327.7414.557
61. Darwish AM, Hassanin MS, and Abou Sekkin IA. Epidemiology and risk factors associated with laparoscopically diagnosed typical and atypical endometriosis among Egyptian women. Middle East Fertil Soc J. (2006) 11:196–201.
62. Ragab A, Shams M, Badawy A, Alsammani MA, and Ragab A. Prevalence of endometriosis among adolescent school girls with severe dysmenorrhea: a cross sectional prospective study. Int J Health Sci. (2015) 9:273–81. doi: 10.12816/0024694
63. Saleh HA, and Shawky Moiety FM. Polycystic ovarian syndrome and congenital uterine anomalies: the hidden common player. Arch Gynecol Obstet. (2014) 290:355–60. doi: 10.1007/s00404-014-3193-9
64. Sanad AS. Prevalence of polycystic ovary syndrome among fertile and infertile women in Minia Governorate, Egypt. Int J Gynecol Obstet. (2014) 125:81–2. doi: 10.1016/j.ijgo.2013.09.025
65. Siam S. Gynecologic laparoscopy and reproductive failure: review of 4103 infertile Egyptian women. Middle East Fertil Soc J. (2014) 19:102–6. doi: 10.1016/j.mefs.2013.05.011
66. Aliani F, Ashrafi M, Arabipoor A, Shahrokh-Tehraninejad E, Jahanian Sadatmahalleh S, and Akhond MR. Comparison of the symptoms and localisation of endometriosis involvement according to fertility status of endometriosis patients. J Obstet Gynaecol. (2018) 38:536–42. doi: 10.1080/01443615.2017.1374933
67. Ansarin H, Aziz-Jalali MH, Rasi A, and Soltani-Arabshahi R. Clinical presentation and etiologic factors of hirsutism in premenopausal Iranian women. Arch Iran Med. (2007) 10:7–13.
68. Ashrafi M, Jahanian Sadatmahalleh S, Akhoond MR, and Talebi M. Evaluation of risk factors associated with endometriosis in infertile women. Int J Fertil Steril. (2016) 10:11–21. doi: 10.22074/ijfs.2016.4763
69. Asgharnia M, Mirblook F, and Soltani MA. The prevalence of polycystic ovary syndrome (PCOS) in high school students in Rasht in 2009 according to NIH criteria. Int J Fertil Steril. (2011) 4:156–9.
70. Esmaeilzadeh S, Delavar MA, Amiri M, Khafri S, and Pasha NG. Polycystic ovary syndrome in Iranian adolescents. Int J Adolesc Med Health. (2014) 26:559–65. doi: 10.1515/ijamh-2013-0335
71. Hashemipoura M, Faghihimanib S, Zolfaghary B, Hovsepian S, Ahmadi F, and Haghighi S. Prevalence of polycystic ovary syndrome in girls aged 14–18 years in Isfahan, Iran. Horm Res. (2004) 62:278–82. doi: 10.1159/000081842
72. Kazemi Jaliseh H, Ramezani Tehrani F, Behboudi-Gandevani S, Hosseinpanah F, Khalili D, Cheraghi L, et al. Polycystic ovary syndrome is a risk factor for diabetes and prediabetes in middle-aged but not elderly women: a long-term population-based follow-up study. Fertil Steril. (2017) 108:1078–84. doi: 10.1016/j.fertnstert.2017.09.004
73. Maghsudi A, Mobarakeh M, and Rashidi I. Adenomyosis among samples from hysterectomy due to abnormal uterine bleeding in Ahwaz, southern Iran. Adv Biomed Res. (2012) 1:49–52. doi: 10.4103/2277-9175.100156
74. Mehrabian F, Khani B, Kelishadi R, and Ghanbari E. The prevalence of polycystic ovary syndrome in Iranian women based on different diagnostic criteria. Endokrynol Pol. (2011) 62:238–42.
75. Rashidi H, Ramezani Tehrani F, Bahri Khomami M, Tohidi M, and Azizi F. To what extent does the use of the Rotterdam criteria affect the prevalence of polycystic ovary syndrome? A community-based study from the Southwest of Iran. Eur J Obstet Gynecol Reprod Biol. (2014) 174:100–5. doi: 10.1016/j.ejogrb.2013.12.018
76. Salehpour S, Shirvani HE, and Entezari A. Evaluation of the prevalence of polycystic ovarian syndrome among adolescent (15-18 years old) girls in Tehran during 2005-2006. Int J Fertil Steril. (2010) 4:122–7.
