
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oral. Health, 26 February 2025
Sec. Oral Epidemiology
Volume 6 - 2025 | https://doi.org/10.3389/froh.2025.1535708
This article is part of the Research TopicOral Microbiota and Immune Response in the Elderly: Implications for Systemic HealthView all 3 articles
 Xiaoming Zhang1*†
Xiaoming Zhang1*† Rui Zeng2,†
Rui Zeng2,† Dongmei Ye2
Dongmei Ye2 Mengxia Shi2
Mengxia Shi2 Aizhang Zhu3,4
Aizhang Zhu3,4 Lihuan Chen5
Lihuan Chen5 Tenghui Fan2
Tenghui Fan2 Ke Zhu2
Ke Zhu2 Fayi Xie2
Fayi Xie2 Wan Zhu2
Wan Zhu2 Yufei Zeng3,4
Yufei Zeng3,4 Jiang Wang3,4*
Jiang Wang3,4* Wenwu Zhang1*
Wenwu Zhang1*
Background: The association between tooth loss trajectories and all-cause mortality has not been sufficiently explored. This study aims to examine the relationship between tooth loss trajectories and all-cause mortality in Chinese adults aged 65 years and older.
Methods: This study included 3,726 participants from the Chinese Longitudinal Healthy Longevity Study (CLHLS). The inclusion criteria required participants to be aged 65 years or older, with complete data on tooth count at baseline and at least one follow-up survey. Participants were excluded if they had missing data on death, time to death, or if their they reported tooth count showed an abnormally high increase. The mean age of participants was 85.16 ± 10.7 years. To identify distinct trajectories of tooth loss, growth mixture models (GMM) were employed. Cox regression analysis was utilized to assess the association between tooth loss trajectories and all-cause mortality. Sensitivity analyses were conducted to test the robustness of the findings, while subgroup analyses were performed to explored potential variations in association across different demographic groups.
Results: The prevalence of edentulism at baseline was 37.13%, with a cumulative incidence of 15.8% over 10-year period. Three distinct tooth loss trajectories were identified during follow-up of 9.41 years: (1) progressively mild loss: comprising 312 participants (8.37%); (2) progressively severe loss, comprising 505 participants (13.55%); and (3) edentulism group, comprising 2,909 participants (78.07%). The median follow-up times for each group were 5.91 years, 3.44 years, and 1.84 years, respectively. During the follow-up period, the number of deaths were 114 (36.54%) in the progressively mild loss group, 274 (54.26%) in the progressively severe loss group, and 2,284 (78.51%) in the edentulism group. Compared to the progressively mild loss group, the hazard ratio (HR) for all-cause mortality was 1.29 (95% CI, 1.01–1.64) in the progressively severe loss group, and 1.60 (95% CI, 1.28–1.99) in the edentulism group.
Conclusions: This study identified three distinct tooth loss trajectories among older Chinese adults, with the edentulism group exhibiting the strongest association with all-cause mortality. These findings highlight the crucial importance of maintaining oral health and preserving natural teeth to promote longevity and improve overall health outcomes in older adults.
The global aging population is rapidly increasing, with the number of older adults expected to reach 1.5 billion by 2050 (1). This demographic shift presents significant challenges in managing the health and well-being of elderly individuals, particularly in relation to chronic diseases that commonly arise with aging. Chronic conditions, including cardiovascular diseases, diabetes, and cognitive decline, are prevalent among older adults and are often exacerbated by poor oral health (2). Oral health is a crucial determinant of overall health in older populations, as it directly affects nutrition, social functioning, and systemic health (3).
The importance of oral health among older adults has received increasing attention in geriatric research. Oral diseases, such as periodontitis, dental caries, and gingival inflammation, are prevalent in aging individuals and can lead to significant functional impairments (4). Tooth loss, in particular, is one of the most common consequences of poor oral health in the elderly. Research has demonstrated that tooth loss is not only a marker of oral health but also a predictor of broader health outcomes, including malnutrition, frailty, and cognitive impairment (5, 6). Furthermore, tooth loss is linked to a decline in quality of life, as it affects speech, mastication, and overall self-esteem (7).
Recent research has increasingly emphasized the bidirectional relationship between oral health and systemic health in older adults (8). Chronic oral conditions, such as untreated periodontitis, have been associated with systemic inflammation, which may exacerbate the development of chronic diseases, including diabetes, cardiovascular disease, and even dementia. Conversely, the presence of these systemic diseases can further accelerate the deterioration of oral health, creating a vicious cycle that exacerbates both oral and general health. Therefore, maintaining oral health in older adults is essential not only for preserving dental function but also for preventing or mitigating the onset of other age-related diseases.
Tooth loss has been recognized as a significant risk factor for mortality in older populations (9). Several studies have documented that individual with fewer remaining teeth, or those with complete edentulism, face a higher risk of mortality compared to those with more teeth (10–12). A dose-response relationship has been observed, indicating that the more teeth a person loses, the greater their risk of death (13). Furthermore, recent studies have shown that rapid tooth loss, as opposed to gradual loss, is associated with greater functional disability (14). This finding points to the importance of understanding the trajectory of tooth loss over time, as it may provide valuable insights into the mechanisms through which oral health affects overall mortality risk.
Despite these findings, most studies have primarily focused on the relationship between tooth loss and mortality, typically examining only the baseline number of remaining teeth and its association with mortality outcomes. Few studies have investigated how tooth loss evolves over time or how these trajectories may impact all-cause mortality. The lack of longitudinal research in this area creates a gap in understanding the dynamic nature of oral health in aging populations. Therefore, the aim of this study is to investigate the trajectories of tooth loss over time and explore their association with all-cause mortality, addressing the critical question of whether distinct trajectories of tooth loss exist and how they affect mortality risk.
Data for this study came from the Chinese Longitudinal Healthy Longevity Study (CLHLS), a longitudinal survey of older adults aged 65 years and above, as well as their adult children aged 35–64 years. Participants were categorized into two group based on survival status: surviving respondents and deceased individuals. The CLHLS collects data on a range of factors, including demographic characteristics, socio-economic background, family structure, health status, quality of life self-assessment, cognitive function, and personality and psychological characteristics. The survey also gathers information on the time and cause of death for deceased respondents. CLHLS employs a multistage cluster random sampling method, covering 866 counties and cities across 23 provinces in China. The survey was initially conducted in 1998, with follow-up surgeys in 2000, 2002, 2005, 2008–2009, 2011–2012, 2014, and 2017–2018. For further information, please visit the CLHLS website (https://opendata.pku.edu.cn/dataverse/CHADS). Written informed consent was obtained from all participants prior to their involvement in the study. The study was approved by the Peking University Biomedical Ethics Committee (IRB00001052-13074).
This study utilized longitudinal data from four waves of the CLHLS, beginning with the initial survey conducted in 2008–2009, followed by subsequent follow-ups in 2011–2012, 2014, and 2017–2018. Participants were excluded if they were younger than 65 years or had missing age data (n = 391), missing baseline data on tooth number (n = 36), missing tooth data at both the first and second follow-ups (n = 8,322), abnormally increased tooth number (n = 3,303), or missing death or time-to-death information (n = 1,176). Of the original cohort of 16,954 participants, ranging in age from 39 to 116 years, a total of 3,726 participants were included in the main analysis (Figure 1). To assess the potential impact of selection bias, we compared baseline characteristics and outcome prevalence between the analyzed and excluded participants, as shown in Supplementary Table 3. Additionally, the baseline characteristics of participants lost to follow-up and those included in the study are presented in Supplementary Table 4.
The number of natural teeth was assessed using the question, “How many natural teeth do you have? (excluding false teeth).” Participants who reported having 0 natural teeth were classified as having edentulism. To distinguish between edentulism and edentulism trajectory groups, all participants with edentulism were uniformly categorized into the “edentulism group.” Oral health data, including the number of natural teeth, were self-reported at baseline and during each follow-up every three years. Previous studies have confirmed that self-reported tooth number is a valid and reliable alternative to clinical examinations, as corroborated by comparative studies between clinical and self-reported data (15). Oral health status was self-reported by older adults, with interviewers available to assist participants in confirming the number of natural teeth and dentures used. Despite this, some individuals reported an abnormal increase in the number of natural teeth at subsequent surveys, which led to their exclusion from the analysis. An “abnormally increased tooth number” was defined as a reported increase in natural teeth exceeded the number reported in the previous survey wave. The three follow-up surveys, conducted in 2011–2012, 2014, and 2017–2018, utilized the same questionnaire and measurement methods as the baseline survey, ensuring consistency in data collection across all waves. For participants with exposure data in the first and second waves but missing data in the third wave, exposure was determined by establishing a trajectory using the first and second waves. Similarly, for participants with exposure data in the first and third waves, exposure was established using data from the first, second, and third waves (this methodology also applies to participants with data from only the first and third waves).
Death was primarily determined based on death certificates provided by local authorities. In cases where a death certificate was unavailable, certificates supplied by the deceased's relatives were used. The definitions for outcome events, missing outcomes, and censoring were defined as follows: (1) Occurrence of outcome events: refers to individuals who clearly experienced an outcome event, such as death, during the study period; (2) Missing outcome events: refers to individuals who had missing death data in all subsequent follow-ups after trajectory grouping was identified, meaning they did not provide complete outcome event information during the study; (3) Censoring: refers to individuals who remained free of an outcome event during the follow-up closest to the study cutoff, meaning their outcome event status is unknown but no event occurred during the effective follow-up period after trajectory grouping was determined. In terms of outcome measurement timeline: (1) For participants with exposure data from the first and second waves but no data in the third wave, the second wave visit was used as the start of follow-up; (2) For participants with exposure data from the first and third waves, the third wave visit was used as the start of follow-up. For calculating follow-up time: (a) For participants with an outcome event, follow-up time was defined as the time at which the event occurred during follow-up minus the start of follow-up; (b) For censoring, follow-up time was defined as the time at the follow-up closest to the study cutoff minus the start of follow-up.
Covariates were selected based on a thorough review of the literature, considering of factors that could potentially confound the relationship between tooth number and all-cause mortality. The selected covariates included age (continuous variable) (16), gender (16), education level (17), marital status (18), residence (16), household income (19), living state (20), Current exercise habits (21), smoking (22), drinking (23), BMI category (23), comorbidity (24), and cognitive impairment (25). Detailed information on these covariates is provided in Supplementary Table 1, while missing covariates are depicted in Supplementary Figure 1.
To address missing data in the CLHLS database, we applied the Template method (using the R package “VIM”) (26) and multiple imputation via Predictive Mean Matching (using the R package “mice”) (27). The imputation process consisted of five cycles (m = 5). A density plot was generated to compare the distributions of imputed data across both methods. The final imputed datasets were subsequently used for all analyses. Descriptive statistics are presented as mean (SD) for continuous variables and number (percentage) for categorical variables. We used the Kruskal–Wallis rank sum test for continuous variables and chi-square tests for categorical variables to compare baseline characteristics between the tooth trajectory groups identified through Growth Mixture Models (GMM). GMM was employed to explore different tooth number trajectories (28). GMM is a multilevel statistical technique that enables researchers to identify different distinct subpopulations within a population and estimate different growth trajectories for each subpopulation. GMM integrates the flexibility of multilevel modeling, which account for variation between individuals and time points, with latent class analysis, which identifies distinct subgroup within the population. This combination allows GMM to not only capture average growth trends at the group level but also to uncover and explain heterogeneity within the group.
The GMM was developed by initially fitting a single category and subsequently increasing the number of categories. Optimal models were selected based on the comparison of fit indices, interpretability, and practical significance. The Akaike Information Criteria (AIC), Bayesian Information Criteria (BIC), Sample Size Adjusted BIC (aBIC), entropy index, LoMendel-Rubin Likelihod Ratio Test (LMR), and Bootstrapped Likelihood Ratio Test (BLRT) were include in our study (29). Smaller value of AIC, BIC, and aBIC indicate better model fit. The LMR and BLRT are used to assess whether a significant difference exists between k and k-1 class models, with smaller the p-values suggesting that the k-class model is superior to the k-1 class model (30). Higher entropy value, closer to 1, reflect greater classification accuracy, meaning the model more accurately assigns individuals to the appropriate subpopulations (31). The fit indices presented in Supplementary Table 2 show a gradual decrease in information indices as the number of categories increases, making it difficult to determine the optimal model based solely on these indices. To address this, we utilized the scree plot for model selection (32), with the BIC results displayed in Supplementary Figure 2. The plot reveals three distinct inflection points, supporting the choice of a three-category model. After considering both statistical performance and clinical significance, we ultimately selected the three-category model for its interpretability and clinical significance in clinical settings.