77. Tehrani FR, Rashidi H, and Azizi F. The prevalence of idiopathic hirsutism and polycystic ovary syndrome in the Tehran Lipid and Glucose Study. Reprod Biol Endocrinol. (2011) 9:144. doi: 10.1186/1477-7827-9-144
78. Tehrani FR, Simbar M, Tohidi M, Hosseinpanah F, and Azizi F. The prevalence of polycystic ovary syndrome in a community sample of Iranian population: Iranian PCOS prevalence study. Reprod Biol Endocrinol. (2011) 9:39. doi: 10.1186/1477-7827-9-39
79. Azargoon A, Mirmohammadkhani M, and Borjian S. The prevalence of polycystic ovarian syndrome, metabolic abnormalities and its association with obesity in adolescents: a cross-sectional study in an urban population in Iran. Acta Med Iran. (2020) 58:388–93. doi: 10.18502/acta.v58i8.4589
80. Mousa BA. The prevalence of PCOS in infertile women according to clinical features and its associated hormonal changes in Al-Hilla city, Iraq. Indian J Public Heal Res Dev. (2019) 10:2607–11. doi: 10.5958/0976-5506.2019.03258.3
81. Pity IS Jalal JA Hassawi BA. Hysterectomy: a clinicopathologic study. Tikrit Med J. (2011) 17:7–16.
82. Eisenberg VH, Weil C, Chodick G, and Shalev V. Epidemiology of endometriosis: a large population-based database study from a healthcare provider with 2 million members. BJOG An Int J Obstet Gynaecol. (2018) 125:55–62. doi: 10.1111/1471-0528.14711
83. Margolin E, Zhornitzki T, Kopernik G, Kogan S, Schattner A, and Knobler H. Polycystic ovary syndrome in post-menopausal women - marker of the metabolic syndrome. Maturitas. (2005) 50:331–6. doi: 10.1016/j.maturitas.2004.09.005
84. Al-Jefout M, Nesheiwat A, Odainat B, Alnawaiseh R, and Nedal S. Questionnaire-based prevalence of endometriosis and its symptoms in Jordanian women. Biomed Pharmacol J. (2017) 10:699–706. doi: 10.13005/bpj/1158
85. Saleh SSS, Fram K, and Sumrein I. Indications for a hysterectomy at Jordan University Hospital; a teaching hospital experience. Jordan Med J (2013) 47:201–9. doi: 10.12816/0025815
86. Obermeyer CM, Armenian HK, and Azoury R. Endometriosis in Lebanon: a case-control study. Am J Epidemiol. (1986) 124:762–7. doi: 10.1093/oxfordjournals.aje.a114452
87. Zreik RS, and Nasrallah MP. The prevalence of endocrinopathies among Lebanese women presenting with hirsutism to an endocrine clinic. J Med Liban. (2014) 62:27–32. doi: 10.12816/0002624
88. Eggert J, Li X, and Sundquist K. Country of birth and hospitalization for pelvic inflammatory disease, ectopic pregnancy, endometriosis, and infertility: a nationwide study of 2 million women in Sweden. Fertil Steril. (2008) 90:1019–25. doi: 10.1016/j.fertnstert.2007.07.1345
89. Al Khaduri M, Al Farsi Y, Al Najjar TAA, and Gowri V. Hospital-based prevalence of polycystic ovarian syndrome among Omani women. Middle East Fertil Soc J. (2014) 19:135–8. doi: 10.1016/j.mefs.2013.06.006
90. Varghese U, and Varughese S. Prevalence of polycystic ovarian syndrome in the Buraimi region of Oman. Brunei Int Med J. (2012) 8:248–52.
91. Musmar S, Afaneh A, and Mo'alla H. Epidemiology of polycystic ovary syndrome: a cross sectional study of University students at An-Najah national University-Palestine. Reprod Biol Endocrinol. (2013) 11:1. doi: 10.1186/1477-7827-11-47
92. Dargham SR, Ahmed L, Kilpatrick ES, and Atkin SL. The prevalence and metabolic characteristics of polycystic ovary syndrome in the Qatari population. PLoS ONE. (2017) 12:e0181467. doi: 10.1371/journal.pone.0181467
93. Sharif E, Rahman S, Zia Y, and Rizk NM. The frequency of polycystic ovary syndrome in young reproductive females in Qatar. Int J Womens Health. (2017) 9:1–10. doi: 10.2147/IJWH.S120027
94. Abbas HY, Awad IA, Alharbi E, Alaameri H, Althubaiti S, and Ashkar L. Prevalence and Incidence of Uterine Fibroid at King Abdulaziz University Hospital Saudi Arabia. Clin Med Diagnostics. (2016) 6:45–8. doi: 10.5923/j.cmd.20160603.01
95. Al-Kadri HM, Al-Turki HA, and Saleh AM. Short and long term complications of abdominal and vaginal hysterectomy for benign disease. Saudi Med J. (2002) 23:806–10.