Cox proportional hazards regression was used to assess the association between tooth number trajectories and the risk of all-cause mortality, reporting hazard ratios (HRs) with 95% confidence intervals (CIs). Three models were developed: Model 1 adjusted for age and gender; Model 2 was further adjusted for education level and marital status, while Model 3 included additionally adjustments for smoking, drinking, current exercise behavior, BMI category, comorbidities, cognitive impairment, and baseline number of teeth. To test the robustness of our findings, we performed a sensitivity analyses by excluding 453 participants with heart disease or stroke at baseline to mitigate potential bias from systemic diseases.
We also stratified all-cause mortality by several factors, including age (<80, ≥80), gender (male, female), residence (urban, rural), household income in the previous year (≤10,000, 10,000–≤50,000, >50,000), marital status (married or partnered, other marital status), education level (years) [no school (0 years), primary school (1–6 years), high school or above (at least 7 years)], living situation (with family members or in an institution, alone), smoking status (never, ever, current), drinking status (never, ever, current), current exercise (no, yes), BMI category (<18.5, 18.5–<24, ≥24), comorbidity (none, 1, ≥2), and cognitive impairment (no, yes). This stratification was performed to assess whether potential confounding variables influenced the association between Tooth Loss Trajectories and all-cause mortality and to examine interactions between tooth Loss trajectories and the stratified variables. Statistical analyses were conducted using R version 4.4.1 and EmpowerRCH version 4.0 software, with significance set at p < 0.05.
Table 1 summarizes the demographic and baseline characteristics of the participants, categorized by tooth number trajectories. A total of 3,726 participants were included in the final analysis, with 2,129 (57.14%) being female. The mean age was 85.16 ± 10.70 years, and the average BMI was 20.43 ± 3.51. A subset of 1,130 participants (30.33%) reported engaging in current exercise, while 698 (18.73%) were current drinkers and 701 (18.81%) were smokers. In terms of health conditions, 1,952 (52.39%) had no chronic diseases, 1,138 (30.54%) had one chronic diseases, and 636 (17.07%) had two or above of chronic diseases. Cognitive impairment was reported in 1,465 (39.32%) participants, and 2,672 (71.71%) had died from any cause by the end of the study. The prevalence of edentulism at the baseline survey in 2008 was 37.13%, and the cumulative incidence of edentulism over 10 years was 15.8%. In Figure 2, the blue box represents the baseline prevalence of edentulism in 2008, while the dark blue dashed lines indicate the cumulative incidence of edentulism over the 10-year period.
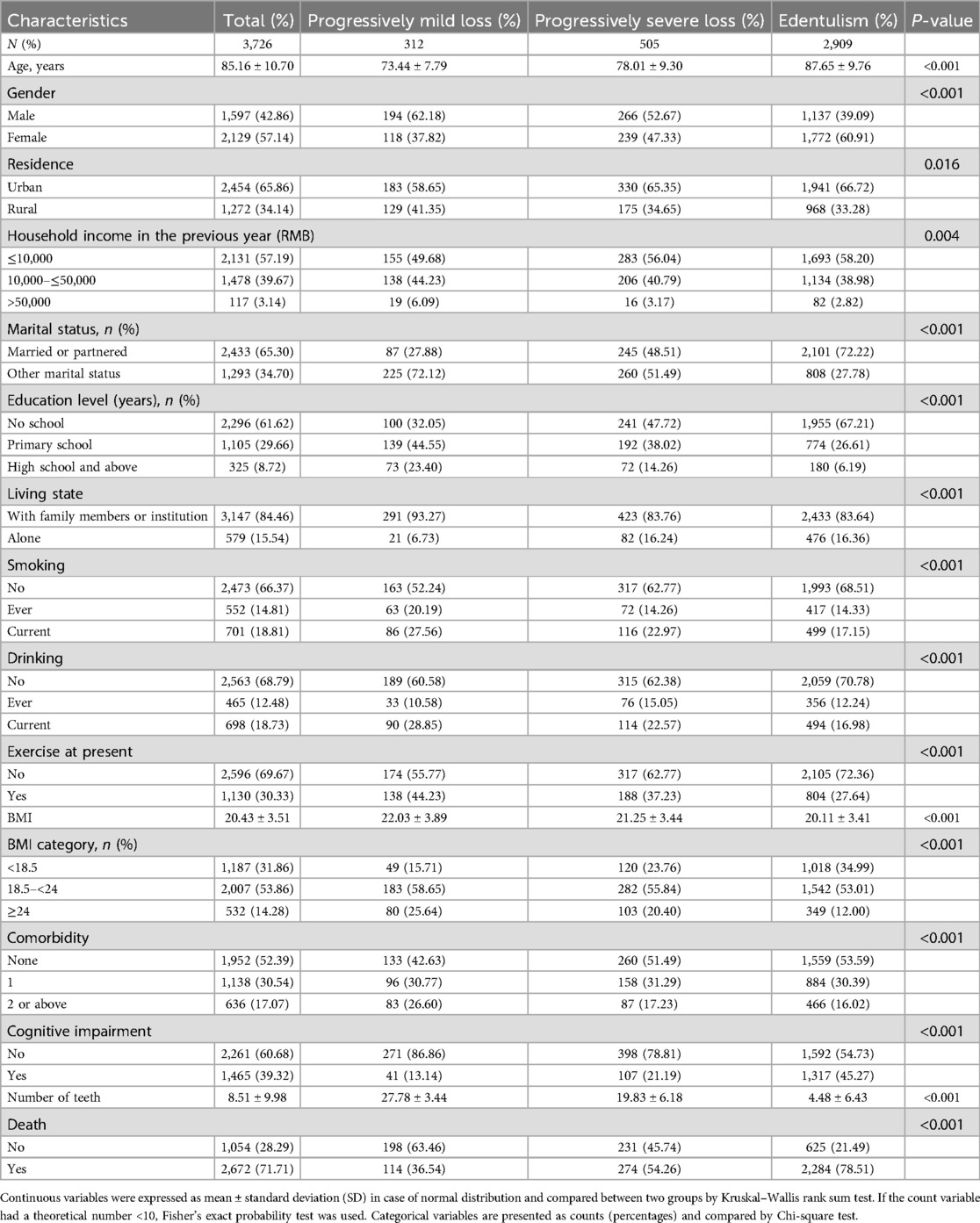
Table 1. Baseline characteristics of study participants by tooth loss trajectories in older adults in China, CLHLS 2008–2014 (N = 3,726).
Compared to participants with progressively mild tooth loss, those in the progressively severe loss and edentulism groups were older, had a lower BMI, were more likely to be female, resided in urban areas, had lower incomes, were married, and had lower rates of cohabitation with family members or institutionalized care. This group also exhibited lower rates of smoking, drinking, and exercisers, but had a higher prevalence of hypertension and cognitive impairment. The characteristics and outcome prevalence between the analyzed and excluded participants are shown in Supplementary Tables 3 and 4. No significant differences were found in gender, living status, BMI category, and comorbidity between the analyzed and excluded participants, However, differences were found in other characteristics and outcome prevalence (p < 0.05). Except for age, residence, income, marital status, and cognitive impairment, the standardized mean difference (SMD) for other characteristics was less than 0.1. For example, the SMD for death was 0.04 (range 0.00–0.08), with 73.56% of excluded participants and 71.71% of those in the study having died, representing 7,076 and 2,672 participants, respectively.
The Growth Mixture Model (GMM) analysis identified distinct latent classes based on the trajectory of tooth loss, with varying classifications. As shown in Supplementary Table 2, the likelihood ratio tests (LMR and BLRT) indicated that the best models were those with 3, 4, and 5 classes. However, because BIC decreases as the number of classes increases without reaching a minimum value, we plotted lithotripsy curve to determine the optimal number of classes (Supplementary Figure 2). The 3-class model was selected as the final model due to its superior fit [AIC = 55,159.909, BIC = 55,247.032, ABIC = 55,202.547, Entropy = 0.959, LMR (p) < 0.001, BLRT (p) < 0.001]. Over 9.41 years of follow-up, three distinct trajectory groups were identified: (1) progressively mild loss (312 participants, 8.37%), (2) progressively severe loss (505 participants, 13.55%), and (3) edentulism (2,909 participants, 78.07%) as shown in Figure 3. The median follow-up times for the progressively mild loss, progressively severe loss, and edentulism groups were 5.91, 3.44, and 1.84 years, respectively. The survival curves across the three groups reveal significant differences in outcome prevalence (P < 0.0001 for all comparisons), as shown in Supplementary Figure 4. The edentulism group exhibited the highest prevalence of outcomes, while the progressively mild loss group showed the lowest. The number of deaths in each group was as follows: progressively mild loss, 114 (36.54%); progressively severe loss, 274 (54.26%); and edentulism, 2,284 (78.51%). The mean age (±SD) for the participants in the progressively mild loss, progressively severe loss, and edentulism groups was 73.44 ± 7.79, 78.01 ± 9.30, and 87.65 ± 9.76 years, respectively, as shown in Table 1. Among those who died, the mean age (±SD) at death for each trajectory group was 77.48 ± 9.08, 81.31 ± 9.32, and 90.8 ± 8.56 years, respectively.
Table 2 presents the results of the univariate analysis conducted over the the period from 2008 to 2018. Significant associations were observed between all-cause mortality and several variables, including age, marital status, education level, smoking, drinking, exercise, Body Mass Index (BMI) category, comorbidity, and cognitive impairment. Although gender did not show a significant univariate association with mortality, it was included as a confounder in the multivariate models. Univariate analysis adjusted for age is presented in Supplementary Table 5.
After adjusting for age (continuous) and gender, cox regression analysis indicated that both the progressively severe loss and edentulism groups had significantly higher risks of all-cause mortality compared to the progressively mild loss group (HR for progressively severe loss = 1.27, 95% CI: 1.00–1.60; HR for edentulism = 1.63, 95% CI: 1.30–2.01). After adjusting for potential confounders, including age (continuous), gender, education level, marital status, smoking, drinking, current exercise, BMI category, comorbidity, cognitive impairment, and baseline number of teeth, the hazard ratios (HRs) for the progressively severe loss and edentulism groups increased further (HR = 1.29, 95% CI: 1.01–1.64; HR = 1.60, 95% CI: 1.28–1.99, respectively), as shown in Table 3. Consistent results were also observed in the sensitivity analysis (Supplementary Table 6).
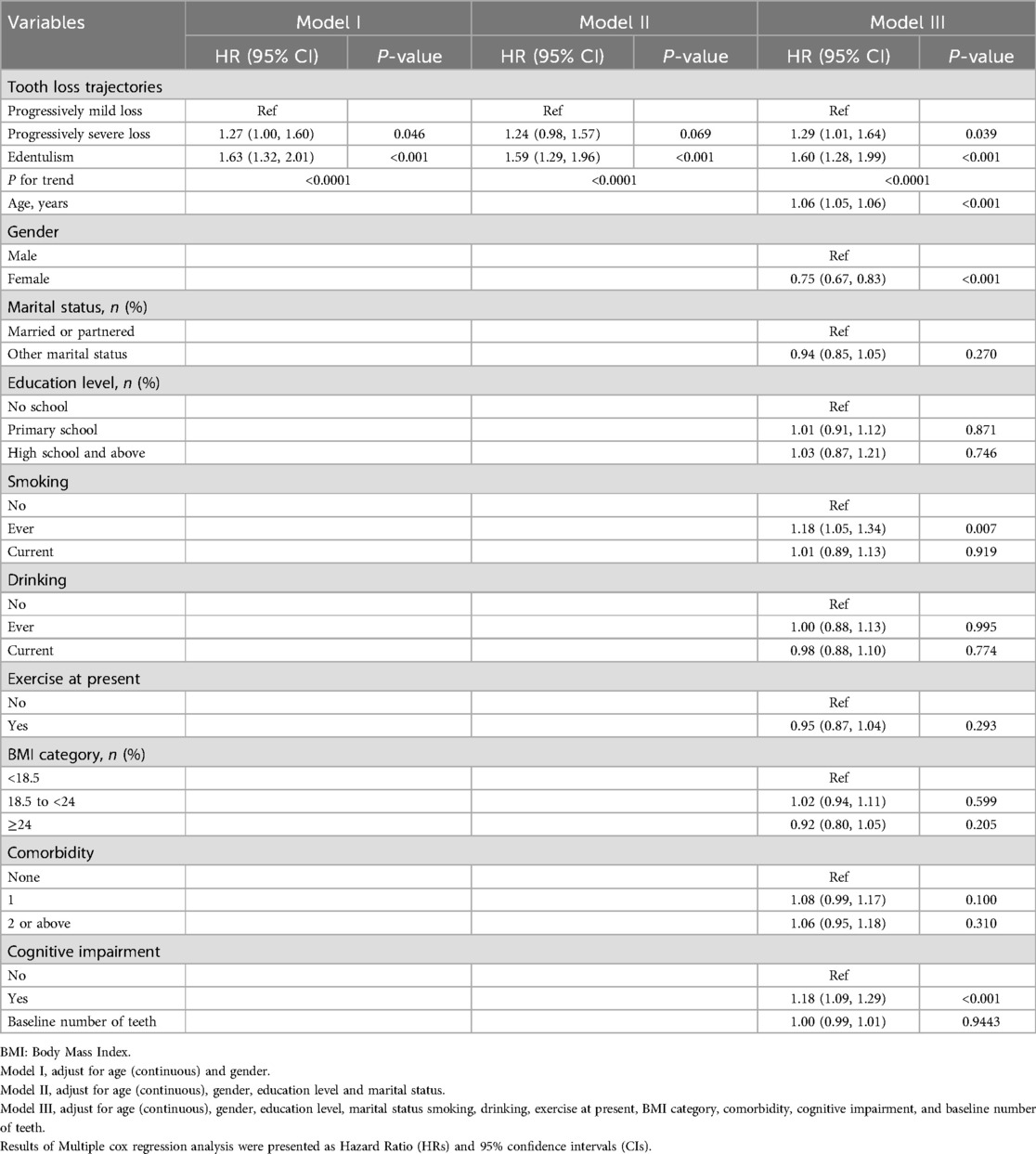
Table 3. Multiple Cox regression analysis association (HRs, 95% CI) between tooth loss trajectories and all-cause mortality.
Subgroup analyses based on the three tooth loss trajectory groups revealed no interaction between tooth loss trajectories and the subgroup variables (Supplementary Figure 3).
This study is the first to examine the association between tooth loss trajectories and all-cause mortality in older Chinese adults. Three distinct trajectories were identified: Progressively Mild Loss (8.24%), Progressively Severe Loss (14.08%), and Edentulism group (77.69%). Compared to individuals with progressively mild tooth loss, those with progressively severe loss or edentulism group were found to have a significantly higher risk of all-cause mortality. These findings emphasize the importance of maintaining oral health and preserving natural teeth to promote longevity among older adults.