96. Al-Ruhaily AD, Malabu UH, and Sulimani RA. Hirsutism in Saudi females of reproductive age: a hospital-based study. Ann Saudi Med. (2008) 28:28–32. doi: 10.5144/0256-4947.2008.28
97. Rouzi AA, Sahly N, Kafy S, Sawan D, and Abduljabbar H. Prevalence of endometriosis at a university hospital in Jeddah, Saudi Arabia. Clin Exp Obs Gynecol. (2015) 42:785–6. doi: 10.12891/ceog1993.2015
98. Sait K, Alkhattabi M, Boker A, and Alhashemi J. Hysterectomy for benign conditions in a university hospital in Saudi Arabia. Ann Saudi Med. (2008) 28:282–6. doi: 10.5144/0256-4947.2008.282
99. Ege S, Peker N, and Bademkiran MH. The prevalence of uterine anomalies in infertile patients with polycystic ovary syndrome: a retrospective study in a tertiary center in Southeastern Turkey. J Turkish Soc Obstet Gynecol. (2020) 16:224–7. doi: 10.4274/tjod.galenos.2019.62589
100. Guzel AI, Topcu HO, Ekilinc S, Tokmak A, Kokanali MK, Cavkaytar S, et al. Recurrence factors in women underwent laparoscopic surgery for endometrioma. Minerva Chir. (2014) 69:277–82. Available online at: https://www.minervamedica.it/en/journals/minerva-surgery/article.php?cod=R06Y2014N05A0277
101. Yeniel O, Cirpan T, Ulukus M, Ozbal A, Gundem G, Ozsener S, et al. Adenomyosis: prevalence, risk factors, symptoms and clinical findings. Clin Exp Obstet Gynecol (2007) 34:163–7.
102. Al-Jefout M, Alawar S, Balayah Z, Alhareb A, Al Ameri F, Alhosani M, et al. Self-reported prevalence of endometriosis and its symptoms in the United Arab Emirates (UAE). Biomed Pharmacol J. (2018) 11:265–75. doi: 10.13005/bpj/1371
103. Gatee OB, Al Attia HM, and Salama IA. Hirsutism in the United Arab Emirates: a hospital study. Postgrad Med J. (1996) 72:168–71. doi: 10.1136/pgmj.72.845.168
104. Lofti G, Isaac B, Nasir R, and Paulose L. Concurrent, prospective, analytical cohort study of endometriosis patients at Latifa Hospital – Dubai, UAE. J Endometr Pelvic Pain Disord. (2016) 8:19–23. doi: 10.5301/je.5000234
105. Saidunnisa B, Atiqulla S, Ayman G, Mohammad B, Housam R, and Khaled N. Prevalence of polycystic ovarian syndrome among students of rak medical and health sciences University united arab emirates. Int J Med Pharm Sci. (2016) 6:109–18.
106. Zaid SMO, and Ben Thabet MA. Histopathological findings in hysterectomy specimens: a retrospective study. Middle East J Intern Med. (2017) 10:1–8.
107. Rahsepar M, Mahjoub S, Esmaeilzadeh S, Kanafchian M, and Ghasemi M. Evaluation of vitamin D status and its correlation with oxidative stress markers in women with polycystic ovary syndrome. Int J Reprod Biomed. (2017) 15:345–50. doi: 10.29252/ijrm.15.6.345
108. Noorbala MT, and Kefaie P. The prevalence of hirsutism in adolescent girls in Yazd, central Iran. Iran. Red Crescent Med J. (2010) 12:111–7.
109. World Health Assembly Resolution 58.33. Sustainable Health Financing, Universal Coverage and Social Health Insurance. World Health Organization (2005).