At baseline, the prevalence of edentulism in this study was 37.13%. In contrast, previous studies utilizing the China Health and Retirement Longitudinal Study (CHARLS) database reported a prevalence of 15.4% among adults aged 45 and older and 30.5% among the elderly population (33). The discrepancy is likely due to the higher proportion of very elderly individuals in the CLHLS cohort compared to CHARLS. Generally, the prevalence of edentulism increases with age. The cumulative incidence of edentulism over 10 years was 15.8%, a figure of notable public health significance. While high-income countries,such as the United States, have seen a decline in edentulism due to better access to dental care and public health initiatives (34), the prevalence remain high in low- and middle-income countries, including China, particularly in rural and underserved areas. Disparities in dental care access, oral health education, and preventive measures likely contribute to the elevated rates observed in this study. Addressing these disparities requires targeted public health initiatives to enhance oral health awareness and improve access to affordable dental care services, particularly for older adults.
The novel contribution of this study lies in its dynamic depiction of tooth loss trajectories, which we categorized into three distinct patterns: slow decline, rapid decline, and edentulism. While previous research has predominantly focused on baseline tooth count and its correlation with mortality (9, 10), our trajectory-based approach reveals that individuals experiencing rapid tooth loss or edentulism are at a significantly higher risks of all-cause mortality, with the edentulism group exhibiting the highest risk. These findings emphasize the critical importance of early interventions aimed at stabilizing tooth count, which plays a critical role in promoting healthy aging. According to the WHO, retaining at least 20 teeth significantly reduces the risk of adverse clinical outcomes in older populations. Our results are consistent with recent studies (14) linking tooth loss trajectories to negative health outcomes, further highlighting the significance of oral health in supporting overall well-being among older adults.
When comparing our study to Huang et al.'s work, we noted several differences and similarities in the trajectories. Huang et al. Classified participants into four trajectories, while we identified three. This discrepancy may stem from their failure to account for participants with abnormally increased tooth numbers, which likely lead to a larger sample and a different categorization approach. Additionally, Huang et al.'s study may did not clearly delineate the exposure and outcome measurement periods, which resulted in an overlap between the long-term exposure and outcome data. This methodological limitation may complicates the interpretation of reverse causality. Despite these methodological differences, both studies found significant heterogeneity in tooth loss trajectories among older populations.
Tooth loss and edentulism significantly increase the risk of mortality through several interconnected mechanisms. A key factor is impaired masticatory function, which restricts edentulous individuals' ability to maintain a balanced diet, particularly with respect to consuming fiber- and vitamin-rich foods such as fruits, vegetables, and meat (35). These dietary deficiencies lead to inadequate intake of essential nutrients necessary for healthy aging and the prevention of chronic diseases (36). Furthermore, poor oral health in edentulous individuals disrupts the oral microbiota, increasing pathogenic bacteria while reducing beneficial microbes. This imbalance fosters chronic inflammation and bacteremia, thereby exacerbating systemic inflammation, a well-established risk factor for cardiovascular diseases (CVD), which are strongly associated with elevated all-cause mortality (37, 38). In addtion, prosthodontic treatments, including dentures and implants, may introduce potential risks. In China, many older adults rely on low-cost dental services of variable quality, which may result in complications such as infections and implant failures. Even in well-established medical facilities, the surgical risks for elderly patients remain substantial, further influencing long-term health outcomes and mortality (39). Moreover, the co-occurrence of tooth loss with aging-related multimorbidity amplifies the burden of chronic diseases and functional decline, which contributes to an elevated mortality risk. Tooth loss may also reflect the aging process itself, which is intrinsically linked to progressive health deterioration. Socioeconomic factors further exacerbate these risks. Lower socioeconomic status is associated with inadequate access to dental care, reduced health literacy, and disparities in healthcare services. Additionally, tooth loss may reduce social status and income, creating a feedback loop that intensifies health risks and mortality (40). Finally, edentulism has a negative impact on psychosocial well-being, including self-esteem and social engagement. Affected individuals often experience social withdrawal and depression, further diminishing their quality of life and increasing their risk of mortality. Consequently, tooth loss and its associated sequelae render older adults a high-risk population for all-cause mortality.
Our study provides several important clinical implications. First, we observed a significantly increased risk of all-cause mortality in the edentulism and progressively severe tooth loss groups compared to the progressively mild loss group, highlighting the significant association between tooth loss trajectories and all-cause mortality. While previous literature has demonstrated that tooth loss impairs masticatory function and negatively affects mental health and social participation (3), our findings underscore the urgent need for preventive measures. Public health initiatives should prioritize promoting good oral hygiene practices, such as regular brushing with fluoridated toothpaste, interdental cleaning, and routine dental check-ups. Moreover, maintaining overall oral and nutritional health may offer more benefits than focusing solely on tooth retention. Second, the prevalence of edentulism in our study was 37.13%, indicating a high cumulative incidence and a significant proportion of older adults at risk for developing edentulism. These findings highlight the importance of addressing edentulism as a public health concern. In addition to its impact on oral function, edentulism has been linked to increased risks of cardiovascular multimorbidity and all-cause mortality (23). Strengthened oral health education and implementing early intervention strategies by healthcare providers could help prevent the progression of edentulism. Active management of oral health may, therefore, serve as an effective strategy to mitigating mortality risks. Finally, our subgroup analysis showed no statistical interaction between tooth loss trajectories and the risk of all-cause mortality, suggesting that the observed results are generally applicable across different subgroups. Notably, older adults over 80 years of age, those with lower income, lower education levels, current drinking habits, overweight, two or more chronic diseases, or cognitive impairment face higher mortality risks. These elevated risks may be attributed to barriers such as mobility limitations, insufficient oral health knowledge, or cognitive decline. Socioeconomic disadvantages are also strongly associated with higher rates of dental caries (23), a key risk factor for edentulism. Therefore, governments and communities should implement targeted support, such as door-to-door oral care, specialized educational programs, and financial assistance, to help vulnerable populations improve their oral health. For individuals with cognitive impairment or mobility challenges, family support in arranging regular check-ups and treatment, as well as tailored oral care guidance, can significantly enhance healthy aging outcomes. While current evidence from long-term studies on tooth loss and all-cause mortality remains limited, future research involving diverse populations and longitudinal cohorts may yield more robust insights. Such studies could ultimately support the notion of maintaining natural teeth as a key determinant of a healthier and longer lifespan for older adults.
This study has several notable strengths. It identifies distinct tooth loss trajectories and their associations with all-cause mortality, providing valuable insights into disease progression and its long-term implications. Additionally, the study's large, nationally representative sample, with up to 10 years of follow-up, may strengthens its generalizability to the broader population of older Chinese adults. However, several limitations must be acknowledged. First, oral health conditions were self-reported, which may introduce inaccuracies. Although interviewers assisted participants in verifying the number of natural teeth and dentures, some individuals inaccurately reported increases in their number of natural teeth during follow-up, resulting in their exclusion from the analysis. This exclusion may have reduced the sample size and potentially affected the stability of the results. Fortunately, comparisons of baseline characteristics and outcome prevalence between the analyzed and excluded participants offer valuable evidence to assess the potential impact of selection bias. Second, the factors examined in our study are often interconnected with aging and systemic health, which could potentially contribute to mortality and introduce confounding effects. Third, our study specifically focused on natural teeth and did not consider dentures, which may also influence both oral health and overall mortality. Finally, as an observational study, residual confounding cannot be entirely ruled out, despite our efforts to adjust for potential biases in the analysis.
In conclusion, our study confirms three distinct tooth loss trajectories among Chinese older adults, with edentulism showing the strongest association with all-cause mortality. Our findings suggest that maintaining oral health and preventing tooth loss are essential for enhancing quality of life and promoting healthy aging. Edentulism, in particular, emerges as a significant public health concern, not only due to its impact on oral function but also its association with increased risks of cardiovascular disease (CVD), multimorbidity, and mortality. To address these challenges, public health initiatives should prioritize improving oral health education and ensuring access to dental care, especially for older populations. Community-based programs that offer regular dental check-ups, preventive care, and early interventions can help mitigate tooth loss. Special attention should be given to vulnerable populations, including those with low socioeconomic status, cognitive impairment, or limited access to healthcare. Public health campaigns should aim to raise awareness about the importance of oral hygiene and the role it plays in overall health, alongside providing targeted interventions to reduce the risk of tooth loss and its associated outcomes. While our study contributes valuable insights into the relationship between tooth loss and mortality, further research, especially long-term studies in diverse populations, is essential to establish definitive causal links and inform more effective public health strategies for promoting healthier aging.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Peking University Biomedical Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
XZ: Conceptualization, Writing – original draft, Writing – review & editing, Data curation, Investigation. RZ: Writing – original draft, Writing – review & editing, Data curation, Validation, Visualization. DY: Writing – review & editing, Methodology, Software, Validation. MS: Writing – review & editing, Methodology, Software. AZ: Writing – review & editing, Supervision, Validation. LC: Writing – review & editing, Methodology, Software. TF: Writing – review & editing, Investigation, Supervision. KZ: Writing – review & editing, Formal Analysis. FX: Writing – review & editing, Formal Analysis. WaZ: Writing – review & editing, Investigation. YZ: Writing – review & editing, Visualization. JW: Writing – original draft, Writing – review & editing, Conceptualization, Supervision. WeZ: Writing – original draft, Writing – review & editing, Conceptualization, Supervision.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We thank the staffs from the people's hospital of Baoan Shenzhen. We thank the research teams of CLHLS for the study concept and design.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/froh.2025.1535708/full#supplementary-material
CLHLS, Chinese Longitudinal Healthy Longevity Study; GMM, Growth mixture models; AIC, Akaike Information Criteria; BIC, Bayesian Information Criteria; aBIC, Sample Size Adjusted BIC; LMR, LoMendel-Rubin Likelihod Ratio Test; BLRT, Bootstrapped Likelihood Ratio Test; BMI, Body Mass Index; CHARLS, China Health and Retirement Longitudinal Study; CVD, cardiovascular diseases.