110. Asbu EZ, Masri MD, and Kaissi A. Health status and health systems financing in the MENA region: roadmap to universal health coverage. Glob Heal Res Policy. (2017) 2:1–13. doi: 10.1186/s41256-017-0044-9
111. Saavalainen L, Tikka T, But A, Gissler M, Haukka J, Tiitinen A, et al. Trends in the incidence rate, type and treatment of surgically verified endometriosis–a nationwide cohort study. Obstet Gynecol Scand. (2018) 97:59–67. doi: 10.1111/aogs.13244
112. Moini A, Malekzadeh F, Amirchaghmaghi E, Kashfi F, Akhoond MR, Saei M, et al. Risk factors associated with endometriosis among infertile Iranian women. Arch Med Sci. (2013) 9:506–14. doi: 10.5114/aoms.2013.35420
113. Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, and Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. (2007) 22:266–71. doi: 10.1093/humrep/del339
114. Upson K, and Missmer SA. Epidemiology of adenomyosis. Semin Reprod Med. (2020) 38:89–107. doi: 10.1055/s-0040-1718920
115. Ng SW, Zaghloul S, Ali HI, Harrison G, and Popkin BM. The prevalence and trends of overweight, obesity andnutrition-related non-communicable diseases in the Arabian Gulf States. Obes Rev. (2011) 12:1–13. doi: 10.1111/j.1467-789X.2010.00750.x
116. Sulaiman N, Elbadawi S, Hussein A, Abusnana S, Madani A, Mairghani M, et al. Prevalence of overweight and obesity in United Arab Emirates Expatriates: the UAE National Diabetes and Lifestyle Study. Diabetol Metab Syndr. (2017) 9:88. doi: 10.1186/s13098-017-0287-0
117. Gambineri A, Pelusi C, Vicennati V, Pagotto U, and Pasquali R. Obesity and the polycystic ovary syndrome. Int J Obes. (2002) 26:883–96. doi: 10.1038/sj.ijo.0801994
118. Aljefree N, and Ahmed F. Prevalence of cardiovascular disease and associated risk factors among adult population in the Gulf region: a systematic review. Adv Public Heal. (2015) 2015:235101. doi: 10.1155/2015/235101
119. Hamoudi R, Saheb Sharif-Askari N, Saheb Sharif-Askari F, Abusnana S, Aljaibeji H, Taneera J, et al. Prediabetes and diabetes prevalence and risk factors comparison between ethnic groups in the United Arab Emirates. Sci Rep. (2019) 9:17437. doi: 10.1038/s41598-019-53505-7
120. Shin S, and Jee H. Prevalence of metabolic syndrome in the Gulf Cooperation Council countries: meta-analysis of cross-sectional studies. J Exerc Rehabil. (2020) 16:27–35. doi: 10.12965/jer.1938758.379
121. Casarini L, and Brigante G. The polycystic ovary syndrome evolutionary paradox: a genome-wide association studies-based, in silico, evolutionary explanation. J Clin Endocrinol Metab. (2014) 99:E2412–20. doi: 10.1210/jc.2014-2703
122. Afifi L, Saeed L, Pasch LA, Huddleston HG, Cedars MI, Zane LT, et al. Association of ethnicity, Fitzpatrick skin type, and hirsutism: a retrospective cross-sectional study of women with polycystic ovarian syndrome. Int J Women's Dermatol. (2017) 3:21–5. doi: 10.1016/j.ijwd.2017.01.006
123. Escobar-Morreale HF, Luque-Ramírez M, and San Millán JL. The molecular-genetic basis of functional hyperandrogenism and the polycystic ovary syndrome. Endocr Rev. (2005) 26:251–82. doi: 10.1210/er.2004-0004
124. Silfen ME, Denburg MR, Manibo AM, Lobo RA, Jaffe R, Ferin M, et al. Early endocrine, metabolic, and sonographic characteristics of Polycystic Ovary Syndrome (PCOS): comparison between nonobese and obese adolescents. J Clin Endocrinol Metab. (2003) 88:4682–8. doi: 10.1210/jc.2003-030617
125. Ghiasi M, Kulkarni M, and Missmer S. Is endometriosis more common and more severe than it was 30 years ago? J Minim Invasive Gynecol. (2019) 27:452–61. doi: 10.1016/j.jmig.2019.11.018
126. Treloar SA, O'Connor DT, O'Connor VM, and Martin NG. Genetic influences on endometriosis in an Australian twin sample. Fertil Steril. (1999) 71:701–10. doi: 10.1016/S0015-0282(98)00540-8
127. Rudan I, Rudan D, Campbell H, Carothers A, Wright A, Smolej-Narancic N, et al. Inbreeding and risk of late onset complex disease. J Med Genet. (2003) 40:925–32. doi: 10.1136/jmg.40.12.925
128. Saha R, Pettersson HJ, Svedberg P, Olovsson M, Bergqvist A, Marions L, et al. Heritability of endometriosis. Fertil Steril. (2015) 51:3–7. doi: 10.1016/j.fertnstert.2015.06.035
129. Matalliotakis M, Zervou MI, Matalliotaki C, Rahmioglu N, Koumantakis G, Kalogiannidis I, et al. The role of gene polymorphisms in endometriosis. Mol Med Rep. (2017) 16:5881–6. doi: 10.3892/mmr.2017.7398
130. Rahmioglu N, Karina B, Paraskevi C, Rebecca D, and Zondervan KT. Large-scale genome-wide association meta-analysis of endometriosis reveals 13 novel loci and genetically-associated comorbidity with other pain conditions. Biorxiv. (2018). p. 406967. doi: 10.1101/406967
131. Angioni S, D'alterio MN, Coiana A, Anni F, Gessa S, and Deiana D. Genetic characterization of endometriosis patients: review of the literature and a prospective cohort study on a mediterranean population. Int J Mol Sci. (2020) 21:1765. doi: 10.3390/ijms21051765
132. Zimmermann A, Bernuit D, Gerlinger C, Schaefers M, and Geppert K. Prevalence, symptoms and management of uterine fibroids: an international internet-based survey of 21,746 women. BMC Womens Health. (2012) 12:6. doi: 10.1186/1472-6874-12-6
133. Vollenhoven BJ, Lawrence AS, and Healy DL. Uterine fibroids: a clinical review. BJOG An Int J Obstet Gynaecol. (1990) 97:285–98.