1. Jarzebski MP, Elmqvist T, Gasparatos A, Fukushi K, Eckersten S, Haase D, et al. Ageing and population shrinking: implications for sustainability in the urban century. npj Urban Sustain. (2021) 1(1):17. doi: 10.1038/s42949-021-00023-z
2. Guo D, Shi Z, Luo Y, Ding R, He P. Association between oral health behavior and chronic diseases among middle-aged and older adults in Beijing, China. BMC Oral Health. (2023) 23(1):97. (eng. 2.6). doi: 10.1186/s12903-023-02764-y
3. Chan CCK, Chen H, McGrath C, Klineberg I, Wong GHY, Chen H. Impact of social wellbeing on tooth loss and cognition: a scoping review. J Dent. (2024) 150:105376. (eng. 4.8). doi: 10.1016/j.jdent.2024.105376
4. Abe T, Tominaga K, Saito H, Shimizu J, Maeda N, Matsuura R, et al. Effect of oral health on functional disability and mortality in older adults in Japan: a cohort study. Lancet Healthy Longev. (2024) 5:100636. (eng. 13.4). doi: 10.1016/j.lanhl.2024.08.005
5. Li L, Zhang Q, Yang D, Yang S, Zhao Y, Jiang M, et al. Tooth loss and the risk of cognitive decline and dementia: a meta-analysis of cohort studies. Front Neurol. (2023) 14:1103052. (eng. 2.7). doi: 10.3389/fneur.2023.1103052
6. Komiyama T, Gallagher JE, Hattori Y. Relationship between tooth loss and progression of frailty: findings from the English longitudinal study of aging. Arch Gerontol Geriatr. (2024) 127:105572. (eng. 3.5). doi: 10.1016/j.archger.2024.105572
7. Gerritsen AE, Allen PF, Witter DJ, Bronkhorst EM, Creugers NHJ. Tooth loss and oral health-related quality of life: a systematic review and meta-analysis. Health Qual Life Outcomes. (2010) 8:126. (eng. 3.2). doi: 10.1186/1477-7525-8-126
8. Kang J, Wu B, Bunce D, Ide M, Aggarwal VR, Pavitt S, et al. Bidirectional relations between cognitive function and oral health in ageing persons: a longitudinal cohort study. Age Ageing. (2020) 49(5):793–9. (eng. 6.0). doi: 10.1093/ageing/afaa025
9. Yu Y-H, Cheung WS, Steffensen B, Miller DR. Number of teeth is associated with all-cause and disease-specific mortality. BMC Oral Health. (2021) 21(1):568. (eng. 2.6). doi: 10.1186/s12903-021-01934-0
10. Shen R, Chen S, Shen J, Lv L, Wei T. Association between missing teeth number and all-cause and cardiovascular mortality: NHANES 1999–2004 and 2009–2014. J Periodontol. (2024) 95(6):571–81. (eng. 4.2). doi: 10.1002/JPER.23-0277
11. Kim SY, Lee CH, Yoo DM, Kwon MJ, Kim JH, Kim J-H, et al. Is the number of missing teeth associated with mortality? A longitudinal study using a national health screening cohort. Front Med (Lausanne). (2022) 9:837743. (eng. 3.1). doi: 10.3389/fmed.2022.837743
12. Goto Y, Wada K, Uji T, Koda S, Mizuta F, Yamakawa M, et al. Number of teeth and all-cause and cancer mortality in a Japanese community: the Takayama study. J Epidemiol. (2020) 30(5):213–8. doi: 10.2188/jea.JE20180243
13. Peng J, Song J, Han J, Chen Z, Yin X, Zhu J, et al. The relationship between tooth loss and mortality from all causes, cardiovascular diseases, and coronary heart disease in the general population: systematic review and dose-response meta-analysis of prospective cohort studies. Biosci Rep. (2019) 39(1):BSR20181773. (eng. 3.8). doi: 10.1042/BSR20181773
14. Huang G, Cao G. Tooth loss trajectories and their association with functional disability among older Chinese adults: results from the Chinese longitudinal healthy longevity survey. J Evid Based Dent Pract. (2022) 22(4):101771. (eng. 4.1). doi: 10.1016/j.jebdp.2022.101771
15. Margozzini P, Berríos R, Cantarutti C, Veliz C, Ortuno D. Validity of the self-reported number of teeth in Chilean adults. BMC Oral Health. (2019) 19(1):99. (eng. 2.6). doi: 10.1186/s12903-019-0794-5
16. GBD 2021 Causes of Death Collaborators. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the global burden of disease study 2021. Lancet. (2024) 403(10440):2100–32. (eng. 98.4). doi: 10.1016/S0140-6736(24)00367-2
17. Zhou S, Zou G, Chen X, Yu H, Wang J, Fang P, et al. Educational attainment and mortality: results from the sixth population census in China. J Glob Health. (2019) 9(2):020604. (eng. 4.5). doi: 10.7189/jogh.09.020604
18. Leung CY, Huang H-L, Abe SK, Saito E, Islam MR, Rahman MS, et al. Association of marital status with total and cause-specific mortality in Asia. JAMA Netw Open. (2022) 5(5):e2214181. (eng. 10.5). doi: 10.1001/jamanetworkopen.2022.14181
19. Stringhini S, Carmeli C, Jokela M, Avendaño M, Muennig P, Guida F, et al. Socioeconomic status and the 25 × 25 risk factors as determinants of premature mortality: a multicohort study and meta-analysis of 1·7 million men and women. Lancet. (2017) 389(10075):1229–37. (eng. 98.4). doi: 10.1016/S0140-6736(16)32380-7
20. Meijer M, Röhl J, Bloomfield K, Grittner U. Do neighborhoods affect individual mortality? A systematic review and meta-analysis of multilevel studies. Soc Sci Med. (2012) 74(8):1204–12. (eng. 4.9). doi: 10.1016/j.socscimed.2011.11.034
21. Tarp J, Dalene KE, Fagerland MW, Steene-Johannesen J, Hansen BH, Anderssen SA, et al. Physical activity volume, intensity, and mortality: harmonized meta-analysis of prospective cohort studies. Am J Prev Med. (2024) 67:887–96. (eng. 4.3). doi: 10.1016/j.amepre.2024.07.022
22. Dietrich T, Walter C, Oluwagbemigun K, Bergmann M, Pischon T, Pischon N, et al. Smoking, smoking cessation, and risk of tooth loss: the EPIC-Potsdam study. J Dent Res. (2015) 94(10):1369–75. (eng. 5.7). doi: 10.1177/0022034515598961
23. Jepsen S, Blanco J, Buchalla W, Carvalho JC, Dietrich T, Dörfer C, et al. Prevention and control of dental caries and periodontal diseases at individual and population level: consensus report of group 3 of joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J Clin Periodontol. (2017) 44(Suppl 18):S85–93. (eng. 5.8). doi: 10.1111/jcpe.12687
24. Moqri M, Snyder M. Organ-specific aging and the risk of chronic diseases. Nat Med. (2023) 29(5):1068–9. (eng. 58.7). doi: 10.1038/s41591-023-02338-z
25. Hayat SA, Luben R, Dalzell N, Moore S, Hogervorst E, Matthews FE, et al. Understanding the relationship between cognition and death: a within cohort examination of cognitive measures and mortality. Eur J Epidemiol. (2018) 33(11):1049–62. (eng. 7.7). doi: 10.1007/s10654-018-0439-z
26. Templ M, Alfons A, Filzmoser P. Exploring incomplete data using visualization techniques. Adv Data Anal Classif. (2012) 6(1):29–47. doi: 10.1007/s11634-011-0102-y
27. Zhang Z. Multiple imputation with multivariate imputation by chained equation (MICE) package. Ann Transl Med. (2016) 4(2):30. (eng). doi: 10.3978/j.issn.2305-5839.2015.12.63
28. Muthén B, Shedden K. Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics. (1999) 55(2):463–9. (eng. 1.4). doi: 10.1111/j.0006-341X.1999.00463.x
29. Wickrama K, Lee TK, O’Neal CW, Lorenz F. Higher-Order Growth Curves and Mixture Modeling with Mplus: A Practical Guide. 2nd ed. New York: Routledge (2021). p. 346. ISBN: 9781003158769.
30. Kim S-Y. Determining the number of latent classes in single- and multi-phase growth mixture models. Struct Equ Modeling. (2014) 21(2):263–79. (eng. 2.5). doi: 10.1080/10705511.2014.882690
31. Wang M-C, Deng Q, Bi X, Ye H, Yang W. Performance of the entropy as an index of classification accuracy in latent profile analysis: a Monte Carlo simulation study. Acta Psychol Sin. (2017) 49(11):1473–82. doi: 10.3724/SP.J.1041.2017.01473
32. Petras H, Masyn K. General growth mixture analysis with antecedents and consequences of change. In: Piquero AR, Weisburd D, editors. Handbook of Quantitative Criminology. New York, NY: Springer New York (2010). p. 69–100.
33. Li Y, Huang C-L, Lu X-Z, Tang Z-Q, Wang Y-Y, Sun Y, et al. Longitudinal association of edentulism with cognitive impairment, sarcopenia and all-cause mortality among older Chinese adults. BMC Oral Health. (2023) 23(1):333. (eng. 2.6). doi: 10.1186/s12903-023-03015-w
34. Slade GD, Akinkugbe AA, Sanders AE. Projections of U.S. edentulism prevalence following 5 decades of decline. J Dent Res. (2014) 93(10):959–65. (eng. 5.7). doi: 10.1177/0022034514546165
35. Xu K-H, Li L, Jia S-L, Li Q, Hao J-X, Ma S, et al. Association of tooth loss and diet quality with acceleration of aging: evidence from NHANES. Am J Med. (2023) 136(8):773–9. (eng. 5.1). doi: 10.1016/j.amjmed.2023.04.008
36. Zhang J, Xu G, Xu L. Number of teeth and denture use are associated with frailty among Chinese older adults: a cohort study based on the CLHLS from 2008 to 2018. J Nutr Health Aging. (2023) 27(11):972–9. (eng. 4.3). doi: 10.1007/s12603-023-2014-x
37. Teeuw WJ, Slot DE, Susanto H, Gerdes VEA, Abbas F, D'Aiuto F, et al. Treatment of periodontitis improves the atherosclerotic profile: a systematic review and meta-analysis. J Clin Periodontol. (2014) 41(1):70–9. (eng. 5.8). doi: 10.1111/jcpe.12171
38. Lockhart PB, Bolger AF, Papapanou PN, Osinbowale O, Trevisan M, Levison ME, et al. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association?: a scientific statement from the American Heart Association. Circulation. (2012) 125(20):2520–44. (eng. 35.5). doi: 10.1161/CIR.0b013e31825719f3
39. Dickey BL, Gore LR, Slebos R, Sirak B, Isaacs-Soriano KA, Kennedy K, et al. A cross-sectional study of the association of dental health factors with progression and all-cause mortality in men diagnosed with HPV-associated oropharyngeal cancer. BMC Oral Health. (2024) 24(1):433. (eng. 2.6). doi: 10.1186/s12903-024-04047-6
Keywords: all-cause mortality, tooth loss trajectories, older Chinese adults, Growth mixture models (GMM), CLHLS
Citation: Zhang X, Zeng R, Ye D, Shi M, Zhu A, Chen L, Fan T, Zhu K, Xie F, Zhu W, Zeng Y, Wang J and Zhang W (2025) Tooth loss trajectories and their association with all-cause mortality among older Chinese adults. Front. Oral Health 6:1535708. doi: 10.3389/froh.2025.1535708
Received: 27 November 2024; Accepted: 10 February 2025;
Published: 26 February 2025.
Edited by:
Oliver Laugisch, Philipps University, GermanyReviewed by:
Atsushi Takayama, Kyoto University, JapanCopyright: © 2025 Zhang, Zeng, Ye, Shi, Zhu, Chen, Fan, Zhu, Xie, Zhu, Zeng, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoming Zhang, emhhbmdtdXhpMDMxMEAxNjMuY29t; Jiang Wang, OTkyMDA3MDA4MkBqZ3N1LmVkdS5jbg==; Wenwu Zhang, end3NUBzaW5hLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.