134. Bougie O, Yap MI, Sikora L, Flaxman T, and Singh S. Influence of race/ethnicity on prevalence and presentation of endometriosis: a systematic review and meta-analysis. BJOG An Int J Obstet Gynaecol. (2019) 126: 1104–15. doi: 10.1111/1471-0528.15692
135. Williams C, Long AJ, Noga H, Allaire C, Bedaiwy MA, Lisonkova S, et al. East and South East Asian ethnicity and moderate-to-severe endometriosis. J Minim Invasive Gynecol. (2019) 26:507–15. doi: 10.1016/j.jmig.2018.06.009
136. Gerlinger C, Faustmann T, Hassall JJ, and Seitz C. Treatment of endometriosis in different ethnic populations: a meta-analysis of two clinical trials. BMC Womens Health. (2012) 12:9. doi: 10.1186/1472-6874-12-9
137. Hammoud MM, White CB, and Fetters MD. Opening cultural doors: providing culturally sensitive healthcare to Arab American and American Muslim patients. Am J Obstet Gynecol. (2005) 193:1307–11. doi: 10.1016/j.ajog.2005.06.065
138. Smith GD, Chaturvedi N, Harding S, Nazroo J, and Williams R. Ethnic inequalities in health: a review of UK epidemiological evidence. Crit Public Health. (2000) 10:375–408. doi: 10.1080/09581590010005331
139. DeJong J, Jawad R, Mortagy I, and Shepard B. The sexual and reproductive health of young people in the Arab countries and Iran. Reprod Health Matters. (2005) 13:49–59. doi: 10.1016/S0968-8080(05)25181-9
140. World Health Organization. Cross-Cutting Gender Issues in Women's Health in the Eastern Mediterranean Region (2007).
141. Williams DR, Mohammed SA, Leavell J, and Collins C. Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann N Y Acad Sci. (2010) 1186:69–101. doi: 10.1111/j.1749-6632.2009.05339.x
142. Davidson PM, McGrath SJ, Meleis AI, Stern P, DiGiacomo M, Dharmendra T, et al. The health of women and girls determines the health and well-being of our modern world: a white paper from the international council on women's health issues. Health Care Women Int. (2011). doi: 10.1080/07399332.2011.603872
143. Lawrence DS, and Hirsch LA. Decolonising global health: transnational research partnerships under the spotlight. Int Health. (2020) 12:518–23. doi: 10.1093/inthealth/ihaa073
Keywords: gynecological disease, epidemiology, global health, Middle East, polycystic ovary syndrome, endometriosis, uterine fibroids, adenomyosis
Citation: Mousa M, Al-Jefout M, Alsafar H, Kirtley S, Lindgren CM, Missmer SA, Becker CM, Zondervan KT and Rahmioglu N (2021) Prevalence of Common Gynecological Conditions in the Middle East: Systematic Review and Meta-Analysis. Front. Reprod. Health 3:661360. doi: 10.3389/frph.2021.661360
Received: 30 January 2021; Accepted: 22 February 2021;
Published: 06 April 2021.
Edited by:
Laura Buggio, IRCCS Ca 'Granda Foundation Maggiore Policlinico Hospital, ItalyReviewed by:
Umberto Leone Roberti Maggiore, Istituto Nazionale dei Tumori (IRCCS), ItalyCopyright © 2021 Mousa, Al-Jefout, Alsafar, Kirtley, Lindgren, Missmer, Becker, Zondervan and Rahmioglu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nilufer Rahmioglu, bmlsdWZlci5yYWhtaW9nbHVAd3JoLm94LmFjLnVr; orcid.org/0000-0002-5169-8571
†These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.